Abstract
Chronic fatigue syndrome (CFS) and Myalgic Encephalomyelitis (ME) are debilitating conditions found globally. Yet, most studies on these illnesses include patients from the United States (U.S) and the United Kingdom (U.K.). The current study compares impairment levels of 124 patients living in Japan with 210 patients from the U.S. All patients are from tertiary-care settings that specialize in ME/CFS. The DePaul Symptom Questionnaire and Medical Outcomes Short-Form 36 were completed and used to assess the participants’ symptoms and functional abilities. The U.S. sample showed more impairment in neurocognitive, gastrointestinal and post-exertional malaise symptoms when compared to the Japanese sample. Japanese women demonstrated significantly worse impairment in physical, role-physical, and mental health functioning than Japanese men. Interestingly, Japanese women reported similar functional impairment levels to both men and women in the U.S., despite being less likely to receive disability benefits. These findings may be due to national differences in disability status and gender parity.
INTRODUCTION
Chronic fatigue syndrome (CFS) and Myalgic Encephalomyelitis (ME) are debilitating conditions that include symptoms of neurocognitive difficulties, post-exertional malaise, and unrefreshing sleep. [1] Previous studies estimate that these illnesses’ prevalences are between 0.4% and 1%. [2, 3] Based upon the recommendation of one of this article’s reviewers, this article will refer to these conditions as ME/CFS. Notably, the majority of investigations on the prevalence and symptomology of ME/CFS originate from the United States (U.S.) and the United Kingdom (U.K.). [4] This consequently limits the understanding of the conditions to these countries. Nevertheless, a cross-national comparison study on ME/CFS between the U.S. and U.K. suggests that these illnesses may affect patients differently based on their country of residence. Zdunek et al. [5] found that patients with ME/CFS from the U.K reported working full time and endorsed significantly worse mental health functioning and role-emotional functioning, than the U.S. sample. Additionally, patients from the U.K. experienced more severe symptoms of pain, neurocognitive difficulties, and immune manifestations. [5] Different cultural perceptions about these illnesses and their legitimacy might have driven the discrepancies found between the U.S. and U.K.
Some research has also compared impairment of patients with ME/CFS from other Western countries. In a cross-national examination between the U.S. and Spain, those in Spain were less likely to be on disability, and reported worse impairment in physical, bodily pain, role-emotional, and mental health functioning. [6] The Spanish sample further demonstrated overall higher levels of symptomology. The complexity of the Spanish disability benefits system and healthcare policies may have contributed to these findings [6]. In the studies done by Zdunek et al. [5] and Bhatia et al. [6], findings suggested that country of residence influences these illnesses’ severity. More investigations between international samples can provide a better understanding of protective or harmful factors for patients with ME/CFS.
Studies on this illness in Asian populations, particularly Japan are scarce [4,7]. Kawakami et al. [8] conducted one of the first Japanese ME/CFS investigations and found a 1.5% CFS prevalence rate in a community sample. A follow-up study used physician reports to be more accurate and identified a 1% CFS prevalence rate in Japan. [7] A meta-analysis also confirmed that the rates found in Japan are comparable to those found in the U.S. and U.K. [4] Despite this similarity, the impact and severity of these illnesses among Japanese patients remains relatively unknown, specifically compared to U.S. patients.
The existing ME/CFS research from Japan suggests that patients face specific societal challenges resulting from their conditions. Japanese medical professionals are often reluctant to diagnose this condition, and patients indicated they were frustrated with the healthcare system. [9,10] Researchers also found that patients feel isolated and misunderstood by their employers, coworkers, and social circles. [9, 10] A lack of social support can correlate with significant fatigue and worse impairment in mental health and physical functionality in ME/CFS [11,12]. Japanese patients may be at risk of experiencing these outcomes. Furthermore, those with ME/CFS are excluded from disability benefits in Japan [13, 14], creating financial difficulties for Japanese citizens with these conditions. When the Japanese Ministry for Health, Labor, and Welfare commissioned a national survey for patients with ME/CFS, 70% of respondents reported they were unable to perform housework and relied on family assistance for daily living needs [13]. By excluding these illnesses from disability status, patients’ families also assume medical costs and other financial responsibilities [13]. Respondents indicated that financial hardships and medical expenses are a substantial source of stress and exacerbate symptoms [13]. By examining ME/CFS manifestations in Japanese populations, healthcare and policy practices can better support those experiencing these conditions.
Comparing illness profiles between two countries can provide insights on protective and harmful factors to patients with ME/CFS. The current study assesses differences in impairment and symptomatology between tertiary-care patients from Japan and the U.S. The current study hypothesizes that the lack of being able to access disability status for those with ME/CFS from Japan in comparison to those with ME/CFS from the U.S. results in more functional impairment and symptoms.
METHODS
Participants
SolveCFS Biobank:
The Chronic Fatigue and Immune Dysfunction Syndrome (CFIDS) Association of America shared data from the SolveCFS BioBank [15] with the DePaul University Research Team. The SolveCFS BioBank gathered clinical information on a cohort of English-speaking individuals over 18 years old; a licensed physician specializing in ME/CFS diagnosed patients using the Fukuda et al. case definition. [16] Participants with a BMI over 40 were excluded from the analysis. A total of 25 participants who had incomplete data were excluded in the analysis. The individuals within the sample lived in the U.S. at the time of the data collection.
A total of 210 participants from the U.S. were included in the present study. The average age of the sample was 49.97 (SD = 12.46). 76.2% of the sample identified as female and 98.1% were White; 52.4% indicated they were married and over half of the sample (65.2%) was on disability. The education level of the patients was high: 87.6% of the sample had at least some college education or graduate degree. 6.2% of the sample reported that they were homemakers.
Japanese Sample:
Japanese participants were recruited from the ME Japan association [14], and associated physician clinics specializing in ME/CFS. A total of 136 participants were recruited. Diagnostic criteria were not applied due to previously mentioned issues with obtaining a diagnosis for these conditions in Japan. Thus, the sample is based on self-report measures and could be classified as patients with profound fatigue. Of the 136 patients, 6 were excluded due to incomplete data. An additional 2 participants were excluded due to being younger than 18 years old. Participants with a BMI over 40 were excluded from the analyses. The final sample includes 124 Japanese participants who were treated and recruited in a tertiary setting for chronic fatigue and associated symptoms with ME/CFS.
The mean age of the Japanese sample was 46.3 (SD = 13.3) years, and over half the sample was female (59.6%). There was no racial information available for this sample, but as the questionnaires were filled out in Japanese, we assume most respondents were Japanese. 48.4% report they were separated from their partners, while 33.9% reported being single. The majority of the sample (88.7%) had at least some college education or professional degrees. Additionally, 19.2% of the Japanese sample reported being homemakers. Less than a quarter of the sample (22.6%) reported being on disability while 21.8% report that they were currently working, and 17.7% indicated they were unemployed and looking for work.
Instruments
DePaul Symptom Questionnaire (DSQ):
The DePaul Symptom Questionnaire (DSQ) is a self-report instrument used to assess ME/CFS symptomatology. The instrument includes 54-items covering the span of several ME/CFS symptom domains: fatigue, post-exertional malaise, sleep, pain, neurocognitive, autonomic, neuroendocrine, and immune. [17] Participants were asked to rate the frequency and severity of each symptom over the past six months on a five-point Likert scale. For each symptom, a composite score was generated by multiplying the frequency and severity symptom scores by 25 and averaging the sum, resulting in a 100-point scale. Higher scores indicated a higher level of symptomatology and severity of symptoms. The DSQ has been found to have good test-retest reliability as a standardized method to identify individuals with ME/CFS [18].
36-Item Short-Form Health Survey:
The SF-36 is a short form self-report measure on functional status related to health. [19] The SF-36 assesses functioning on eight subscales domains: physical functioning, role physical, bodily pain, general health, social functioning, vitality, role emotional, and mental health. Scores range from a 0–100 scale, where a higher score indicates better functioning. The measure has good discriminant validity as a measure of mental health and physical functioning, and is both psychometrically and clinically valid in English and Japanese [19–22].
RESULTS
Table 1 provides the demographic differences between the samples. Gender was significantly different between the samples, as women constituted a larger proportion of the U.S. sample (76.2%) than the Japanese sample (59.6%). There was also a significant difference of age between the two samples, with the U.S. sample being significantly older than the Japanese sample. Additionally, a greater portion of the U.S. sample (65.2%) was on disability compared to the Japanese sample (22.6%). Age was used as a covariate in the subsequent analysis. We did not control for gender and disability status but instead these two variables were used as fixed effects. This is because both variables are dichotomous in nature, and previous studies have highlighted that both gender and disability separately affect health outcomes, but their interactions were not examined. [23,24] In addition, our hypotheses involved both gender status and disability status, thus our study explored the relationship of both variables in our analyses.
Table 1:
Demographic Comparisons.
| Japan (N=124) | U.S. (N=210) | Sig. | |
|---|---|---|---|
| M (SD) | M (SD) | ||
| Age | 46.28 (13.31) | 49.97 (12.46) | * |
| Gender | % (n) | % (n) | |
| Female | 59.6 (74) | 76.2 (160) | ** |
| Male | 40.4 (50) | 23.8 (50) | |
| Disability Status | |||
| On disability | 22.6 (32) | 65.2 (137) | *** |
| Not on disability | 77.4 (92) | 34.8 (73) | |
| Education Level | |||
| High School/GED | 9.8 (12) | 12.0 (25) | |
| Some college | 23.0 (28) | 20.1 (42) | |
| College degree or higher | 67.2 (82) | 67.9 (142) |
As indicated in (Table 2), the U.S. sample reported significantly higher symptomology for post-exertional malaise, neurocognitive, and gastro-intestinal domains than the sample from Japan. Within the post-exertional malaise domain, the U.S. sample had significantly more problems with the item “dead, heavy feeling”. Within the neurocognitive domain, the U.S. sample had significantly worse scores among 8 items such as “difficulty remembering things” and “difficulty expressing thoughts”. Finally, within the gastro-intestinal domain, the U.S. sample had significantly worse scores for “irritable bowel disease” and “feeling nausea”.
Table 2:
DSQ Scores Compared Between Japan and U.S. Samples.
| Japan n=124 M (SD) |
U.S. n =210 M (SD) |
ηp2 | Sig | |
|---|---|---|---|---|
| Fatigue/ Extreme tiredness | 76.2 (22.5) | 79.9 (16.9) | .011 | |
| Post-Exertional Malaise | 63.9 (24.8) | 68.6 (21.7) | .012 | * |
| Dead, heavy feeling | 56.4 (35.3) | 70.3 (26.6) | .053 | *** |
| Next day soreness | 69.8 (28.2) | 69.3 (26.7) | .000 | |
| Mentally tired after the slightest effort | 55.8 (29.5) | 58.7 (27.9) | .002 | |
| Minimum exercise makes you physically tired | 69.1 (27.5) | 74.3 (24.2) | .013 | |
| Physically drained or sick after mild activity | 68.8 (27.5) | 70.3 (26.4) | .002 | |
| Neurocognitive | 48.4 (28.4) | 58.5 (23.0) | .036 | *** |
| Muscle twitches | 20.9 (23.7) | 31.1 (25.4) | .039 | *** |
| Sensitivity to noise | 34.2 (33.3) | 58.5 (29.9) | .125 | *** |
| Sensitivity to bright lights | 46.8 (32.0) | 56.3 (29.6) | .022 | ** |
| Difficulty remembering things | 44.8 (33.9) | 66.4 (25.5) | .112 | *** |
| Difficulty paying attention | 63.1 (33.2) | 56.3 (25.7) | .012 | |
| Difficulty expressing thoughts | 47.3 (33.4) | 62.3 (25.9) | .056 | *** |
| Difficulty understanding things | 39.4 (32.8) | 48.8 (28.0) | .023 | ** |
| Unable to focus vision and/or attention | 50.8 (34.0) | 60.6 (29.2) | .025 | ** |
| Absent mindedness | 44.7 (32.2) | 59.2 (28.9) | .048 | *** |
| Loss of depth perception | 16.9 (27.2) | 23.1 (29.6) | .011 | |
| Sleep | 59.4 (24.5) | 58.8 (19.4) | .000 | |
| Unrefreshed waking up | 69.7 (25.3) | 79.1 (21.4) | .044 | *** |
| Difficulty falling asleep | 66.2 (49.3) | 58.6 (31.0) | .009 | |
| Difficulty staying asleep | 56.9 (30.0) | 54.1 (30.5) | .003 | |
| Waking up early in the morning (e.g. 3am) | 44.5 (32.7) | 45.2 (32.7) | .000 | |
| Sleeping all day & staying awake all night | 19.4 (27.9) | 16.8 (26.5) | .000 | |
| Need to nap daily | 59.7 (31.9) | 56.5 (33.0) | .002 | |
| Gastro-Intestinal | 32.9 (26.4) | 40.1 (26.3) | .020 | ** |
| Bladder problems | 25.2 (32.7) | 30.6 (33.8) | .005 | |
| Irritable bowel issues | 30.5 (34.6) | 42.9 (33.8) | .033 | ** |
| Feeling nausea | 24.6 (26.9) | 32.9 (26.9) | .032 | ** |
| Orthostatic Intolerance | 33.2 (22.7) | 32.6 (20.1) | .000 | |
| Feeling unsteady on feet | 39.1 (30.9) | 37.5 (26.4) | .000 | |
| Shortness of breath | 39.9 (31.9) | 36.4 (29.1) | .000 | |
| Dizziness or fainting | 36.2 (30.2) | 36.0 (28.1) | .000 | |
| Irregular heartbeats | 26.2 (26.9) | 27.5 (25.2) | .001 | |
| Neuroendocrine | 36.7 (25.3) | 34.3 (23.6) | .001 | |
| Losing or gaining weight without effort | 29.8 (31.8) | 35.7 (35.6) | .008 | |
| Poor appetite | 31.0 (30.3) | 21.1 (23.6) | .029 | *** |
| Sweating hands | 18.2 (23.7) | 9.6 (20.4) | .027 | *** |
| Night sweats | 26.2 (24.9) | 32.9 (31.1) | .013 | * |
| Cold Limbs | 56.6 (32.6) | 41.9 (31.9) | .045 | *** |
| Feeling chills or shivers | 33.1 (29.7) | 30.1 (27.8) | .001 | |
| Feeling hot or cold for no reason | 36.5 (32.1) | 43.2 (30.8) | .013 | |
| Feeling of having a low temperature | 20.9 (30.4) | 22.3 (27.1) | .001 | |
| Feeling of having a high temperature | 32.5 (30.6) | 28.5 (28.9) | .003 | |
| Alcohol intolerance | 30.5 (35.9) | 34.9 (37.7) | .004 | |
| Immune | 36.5 (24.9) | 35.5 (20.3) | .000 | |
| Flu-like symptoms | 27.4 (30.1) | 47.3 (30.8) | .097 | *** |
| Sore throat | 41.4 (30.8) | 35.7 (24.9) | .007 | |
| Fever | 39.0 (30.4) | 16.3 (22.7) | .142 | *** |
| Tender lymph nodes | 38.1 (32.3) | 41.7 (28.6) | .005 | |
| Smells, foods, meds, or chemicals make you sick | 30.7 (33.8) | 46.0 (35.7) | .044 | |
| Pain | 56.8 (31.6) | 49.7 (28.3) | .002 | |
| Pain or aching muscles | 62.1 (33.0) | 63.3 (28.1) | .000 | |
| Pain or aching joints | 51.5 (35.7) | 56.1 (32.6) | .004 | |
| Bloating | 32.2 (32.6) | 38.6 (29.4) | .000 | |
| Eye pain | 32.9 (30.5) | 25.6 (28.1) | .013 | * |
| Stomach pain | 36.0 (28.9) | 38.2 (30.8) | .003 | |
| Chest pain | 25.3 (27.6) | 25.5 (25.9) | .000 | |
| Headaches | 48.9 (31.0) | 48.5 (26.3) | .001 |
As indicated in (Table 3), there was a significant difference between the countries in the SF-36 subscales for Physical functioning, Role-Physical functioning, and Mental Health functioning. The U.S. sample had lower physical functioning and role-physical functioning scores, and higher mental health functioning scores than the Japanese sample.
Table 3:
SF-36 Score Comparisons between the U.S. & Japanese Samples.
| Japan (n =123) M (SD) |
U.S. (n =210) M (SD) |
ηp2 | Sig. | |
|---|---|---|---|---|
| Physical Functioning | 45.2 (27.6) | 38.2 (23.8) | .017 | * |
| Role Physical | 16.5 (33.3) | 3.6 (12.9) | .071 | *** |
| Bodily Pain | 44.3 (26.8) | 46.7 (24.3) | .002 | |
| General Health Functioning | 26.3 (14.5) | 26.1 (17.4) | .000 | |
| Social Functioning | 29.1 (28.8) | 30.0 (26.2) | .000 | |
| Mental Health Functioning | 57.3 (22.6) | 67.0 (18.9) | .050 | *** |
| Role-Emotional | 64.0 (43.9) | 70.0 (42.2) | .005 | |
| Vitality | 18.6 (18.0) | 15.4 (14.7) | .009 |
Due to the discrepancies in gender and disability status among patients with ME and CFS, further investigation on these factors were explored between the two countries. As symptomatology was more equivalent between countries than functioning, we examined the impact of our fixed effects (country, gender, disability) on physical functioning, role physical functioning, and mental health. Age was included as a covariate due to significant differences between the two sample populations.
Role-Physical Functioning
There was a significant three-way interaction effect among country, gender, and disability status for Role-Physical functioning, F (1,309) = 4.59, p < .05, d = 0.25. As indicated on Figure 1a, among those on disability, individuals from the U.S. had worse scores than those from Japan. In addition, also on this figure, and Japanese women had significantly higher scores than Japanese men, and both men and women from the U.S. A different set of relations is evident in Figure 1b, that depicts those who were not on disability. For males, those from the U.S. had significantly lower scores than those from Japan.
Figure 1a.
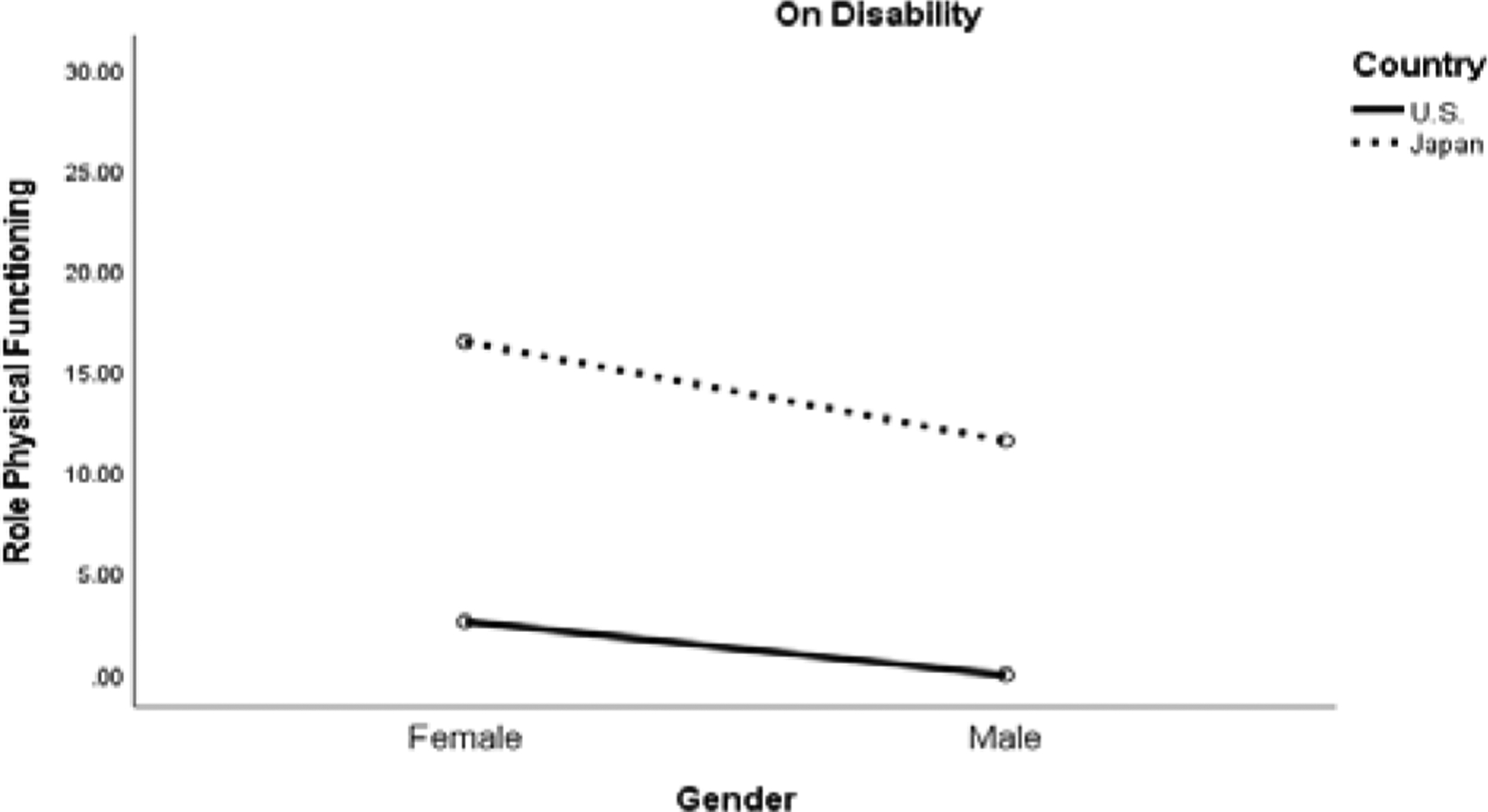
Role Physical Functioning Between Patients on Disability in the U.S. and Japan Samples.
Figure 1b.

Role Physical Functioning Between Patients Not on Disability in the U.S. and Japan Samples.
Physical Functioning
There was a significant main effect of gender on physical functioning F(1,309) = 5.32, p < .05, d = 0.26, with women reporting worse physical functioning scores than men. As expected, those on disability reported worse physical functioning scores than participants not on disability.
Mental Health Functioning
There was a significant three-way interaction effect among country, gender, and disability status for Mental Health functioning, F(1,309) = 9.024, p < .01, d = .35 (see Figures 2a,b). There was also an interaction effect of country and disability status F (1,309) = 4.22, p < .05, d = .24. For those on disability, Japanese men had significantly lower mental health scores than U.S. men and from both women in the U.S. and Japan. Finally, for those not on disabilities, Japanese women scored significantly lower than U.S. women. However, for those not on disability, scores for men in the US were not significantly different from scores of men in Japan, but had significantly lower scores than women in the U.S.
Figure 2a.

Mental Health Functioning Among Patients on Disability Between the U.S. and Japan Samples.
Figure 2b.
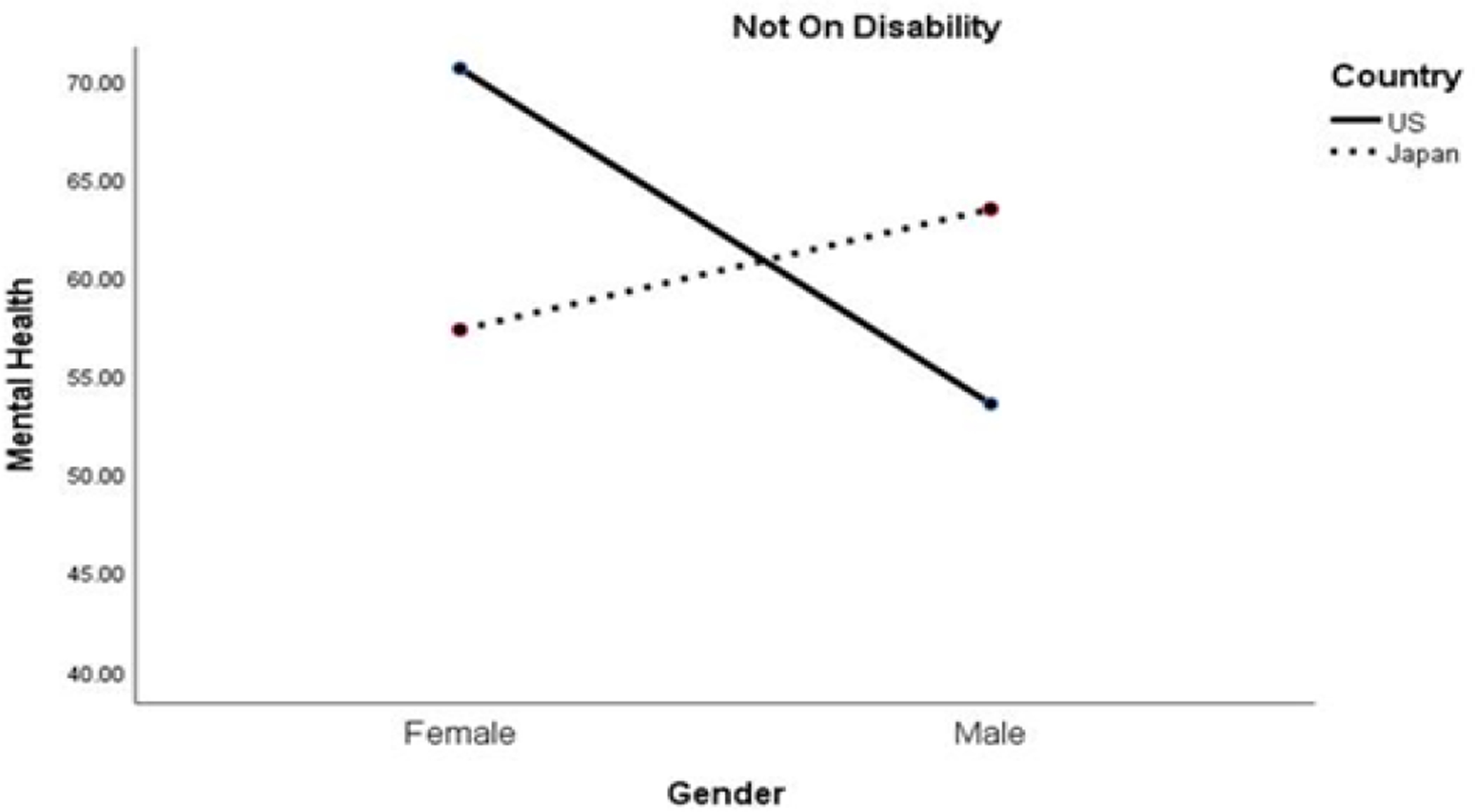
Mental Health Functioning Among Patients Not on Disability Between the U.S. and Japan Samples.
DISCUSSION
The present study compared a U.S. patient sample diagnosed with ME/CFS using the Fukuda et al. [16] criteria with a Japanese patient sample with chronic fatigue (of which most patients were presumed to have ME/CFS). Our study suggests that patients from the U.S. were more severely impaired in the domains of post-exertional malaise, neurocognitive symptoms, and gastrointestinal symptoms. Patients from both the U.S. and Japan experienced comparably severe sleep difficulties, orthostatic intolerance, and problems within neuroendocrine and immune symptom domains. Among the patients who were not on disability, Japanese patients evidenced higher functioning in the role physical area than any other group. For physical functioning, women in both the U.S and Japan samples evidenced worse functioning than men. In the mental health subscale, Japanese men who were on disability had the worse functioning. These are somewhat complicated findings, yet, they suggest that gender, disability, and country of residence are essential factors in ME/CFS. Japanese disability and welfare policies, in addition to cultural gender roles, could explain the differences between the two countries.
Previous cross-national comparisons between patients with ME/CFS have found that those in the U.K. [5] and Spain [6] had worse functioning than samples from the U.S. In the current study, Japanese patients reported overall better symptomology and functioning, compared to the U.S. sample. This discrepancy may reflect the cultural differences between the two countries. Japanese culture tends to be more collectivistic and family-oriented, whereas Western cultures tend to be individualistic and value personal autonomy. [25, 26] As such, in collectivist cultures, secondary control coping mechanisms (i.e., changing the individual’s feelings and thoughts to adjust to the objective environment) are more emphasized. [27, 28] While in individualistic cultures, primary control coping (i.e., changing the existing environment to fit the individual’s need) is more prevalent. [27, 28] The discrepancies between the Japanese and U.S. responses may indicate that a collectivistic culture may serve as a protective factor for patients. Thus, this may reduce symptomology and functional limitations, despite the lack of accessibility for disability benefits.
The differences may also relate with the countries’ differences in accessibility to disability benefits. Japanese patients were less likely to be on disability, which corresponds with ME/CFS not qualifying citizens for disability benefits in Japan. Moreover, this is consistent with the finding that only 2% to 5% of Japan’s population are on disability, [29,30] which is lower than the percentage of those on disability in the U.S. Individuals with ME/CFS in Japan may be more likely to be unemployed; other individuals presumably may need to work at a reduced capacity. Our data indicated that nearly 18% of the Japanese sample are unemployed and/or are looking for work, and nearly 19% are also housekeeping. A review done by Taylor and Kielhofner [31] highlighted the difficulties of maintaining employment for individuals with ME/CFS; patients’ symptomology and reduced limited capacity often create difficulties in the workplace. The current study’s Japanese patients may be experiencing similar barriers.
The current findings also illustrate gender as an operative factor for Japanese patients. Japanese women who are not on disability status have worse mental health functioning than both women who are on disability and those who are not on disability from the U.S. This suggests that Japanese women in Japan who are unable to receive disability and cannot work internalize feelings of being a burden to their families. Most individuals with ME/CFS in Japan depend on family assistance in their daily activities and financial situation. [13] This may lead to feelings of inadequacy or stresses over financial hardship. Working beyond one’s capacity may further effect the severity of the illness. Other difficulties that could contribute to poor mental health functioning include the lack of medical specialists in Japan, isolation due to the inability to participate in social activities, and the lack of understanding about the disease from health providers, coworkers, friends, and family members. [13] Moreover, those with ME/CFS in Japan are less likely to be receiving support from the health care system due to the illness stigma [9,10].
Overall, women from both countries evidenced the lowest physical functioning. Disability status, however, had an interesting role in the illness profile for ME/CFS. Among those who are on disability, Japanese women had the highest functioning in the role-physical assessment. For Japanese men, those who were not on disability experienced the highest levels of role physical functioning, but those who were on disability experienced the lowest mental health functioning. These findings suggest that Japanese men not on disability have better role functioning, but once on disability, their mental health seems to be negatively impacted. This could reflect on social roles for Japanese men, where the ability to work is important to this culture. The findings also indicate that Japanese women have higher mental health scores than their male counterparts, but have lower role physical functioning when not on disability. This may be a result of Japanese women having to balance employment and domestic responsibilities.
It is important to consider the current study’s limitations. First, the SolveCFS Biobank required a physician diagnosis whereas the sample from Japan did not, thus there are important differences in selection criteria for the two countries. Moreover, ME/CFS is eligible for disability in the U.S., but not in Japan. Therefore, disability benefits were more accessible to the participants in the SolveCFS Biobank sample. The two samples also originate from tertiary care settings, thus the scope of the findings are specific to those who have resources to seek healthcare and treatment. In addition, this study is limited by the lack of biomarkers for evaluating the illness, and therefore, the ultimate diagnosis is usually based on individual physicians, with different criteria based on disparate criteria. Future studies should aim to recruit more homogenous samples from the U.S. and Japan. A stronger comparison of this illness among the two countries may include patients from both community and tertiary care settings; utilize a singular case definition and diagnosis criterion for participants; and comparable demographics between the samples.
The current study suggests Japanese women experience ME/CFS in different ways than their male counterparts, but to fully understanding these differences requires more research. Lastly, the illness and economic burden of ME/CFS in Japan is likely harmful on this vulnerable community. Their conditions are ineligible for disability benefits and the stress and hardship of employment among Japanese individuals with ME/CFS is evident. There is a need for the Japanese Ministry for Health, Labor, and Welfare to reevaluate its disability policy and consider making ME/CFS eligible for disability benefits among Japanese citizens.
ACKNOWLEDEGEMENT
This research study was supported by the National Institutes of Health [Grant Number 1R01AI105781-01A1].
REFERENCES
- 1.Jason LA, McManimen S, Sunnquist M, Newton JL, Strand EB. Clinical criteria versus a possible research case definition in chronic fatigue syndrome/myalgic encephalomyelitis. Fatigue: biomedicine, health & behavior. 2017; 5(2):89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jason LA, Richman JA, Rademaker AW, Jordan KM, Plioplys AV, et al. A community-based study of chronic fatigue syndrome. Archives of internal medicine. 1999; 159(18):2129–2137. [DOI] [PubMed] [Google Scholar]
- 3.Son CG. Review of the prevalence of chronic fatigue worldwide. The Journal of Korean Medicine. 2012; 33(2):25–33. [Google Scholar]
- 4.Lim EJ, Ahn YC, Jang ES, Lee SW, Lee SH, et al. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). Journal of translational medicine. 2020;18(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zdunek M, Jason LA, Evans M, Jantke R, Newton JL. A cross cultural comparison of disability and symptomatology associated with CFS. International journal of psychology and behavioral sciences. 2015; 5(2):98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhatia S, Olczyk NA, Jason L, Alegre J, Fuentes-Llanos J, et al. A Cross-National Comparison of Myalgic Encephalomyelitis and Chronic Fatigue Syndrome at Tertiary Care Settings from the US and Spain. Am. J. Soc. Sci. Humanit 2020; 5:104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamaguchi M, Kawahito Y, Takeda N, Kato T, Kojima T. Characteristics of chronic fatigue syndrome in a Japanese community population. Clinical rheumatology. 201; 30(7): 895–906. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami N, Iwata N, Fujihara S, Kitamura T. Prevalence of chronic fatigue syndrome in a community population in Japan. The Tohoku journal of experimental medicine. 1998; 186(1): 33–41. [DOI] [PubMed] [Google Scholar]
- 9.Hosoda M Living with a Misunderstood Disease: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome in Japan. Eubios Journal of Asian and International Bioethics; EJAIB. 2013; 23(3): 70–72. [Google Scholar]
- 10.Hosoda M, Hosoda M. Supporting Patient-led Initiatives to Improve Healthcare: An Investigation of Cancer and ME/CFS Support Groups and Prefectural Medical Councils in Japan. International Journal of Patient-Centered Healthcare (IJPCH). 2019; 9(2):20–28. [Google Scholar]
- 11.Jason LA, Witter E, Torres-Harding S. Chronic fatigue syndrome, coping, optimism and social support. Journal of Mental Health. 2003; 12(2):109–18. [Google Scholar]
- 12.Prins JB, Bos E, Huibers MJ, Servaes P, Van Der Werf SP, et al. Social support and the persistence of complaints in chronic fatigue syndrome. Psychotherapy and psychosomatics. 2004; 73(3):174–182. [DOI] [PubMed] [Google Scholar]
- 13.Shinohara M ME/CFS patient survey report: Ministry of Health survey reveals harsh reality of ME/CFS patients in Japan. 2015. Bethesda: International Association for CFS/ME. [Google Scholar]
- 14.ME in Japan. (2020, May 24). Retrieved June 26, 2020.
- 15.You + M.E Registry. 2020.
- 16.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, et al. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Annals of internal medicine. 1994; 121(12):953–959. [DOI] [PubMed] [Google Scholar]
- 17.Jason LA, Sunnquist M. The development of the depaul symptom questionnaire: Original, expanded, brief, and pediatric versions. Frontiers in pediatrics. 2018; 6: 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jason LA, So S, Brown AA, Sunnquist M, Evans M. Test–retest reliability of the DePaul Symptom Questionnaire. Fatigue: biomedicine, health & behavior. 2015; 3(1): 16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical care. 1992; 30(60): 473–483. [PubMed] [Google Scholar]
- 20.McHorney CA, Ware JE Jr, Raczek AE. Psychometric and Clinical Tests of Validity in Measuring Physical and Mental Health Constructs. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993; 31(3): 247–263. [DOI] [PubMed] [Google Scholar]
- 21.Baba S, Katsumata Y, Okamoto Y, Kawaguchi Y, Hanaoka M, et al. Reliability of the SF-36 in Japanese patients with systemic lupus erythematosus and its associations with disease activity and damage: a two-consecutive year prospective study. Lupus. 2018; 27(3): 407–416. [DOI] [PubMed] [Google Scholar]
- 22.Fukuhara S, Bito S, Green J, Hsiao A, Kurokawa K. Translation, adaptation, and validation of the SF-36 Health Survey for use in Japan. Journal of clinical epidemiology. 1998; 51(11): 1037–1044. [DOI] [PubMed] [Google Scholar]
- 23.Jason LA, Benton MC, Valentine L, Johnson A, Torres-Harding S. The economic impact of ME/CFS: individual and societal costs. Dynamic Medicine. 2008; 7(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richman JA, Jason LA. Gender biases underlying the social construction of illness states: The case of chronic fatigue syndrome. Current Sociology. 2001; 49(3): 15–29. [Google Scholar]
- 25.Jason LA, Luna RD, Alvarez J, Stevens E. Collectivism and individualism in Latino recovery homes. Journal of ethnicity in substance abuse. 2018; 17(3): 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triandis HC, Bontempo R, Villareal MJ, Asai M, Lucca N. Individualism and collectivism: Cross-cultural perspectives on self-in-group relationships. Journal of personality and Social Psychology. 1988; 54(2): 323–338. [Google Scholar]
- 27.Lam AG, Zane NW. Ethnic differences in coping with interpersonal stressors: A test of self-construals as cultural mediators. Journal of Cross-Cultural Psychology. 2004; 35(4): 446–459. [Google Scholar]
- 28.McCarty CA, Weisz JR, Wanitromanee K, Eastman KL, Suwanlert S,et al. Culture, coping, and context: Primary and secondary control among Thai and American youth. Journal of Child Psychology and Psychiatry. 1999; 40(5): 809–818. [PubMed] [Google Scholar]
- 29.Heyer K Between equality and difference: The politics of disability in Japan. Japanstudien. 2000; 11(1): 105–133. [Google Scholar]
- 30.Meyer HD. Framing disability: Comparing individualist and collectivist societies. Comparative sociology. 2010; 9(2): 165–181. [Google Scholar]
- 31.Taylor RR, Kielhofner GW. Work-related impairment and employment-focused rehabilitation options for individuals with chronic fatigue syndrome: A review. Journal of Mental Health. 2005; 14(3): 253–267. [Google Scholar]


