Abstract
Ataxia-telangiectasia mutated and Rad3-related (ATR) is master regulator of the DNA-damage response that, through multiple mechanisms, can promote cancer cell survival in response to replication stress from sources including chemotherapy and radiation. Elimusertib (BAY-1895344) is an orally available small molecule ATR inhibitor currently in preclinical and clinical development for cancer treatment. To support these studies and define elimusertib pharmacokinetics, we developed a high-performance liquid chromatography mass spectrometry method for its quantitation. A 50 μL volume of plasma was subjected to acetonitrile protein precipitation, followed by chromatographic separation using a Phenomenex Polar-RP column (4 μm, 2 x 50 mm) and a gradient mobile phase consisting of 0.1% formic acid in acetonitrile and water, during a 7 minute run time. Mass spectrometric detection was achieved using a SCIEX 4000 triple-stage mass spectrometer with electrospray positive-mode ionization. With a stable isotopic internal standard, the assay was linear from 30 to 5,000 ng/mL and proved to be both accurate (93.5-108.2%) and precise (<6.3 %CV) fulfilling criteria from the Food and Drug Administration (FDA) guidance on bioanalytical method validation. This LC-MS/MS assay will support several ongoing clinical studies by defining elimusertib pharmacokinetics.
Keywords: ATR inhibitor, ATR, tandem mass spectrometry, assay, validation, chromatography
1. Introduction
Ataxia telangiectasia and Rad3-related (ATR) is an apical serine/threonine kinase that initiates and regulates the cellular DNA damage response (DDR) (Awasthi, et al. 2015, Li, et al. 2016, Qiu, et al. 2018, Smith, et al. 2010). DNA damage and replication stress, caused by sources including genomic instability in cancer cells or by cytotoxic cancer treatments, can result in single-strand breaks (SSB) which are recognized by ATR and allow its activation by phosphorylation (Awasthi, et al. 2015, Bouwman and Jonkers 2012). Signaling cascades initiated by activated ATR initiates several pathways that ultimately promote cell survival by limiting further damage and prevent premature cellular division that can result in mitotic catastrophe and cell death (Canman 2001). Several mechanisms are involved in this process including stabilization of replication forks and direct activation of Chk1, which can induce cell-cycle arrest (Smith, et al. 2010). Ultimately, ATR allows cells the ability to survive the stressors of unstable genomes and cancer treatment, and as a result has become an attractive therapeutic target for pharmacological inhibition (Cheung-Ong, et al. 2013, Gourley, et al. 2019, Reaper, et al. 2011).
Although none of these agents, collectively referred to as ATR inhibitors (ATRi), are currently approved by regulatory agencies, several are in various phases of clinical development including ceralasertib (AZD6738) by AstraZeneca, berzosertib (M6620, VX-970), M1774 and M4344 by EMD Serono, and elimusertib by Bayer (Dillon, et al. 2018, Mei, et al. 2019, Yap, et al. 2020a). ATRi are commonly studied in combination with a primary DNA damaging agent, including chemotherapies and radiotherapy, in an effort to enhance the effects of the cytotoxic. Preclinical studies have shown that in cancers with defects in DDR components, in particular ATM (ataxia telangiectasia mutated), ATRi monotherapy could produce synthetic lethality (Dunne, et al. 2017, Middleton, et al. 2018, Toledo, et al. 2011, Yap, et al. 2020b). ATRi have also been used preclinically as pharmacological probes to investigate the role ATR plays in cancer development and maintenance as well as the understanding how it functions in response to DNA damage by chemotherapies and radiation (Kwok, et al. 2016, Kwok, et al. 2015, Vendetti, et al. 2018, Vendetti, et al. 2017). ATR also appears to have a role in the immunological response to cancer treatment and ATRi can enhance CD8+ T cell antitumor responses following conformal radiation, (Dillon, et al. 2019, McLaughlin, et al. 2020, Sheng, et al. 2020, Sun, et al. 2018, Vendetti, et al. 2018). In an increasingly crowded ATRi class, methods to accurately characterize the pharmacokinetics, drug-drug interactions and exposure-response relationships are important to distinguish the various ATRi as well a to support their safe and effective development (Alavijeh and Palmer 2004, Singh 2006).
Elimusertib is a potent orally available ATRi that has entered clinical development with the results of a Phase 1 first-in-human clinical trial being recently reported and several preclinical studies demonstrating effectiveness as a monotherapy or as part of a combination regimen in several tumor cell lines (Lucking, et al. 2020, Wengner, et al. 2020, Yap, et al. 2020b). These reports describe the clinical and preclinical PK of elimusertib but the analytical methods description are limited in a manner not allowing replication. To support both future clinical trials of elimusertib, a simple, accurate, and precise liquid chromatography-tandem mass spectroscopy (LC-MS/MS) assay for quantitating elimusertib in human plasma was developed and validated according to the FDA Bioanalytical Method Validation Guidance (U.S. Department of Health and Human Services Food and Drug Administration 2018). Assay application was demonstrated with a patient samples from an ongoing clinical trial (NCT04535401) investigating elimusertib combined with FOLFIRI (irinotecan, fluorouracil, and leucovorin). The assay described here is supporting multiple NCI sponsored clinical trials of elimusertib and will the continued development of this novel cancer treatment.
2. Experimental
2.1. Chemicals and reagents
Elimusertib (99.9% D0/(D4+D0); 100.1% purity) and [13C,2H3]-elimusertib (IS, (99.9% D4/(D4+D0))) were manufactured and provided by Bayer (Leverkusen, Germany), see Suppl.Fig. 1A and B for structures. Acetonitrile and water (all HPLC grade) were purchased from Fisher Scientific (Fairlawn, NJ, USA). Dimethyl sulfoxide and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Control EDTA and heparinized human plasma was purchased from Lampire Biological Laboratories (Pipersville, PA, USA). Nitrogen for mass spectrometric applications was purified with a nitrogen generator (Parker Balston, Haverhill, MA, USA).
2.2. Chromatography
The LC system consisted of an Agilent (Palo Alto, CA, USA) 1200 SL autosampler and binary pump flowing into a Phenomenex (Torrance, CA USA) Synergi Polar-RP 80Å (4 μm, 50 x 2.0 mm) column using a gradient style elution program. Needle wash consisted of acetonitrile:isopropanol:acetone (40:40:20, v/v/v). Mobile phase solvent A consisted of 0.1% formic acid in water and mobile phase solvent B consisted of 0.1% formic acid in acetonitrile. The initial mobile phase composition was 90% solvent A and 10% solvent B. From 0 to 3.0 min, at a 0.5 mL/min flow rate, solvent A was decreased to 5% and conditions held until 3.1 min. Between 3.1 and 5.0 min, solvent A was maintained at 5% and the flowrate increased to 1.0 mL/min and held constant until 5.1 min, followed by a return to the initial composition at a flow rate of 1.0 mL/min until 7.0 min. The total run time was 7 min with an injection volume of 2 μL, resulting in a retention time of 2.17 min. Between 0 to 1 min and 3 to 7 min the HPLC flow was diverted to waste.
2.3. Mass spectrometry
Detection was accomplished with a SCIEX 4000 hybrid linear ion trap tandem mass spectrometer (Framingham, MA) utilizing electrospray ionization in positive-ion multiple reaction monitoring (MRM) mode. The settings of the mass spectrometer in positive mode scanning parameters were as follows: curtain gas 40, IS voltage 5000 V, probe temperature 550 °C, GS1 40, GS2 40, DP of 50 V, EP of 40 V, CE of 50 V, and an exit potential of 15 V. The temperature of the autosampler was 4 °C. The MRM m/z transitions monitored were: 376.3>318.2 (quantitative) and 376.3>277.3 (qualitative) for elimusertib and 380.3>322.2 for [13C, 2H3]-elimusertib. Control of the LC system and mass spectrometer as well as data collection was accomplished with Analyst software (version 1.6.2).
2.4. Preparation of calibration standards and quality control samples
Stock solutions of elimusertib and [13C,2H3]-elimusertib were created by dissolving 1 mg/mL in DMSO and sequential dilution into working stocks of 0.1 mg/mL DMSO and storing all solutions at −80 °C. On the day of the assay, elimusertib working stock was diluted in 10-fold steps in DMSO to obtain 10 and 1 μg/mL solutions. These solutions were diluted in human plasma to produce the following elimusertib concentrations: 30, 50, 100, 300, 500, 1,000, 3,000 and 5,000 ng/mL. For each calibration series, zero and blank samples were also prepared from control plasma. Quality control (QC) stock solutions were prepared independently and stored at −80 °C. These solutions were diluted in human plasma to produce the following QC samples of either: Lower Limit of Quantitation (LLOQ) 30 ng/mL, QC Low (QCL) 40 ng/mL; QC Mid (QCM) 200 ng/mL, and QC High (QCH) 4,000 ng/mL. On the day of the assay, [13C,2H3]-elimusertib was diluted to 1 μg/mL in DMSO as an IS working stock. The maximum composition of DMSO in calibration standards and QCs was ≤ 5%.
2.5. Sample preparation
A volume of 50 μL of the standard, QC, or sample plasma was pipetted into a microfuge tube and 10 μL 0.001 mg/mL [13C,2H3]-elimusertib was added. A total of 200 μL of acetonitrile was then added followed by vortexing for 1 min on a Vortex Genie-2 set at 10 (Model G-560 Scientific Industries, Bohemia, NY, USA). Samples were centrifuged at 13,500 × g at room temperature for 5 min. Supernatants were transferred to autosampler vials followed by injection of 2 μL into the LC-MS/MS system.
2.6. Validation procedures
2.6.1. Calibration curve and lower limit of quantitation (LLOQ)
Calibration standards and blanks were prepared in human potassium EDTA plasma (see paragraph 2.4 and 2.5) and analyzed in triplicate to establish the calibration range with acceptable accuracy and precision.
2.6.2. Accuracy and precision
The accuracy and precision of the assay were determined by analyzing samples in human EDTA plasma at the LLOQ, QCL, QCM, and QCH concentrations in 6 replicates each in 3 analytical runs, together with independently prepared, triplicate calibration curves.
2.6.3. Selectivity and specificity
To investigate whether endogenous matrix constituents interfered with the assay, six individual batches of control, drug-free human EDTA plasma were processed and analyzed according to the described procedure. Responses of analytes at the LLOQ concentrations were compared with the response of the blank samples. The crosstalk of elimusertib into the IS signal, and vice versa, were characterized by monitoring MRM channels of separately spiked samples of 50,000 ng/mL elimusertib or IS and quantitating the response in the other channel. Because elimusertib is being studied in combination with several other chemotherapeutics, we evaluated the impact of 5-fluorouracil (10,000 ng/mL), irinotecan (2,000 ng/mL), gemcitabine (10,000 ng/mL), dFdU (50,000 ng/mL), and topotecan (1,000 ng/mL) on the quantitation of elimusertib QCM samples (N=5), and their response in blank samples.
Carry-over was assessed by separately injecting plasma samples with 5,000 ng/mL elimusertib or IS, followed by serial plasma blank injections.
2.6.4. Extraction recovery and matrix effect
We determined the extraction recovery of elimusertib from EDTA plasma by comparing the absolute response of an extract of control plasma to which the analyte had been added after protein precipitation, with the absolute response of an extract of plasma to which the same amount had been added before protein precipitation. The matrix effect by plasma matrix components was defined as the change of the absolute response of an extract of control plasma to which analyte had been added after the protein precipitation relative to the absolute response of solvent to which the same amount of the analyte had been added. Experiments were performed in replicates of four at the QCL, QCM and QCH concentrations.
2.6.5. Stability
Stock solution stabilities were determined using freshly prepared stock solutions in DMSO and comparing them to stock solutions left on the benchtop at RT for 6 h (benchtop stability) or stored at −80 °C for 12 months (long term stability). Stock solutions were diluted to 100 ng/mL (benchtop stability) or 1,000 ng/mL (long term freezer stability) in control plasma and prepared in replicates of 4. Concentrations used to assess stability were derived by the same calibration curve and QC series.
The stability of elimusertib in human EDTA plasma at −80 °C was determined by assaying samples before and after storage for 12 months. The effect of 3 freeze/thaw cycles analyte concentrations on plasma was evaluated by assaying samples after they had been frozen (−80 °C) and thawed on 3 separate days and comparing the results with those of freshly prepared samples. The stability of elimusertib in plasma during sample preparation was evaluated by assaying samples before and after 4 h of storage at room temperature and ambient light. All stability testing in plasma was performed in replicates of four at the QCL, QCM and QCH concentrations. To evaluate the stability of elimusertib in reconstituted samples in the autosampler, QC samples and calibration curves were re-injected approximately 72 h after the first injection and compared with concentrations derived from the second injection with those derived from the first injection using the initial duplicate calibration curve, as well as relative to a fresh duplicate calibration curve. The results of the second runs were expressed as a percentage of their respective values in the first runs.
2.6.6. Additional validation items
The impact of hemolysis was assessed by adding 10% (v/v) pre-hemolyzed whole blood to blanks, LLOQ, QCL, and QCH samples (n=4) and incubating at room temperature for 4 h. Heparinized plasma was evaluated as an alternative anticoagulated matrix to the standard EDTA plasma by analyzing QCL, QCM, and QCH samples (n=6) prepared in EDTA plasma against a heparinized plasma based triplicate curve. The ability to analyze urine was evaluated by adding 10% (v/v) human urine to LLOQ and QCH samples (n=4). Dilution integrity was shown by preparing plasma samples (n=3) at 30,000 ng/mL and analysis after 100-fold dilution (to 300 ng/mL) with control plasma. In case extra assurance is desired by means of a qualifier ion, MRM m/z transition 376.3>277.3 was monitored . The potential for phospholipids to interfere in analysis was also assessed by monitoring known phospholipid MRM channels in a QCH sample (Little, et al. 2006, Zhang and Wujcik 2009).
2.7. Application of the assay
To demonstrate the applicability of the assay, we analyzed samples from a patient enrolled in a clinical trial (NCT04535401) of elimusertib in combination with FOLFIRI (irinotecan, fluorouracil, and leucovorin) This patient had signed informed consent on an institutional review board approved protocol. The patient received a 20 mg PO dose of elimusertib and blood samples were collected between 0 and 6.5 hours. Pharmacokinetic parameters were determined non-compartmentally.
3. Results and Discussion
3.1. Validation of the assay
3.1.1. Chromatography
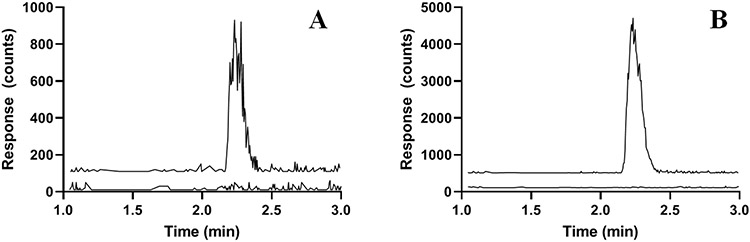
The retention time of elimusertib was 2.17 min which corresponds to a capacity factory of 0.84 in conjunction with a void time of 1.18 min. Representative chromatograms of the LLOQ and IS in human plasma are displayed in Fig. 1A and B.
Fig. 1.
Representative chromatograms of: A) elimusertib (m/z 376.3>318.2; 2.2 min) added to control human plasma at the LLOQ concentration of 30 ng/mL (top trace with an offset of 100 counts) and control human plasma (bottom trace); B) [13C2H3]-elimusertib (m/z 380.3>322.2; 2.2 min) added to control human plasma at a concentration of 500 ng/mL (top trace with an offset of 500 counts) and control human plasma (bottom trace with an offset of 100 counts)
3.1.2. Calibration curve and LLOQ
The selected assay range of 30-5,000 ng/mL fulfilled the FDA criteria for the LLOQ concentration and the calibration curve (calibrators within ± 15% of nominal concentrations, with LLOQ within ± 20%, and 75% and a minimum of six non-zero calibrators meeting these criteria in each run) (U.S. Department of Health and Human Services Food and Drug Administration 2018). Accuracies and precisions for each concentration from triplicate calibration curves prepared on three separate days are reported in Suppl.Table 1. A representative calibration curve and the corresponding correlation and regression coefficient and corresponding accuracies are shown in Suppl.Fig. 2A and B.
3.1.3. Accuracy and precision
QC based accuracies ranged from 96.7 to 105.7%. The intra- and inter-assay precisions for the tested concentrations (LLOQ, QCL, QCM, QCH) were all within the defined acceptance criteria (accuracy: ± 15% of nominal concentrations; except ± 20% at LLOQ, precision: within-run and between runs: ± 15% CV, except ± 20% CV at LLOQ) (Table 1) (U.S. Department of Health and Human Services Food and Drug Administration 2018).
Table 1.
Assay performance data for the quantitation of LLOQ, QCL, QCM, and QCH of elimusertib concentrations in human plasma.
| Concentration (ng/mL) |
Intra-assay Accuracy (%) |
Inter-assay Accuracy (%) |
Intra-assay precision (%) |
Inter-assay precision (%) |
|---|---|---|---|---|
| 30 (LLOQ) | 92.9-99.6 | 96.7 | 3.9 | 3.1 |
| 40 (QCL) | 93.4-103.5 | 98.1 | 2.8 | 5.1 |
| 200 (QCM) | 103.3-107.5 | 105.7 | 1.3 | 2.0 |
| 4000 (QCH) | 102.8-106.6 | 105.1 | 1.9 | 1.7 |
n=18; 6-fold results, each in 3 separate runs, for each concentration. LLOQ, lower limit of quantitation; QCL, QC Low; QCM, QC Mid; QCH, QC High.
3.1.4. Selectivity and specificity
Chromatograms of six individual control plasma samples contained no co-eluting peaks >20% of the analyte areas at the LLOQ concentration (interference ≤10.1%) and no co-eluting peaks >5% of the IS area (interference ≤0.30%).
The crosstalk of the 50,000 ng/mL IS into the elimusertib channel was 0.02%. Scaling this to the assay IS concentration of 200w into the IS MRM cannel and which would correspond to 0.35 ng/mL at the 5,000 ng/mL ULOQ (0.18% of the applied IS concentration). In the presence of high concentrations of 5-fluorouracil, irinotecan, gemcitabine, dFdU, and topotecan elimusertib could be quantitated accurately (104.1%) and precise CV 8.6%). The response of these compounds in control plasma resulted in a response of less than 0.5% of the LLOQ.
Carryover in the developed assay resulted in 0.061, 0.005, and 0.003% of the original 5,000 ng/mL sample signal in sequential blank plasma injections (Suppl.Fig. 3). Defining the maximum carryover as 20% of the LLOQ area, this would mean that any sample with a concentration of more than approximately 9700 ng/mL could result in unacceptable carryover into the next sample.
3.1.5. Extraction recovery and matrix effect
The recoveries of elimusertib ranged from 106.7% to 113.1% (CV 4.5% to 5.7%). The values above 100% are a result of intrinsic analytical or technician variability and because the reported values are a calculated ratio of 2 measurement results. Matrix effect ranged from −1.5% to 3.7% (CV 3.4% to 4.9%) (Table 2).
Table 2.
Recoveries of elimusertib from human plasma and respective matrix effects in human plasma extract, with coefficients of variation (CV).
| Concentration (μg/mL) |
Recovery (%) |
CV (%) |
Matrix effect (%) |
CV (%) |
|---|---|---|---|---|
| 40 (QCL) | 106.7 | 4.8 | 3.7 | 4.2 |
| 200 (QCM | 110.0 | 5.7 | 1.6 | 4.9 |
| 4000 (QCH) | 113.1 | 4.5 | −1.5 | 3.4 |
n=4, for each concentration. QCL, QC Low; QCM, QC Mid; QCH, QC High
3.1.6. Stability
Stock solution stability of elimusertib in DMSO after 6 h at RT was 100.8% (8.7% CV) and after 12 months at −80 °C was 91.8 % (5.7 % CV) with both experiments utilizing replicates of 4. The stability of elimusertib in human plasma is reported in Table 3. Stability in plasma after 3 freeze thaw cycles (−80 °C to RT) was between 100.1 to 101.1% (CV ≤4.4%) using replicates of 4. Benchtop stability in plasma after 4 h at RT with ambient light ranged between 102.0 and 105.2% (CV ≤6.4%) using replicates of 4. Long-term stability of elimusertib in plasma at −80 °C was adequate with recovery between 92.9 to 103.9% using replicates of 4.. The ratio of analyte to IS of plasma extracts of elimusertib at QC levels, when reconstituted and kept in the autosampler for 72 h, ranged from 96.1 to 102.4% (CV ≤6.6%) of the initial response, while with fresh calibration curves, the range was 91.4 to 95.3% (CV ≤5.2%) of the initial response while also passing the requirements set by the FDA (accuracy ± 15% of nominal) (U.S. Department of Health and Human Services Food and Drug Administration 2018). These autosampler stability experiments utilized replicates of 6.
Table 3.
Stability of elimusertib in human plasma under varying conditions.
| Storage Condition | Concentration (ng/mL) |
Stability (%) |
CV (%) |
|---|---|---|---|
| 4 h ambient temp | 40 (QCL) | 105.2 | 6.4 |
| 200 (QCM) | 102.0 | 2.1 | |
| 4000 (QCH) | 104.5 | 2.0 | |
| 3 freeze-thaw cycles −80 °C | 40 (QCL) | 100.4 | 4.4 |
| 200 (QCM) | 101.1 | 3.7 | |
| 4000 (QCH) | 100.1 | 1.2 | |
| 72 h extract reinjection vs reinjected calibrators | 40 (QCL) | 96.3 | 6.6 |
| 200 (QCM) | 103.3 | 4.1 | |
| 4000 (QCH) | 102.4 | 3.6 | |
| 72 h extract reinjection vs fresh calibrators | 40 (QCL) | 93.6 | 5.0 |
| 200 (QCM) | 91.4 | 3.9 | |
| 4000 (QCH) | 92.3 | 3.7 | |
| 12 months at −80 °C | 40 (QCL) | 103.9 | 5.9 |
| 200 (QCM) | 100.6 | 4.1 | |
| 4000 (QCH) | 92.9 | 4.9 |
QCL, QC Low; QCM, QC Mid; QCH, QC High. Stability experiments were performed in replicates of n=4, except for autosampler stability which were done in replicates of n=6.
3.1.7. Additional validation items
Hemolyzed plasma did not affect quantitation with an accuracy of 99.6-103.0% (CV ≤6.4%) among tested concentrations. Heparinized plasma was free of interference and calibrators prepared in heparinized plasma used to quantitate QC samples prepared in EDTA plasma resulted in adequate performance with an accuracy of 89.2 to 93.7% (CV ≤ 4.0%). Urine, diluted 1:10 v/v with control plasma, did not impact quantitation with an accuracy of 106.1 to 112.0% (CV ≤6.4%), suggesting urine samples can be analyzed after 10-fold dilution in control plasma. Dilution integrity of the assay was confirmed with 104.8% accuracy (CV of 5.4%). The qualifier transition (m/z 376.3>277.3) had an intensity of ~65% of the quantifier transition m/z 376.3>318.2, with a suggested tolerance of ±20% . Across QCL, QCM and QCH samples, N = 6 each, the mean relative ion intensity of the qualifier was 65.8% (range 57.3-70.7%), well within tolerance. Endogenous phospholipids did not co-elute with phospholipid MRM channels, limiting the risk of interference (Suppl.Fig. 4).
3.2. Development
Development began with mass spectroscopic tuning to optimize the parent and product ions for both elimusertib and IS. Infusion solvents of 50/50 water/methanol (v/v) or 50/50 water/acetonitrile (v/v) resulted in similar ionization intensity. Spectra from MS scan in positive ionization mode revealed modestly large m/z 376.3 and 380.3 protonated ion intensities for elimusertib and IS, respectively. Product ion scans for the parent compound revealed a predominant product ion trace of m/z 318.2 resulting in a selected elimusertib MRM channel of m/z 376.3>318.2. A similar fragmentation pattern in the IS resulted in selecting an MRM channel of m/z 380.3>322.2. For qualitative purposes, a less sensitive elimusertib product ion was selected and included in the MS method. Acetonitrile was selected as the organic mobile phase component due to its capacity to reduce backpressure.
The LC method was first explored by injections of neat standards into the LC-MS/MS system using a Phenomenex Kinetex C18, 2.6 μ, 2.1 x 50 mm column. Although peak shapes were reproducible, the compound was highly sensitive to the composition of organic mobile phase with limited retention at an isocratic 75:25 (v/v) water:acetonitrile, 0.1% formic acid mobile phase. A simple gradient program was utilized that allowed ample retention, 1.74 min, where from 0 to 2.5 min the organic mobile phase increased from 10% to 90% with a flow rate of 0.5 mL/min, followed by re-equilibration for an additional 1.5 min at the initial 90% aqueous conditions (total run time 4 min) at flow rate of 1.0 mL/min. This LC method was used to compare Phenomenex Hydro RP (50 mm) and Phenomenex Polar RP (50 mm) columns and both resulted in increased retention and minimally better peak shape (reduced tailing). Although the Hydro RP column was initially chosen for development, batch variance in the column produced inconsistent retention and thus the Polar RP column was chosen for further validation.
Analytical carry-over was identified upon further testing which was minimized by two changes to the method. First, a longer wash and equilibration steps were integrated into the LC method. Secondly, the needle wash was changed from plain acetonitrile to acetonitrile:isopropanol:acetone 40:40:20 (v/v/v). Together, these steps reduced carry-over.
An LLOQ of 10 ng/mL was initially targeted which demonstrated an approximate 5:1 signal to noise. To enhance robustness, the LLOQ was raised to 30 ng/mL. The 30-5,000 ng/mL assay range is expected to cover expected clinical concentrations based on available clinical PK data of the first-in-human elimusertib trial (Yap, et al. 2020b). Our assay ULOQ of 5,000 ng/mL would comfortably capture the multiple dose Cmax of 3,255 ng/mL (n=1) at the highest investigated dose of 80 mg BID as well as the mean (n=2) 1,840 ng/mL multiple-dose Cmax for the 40 mg BID MTD. The assay LLOQ of 30 ng/mL was able easily capture the complete profile of the our patient at the lowest dose of 20 mg, see next section.
3.3. Application of the assay
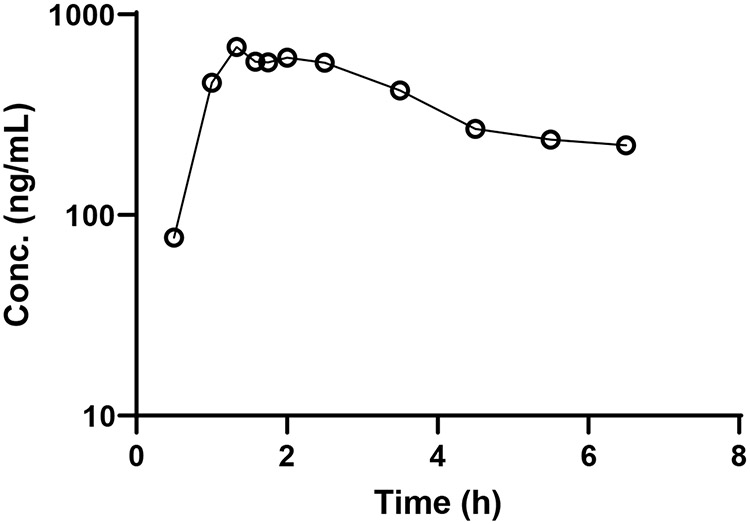
All concentrations between 0.5 and 6.5 h post-administration were within the assay range (Fig. 2). The patient had a Cmax of 688 ng/mL occurring 80 min post dose which agrees with the previously reported Cmax 569 ng/mL when elimusertib is administered alone (Yap, et al. 2020b). The derived AUC0-6.5 was 2,366 ng•h/mL and AUC0-∞ was 4,744 ng•h/mL. The AUC0-12 for the dosing interval was calculated to be 3,345 ng•h/mL which is relatively similar to the previously reported mean AUC0-12 of 4,060 ng•h/mL at the same dose level. The calculated half-life from this patient was 7.4 h which was slightly shorter than the previously reported 8.6 to 17.8 h range of half-lives. Overall, the assay proved appropriate to accurately quantitate elimusertib in plasma.
Fig. 2.
Elimusertib (○) plasma concentrations in a patient administered 20 mg of elimusertib PO.
4. Conclusion
We developed and validated a clinically relevant LC-MS/MS assay to quantitate elimusertib in plasma that is both accurate and reliable which will support several clinical trials (Clinicaltrails.gov identifiers NCT04491942, NCT04616534, NCT04514497, and NCT04535401). In summary, the assay reported here was developed and validated to the criteria of the most recent FDA guidance and proved able to accurately capture clinically relevant concentrations. This method will offer investigators a reliable, accurate and precise method of quantitating elimusertib and determining its PK in both clinical and preclinical settings.
Supplementary Material
Funding
Support: AFPE funding, Tobacco grant, Grants UM1 CA186690 and U24 CA247643 (NCI-CTEP) and R50 CA211241 (NCI). This project used the UPMC Hillman Cancer Center’s Cancer Pharmacokinetics and Pharmacodynamics Facility (CPPF) and was supported in part by award P30 CA47904.
5 References
- Commission Decision (2002/657/EC) of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results: Brussels, Belgium, 2002. [Google Scholar]
- Alavijeh MS and Palmer AM The pivotal role of drug metabolism and pharmacokinetics in the discovery and development of new medicines. IDrugs 2004; 7 (8): 755–63. [PubMed] [Google Scholar]
- Awasthi P, Foiani M and Kumar A ATM and ATR signaling at a glance. J Cell Sci 2015; 128 (23): 4255–62. DOI: 10.1242/jcs.169730. [DOI] [PubMed] [Google Scholar]
- Bouwman P and Jonkers J The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer 2012; 12 (9): 587–98. DOI: 10.1038/nrc3342. [DOI] [PubMed] [Google Scholar]
- Canman CE Replication checkpoint: preventing mitotic catastrophe. Curr Biol 2001; 11 (4): R121–4. DOI: 10.1016/s0960-9822(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Cheung-Ong K, Giaever G and Nislow C DNA-damaging agents in cancer chemotherapy: serendipity and chemical biology. Chem Biol 2013; 20 (5): 648–59. DOI: 10.1016/j.chembiol.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Dillon MT, Bergerhoff KF, Pedersen M, Whittock H, Crespo-Rodriguez E, Patin EC, Pearson A, Smith HG, Paget JTE, Patel RR, Foo S, Bozhanova G, Ragulan C, Fontana E, Desai K, Wilkins AC, Sadanandam A, Melcher A, McLaughlin M and Harrington KJ ATR Inhibition Potentiates the Radiation-induced Inflammatory Tumor Microenvironment. Clinical Cancer Research 2019; 25 (11): 3392–3403. DOI: 10.1158/1078-0432.ccr-18-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon MT, Boylan Z, Smith D, Guevara J, Mohammed K, Peckitt C, Saunders M, Banerji U, Clack G, Smith SA, Spicer JF, Forster MD and Harrington KJ PATRIOT: A phase I study to assess the tolerability, safety and biological effects of a specific ataxia telangiectasia and Rad3-related (ATR) inhibitor (AZD6738) as a single agent and in combination with palliative radiation therapy in patients with solid tumours. Clin Transl Radiat Oncol 2018; 12: 16–20. DOI: 10.1016/j.ctro.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne V, Ghita M, Small DM, Coffey CBM, Weldon S, Taggart CC, Osman SO, McGarry CK, Prise KM, Hanna GG and Butterworth KT Inhibition of ataxia telangiectasia related-3 (ATR) improves therapeutic index in preclinical models of non-small cell lung cancer (NSCLC) radiotherapy. Radiother Oncol 2017; 124 (3): 475–481. DOI: 10.1016/j.radonc.2017.06.025. [DOI] [PubMed] [Google Scholar]
- Gourley C, Balmana J, Ledermann JA, Serra V, Dent R, Loibl S, Pujade-Lauraine E and Boulton SJ Moving From Poly (ADP-Ribose) Polymerase Inhibition to Targeting DNA Repair and DNA Damage Response in Cancer Therapy. J Clin Oncol 2019; 37 (25): JCO1802050. DOI: 10.1200/JCO.18.02050. [DOI] [PubMed] [Google Scholar]
- Kwok M, Davies N, Agathanggelou A, Smith E, Oldreive C, Petermann E, Stewart G, Brown J, Lau A, Pratt G, Parry H, Taylor M, Moss P, Hillmen P and Stankovic T ATR inhibition induces synthetic lethality and overcomes chemoresistance in TP53- or ATM-defective chronic lymphocytic leukemia cells. Blood 2016; 127 (5): 582–95. DOI: 10.1182/blood-2015-05-644872. [DOI] [PubMed] [Google Scholar]
- Kwok M, Davies N, Agathanggelou A, Smith E, Petermann E, Yates E, Brown J, Lau A and Stankovic T Synthetic lethality in chronic lymphocytic leukaemia with DNA damage response defects by targeting the ATR pathway. Lancet 2015; 385 Suppl 1: S58. DOI: 10.1016/S0140-6736(15)60373-7. [DOI] [PubMed] [Google Scholar]
- Li Z, Pearlman AH and Hsieh P DNA mismatch repair and the DNA damage response. DNA Repair (Amst) 2016; 38: 94–101. DOI: 10.1016/j.dnarep.2015.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little JL, Wempe MF and Buchanan CM Liquid chromatography-mass spectrometry/mass spectrometry method development for drug metabolism studies: Examining lipid matrix ionization effects in plasma. J Chromatogr B Analyt Technol Biomed Life Sci 2006; 833 (2): 219–30. DOI: 10.1016/j.jchromb.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Lucking U, Wortmann L, Wengner AM, Lefranc J, Lienau P, Briem H, Siemeister G, Bomer U, Denner K, Schafer M, Koppitz M, Eis K, Bartels F, Bader B, Bone W, Moosmayer D, Holton SJ, Eberspacher U, Grudzinska-Goebel J, Schatz C, Deeg G, Mumberg D and von Nussbaum F Damage Incorporated: Discovery of the Potent, Highly Selective, Orally Available ATR Inhibitor BAY 1895344 with Favorable Pharmacokinetic Properties and Promising Efficacy in Monotherapy and in Combination Treatments in Preclinical Tumor Models. J Med Chem 2020; 63 (13): 7293–7325. DOI: 10.1021/acs.jmedchem.0c00369. [DOI] [PubMed] [Google Scholar]
- McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA and Harrington KJ Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer 2020; 20 (4): 203–217. DOI: 10.1038/s41568-020-0246-1. [DOI] [PubMed] [Google Scholar]
- Mei L, Zhang J, He K and Zhang J Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand. J Hematol Oncol 2019; 12 (1): 43. DOI: 10.1186/s13045-019-0733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton FK, Pollard JR and Curtin NJ The Impact of p53 Dysfunction in ATR Inhibitor Cytotoxicity and Chemo- and Radiosensitisation. Cancers (Basel) 2018; 10 (8): 1–13. DOI: 10.3390/cancers10080275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Oleinick NL and Zhang J ATR/CHK1 inhibitors and cancer therapy. Radiother Oncol 2018; 126 (3): 450–464. DOI: 10.1016/j.radonc.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaper PM, Griffiths MR, Long JM, Charrier JD, Maccormick S, Charlton PA, Golec JM and Pollard JR Selective killing of ATM- or p53-deficient cancer cells through inhibition of ATR. Nat Chem Biol 2011; 7 (7): 428–430. DOI: 10.1038/nchembio.573. [DOI] [PubMed] [Google Scholar]
- Sheng H, Huang Y, Xiao Y, Zhu Z, Shen M, Zhou P, Guo Z, Wang J, Wang H, Dai W, Zhang W, Sun J and Cao C ATR inhibitor AZD6738 enhances the antitumor activity of radiotherapy and immune checkpoint inhibitors by potentiating the tumor immune microenvironment in hepatocellular carcinoma. J Immunother Cancer 2020; 8 (1): 1–13. DOI: 10.1136/jitc-2019-000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SS Preclinical pharmacokinetics: an approach towards safer and efficacious drugs. Curr Drug Metab 2006; 7 (2): 165–82. DOI: 10.2174/138920006775541552. [DOI] [PubMed] [Google Scholar]
- Smith J, Tho LM, Xu N and Gillespie DA The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv Cancer Res 2010; 108: 73–112. DOI: 10.1016/B978-0-12-380888-2.00003-0. [DOI] [PubMed] [Google Scholar]
- Sun LL, Yang RY, Li CW, Chen MK, Shao B, Hsu JM, Chan LC, Yang Y, Hsu JL, Lai YJ and Hung MC Inhibition of ATR downregulates PD-L1 and sensitizes tumor cells to T cell-mediated killing. Am J Cancer Res 2018; 8 (7): 1307–1316. [PMC free article] [PubMed] [Google Scholar]
- Toledo LI, Murga M, Zur R, Soria R, Rodriguez A, Martinez S, Oyarzabal J, Pastor J, Bischoff JR and Fernandez-Capetillo O A cell-based screen identifies ATR inhibitors with synthetic lethal properties for cancer-associated mutations. Nat Struct Mol Biol 2011; 18 (6): 721–727. DOI: 10.1038/nsmb.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services Food and Drug Administration. Guidance for Industry-Bioanalytical Method Validation, U.S.Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Veterinary Medicine (CVM), 2018. [Google Scholar]
- Vendetti FP, Karukonda P, Clump DA, Teo T, Lalonde R, Nugent K, Ballew M, Kiesel BF, Beumer JH, Sarkar SN, Conrads TP, O'Connor MJ, Ferris RL, Tran PT, Delgoffe GM and Bakkenist CJ ATR kinase inhibitor AZD6738 potentiates CD8+ T cell-dependent antitumor activity following radiation. J Clin Invest 2018; 128 (9): 3926–3940. DOI: 10.1172/JCI96519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendetti FP, Leibowitz BJ, Barnes J, Schamus S, Kiesel BF, Abberbock S, Conrads T, Clump DA, Cadogan E, O'Connor MJ, Yu J, Beumer JH and Bakkenist CJ Pharmacologic ATM but not ATR kinase inhibition abrogates p21-dependent G1 arrest and promotes gastrointestinal syndrome after total body irradiation. Sci Rep 2017; 7: 41892. DOI: 10.1038/srep41892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wengner AM, Siemeister G, Lucking U, Lefranc J, Wortmann L, Lienau P, Bader B, Bomer U, Moosmayer D, Eberspacher U, Golfier S, Schatz CA, Baumgart SJ, Haendler B, Lejeune P, Schlicker A, von Nussbaum F, Brands M, Ziegelbauer K and Mumberg D The Novel ATR Inhibitor BAY 1895344 Is Efficacious as Monotherapy and Combined with DNA Damage-Inducing or Repair-Compromising Therapies in Preclinical Cancer Models. Mol Cancer Ther 2020; 19 (1): 26–38. DOI: 10.1158/1535-7163.MCT-19-0019. [DOI] [PubMed] [Google Scholar]
- Yap TA, O'Carrigan B, Penney MS, Lim JS, Brown JS, de Miguel Luken MJ, Tunariu N, Perez-Lopez R, Rodrigues DN, Riisnaes R, Figueiredo I, Carreira S, Hare B, McDermott K, Khalique S, Williamson CT, Natrajan R, Pettitt SJ, Lord CJ, Banerji U, Pollard J, Lopez J and de Bono JS Phase I Trial of First-in-Class ATR Inhibitor M6620 (VX-970) as Monotherapy or in Combination With Carboplatin in Patients With Advanced Solid Tumors. J Clin Oncol 2020a; 38 (27): 3195–3204. DOI: 10.1200/JCO.19.02404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap TA, Tan DS, Terbuch A, Caldwell R, Guo C, Goh BC, Heong V, Haris NRM, Bashir S, Drew Y, Hong DS, Meric-Bernstam F, Wilkinson G, Hreiki J, Wengner AM, Bladt F, Schlicker A, Ludwig M, Zhou Y, Liu L, Bordia S, Plummer R, Lagkadinou E and de Bono JS First-in-Human Trial of the Oral Ataxia Telangiectasia and Rad3-Related Inhibitor BAY 1895344 in Patients with Advanced Solid Tumors. Cancer Discov 2020b; 11 (1): 80–91. DOI: 10.1158/2159-8290.CD-20-0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G and Wujcik CE Overcoming ionization effects through chromatography: a case study for the ESI-LC-MS/MS quantitation of a hydrophobic therapeutic agent in human serum using a stable-label internal standard. J Chromatogr B Analyt Technol Biomed Life Sci 2009; 877 (22): 2003–10. DOI: 10.1016/j.jchromb.2009.05.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.