Abstract
Patients with localized juvenile periodontitis (LJP) have elevated levels of immunoglobulin G2 (IgG2) in their sera. This is also observed in vitro when peripheral blood leukocytes from LJP patients are stimulated with pokeweed mitogen. In previous studies, we showed that lymphocytes from subjects with no periodontitis (NP subjects) produced substantial amounts of IgG2 when they were cultured with monocytes from LJP patients (LJP monocytes). These observations indicate that monocytes or monocyte-derived mediators are positive regulators of the production of IgG2. The present study was initiated to determine if secreted factors from LJP monocytes were capable of enhancing IgG2 production and to determine if prostaglandin E2 (PGE2), which LJP monocytes produce at elevated levels, enhances IgG2 production. Experiments in a transwell system and with monocyte-conditioned media indicated that cell-cell contact was not necessary for LJP monocytes to augment the production of IgG2 by T and B cells from NP subjects. Moreover, the production of IgG2 was selectively induced by the addition of PGE2 or platelet-activating factor (PAF), another lipid cytokine, which can elevate PGE2 synthesis. Furthermore, IgG2 production was abrogated when cells were treated with indomethacin, a cyclooxygenase inhibitor that blocks the synthesis of PGE2, or the PAF antagonists CV3988 and TEPC-15. The effects of indomethacin were completely reversed by PGE2, indicating that this is the only prostanoid that is essential for the production of IgG2. Similarly, PGE2 reversed the effects of a PAF antagonist, suggesting that the effects of PAF are mediated through the induction of PGE2 synthesis. Together, these data indicate that PGE2 and PAF are essential for the production of IgG2.
Recent results suggest that early-onset periodontitis (EOP) patients have a genetic predisposition to develop disease early in life (22). The clinical manifestations of EOP are variable. Even in the same family, some patients may have a localized form restricted to first molars and incisors (localized juvenile periodontitis [LJP]) and others a severe generalized form. It is likely that these differences in clinical expression are related to several factors, including differences in microbial flora and differences in the host response. Serum total immunoglobulin G2 (IgG2) levels in LJP patients are significantly elevated over those in race- and age-matched controls with no periodontitis (NP controls) (21). Much of this IgG2 antibody is directed against Actinobacillus actinomycetemcomitans serotype b, a putative etiologic agent for EOP, and Porphyromonas gingivalis, which is also associated with EOP (8, 20, 36, 37). The immunodominant antigens of these organisms are the serotype-specific carbohydrates, and, for A. actinomycetemcomitans, the carbohydrate is a high-molecular-weight form of lipopolysaccharide (LPS) (7, 8, 35, 36). Compared to NP subjects, EOP patients frequently have very high titers of IgG2 reactive with the high-molecular-weight A. actinomycetemcomitans LPS, and this antibody is associated with less-severe disease (6). This IgG2 antibody appears to be an effective opsonin when the appropriate Fcγ receptor is present (2, 34), which suggests that the ability to mount a high IgG2 response may help control infection and localize the disease (8, 15, 26).
We are interested in elucidating the biological mechanisms responsible for the increased IgG2 production in LJP patients, and we have developed an in vitro culture system to help address this issue. When peripheral blood leukocytes (PBL) are cultured in the presence of pokeweed mitogen (PWM; a polyclonal activator), two- to threefold more IgG2 is produced by cells from LJP patients than by cells from NP subjects (38). This increase is consistent with the ratio of IgG2 in the sera of LJP patients to that in sera of NP subjects (21). Although purified T and B cells from LJP subjects produce large amounts of IgG2 in the absence of monocytes, T and B cells from NP subjects (NP T and B cells) require monocyte help to produce IgG2. However, if NP T and B cells are cultured in the presence of monocytes from an LJP subject (LJP monocytes), their levels of IgG2 production approach that of LJP PBL. These observations suggest that the monocytes of LJP and NP subjects are phenotypically different. It is possible that these differences arise because the cells are exposed to different in vivo environments. Alternatively, the LJP monocyte phenotype may stem from genetic abnormalities in these patients. Interestingly, the myeloid cells of LJP patients are known to be somewhat abnormal. Among these abnormalities is the production of unusually large amounts of prostaglandin E2 (PGE2) by both resting and LPS-activated LJP monocytes (30). These observations prompted the hypothesis that PGE2 could be responsible, at least in part, for the increased IgG2 production that is observed in LJP patients.
Our data indicate that LJP monocytes do indeed secrete soluble factors that promote the production of IgG2. Addition of PGE2 or platelet-activating factor (PAF), which can induce the synthesis of PGE2, selectively induced the production of IgG2 by NP PBL. Furthermore, IgG2 production was abrogated when cells were treated with indomethacin or PAF antagonists and the effects of the antagonists were reversed by the addition of PGE2. In short, the data reported here indicate that PGE2 is essential for the production of IgG2.
MATERIALS AND METHODS
Human subjects.
Subjects for study were obtained by the Clinical Research Center for Periodontal Disease, School of Dentistry, Medical College of Virginia, Virginia Commonwealth University, Richmond, Va. Patients with LJP were 30 years old or less and had localized patterns of severe periodontal destruction limited to the first molar or incisor teeth and up to two additional teeth. The NP control subjects were age matched and had no evidence of attachment loss, except for recession on the buccal surfaces of anterior teeth at no more than one site and no pocket greater than 3 mm. Included were 33 black LJP subjects, 28 black NP subjects, and 29 white NP subjects. Different LJP and NP subjects were used to replicate data within an experimental group.
Lymphocyte separation.
PBL were obtained from heparinized blood of LJP or NP subjects by density centrifugation using lymphocyte separation medium (Organon Teknika Corporation, Durham, N.C.). The blood cells were centrifuged at 400 × g for 20 min, and the PBL were collected from the interface and washed three times in Hanks' balanced salt solution. After being washed the PBL were suspended in RPMI 1640 supplemented with fetal calf serum (FCS) and antibiotics for cell culture as explained in “Cell culture” below.
Magnetic cell separation. (i) Antibody labeling.
A magnetic cell separation system (Miltenyi Biotec GmbH, Gladbach, Germany) was used to separate lymphocytes and monocytes. PBL suspended in 5% FCS (Hyclone, Logan, Utah) in phosphate-buffered saline (PBS) (FCS-PBS) were incubated with superparamagnetic beads conjugated with antibodies against CD3, CD4, and CD8 molecules (for T-cell depletion), CD19 molecules (for B-cell depletion), or CD14 molecules (for monocyte depletion). All antibodies were purchased from Miltenyi Biotec GmbH. For each 107 cells, 10 μl of the stock solution provided by Miltenyi Biotec GmbH was used. The cells were washed once with 10 ml of FCS-PBS, suspended in 2 ml of FCS-PBS, and loaded in magnetic columns that were seated in a strong magnetic field.
(ii) Depletion of T cells, B cells, and monocytes.
The columns were first incubated with 70% ethanol for 30 min to minimize microbial contamination and then washed with sterile FCS-PBS. The magnetic microbead-labeled cells were loaded, and the columns were then washed with 20 ml of FCS-PBS. The labeled cells were trapped in the columns, and the cells in the eluates were used in these studies. The T and B cells were defined as the cells remaining after monocytes were depleted using anti-CD14. Monocytes were defined as the cells remaining after depletion of both T and B cells using anti-CD3, -CD4, -CD8, and -CD19.
Analysis of cell population by flow cytometry.
Cells in the eluates were analyzed using flow cytometry (FACScan; Becton Dickinson, San Jose, Calif.). Cells (0.25 × 106) were incubated with 5 μl of fluorescein isothiocyanate- (FITC) or phycoerythrin (PE)-conjugated antibodies against CD3, -19 or -14 (PharMingen, San Diego, Calif.) for 30 min on ice in the dark. The cells were washed once with PBS containing 5% bovine serum albumin and resuspended with 750 μl of PBS (Gibco, Grand Island, N.Y.). The cells were then identified on the basis of FITC or PE emission and light scatter analysis. Approximately 10,000 cells were included in each analysis. Typically the depletion of T cells and B cells was in excess of 95%, and depletion of the monocytes was typically over 90%.
Cell culture.
PBL (106) were resuspended in RPMI 1640 (Mediatech, Herndon, Va.) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 2 mM glutamine, 100 U of penicillin/ml, and 100 mg of streptomycin sulfate (Sigma, St. Louis, Mo.)/ml and cultured in 75-mm tubes (Falcon, Lincoln Park, N.J.) in a volume of 1 ml/tube at 37°C in a humidified atmosphere of 5% CO2. Nine microliters of PWM was added to the cell cultures to induce antibody production. The cultures were incubated for 7 days, and then supernatant fluids were collected and assayed for IgG2. In some experiments, cells were cultured in the presence of PGE2 (Sigma), PAF (Sigma), CV3988 (Biomol, Plymouth Meeting, Pa.), indomethacin (Sigma), or TEPC-15 (Sigma). In these cases, a control culture consisting of a vehicle or nonspecific antibody control was included. Each experimental group was run in triplicate.
Transwell experiments.
The transwell system consists of a semipermeable membrane (pore size of 0.2 μm) that separates two chambers of a tissue culture plate (Costar, Cambridge, Mass.). The membrane allows cell culture media to pass between the chambers while preventing contact of the monocytes with the T and B cells. The transwell cultures consisted of 0.8 × 106 T and B lymphocytes in the lower chamber and 0.2 × 106, 0.4 × 106, or 0.8 × 106 monocytes in the upper chamber. The cells were cultured in the presence of PWM for 7 days, and the supernatant fluids were collected and assayed for IgG2 as outlined below.
Preparation of monocyte-conditioned media.
Monocytes (0.2 × 106) were cultured in the presence of 9 μl of PWM, and the supernatant fluids were collected after 3 days. For cell culture experiments, 500 μl of the supernatant fluids was added to 500 μl of fresh medium that contained 0.8 × 106 T and B cells depleted of monocytes and fresh PWM. The cells were cultured for 7 days, and the culture supernatant fluids were collected and assayed for IgG.
IgG assays.
IgG levels in supernatant fluids were determined with a sandwich enzyme-linked immunosorbent assay. Briefly, Fc-specific goat anti-mouse antibody (10 μl/ml) (ICN, Aurora, Ohio) was placed in a carbonate buffer (pH 9.6) and used to coat the microtiter plates (Dynex, Chantilly, Va.). Mouse anti-human IgG1 and IgG2 monoclonal antibodies (1 μg/ml) (IgG1 was from Zymed Laboratories, Inc., San Francisco, Calif., and IgG2 was from Sigma) were then added to the plates to serve as capture antibodies. Plates were incubated overnight at 4°C, followed by a 1-h application of 5% bovine serum albumin–PBS to minimize nonspecific binding at room temperature. A known standard for human IgG1 and IgG2 antibodies and a control (pooled human sera) (The Binding Site, San Diego, Calif.) and the culture supernatant fluids were applied to different wells in plates and incubated for 1 h at room temperature. Horseradish peroxidase-conjugated goat anti-human Ig (Sigma) was added, and the plates were incubated for 1 h at room temperature. Horseradish peroxidase-conjugated goat anti-human Ig (Sigma) was added, and the plates were incubated for 1 h at room temperature. The plates were washed three times with PBS containing 0.05% Tween 20 between each step. The color was developed using O-phenylenediamine dihydrochloride (Sigma) as the substrate. The optical density at 450 nm was measured using a microplate reader (Dynex). The IgG concentration in each test sample was calculated using the standard curve generated in each assay.
Statistical analysis.
All experiments were replicated with at least three different NP or LJP subjects. In each experiment, the cultures were set up in triplicate and the mean and standard deviation of each set of replicates were determined. Groups of NP and LJP subjects differ in the level of IgG2 produced, but considerable individual-to-individual variation exists. We found that the use of percentages helped normalize the data and allowed us to use smaller numbers of subjects for individual experiments. The control IgG2 response (defined as 100%) induced in the PBL by PWM (alone) is indicated in the table footnotes and figure legends so that the actual IgG2 level under each treatment can be readily calculated. Statistical significance was determined through t tests, with P < 0.05 as the cutoff for significance.
RESULTS
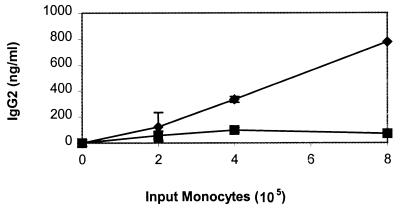
In a previous study, we determined that monocytes from LJP patients could enhance the production of IgG2 by T and B cells from periodontally healthy subjects (38). As monocytes are known to secrete many immunoregulatory cytokines, experiments were performed to determine if LJP monocytes could exert this effect in the absence of cell-cell contact. Two approaches were used. In the first approach, a transwell system provided a physical barrier between LJP or NP monocytes and T and B cells from NP subjects (Fig. 1). In the absence of monocytes, the NP T and B cells produced minimal amounts of IgG2 (data not shown). However, the addition of LJP monocytes induced a dose-dependent increase in IgG2 production by the NP T and B cells. In contrast, when NP monocytes were added to the cultures, only a modest level of IgG2 production was observed. In the second approach, NP T and B cells were cultured in the presence of conditioned media from LJP or NP monocytes (Table 1). T and B cells cultured in the presence of NP monocyte-conditioned media produced the same amount of IgG2 as NP PBL. In contrast, LJP monocyte-conditioned media induced an approximately threefold increase in IgG2 production. Together, these data indicate that LJP monocytes secrete one or more factors that promote the production of IgG2.
FIG. 1.
Cell-cell contact is not required for the induction of IgG2 by LJP monocytes. Monocytes and lymphocytes (T and B cells) were added to separate chambers of a transwell plate. PWM was added to stimulate the production of IgG2. The cells were cultured for 7 days, and then the supernatant fluids were harvested and IgG2 was quantified. The data shown are compiled from six independent experiments with different LJP (diamonds) and NP (squares) subjects.
TABLE 1.
Effect of monocyte supernatant fluids on IgG2 productiona
| Cultured NP lymphocytes | Origin of Mφb fluids | Mean IgG2 concn (ng/ml) ± SE | n | P |
|---|---|---|---|---|
| PBL | None | 136 ± 10 | 6 | |
| T + B | NP Mφ | 146 ± 20 | 6 | NSd |
| T + B | LJP Mφ | 422 ± 49c | 5 | <0.01 |
The final culture contained 50% fresh medium and 50% supernatant fluid.
Mφ, macrophage.
Significantly different from PBL cultured in the absence of macrophage supernatant fluids.
NS, not significantly different from PBL cultured in the absence of macrophage supernatant fluids.
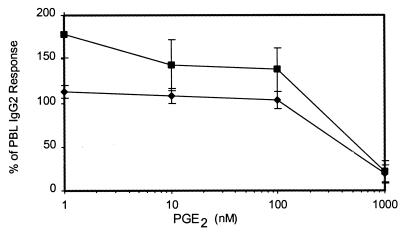
Several lines of evidence indicate that the periodontal tissues of LJP patients contain elevated amounts of PGE2 (9, 24, 25). Cells of the macrophage/monocyte lineage are a major source of PGE2, and a recent study indicates that peripheral blood monocytes from LJP patients produce more PGE2 than cells from periodontally healthy subjects (30). Therefore, experiments were performed to determine if IgG2 production could be enhanced by PGE2. PBL from NP and LJP subjects were cultured in the absence and presence of various doses of PGE2. Although high doses (>10−6 M) were inhibitory, IgG2 production by NP PBL was enhanced by submicromolar levels of PGE2 (Fig. 2). In contrast, LJP PBL produced similar amounts of IgG2 in the presence and absence of PGE2.
FIG. 2.
PGE2 promotes the production of IgG2 by NP PBL but not LJP PBL. PBL from NP and LJP subjects were cultured with PWM in the presence of various doses of PGE2. After 7 days, the supernatant fluids were collected and IgG2 was quantified as described in Materials and Methods. The results were normalized by representing data as percentages of the IgG2 response in NP or LJP PBL cultured in the absence of PGE2. Thus, the control response in the absence of PGE2 equals 100%. For NP subjects, the 100% response was 442 ng of IgG2/ml and individual results ranged from 200 to 1,000 ng/ml. For LJP subjects, the 100% response was 1,200 ng of IgG2/ml and individual results ranged from 550 to 1,430 ng/ml. These results were compiled from seven different LJP subjects (diamonds) and five different NP subjects (squares). Means ± standard errors are shown.
Monocytes are also a source of PAF, a phospholipid cytokine that regulates cellular function by binding to a G protein-linked receptor (33). In a previous study, we demonstrated that PAF induces murine macrophages to produce PGE2 (3). Therefore, an experiment was performed to determine if PAF could also promote the production of IgG2. PBL from NP and LJP subjects were cultured in the absence and presence of various doses of PAF. As is shown in Table 2, PAF augmented IgG2 production by NP PBL but not by LJP PBL. This was the same pattern as that observed when LJP and NP PBL were cultured with PGE2 (Fig. 2). Together, these data indicated that IgG2 production was positively regulated by the lipid cytokines PAF and PGE2. Furthermore, these effects appeared to be specific for IgG2, as PGE2 and PAF had no effect on IgG1 production (Table 3).
TABLE 2.
Induction of IgG2 by PAFa
| PAF concn (nM) | NP PBL
|
LJP PBL
|
||||
|---|---|---|---|---|---|---|
| IgG2 induction (%) | n | P | IgG2 induction (%) | n | P | |
| Control | 100 | 7 | 100 | 4 | ||
| 10 | 144 ± 15b | 7 | <0.03 | 98 ± 4 | 4 | NSc |
| 100 | 123 ± 3b | 7 | <0.01 | 105 ± 5 | 4 | NS |
| 1,000 | 122 ± 22 | 6 | NS | 103 ± 3 | 4 | NS |
The data were normalized by presenting the results as the percentages of the IgG2 response of NP or LJP PBL cultured in the absence of PAF. Thus, the control response in the absence of PAF equals 100%. The 100% response for NP subjects was 339 ng of IgG2/ml, and individual results ranged from 160 to 570 ng/ml. For LJP subjects, the 100% response was 1,020 ng of IgG2/ml, and individual results ranged from 480 to 1,800 ng/ml. Means ± standard errors are shown.
Significantly different from control PBL.
NS, not significantly different from PBL.
TABLE 3.
Selective induction of IgG2 by PGE2 and PAFa
| Culture conditions | IgG1
|
IgG2
|
||||
|---|---|---|---|---|---|---|
| n | Induction (% of PBL response) | P | n | Induction (% of PBL response) | P | |
| PBL | 4 | 100 | 5 | 100 | ||
| PBL + PGE2 | 4 | 83 ± 15 | NSc | 5 | 208 ± 24b | 0.01 |
| PBL + PAF | 4 | 83 ± 13 | NS | 7 | 123 ± 3b | 0.05 |
PAF (100 nM) or PGE2 (1 nM) was added to PBL from an NP subject. The data were normalized by presenting the results as the percentages of the IgG2 response of NP PBL cultured in the absence of PAF or PGE2. The 100% responses for NP IgG1 and IgG2 were 821 ± 28 and 486 ± 112 ng/ml, respectively. All data are expressed as the means ± standard errors.
Significantly different from control PBL cultured in the absence of PGE2 or PAF.
NS, not significantly different from control PBL cultured in the absence of PGE2 or PAF.
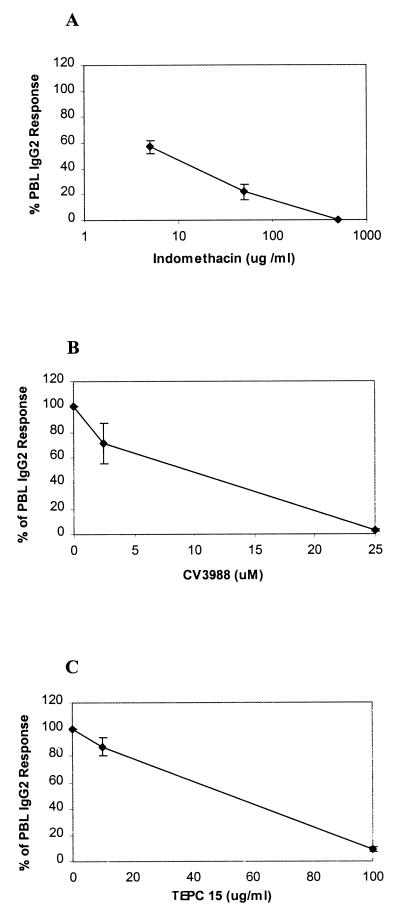
As IgG2 production was augmented by PGE2 and PAF, we reasoned that inhibitors which blocked the production or action of these lipid cytokines might also block the production of IgG2. Indomethacin, an inhibitor of the cyclooxygenase enzymes, blocks the production of PGE2 and other prostanoids. When LJP PBL were treated with indomethacin, a concentration-dependent reduction in IgG2 production was observed (Fig. 3A). The production of IgG2 was completely inhibited when PBL were treated with 500 μg of indomethacin/ml. Similarly, CV3988, a PAF receptor antagonist, reduced IgG2 production in a concentration-dependent manner in LJP PBL (Fig. 3B). The same effect was observed when LJP PBL were treated with TEPC-15 (Fig. 3C), an antibody directed against phosphorylcholine which is expected to bind the phosphorylcholine portion of the PAF molecule and to represent an anti-PAF. This expectation was supported by the observation that TEPC-15 binds proteins haptenated with phosphorylcholine and that PAF specifically inhibits TEPC-15 binding to the hapten (unreported observation). Hence, we anticipated that TEPC-15 would bind to PAF and prevent it from interacting with the PAF receptor. At the highest concentrations, CV3988 and TEPC-15 completely inhibited IgG2 production. The same inhibitory effects were seen when indomethacin, CV3988, or TEPC-15 was added to PWM-stimulated PBL cultures from NP subjects (data not shown). Together, these results suggest that the lipid cytokines PAF and PGE2 are essential for the production of IgG2.
FIG. 3.
PAF and PGE2 are essential for PWM-stimulated IgG2 production. PBL from LJP subjects were cultured with PWM in the presence of various doses of indomethacin (cyclooxygenase inhibitor that blocks PGE2 production), CV3988 (PAF receptor antagonist), or TEPC-15 (anti-PAF antibody). After 7 days of culture, the supernatant fluids were harvested and IgG2 was quantified. The data are presented as the percentages of IgG2 produced by cells that were cultured in the absence of the inhibitors. In all cases, vehicle controls (A and B) or an isotype control (C) was included and had no effect on IgG2 production. Means ± standard errors are shown. (A) indomethacin. Four LJP subjects were studied. The control (100%) response for LJP subjects was 430 ng of IgG2/ml, and individual results ranged from 210 to 700 ng/ml. (B) CV3988. Three LJP subjects were studied; the 100% response was 763 ng of IgG2/ml, and individual results were 1,100, 710, and 480 ng/ml. (C) TEPC-15. Three LJP subjects were studied; the 100% response was 1,355 ng of IgG2/ml, and individual results were 1,730, 1,420, and 915 ng/ml. The isotype control for TEPC-15 was a nonspecific IgA (obtained from PharMingen), which had no inhibitory effect on IgG2 production.
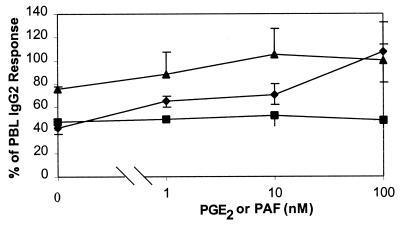
As indomethacin is a cyclooxygenase inhibitor, it is not specific for PGE2 but rather blocks the production of a family of potentially immunoregulatory molecules, the prostanoids. Therefore, experiments were performed to determine if PGE2 alone could reverse the inhibitory effects of indomethacin on IgG2 production. As shown in Fig. 4, PGE2 completely reversed the suppressive effects of indomethacin. In contrast, PAF had no effect on the inhibition of IgG2 production by indomethacin. In another set of experiments, cells were treated with 5 μM CV3988 (PAF antagonist) in the presence and absence of PGE2 (Fig. 4). Addition of 1 nM PGE2 completely reversed the effects of CV3988 (P < 0.04; paired t test of geometric means), and the data at 10 and 100 nM were consistent with reversal but did not reach statistical significance at these doses. Together, these data indicate that PGE2 is the essential prostanoid in IgG2 production and that the effects of PAF may be mediated through its ability to induce the production of PGE2.
FIG. 4.
PGE2 reverses the effects of indomethacin and CV3988. PBL from LJP subjects were stimulated with PWM in the presence of indomethacin (5 μg/ml) or CV3988 (5 μM). After 7 days of culture, the supernatant fluids were harvested and IgG2 was quantified as described in Materials and Methods. The data are presented as the percentages of IgG2 produced by cells that were cultured in the absence of inhibitors, PAF, or PGE2. Squares, indomethacin plus PAF (n = 5); diamonds, indomethacin plus PGE2 (n = 5); triangles, CV3988 plus PGE2 (n = 3).
DISCUSSION
In previous studies, we demonstrated that subjects with LJP have elevated levels of IgG2 in serum and that this phenomenon can also be seen in a PWM-stimulated in vitro culture system (21, 38). The increase in IgG2 production appears to be determined by LJP monocytes, which induce high levels of IgG2 production when they are added to T and B cells from healthy subjects (38). Experiments with transwell cultures and macrophage-conditioned media, reported in the present study, indicated that LJP macrophages produced soluble factors that promoted IgG2 production (Fig. 1 and Table 1). It is known that monocytes from LJP patients produce abnormally large amounts of PGE2 (30). Therefore, the transwell experiments were followed by studies to determine if PGE2 enhances IgG2 production. When PBL from NP subjects were cultured in the presence of exogenous PGE2 or PAF, a concentration-dependent increase in IgG2 production was observed. Furthermore, IgG2 production was abrogated when cells were treated with indomethacin or PAF antagonists and the effects of the antagonists were completely reversed by addition of PGE2. The effects of PGE2 and PAF on IgG2 production appear to be selective, as PGE2 and PAF have no effect on IgG1 production (Table 3). In brief, the data reported here support the concept that PGE2 and PAF are essential for the production of IgG2 and that PGE2 selectively promotes IgG2 production.
Like PGE2, PAF is a proinflammatory lipid that regulates cell behavior through a G protein-linked receptor (33). The production of IgG2 was abrogated by both a chemical PAF receptor antagonist (CV3988) and the TEPC-15 monoclonal antibody, which apparently binds phosphorylcholine on the PAF molecule and blocks its access to the receptor. PAF receptors are expressed by a variety of cells, including B cells, T cells, and macrophages (4, 33). Hence, PAF could modulate the behavior of any or all of these cells. Nevertheless, it appears that the effect of PAF on IgG2 production by B lymphocytes is indirect. Although PAF reversed the effects of the PAF receptor antagonist (data not shown), it had no effect on the inhibition of IgG2 production by indomethacin. However, PGE2 reversed the effects of both indomethacin and the PAF receptor antagonist. These data suggest that the effects of PAF on IgG2 production may be mediated through PGE2. Indeed, we previously demonstrated that PAF induces the production of PGE2 by murine macrophages through the activation of a secretory phospholipase A2 (sPLA2) (3). Interestingly, there are higher levels of sPLA2 activity in the sera of LJP subjects than in the sera of healthy subjects (S. E. Barbour, J. C. Gunsolley, H. A. Schenkein, and J. G. Tew, unpublished observations). It is tempting to speculate that there are elevated levels of PAF in LJP subjects which set off a cascade of events including the activation of sPLA2 and PGE2 production and culminate in increased secretion of IgG2 by B cells. If this is the case, then the elevated levels of IgG2 in the sera of LJP subjects that are observed may be attributed (at least in part) to the levels of PGE2 and PAF that are produced by monocytes in these subjects.
Interestingly, IgG2 production by LJP PBL was not affected by PAF or PGE2 (Fig. 2 and Table 2). This observation suggests that LJP PBL already produce quantities of the lipid mediators that are sufficient for IgG2 production and is consistent with a report indicating that monocytes from LJP patients make high levels of PGE2 (30). The observation that indomethacin, an inhibitor of both cyclooxygenase-1 and cyclooxygenase-2, completely suppressed IgG2 production by PWM-stimulated PBL suggests that cyclooxygenase products are positive regulators of IgG2 production. Although macrophages produce several cyclooxygenase products, the most abundant of these is PGE2. PGE2 completely reversed the effects of indomethacin, suggesting that it is the only cyclooxygenase product that is essential for the production of IgG2. However, it remains possible that IgG2 production is regulated by other eicosanoids. For example, macrophages produce leukotriene C4 and other products of the lipooxygenase enzymes that could regulate the production of IgG2. We will address this possibility in future studies.
Both macrophages and neutrophils are known to produce copious quantities of PAF (33). In LJP subjects, it is possible that these cells produce large amounts of PAF that drive the production of IgG2. However, the availability of PAF is also regulated by the rate of its catabolism. PAF is catabolized by PAF acetylhydrolase (PAF-AH), a form of PLA2. One form of PAF-AH is produced by macrophages and secreted into the serum (31). In our studies, PGE2 was somewhat more effective than PAF in augmenting IgG2 production, and this may be due to the presence of PAF-AH in the sera that were used in the cultures. The amount of PAF-AH secretion increases as monocytes mature into macrophages (12). Several lines of evidence indicate that the peripheral blood mononuclear cells of LJP subjects exhibit somewhat less-mature phenotypes than cells from healthy subjects (23). Hence, it is possible that these less-mature cells secrete less PAF-AH into the sera of LJP subjects. Indeed, we have preliminary data indicating that the mononuclear cells of LJP subjects contain less PAF-AH activity than cells from subjects who are periodontally healthy (S. E. Barbour, J. B. Zhang, Y. Ishihara, H. A. Schenkein, and J. G. Kew, unreported observation). Therefore, it is possible that there is more PAF available in LJP subjects and that this promotes the production of IgG2. This hypothesis is consistent with other studies indicating that the mononuclear cells of LJP subjects are phenotypically distinct from the cells of periodontally healthy subjects (10, 17, 23, 30, 32).
The mechanism of the induction of IgG2 production by PGE2 is not certain at present. As macrophages, T cells, and B cells express receptors for PGE2, it is possible that any or all of these cells could be affected by the eicosanoid (14, 16, 18). In murine systems, it is well established that PGE2 promotes isotype switching to IgE and IgG1 (19, 28). In part, this can be attributed to the direct binding of PGE2 to receptors on B cells (13). This interaction has been demonstrated to induce the production of germ line ε transcripts that then promote class switching to IgE (27). Furthermore, in both human and murine systems, the interaction of PGE2 with naive T cells induces differentiation to the Th2 phenotype (1, 11, 19, 29). These T cells produce interleukin-4 (IL-4), IL-5, and the other Th2 cytokines that promote the production of IgE and IgG1 (19). Given these data, it is challenging to envisage a role for PGE2 in the production of IgG2. However, one recent study indicates that subnanomolar concentrations of PGE2 enhance the production of gamma interferon (IFN-γ) by Th1 cells (5). These data are intriguing because IFN-γ is the cytokine most closely associated with IgG2 production (17, 20–22). Hence, it is possible that PGE2 interacts with receptors in preexisting Th1 cells or B cells to induce the production of IFN-γ, which, in turn, elicits IgG2. Obviously, further experiments are necessary to clearly delineate the role of PGE2 in IgG2 production in our experimental system.
The production of IgG2 is almost completely abrogated by both indomethacin and the PAF receptor antagonist CV3988, suggesting that lipid mediators are necessary for the production of this antibody. However, although PGE2 and PAF helped induce IgG2 production by NP PBL, they were not as effective as the conditioned media from LJP monocytes (compare Table 1, Figure 2, and Table 2). These observations indicate that PGE2 and PAF are essential but that other cytokines are also important for optimal production of IgG2. Preliminary studies indicate that IL-1α, IL-1β, IL-6, and IL-12 are also critical for maximal IgG2 production and that PWM-stimulated monocytes from LJP subjects make more IL-1β than cells from NP subjects (J. B. Zhang, Y. Ishihara, S. E. Barbour, H. A. Schenkein, and J. G. Kew, unpublished observations). However, the ILs appear to induce both IgG1 and IgG2 and therefore are not selective in their actions. Future studies will be directed toward understanding how the selective actions of PGE2 and PAF and the nonselective actions of the interleukins are integrated to induce the synthesis of IgG2.
ACKNOWLEDGMENT
This work was supported by a grant from the National Institute of Dental and Craniofacial Research (grant no. DE10703).
REFERENCES
- 1.Abe N, Katamura K, Shintaku N, Fukui T, Kiyomasu T, Iio J, Ueno H, Tai G, Mayumi M, Furusho K. Prostaglandin E2 and IL-4 provide naive CD4+ T cells with distinct inhibitory signals for the priming of IFN-gamma production. Cell Immunol. 1997;181:86–92. doi: 10.1006/cimm.1997.1180. [DOI] [PubMed] [Google Scholar]
- 2.Baker P J, Wilson M E. Opsonic IgG antibody against Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. Oral Microbiol Immunol. 1989;4:98–105. doi: 10.1111/j.1399-302x.1989.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 3.Barbour S E, Dennis E A. Antisense inhibition of group II phospholipase A2 expression blocks the production of prostaglandin E2 by P388D1 cells. J Biol Chem. 1993;268:21875–21882. [PubMed] [Google Scholar]
- 4.Bastien Y, Toledano B J, Mehio N, Cameron L, Lamoukhaid B, Renzi P, Hamid Q, Mazer B D. Detection of functional platelet-activating factor receptors on human tonsillar B lymphocytes. J Immunol. 1999;162:5498–5505. [PubMed] [Google Scholar]
- 5.Bloom D, Jabrane-Ferrat N, Zeng L, Wu A, Li L, Lo D, Turck C W, An S, Goetzl E J. Prostaglandin E2 enhancement of interferon-gamma production by antigen-stimulated type 1 helper T cells. Cell Immunol. 1999;194:21–27. doi: 10.1006/cimm.1999.1479. [DOI] [PubMed] [Google Scholar]
- 6.Califano J V, Gunsolley J C, Nakashima K, Schenkein H A, Wilson M E, Tew J G. Influence of anti-Actinobacillus actinomycetemcomitans Y4 (serotype b) lipopolysaccharide on severity of generalized early-onset periodontitis. Infect Immun. 1996;64:3908–3910. doi: 10.1128/iai.64.9.3908-3910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Califano J V, Schenkein H A, Tew J G. Immunodominant antigens of Actinobacillus actinomycetemcomitans serotype b in early-onset periodontitis patients. Oral Microbiol Immunol. 1992;7:65–70. doi: 10.1111/j.1399-302x.1992.tb00511.x. [DOI] [PubMed] [Google Scholar]
- 8.Califano J V, Schifferle R E, Gunsolley J C, Best A M, Schenkein H A, Tew J G. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J Periodontol. 1999;70:730–735. doi: 10.1902/jop.1999.70.7.730. [DOI] [PubMed] [Google Scholar]
- 9.Damare S M, Wells S, Offenbacher S. Eicosanoids in periodontal diseases: potential for systemic involvement. Adv Exp Med Biol. 1997;433:23–35. doi: 10.1007/978-1-4899-1810-9_5. [DOI] [PubMed] [Google Scholar]
- 10.Daniel M A, McDonald G, Offenbacher S, Van Dyke T E. Defective chemotaxis and calcium response in localized juvenile periodontitis neutrophils. J Periodontol. 1993;64:617–621. doi: 10.1902/jop.1993.64.7.617. [DOI] [PubMed] [Google Scholar]
- 11.Demeure C E, Yang L-P, Desjardins C, Raynauld P, Delespesse G. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol. 1997;27:3526–3531. doi: 10.1002/eji.1830271254. [DOI] [PubMed] [Google Scholar]
- 12.Elstad M R, Stafforini D M, McIntyre T M, Prescott S M, Zimmerman G A. Platelet activating factor acetylhydrolase increases during macrophage differentiation. J Biol Chem. 1989;264:8467–8470. [PubMed] [Google Scholar]
- 13.Fedyk E R, Harris S G, Padilla J, Phipps R P. Prostaglandin receptors of the EP2 and EP4 subtypes regulate B lymphocyte activation and differentiation to IgE-secreting cells. Adv Exp Med Biol. 1997;433:153–157. doi: 10.1007/978-1-4899-1810-9_31. [DOI] [PubMed] [Google Scholar]
- 14.Fedyk E R, Ripper J M, Brown D M, Phipps R P. A molecular analysis of PGE receptor (EP) expression on normal and transformed B lymphocytes: coexpression of EP1, EP2, EP3β and EP4. Mol Immunol. 1996;33:33–45. doi: 10.1016/0161-5890(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 15.Gunsolley J C, Burmeister J A, Tew J G, Best A M, Ranney R R. Relationship of serum antibody to attachment level patterns in young adults with juvenile periodontitis or generalized severe periodontitis. J Periodontol. 1987;58:314–320. doi: 10.1902/jop.1987.58.5.314. [DOI] [PubMed] [Google Scholar]
- 16.Holter W, Spiegel A M, Howard B H, Weber S, Brann M R. Expression of GTP-binding proteins and prostaglandin E2 receptors during human T cell activation. Cell Immunol. 1991;134:287–295. doi: 10.1016/0008-8749(91)90303-s. [DOI] [PubMed] [Google Scholar]
- 17.Katsuragi K, Takashiba S, Kurihara H, Murayama Y. Molecular basis of leukocyte adhesion molecules in early-onset periodontitis patients with decreased CD11/CD18 expression on leukocytes. J Periodontol. 1994;65:949–957. doi: 10.1902/jop.1994.65.10.949. [DOI] [PubMed] [Google Scholar]
- 18.Katsuyama M, Ikegami R, Karahashi H, Amano F, Sugimoto Y, Ichikawa A. Characterization of the LPS-stimulated expression of EP2 and EP4 prostaglandin E receptors in mouse macrophage-like cell line, J774.1. Biochem Biophys Res Commun. 1998;251:727–731. doi: 10.1006/bbrc.1998.9540. [DOI] [PubMed] [Google Scholar]
- 19.Lewis R A. Interactions of eicosanoids and cytokines in immune regulation. Adv Prostaglandin Thromboxane Leukot Res. 1990;20:170–178. [PubMed] [Google Scholar]
- 20.Lu H, Califano J V, Schenkein H A, Tew J G. Immunoglobulin class and subclass distribution of antibodies reactive with the immunodominant antigen of Actinobacillus actinomycetemcomitans serotype b. Infect Immun. 1993;61:2400–2407. doi: 10.1128/iai.61.6.2400-2407.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu H, Wang M, Gunsolley J C, Schenkein H A, Tew J G. Serum immunoglobulin G subclass concentrations in periodontally healthy and diseased individuals. Infect Immun. 1994;62:1677–1682. doi: 10.1128/iai.62.5.1677-1682.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marazita M L, Burmeister J A, Gunsolley J C, Koertge T E, Lake K, Schenkein H A. Evidence for autosomal dominant inheritance and race-specific heterogeneity in early-onset periodontitis. J Periodontol. 1994;65:623–630. doi: 10.1902/jop.1994.65.6.623. [DOI] [PubMed] [Google Scholar]
- 23.Nemoto E M, Nakamura S, Shoji S, Horiuchi H. Circulating promyelocytes and low levels of CD16 expression on polymorphonuclear leukocytes accompany early-onset periodontitis. Infect Immun. 1997;65:3906–3912. doi: 10.1128/iai.65.9.3906-3912.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Offenbacher S, Heasman P A, Collins J G. Modulation of host PGE2 secretion as a determinant of periodontal disease expression. J Periodontol. 1993;64:432–444. doi: 10.1902/jop.1993.64.5s.432. [DOI] [PubMed] [Google Scholar]
- 25.Offenbacher S, Odle B M, Van Dyke T E. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 26.Ranney R R, Yanni N R, Burmeister J A, Tew J G. Relationship between attachment loss and precipitating serum antibody to Actinobacillus actinomycetemcomitans in adolescents and young adults having severe periodontal destruction. J Periodontol. 1982;53:1–7. doi: 10.1902/jop.1982.53.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Roper R L, Brown D M, Phipps R P. Prostaglandin E2 promotes B lymphocyte Ig isotype switching to IgE. J Immunol. 1995;154:162–170. [PubMed] [Google Scholar]
- 28.Roper R L, Conrad D H, Brown D M, Warner G L, Phipps R P. Prostaglandin E2 promotes IL-4-induced IgE and IgG1 synthesis. J Immunol. 1990;145:2644–2651. [PubMed] [Google Scholar]
- 29.Roper R L, Phipps R P. Prostaglandin E2 regulation of the immune response. Adv Prostaglandin Thromboxane Leukot Res. 1994;22:101–11. :101–111. [PubMed] [Google Scholar]
- 30.Shapira L, Soskolne W A, Sela M N, Offenbacher S, Barak V. The secretion of PGE2, IL-1 beta, IL-6, and TNF alpha by adherent mononuclear cells from early onset periodontitis patients. J Periodontol. 1994;65:139–146. doi: 10.1902/jop.1994.65.2.139. [DOI] [PubMed] [Google Scholar]
- 31.Stafforini D M, Prescott S M, McIntyre T M. Human plasma platelet-activating factor acetylhydrolase. Purification and properties. J Biol Chem. 1987;262:4223–4230. [PubMed] [Google Scholar]
- 32.Van Dyke T E, Warbington M, Gardner M, Offenbacher S. Neutrophil surface protein markers as indicators of defective chemotaxis in LJP. J Periodontol. 1990;61:180–184. doi: 10.1902/jop.1990.61.3.180. [DOI] [PubMed] [Google Scholar]
- 33.Venable M E, Zimmerman G A, McIntyre T M, Prescott S M. Platelet-activating factor: a phospholipid autacoid with diverse actions. J Lipid Res. 1993;34:691–702. [PubMed] [Google Scholar]
- 34.Wilson M E, Bronson P M, Hamilton R G. Immunoglobulin G2 antibodies promote neutrophil killing of Actinobacillus actinomycetemcomitans. Infect Immun. 1995;63:1070–1075. doi: 10.1128/iai.63.3.1070-1075.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson M E, Hamilton R G. Immunoglobulin G subclass response of localized juvenile periodontitis patients to Actinobacillus actinomycetemcomitans Y4 lipopolysaccharide. Infect Immun. 1992;60:1806–1812. doi: 10.1128/iai.60.5.1806-1812.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson M E, Schifferle R E. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect Immun. 1991;59:1544–1551. doi: 10.1128/iai.59.4.1544-1551.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zambon J J. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol. 1985;12:1–20. doi: 10.1111/j.1600-051x.1985.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 38.Zhang J B, Quinn S M, Rausch M, Gunsolley J C, Schenkein H A, Tew J G. Hyper-immunoglobulin G2 production by B cells from patients with localized juvenile periodontitis and its regulation by monocytes. Infect Immun. 1996;64:2004–2009. doi: 10.1128/iai.64.6.2004-2009.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]