Abstract
Filamentous microorganisms are used as molecular factories in industrial biotechnology. In 2007, a new approach to improve productivity in submerged cultivation was introduced: microparticle‐enhanced cultivation (MPEC). Since then, numerous studies have investigated the influence of microparticles on the cultivation. Most studies considered MPEC a morphology engineering approach, in which altered morphology results in increased productivity. But sometimes similar morphological changes lead to decreased productivity, suggesting that this hypothesis is not a sufficient explanation for the effects of microparticles. Effects of surface chemistry on particles were paid little attention, as particles were often considered chemically‐inert and bioinert. However, metal oxide particles strongly interact with their environment. This review links morphological, physical, and chemical properties of microparticles with effects on culture broth, filamentous morphology, and molecular biology. More precisely, surface chemistry effects of metal oxide particles lead to ion leaching, adsorption of enzymes, and generation of reactive oxygen species. Therefore, microparticles interfere with gene regulation, metabolism, and activity of enzymes. To enhance the understanding of microparticle‐based morphology engineering, further interactions between particles and cells are elaborated. The presented description of phenomena occurring in MPEC eases the targeted choice of microparticles, and thus, contributes to improving the productivity of microbial cultivation technology.
Keywords: enzyme immobilization, filamentous microorganisms, gene regulation, metal oxides, microparticle‐enhanced cultivation
Abbreviations
- APTES
3‐aminopropyltriethoxysilane
- CPO
chloroperoxidase
- FUR
ferric uptake regulator
- MOP
metal oxide particles
- MPEC
microparticle‐enhanced cultivation
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX
NADPH oxidases
- ROS
reactive oxygen species
1. INTRODUCTION
Filamentous microorganisms are widely used in large‐scale industrial bioprocesses. Transforming matter on a molecular basis, filamentous microorganisms, for example, are used to produce antibiotics, chemicals, pharmaceuticals, food, colorants, and flavors [1]. With the ability to synthesize such important goods from low‐cost carbon and nitrogen sources, including industrial waste material, their importance constantly grows to satisfy the needs of a growing human population with limited resources. The annual market volume for microbial fermentation technology already exceeded $ 1.7 trillion in the year 2020 and it is constantly growing [2]. Therefore, techniques to increase yields of bioprocesses are of high scientific and economic interest, for example, the addition of microparticles to a submerged culture of filamentous microorganisms. This upcoming technique is called microparticle‐enhanced cultivation (MPEC) [3].
In 2007, the first experiments by Kaup et al. [3] revealed the potential of metal oxide particles (MOP) to influence bioprocesses with filamentous microorganisms. Since then numerous researches have shown that MOP, when added to the cultivation medium, influence the morphology and metabolism of microorganisms. Therefore, MPEC is often considered a morphology engineering approach [4]. The common working hypothesis of most MPEC experiments is that microparticle addition leads to reduced size of dense hyphal aggregates, called pellets, conceivably resulting in higher product titers, due to improved diffusion of oxygen and nutrients [5]. In contradiction to this hypothesis, a comparison of MPEC experiments (see Table 1) has shown that the effects of microparticle addition to submerged culture of filamentous microorganisms are highly application‐specific and particle‐specific, and that the effects of MOP addition are not limited to influencing the morphology of microorganisms (see Figure 1). Pellet size is not necessarily reduced by microparticle addition [6]. Documented MPEC effects include strong effects on morphogenesis; adoption of the microorganism transcriptome in the presence of MOP, including almost all functional gene classes [7]; changes in product titers [8]; variation in the pH of fermentation broth [9]; modified oxygen uptake rate [10] and respiratory quotient [11]; adopted exopolysaccharide composition [12]; and modified enzyme production rates [13].
TABLE 1.
Recent MPEC experiments, including the microorganisms and particles worked with
| Year | Author(s) | Microorganism species | Microparticles |
|---|---|---|---|
| fungi | |||
| 2007 | Kaup et al. [3] | Caldariomyces fumago, 9 other | Talc |
| 2010 | Driouch et al. [63] | Aspergillus niger | Alumina, Talc |
| 2011 | Driouch et al. [91] | Aspergillus niger | Titanate |
| 2011 | Driouch et al. [144] | Aspergillus niger | Alumina, Talc |
| 2012 | Wucherpfennig et al. [145] | Aspergillus niger | Talc |
| 2013 | Gonciarz, and Bizukojć [146] | Aspergillus terreus | Talc |
| 2014 | Etschmann et al. [14] | Aspergillus niger | Talc, 16 other |
| 2014 | Etschmann et al. [14] | Trichoderma viride | FeO, 17 other |
| 2014 | Gao et al. [147] | Mortierella isabellina | Talc |
| 2015 | Coban et al. [148] | Aspergillus ficuum | Alumina, Talc |
| 2015 | Coban, and Demirci [149] | Rhizopus oryzae | Alumina, Talc |
| 2016 | Yang et al. [4] | Curvularia sp. IFB‐Z10 | Alumina, Silica, Talc |
| 2016 | Yatmaz et al. [8] | Aspergillus sojae | Alumina, Talc |
| 2016 | Gonciarz et al. [150] | Aspergillus terreus | Talc |
| 2017 | Karahalil et al. [151] | Aspergillus sojae | Alumina |
| 2017 | Germec et al. [152] | Aspergillus sojae | Alumina, Talc, Titanate |
| 2018 | Kurt et al. [11] | Aspergillus niger | Talc |
| 2018 | Kowalska et al. [6] | Penicillium rubens, 3 other | Alumina |
| 2018 | Singh [13] | Aspergillus oryzae | Alumina, Talc |
| 2018 | Tao et al. [12] | Grifola frondosa | Talc |
| 2018 | Dong et al. [153] | Trichoderma viride | Alumina |
| 2019 | Kowalska et al. [10] | Mucor racemosus, 3 other | Alumina |
| 2019 | Boruta, and Bizukojć [154] | Aspergillus terreus | Alumina |
| 2019 | Li et al. [155] | Shiraia bambusciola | Bamboo Charcoal Powder |
| 2020 | Du et al. [9] | Chaetomium globosum | Glass Beads, Silica, Talc |
| 2020 | Saberi et al. [156] | Aspergillus terreus | Talc |
| 2020 | Gürler et al. [157] | Aspergillus sojae | Alumina, Talc |
| 2020 | Yatmaz et al. [64] | Aspergillus sojae | Alumina, Talc |
| 2020 | Niu et al. [158] | Aspergillus nidulans | Glass Beads, Talc |
| Bacteria | |||
| 2017 | Holtmann et al. [23] | Streptomyces | Silica |
| 2017 | Walisko et al. [28] | Lechevalieria aerocolonigenes | Glass Beads, Talc |
| 2020 | Schrinner et al. [56] | Lentzea aerocolonigenes | Glass Beads |
| 2020 | Kuhl et al. [7] | Streptomyces albus | Talc |
FIGURE 1.
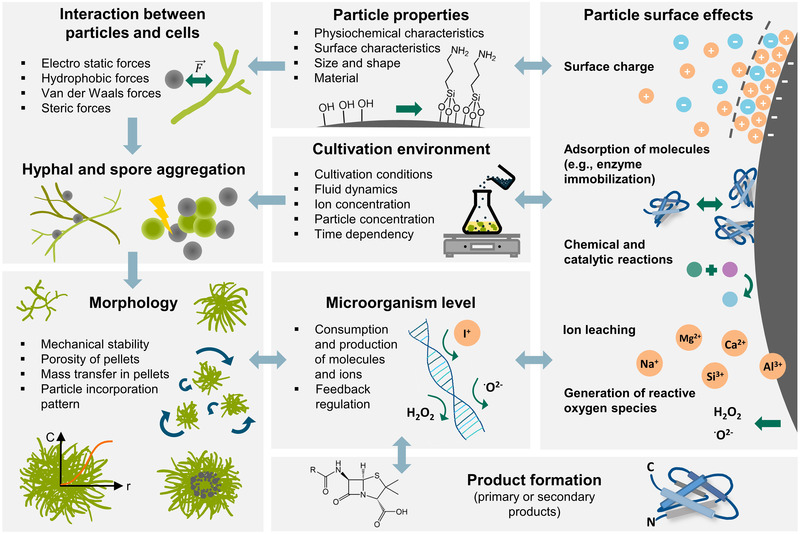
Scheme representing the aspects of microparticle‐enhanced cultivation (MPEC) described in the article
PRACTICAL APPLICATION
Adding microparticles to filamentous cultivations is a promising approach to increase the production yield of biotechnological processes. Applicable metal oxide particles are inexpensive bulk products. Previous studies often reduced this cultivation strategy to a morphology engineering approach. Contrary, this review describes physical and chemical molecular level interactions of particles with cultivation broth and filamentous microorganisms on extracellular, subcellular, and cellular level and the particle characteristics such effects heavily rely on. Consequently, this review facilitates the application of tailor‐made microparticles and enables to exploit the full potential of microparticle‐enhanced cultivation.
Yatmaz et al. [8] demonstrated that the effects of MPEC are application‐specific and depend on microparticle characteristics. They tested three factors (particle type, particle concentration, and cultivation medium) against each other and measured their influence on enzyme activity and biopellet size. These experiments were conducted with Aspergillus sojae and talc or alumina microparticles at various concentrations on carob pod or glucose cultivation media. Although biopellet size decreased in all cases, enzyme activity was affected in a cultivation medium‐, particle type‐, and concentration‐specific manner which cannot be directly and solely associated with the changes in morphology. The experiment further shows that decreased biopellet size does not necessarily result in increased enzyme activity because the addition of microparticles can result in decreased pellet size in conjunction with decreased enzyme activity.
In the MPEC experiments mainly talc and alumina particles were used (see Table 1), in a size range from 2 to 150 µm. Furthermore titania or silica microparticles within the same size range and glass beads (macroparticles: 0.5‐2 mm) were used. Although all of these particles comprised metal oxides, they might have strongly varied in morphological characteristics and physiochemical properties. 1 Owing to the variation in particle characteristics, the physical and biochemical interactions of these particles with their environment and microorganisms strongly vary.
The advance of MPEC as a technology to increase the rentability of microbial cultivation processes depends on understanding molecular level interactions from a physical, chemical, and biological point of view. In an interdisciplinary attempt, past MPEC experiments were analyzed and the theoretical framework of interaction was examined to understand how metal oxide particles and filamentous microorganisms in bioprocesses interact. Thereby, it is shown how molecular interactions on MOP depend on particle characteristics. Further, it is explained, how the usage of defined and specially designed particles might enable the regulation of molecular and cellular interactions in future microparticle‐enhanced cultivations.
2. PARTICLE PROPERTIES
Etschmann et al. [14] mentioned the heterogeneity in the properties of particles (size, shape, and surface properties) as the reason for the lack of a mechanistic understanding of the effect of particles in MPEC. In this context, particle morphology and surface chemistry generally influence chemical and physical processes at the particle surface (e.g., ion release, chemical reactions, interaction forces, and adsorption). Past studies have mostly reported only the particle size and the material; therefore, a more comprehensive characterization of the particles could lead to increased comparability, and a deeper understanding of mechanisms of action and targeted utilization of molecular interactions in the future. In the following, a brief overview of important particle properties, including their measurement methods, is given.
2.1. Characterization and control of the particle morphology
Through the targeted selection of manufacturing processes, tailor‐made particles could be produced in the future, assuming that the optimal particle properties for use in MPEC cultivations are known. The manufacturing processes of particles can be divided into top‐down and bottom‐up processes, wherein the type of process determines the properties of the particles. In top‐down processes, smaller particles are produced from coarser particles by mechanical processes such as comminution, whereas in bottom‐up processes (e.g., precipitation, crystallization, sol‐gel processes), particles are produced from atomic or molecular building blocks. The Stöber synthesis as a sol‐gel process can be used to produce monodisperse, spherical silica particles with a high purity and defined size [15]. By modifying the Stöber synthesis, Zhang et al. [16] could produce silica particles up to a size of 4.5 m. In addition, alumina is produced in the Bayer process by the precipitation of gibbsite and its calcination [17]. In contrast to Bayer process, alumina with a higher chemical purity and different morphologies can be obtained via hydrothermal synthesis (crystallization) [18].
For the analysis of particle size distribution in the lower micrometer range, methods such as laser diffraction, sedimentation methods as well as static or dynamic image analysis methods are suitable [19]. Particle shape in particular has a strong influence on the measured particle size, depending on the measurement method used. Image analysis methods offer an advantage here, as they can determine the shape as well as the particle size. Figure 2 (A) ‐ (C) illustrates the strong differences in particle shapes between the exemplarily selected particles of talc (plate‐shaped), alumina (polygonal) and glass (spherical). Electron microscopy images of Yekeler et al. [20] show that the shape of talc particles depends on the type of mill that is used. The fracture mechanism, which depends on the mill type, determines not only the shape but also the surface roughness of the particles [20, 21]. In this context, talc particles have rough breaking edges due to their layer structure and top down production process (milling), whereas alumina crystals and glass particles have comparatively smooth surfaces (Figure 2, (D) ‐ (F)). The roughness, together with the particle size and shape, influences the specific surface area of the particles, which can be determined by the Brunauer, Emmet and Teller (BET) method [22]. When using porous solids, such as the fractured porous SiO particles used by Holtmann et al. [23], the internal particle surface area needs to be determined additionally by means of porosity measurements (mercury porosimetry, capillary condensation method).
FIGURE 2.
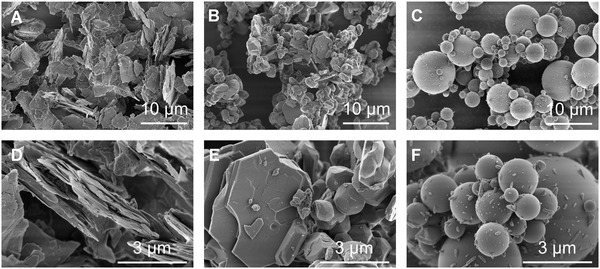
Scanning electron microscopy images showing the surface structure of: (A, D) talc (350 mesh); (B, E) aluminum oxide (x = 9.72 ); (C, F) glass beads (x = 7.9 ). The smooth surface of glass beads (C, F) contains production impurities. 10,000 x magnification (A‐C) and 50,000 x magnification (D‐F)
2.2. Physiochemical characterization of microparticles
Besides particle morphology, pysiochemical characteristics define the interaction of microparticles with their environment. Being inorganic and of non‐metallic nature, alumina, silica, or titania are ceramics, whereas talc, being of natural origin, is a mineral. All these substances comprise metal oxides but vary in their elementary composition and they may exist in different forms of crystal lattices with different thermodynamic stability [24]. The crystal lattice further affects the surface energy [25] as well as the band gap energy [26]. The crystal lattice of ceramics can be influenced by the production process and aluminas with various crystal lattices are commercially available for catalytic applications [24]. X‐ray diffraction allows the analysis of the crystal lattice [24]. Information on the electronic structure and chemical composition (including impurities) can for example be obtained by X‐ray photoelectron spectroscopy or UV‐photoelectron spectroscopy [27].
An important factor in terms of electrostatic interactions, surface chemistry, and adsorption effects is the zeta potential of particles. In MPEC applications, the zeta potential probably plays an extremely important role. For example, Walisko et al. [28] demonstrate that talcs of various zeta potentials result in highly different antibiotic production by Lechevalieria aerocolonigenes. The zeta potential is the electrical potential at the slipping plane, as particles move relative to a liquid [29]. It relies on the particle substance and the electrolytes present in the particle surrounding liquid [30]. PH value and concentration of ions in the medium determine size and sign of the zeta potential of metal oxide surfaces [31]. Owing to the layered structure of phyllosilicate minerals, talc exhibits different zeta potentials on the planes and on the edges of the layers [29]. The edges possess a more pH dependent charge than the planes, making the zeta potential and electrostatic behavior of talc also a function of milling grade. Various methods exist to measure the zeta potential of particles, including electrophoretic or electroacoustic methods [32].
To characterize and compare particles in terms of surface electric charge the closely related point of zero charge is more suitable than the zeta potential. The point of zero charge is defined as the isoelectric point (pH at which the zeta potential of a certain particle is zero) in presence of so‐called inert electrolytes, certain standardized electrolytes with little shifting effect on zeta potential. Zeta potential and isoelectric point of metal oxides are strongly influenced by the adsorption of multivalent inorganic ions, ionic surfactants and polyelectrolytes. Thus, the zeta potential and isoelectric point are application specific parameters, whereas the point of zero charge is a particle property measured in a standardized environment to ensure comparability [33].
Particles further vary in hydrophobic/hydrophilic share of the surface, and its acidity and basicity [25]. These parameters are connected with the presence of interfacial groups and the depth of the surface layer. Surface characterization is for example possible by atomic force microscopy (AFM), X‐ray photoelectron spectroscopy (XPS), or reaction chemistry [25, 27]. Surface basicity can be analyzed with UV‐absorption and luminescence spectroscopy [24]. Surface characteristics can be modified by the adsorption of molecules on the particle surface, that is, by chemical surface modification. Therefore, particle surface characterization provides details of the initial state of the particle surface. However, surface parameters may not remain constant during a cultivation process (see Section 4.2).
For MPEC applications it is important to note that commonly used terms such as alumina or talc refer to groups of substances and denote groups of substances with varying physiochemical characteristics. The term alumina is used for various aluminas and alumina‐hydrates of different crystal lattice and chemical potential [24]. The term talc is used for hydrous magnesium silicate of varying purity and for a mixture of various silicates including talc, called industrial talc [34]. The elementary composition and purity of talc strongly vary and depend on its natural origin, for example iron, calcium, and aluminum are always present in various concentrations [35].
2.3. Particle surface modification
Modification of the particle surface allows adjustment of the desired chemical surface properties without changing other particle properties such as shape or density [14]. This approach was pursued in cultivations of a filamentous bacteria by Walisko et al. [28], who produced various modified talc particles using a layer‐by‐layer process. These particles differed in their zeta potentials as well as hydrophobicity, which resulted in varying product concentrations. In addition to the layer‐by‐layer technology [36], the surface chemistry of talc and thus its adsorption capacity can be adjusted by the attachment of surfactants [37]. Another possibility for surface modification of talc is calcination, which led to altered adsorption of chloroperoxidase and affected the activity of the immobilized enzyme in the case of Aoun et al. [38].
A large number of studies from different application areas exist, where materials such as AlO [39] or glass [40, 41, 42, 43, 44] were chemically modified with the 3‐aminopropyltriethoxysilane (APTES) molecule (see Figure 1; Particle properties). The binding of APTES creates amino groups on the particle surface in the first step, to which various carboxylic acids can be covalently bonded in the second step [39]. This two‐step modification method allows the generation of both hydrophobic and hydrophilic surface properties while retaining constant particle properties otherwise. Thus, this approach is particularly suitable for future studies to investigate the role of surface chemistry of particles and their interactions with filamentous microorganisms. Fundamentally similar issues are being addressed in the area of microbial adhesion and associated biofilm formation. In this field, approaches to control the adhesion behavior of cells on surfaces already exist [42, 45, 46], which could potentially be applied to MPEC cultivations in the future. One example is the systematic study of the adhesion behavior of different bacteria as well as the adsorption of two proteins on synthetic surfaces by varying the chemical properties of attached molecules [41]. In summary, the methods presented can be used to create a targeted surface chemistry on the particles, which in turn influences various chemical and physical processes on and between surfaces (see Sections 3 and 5).
3. SURFACE CHEMISTRY EFFECTS OF METAL OXIDE PARTICLES
The surface chemistry potential of metal oxides, as applied in MPEC, is well researched and metal oxides are used as catalysts in various technical applications in many industries. Therefore, various surface chemistry effects of MOP are known which probably play an important role in MPEC applications. Surface chemistry effects on MOP can be regulated by modification of particle characteristics. Variation in particle characteristics and subsequently variation of surface chemistry cause application‐ and particle specific effects of MOP addition to submerged culture of filamentous microorganisms.
Morphological and physiochemical particle characteristics determine the surface chemistry potential of the applied MOP (see Section 2). Particles consist of the body of the particle which is surrounded by the particle surface, which forms an interface of the solid phase of the particle bulk and the liquid phase of the cultivation medium. Because MOP in cultivation media provide a liquid/solid interface, they are heterogenous catalysts [24]. The catalytic activity is proportional to the size of the catalytic interface which is determined by the specific surface area of the particles. At the same time, particles with similar physiochemical characteristics may show a variation of surface chemistry effects due to variation of morphological parameters.
By design, past MPEC‐experiments often targeted the effects of particle application on product titers by means of morphology engineering. Therefore, extremely few experiments had a design suitable to link observed effects of particle application to specific surface chemistry effects. Future experiments with variation in certain particle characteristics will be a first step to benefit from utilization of surface chemistry effects in MPEC applications and toward exclusion of unwanted side effects of uncontrolled molecular level interactions. The following sections explain the surface chemistry effects we expect to have the most significant impact in MPEC applications and which can most easily be influenced by variation of particle properties.
3.1. Adsorption of biomolecules on microparticles
In MPEC, adsorption effects must be considered. Biomolecules from liquid phases (cultivation medium) adsorb at solid phases (particle surface) by physisorption (by adhesion forces) or chemisorption (covalently bound) [47]. Adsorbed substances may get transformed, because the strong forces of chemisorption can break covalent bonds [27]. Heterogenous catalysis happens if two adsorbed substances react (Langmuir‐Hinshelwood mechanism) or an adsorbed molecule reacts with a free molecule (Eley‐Rideal mechanism) [48]. Adsorption effects and related catalytic activity in MPEC applications might significantly affect biomolecule availability.
MOP further adsorb metal ions [49, 50]. Metal ions play an important role in the metabolism of filamentous microorganisms as micro‐nutrients or signal molecules (see Section 5.1.3). Also enzymes get adsorbed at the microparticle surface, depending on parameters like particle charge, functional groups on the particle surface, zeta potential, and pH [51]. Immobilized enzymes can no longer freely move in the liquid phase; accessibility of the active center and diffusion rate of substrate molecules get modified; and the pH optimum of enzyme activity shifts. Enzyme immobilization has a strong impact on enzyme activity, and uncontrolled enzyme immobilization may result in significant reduction or increase in enzyme activity by several folds [52].
Enzyme immobilization probably plays a major role in MPEC applications. Kaup et al. [3], who introduced the term MPEC, conducted an experiment with talc microparticles and choloroperoxidase (CPO)‐producing Caldariomyces fumago. They observed a decrease in CPO activity at high talc concentration and proposed immobilization of CPO onto talc microparticles as an explanation. They further referred to Aoun et al. [38] who described the effects on enzyme activity of enzyme immobilization onto talc. Aoun et al. [38] showed that the immobilization of CPO onto talc led to specific enzyme activity, ranging from total inhibition to doubled activity compared with native CPO. The activity of immobilized CPO varied with the type of talc, pH at immobilization time, and pH at biocatalysis time. Up to now, the role of enzyme immobilization in MPEC has not been examined any further. The important role of immobilization of enzymes and other proteins in MPEC is theoretically examined in Section 5.3.
3.2. Ion leaching of microparticles
Etschmann et al. [14] showed that talc, silica and alumina particles leach ions. In this study, these ions affected the cultivation process, even without the microparticles being present. Ion leaching is a thermodynamically driven process, and it automatically happens depending on the ionicity of the particle. MOP vary in ionicity. The ionicity of metal oxides calculated from the electronegativity of the respective metal is called ionicity by Pauling. If ionicity by Pauling is 0.5, it can be concluded that next to covalent bindings also ionic oxides exist in the structure of the metal oxide. Such is for example the case for MgO (0.68), TiO (0.59), and AlO (0.57) [24].
Besides the main particle component, impurities in particles also get leached. For example, different talc preparations provide different ions in various concentrations [22]. The most common leaching products of talc, depending on its origin, are Fe, Ca and Mg [53, 54]. Glass particles, too, provide various ions. The composition of commercially widespread soda‐lime glass not only comprises SiO but also includes significant amounts of NaO, CaO, and MgO. Therefore, soda‐lime glass particles leach the corresponding Na, Ca, and Mg ions [55]. Glass particles of this composition were already used in MPEC experiments [28, 56].
Metal ions are able to influence cellular processes and gene regulation of microorganisms profoundly [57]. Leached ions may also promote unwanted chemical reactions, including the Fenton‐reaction. Section 5.1 elaborates possible effects of ion leaching and explains how geoactive filamentous fungi further actively leach ions from MOP.
3.3. Generation of reactive oxygen species
Metal oxides are capable to promote the generation of reactive oxygen species (ROS) by released metal ions which act as catalysts, by catalytic reactions on the particle surface with molecular oxygen, or by reaction of surface defect sites with molecular oxygen, carbon dioxide or water [58]. Metal oxides applied in MPEC applications might therefore change ROS concentrations in the cultivation medium. ROS species may start oxidative chain reactions in the cultivation medium, induce oxidative stress in microorganisms, and interfere with microbial regulation mechanisms (see Section 5.2).
If the respective metal oxide has a narrow band gap, it may act as a photocatalyst too. For example, TiO is a strong photocatalyst. Linked to photocatalytic ROS generation, it is capable of photocatalytic degradation of organic molecules to CO and water in a process called chalking cycle [59]. If semiconductive metal oxides such as TiO or FeO are used in MPEC, special attention must be paid to light irradiation of the microparticles.
4. INTERACTION BETWEEN PARTICLES AND CELL WALLS
To explain the aggregation behavior of spores and the associated pellet formation, all interaction and fluid forces must be considered in combination [60]. Depending on the physiological state of the spores and the strength of the individual interaction forces, coagulative or non‐coagulative pellet formation occurs [61]. In the case of pellet formation by hyphal aggregation, the chemical composition of the hyphal cell wall plays an important role [62]. However, the influence of surface interactions with particles has not been a major focus of MPEC research in the past; therefore, generalized relationships for the mechanism of action of particles are lacking [5].
Using image analysis over time, Driouch et al. [63] could show that the addition of talc particles after inoculation suppressed the initial aggregation phase of Aspergillus niger spores. As a result, loose mycelium was formed, whereas the addition of talc particles at a later cultivation time did not result in any change in morphology. Also Yatmaz et al. [64] indicated that the particles used seemed to disturb the spore aggregation of Aspergillus sojae. These results show that the interaction forces of spores with particle surfaces must also be considered. Because MPEC studies in the past mainly used Aspergilli, whose pellet formation is based on the aggregation of spores, four filamentous fungi with different aggregation behavior were studied inshake flasks by Kowalska et al. [6]. The results showed, independently of the strain, that the effect of particles depends on the size of mycelial objects. When the particles have an affinity for the spores or smaller mycelial objects, the particles can promote aggregation through a scaffolding function. For larger mycelial objects, particles tend to cause a decrease in pellet size owing to obstruction of aggregation or destruction of aggregates. In addition to these general statements, different influences on the four strains were observed. For the strains Aspergillus terreus (spore‐aggregative species) and Chaetomium globosum (no clear assignment of aggregative behavior), the addition of particles led to a reduction in pellet size. In the case of the non‐aggregating Mucor racemosus, addition of AlO particles promoted pellet formation. In contrast, Penicillium rubens as a hyphal aggregating strain showed accelerated aggregation of hyphae and more stable pellets after addition of particles.
For the process of pellet formation from spores, electrostatic, hydrophobic, and cell wall‐specific interaction forces have already been comprehensively described in the literature as crucial influencing variables [61, 65]. Overall, it can be assumed that in MPEC the particle surfaces have an influence on the aggregation behavior through interaction with the cell walls. This potential influence needs to be elaborated in the future to better explain the often strongly altered morphology of pellets. Thereby, it is known that the complex process of adhesion of cells to surfaces is determined, next to surface characteristics, by the properties of the cell itself and environmental factors [66, 67]. Furthermore, a broad field of studies on the adhesion of biological cells to surfaces already exists [46, 68, 69, 70]. The combination of both research areas will allow the development of a more fundamental understanding of the role of interaction forces between particles and biological cells in MPEC.
4.1. Interaction forces in MPEC
As a result of the sum of all forces occurring on a surface (electrostatic, hydrophobic, van der Waals, and steric interactions), attraction or repulsion can take place between two particulate objects (spores, hyphae, particles). An attractive total force leads to the formation of aggregates comprising one or more particle species (hetero‐aggregation), whereas repulsive forces prevent aggregation. Depending on the sign of the zeta potentials (see Section 2.2) of two surfaces, repulsive or attractive Coulomb forces result of electrostatic interactions. Attractive forces arise between particles and biological cells at opposing zeta potentials [71], which may lead to hetero‐aggregation between particles and cells (spores and hyphae). In principle, biological cells are often negatively charged; thus, the adhesion of cells to positively charged surfaces is enhanced [68]. In contrast, at high pH, the negative charge of spores results in repulsive forces, and thus suppression of spore aggregation [72]. Lee et al. [73] showed that spore adhesion was prevented on strongly negatively charged surfaces. In addition to pH, the magnitude of the zeta potential is dependent on the ion concentration in the medium [72] and the strain, because spores of different fungal strains have different chemical surface components [74]. In the case of Aspergillus niger spores, Wargenau et al. [72] indicated the carboxyl groups present in the pigment melanin as an origin of the negative charges, which was confirmed by decreasing the negative charges in spores with knocked out melanin formation [75]. Different metal ions can be bound by functional groups in pigmented cells [61], in which Mg and Zn showed the highest ability to desorb Cu which was previously adsorbed on melanin [76]. According to Marcelja [77], the specific binding of divalent cations has an influence on the electrostatic and hydrophobic interactions. Owing to the release of ions from particles (see Section 3.2), it can be assumed that the electrostatic interactions between spores are influenced both by the binding of metal ions to pigment‐containing cells and by changes in the ionic strength of the medium.
In addition to electrostatic interactions, Lewis acid‐base interactions cause hydrophobic attraction and hydrophilic repulsion between biosurfaces [78]. In case of spore aggregation [60, 79] or adhesion of cells to surfaces [70] hydrophobic interactions play an important role. The hydrophobic properties of spores of filamentous fungi arise from the formation of proteins called hydrophobins, which attach to cell walls by self‐assembly [80]. As a third type of interaction, the attractive van der Waals forces occur, which decrease rapidly with increasing distance [46]. In addition, steric interaction forces can arise in bacteria due to bacterial nanofibers (pili) [46]. A comprehensive review of adhesion as well as other adhesives of fungi is given by Epstein and Nicholson [69].
4.2. Changes in cell wall and particle surface properties over time
For the formation and the magnitude of the complex interaction forces between particles and cell walls, the condition of the respective surface is crucial. Due to different chemical and biological processes during cultivation, the surface properties, and thus interactions can change significantly over time. In the following, some examples are briefly presented that might be relevant in MPEC cultivations.
The correlation of physicochemical properties of a solid surface and the adhesion behavior of cells is complicated by the formation of a conditioning film on the particle surface [81]. The conditioning film is formed after the addition of a solid to an aqueous medium due to the adsorption of organic molecules on the particle surface, which can lead to altered roughness, charge, hydrophobicity, and surface tension of the substrate [82]. Specifically, monomeric hydrophobins formed by fungi can attach to surfaces in the soluble state owing to their amphipathic structure; thus, hydrophobic substrates can be converted to hydrophilic substrates and vice versa [83]. In addition to the particle surface, the initial composition of the cell walls also changes during spore germination [61], which initially comprise chitin, glucans, hydrophobins, melanin, ions, proteins, and lipids [84]. In the model approach summarized by Zhang and Zhang [61], the spores lose their layer of melanin and hydrophobins as they swell until finally polysaccharides are present on the surface. After inoculation with spores, both hydrophobic and electrostatic forces act initially, with the hydrophobic portions gradually decreasing due to loss of hydrophobins. The polysaccharides from the cell wall now present on the surface then form specific interactions between the spores due to salt bridges. In summary, the previously mentioned examples show that the interactions between particles and cells can change over the cultivation period. As a consequence, the exclusive characterization of particle‐cell interactions only at the beginning of cultivation may lead to not explainable or contradictory results.
4.3. Quantification and modeling of interaction forces
For the theoretical explanation of the adhesion behavior of biological cells to solids, both simple thermodynamic approaches [41, 43] and more complex models such as conventional or extended Derjaguin‐Landau‐Verwey‐Overbeek (DLVO) theory [71, 72, 85] are used in the literature. A good summary of the previously mentioned theories can be found in the studies of Perni et al. [67], and Hori, and Matsumoto [46]. Experimental methods for characterizing the solid surfaces are associated with the mathematical models. These include, for example, the measurement of the contact angle to calculate the surface energies or the determination of the zeta potential. In contrast, atomic force microscopy allows direct measurement of the sum of all interaction forces between two cells [86, 87] or a cell and a solid interface [88, 89, 90].
5. EFFECTS OF MPEC ON SUBCELLULAR STAGE
As already mentioned, MPEC of filamentous microorganisms leads to a broad spectrum of effects, including changes in metabolism and morphology. Latest studies revealed a strong connection between both these aspects with profound changes in cellular gene expression [7].
Kuhl et al. [7] conducted MPEC experiments with Streptomyces albus and talc. They investigated the effects of talc on the production of pamamycin derivatives and its precursors as well as the effects on the morphology of the microorganism. Therefore, they also conducted transcriptomic analysis to examine the underlying molecular changes. Talc supply led to decreased production of low‐molecular‐weight pamamycin derivatives but increased production of high‐molecular‐weight pamamycins; the corresponding pamamycin precursor CoA‐esters were analogously down‐ or upregulated. As a result of the transcriptomic analysis, 56 % alteration of the total gene activity as a reaction to talc supplementation was identified, revealing the enormous impact of MOP on the microorganisms molecular activity. In total, 3341 genes were found to be altered in their expression level, including genes involved in amino acid biosynthetic pathways and amino acid degradation, poyketide metabolism, carbon source metabolism, fatty acid degradation, and secondary metabolite biosynthesis, as well as genes related to stress and cell death. Overall, 55 genes addressing particularly morphological purposes were overexpressed, for example, morphological regulators, resulting in accelerated morphological development [7]. Reduction of pellet size was also observed by Kuhl et al. [7], most probably a consequence of the sum of the described effects ‐ interaction of microparticles, spores, and cell walls; surface chemistry effects; and microbial adaption processes (compare Figure 1).
Consequently, the study by Kuhl et al. [7] illustrates that it might be a strong simplification to attribute altered product synthesis and cellular morphology only to structural changes caused by particle incorporation into pellets and disruption of spore aggregation [28, 63, 91]. Rather, it seems likely that both morphology and product metabolism strongly correlate with an altered gene activity on subcellular stage. Thus, the following sections describe how the presence of MOP might affect the gene regulation with respect to certain molecular effects and mechanisms presented in previous chapters.
5.1. The role of metal ions in cell biology
All living species need metals in certain concentrations to fulfill metabolic activities such as the production of organic acids, enzymes, and metabolites [92]. Thus, the presence of soluble metal ions in microbial cultivations can influence cellular processes and gene regulation of microorganisms profoundly [57]. As described in Section 3.2, metal ion leaching occurs when MOP are supplemented in MPEC. In dependence of the chemical composition and stability, several ion species were proven to be involved in leaching processes from metal oxide species [53, 54, 55]. It is noteworthy that in various experiments, as those described in the following section, metal ion concentrations of merely 5 mg were sufficient to evoke fundamental changes in bacterial metabolisms [93]. Such concentrations concur with quantities already detected in metal ion leached supernatants [14] and interestingly, even to some extent exceed concentrations which can be found in minimal cultivation media [3, 91]. Owing to the profound complexity, the relevance of metal ions in connection with MPEC for microorganisms will be shown in the following by using selected examples.
5.1.1. Function of metal ions in gene regulation
Gene expression is generally regulated in different subcellular stages [57], ranging through all metabolic pathways from DNA to RNA synthesis, resulting in the protein biosynthesis. Because all subcellular stages require the supply of appropriate metal ion cofactors [94], metal ions play a specific role in the structure, function and metabolism of nucleic acids. Within this context, RNA analysis of numerous prokaryotic and eukaryotic cells have already identified strong bonds between RNA and cations such as Mg, Ca, Fe, Al, Cu and Zn. Furthermore, transition metals, like zinc, participate in nucleic acid synthesis pathways and stabilize the tertiary structure of RNA [95].
Starting on the genetic stage, Cuero et al. [96] observed an increased DNA concentration as a consequence of zinc supplemented cultivations of the investigated fungal microorganisms. At the transcriptional level, metal ion supplementations (iron, copper, and zinc) led to an enrichment of the total RNA concentration within microorganisms based on the type and mixture of supplemented ions. Furthermore, enhanced levels of metalloenzymes were reported due to the presence of divalent metal ions in fungi leading to altered fungal growth and product synthesis [93]. Thus, the studies of Cuero and Ouellet [93] and Cuero et al. [95, 96] already postulated a general stimulation of gene transcription by metal ion supplementation, resulting in altered patterns of gene expression of various filamentous species. Furthermore, they postulated that some of the affected genes might be involved in fungal enzymatic mechanisms related to both growth and/or production of secondary metabolites [95]. In line with these suggestions, Cuero and Ouellet [93] and Cuero et al. [95] also demonstrated the stimulating effect of metal ions by increased biomass concentrations and product titers in the considered cultivations. The hypothesis as well as the experimental proof show a broad similarity with the investigations of Kuhl et al. [7].
Consequently, the changes in gene regulation due to metal ions can also alter the production of numerous secondary metabolites. This also includes products of medical and industrial interest, for example, the since the 1930s known iron regulated production of the diphtheria toxin [97, 98, 99]. Therefore, optimization of metal ion supplementation is a crucial factor for increased product titers in several processes such as cultivations of Bacilli or Actinomycetes [57, 100]. Metal ions are also of great relevance for the biological function of many enzymes, a direct product of gene expression. Thereby, metal ions can interact with the enzymes in form of metal‐, ligand‐, and enzyme‐bridge complexes. Here, the role of metals can be found as electron donors or acceptors. tThus, metal ions play an important role in enzyme activity [101].
5.1.2. Fungal bioleaching and biosorption of metal ions
In the previously described MPEC processes, microorganisms are confronted with various ion leaching products of thermodynamically driven leaching processes. To actively overcome nutrient limitations, evolution provided microorganisms with the ability to force ion leaching processes and to directly dissolve metals from substrates, known as bioleaching and biosorption. Heterotrophic fungi such as Aspergillus and Penicillium are predestined to use bioleaching as a tool for nutrition supply because they are good producers of citric acid, oxalic acid, and glucuronic acid. These organic acids as well as amino acids and other metabolites, for example, siderophores, are proven metal leaching agents owing to their capability to form chelates or complexes with metal ions. Thereby, ions can be acquired in limited or insoluble environments and the pH of the environment can be decreased, which is also favorable for the leaching process [92].
Burgstaller, and Schinner [102] described bioleaching in detail, which in summary contains a four‐step mechanism: (i) acidolysis; (ii) complexolysis; (iii) redoxolysis; and (iv) bioaccumulation [102, 103]. Bioleaching requires a mixture of both direct physical force (e.g., via extension of hyphae) and the excretion of dissolving agents [94, 102, 103, 104, 105]. In this context, it was observed that fungal hyphae were able to “dig” tunnels into the surfaces of different ores to selectively extract nutrients during bioleaching. Jongmans et al. [106] entitled this phenomenon as “rock‐eating fungi”. While spores germinate, fungal mycelia can degrade particle textures through the physical destruction force caused by hyphal extension; releasing a new nutrient source [103, 107]. Depending on the cultivated strain and the substrate, metals such as iron, sodium, chlorine, potassium, and calcium leached from the particles, whereas elements such as oxygen, magnesium, aluminum, silicon, and phosphorous persisted [92, 104]. It was shown that free aluminum ions were actively accumulated or passively immobilized onto cell walls via biosorption in Streptomyces [108]. Moreover, Kuhl et al. [7] found genes linked to siderophore production significantly upregulated in MPEC cultivation of Streptomyces albus by talc microparticle supplementation. Thus, microparticles incorporated into pellets during MPEC [28, 63] might also be a target of active bioleaching in terms of containing several analogous ions of interest (see Section 5.1).
5.1.3. The role of calcium and magnesium in microorganisms
Adding calcium ions to fungal cultivations is known to have an enormous impact on the organism. In terms of metabolite synthesis, Aspergillus niger showed a higher product titer, with enhanced biomass concentration and the morphology shifted to a more pelleted form. Additionally, the micromorphology showed hyper‐branched structures, along with a bulbous cellular appearance and altered cell wall structure [109].
As a key messenger in signaling pathways, calcium function is conserved along fungi and other eukaryotes and plays an important role in altering gene expression in response to stress situations. Important for calcium homeostasis in fungi is calcineurin, an enzyme for the regulation of several calcium transporters, as well as transcription factors. Considering a whole signaling cascade, calcineurin and its calcium regulation function significantly influences the growth, differentiation, stress response, mating, budding, and tolerance of fungi toward antifungal drugs [110]. Without such feedback control systems, inadequate calcium accumulation would lead to a deregulation of important cellular processes and thus affect cell viability [110, 111]. The entry of calcium into fungi and other species occurs via voltage‐gated Ca channels, which in organisms such as Aspergillus nidulans matters in certain physiological processes such as conidiation, hyphal polarity, and assembly of cell wall components [112]. Accordingly, Jackson and Heath [113] demonstrated high calcium ion gradients in the tips of fungi and debated its function for the growth, physiology, and differentiation of hyphae. With the increase in extracellular Ca concentrations within a range of 0.4–400 mg , the hyphal extension rate increased. A limitation of extracellular calcium led to diminished or ceased extension rates in several organisms as well as abnormal hyphal morphogenesis, whereas high Ca concentrations ( 2 g ) inhibit growth. Thus, exterior calcium ions also influence the internal distribution [113]. Furthermore, Ca was identified to stabilize enzymes such as trypsin and modulate its activity spectrum [114], which may play an important role in enzyme activity in the culture broth due to potential MPEC calcium leaching from glass or talc.
Magnesium, a major component of talc, was additionally identified to have a positive effect on calcium tolerance in Aspergillus nidulans accompanied by enhanced growth, indicating an existing crosstalk of Mg and Ca homeostatic pathways [111]. Furthermore, Mg ions are important cofactors for enzymes, which are crucial for fungal growth and secondary metabolism. The mode of action was exemplified in experiments, in which a supplementation of 0.048 g magnesium ions was sufficient to significantly decrease the yield of the mycotoxin trichothecenes in a culture of Fusarium graminearum [115].
5.1.4. The role of iron in microorganisms
Iron is one of the most important essential micronutrients in the cellular metabolism, participating as a cofactor for numerous enzymes (e.g., oxidoreductases) and secondary metabolism pathways. A limitation of iron was shown to provoke impaired cellular differentiation and secondary metabolite production, for example, in strains of Streptomyces [116, 117, 118]. Ferrous ions as culture supplement were observed to significantly influence the size and density of pellets, leading to changed biomass production and altered the volumetric activity of various enzymes [119, 120]. Such alterations in enzyme activity can on the one hand be the result of altered enzyme production, as it is often assumed by volumetric activity measurements [119, 120]. On the other hand, ferrous ions leached in MPEC experiments may act as enzyme activators or inhibitors, as various metal ions (ferrous ions included) were shown to distinctly influence enzyme activities [121]. Also, allosteric metallosensor proteins play an essential role in microorganisms in order to recognize metal ions, inducing transcriptional response for homeostatic actions and metal resistance [122]. One exceptional important protein is the metallosensor FUR (ferric uptake regulator), a transcriptional repressor in a broader transcriptional network of iron homeostasis [123]. The role of FUR in the citric acid cycle is a further example of its diverse regulating function, just like its part in the enzymatic defense against ROS in many bacterial pathogens. Additionally, FUR was identified to be required as an activator for the expression of several genes [94]. Due to the low solubility of Fe ions, their availability is often limited in biological systems, whereas Fe ions in high concentrations are toxic [116]. Consequently, a disturbed uptake of iron into biological systems was shown to provoke globally reduced growth in considered species [124].
5.2. The role of ROS in MPEC experiments
As discussed in Section 3.3, certain MOP may cause the formation of ROS as a consequence of ion leaching in MPEC or photocatalytic activity during interaction with semiconductors. Reduced metal ions, such as Cu or Fe, can induce the Fenton reaction, causing the formation of hydroxyl radicals and hydroxide ions by hydrogen peroxide [125]. Owing to their unpaired electrons, ROS are highly reactive small molecules, potentially causing photooxidative damage to several cellular structures such as DNA, lipids, and proteins. Furthermore, ROS can lead to the inactivation of the previously mentioned FUR system. Subsequently, it is obligatory for organisms to tightly regulate the intracellular metal ion concentration [94]. Contrarily, ROS also act as signaling molecules in processes, such as growth, development, or cell death [125, 126].
Extracellular ROS can either directly act on fungal cell walls, addressing plasma membrane receptors and ion channels or alternatively pass the membrane to activate internal cellular signaling and downstream signaling pathways [127]. Moreover, ROS from foreign organisms can induce host‐signaling pathways [128], which leads to the assumption that ROS caused by leached metal ions have the potential to affect microorganisms in MPEC experiments. At least for prokaryotic cells, it was proven that metal ions affect the expression of peroxide stress genes [57]. Eventually, an increased ROS level was observed to cause increased product titers in cultivations of Aspergillus species, assuming that secondary metabolite synthesis has a compensatory response to ROS stress in microorganisms [128, 129].
ROS synthesis in living systems is realized by distinct NADPH oxidases (NOX) or their homologs, which are, among other things, directly regulated by calcium [130]. The overall function of NOX is important for fungal development [125], involving participation in the multicellular differentiation and development in animals, plants, and fungi [130]. With respect to the latter, the NOX machinery is important for hyphal branching, morphogenesis, (ascospore) germination, and sexual differentiation [128, 130, 131]. Furthermore, NOX‐dependent variation of ROS concentration leads to aberrant hyphal growth [131]. It is noteworthy that for the regulation of polarized hyphal growth a ROS gradient at the proximal tip is an important feature to establish and maintain hyphal polarity [132, 133]. In addition, ROS may promote the calcium entrance into cells by activating calcium channels causing calcium accumulation in hyphal tips [133]. Similar to metal ion regulation, fungi own a battery of enzymatic ROS detoxification systems to handle ROS‐induced oxidative stress [130]. Among secondary metabolites with antioxidant functions, antioxidant enzymes (e.g., catalase and superoxide dismutase) as well as mitogen‐activated protein kinases regulate redox homeostasis [129] and may be upregulated in case of MPEC‐mediated effects of ROS.
5.3. Adsorption effects in MPEC experiments
Owing to a high specific surface area, microparticles used in MPEC are predestined to show good adsorption properties toward molecules in culture broths [56]. Because biotechnological cultivations form systems of several compounds, the adsorption of each compound profoundly influences the MPEC system and its outcome in different aspects. Therefore, protein immobilization and the adsorption of substrates or products are exemplified in the following, as they probably have a major impact.
In general, the adsorption of proteins depends on particle properties (see Section 3.1), which can be influenced by the methods described in Section 2.3, and process parameters such as cultivation medium composition, pH, and temperature [51]. Talc, as an effective MPEC supplement, is generally considered to be a good adsorption scaffold, in which the ratio between hydrophilic (lateral) and hydrophobic (basal) surfaces is of great importance for the adsorption dynamics [37]. A well‐described example for the effects of enzyme immobilization is the enzyme chloroperoxidase (CPO), which was not only used for adsorption experiments with talc [38] but also used in first MPEC experiments described by Kaup et al. [3]. The cultivation of Caldariomyces fumago with talc particles led to the formation of dispersed mycelium and affected CPO formation and CPO activity in the culture supernatant [3]. Kaup et al. [3] hypothesized that increased oxygen and nutrient transfer may have led to higher metabolic productivity. But the authors also proposed that further significant factors may have been CPO immobilization, as well as the subsequent alteration of enzyme activity, as described by Aoun et al. [38] (see Section 3.1). Besides affecting enzyme activity, adsorption effects can also influence the availability of compounds and the properties of the enzyme's environment [134].
Furthermore, the aspect of feedback regulation may be of great relevance. Cells have developed intricate mechanisms to regulate the kinetics of metabolic pathways via both positive and negative feedback and forward regulation loops [135]. In case of high product titers, feedback inhibition intervenes to stop further product synthesis. Adsorption of biotechnological products may prevent feedback inhibition leading to enhanced product titers. Furthermore, a potential degradation or metabolization of the product can be prevented [134].
The targeted adsorption of further culture compounds is favorable in many aspects. A common strategy to scale up adsorption performance is the supplementation of adsorber resins, which enlarges the adsorptive particle surface up to several magnitudes [56]. In cultivation broths, adsorber concentrations were shown to correlate with a decrease of nutrient concentrations, leading to the diminishment of particular molecules, for example, carbon source or amino acids [136], which can be precursors for the product of interest. Contrary, microorganisms might directly desorb what they need from the surface [56]. This aspect particularly emphasizes the role of microparticles in filamentous cultivations, which can be found embedded in pellets [28, 91]. In favor of this idea is the observation that resins only enhance product titers if they are in physical contact to the microorganisms of interest [136]. As adsorption is a reversible process, also desorption processes due to decreasing concentration of the compound in the liquid phase must be considered. Lastly, if substrates or metabolites are available in excess, they can disturb the product formation allosterically or via substrate inhibition. Such substrate inhibitions can cause downregulation of the specific synthesis pathway [135], which can potentially be circumvented by adsorption. Moreover, adsorption of possibly toxic metabolites or products enables their further synthesis without inhibiting effects on the production organisms themselves.
In conclusion, the large specific surface area of microparticles in MPEC provides the framework to adsorptively interact with the cellular metabolism in terms of immobilization of several cultivation compounds, including immobilization of enzymes which can affect enzyme activity by several folds [52].
6. CONCLUDING REMARKS
Numerous studies demonstrated that MPEC of filamentous microorganisms lead to altered cellular morphologies and product formation. Most of these studies reduced MPEC to a morphology engineering approach and assumed no interference of microparticles with the cultivation medium or the metabolism of microorganisms [5, 137, 138]. However, there is recent evidence that applied microparticles such as talc, alumina, silica, and titanate strongly influence the cultivation medium as well as the subcellular and cellular level of filamentous microorganism. In this review, knowledge from particle technology, chemistry, and molecular biology is combined to draw a holistic picture on the effects of MPEC. We propose that the initial step toward a targeted application of MPEC is the morphological characterization as well as physiochemical characterization of microparticles. First, particle properties determine interaction forces between particles and cells, which further lead to particle‐cell aggregation. The mechanisms underlying theses aggregation processes and their effects on cellular morphology are elaborated in this review. Second, particle properties determine processes on the surface, including for example the adsorption of biomolecules, the release of ions, or the generation of ROS. Based on these processes, it has been demonstrated that microparticles and filamentous microorganisms interact on a molecular level, which has barely been considered to date. For example, leached metal ions and generated ROS might significantly interact and interfere with cellular processes and gene regulation of microorganisms. Moreover, we propose that adsorbed biomolecules alter the conversion of metabolites. Particularly, the adsorption of enzymes by microparticles is already known to strongly up‐ or downregulate the enzymatic activity. Further, adsorption of products or substrates might influence feedback inhibition and consumption rates.
To exploit the full potential of MPEC, all interactions among particles, microorganisms, and medium need to be considered. We propose strong molecular interactions between particles and filamentous microorganisms, which also include various mechanisms underlying the influence of microparticles on filamentous morphology. These interactions can increase or decrease the generation of desired products and depend on particle properties. Thus, this review can be seen as an initial step toward the targeted design and use of microparticles to control the growth and metabolism of filamentous microorganisms.
For future MPEC experiments, the following principles are proposed:
Experiments should be conducted with well‐defined and characterized microparticles. This helps to identify the effects of microparticles and make experiments more comparable. Special attention should be paid to specific surface area, zeta potential, particle composition, ionicity, and surface functions.
Experiments with surface‐modified microparticles should be conducted to deepen the knowledge on the aggregation between microparticles and filamentous microorganisms.
Metabolomics, proteomics, and transcriptomics shall be executed, ideally at different cultivation times. This enables effects of microparticles to be linked with molecular biology.
Microparticles can be added at different cultivation times to refine the understanding of temporal effects such as particle‐cell aggregation and interference of surface chemistry effects with extracellular, subcellular, and cellular processes.
As metal oxide microparticles have the potential to strongly interact with cells on molecular level, they exhibit toxic effects and pose a serious risk for human health and the environment [139, 140, 141, 142, 143]. It must be considered that microparticles continuously degrade in size and increase in toxicity, owing to natural processes. As MPEC, a technology to contribute to profitable biotechnological cultivation processes and a sustainable and circular economy, is promising, future industrial MPEC applications must responsibly consider the risks posed by MOP on human health and nature and ensure that no particles contaminate the product or environment.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
AUTHOR CONTRIBUTIONS
Conceptualization: Andreas Reiner Laible (lead), Anna Dinius, Marcel Schrader, and Stefan Schmideder (supporting); Investigation: Andreas Reiner Laible (lead), Anna Dinius, Marcel Schrader, and Stefan Schmideder (supporting); Writing–original draft: Andreas Reiner Laible (1, 2.2, 3.x), Anna Dinius (5.x), Marcel Schrader (2., 2.1, 2.3, 4.x), Stefan Schmideder (abstract, 6); Writing–review & editing: Andreas Reiner Laible, Marcel Schrader, Anna Dinius, Stefan Schmideder (equal) and Rainer Krull, Arno Kwade, Heiko Briesen (supporting); Visualization (graphical): Anna Dinius and Marcel Schrader; Supervision: Stefan Schmideder; Funding acquisition & Project administration: Rainer Krull, Arno Kwade, Heiko Briesen.
ACKNOWLEDGEMENTS
Special thanks go to Dr. rer. nat. Bogdan Semenenko and Karl Vorländer for scanning electron microscopic measurements. The authors thank the Deutsche Forschungsgemeinschaft for financial support for this study within the SPP 1934 DiSPBiotech–315384307 and 315457657. Open access funding was enabled and organized by Projekt DEAL.
Laible, A. R. , Dinius, A. , Schrader, M. , Krull, R. , et al. Effects and interactions of metal oxides in microparticle‐enhanced cultivation of filamentous microorganisms. Eng. Life Sci. 2022, 22, 725–743. 10.1002/elsc.202100075
Footnotes
Glass particles, being amorphous mixtures of metal oxides, are further referred to as metal oxide particles also, owing to similar chemical and physical properties.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Meyer, V. , Basenko, E. Y. , Benz, J. P. , Braus, G. H. et al. Growing a circular economy with fungal biotechnology: a white paper. Fungal. Biol. Biotechnol. 2020, 7. 10.1186/s40694-020-00095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Research, Markets Microbial Fermentation Technology ‐ Global Market Trajectory & Analytics. Research and Markets, Guinness Centre, Taylors Lane, Dublin 8, D08 AH31, Ireland., 2020.
- 3. Kaup, B. A. , Ehrich, K. , Pescheck, M. , Schrader, J. Microparticle‐enhanced cultivation of filamentous microorganisms: increased chloroperoxidase formation by caldariomyces fumago as an example. Biotechnol. Bioeng. 2007, 99, 491–498. [DOI] [PubMed] [Google Scholar]
- 4. Yang, J. , Jiao, R. H. , Yao, L. Y. , Han, W. B. et al. Control of fungal morphology for improved production of a novel antimicrobial alkaloid by marine‐derived fungus Curvularia sp. IFB‐Z10 under submerged fermentation. Process Biochem. 2016, 51, 185–194. [Google Scholar]
- 5. Antecka, A. , Bizukojc, M. , Ledakowicz, S. Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World. J. Microbiol. Biotechnol. 2016, 32, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kowalska, A. , Boruta, T. , Bizukojć, M. Morphological evolution of various fungal species in the presence and absence of aluminum oxide microparticles: comparative and quantitative insights into microparticle‐enhanced cultivation (MPEC). Microbiology open 2018, 7, e00603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuhl, M. , Gläser, L. , Rebets, Y. , Rückert, C. et al. Microparticles globally reprogram streptomyces albus toward accelerated morphogenesis, streamlined carbon core metabolism, and enhanced production of the antituberculosis polyketide pamamycin. Biotechnol. Bioeng. 2020, 10.1002/bit.27537. [DOI] [PubMed] [Google Scholar]
- 8. Yatmaz, E. , Karahalil, E. , Germec, M. , Ilgin, M. et al. Controlling filamentous fungi morphology with microparticles to enhanced ‐mannanase production. Bioprocess Biosyst. Eng. 2016, 39, 1391–1399. [DOI] [PubMed] [Google Scholar]
- 9. Du, L. , Gao, B. , Liang, J. , Wang, Y. et al. Microparticle‐enhanced chaetomium globosum DX‐THS3 ‐d‐glucuronidase production by controlled fungal morphology in submerged fermentation. 3 Biotech. 2020, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kowalska, A. , Boruta, T. , Bizukojć, M. Kinetic model to describe the morphological evolution of filamentous fungi during their early stages of growth in the standard submerged and microparticle‐enhanced cultivations. Eng. Life Sci. 2019, 19, 557–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kurt, T. , Marbà‐Ardébol, A. M. , Turan, Z. , Neubauer, P. et al. Rocking aspergillus: morphology‐controlled cultivation of aspergillus niger in a wave‐mixed bioreactor for the production of secondary metabolites. Microb. Cell Fact. 2018, 17, 10.1186/s12934-018-0975-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tao, T. L. , Cui, F. J. , Chen, X. X. , Sun, W. J. et al. Improved mycelia and polysaccharide production of grifola frondosa by controlling morphology with microparticle talc. Microb. Cell Fact. 2018, 17, 10.1186/s12934-017-0850-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singh, B. Engineering fungal morphology for enhanced production of hydrolytic enzymes by Aspergillus oryzae SBS50 using microparticles. 3 Biotech 2018, 8, 10.1007/s13205-018-1308-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Etschmann, M. M. W. , Huth, I. , Walisko, R. , Schuster, J. , Krull, R. , Holtmann, D. , Wittmann, C. , Schrader, J. Improving 2‐phenylethanol and 6‐pentyl‐α‐pyrone production with fungi by microparticle‐enhanced cultivation (MPEC). Yeast 2015, 32, 145–157. [DOI] [PubMed] [Google Scholar]
- 15. Ghimire, P. P. , Jaroniec, M. Renaissance of stöber method for synthesis of colloidal particles: new developments and opportunities. J. Colloid Interface Sci. 2021, 584, 838–865. [DOI] [PubMed] [Google Scholar]
- 16. Zhang, S. , Li, G. L. , Cong, H. L. , Yu, B. Size control of monodisperse silica particles by modified Stöber method. Integr. Ferroelectr. 2017, 178, 52–57. [Google Scholar]
- 17. Hind, A. R. , Bhargava, S. K. , Grocott, S. C. The surface chemistry of bayer process solids: a review. Colloids Surf. A 1999, 146, 359–374. [Google Scholar]
- 18. Suchanek, W. L. Hydrothermal synthesis of alpha alumina (‐Al2O3) powders: study of the processing variables and growth mechanisms. J. Am. Ceram. Soc. 2010, 93, 399–412. [Google Scholar]
- 19. Merkus, H. G. Sedimentation techniques. In: Particle Size Measurements. Springer, 2009. p. 319–348. [Google Scholar]
- 20. Yekeler, M. , Ulusoy, U. , Hiçyılmaz, C. Effect of particle shape and roughness of talc mineral ground by different mills on the wettability and floatability. Powder Technol. 2004, 140, 68–78. [Google Scholar]
- 21. Ulusoy, U. Application of ANOVA to image analysis results of talc particles produced by different milling. Powder Technol. 2008, 188, 133–138. [Google Scholar]
- 22. Malandrini, H. , Clauss, F. , Partyka, S. , Douillard, J. Interactions between talc particles and water and organic solvents. J. Colloid Interface Sci. 1997, 194, 183–193. [DOI] [PubMed] [Google Scholar]
- 23. Holtmann, D. , Vernen, F. , Müller, J. M. , Kaden, D. et al. Effects of particle addition to Streptomyces cultivations to optimize the production of actinorhodin and streptavidin. Sustain. Chem. Pharm. 2017, 5, 67–71. [Google Scholar]
- 24. Busca, G. Heterogeneous catalysts. Heterogeneous Catalytic Materials 2014, 10.1016/b978-0-444-59524-9.00001-8. [DOI]
- 25. Allcock, H. R. Introduction to Materials Chemistry. John Wiley & Sons, 2019. [Google Scholar]
- 26. Grundmann, M. Physics of Semiconductors. vol. 11. Springer, 2016. [Google Scholar]
- 27. Hofmann, A. Physical Chemistry Essentials. Springer, 2018, 10.1007/978-3-319-74167-3. [DOI]
- 28. Walisko, J. , Vernen, F. , Pommerehne, K. , Richter, G. , et al. Particle‐based production of antibiotic rebeccamycin with Lechevalieria aerocolonigenes. Process Biochem. 2017, 53, 1–9. [Google Scholar]
- 29. Alvarez‐Silva, M. , Mirnezami, M. , Uribe‐Salas, A. , Finch, J. A. Point of zero charge, isoelectric point and aggregation of phyllosilicate minerals. Can. Metall. Q. 2010, 49, 405–410. [Google Scholar]
- 30. Gulicovski, J. J. , Çerović, L. S. , Milonjić, S. K. Point of zero charge and isoelectric point of alumina. Mater. Manuf. Processes 2008, 23, 615–619. [Google Scholar]
- 31. Wills, B. A. , Finch, J. Wills' Mineral Processing Technology. 8th ed. Butterwood‐Heinemann, 2015. [Google Scholar]
- 32. Higashitani, K. , Makino, H. , Matsusaka, S. Powder Technology Handbook. CRC Press, 2019. [Google Scholar]
- 33. Kosmulski, M. The pH dependent surface charging and points of zero charge. VIII. Update. Adv. Colloid Interface Sci. 2020, 275, 102064. [DOI] [PubMed] [Google Scholar]
- 34. Ciullo, P. A. , Anderson, J. Industrial talc. J. Coat. Technol. 2002, 74, 15–19. [Google Scholar]
- 35. Ferrer, J. , Villarino, M. A. , Tura, J. M. , Traveria, A. et al. Talc preparations used for pleurodesis vary markedly from one preparation to another. Chest 2001, 119, 1901–1905. [DOI] [PubMed] [Google Scholar]
- 36. Peyratout, C. S. , Dähne, L. Tailor‐made polyelectrolyte microcapsules: from multilayers to smart containers. Angew. Chem. Int. Ed. 2004, 43, 3762–3783. [DOI] [PubMed] [Google Scholar]
- 37. Yakovleva, A. A. Analysis of techniques for improving the adsorption capacity of talc. Prot. Met. Phys. Chem. Surf. 2019, 55, 439–444. [Google Scholar]
- 38. Aoun, S. , Chebli, C. , Baboulene, M. Noncovalent immobilization of chloroperoxidase onto talc: catalytic properties of a new biocatalyst. Enzyme Microb. Technol. 1998, 23, 380–385. [Google Scholar]
- 39. Kockmann, A. , Hesselbach, J. , Zellmer, S. , Kwade, A. et al. Facile surface tailoring of metal oxide nanoparticles via a two‐step modification approach. RSC Adv. 2015, 5, 60993–60999. [Google Scholar]
- 40. Ozmen, M. , Can, K. , Akin, I. et al. Surface modification of glass beads with glutaraldehyde: characterization and their adsorption property for metal ions. J. Hazard. Mater. 2009, 171, 594–600. [DOI] [PubMed] [Google Scholar]
- 41. Cunliffe, D. , Smart, C. , Alexander, C. , Vulfson, E. Bacterial adhesion at synthetic surfaces. Appl. Environ. Microbiol. 1999, 65, 4995–5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jradi, K. , Daneault, C. , Chabot, B. Chemical surface modification of glass beads for the treatment of paper machine process waters. Thin Solid Films 2011, 519, 4239–4245. [Google Scholar]
- 43. Katsikogianni, M. G. , Missirlis, Y. F. Bacterial adhesion onto materials with specific surface chemistries under flow conditions. J. Mater. Sci. Mater. Med. 2010, 21, 963–968. [DOI] [PubMed] [Google Scholar]
- 44. Verné, E. , Vitale‐Brovarone, C. , Bui, E. , Bianchi, C. L. et al. Surface functionalization of bioactive glasses. J. Biomed. Mater. Res. Part A 2009, 90A, 981–992. [DOI] [PubMed] [Google Scholar]
- 45. Achinas, S. , Charalampogiannis, N. , Euverink, G. J. W. A brief recap of microbial adhesion and biofilms. Appl. Sci. 2019, 9, 2801. [Google Scholar]
- 46. Hori, K. , Matsumoto, S. Bacterial adhesion: from mechanism to control. Biochem. Eng. J. 2010, 48, 424–434. [Google Scholar]
- 47. Ross, J. R. Contemporary Catalysis: Fundamentals and Current Applications. Elsevier, 2018. [Google Scholar]
- 48. Bhargava, S. K. , Tardio, J. , Prasad, J. , Föger, K. et al. Wet oxidation and catalytic wet oxidation. Ind. Eng. Chem. Res. 2006, 45, 1221–1258. [Google Scholar]
- 49. Huang, P. , Fuerstenau, D. W. The effect of the adsorption of lead and cadmium ions on the interfacial behavior of quartz and talc. Colloids Surf. A 2001, 177, 147–156. [Google Scholar]
- 50. Kim, M. S. , Hong, K. M. , Chung, J. G. Removal of Cu (II) from aqueous solutions by adsorption process with anatase‐type titanium dioxide. Water Res. 2003, 37, 3524–3529. [DOI] [PubMed] [Google Scholar]
- 51. Derr, L. Interactions between enzymes and oxide colloidal particles and their influence on enzymatic activity, 2016.
- 52. Dwevedi, A. Enzyme Immobilization, 2016.
- 53. Barani, K. , Aghazadeh, V. Removal of impurities from talc ore by leaching method. J. Chem. Technol. Metall. 2018, 53, 296–300. [Google Scholar]
- 54. Castillo, L. A. , Barbosa, S. E. , Maiza, P. , Capiati, N. J. Integrated process for purification of low grade talc ores. Part. Sci. Technol. 2014, 32, 1–7. [Google Scholar]
- 55. Guiheneuf, V. , Delaleux, F. , Riou, O. , Logerais, P. O. et al. Investigation of damp heat aging on soda‐lime glass for photovoltaic applications. In: 32nd European Photovoltaic Solar Energy Conference and Exhibition , 2016.
- 56. Schrinner, K. , Veiter, L. , Schmideder, S. , Doppler, P. et al. Morphological and physiological characterization of filamentous lentzea aerocolonigenes: comparison of biopellets by microscopy and flow cytometry. PLOS ONE 2020, 15, e0234125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Silver, S. , Walden, W. Metal Ions in Gene Regulation. Springer Science & Business Media, 2012. [Google Scholar]
- 58. Schoonen, M. A. , Cohn, C. A. , Roemer, E. , Laffers, R. et al. Mineral‐induced formation of reactive oxygen species. Rev. Mineral. Geochem. 2006, 64, 179–221. [Google Scholar]
- 59. Winkler, J. Titandioxid. 2nd ed. Vincentz Network GmbH & Co KG, 2013. [Google Scholar]
- 60. Dynesen, J. , Nielsen, J. Surface Hydrophobicity of aspergillus nidulans conidiospores and its role in pellet formation. Biotechnol. Prog. 2003, 19, 1049–1052. [DOI] [PubMed] [Google Scholar]
- 61. Zhang, J. , Zhang, J. The filamentous fungal pellet and forces driving its formation. Crit. Rev. Biotechnol. 2015, 36, 1066–1077. [DOI] [PubMed] [Google Scholar]
- 62. Miyazawa, K. , Yoshimi, A. , Abe, K. The mechanisms of hyphal pellet formation mediated by polysaccharides, ‐1,3‐glucan and galactosaminogalactan, in Aspergillus species. Fungal. Biol. Biotechnol. 2020, 7, 10.1186/s40694-020-00101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Driouch, H. , Sommer, B. , Wittmann, C. Morphology engineering of aspergillus niger for improved enzyme production. Biotechnol. Bioeng. 2010, n/a–n/a. [DOI] [PubMed] [Google Scholar]
- 64. Yatmaz, E. , Germec, M. , Karahalil, E. , Turhan, I. Enhancing ‐mannanase production by controlling fungal morphology in the bioreactor with microparticle addition. Food Bioprod. Process. 2020, 121, 123–130. [Google Scholar]
- 65. Veiter, L. , Rajamanickam, V. , Herwig, C. The filamentous fungal pellet—relationship between morphology and productivity. Appl. Microbiol. Biotechnol. 2018, 102, 2997–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. An, Y. H. , Friedman, R. J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998, 43, 338–348. [DOI] [PubMed] [Google Scholar]
- 67. Perni, S. , Preedy, E. C. , Prokopovich, P. Success and failure of colloidal approaches in adhesion of microorganisms to surfaces. Adv. Colloid Interface Sci. 2014, 206, 265–274. [DOI] [PubMed] [Google Scholar]
- 68. Berne, C. , Ellison, C. K. , Ducret, A. , Brun, Y. V. Bacterial adhesion at the single‐cell level. Nat. Rev. Microbiol. 2018, 16, 616–627. [DOI] [PubMed] [Google Scholar]
- 69. Epstein, L. , Nicholson, R. Adhesion and adhesives of fungi and oomycetes. In: Biological Adhesives. Springer, 2016. p. 25–55. [Google Scholar]
- 70. Desrousseaux, C. , Sautou, V. , Descamps, S. , Traoré, O. Modification of the surfaces of medical devices to prevent microbial adhesion and biofilm formation. J. Hosp. Infect. 2013, 85, 87–93. [DOI] [PubMed] [Google Scholar]
- 71. Schwarz, M. , Kloß, S , Stöckel, S. et al. Pioneering particle‐based strategy for isolating viable bacteria from multipart soil samples compatible with Raman spectroscopy. Anal. Bioanal. Chem. 2017, 409, 3779–3788. [DOI] [PubMed] [Google Scholar]
- 72. Wargenau, A. , Kampen, I. , Kwade, A. Linking aggregation ofAspergillus nigerspores to surface electrostatics: a theoretical approach. Biointerphases 2013, 8, 7. [DOI] [PubMed] [Google Scholar]
- 73. Lee, I. , Chung, E. , Kweon, H. , Yiacoumi, S. , Tsouris, C. Scanning surface potential microscopy of spore adhesion on surfaces. Colloids Surf. B 2012, 92, 271–276. [DOI] [PubMed] [Google Scholar]
- 74. Fisher, D. J. Charges on fungal spores. Pestic. Sci. 1973, 4, 845–860. [Google Scholar]
- 75. Priegnitz, B. E. , Wargenau, A. , Brandt, U. et al. The role of initial spore adhesion in pellet and biofilm formation in aspergillus niger. Fungal Genet. Biol. 2012, 49, 30–38. [DOI] [PubMed] [Google Scholar]
- 76. Gadd, G. M. , de Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 1988, 29, 610–617. [Google Scholar]
- 77. Marcelja, S. Electrostatics of membrane adhesion. Biophys. J. 1992, 61, 1117–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. van Oss, C. J. Hydrophobicity and hydrophilicity of biosurfaces. Curr. Opin. Colloid Interface Sci. 1997, 2, 503–512. [Google Scholar]
- 79. Mamane‐Gravetz, H. , Linden, K. G. Relationship between physiochemical properties, aggregation and u.v. inactivation of isolated indigenous spores in water. J. Appl. Microbiol. 2005, 98, 351–363. [DOI] [PubMed] [Google Scholar]
- 80. Wösten, H. A. B. Hydrophobins: multipurpose proteins. Annu. Rev. Microbiol. 2001, 55, 625–646. [DOI] [PubMed] [Google Scholar]
- 81. Linke, D. , Goldman, A. Bacterial Adhesion: Chemistry, Biology and Physics. vol. 715. Springer Science & Business Media, 2011. [Google Scholar]
- 82. Hwang, G. , Kang, S. , El‐Din, M. G. , Liu, Y. Impact of conditioning films on the initial adhesion of Burkholderia cepacia. Colloids Surf. B 2012, 91, 181–188. [DOI] [PubMed] [Google Scholar]
- 83. Wösten, H. A. B. , Scholtmeijer, K. Applications of hydrophobins: current state and perspectives. Appl. Microbiol. Biotechnol. 2015, 99, 1587–1597. [DOI] [PubMed] [Google Scholar]
- 84. Feofilova, E. P. The fungal cell wall: modern concepts of its composition and biological function. Microbiology 2010, 79, 711–720. [PubMed] [Google Scholar]
- 85. Wyness, A. J. , Paterson, D. M. , Defew, E. C. , Stutter, M. I. , Avery, L. M. The role of zeta potential in the adhesion of E. coli to suspended intertidal sediments. Water Res. 2018, 142, 159–166. [DOI] [PubMed] [Google Scholar]
- 86. Wargenau, A. , Kwade, A. Determination of adhesion between single aspergillus niger spores in aqueous solutions using an atomic force microscope. Langmuir 2010, 26, 11071–11076. [DOI] [PubMed] [Google Scholar]
- 87. Alsteens, D. , Van Dijck, P. , Lipke, P. N. , Dufrêne, Y. F. Quantifying the forces driving cell–cell adhesion in a fungal pathogen. Langmuir 2013, 29, 13473–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bowen, W. R. , Lovitt, R. W. , Wright, C. J. Atomic force microscopy study of the adhesion of saccharomyces cerevisiae. J. Colloid Interface Sci. 2001, 237, 54–61. [DOI] [PubMed] [Google Scholar]
- 89. Viljoen, A. , Mignolet, J. , Viela, F. , Mathelié‐Guinlet, M. et al. How microbes use force to control adhesion. J. Bacteriol. 2020, 202, ed. by Margolin, W. , 10.1128/jb.00125-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Papi, M. , Maiorana, A. , Bugli, F. , Torelli, R. et al. Detection of biofilm‐grown aspergillus fumigatus by means of atomic force spectroscopy: ultrastructural effects of alginate lyase. Microsc. Microanal. 2012, 18, 1088–1094. [DOI] [PubMed] [Google Scholar]
- 91. Driouch, H. , Hänsch, R. , Wucherpfennig, T. , Krull, R. et al. Improved enzyme production by bio‐pellets of aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol. Bioeng. 2011, 109, 462–471. [DOI] [PubMed] [Google Scholar]
- 92. Shah, S. S. , Palmieri, M. C. , Sponchiado, S. R. P. , Bevilaqua, D. Enhanced bio‐recovery of aluminum from low‐grade bauxite using adapted fungal strains. Braz. J. Microbiol. 2020, 51, 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cuero, R. , Ouellet, T. Metal ions modulate gene expression and accumulation of the mycotoxins aflatoxin and zearalenone. J. Appl. Microbiol. 2005, 98, 598–605. [DOI] [PubMed] [Google Scholar]
- 94. Troxell, B. , Hassan, H. M. Transcriptional regulation by ferric uptake regulator (Fur) in pathogenic bacteria. Front. Cell. Infect. Microbiol. 2013, 3, 10.3389/fcimb.2013.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Cuero, R. , Ouellet, T. , Yu, J. , Mogongwa, N. Metal ion enhancement of fungal growth, gene expression and aflatoxin synthesis in aspergillus flavus: RT‐PCR characterization. J. Appl. Microbiol. 2003, 94, 953–961. [DOI] [PubMed] [Google Scholar]
- 96. Cuero, R. , Duffus, E. , Williams, M. , Baca, D. , Navarro, M. Effects of zinc and associated mycoflora on fungal growth and toxin production: F. moniliforme, F. graminearum, aspergillus flavus. Mycotoxins and Phycotoxins–Developments in Chemistry, Toxicology and Food Safety. 1998, 311–319. [Google Scholar]
- 97. Silver, S. , Ji, G. Newer systems for bacterial resistances to toxic heavy metals. Environ. Health Perspect. 1994, 102, 107–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pappenheimer, A. M. et al. Studies in diphtheria toxin production. I: the effect of iron and copper. Br. J. Exp. Pathol. 1936, 17, 335. [Google Scholar]
- 99. Pappenheimer, A. M. Diphtheria toxin. Annu. Rev. Biochem. 1977, 46, 69–94. [DOI] [PubMed] [Google Scholar]
- 100. Weinberg, E. D. Roles of trace metals in transcriptional control of microbial secondary metabolism. Biol. Met. 1990, 2, 191–196. [DOI] [PubMed] [Google Scholar]
- 101. Riordan, J. F. The role of metals in enzyme activity. Ann. Clin. Lab. Sci. 1977, 7, 119–129. [PubMed] [Google Scholar]
- 102. Burgstaller, W. , Schinner, F. Leaching of metals with fungi. J. Biotechnol. 1993, 27, 91–116. [Google Scholar]
- 103. Vakilchap, F. , Mousavi, S. , Shojaosadati, S. Role of aspergillus niger in recovery enhancement of valuable metals from produced red mud in bayer process. Bioresour. Technol. 2016, 218, 991–998. [DOI] [PubMed] [Google Scholar]
- 104. Pinzari, F. , Cuadros, J. , Napoli, R. , Canfora, L. et al. Routes of phlogopite weathering by three fungal strains. Fungal. Biol. 2016, 120, 1582–1599. [DOI] [PubMed] [Google Scholar]
- 105. Santhiya, D. , Ting, Y. P. Use of adapted aspergillus niger in the bioleaching of spent refinery processing catalyst. J. Biotechnol. 2006, 121, 62–74. [DOI] [PubMed] [Google Scholar]
- 106. Jongmans, A. G. , van Breemen, N. , Lundström, U. , van Hees, P. A. W. et al. Rock‐eating fungi. Nature 1997, 389, 682–683. [Google Scholar]
- 107. Kolo, K. , Keppens, E. , Préat, A. , Claeys, P. Experimental observations on fungal diagenesis of carbonate substrates. J. Geophys. Res. 2007, 112, 10.1029/2006jg000203. [DOI] [Google Scholar]
- 108. Tassist, A. , Lounici, H. , Abdi, N. , Mameri, N. Equilibrium, kinetic and thermodynamic studies on aluminum biosorption by a mycelial biomass (Streptomyces rimosus). J. Hazard. Mater. 2010, 183, 35–43. [DOI] [PubMed] [Google Scholar]
- 109. Pera, L. M. , Callieri, D. A. Influence of calcium on fungal growth, hyphal morphology and citric acid production inaspergillus niger. Folia Microbiol. 1997, 42, 551–556. [DOI] [PubMed] [Google Scholar]
- 110. Tisi, R. , Rigamonti, M. , Groppi, S. , Belotti, F. Calcium homeostasis and signaling in fungi and their relevance forpathogenicity of yeasts and filamentous fungi. AIMS Mol. Sci. 2016, 3, 505–549. [Google Scholar]
- 111. Manoli, M. , Espeso, E. A. Modulation of calcineurin activity in Aspergillus nidulans : the roles of high magnesium concentrations and of transcriptional factor CrzA. Mol. Microbiol. 2019, 111, 1283–1301. [DOI] [PubMed] [Google Scholar]
- 112. Wang, S. , Cao, J. , Liu, X. , Hu, H. et al. Putative calcium channels CchA and MidA play the important roles in conidiation, hyphal polarity and cell wall components in aspergillus nidulans. PLoS ONE 2012, 7, ed. by Goldman, G. H. , e46564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jackson, S. , Heath, I. Roles of calcium ions in hyphal tip growth. Microbiol. Mol. Biol. Rev. 1993, 57, 367–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Sipos, T. , Merkel, J. R. Effect of calcium ions on the activity, heat stability, and structure of trypsin. Biochemistry 1970, 9, 2766–2775. [DOI] [PubMed] [Google Scholar]
- 115. Pinson‐Gadais, L. , Richard‐Forget, F. , Frasse, P. , Barreau, C. et al. Magnesium represses trichothecene biosynthesis and modulates Tri5, Tri6, and Tri12 genes expression in fusarium graminearum. Mycopathologia 2007, 165, 51–59. [DOI] [PubMed] [Google Scholar]
- 116. Andrews, S. C. , Robinson, A. K. , Rodríguez‐Quiñones, F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003, 27, 215–237. [DOI] [PubMed] [Google Scholar]
- 117. Locatelli, F. M. , Goo, K. S. , Ulanova, D. Effects of trace metal ions on secondary metabolism and the morphological development of streptomycetes. Metallomics 2016, 8, 469–480. [DOI] [PubMed] [Google Scholar]
- 118. Traxler, M. F. , Seyedsayamdost, M. R. , Clardy, J. , Kolter, R. Interspecies modulation of bacterial development through iron competition and siderophore piracy. Mol. Microbiol. 2012, 86, 628–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Couri, S. , Pinto, G. A. S. , Senna, L. F. d. , Martelli, H. L. Influence of metal ions on pellet morphology and polygalacturonase synthesis by Aspergillus niger 3T5B8. Braz. J. Microbiol. 2003, 34, 16–21. [Google Scholar]
- 120. Shah, V. , Dobiášová, P , Baldrian, P. , Nerud, F. et al. Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus trametes versicolor. J. Hazard. Mater. 2010, 178, 1141–1145. [DOI] [PubMed] [Google Scholar]
- 121. Jernejc, K. , Legisa, M. The influence of metal ions on malic enzyme activity and lipid synthesis in Aspergillus niger. FEMS Microbiol. Lett. 2002, 217, 185–190. [DOI] [PubMed] [Google Scholar]
- 122. Reyes‐Caballero, H. , Campanello, G. C. , Giedroc, D. P. Metalloregulatory proteins: metal selectivity and allosteric switching. Biophys. Chem. 2011, 156, 103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ma, Z. , Jacobsen, F. E. , Giedroc, D. P. Coordination chemistry of bacterial metal transport and sensing. Chem. Rev. 2009, 109, 4644–4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Barona‐Gómez, F. , Lautru, S. , Francou, F. X. , Leblond, P. et al. Multiple biosynthetic and uptake systems mediate siderophore‐dependent iron acquisition in Streptomyces coelicolor A3(2) and streptomyces ambofaciens ATCC 23877. Microbiology 2006, 152, 3355–3366. [DOI] [PubMed] [Google Scholar]
- 125. Kim, H. J. Exploitation of reactive oxygen species by fungi: roles in host‐fungus interaction and fungal development. J. Microbiol. Biotechnol. 2014, 24, 1455–1463. [DOI] [PubMed] [Google Scholar]
- 126. Tripathy, B. C. , Oelmüller, R. Reactive oxygen species generation and signaling in plants. Plant. Signal. Behav. 2012, 7, 1621–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Nishida, M. , Sawa, T. , Kitajima, N. , Ono, K. et al. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat. Chem. Biol. 2012, 8, 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Scott, B. , Eaton, C. J. Role of reactive oxygen species in fungal cellular differentiations. Curr. Opin. Microbiol. 2008, 11, 488–493. [DOI] [PubMed] [Google Scholar]
- 129. Reverberi, M. Di Mario, F., Tomati, U. ‐Glucan synthase induction in mushrooms grown on olive mill wastewaters. Appl. Microbiol. Biotechnol. 2004, 66, 217–225. [DOI] [PubMed] [Google Scholar]
- 130. Aguirre, J. , Ríos‐Momberg, M. , Hewitt, D. , Hansberg, W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005, 13, 111–118. [DOI] [PubMed] [Google Scholar]
- 131. Harris, S. D. , Momany, M. Polarity in filamentous fungi: moving beyond the yeast paradigm. Fungal Genet. Biol. 2004, 41, 391–400. [DOI] [PubMed] [Google Scholar]
- 132. Tanaka, A. , Takemoto, D. , Hyon, G. S. , Park, P. et al. NoxA activation by the small GTPase RacA is required to maintain a mutualistic symbiotic association between Epichloë festucae and perennial ryegrass. Mol. Microbiol. 2008, 68, 1165–1178. [DOI] [PubMed] [Google Scholar]
- 133. Semighini, C. P. , Harris, S. D. Regulation of apical dominance in aspergillus nidulans hyphae by reactive oxygen species. Genetics 2008, 179, 1919–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Rodrigues, R. C. , Ortiz, C. , Berenguer‐Murcia, n. , Torres, R. et al. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [DOI] [PubMed] [Google Scholar]
- 135. Locasale, J. W. New concepts in feedback regulation of glucose metabolism. Curr. Opin. Syst. Biol. 2018, 8, 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Phillips, T. , Chase, M. , Wagner, S. , Renzi, C. et al. Use of in situ solid‐phase adsorption in microbial natural product fermentation development. J. Ind. Microbiol. Biotechnol. 2013, 40, 411–425. [DOI] [PubMed] [Google Scholar]
- 137. Karahalil, E. , Coban, H. B. , Turhan, I. A current approach to the control of filamentous fungal growth in media: microparticle enhanced cultivation technique. Crit. Rev. Biotechnol. 2018, 39, 192–201. [DOI] [PubMed] [Google Scholar]
- 138. Böl, M. , Schrinner, K. , Tesche, S. , Krull, R. Challenges of influencing cellular morphology by morphology engineering techniques and mechanical induced stress on filamentous pellet systems—A critical review. Eng. Life Sci. 2020, 21, 51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Navarro, E. , Baun, A. , Behra, R. , Hartmann, N. B. et al. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [DOI] [PubMed] [Google Scholar]
- 140. Limo, M. J. , Sola‐Rabada, A. , Boix, E. , Thota, V. et al. Interactions between metal oxides and biomolecules: from fundamental understanding to applications. Chem. Rev. 2018, 118, 11118–11193. [DOI] [PubMed] [Google Scholar]
- 141. Khan, I. , Saeed, K. , Khan, I. Nanoparticles: properties, applications and toxicities. Arabian J. Chem. 2019, 12, 908–931. [Google Scholar]
- 142. Kasper, C. , Bloh, J. Z. , Wagner, S. , Bahnemann, D. W. et al. Untersuchungen zur zytotoxizität von photokatalytisch aktiven titandioxid‐nanopartikeln. Chem. Ing. Tech. 2010, 82, 335–341. [Google Scholar]
- 143. Xiong, S. , Tang, Y. , Ng, H. S. , Zhao, X. et al. Specific surface area of titanium dioxide (TiO2) particles influences cyto‐ and photo‐toxicity. Toxicology 2013, 304, 132–140. [DOI] [PubMed] [Google Scholar]
- 144. Driouch, H. , Roth, A. , Dersch, P. , Wittmann, C. Filamentous fungi in good shape: microparticles for tailor‐made fungal morphology and enhanced enzyme production. Bioeng. Bugs 2011, 2, 100–104. [DOI] [PubMed] [Google Scholar]
- 145. Wucherpfennig, T. , Lakowitz, A. , Driouch, H. , Krull, R. et al. Customization of aspergillus niger morphology through addition of talc micro particles. J. Vis. Exp. 2012, 10.3791/4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Gonciarz, J. , Bizukojć, M. Adding talc microparticles toAspergillus terreus ATCC 20542 preculture decreases fungal pellet size and improves lovastatin production. Eng. Life Sci. 2013, 14, 190–200. [Google Scholar]
- 147. Gao, D. , Zeng, J. , Yu, X. , Dong, T. et al. Improved lipid accumulation by morphology engineering of oleaginous fungus Mortierella isabellina. Biotechnol. Bioeng. 2014, 111, 1758–1766. [DOI] [PubMed] [Google Scholar]
- 148. Coban, H. B. , Demirci, A. , Turhan, I. Microparticle‐enhanced aspergillus ficuum phytase production and evaluation of fungal morphology in submerged fermentation. Bioprocess Biosyst. Eng. 2015, 38, 1075–1080. [DOI] [PubMed] [Google Scholar]
- 149. Coban, H. B. , Demirci, A. Enhancement and modeling of microparticle‐added Rhizopus oryzae lactic acid production. Bioprocess Biosyst. Eng. 2015, 39, 323–330. [DOI] [PubMed] [Google Scholar]
- 150. Gonciarz, J. , Kowalska, A. , Bizukojć, M. Application of microparticle‐enhanced cultivation to increase the access of oxygen to Aspergillus terreus ATCC 20542 mycelium and intensify lovastatin biosynthesis in batch and continuous fed‐batch stirred tank bioreactors. Biochem. Eng. J. 2016, 109, 178–188. [Google Scholar]
- 151. Karahalil, E. , Demirel, F. , Evcan, E. , Germeç, M. et al. Microparticle‐enhanced polygalacturonase production by wild type aspergillus sojae. 3 Biotech 2017, 7, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Germec, M. , Yatmaz, E. , Karahalil, E. , Turhan, r. Effect of different fermentation strategies on ‐mannanase production in fed‐batch bioreactor system. 3 Biotech 2017, 7, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Dong, M. , Wang, S. , Xu, F. , Li, Q. et al. Addition of aluminum oxide microparticles to trichoderma viride my preculture enhances cellulase production and influences fungal morphology. Eng. Life Sci. 2018, 18, 353–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Boruta, T. , Bizukojć, M. Application of aluminum oxide nanoparticles in aspergillus terreus cultivations: evaluating the effects on lovastatin production and fungal morphology. Biomed. Res. Int. 2019, 2019, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Li, X. P. , Ma, Y. J. , Wang, J. W. Adding bamboo charcoal powder to Shiraia bambusicola preculture improves hypocrellin A production. Sustain. Chem. Pharm. 2019, 14, 100191. [Google Scholar]
- 156. Saberi, A. , Jalili, H. , Nikfarjam, A. , Koohsorkhi, J. et al. Monitoring of aspergillus terreus morphology for the lovastatin production in submerge culture by impedimetry. Biochem. Eng. J. 2020, 107615. [Google Scholar]
- 157. Gürler, H. N. , Erkan, S. B. , Ozcan, A. , Yılmazer, C. et al. Scale‐up processing with different microparticle agent for ‐mannanase production in a large‐scale stirred tank bioreactor. J. Food Process. Preserv. 2020, 10.1111/jfpp.14915. [DOI] [Google Scholar]
- 158. Niu, K. , Wu, X. P. , Hu, X. L. , Zou, S. P. et al. Effects of methyl oleate and microparticle‐enhanced cultivation on echinocandin B fermentation titer. Bioprocess Biosyst. Eng. 2020, 43, 2009–2015. [DOI] [PubMed] [Google Scholar]
- 159. Bush, J. , Long, B. , Catino, J. , Bradner, W. Production and biological activity of rebeccamycin, a novel antitumor agent. J. Antibiot. 1987, 40, 668–678. [DOI] [PubMed] [Google Scholar]
- 160. Long, B. H. , Rose, W. C. , Vyas, D. M. , Matson, J. A. et al. Discovery of antitumor indolocarbazoles: rebeccamycin, NSC 655649, and fluoroindolocarbazoles. Curr. Med. Chem. Anti‐Cancer Agents 2002, 2, 255–266. [DOI] [PubMed] [Google Scholar]
- 161. Nouioui, I. , Carro, L. , García‐López, M. , Meier‐Kolthoff, J. P. et al. Genome‐based taxonomic classification of the phylum actinobacteria. Front. Microbiol. 2018, 9, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


