Abstract
A high proportion of those with schizophrenia experience treatment non-response, placing them at higher risk for mortality and suicide attempts, compared to treatment responders. The clinical, social, and economic burden of treatment-resistant schizophrenia (TRS) are substantial. Previous genomic and epidemiological studies of TRS were often limited by sample size or lack of comprehensive genomic data. We aimed to systematically understand the clinical, demographic, and genomic correlates of TRS using epidemiological and genetic epidemiological modelling in a Swedish national population sample (n = 24,706) and then in a subgroup with common variant genetic risk scores, rare copy-number variant burden, and rare exonic burden (n = 4936). Population-based analyses identified increasing schizophrenia family history to be significantly associated with TRS (highest quartile of familial burden vs. lowest: adjusted odds ratio (aOR): 1.31, P = 4.8 × 10−8). In males, a decrease of premorbid IQ of one standard deviation was significantly associated with greater risk of TRS (minimal aOR: 0.94, P = 0.002). In a subset of cases with extensive genomic data, we found no significant association between the genetic risk scores of four psychiatric disorders and two cognitive traits with TRS (schizophrenia genetic risk score: aOR = 1.07, P = 0.067). The association between copy number variant and rare variant burden measures and TRS did not reach the pre-defined statistical significance threshold (all P ≥ 0.005). In conclusion, direct measures of genomic risk were not associated with TRS; however, premorbid IQ in males and schizophrenia family history were significantly correlated with TRS and points to new insights into the architecture of TRS.
Introduction
Antipsychotic medications are the mainstay of treatment for schizophrenia (SCZ). However, 30–60% experience treatment non-response [1] and are at high risk for a multitude of poor outcomes, including higher rates of mortality [2]. Suicide attempts are markedly higher in persons with treatment-resistant schizophrenia (TRS) compared to treatment-responsive SCZ (27% vs. 15%) [3]. The costs associated with TRS are 60x higher than the general population, and 10x higher than others with SCZ (mostly due to hospitalisations, 75 vs. 5.3 days/year) [4]. A Danish national study of 8044 individuals with SCZ (21% with TRS) highlighted younger age, living in a rural area, and previous suicide attempts as correlates of TRS [3]. Clinical cohort studies (all with fewer than 100 SCZ cases) have shown that those with TRS have more pronounced cognitive deficits than those who respond to antipsychotic therapy [5, 6]. The clinical, societal, and economic burdens of TRS are substantial.
TRS is defined as treatment failure despite appropriate dosing of two antipsychotic medications (from different chemical classes) for ≥ 4 or ≥ 6 weeks duration [7, 8]. Prescription of clozapine has also been used to identify TRS [2, 3] but, given that clozapine is underused in SCZ [9], this definition alone would not capture all individuals with TRS. Other investigators used “clozapine eligibility” (i.e., antipsychotic polypharmacy for ≥ 90 days [3]) as a marker for TRS.
A genomic basis for treatment response in SCZ was postulated following evidence that SCZ cases with a family history of psychosis were more likely to have poor response to antipsychotics [10, 11]. To add precision, a number of TRS studies evaluated the predictive capacity of SCZ genetic risk scores (GRS) [12–15]. GRS captures the overall, genome-wide, common variant liability to SCZ [16]. The results have been mixed. Two studies found similar mean SCZ GRS in SCZ cases with and without TRS (227 TRS cases vs. 385 non-TRS cases, P = 0.181 and 181 TRS cases vs. 681 non-TRS cases, adjusted hazard ratio = 1.13, 95% confidence interval = 0.95–1.35) [13, 14]. The two nominally positive studies used TRS surrogate measures: one study found higher mean SCZ GRS comparing those with (n = 434) and without (n = 370) a history of clozapine treatment (P = 0.02), and a study of first-episode psychosis found higher SCZ GRS in 179 treatment non-responders (n = 179) vs. 154 responders (OR = 1.91, P = 0.0034) [15]. A few studies have evaluated the impact of rare genetic variation on TRS. Increased numbers of rare copy-number variant (CNV) duplications have been associated with TRS (227 TRS cases vs. 385 non-TRS cases, P = 0.002) [13]. Rare, disruptive protein-coding variation has also been implicated in TRS: an excess of rare disruptive variants in antipsychotic gene targets was shown in 531 TRS cases (P = 0.0067) [17] and SCZ antipsychotic non-responders had an enrichment of rare, damaging variants in two synaptic gene sets (160 TRS cases vs. 156 SCZ antipsychotic responders, P = 0.001) [18].
While these genomic studies indicate a potential role for common and rare genetic variation in TRS, existing studies were all small ( < 600 TRS cases). A complete evaluation of the impact of genetic risk on TRS should evaluate common variant GRS for SCZ but also using burden measures for CNV and rare variation within the same cohort. Finally, to the best of our knowledge, whether genetic scores for other psychiatric disorders or complex traits play a role in TRS has not been investigated.
Given the clinical importance of TRS, we sought to comprehensively understand the predictors and correlates of TRS. We applied a novel design whereby we conducted comprehensive epidemiological and genetic epidemiological modelling of TRS in a Swedish national frame (n = 24,706 SCZ cases) and then explored TRS in a subgroup with comprehensive genotyping, including common variant genetic risk scores, rare CNV burden, and rare exonic burden (n = 4 936 SCZ cases). This approach combines the strength of comprehensive national registers with directly assessed genomic assays.
Methods
Study design and population
For the first step involving a population-based cohort, the source population was SCZ cases born in Sweden, and alive and aged 18–64 years on 1 July, 2005 [19], coinciding with the start of the Swedish National Prescribed Drug Register [20] (n = 24,706). Participants were followed from this baseline until whichever came first: emigration, death, or end of the register data period (31 December, 2013). Register data on the population-based Swedish SCZ cases was available up to 2013. The use of the population-based cohort was approved by the Regional Ethics Board of Stockholm. No informed consent is required for register-based studies using anonymized data.
For the second step, we used a sample of the Sweden Schizophrenia Study (S3) [21] of 4936 SCZ cases, born in Sweden, with genomic data. Ethical permission for S3 was obtained from the Karolinska Institutet Ethical Review Committee in Stockholm, Sweden and all subjects provided written informed consent.
Data sources
For both study cohorts, we linked multiple Swedish National Registers (Supplementary Fig. S1) using the Swedish personal identity number, which is assigned at birth. The Sweden National Patient Register [22] provided admission dates and diagnoses for inpatient and outpatient psychiatric specialist treatment. The Swedish National Prescribed Drug Register [20] provided all redeemed prescriptions in outpatient care. The Multi-Generation Register [23] contains information on first-degree relatives. Year 9 grades, defined as the average grade point earned by the individuals at the end of school year 9 (around age 16), were extracted from the National School Registry (since 1988). The Military Conscription Register provided premorbid intelligence quotient (IQ) scores for males at around age 18 years old between 1967 and 2010. The Cause of Death Register [24] provided date of death.
Definitions of TRS
We employed two definitions for TRS: Definition 1 was defined as any of those in the study who redeemed a clozapine prescription between 1 July, 2005–31 December, 2013 [3]. Those meeting Definition 1 were compared to those not meeting Definition 1 (i.e., SCZ who did not redeem clozapine). Definition 2 was defined as those meeting either Definition 1 or having received antipsychotic polypharmacy (i.e., ≥2 antipsychotics) simultaneously for ≥ 90 days between 1 July, 2005–31 December, 2013 [3]. Those meeting Definition 2 were compared to those not meeting Definition 2 (i.e., SCZ who did not redeem clozapine nor antipsychotic polypharmacy for ≥90 days). These definitions have been used extensively before [2, 3, 12–14]. A recent validation study reported using multiple TRS definitions was necessary to increase the probability of correctly classifying all true TRS cases [25].
Clinical and demographic factors associated with TRS
The following factors were investigated for an association with TRS: sex, age, number of psychiatric treatment contacts (inpatient or outpatient contacts from 1 July, 2005 to 31 December, 2013), comorbid psychiatric disorders (Supplementary Table S1 for International Classification of Diseases (ICD) codes), educational attainment (number of education years standardised to birth year and sex), year 9 grades (standardised to year of testing and sex), and premorbid IQ in males around age 18 years (standardised to testing year). The weeks of antipsychotic drug use (Supplementary Table S2 for drug names) was reported: adequate antipsychotic dose, clozapine use, and polypharmacy ( ≥2 concurrent antipsychotic prescriptions). Antipsychotic drug use was also reported by type of antipsychotic (oral and injectable).
We generated a quantitative family history score of SCZ, as previously described [26]. Briefly, we summarised the SCZ family history in first-degree relatives (parents, siblings, or children) for each index case accounting for family size and the age and sex distributions of the relatives of the index case. The SCZ family history score can be interpreted as the deviation from the expected family history in standard deviation units. Further details are provided in the Supplementary Information.
Genetic risk scores (GRS), common variant burden
We included SCZ GRS because those with a family history of psychosis were more likely to have poor response to antipsychotics [10, 11]. Additionally, we selected GRS of bipolar disorder, major depressive disorder, and autism spectrum disorder because of significant genetic correlations with SCZ [27–29]. Intelligence GRS was included because of its negative genetic correlation with SCZ [30] and IQ is an important SCZ clinical feature [31, 32]. Educational attainment has a mixed genetic correlation with SCZ [33, 34]. We obtained genome-wide association study results for SCZ [28], major depressive disorder [35], bipolar disorder [36], and autism spectrum disorder [37] from the Psychiatric Genomics Consortium (PGC) and elsewhere for educational attainment [38] and IQ [39] to create training sets. Swedish samples are part of many of these studies and we used a training set that excluded Swedish samples.
Rare copy-number variant burden
Full details of CNV processing are provided elsewhere [40], and summarised in the Supplementary Information. CNV burden is reported as the total burden and then separately for duplications and deletions for the following: (1) CNV size (in kilobases, kb), (2) total number, (3) number located in brain-expressed genes, and (4) number identified from a CLOZUK study [41], where the sample consisted of TRS cases recruited in the United Kingdom and Wellcome Trust Case Control Consortium 2 controls.
Rare exonic burden
Whole-exome sequencing details were reported previously [42, 43] and summarised in the Supplementary Information. We computed the number of ultra-rare damaging and disruptive variants (ddURVs) and were never observed in the Exome Aggregation Consortium [44]. A small number of outliers were removed [42], leaving 4 618 SCZ cases with whole-exome data.
Statistical analyses
For the national cohort, we generated three multivariable logistic regression models to test the association between candidate predictors and TRS. Model 1 included candidate predictors in association with TRS (n = 23,066 with full data). Model 2 (Year 9 grades; n = 3989) and model 3 (Premorbid IQ; n = 8238 males) were assessed separately due to less available data and were Z-score standardised to year of testing (both) and sex (year 9 grades only). We ran separate models for Models 2 and 3: minimally adjusted (including only the exposure) and a fully adjusted model (including all relevant predictors from Model 1: psychiatric treatment contacts, psychiatric comorbidities, antipsychotic usage, education years, and SCZ family history score).
For the genomic subset, we used multivariable logistic regression to test the association between genomic predictors and TRS, using either TRS definition. Separate models were evaluated and each included the first five-ancestry principal components: (1) common genetic burden scores included six GRS; (2) rare CNV burden: (i) all CNVs, (ii) duplications-only, or (iii) deletions-only; and (3) rare exome burden included the total count of ddURVs, count of highly conserved damaging or disruptive variants, and the count of synonymous variants (as a technical covariate).
All analyses were performed using R (version 3.4.1) and R-Studio (version 1.0.143). Odds ratios (OR) and 95% confidence intervals (95% CI) are reported. Following recent recommendations [45], and due to the number of tests performed, the significance level was P < 0.005. All statistical tests were two-sided. For further methods, see the Supplementary Information.
Results
For the population-based cohort study, 24,706 SCZ cases born in Sweden and 18–64 years of age at the start of the Drug Register were followed from study baseline (1 July, 2005) until emigration, death or end of follow-up. In the genomic subset, 4936 Swedish SCZ cases provided a sample and had register data up to 31 December, 2014.
Population-based analyses
Twenty-four thousand seven-hundred and six individuals fulfilled criteria for inclusion in the population-based cohort analyses, of whom 21,561 (87.2%) were followed to the end of the study (3029 [12.3%] cases died and 116 [0.5%] cases emigrated). Individuals were followed for a median of 8.5 years, with 4813 (19.5%) SCZ cases meeting TRS Definition 1 (clozapine treatment) and 13,779 (55.8%) SCZ cases meeting TRS Definition 2 (clozapine or polypharmacy, Table 1). Cases who met either TRS definition differed from those with SCZ not meeting the TRS definitions were proportionally more often male, younger, and had more treatment contacts and suicide attempts.
Table 1.
Register-based characteristics of individuals with treatment-resistant schizophrenia and schizophrenia without treatment-resistance in Sweden from the population-based analyses (n = 24,706) using two definitions of treatment-resistance
| Definition 1: clozapine-SCZ |
Definition 2: clozapine or polypharmacy-SCZ |
|||
|---|---|---|---|---|
| Variable | Clozapine | No clozapine | Clozapine or polypharmacy | Neither clozapine nor polypharmacy |
|
| ||||
| N | 4813 | 19,893 | 13,779 | 10,927 |
| Male sex | 2996 (62.2%) | 11,178 (56.2%) | 8000 (58.1%) | 6174 (56.5%) |
| Age at 1 July, 2005 | 42.8 years (34.2, 51.5) | 47.5 years (38.1, 55.6) | 44.7 years (35.6, 53.3) | 48.8 years (39.6, 56.5) |
| Psychiatric treatment contacts | ||||
| Inpatient | 1 (0, 5) | 1 (0, 3) | 2 (0, 5) | 0 (0, 2) |
| Outpatient | 10 (4, 21) | 9 (4, 18) | 12 (5, 23) | 7 (2, 13) |
| Comorbid psychiatric diagnoses | ||||
| Major depressive disorder | 844 (17.5%) | 4372 (21.9%) | 2976 (21.2%) | 2240 (20.4%) |
| Suicide attempt | 678 (14.1%) | 2329 (11.7%) | 2082 (15.1%) | 925 (8.5%) |
| Substance use disorder | 654 (13.6%) | 3396 (17.0%) | 2395 (17.4%) | 1655 (15.1%) |
| Antipsychotic usage (duration in weeks)a | ||||
| Adequate antipsychotic dose | 14.9 (52.8) | 20.5 (71.5) | 26.0 (76.2) | 11.2 (55.5) |
| Clozapine | 316.9 (175.4) | 0 | 110.8 (183.3) | 0 |
| Polypharmacy | 143.9 (158.5) | 70.9 (124.9) | 151.8 (150.9) | 1.28 (2.8)b |
| Antipsychotic usage (different medications) | ||||
| Oral antipsychotics | 2 (1, 4) | 2 (1, 3) | 3 (2, 4) | 1 (0, 2) |
| Injectable antipsychotics | 0 (0,1) | 0 (0,1) | 0 (0,1) | 0 (0,1) |
| Year 9 grade at age 16 years, Z-scorea | −0.45 (1.2) | −0.42 (1.2) | −0.46 (1.2) | −0.37 (1.2) |
| Premorbid IQ in males at age 18 years, Z-scorea | −0.58 (1.1) | −0.54 (1.0) | −0.58 (1.1) | −0.51 (1.1) |
| Educational attainment years, Z-scorea | −0.55 (0.8) | −0.38 (0.9) | −0.50 (0.8) | −0.31 (0.9) |
| Schizophrenia family history score, Z-scorea | 1.1 (3.3) | 0.81 (2.9) | 0.95 (3.2) | 0.74 (2.8) |
Data are n (%), median (interquartile range)
Mean (SD)
Individuals with polypharmacy 90 < days and no clozapine
Multivariable logistic regression analyses for the population cohort included 23,066 individuals (Fig. 1 and Supplementary Table S3, Model 1), with the following factors being statistically significantly associated with increasing risk of TRS, using either TRS definition: male sex, younger age, and increased number of inpatient contacts. Those defined by Definition 1 (clozapine) had significantly increased risk of TRS with an increasing number of oral antipsychotic products (OR: 1.29, 95% CI: 1.25–1.32, P < 2 × 10−16), and decreased risk with increasing number of injectable antipsychotics (OR: 0.77, 95% CI: 0.73–0.80, P < 2 × 10−16). There is the potential for reverse causality, given more oral and injectable antipsychotics are part of most clinical treatment regimens for TRS. Individuals meeting TRS Definition 2 (clozapine or polypharmacy) had significantly increased risk for suicide attempts (OR: 1.34, 95% CI: 1.20–1.47, P = 2.5 × 10−7). A one standard deviation decrease in educational attainment was significantly associated with increased risk of TRS by both definitions. Those with a higher SCZ family history score had significantly increased risk for TRS, by either TRS definition (TRS definition 2: highest quartile of SCZ familial burden vs. lowest: adjusted odds ratio: 1.31, 95% CI: 1.19–1.42, P = 4.8 × 10−8).
Fig. 1.
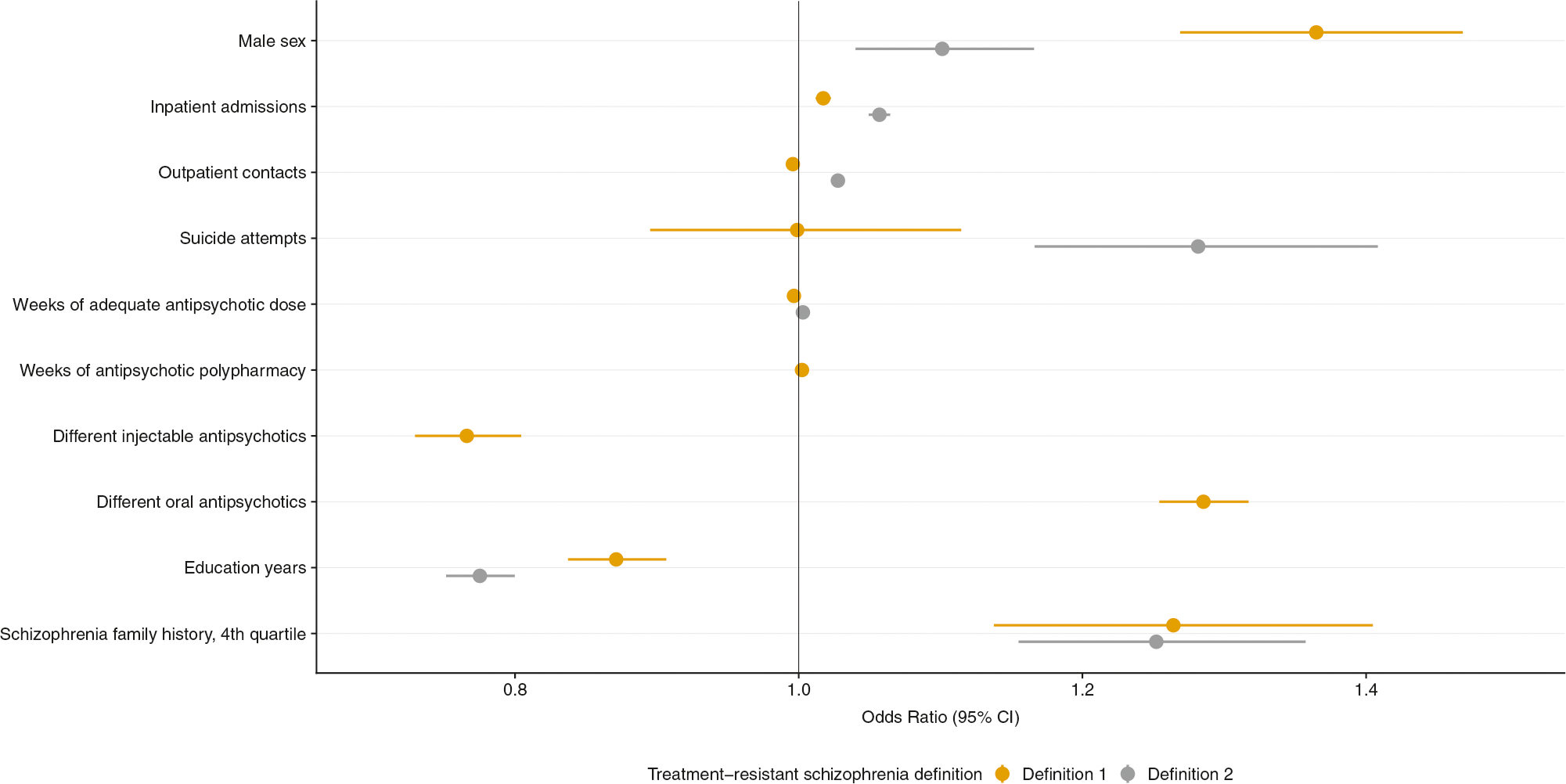
Multivariable logistic regression analyses of clinical and demographic factors associated with treatment-resistant schizophrenia within a Swedish National population-based sample (n = 23,066). The outcome is either treatment-resistant schizophrenia Definition 1 (clozapine) or Definition 2 (clozapine or polypharmacy). Results are presented as a single multivariable logistic regression model including all covariates listed and age, major depressive disorder, and substance use disorder. Odds ratios and 95% confidence intervals (CI) are reported. All factors are significantly associated with increased risk of treatment-resistance in schizophrenia (P ≤ 0.005), apart from suicide attempts with treatment-resistant schizophrenia Definition 1. This figure visualises the content of Supplementary Table S3 (Model 1)
In Model 2 for the population-based analyses, we assessed year 9 grades in 3989 SCZ cases (Fig. 2a and Supplementary Table S3) and found no significant association between year 9 grades and risk of TRS, either minimally or full adjusted and by either TRS definition. Finally, model 3 assessed 8238 males with available conscription data and found a one standard deviation decrease in premorbid IQ was significantly associated with TRS risk (TRS Definition 2: 0.94, 95% CI: 0.90–0.98, P = 0.002, Fig. 2b and Supplementary Table S3). However, there was less certainty regarding this effect, upon adjusting for confounders, which also reduced the number of individuals included in the model (n = 8217, OR: 0.95, 95% CI: 0.91–1.00, P = 0.050).
Fig. 2.
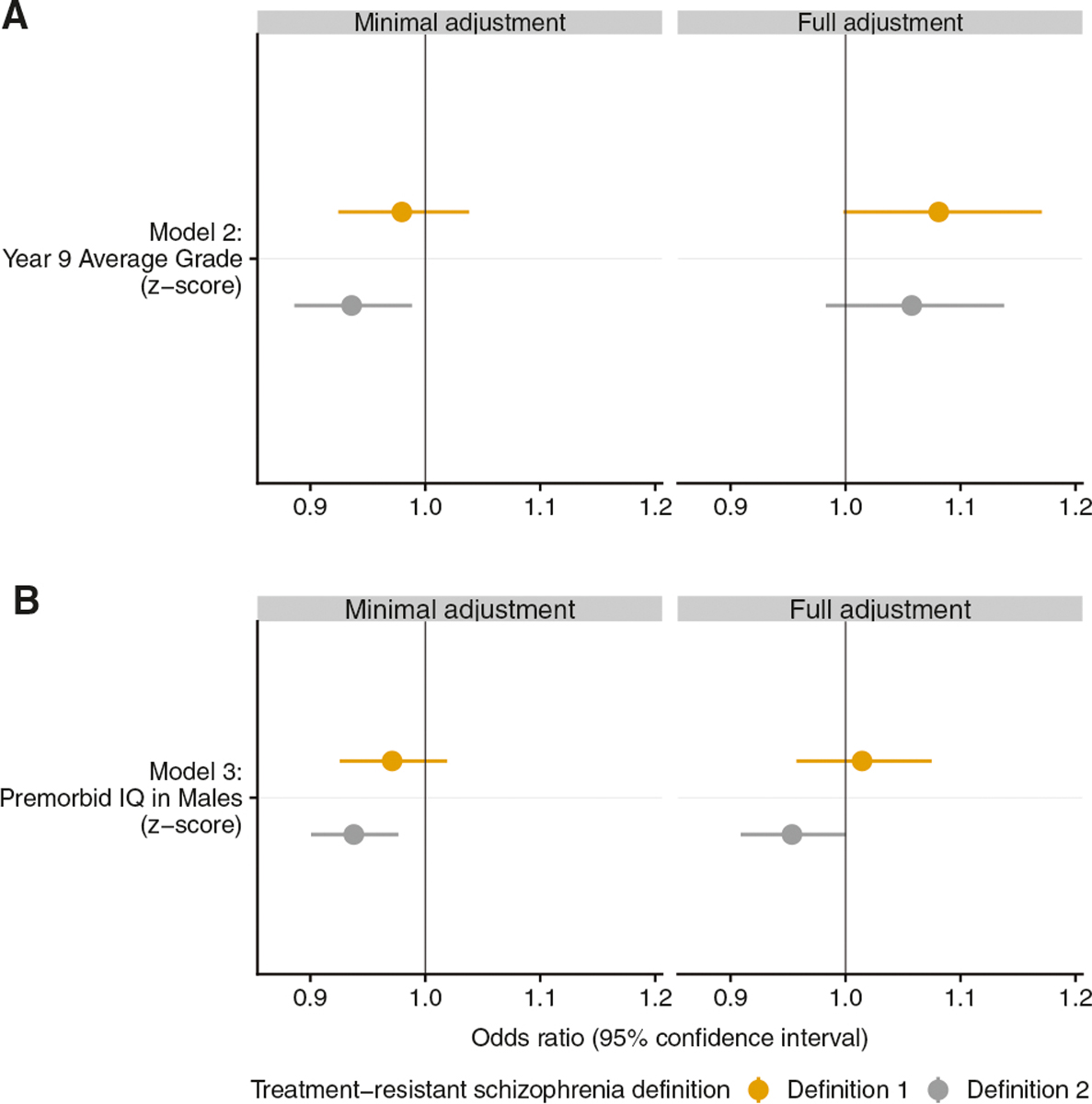
Multivariable logistic regression analyses of cognitive measures associated with treatment-resistant schizophrenia within a Swedish National population-based sample. Model 2 includes the exposure year 9 grades (minimal adjustment n = 3989, full adjustment n = 3960) and model 3 includes premorbid IQ in males (minimal adjustment n = 8238, full adjustment n = 8217). Minimal adjustment includes only the exposure of interest, whereas full adjustment includes all relevant clinical and demographic predictors (Psychiatric treatment contacts, psychiatric comorbidities, antipsychotic usage, education years, and SCZ family history score). The outcome is either treatment-resistant schizophrenia Definition 1 (clozapine) or Definition 2 (clozapine or polypharmacy). Odds ratios and 95% confidence intervals (CI) are reported. This figure visualises the content of Supplementary Table S3 (Models 2 and 3)
Genomic analyses
Four-thousand nine-hundred thirty-six individuals with SCZ were included in this study aim (Supplementary Table S4), and 4170 individuals (84.5%) were followed to the end of the study as 766 patients died (15.5%) during the follow-up period. One-thousand two-hundred sixty-one (25.5%) patients met TRS definition 1 (clozapine), and 2997 (60.7%) met definition 2 (clozapine or polypharmacy). Reassuringly, similar differences in the clinical and demographic characteristics of TRS cases with genomic data were observed compared to those from the national cohort.
We performed multivariable logistic regression analyses in the Swedish genomic subset to test the association between a comprehensive catalogue of genomic factors and TRS. We assessed GRS of four psychiatric disorders (SCZ, BIP, MDD, ASD) and two cognitive traits (IQ and educational attainment). However, no GRS reached our stringent significance threshold (P ≤ 0.005) as a correlate of TRS (Fig. 3a and Supplementary Table S5). We assessed the association between TRS and CNV burden measures, including a composite measure of all CNVs and stratified by duplications or deletions individually, however, none reached the statistical significance threshold (all P ≥ 0.1, Fig. 3b and Supplementary Table S5). When assessing the rare variant burden measures, those meeting the TRS 2 definition tended to have more highly conserved disruptive rare variants but this was not statistically significant (OR: 1.17, 95% CI: 1.04–1.32, P = 0.019, Fig. 3c and Supplementary Table S5). All other rare variant burden measures, including damaging or disruptive URVs, were not significantly associated with TRS. We performed a separate logistic regression model testing the association between sextiles of all six GRS and TRS, with adjustment for the first five principal components (Supplementary Fig. S2) but no sextile of GRS was significantly associated with TRS (all P ≥ 0.005).
Fig. 3.
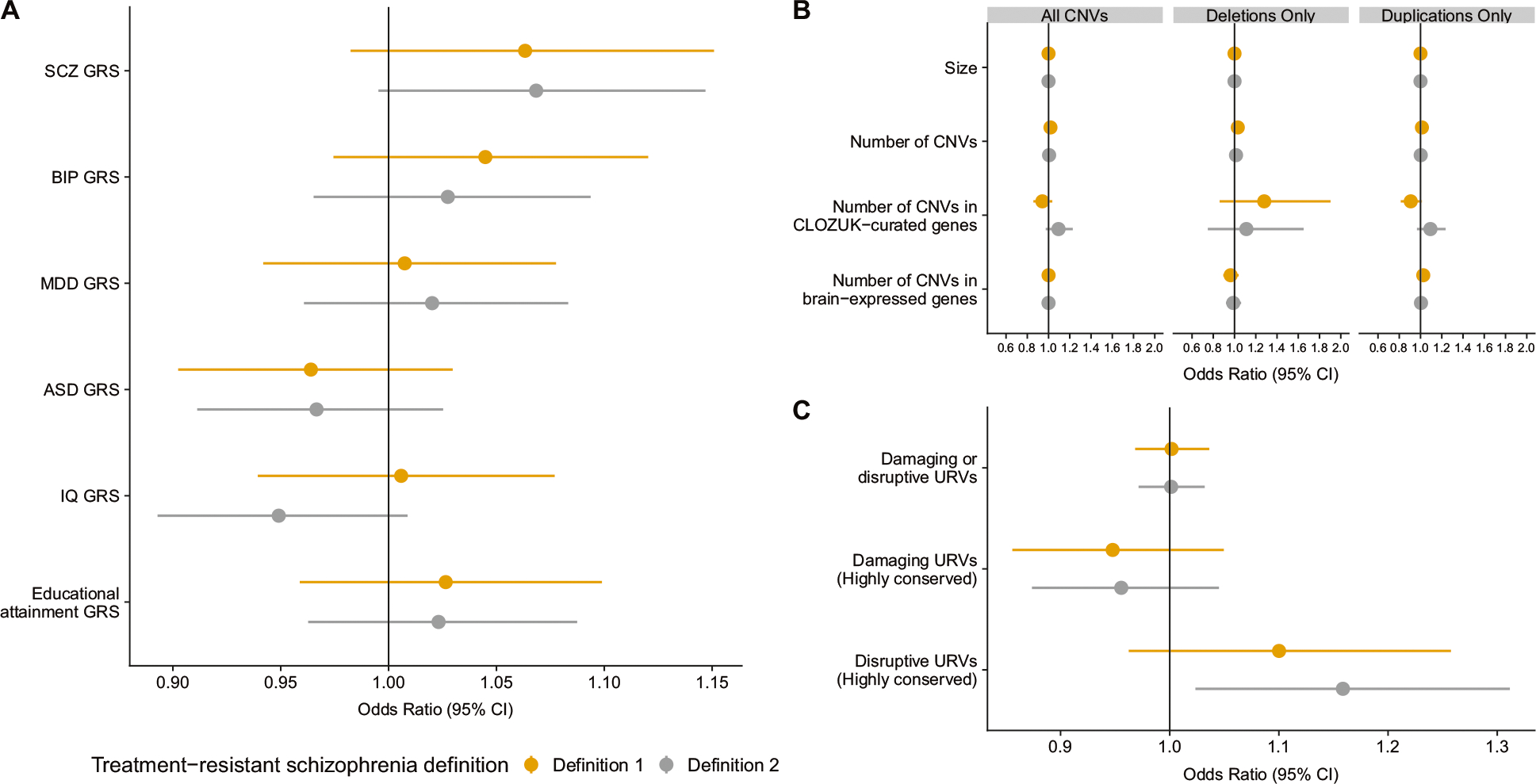
Multivariable logistic regression analyses using a genetic scores, b copy number variant measures (CNV), and c exome burden measures in association with treatment-resistant in schizophrenia (n = 4936). Panel a includes all six genetic risk scores (GRS): schizophrenia (SCZ), bipolar disorder (BIP), major depressive disorder (MDD), autism spectrum disorder (ASD), IQ, and educational attainment. Panel b includes three separate models of rare CNV measures: (1) by total number of CNVs, (2) total deletions, and (3) total duplications. Panel c includes three measures of ultra-rare variant (URVs) burden measures in one model. Each model is adjusted for the first five-ancestry principal components, with panel c additionally adjusted for the count of synonymous variants. Odds ratios and 95% confidence intervals (CI) are reported. No factors were significantly associated with treatment-resistance in schizophrenia (P > 0.005). This figure visualises the content of Supplementary Table S5
Discussion
We report the results of a combined population-based register study and genomic study examining the link between various clinical, demographic, and genomic correlates and TRS utilising two samples from Sweden: 24,706 Swedish SCZ cases with register data and 4936 Swedish SCZ cases with genomic data. Using population register data, we found that higher SCZ family history score was significantly associated with an increased risk of TRS. In the subset of TRS male cases with available military conscription data containing a direct measurement of premorbid intelligence, we identified lower premorbid IQ was significantly associated with increased risk of TRS compared to non-TRS. These findings have not been previously associated with TRS using a large, population-based cohort of this size. We additionally replicated known factors associated with TRS, including male sex [4], increased inpatient and outpatient specialist treatment contacts [4], increased suicide attempts [3], and decreased educational attainment [3].
Low IQ predicts poorer functional outcomes in SCZ [31, 32, 46], and may be related to the cognitive reserve hypothesis [47]. Individuals with SCZ and higher cognitive reserve (or intellectual functioning) might be better able to cope with psychotic symptoms because of either superior reasoning or by inhibiting abnormal neural processes associated with psychosis [47]. In a subset of 8238 males with SCZ, we found that those with TRS had significantly lower premorbid-IQ compared to non-TRS cases. Small studies of cognitive functioning in TRS have demonstrated that those with TRS perform significantly worse on cognitive tasks, compared to non-TRS cases [5, 6]. Our finding of lower premorbid IQ in TRS compared to non-TRS cases could be important in efforts to design novel drug treatments improving cognition.
A striking finding was that genomic measures that have been significantly associated with increased risk for SCZ were not associated with TRS. These included genomic measures of SCZ, bipolar disorder, autism spectrum disorder, major depressive disorder, IQ, and educational attainment, which have all been associated with SCZ [27–30, 33, 34]. This is notable given the large size of our sample and as previous genomic studies of TRS included < 600 TRS cases and evaluated a single-genomic burden measure (i.e., only common or only rare variation). However, even by significantly increasing the sample size and a full complement of common (GRS) and rare (CNV and whole-exome sequencing) genomic burden measures, we did not find any significant associations with TRS.
Intriguingly, although direct measures of genomic risk were not significant, SCZ family history was significantly associated with TRS. Family history captures the shared environmental and genetic risks and family history scores provide a more nuanced quantitative measure instead of the traditional presence/absence [26]. Family history is a well-established risk factor for SCZ [48], and has also been investigated in regards to TRS. Family history investigations of TRS have included < 200 TRS cases but have demonstrated that a SCZ family history predicted likelihood of non-recovery in 150 SCZ cases (OR = 2.2) [49]. A family history of psychosis also predicted unfavourable antipsychotic response in 165 SCZ non-responders compared to 188 responders (OR = 8.9, P = 0.003) [10], with attenuated findings following adjustment for covariates from a separate cohort of 186 SCZ cases (OR = 2.83, 95% CI = 1.36–5.91; P = 0.005) [11]. A Danish study of 8044 SCZ individuals (1703 TRS), which is the only other large, population-based assessment of TRS, showed a similar effect size to our study for a binary SCZ family history measure but was not significant (Hazard ratio = 1.03, 95% CI = 0.91–1.17) [3]. Instead of a dichotomised assessment of SCZ family history, we employed a continuous SCZ family history score in the population-based cohort study, which was able to consider age and sex, and allows for discrimination across the full spectrum of family histories in participants [50]. The implication of a significant association for SCZ family history score and TRS, but not SCZ GRS or other genomic measures, could imply that a shared environmental risk for TRS requires further exploration. The lack of a significant association in previous studies investigating genomic measures and TRS have often cited sample size as the culprit (N < 600 TRS cases). However, we increased the TRS case sample size two-to fivefold and did not find a significant association. The reasons for this are not entirely clear but the possibility to generate a TRS-specific GRS, using only SCZ and no healthy controls, may prove helpful.
A major strength of this study was the combination of a large population-based cohort and a subset with comprehensive genomic data, all from one country. We used multiple definitions of TRS to attempt to capture all TRS cases. Clozapine is under-prescribed or might not be a valid treatment option for many people; therefore, using only clozapine prescription would not capture all TRS cases. The employment of multiple definitions of TRS was recently reported to be necessary for correctly identifying TRS in register-based studies [25]. The lack of SCZ clinical scores [7], which should ideally be included in the identification of TRS, is a limitation of this study, as these scores were not available in the registers. Medication non-compliance can contribute to TRS [51]; however, compliance could not be assessed given that there is no objective means of assessing actual drug consumption (e.g., medication serum levels or pill counts) in the Swedish National Drug Register, and points to a future research objective. Drugs dispensed during hospital visits are not captured by the Swedish National Drug Register [20], and thus the number of antipsychotics may be underestimated, meaning a possibly biased TRS definition. However, these reasons provide additional rationale for using multiple TRS definitions to increase the confidence in adequately capturing TRS. The TRS definitions in our population-based cohort resulted in ~20–50% of our study meeting the TRS criteria, in line with past research [1, 3].
Conclusions
We sought to comprehensively understand the predictors and correlates of treatment-resistant schizophrenia within a Swedish national population sample and within a subgroup with comprehensive genotyping. Our study indicated that lower premorbid IQ in males and higher SCZ family history burden was associated with TRS, whereas no genomic factors were robustly associated with treatment resistance. Future studies incorporating a treatment-resistant SCZ-specific GRS may be important.
Code availability
Custom written R scripts used for statistical analyses can be provided upon request.
Supplementary Material
Acknowledgements
This work was supported by the Swedish Research Council (Vetenskapsrådet, award D0886501 to PFS). The Sweden Schizophrenia Study was supported by NIMH R01 MH077139. KK received funding from the European Union’s Horizon 2020 Research and Innovation programme under the Marie Skłodowska-Curie grant agreement (No. 793530) and from the Government of Canada Banting Postdoctoral Fellowship Programme.
Footnotes
Compliance with ethical standards
Conflict of interest PFS reports the following potential competing financial interests: Current: Lundbeck (advisory committee, grant recipient); Past 3 years: Pfizer (scientific advisory board), Element Genomics (consultation fee), and Roche (speaker reimbursement). HL has served as a speaker for Evolan Pharma and Shire, and has received research grants from Shire; all outside the submitted work. All other authors report no disclosures related to this work.
Supplementary information The online version of this article (https://doi.org/10.1038/s41380-019-0575-1) contains supplementary material, which is available to authorised users.
References
- 1.Barnes T Schizophrenia Consensus Group of British Association for Psychopharmacology. Evidence-based guidelines for the pharmacological treatment of schizophrenia: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2011;25:567–620. [DOI] [PubMed] [Google Scholar]
- 2.Wimberley T, MacCabe JH, Laursen TM, Sørensen HJ, Astrup A, Horsdal HT, et al. Mortality and self-harm in association with clozapine in treatment-resistant schizophrenia. Am J Psychiatry. 2017;174:990–8. [DOI] [PubMed] [Google Scholar]
- 3.Wimberley T, Stovring H, Sorensen HJ, Horsdal HT, MacCabe JH, Gasse C, et al. Predictors of treatment resistance in patients with schizophrenia: A population-based cohort study. Lancet Psychiatry. 2016;3:358–66. [DOI] [PubMed] [Google Scholar]
- 4.Kennedy JL, Altar CA, Taylor DL, Degtiar I, Hornberger JC. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29:63–76. [DOI] [PubMed] [Google Scholar]
- 5.de Bartolomeis A, Balletta R, Giordano S, Buonaguro EF, Latte G, Iasevoli F. Differential cognitive performances between schizophrenic responders and non-responders to antipsychotics: correlation with course of the illness, psychopathology, attitude to the treatment and antipsychotics doses. Psychiatry Res. 2013;210:387–95. [DOI] [PubMed] [Google Scholar]
- 6.Frydecka D, Beszłej JA, Gościmski P, Kiejna A, Misiak B. Profiling cognitive impairment in treatment-resistant schizophrenia patients. Psychiatry Res. 2016;235:133–8. [DOI] [PubMed] [Google Scholar]
- 7.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–96. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Remington G, Mulsant BH, Rajji TK, Uchida H, Graff-Guerrero A, et al. Treatment resistant schizophrenia and response to antipsychotics: A review. Schizophr Res. 2011;133:54–62. [DOI] [PubMed] [Google Scholar]
- 9.Bachmann CJ, Aagaard L, Bernardo M, Brandt L, Cartabia M, Clavenna A, et al. International trends in clozapine use: a study in 17 countries. Acta Psychiatr Scand. 2017;136:37–51. [DOI] [PubMed] [Google Scholar]
- 10.Crespo-Facorro B, de la Foz VOG, Ayesa-Arriola R, Pérez-Iglesias R, Mata I, Suarez-Pinilla P, et al. Prediction of acute clinical response following a first episode of non affective psychosis: Results of a cohort of 375 patients from the Spanish PAFIP study. Prog Neuro-Psychopharmacol Biol Psychiatry. 2013;44:162–7. [DOI] [PubMed] [Google Scholar]
- 11.Hassan AN, De Luca V. The effect of lifetime adversities on resistance to antipsychotic treatment in schizophrenia patients. Schizophr Res. 2015;161:496–500. [DOI] [PubMed] [Google Scholar]
- 12.Frank J, Lang M, Witt SH, Strohmaier J, Rujescu D, Cichon S, et al. Identification of increased genetic risk scores for schizophrenia in treatment-resistant patients. Mol Psychiatry. 2015;20:150–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin AK, Mowry B. Increased rare duplication burden genomewide in patients with treatment-resistant schizophrenia. Psychol Med. 2016;46:469–76. [DOI] [PubMed] [Google Scholar]
- 14.Horsdal HT, Meier SM, Wimberley T, Agerbo E, Gasse C, MacCabe JH. Polygenic risk score for schizophrenia and treatment-resistant schizophrenia. Schizophr Bull. 2017;43:1064–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JP, Robinson D, Yu J, Gallego J, Fleischhacker WW, Kahn RS, et al. Schizophrenia polygenic risk score as a predictor of antipsychotic efficacy in first-episode psychosis. Am J Psychiatry. 2019;176:21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, DeCandia TR, Ripke S, Yang J. Schizophrenia Psychiatric Genome-Wide Association Study Consortium (PGC-SCZ) PF, International Schizophrenia Consortium (ISC) ME, et al. Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44:247–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruderfer DM, Charney AW, Readhead B, Kidd BA, Kähler AK, Kenny PJ, et al. Polygenic overlap between schizophrenia risk and antipsychotic response: A genomic medicine approach. Lancet Psychiatry. 2016;3:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Man WuH, Yue W, Yan H, Zhang Y, Tan L, et al. Effect of damaging rare mutations in synapse-related gene sets on response to short-term antipsychotic medication in chinese patients with schizophrenia. JAMA Psychiatry. 2018;75:1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jedenius E, Tanskanen A, Leval A, Hoti F, Majak M, Taipale H, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wettermark B, Hammar N, Fored CM, Leimanis A, Olausson PO, Bergman U, et al. The new Swedish Prescribed Drug Register Opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16:726–35. [DOI] [PubMed] [Google Scholar]
- 21.Ripke S, O’Dushlaine C, Chambert K, Moran JL, Kahler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013;45:1150–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ekbom A The Swedish Multi-generation Register. Methods Mol Biol. 2010;675:215–20. [DOI] [PubMed] [Google Scholar]
- 24.Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ajnakina O, Horsdal HT, Lally J, MacCabe JH, Murray RM, Gasse C, et al. Validation of an algorithm-based definition of treatment resistance in patients with schizophrenia. Schizophr Res. 2018;197:294–7. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan PF, Wells JE, Joyce PR, Bushnell JA, Mulder RT, Oakley-Browne MA. Family history of depression in clinic and community samples. J Affect Disord. 1996;40:159–68. [DOI] [PubMed] [Google Scholar]
- 27.Purcell SM, Wray NR, Stone JL, Visscher PM, O’Donovan MC, Sullivan PF, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;10:8192–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ripke S, Neale BM, Corvin A, Walters JTR, Farh KH, Holmans PA, et al. Biological insights from 108 schizophrenia-associated genetic loci. Nature. 2014;511:421–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet (Lond, Engl). 2013;381:1371–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hubbard L, Tansey KE, Rai D, Jones P, Ripke S, Chambert KD, et al. Evidence of common genetic overlap between schizophrenia and cognition. Schizophr Bull. 2016;42:832–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leeson VC, Barnes TRE, Hutton SB, Ron MA, Joyce EM. IQ as a predictor of functional outcome in schizophrenia: a longitudinal, four-year study of first-episode psychosis. Schizophr Res. 2009;107:55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Winkel R, Myin-Germeys I, De Hert M, Delespaul P, Peuskens J, van Os J. The association between cognition and functional outcome in first-episode patients with schizophrenia: mystery resolved? Acta Psychiatr Scand. 2007;116:119–24. [DOI] [PubMed] [Google Scholar]
- 33.Escott-Price V, Bracher-Smith M, Menzies G, Walters J, Kirov G, Owen MJ, et al. Genetic liability to schizophrenia is negatively associated with educational attainment in UK Biobank. Mol Psychiatry. 2019; 10.1038/s41380-018-0328-6. [DOI] [PubMed] [Google Scholar]
- 34.Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie SJ, et al. Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112151) and 24 GWAS consortia. Mol Psychiatry. 2016;21:1624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wray NR, Ripke S, Mattheisen M, Trzaskowski M, Byrne EM, Abdellaoui A, et al. Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet. 2018;50:668–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Green EK, Hamshere M, Forty L, Gordon-Smith K, Fraser C, Russell E, et al. Replication of bipolar disorder susceptibility alleles and identification of two novel genome-wide significant associations in a new bipolar disorder case-control sample. Mol Psychiatry. 2013;18:1302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Autism Spectrum Disorders Working Group of The Psychiatric Genomics Consortium. Meta-analysis of GWAS of over 16,000 individuals with autism spectrum disorder highlights a novel locus at 10q24.32 and a significant overlap with schizophrenia. Mol Autism. 2017;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JJ, Wedow R, Okbay A, Kong E, Maghzian O, Zacher M, et al. Gene discovery and polygenic prediction from a genome-wide association study of educational attainment in 1.1 million individuals. Nat Genet. 2018;50:1112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savage JE, Jansen PR, Stringer S, Watanabe K, Bryois J, De Leeuw CA, et al. Genome-wide association meta-analysis in 269,867 individuals identifies new genetic and functional links to intelligence. Nat Genet. 2018;50:912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szatkiewicz JP, O’Dushlaine C, Chen G, Chambert K, Moran JL, Neale BM, et al. Copy number variation in schizophrenia in Sweden. Mol Psychiatry. 2014;19:762–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tansey KE, Rees E, Linden DE, Ripke S, Chambert KD, Moran JL, et al. Common alleles contribute to schizophrenia in CNV carriers. Mol Psychiatry. 2016;21:1085–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Genovese G, Fromer M, Stahl EA, Ruderfer DM, Chambert K, Landen M, et al. Increased burden of ultra-rare protein-altering variants among 4,877 individuals with schizophrenia. Nat Neurosci. 2016;19:1433–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature. 2014;506:185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536:285–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ioannidis JPA. The proposal to lower P value thresholds to .005. JAMA. 2018;319:1429–30. [DOI] [PubMed] [Google Scholar]
- 46.Sumiyoshi C, Fujino H, Yamamori H, Kudo N, Azechi H, Fujimoto M, et al. Predicting work outcome in patients with schizophrenia: Influence of IQ decline. Schizophr Res. 2018;201:172–9. [DOI] [PubMed] [Google Scholar]
- 47.Barnett JH, Salmond CH, Jones PB, Sahakian BJ. Cognitive reserve in neuropsychiatry. Psychol Med. 2006;36:1053–64. [DOI] [PubMed] [Google Scholar]
- 48.Mortensen PB, Pedersen CB, Westergaard T, Wohlfahrt J, Ewald H, Mors O, et al. Effects of family history and place and season of birth on the risk of schizophrenia. N Engl J Med. 1999;340:603–8. [DOI] [PubMed] [Google Scholar]
- 49.Murray RM, Van OsJ. Predictors of outcome in schizophrenia. J Clin Psychopharmacol. 1998;18:2S–4S. [DOI] [PubMed] [Google Scholar]
- 50.Hunt SC, Williams RR, Barlow GK. A comparison of positive family history definitions for defining risk of future disease. J Chronic Dis. 1986;39:809–21. [DOI] [PubMed] [Google Scholar]
- 51.McCutcheon R, Beck K, D’Ambrosio E, Donocik J, Gobjila C, Jauhar S, et al. Antipsychotic plasma levels in the assessment of poor treatment response in schizophrenia. Acta Psychiatr Scand. 2018;137:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


