Abstract
We investigated the alteration of gut microbiota and the associated metabolic risks in hypertensive patients with obstructive sleep apnea (OSA) comorbidity. Fecal and blood samples were collected from 52 hypertensive patients, who were divided into three groups: A (controls, apnea‐hypopnea index[AHI] < 5, n = 15), B (mild OSA, 5 < AHI < 20, n = 17), and C (moderate‐to‐severe OSA, AHI > 20, n = 20). The composition of the gut microbiota was studied through 16s RNA sequencing of variable regions 3–4. Analysis of the results revealed that group C had a significant higher concentration of total cholesterol, low‐density lipoprotein, and IL‐1β compared with group A. The Shannon index showed that bacterial biodiversity was lower in OSA patients. At the phylum level, the ratio of Firmicutes to Bacteroidetes (F/B) was significantly higher in group C than in groups A and B. At the genus level, the relative abundance of short‐chain fatty acids (SCFA)‐producing bacteria (e.g., Bacteroides and Prevotella) was lower while the number of inflammation‐related bacteria (e.g., Lactobacillus) was increased in patients with OSA. We found that the IL‐1β level was negatively correlated with Bacteroidetes. The area under the receiver operating characteristic curve was .672 for F/B ratio in determining hypertensive patients with OSA. In patients with hypertension, OSA was associated with worse gut dysbiosis, as evidenced by decreased levels of short‐chain fatty acids‐producing bacteria and increased number of inflammation‐related bacteria. The differences in gut microbiota discriminate hypertensive patients with OSA from those without and may result in an enhanced inflammatory response and increase the risk of metabolic diseases.
Keywords: gut dysbiosis, hypertension, inflammation, short‐chain fatty acids, sleep apnea
1. INTRODUCTION
The global burden of hypertension, also known as high blood pressure, was projected to rise from 26.4% among the adult population in 2000 to 29.2% in 2025. 1 Globally, hypertension is the leading modifiable cause of cardiovascular disease (CVD) and premature deaths. Despite significant advances in the development of pharmacological treatments globally, the control rates for hypertension remain low. 2 In hypertensive patients, the control of risk factors and appropriate application of therapeutic options including diet and lifestyle changes have been reported to yield promising results. 2 , 3 As a risk factor, obstructive sleep apnea (OSA) is strongly associated with an elevated risk of hypertension and CVD. OSA is a common sleep disorder characterized by repetitive collapse episodes in the upper airway during sleep, accompanied by oxygen desaturation. The estimated global prevalence of OSA ranges from 9% to 38% and it is significantly higher in men, the elderly and obese individuals. 4 The OSA is now recognized as an identifiable cause of hypertension. The prevalence of hypertension among patients with OSA is as high as 42%. 5 Endothelial damage, sympathetic hyperactivity, and proinflammation are among the physiological links between OSA and hypertension. 6 Studies suggest that gut dysbiosis plays an important role in the development of OSA‐induced hypertension. 7 , 8 , 9 Durgan et al. applied a rat model to demonstrate that OSA and a high‐fat diet caused significant changes in the gut microbiota. In addition, transplanting dysbiotic cecal contents from hypertensive OSA rats into normotensive subjects resulted to increased blood pressure. 7 Liu et al. reported that gut dysbiosis developed in hypertensive rats with OSA, and supplementation of probiotic Lactobacillus rhamnosus contributed to reductions in inflammatory cytokines and hypertension severity. 10 These findings suggested the causal role of gut dysbiosis in OSA‐induced hypertension. However, the role of OSA‐associated gut dysbiosis in patients with pre‐existing hypertension, specifically whether gut microbiota varies between OSA‐related hypertension and hypertension without OSA, has not been sufficiently investigated through clinical research. Because OSA‐related hypertension is more likely to be drug‐resistant, 11 it is necessary to identify this distinct population. Clinically, OSA is frequently underdiagnosed by cardiologists and hence undertreated. 12 To address this, we examined changes in gut microbiota in patients with OSA‐related hypertension in comparison to other forms of hypertension. Previous studies have linked OSA, gut dysbiosis, and hypertension to the development of chronic inflammation. 9 , 13 Thus, we assessed the relationship between the gut microbiota and inflammatory markers in OSA‐related hypertension.
2. METHODS
2.1. Patients
This study was approved by the Institutional Review Board and Ethics Committee of Wannan Medical College, China, and was performed in line with the principles outlined in the Declaration of Helsinki. An observational case‐controlled research was conducted at the Vascular Disease Centre of Wannan Medical College and the Third affiliated hospital of Anhui Medical University, from August 2021 to December 2021. During this period, hypertension patients were screened for inclusion. Hypertension was defined as blood pressure ≥140/90 mmHg. Patients diagnosed with secondary hypertension, history of acute or chronic inflammatory diseases were excluded. Patients who had recently (3 months) used antibiotics, probiotics, or oncologic treatment were also excluded. To evaluate the risk of OSA in hypertensive patients, the NoSAS questionnaire, a well‐validated tool for differentiating OSA, was adopted. 14 Patients suspected with OSA underwent a full night of polysomnography (PSG). Fasting blood was collected on the second day after hospitalization. The blood was centrifuged at 3000 rpm for 10 min to obtain plasma. Fecal samples were collected in sterile tubes. All samples were stored in the −80°C fridge until use.
2.2. OSA assessment and grouping
A standard PSG was used to diagnose OSA. After examination for one night, apnea‐hypopnea index (AHI) was computed as the average number of episodes of apnea and hypoxia per hour, and the oxygen desaturation index (ODI) was determined as the number of desaturation episodes per hour. Patients were stratified into three groups based on the value of AHI and the absence of snoring: A (controls, non‐snorers and AHI < 5, n = 15), B (mild OSA, 5 < AHI < 20, n = 17), C (moderate‐to‐severe OSA, AHI > 20, n = 20). In a two‐group analysis, groups B and C were combined as the OSA group (AHI > 5), and group A served as the control group (AHI < 5). The lowest oxygen saturation and overnight mean oxygen saturation were also recorded.
2.3. Cytokine analysis
Interleukin (IL)‐1β (IL‐1β) and tumor necrosis factor α (TNF‐α) levels were measured using commercial ELISA kits (Yubo, Shanghai, China).
2.4. Sampling and 16S RNA sequencing
Fresh fecal samples were collected in a sterile stool tube and immediately stored in a −80° refrigerator. The gut microbiota composition was analyzed using 16s RNA sequencing of variable regions 3–4. Genomic DNA was extracted from samples using the CTAB/SDS method. DNA concentration and purity were determined through 1% agarosegels. The barcode‐specific primer was used to amplify the 16s rRNA genes. All PCR reactions were carried out in 30 μl reactions with 15 μl of PhusionHigh‐Fidelity PCR Master Mix (New England Biolabs). The PCR products were quantified, qualified, and mixed in equidensity ratios, and then purified with the AxyPrepDNA Gel Extraction Kit (AXYGEN, USA). Sequencing libraries were generated using NEB NextUltraDNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer's instructions. The library quality was evaluated on the Qubit@ 2.0 Fluorometer (Thermo Scientific) and Agilent Bioanalyzer 2100 system. The library was then sequenced on an Illumina NovaSeq 600 platform and 250 bp paired‐end reads were generated. These experiments were conducted in line with the manufacturer's recommendations.
The UPARSE software package was used to analyze the sequences, using UPARSE‐OTU and UPARSE‐OTUref algorithms. The Operational Taxonomic Unit (OTU) method was used to analyze alpha and beta diversity. Quantitative analysis of biomarkers within various groups was conducted using the LDA Effect Size (LEfSe) method. 15 The Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) algorithm was used to predict the bacterial functional profiling, 16 and the Kyoto Encyclopedia of Genes and Genomes (KEGG) database was used as a functional reference resource. 17
2.5. Statistics
Data were presented as the mean ± standard deviation (SD). Mann–Whitney U‐test and independent t‐test were used to compare two groups, and one‐way ANOVA followed by Turkey post hoc analyses were performed to compare differences among three groups. Pearson's (two‐tailed) correlation coefficients were calculated to estimate correlations between gut microbial composition (relative abundance) and inflammatory markers. The sensitivity and specificity of the relative abundance of various taxa in detecting OSA status were determined by analyzing the receiver operating characteristic (ROC) curve. Statistical analyses were conducted using the SPSS Software version 22.0 (SPSS Inc., Chicago, IL, USA). P value less than .05 was considered statistically significant.
3. RESULTS
A total of 52 patients were enrolled after matching by age and gender. They were classified into three groups based on the estimated severity of OSA: A (controls, non‐snorers and AHI < 5, n = 15), B (mild OSA, 5 < AHI < 20, n = 17), C (moderate‐to‐severe OSA, AHI > 20, n = 20). There were no significant differences in gender (P = .25), age (P = .94), body mass index (BMI, P = .06) and ratio of diabetes (P = .98). The three groups had similar blood pressure‐lowering medication as well as hypertension duration (Supplementary Table S1). There was no difference in renal function among the three groups. Notably, group C exhibited significantly higher concentrations of total cholesterol (P = .017) and low‐density lipoprotein (P = .014) compared with group A (Figure 1A,B). The levels of triglyceride were comparable among the groups (Table 1).
FIGURE 1.
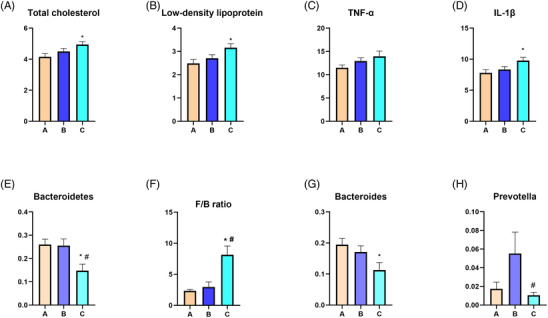
Obstructive sleep apnea changes plasma total cholesterol, low‐density lipoprotein, inflammation markers, and gut microbiota composition. Group C (AHI > 20) had higher plasma levels of total cholesterol (A), low density lipoprotein (B) and IL‐1β compared with group A (AHI < 5). Group C was associated with decreased relative abundance of Bacteroidetes (E) and increased F/B ratio (F) compared with group A and group B (5 < AHI < 20). Group C had lower relative abundance of Bacteroides (G) compared with group A, and decreased Prevotella (H) compared with group B. Data are represented as mean ± SEM. n = 15, 17, and 20 in group A, group B, and group C, respectively. *P < .05 versus group A, # P < .05 versus group B. F/B, The ratio of Firmicutes to Bacteroidetes. AHI, apnea‐hypopnea index; IL‐1β, Interleukin‐1β; SEM, standard error of the mean; TNF‐α, tumor necrosis factor α.
TABLE 1.
Characteristics of the participants
| Group A | Group B | Group C | P value | |
|---|---|---|---|---|
| Gender (female/male) | 3/12 | 4/13 | 1/19 | .25 |
| Age (years) | 51.73 ± 11.09 | 51.12 ± 10.76 | 52.35 ± 10.43 | .94 |
| BMI (kg/m2) | 24.67 ± 3.09 | 26.36 ± 4.88 | 28.19 ± 4.42 | .06 |
| Percentage of diabetes [n (%)] | 3 (20.0) | 3 (17.6) | 4 (20.0) | .98 |
| AHI (events/h) | 1.95 ± 1.35 | 11.10 ± 5.53* | 41.00 ± 12.77* | <.001 |
| ODI (events/h) | 1.24 ± 1.29 | 9.66 ± 6.79 | 35.08 ± 17.07* | <.001 |
| Mean SpO2 (%) | 94.82 ± 1.42 | 89.24 ± 23.82 | 93.13 ± 14.03 | .576 |
| Lowest SpO2 (%) | 88.20 ± 5.39 | 85.20 ± 5.95* | 77.35 ± 11.79* | .005 |
| Total cholesterol (mmol/L) | 4.15 ± .82 | 4.50 ± .76 | 4.94 ± .84* | .022 |
| Low density lipoprotein (mmol/L) | 2.48 ± .64 | 2.71 ± .62 | 3.16 ± .75* | .015 |
| Triglyceride (mmol/L) | 1.51 ± .99 | 2.12 ± 1.35 | 1.87 ± .80 | .279 |
| TNFα (pg/ml) | 11.49 ± 2.28 | 12.93 ± 2.88 | 13.94 ± 3.83 | .186 |
| IL‐1β (pg/ml) | 7.79 ± 1.92 | 8.34 ± 1.94 | 9.78 ± 2.35* | .022 |
Data are mean ± standard deviation or percentages. *Indicates P < .05 versus group A. AHI, apnea‐hypopnea index; BMI, body mass index; IL‐1β, interleukin (IL)‐1β; ODI, oxygen desaturation index; TNF‐α, tumor necrosis factor α.
Analysis of the PSG showed that AHI was significantly higher in groups C and B (P < .05 for all), than in group A. Group C exhibited higher ODI and lower lowest SpO2 compared with group A. However, the mean SpO2 was not significantly different among the three groups (Table 1).
There were no significant differences in levels of TNFα (P = .186) among the groups, but the plasma level of IL‐1β was significantly higher in group C compared with group A (P = .025) (Figure 1C,D) (Table 1).
3.1. Gut microbiota analysis
3.1.1. Richness and diversity
The richness and diversity as well as the structure of the gut microbiota in OSA (AHI > 5) and control patients (AHI < 5) were compared. Notably, the specaccum curve was stable as the sample numbers increased to 50, indicating that the 52 samples were adequate for the evaluation of the gut microbial diversity (Figure 2A). Figure 2 depicts the alpha diversity expressed by the Chao1 index, Observed species index, Shannon index, and Simpson index. The Shannon index was lower in the OSA group compared with the control group (P = .03) (Figure 2D).
FIGURE 2.
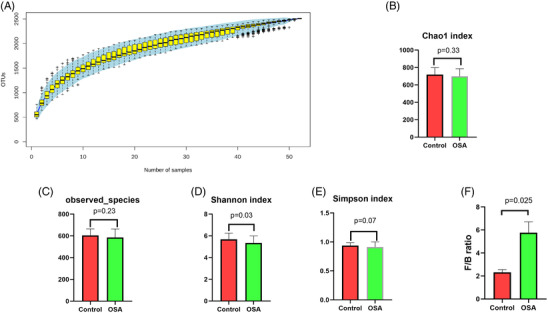
Alpha diversity indices in patients with obstructive sleep apnea. The specaccum curve (A) was stable as the sample numbers increased to 50. Alpha diversity parameters including Chao1 (B), Observed species (C), and Simpson index (E) were not significantly changed in obstructive sleep apnea (OSA) patients, while Shannon index (D) was reduced in OSA patients. Compared with control, OSA patients had a higher value of F/B ratio (F). n = 15 and 37 in control and OSA group, respectively.
3.1.2. Alterations in bacteria abundance
The structure of the gut microbiome varied between groups. Supplementary Table S2 summarizes the most abundant phyla in each group. The one‐way ANOVA was employed to compare the top 10 taxa across three groups. At the phylum level, we found that group C had a lower relative abundance of Bacteroidetes than group A (P = .015) and B (P = .014) (Figure 1E). The Firmicutes to Bacteroidetes (F/B) ratio was significantly higher in group C than in groups A (P < .001) and B (P = .002) (Figure 1F). Compared with the control group, OSA patients had a higher value of F/B ratio (P = .025) (Figure 2F).
The most abundant genera are presented in Supplementary Table S3. At the genus level, group C had a lower relative abundance of Bacteroides than group A (P = .028) (Figure 1G). Prevotella relative abundance was lower in group C than in group B (P = .042) (Figure 1H).
For further characterization, the LEfSe method 15 was employed to identify the main taxa determining differences between control and OSA patients. As demonstrated in Figure 3A, OSA patients were enriched with Megamonas, Lactobacillus, Megasphaera, and Coprococcus at the genus level, while Alistipes, Eubacterium_coprostanoligenes, Blautia, Roseburia, Fusobacteria, and Ruminococcus_gnavus were depleted compared with control. Based on KEGG, these significant differences in the fecal microbiome suggested that group C had enhanced pathways involved in cell motility and metabolic diseases (Figure 3B), whereas group A had inhibited pathways including transport and catabolism, cofactor and vitamin metabolism, and energy metabolism.
FIGURE 3.
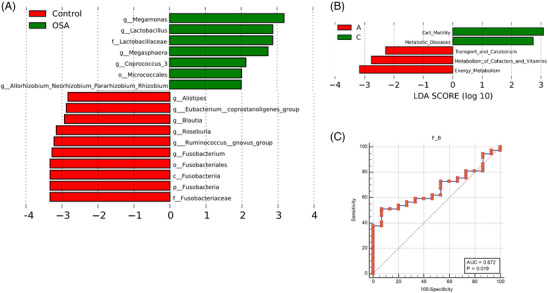
Changes in gut microbiota composition discriminate hypertensive patients with obstructive sleep apnea. (A) LEfSe method identified the most differentially abundant taxons in OSA patients (green) in respect to controls (red); (B) PICRUSt based on the KEGG database predicted different pathways between controls and those with significant OSA. (C) Receiver operating characteristic analysis of F/B cutoff value in detection of OSA patients. KEGG, Kyoto Encyclopedia of Genes and Genomes; LEfSe, linear discriminant analysis Effect Size; OSA, obstructive sleep apnea; PICRUSt, Phylogenetic Investigation of Communities by Reconstruction of Unobserved States.
3.1.3. Association between fecal microbiota and plasma IL‐1β
Using Pearson's test, we found that Bacteroidetes (r = −.641) and Bacteroides (r = −.530) showed a moderate negative correlation with the IL‐1β level (Table 2).
TABLE 2.
Relationship between taxa and plasma levels of interleukin −1β obtained by Pearson's test
| Taxa | P value | r value | |
|---|---|---|---|
| Phyla | Firmicutes | .101 | .232 |
| Bacteroidetes | .001 | −.641 | |
| Proteobacteria | .028 | .309 | |
| Actinobacteria | .580 | .079 | |
| Fusobacteria | .902 | −.018 | |
| Verrucomicrobia | .862 | −.025 | |
| Tenericutes | .430 | −.113 | |
| Epsilonbacteraeota | .459 | −.106 | |
| Patescibacteria | .945 | −.010 | |
| Euryarchaeota | .970 | −.005 | |
| F/B ratio | 0.022 | 0.320 | |
| Genera | Bacteroides | .001 | −.530 |
| Faecalibacterium | .875 | .023 | |
| Escherichia | .069 | .257 | |
| Blautia | .848 | .027 | |
| Roseburia | .390 | .123 | |
| Prevotella9 | .017 | −.333 | |
| Klebsiella | .093 | .238 | |
| Subdoligranulum | .237 | −.114 | |
| Megamonas | .297 | −.149 | |
| Ruminococcus | .811 | −.034 |
The correlation r value more than .4 (less than −.4) is considered moderate correlation.
3.1.4. Discriminative value of gut taxa for OSA
Using ROC analysis, we attempted to discriminate OSA patients from controls to identify predictive features of various taxa (Supplementary Table S4). The ROC‐area under the curve (AUC) value for the BMI was .643, and two taxa were associated with a higher ROC‐AUC value than BMI (Supplementary Figure S1): Epsilonbacteraeota (.651) and Megamonas (.692). The AUC value of F/B in predicting OSA was .672. Gut microbiota F/B value of more than 3.2 showed a 51.4% sensitivity and 93.3% specificity in detecting positive OSA status (Figure 3C).
4. DISCUSSION
Majority of previous studies included both hypertension and non‐hypertension subjects. Because hypertension may interact with gut microbiota, the confounding effects of hypertension could not be ruled out. Contrary to previous studies, the current study investigated gut microbiota alteration in hypertensive patients who also had OSA. The gut microbial community of OSA patients showed alterations, including a decrease in the relative abundance of SCFA‐producing bacteria (e.g., Bacteroides and Prevotella) and an increase in inflammation‐related bacteria (e.g., Lactobacillus). 18 We also observed an increased level of inflammatory cytokines and hyperlipidemia in OSA. The ROC‐AUC value implied that gut microbiome information might be used to identify OSA patients from controls in the hypertensive population. Our findings show that gut microbiota plays a critical role in OSA‐related hypertension, and might be associated with metabolic comorbidities.
Recent studies have reported alterations in gut microbiota with its metabolites in patients with hypertension and OSA. 19 , 20 We previously demonstrated that chronic intermittent hypoxia, one of the key features of OSA, was associated with gut dysbiosis, and increased blood pressure in rats. 21 Besides increasing the risk of incident hypertension, OSA makes it difficult to treat high blood pressure. Clinically, OSA may contribute to poor control of blood pressure, 22 with more than 70% of resistant hypertension patients suffering from OSA. 23 In this study, the Shannon index showed that hypertensive patients with OSA had lower bacterial biodiversity. Moreover, we observed a significantly elevated F/B ratio, which is widely considered a sign of gut microbiota imbalance, and extensively used as a biomarker for assessing pathological status. 24 , 25 Yang et al. demonstrated that in spontaneously hypertensive rats and chronic angiotensin II infusion‐induced hypertension rats, gut dysbiosis manifested as a significant decrease in microbial richness and diversity, as well as increased F/B ratio. 26 Furthermore, they found that these changes were associated with a reduction in SCFA‐producing bacteria. 26 We observed a similar dysbiotic pattern, indicating that OSA‐related hypertension has aggravated gut dysbiosis in comparison with other types of hypertension. Notably, patients with significant OSA (group C) showed reduced relative abundance of Bacteroidetes and higher F/B compared with mild OSA (group B), implying that gut dysbiosis may be associated with OSA severity.
Microbial dysbiosis has been associated with increased permeability of the intestinal epithelial barrier, resulting in local and systemic inflammation and metabolic disorders. 27 , 28 The gut microbiota and its metabolites influence reverse cholesterol transport, adipose tissue inflammation, and plasma lipid levels. 29 In the current study, hypertensive patients with significant OSA showed elevated levels of IL‐1β and dyslipidemia, indicating that OSA increased the risk of metabolic abnormalities. This result might be attributed to OSA‐induced gut dysbiosis, especially the decrease of SCFA‐producing bacteria. The SCFA, which mainly comprised acetate, propionate, and butyrate, attenuates systemic inflammation and lowers blood pressure. 13 , 30 Bacteroidetes, a phylum that produces acetate, were associated with decreased production of inflammatory mediators. 31 In our case, we observed that Bacteroidetes was significantly lower in patients with significant OSA. Also, Bacteroides and Prevotella are propionates‐ and butyrate‐producing bacteria, 32 , 33 , 34 and their abundance was lower in patients with OSA. These changes in gut microbiota composition may contribute to an enhanced inflammatory response. Additionally, the LEfSe analysis revealed that Lactobacillus was enriched in OSA patients. Lactobacillus was associated with inflammatory bowel disease. 18 , 35 Lactobacillus is also positively associated with trimethylamine N‐oxide (TMAO) levels. 36 The TMAO has been associated with the risk of CVDs such as vascular inflammation, 37 atherosclerosis, and heart failure. 38 Moreover, TMAO promotes angiotensin II‐induced vasoconstriction and aggravates angiotensin II‐induced hypertension. 39 Therefore, Lactobacillus enrichment may play a critical role in OSA‐related disorders. We also found that OSA was associated with an increased abundance of Megamonas, which promotes the biosynthesis of lipopolysaccharide, 40 a significant inflammation stimulator. 41 In addition, the present study found that Megasphaera was enriched in OSA patients. Megasphaera was previously linked to fibrotic markers, including type I collagen cross‐linked c‐telopeptide and procollagen type 1 n‐terminal propeptide. 42 These changes in the gut microbial composition may account for OSA‐related CVD risk. Furthermore, some taxa were associated with inflammatory cytokine. Moreover, the predictive functional analysis indicated that patients with significant OSA had enhanced pathways involving cell motility and metabolic diseases, supporting the above results. Taken together, OSA might lead to a disease‐related dybiosis in patients with hypertension.
OSA is a major cause of refractory hypertension, which is more likely to damage the target organs. 43 Therefore, it is important to isolate OSA‐related hypertension from another form of hypertension. However, in clinical practice, OSA is usually misdiagnosed and hence undertreated. 12 Based on the significant alteration of gut microbiota, we postulated that gut taxa might be useful in the detection or exclusion of those hypertension with OSA. We observed that F/B, Epsilonbacteraeota, and Megamonas had a moderate ROC‐AUC value in the determination of OSA. Notably, obesity or overweight is a significant predictor of OSA, 44 while the above taxa indices seemed to be more effective than BMI in detecting OSA.
Our study had several limitations. Firstly, the enrolled sample size was small, and thus the results should be interpreted with caution. Secondly, although several taxa have been linked to inflammation markers, we did not detect bioactive metabolites like SCFAs, lipopolysaccharide, and TMAO. Therefore, the causal effects of gut microbiota on patients with hypertension and OSA need further investigation. Thirdly, multiple factors may contribute to the pathophysiology of hypertension, whereas we only studied OSA‐related gut dysbiosis in hypertension, leaving out other comorbidities. Fourthly, only two inflammatory markers were examined in this study, while additional biomarkers such as IL‐6 and CRP might provide better persuasion. However, the association between inflammatory markers and OSA has been well established previously, 45 which is in line with the present study. Fifthly, the potential effects of metabolic status (e.g., obesity, dyslipidemia, and glucose intolerance) on OSA should be considered. We observed that OSA patients were more likely to have metabolic abnormalities such as dyslipidemia. However, whether gut dysbiosis in patients with OSA is indicative of underlying impaired metabolism (rather than OSA per se) cannot be eliminated.
5. CONCLUSION
In patients with hypertension, OSA is associated with exacerbated gut dysbiosis, including decreased SCFA‐producing bacteria and increased inflammation‐related bacteria. These changes in gut microbiota discriminate hypertension patients with OSA comorbidity and may lead to enhanced inflammatory response and elevated risk of metabolic diseases. Our study suggests that OSA‐associated gut microbiota alteration plays an important pathophysiological role in patients with hypertension, and might be a target for diagnosis and treatment.
AUTHOR CONTRIBUTIONS
Dasheng Lu: conception, analyses, and editing; Shaodong Xu and Ping Dai: writing and construction. Lijuan Wu and Hongxiang Zhang: Data acquisition; Birong Zhou: Supervision. All authors have a significant contribution to this work and contributed to the article and approved the submitted version.
CONFLICTS OF INTEREST
The authors declare they have no conflict of interest.
Supporting information
supplementary Information
supplementary Information
supplementary Information
supplementary Information
supplementary Information
ACKNOWLEDGEMENT
The authors thank the gutMGene database 46 for providing convenient search for target genes and metabolites of gut microbes. This study was supported by grant from the National Natural Science Youth Foundation of China (81800445) and Cultivating Fund for the Key Scientific Research Project of Wannan Medical College (WK2018ZF10).
Lu D, Xu S, Dai P, Wu L, Zhang H, Zhou B. Gut microbiota in hypertensive patients with versus without obstructive sleep apnea. J Clin Hypertens. 2022;24:1598–1605. 10.1111/jch.14598
REFERENCES
- 1. Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217‐223. [DOI] [PubMed] [Google Scholar]
- 2. Mills KT, Bundy JD, Kelly TN, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population‐based studies from 90 countries. Circulation. 2016;134(6):441‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ozemek C, Laddu DR, Arena R, Lavie CJ. The role of diet for prevention and management of hypertension. Curr Opin Cardiol. 2018;33(4):388‐393. [DOI] [PubMed] [Google Scholar]
- 4. Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70‐81. [DOI] [PubMed] [Google Scholar]
- 5. Baguet J‐P, Hammer L, Lévy P, et al. Night‐time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens. 2005;23(3):521‐527. [DOI] [PubMed] [Google Scholar]
- 6. Brown J, Yazdi F, Jodari‐Karimi M, Owen JG, Reisin E. Obstructive sleep apnea and hypertension: updates to a critical relationship. Curr Hypertens Rep. 2022:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durgan DJ, Ganesh BP, Cope JL, et al. Role of the gut microbiome in obstructive sleep apnea‐induced hypertension. Hypertension. 2016;67(2):469‐474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adnan S, Nelson JW, Ajami NJ, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49(2):96‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mashaqi S, Gozal D. Obstructive sleep apnea and systemic hypertension: gut dysbiosis as the mediator? J Clin Sleep Med. 2019;15(10):1517‐1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liu J, Li T, Wu H, et al. Lactobacillus rhamnosus GG strain mitigated the development of obstructive sleep apnea‐induced hypertension in a high salt diet via regulating TMAO level and CD4+ T cell induced‐type I inflammation. Biomed Pharmacother. 2019;112:108580. [DOI] [PubMed] [Google Scholar]
- 11. Gonçalves SC, Martinez D, Gus M, et al. Obstructive sleep apnea and resistant hypertension: a case‐control study. Chest. 2007;132(6):1858‐1862. [DOI] [PubMed] [Google Scholar]
- 12. Costa LE, Uchôa CH, Harmon RR, et al. Potential underdiagnosis of obstructive sleep apnoea in the cardiology outpatient setting. Heart. 2015;101(16):1288‐1292. [DOI] [PubMed] [Google Scholar]
- 13. Zhang L, Ko C‐Y, Zeng Y‐M. Immunoregulatory effect of short‐chain fatty acids from gut microbiota on obstructive sleep apnea‐associated hypertension. Nat Sci Sleep. 2022;14:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marti‐Soler H, Hirotsu C, Marques‐Vidal P, et al. The NoSAS score for screening of sleep‐disordered breathing: a derivation and validation study. Lancet Respir Med. 2016;4(9):742‐748. [DOI] [PubMed] [Google Scholar]
- 15. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457‐D462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang W, Chen L, Zhou R, et al. Increased proportions of Bifidobacterium and the Lactobacillus group and loss of butyrate‐producing bacteria in inflammatory bowel disease. J Clin Microbiol. 2014;52(2):398‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ko CY, Liu QQ, Su HZ, et al. Gut microbiota in obstructive sleep apnea‐hypopnea syndrome: disease‐related dysbiosis and metabolic comorbidities. Clin Sci (Lond). 2019;133(7):905‐917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu D, Wang J, Zhang H, Shan Q, Zhou B. Renal denervation improves chronic intermittent hypoxia induced hypertension and cardiac fibrosis and balances gut microbiota. Life Sci. 2020;262:118500. [DOI] [PubMed] [Google Scholar]
- 22. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342(19):1378‐1384. [DOI] [PubMed] [Google Scholar]
- 23. Logan AG, Perlikowski SM, Mente A, et al. High prevalence of unrecognized sleep apnoea in drug‐resistant hypertension. J Hypertens. 2001;19(12):2271‐2277. [DOI] [PubMed] [Google Scholar]
- 24. Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mariat D, Firmesse O, Levenez F, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. hypertension. 2015;65(6):1331‐1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neroni B, Evangelisti M, Radocchia G, et al. Relationship between sleep disorders and gut dysbiosis: what affects what? Sleep Med. 2021;87:1‐7. [DOI] [PubMed] [Google Scholar]
- 28. Barceló A, Esquinas C, Robles J, et al. Gut epithelial barrier markers in patients with obstructive sleep apnea. Sleep Med. 2016;26:12‐15. [DOI] [PubMed] [Google Scholar]
- 29. Zwartjes MS, Gerdes VE, Nieuwdorp M. The role of gut microbiota and its produced metabolites in obesity, dyslipidemia, adipocyte dysfunction, and its interventions. Metabolites. 2021;11(8):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Natarajan N, Hori D, Flavahan S, et al. Microbial short chain fatty acid metabolites lower blood pressure via endothelial G protein‐coupled receptor 41. Physiol Genomics. 2016;48(11):826‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maslowski KM, Vieira AT, Ng A, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jacobson A, Lam L, Rajendram M, et al. A gut commensal‐produced metabolite mediates colonization resistance to Salmonella infection. Cell Host Microbe. 2018;24(2):296‐307.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esquivel‐Elizondo S, Ilhan Z, Garcia‐Peña E, Krajmalnik‐Brown R. Insights into butyrate production in a controlled fermentation system via gene predictions. mSystems. 2017;2(4):e00051‐00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ren W, Yan H, Yu B, et al. Prevotella‐rich enterotype may benefit gut health in finishing pigs fed diet with a high amylose‐to‐amylopectin ratio. Anim Nutr. 2021;7(2):400‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Moustafa A, Li W, Anderson EL, et al. Genetic risk, dysbiosis, and treatment stratification using host genome and gut microbiome in inflammatory bowel disease. Clin Transl Gastroenterol. 2018;9(1):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hsu CN, Lu PC, Lo MH, et al. Gut microbiota‐dependent trimethylamine N‐oxide pathway associated with cardiovascular risk in children with early‐stage chronic kidney disease. Int J Mol Sci. 2018;19(12):3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu H, Jia K, Ren Z. PRMT5 critically mediates TMAO‐induced inflammatory response in vascular smooth muscle cells. Cell Death Dis. 2022;13(4):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Konieczny RA, Kuliczkowski W. Trimethylamine N‐oxide in cardiovascular disease. Adv Clin Exp Med. 2022;31(8):913‐925. [DOI] [PubMed] [Google Scholar]
- 39. Jiang S, Shui Y, Cui Y, et al. Gut microbiota dependent trimethylamine N‐oxide aggravates angiotensin II‐induced hypertension. Redox Biol. 2021;46:102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Huang R, He K, Duan X, et al. Changes of intestinal microflora in colorectal cancer patients after surgical resection and chemotherapy. Comput Math Methods Med. 2022:2022:1940846. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41. Huuskonen J, Suuronen T, Nuutinen T, Kyrylenko S, Salminen A. Regulation of microglial inflammatory response by sodium butyrate and short‐chain fatty acids. Br J Pharmacol. 2004;141(5):874‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He J, Xu S, Zhang B, et al. Gut microbiota and metabolite alterations associated with reduced bone mineral density or bone metabolic indexes in postmenopausal osteoporosis. Aging (Albany NY). 2020;12(9):8583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matanes F, Khan MB. An update on refractory hypertension. Curr Hypertens Rep. 2022;24(7):225‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Drager LF, Togeiro SM, Polotsky VY, Lorenzi‐Filho G. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol. 2013;62(7):569‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Nadeem R, Molnar J, Madbouly EM, et al. Serum inflammatory markers in obstructive sleep apnea: a meta‐analysis. J Clin Sleep Med. 2013;9(10):1003‐1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cheng L, Qi C, Yang H, et al. gutMGene: a comprehensive database for target genes of gut microbes and microbial metabolites. Nucleic Acids Res. 2022;50(D1):D795‐D800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information
supplementary Information
supplementary Information
supplementary Information
supplementary Information


