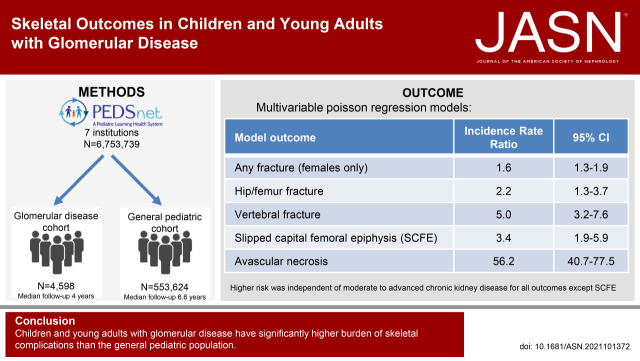
Significance Statement
Children and young adults with glomerular disease have unique risk factors for compromised bone health, but data on skeletal complications are lacking. We leveraged the PEDSnet pediatric health system population of more than 6.5 million children to compare incidence rates of adverse skeletal outcomes in patients with glomerular disease to a general pediatric reference cohort. Children and young adults with glomerular disease had greater risk of vertebral and hip/femur fractures, avascular necrosis/osteonecrosis, and slipped capital femoral epiphysis than those in the reference cohort. For fractures at any body site, girls with glomerular disease were at increased risk compared with peers in the general pediatric population, an effect that CKD does not appear to drive entirely.
Keywords: glomerular disease, fracture, avascular necrosis, osteonecrosis, slipped capital femoral epiphysis, PEDSnet, nephrotic syndrome, young adult, child, musculoskeletal system
Visual Abstract
Abstract
Background
Children with glomerular disease have unique risk factors for compromised bone health. Studies addressing skeletal complications in this population are lacking.
Methods
This retrospective cohort study utilized data from PEDSnet, a national network of pediatric health systems with standardized electronic health record data for more than 6.5 million patients from 2009 to 2021. Incidence rates (per 10,000 person-years) of fracture, slipped capital femoral epiphysis (SCFE), and avascular necrosis/osteonecrosis (AVN) in 4598 children and young adults with glomerular disease were compared with those among 553,624 general pediatric patients using Poisson regression analysis. The glomerular disease cohort was identified using a published computable phenotype. Inclusion criteria for the general pediatric cohort were two or more primary care visits 1 year or more apart between 1 and 21 years of age, one visit or more every 18 months if followed >3 years, and no chronic progressive conditions defined by the Pediatric Medical Complexity Algorithm. Fracture, SCFE, and AVN were identified using SNOMED-CT diagnosis codes; fracture required an associated x-ray or splinting/casting procedure within 48 hours.
Results
We found a higher risk of fracture for the glomerular disease cohort compared with the general pediatric cohort in girls only (incidence rate ratio [IRR], 1.6; 95% CI, 1.3 to 1.9). Hip/femur and vertebral fracture risk were increased in the glomerular disease cohort: adjusted IRR was 2.2 (95% CI, 1.3 to 3.7) and 5 (95% CI, 3.2 to 7.6), respectively. For SCFE, the adjusted IRR was 3.4 (95% CI, 1.9 to 5.9). For AVN, the adjusted IRR was 56.2 (95% CI, 40.7 to 77.5).
Conclusions
Children and young adults with glomerular disease have significantly higher burden of skeletal complications than the general pediatric population.
Numerous studies have demonstrated increased fracture rates and associated morbidity and mortality in adults on dialysis1–6 and adults with moderate to advanced (stage 3–5) CKD.7–12 Pediatric studies have also found a high burden of fracture in children with CKD.13,14 None of these studies evaluated the effect of underlying glomerular disease. With 90% of peak bone mass established by 18 years of age, threats to healthy bone development in childhood have a critical effect on lifelong skeletal health. Glomerular disorders are frequent causes of CKD in children and young adults, although the majority of those with idiopathic nephrotic syndrome have early-stage CKD and normal kidney function. Even in the setting of normal kidney function, however, individuals with glomerular disease have unique and potentially modifiable risk factors for skeletal morbidity, such as disordered vitamin D homeostasis, exposure to medications with adverse skeletal effects such as corticosteroids, and chronic systemic inflammation. Although prior studies have demonstrated reduced bone mineral density in children with nephrotic syndrome15–25 and in young adults with a history of nephrotic syndrome in childhood,26,27 studies addressing fractures and other skeletal complications among these populations are lacking.
Glomerular diseases are rare, and studies of clinical outcomes in children with glomerular disease have been largely limited to case series, with a paucity of population-based data.18 This study was conducted within PEDSnet, a multi-institutional national clinical research network, and leveraged a computable phenotype algorithm that was demonstrated to identify children with glomerular disease reliably,28 allowing for both complete capture of this population and comparison to a reference cohort of general pediatric patients within the network.
The main objective of this retrospective cohort study was to examine the incidence rates of skeletal outcomes, including fracture, avascular necrosis/osteonecrosis (AVN), and slipped capital femoral epiphysis (SCFE), in children and young adults with glomerular disease compared with those among general pediatric patients. In secondary analyses, we examined rates of skeletal outcomes in the subcohort of children with nephrotic conditions, and how moderate to advanced CKD affected rates of skeletal outcomes in glomerular disease. Finally, we investigated the effect of corticosteroid exposure on the risk of skeletal outcomes within the cohort of children with glomerular disease.
Methods
Study Setting
PEDSnet (pedsnet.org) is a multi-institutional clinical research network that aggregates electronic health record (EHR) data from several of the nation’s large children’s health care organizations (Institutional Review Board [IRB] protocol #14–011242). Seven institutions participated in this study: Children’s Hospital of Philadelphia (CHOP), Cincinnati Children’s Hospital Medical Center (CCHMC), Children’s Hospital Colorado, Nationwide Children’s Hospital, Nemours Children’s Health System (a Delaware and Florida health system), Seattle Children’s Hospital, and St. Louis Children’s Hospital/Washington University.29 PEDSnet standardizes EHR data across institutions to the PEDSnet common data model, which is based on the Observational Medical Outcomes Partnership common data model version 5.30 PEDSnet has accrued data for nearly seven million children seen since 2009 from inpatient and outpatient clinical settings. The PEDSnet database includes data from patients who reside in all 50 states; the states contributing the largest volume of data are Colorado, Delaware, Florida, Illinois, Indiana, Kentucky, Missouri, New Jersey, Ohio, Pennsylvania, and Washington. This analysis was reviewed by the IRB at CHOP and CCHMC because, at the time of analysis, CCHMC housed its PEDSnet data separately, whereas data from the other centers were centrally stored at CHOP. This study was determined to meet criteria for exemption by the CCHMC IRB and was determined not to meet the criteria for human subjects research by the CHOP IRB, which was accepted by all other PEDSnet sites.
Cohort Definitions
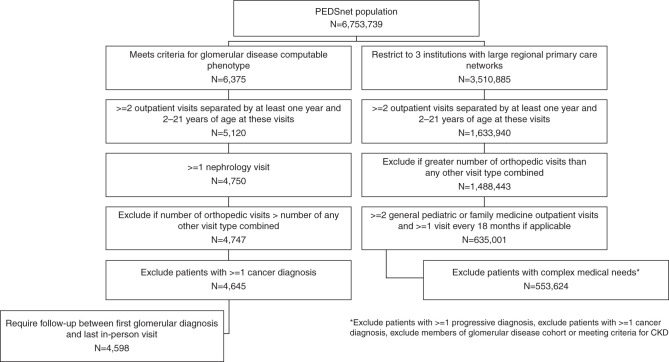
Figure 1 outlines the criteria for derivation of the two central cohorts under study: children with glomerular disease and general pediatric patients receiving primary care. Among the glomerular disease cohort, a subcohort of children with nephrotic conditions was also evaluated.
Figure 1.
Derivation of study cohorts: children with glomerular disease and general pediatric patients receiving primary care.
The cohort of 4598 children with glomerular disease was defined using a computable phenotype algorithm, which was previously developed and evaluated in PEDSnet.28 This algorithm involves two or more glomerular disease diagnosis codes on different dates or one or more glomerular disease diagnosis code(s) and a native kidney biopsy procedure. An associated computational phenotype was used to identify the subcohort of 2238 children and young adults with nephrotic conditions, including idiopathic nephrotic syndrome (without a biopsy), minimal change nephropathy, focal segmental glomerulosclerosis, and membranous nephropathy. Patients were required to have at least one encounter with a nephrology provider or at a nephrology clinic. Follow-up time for this cohort began at the first encounter with a glomerular disease diagnosis and ended at the most recent “in-person” clinical encounter (inpatient, outpatient, or emergency department visit).
To develop a cohort of children and young adults representative of the general pediatric population, we selected patients receiving primary care, excluding those for whom there was evidence in the EHR of severe chronic disease. The general pediatric cohort was defined as follows. First, we restricted the cohort to the three health systems within PEDSnet with large regional primary care networks (CHOP, Nemours, and Nationwide). Second, we required evidence of ongoing primary care, defined as at least two outpatient general pediatric or family medicine encounters and at least one such encounter every 18 months if followed >3 years (approximating recommendations for frequency of well-child visits). Third, we applied the taxonomy from the Pediatric Medical Complexity Algorithm (PMCA)31 to identify and exclude patients with a diagnosis associated with deteriorating health or reduced life expectancy, labeled as “progressive” in the PMCA. In addition, patients meeting the criteria for CKD (defined below) were excluded. Follow-up time for this cohort began at the first outpatient general pediatric encounter and ended at the last outpatient general pediatric encounter.
We applied a set of three additional requirements to both the glomerular disease and general pediatric cohorts. First, we required that all patients have at least 1 year of follow-up, defined as two outpatient visits separated by at least 12 months. The motivation for this criterion was to avoid inclusion of patients who interact with the network for episodic care as the amount of person-time contributed per outcome event would be small and inflate incidence rates. Second, we excluded patients who had more orthopedic-related visits than all other visit types combined. The motivation for this exclusion was to remove patients from the general pediatric cohort with recurrent orthopedic issues because they are not representative of the general population; further, for both cohorts, having more orthopedic than all other visit types could indicate that nonorthopedic care (for kidney disease or primary care) was being received largely outside the network. Third, we implemented a cancer diagnosis exclusion because of established skeletal morbidity in survivors of childhood cancer.32
Assessment of Skeletal Outcomes
We developed diagnosis code sets using the SNOMED terminology for bone fracture, AVN, and SCFE. All code sets used are available at https://github.com/PEDSnet/skeletal_outcomes. For bone fracture, given a concern that some diagnoses in the EHR would represent prevalent rather than incident fractures, we required evidence of an accompanying radiologic, splinting, or casting procedure within 48 hours of the fracture diagnosis. Due to the challenge of distinguishing recurrent skeletal outcome events from a single skeletal outcome event receiving ongoing treatment within a period of several months within the EHR, we took an annualized approach to fracture within each patient year of age in order to avoid inflation of incidence rates due to capturing encounters for follow-up care of the skeletal outcome. For each patient, if their follow-up period included any time within a given year of age, they contributed person-time and a determination of whether they experienced at least one fracture within that year of age. When follow-up began or ended within a year of age, patients contributed the number of days of follow-up within that year of age. We excluded person-time below the age of 2 years for several reasons. Fractures occurring before 18 months, the usual age of walking, have different risk factors.33,34 Measures such as body mass index and eGFR, which we included in our analyses, are not appropriately applied before 2 years of age. Finally, as glomerular disease diagnoses are rare before 2 years of age, sample sizes were small for the first and second year of life.
Primary Analyses
Standard descriptive statistics were used to summarize cohort characteristics, utilizing medians and interquartile ranges for continuous variables and counts and frequencies for categorical variables. Unadjusted incidence was calculated as the number of events per 10,000 person-years. Overall incidence (incidence across the age span of 2–21 years) was calculated as the total number of annualized events divided by the total follow-up for the cohort across the age span. For incidence within a given year of age, the number of annualized events within the year of age was divided by total person-time within that same year of age.
We applied Poisson regression to model the aggregated number of skeletal outcome events within each year of age group, stratified by covariates, using total person-time within each year of age as the offset. It is well established that rates of skeletal outcomes vary across age and sex.35 We therefore adjusted for year of age, sex, and the interaction between age and sex across the analyses, and also performed sex-stratified analyses. To capture any potential nonlinear relationship between the skeletal outcome events and age, we included polynomial terms of age (i.e., age, age squared, and age cubed) in the regression models. We also adjusted for race and obesity because obesity is a known risk factor for adverse skeletal outcomes,36–38 racial differences in bone outcomes have been reported in healthy children and children with CKD,39 and the distribution of race and obesity differed across cohorts. Obesity was operationalized as a non-time-updated binary variable corresponding to whether a patient had three or more body mass index measures at or above the 95th percentile, separated by at least 6 months, between 2 years of age and the end of their follow-up. Race (from the EHR) was categorized as: Asian American, Black or African American, other (American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Multiple, other), unknown, or White. Interactions were retained in the model only when they were significant by the likelihood ratio test. We evaluated certain site-specific fractures (vertebra and hip/femur), for which we did not perform sex-stratified analyses due to the lower incidence of these outcomes. Incidence rate ratios (IRR) with 95% confidence intervals (CI) were reported between cohorts.
Secondary Analyses
CKD was evaluated as a time-updated exposure in the glomerular cohort where person-time before meeting the CKD criteria was considered unexposed and person-time thereafter exposed, if applicable. A patient was defined as meeting the criteria for CKD when there was evidence in the EHR of one or more of the following: a dialysis procedure, a kidney transplant, or two eGFR measures of <60 ml/min per 1.73 m2 separated by 90 days (without a subsequent eGFR>90 ml/min per 1.73 m2, unless this eGFR followed a kidney transplant). The “bedside” estimating equation developed by the Chronic Kidney Disease in Children study was used to calculate eGFR40 on the basis of serum creatinine and the closest associated height measurement within 60 days.
Cox proportional hazard models were applied to investigate whether corticosteroid exposure, operationalized as a fixed covariate of two corticosteroid prescriptions separated by at least 90 days, affected time to first fracture and time to AVN within the glomerular disease cohort, adjusting for sex, age, race, and obesity. In these analyses, patients were right censored at end of follow-up or first date at which CKD criteria were met. The proportional hazard assumption was examined using the Pearson product-moment correlation between the scaled Schoenfeld residuals and log-transformed time for each covariate. Supplemental Table 1 provides a summary of all analyses performed.
Results
Descriptive Characteristics
Descriptive characteristics of each cohort are consistent with clinical expectations (Table 1). For proteinuria, in the glomerular disease cohort and nephrotic subcohort, >80% of patients had at least one positive urine protein measurement compared with 6.9% in the general pediatric cohort. About half of the glomerular cohort had a hypertension diagnosis, and 42.5% had a kidney biopsy. Systemic corticosteroid exposure was seen in 62.7%, 77.4%, and 23.5% of the glomerular, nephrotic, and general pediatric cohorts, respectively. For the nonsteroid immunosuppressants included in Table 1, rates were consistently close to zero in the general pediatric cohort. More than 50% of the glomerular disease cohort and nephrotic subcohort had at least one hospitalization compared with 7.1% of the general pediatric cohort. Obesity was identified in 24.8% of the glomerular disease cohort and 27.2% of the nephrotic subcohort compared with 14.7% in the general pediatric cohort.
Table 1.
Population characteristics
| Cohort | |||
|---|---|---|---|
| Glomerular Disease | Nephrotic Subcohort | General Pediatric | |
| N | 4598 | 2238 | 553,624 |
| Age at index in years | 9.83 (5.78, 14.7) | 7.59 (4.2, 13.4) | 2.9 (2.05, 7.24) |
| Sex | |||
| Boys | 2514 (54.7) | 1233 (55.1) | 279,821 (50.5) |
| Girls | 2084 (45.3) | 1005 (44.9) | 273,803 (49.5) |
| Race | |||
| Black or African American | 860 (18.7) | 507 (22.7) | 175,608 (31.7) |
| Other | 698 (15.2) | 359 (16) | 33,341 (6.02) |
| Unknown | 264 (5.74) | 149 (6.66) | 57,045 (10.3) |
| White | 2582 (56.2) | 1112 (49.7) | 265,874 (48) |
| Asian American | 194 (4.22) | 111 (4.96) | 21,756 (3.93) |
| Ethnicity | |||
| Hispanic or Latinx | 718 (15.6) | 346 (15.5) | 52,900 (9.56) |
| Other | 36 (0.783) | 16 (0.715) | 1866 (0.337) |
| Not Hispanic or Latinx | 3673 (79.9) | 1790 (80) | 453,926 (82) |
| Unknown | 171 (3.72) | 86 (3.84) | 44,932 (8.12) |
| Years of follow-up duration | 4.05 (2.04, 7.17) | 4.47 (2.32, 7.87) | 6.55 (3.29, 10.6) |
| ≥1 nephrology visit | 4438 (96.5) | 2177 (97.3) | 6157 (1.11) |
| Nephrology visits per person-year | 2.71 (1.09, 5.3) | 3.01 (1.43, 5.74) | 0 (0, 0) |
| ≥1 general pediatric visit | 1630 (35.5) | 809 (36.1) | 553,624 (100) |
| General pediatric visits per person-year | 0 (0, 0.715) | 0 (0, 0.755) | 2.65 (1.77, 3.84) |
| ≥1 25-hydroxyvitamin D measurement | 618 (13.4) | 266 (11.9) | 4104 (0.741) |
| Height measured | 4564 (99.3) | 2218 (99.1) | 551,550 (99.6) |
| Z-scored height at index | −0.079 (–0.883, 0.663) | −0.117 (–0.913, 0.575) | 0.262 (–0.767, 0.934) |
| ≥3 BMI >95th percentile | 1139 (24.8) | 609 (27.2) | 81,370 (14.7) |
| BMI measured | 4566 (99.3) | 2218 (99.1) | 551,263 (99.6) |
| Z-scored BMI at index | 0.862 (–0.00043, 1.67) | 1.11 (0.252, 1.86) | 0.232 (–0.522, 0.982) |
| ≥1 serum creatinine measurement | 4380 (95.3) | 2117 (94.6) | 104,448 (18.9) |
| Serum creatinine measurements per person-year | 3.35 (1.09, 9.71) | 2.71 (0.912, 8.04) | 0 (0, 0) |
| Minimum eGFR (ml/min per 1.73 m2) across follow-up, right censored at kidney transplant or dialysis | |||
| <15 | 609 (13.2) | 247 (11) | 222 (0.0401) |
| 15–<30 | 294 (6.39) | 124 (5.54) | 45 (0.00813) |
| 30–<60 | 579 (12.6) | 289 (12.9) | 626 (0.113) |
| 60–<90 | 1095 (23.8) | 492 (22) | 19,889 (3.59) |
| ≥90 | 1422 (30.9) | 802 (35.8) | 59,515 (10.8) |
| Unavailable | 599 (13) | 284 (12.7) | 473,327 (85.5) |
| ≥1 urine protein measurement | 3896 (94.4) | 1904 (94.4) | 125,131 (22.6) |
| Urine protein measurements per person-year | 2.99 (1.11, 6.63) | 3.1 (1.14, 6.78) | 0 (0, 0) |
| ≥1 positive urine protein measurement | 3467 (84) | 1743 (86.4) | 38,291 (6.92) |
| ≥1 hypertension diagnosis | 2323 (50.5) | 1029 (46) | 7536 (1.36) |
| ≥1 hospitalization | 2524 (54.9) | 1327 (59.3) | 39,176 (7.08) |
| Hospitalizations per person-year | 0.146 (0, 0.685) | 0.193 (0, 0.722) | 0 (0, 0) |
| ≥1 kidney biopsy procedure | 1953 (42.5) | 902 (40.3) | 11 (0.00199) |
| ACE inhibitors | 1902 (41.4) | 866 (38.7) | 659 (0.119) |
| Angiotensin II receptor blockers | 616 (13.4) | 316 (14.1) | 140 (0.0253) |
| Aldosterone receptor blockers | 114 (2.48) | 66 (2.95) | 999 (0.18) |
| Systemic corticosteroids | 2883 (62.7) | 1732 (77.4) | 129,886 (23.5) |
| Mycophenolate | 1182 (25.7) | 573 (25.6) | 21 (0.00379) |
| Calcineurin inhibitors | 974 (21.2) | 729 (32.6) | 16 (0.00289) |
| Cyclophosphamide | 324 (7.05) | 150 (6.7) | <11 (<0.00199) |
| Azathioprine | 263 (5.72) | 54 (2.41) | 91 (0.0164) |
| Rituximab | 524 (11.4) | 325 (14.5) | <11 (<0.00199) |
| Eculizumab | 24 (0.522) | <11 (<0.492) | 0 (0) |
| Corticotropin | <11 (<0.239) | <11(<0.492) | 0 (0) |
| Abatacept | 15 (0.326) | <11 (<0.492) | 11 (0.00199) |
All continuous data as median (25th percentile, 75th percentile); all categorical data as n (%). Urine protein measurements exclude one site, for which these measurements are unavailable. All medication and biopsy data are pre kidney transplant, if applicable. BMI, body mass index.
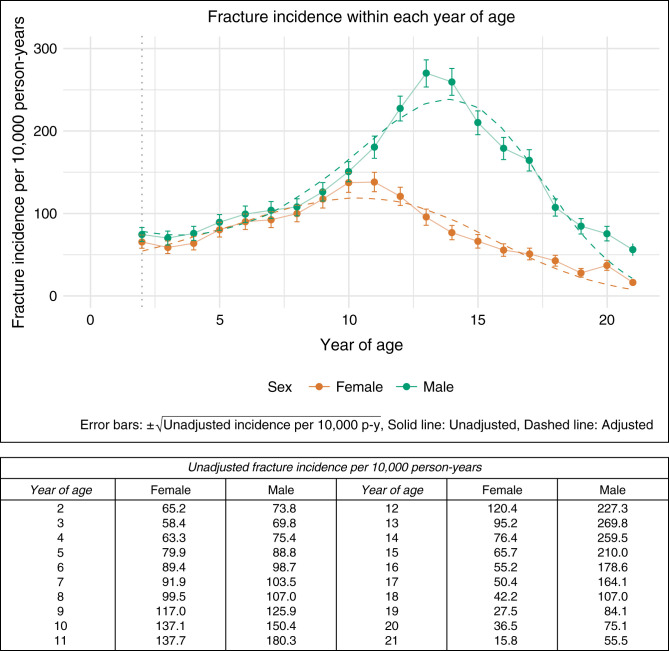
Incidence of Fracture in the General Pediatric Cohort
In the general pediatric cohort, the peak fracture incidence rate for boys was 270 per 10,000 person-years at age 13, whereas for girls, it was 138 per 10,000 person-years at age 11 (Figure 2). In the model for the general pediatric cohort (Supplemental Table 2), the linear, quadratic, and cubic components of age were significant predictors of fracture incidence, as expected given the curvilinear relationship between age and fracture risk, whereby risk is heightened around the time of peak linear growth during puberty. Obesity was associated with an increased incidence of fracture (IRR=1.2; 95% CI, 1.1 to 1.25).
Figure 2.
Age-specific fracture incidence by sex in the general pediatric cohort.
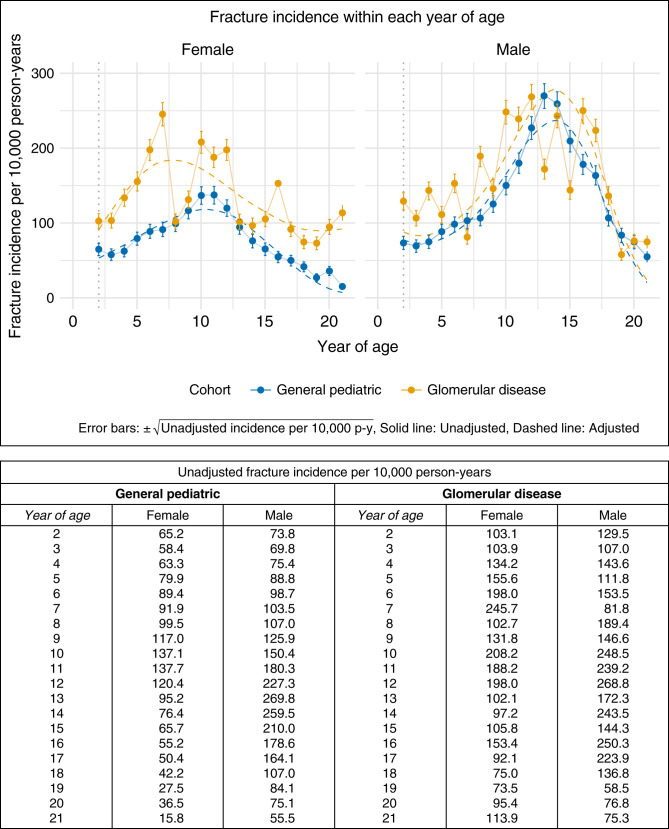
Incidence of Fracture in the Glomerular Disease Cohort Compared with the General Pediatric Cohort
The model comparing the glomerular disease cohort to the general pediatric cohort indicated an interaction between cohort and sex (Supplemental Table 3), whereby the effect of the cohort was strengthened in girls and attenuated in boys. Sex-stratified analyses demonstrated that among girls, the difference in fracture incidence between the cohorts changed across age (P=0.04) with a greater magnitude of difference before 10 years of age (Figure 3, Table 2). To understand whether there was an overall main effect of cohort among girls, we examined a model that excluded this interaction between cohort and age. In this model, girls with glomerular disease were 1.6 (95% CI, 1.3 to 1.9) times more likely to experience a fracture than girls in the general pediatric cohort (Table 3). The difference between the glomerular disease and general pediatric cohorts did not reach significance in the sex-stratified analyses for boys (Table 4). Obesity remained associated with increased incidence of fracture in sex-stratified analyses.
Figure 3.
Age-specific fracture incidence by sex in the glomerular disease cohort versus the general pediatric cohort.
Table 2.
Poisson regression model for incident fracture among girls in the glomerular disease and general pediatric cohorts
| Variable | Incidence Rate Ratio | 95% CI | P Value |
|---|---|---|---|
| Cohort: glomerular disease | 0.986 | 0.182 to 5.336 | 0.99 |
| Age (yr) | 1.129 | 1.003 to 1.27 | 0.04 |
| Age squared | 1.005 | 0.992 to 1.018 | 0.42 |
| Age cubed | 0.999 | 0.999 to 1 | 0.001 |
| Race | |||
| White | 1 | — | — |
| Black or African American | 0.975 | 0.907 to 1.048 | 0.49 |
| Other | 1.339 | 1.221 to 1.467 | <0.001 |
| Asian American | 0.745 | 0.68 to 0.817 | <0.001 |
| Unknown | 0.934 | 0.81 to 1.076 | 0.35 |
| Obese | 1.31 | 1.215 to 1.413 | <0.001 |
| Interaction between cohort and age | 1.356 | 0.792 to 2.321 | 0.27 |
| Interaction between cohort and age squared | 0.957 | 0.91 to 1.007 | 0.09 |
| Interaction between cohort and age cubed | 1.002 | 1 to 1.003 | 0.02 |
Table 3.
Poisson regression model for incident fracture among girls in the glomerular disease and general pediatric cohorts excluding a cohort and age interaction
| Variable | Incidence Rate Ratio | 95% CI | P Value |
|---|---|---|---|
| Cohort: glomerular disease | 1.594 | 1.324 to 1.918 | <0.001 |
| Age (yr) | 1.145 | 1.017 to 1.288 | 0.03 |
| Age squared | 1.003 | 0.991 to 1.016 | 0.59 |
| Age cubed | 0.999 | 0.999 to 1 | 0.003 |
| Race | |||
| White | 1 | — | — |
| Black or African American | 0.975 | 0.906 to 1.049 | 0.5 |
| Other | 1.339 | 1.22 to 1.468 | <0.001 |
| Asian American | 0.745 | 0.68 to 0.817 | <0.001 |
| Unknown | 0.934 | 0.81 to 1.076 | 0.34 |
| Obese | 1.31 | 1.214 to 1.415 | <0.001 |
Table 4.
Poisson regression model for incident fracture among boys in the glomerular disease and general pediatric cohorts
| Variable | Incidence Rate Ratio | 95% CI | P Value |
|---|---|---|---|
| Cohort: glomerular disease | 1.148 | 0.993 to 1.329 | 0.06 |
| Age (yr) | 0.58 | 0.078 to 4.315 | 0.59 |
| Age squared | 0 | 0 to 0 | <0.001 |
| Age cubed | 0.002 | 0.001 to 0.008 | <0.001 |
| Race | |||
| White | 1 | — | — |
| Black or African American | 1.469 | 1.338 to 1.614 | <0.001 |
| Other | 1.636 | 1.507 to 1.777 | <0.001 |
| Asian American | 0.86 | 0.759 to 0.973 | 0.02 |
| Unknown | 1.093 | 0.953 to 1.252 | 0.2 |
| Obese | 1.109 | 1.036 to 1.188 | 0.003 |
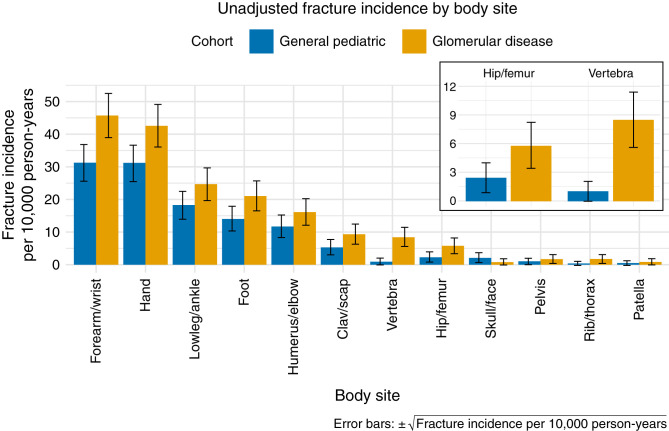
Body Site–Specific Incidence
Figure 4 shows the unadjusted incidence rate of fracture at different body sites. As expected, forearm/wrist and hand fractures were the most frequent in both the glomerular disease and general pediatric cohorts. The location-specific fracture SNOMED-CT diagnosis codes with the highest frequency were as follows: “Closed fracture of distal end of radius” (17222009), “Closed fracture of phalanx of finger” (24424003), “Closed supracondylar fracture of humerus” (58580000), “Closed fracture of lower end of radius AND ulna” (33192001), and “Closed torus fracture of radius” (429655000). In analyses of vertebral fractures adjusted for sex, race, age, and obesity, we found that children and young adults with glomerular disease had a five-fold (95% CI, 3.2 to 7.6) increased incidence rate compared with the general pediatric cohort (Supplemental Table 4). Seventeen patients (0.37%) in the glomerular disease cohort had at least one vertebral fracture (unadjusted incidence 8.5 per 10,000 person-years) compared with 335 (0.06%) in the general pediatric cohort (1.0 per 10,000 person-years). For analyses of hip/femur fractures, the glomerular disease cohort had a 2.2-fold (95% CI, 1.3 to 3.7) increased incidence rate (Supplemental Table 5). Ten (0.22%) patients in the glomerular disease cohort had at least one hip/femur fracture (5.8 per 10,000 person-years) compared with 771 (0.14%) in the general pediatric cohort (2.4 per 10,000 person-years).
Figure 4.
Body site–specific fracture incidence in the glomerular disease and general pediatric cohorts.
Incidence of Fracture in the Nephrotic Subcohort Compared with the General Pediatric Cohort
Restricting the glomerular disease cohort to those with nephrotic conditions did not change the overall pattern of results (Supplemental Table 6). The analysis comparing the nephrotic subcohort to the general pediatric cohort suggested that girls in the nephrotic subcohort were 1.6 times more likely (95% CI, 1.3 to 1.9) to experience a fracture, with no such effect for boys.
Incidence of Fracture in Glomerular Disease: Effect of CKD
Among the glomerular disease cohort, 909 (19.8%) patients met the CKD criteria at any time during their follow-up. A model comparing CKD and non-CKD person-time in the glomerular disease cohort to the general pediatric cohort demonstrated that girls with glomerular disease and CKD were 1.9 (95% CI, 1.3 to 2.6) times more likely than the general pediatric cohort to experience a fracture, and for those without CKD, the risk ratio was 1.6 (95% CI, 1.2 to 1.9; Supplemental Table 7). Patients with glomerular disease and CKD were 9.1 (95% CI, 4.9 to 16.9) times more likely than the general pediatric cohort to experience a vertebral fracture, whereas the IRR for those without CKD was 3.5 (95% CI, 2 to 6.3; Supplemental Table 8). For hip/femur fracture, the IRR was 2.2 (95% CI, 0.7 to 6.7) for glomerular CKD and 2.5 (95% CI, 1.5 to 4.3) for glomerular disease without CKD (Supplemental Table 9).
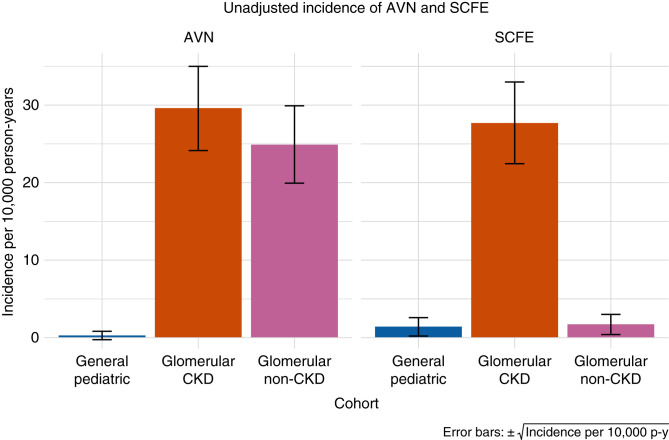
Other Skeletal Outcomes
Figure 5 shows the unadjusted incidence rates of AVN and SCFE for the general pediatric cohort compared with the glomerular disease cohort by CKD and non-CKD person-time.
Figure 5.
AVN and SCFE incidence according to person-time exposed to CKD.
AVN
Thirty (0.65%) patients in the glomerular disease cohort had at least one diagnosis of AVN (unadjusted incidence 26.44 per 10,000 person-years) compared with 66 (0.012%) patients in the general pediatric cohort (0.28 per 10,000 person-years). Adjusted analyses indicated that children with glomerular disease are 56.2 times (95% CI, 40.7 to 77.5) more likely to experience AVN (Supplemental Table 10). The interaction between cohort and sex indicated that the effect of glomerular disease is strengthened in girls and attenuated in boys. The effect of glomerular disease was independent of CKD, with an IRR of 61.9 (95% CI, 36.2 to 105.6) and 50.1 (95% CI, 34.8 to 72.1), respectively, with and without exposure to CKD (Supplemental Table 11).
SCFE
For SCFE, seven (0.15%) patients in the glomerular disease cohort had at least one diagnosis of SCFE (unadjusted incidence 7.6 per 10,000 person-years) compared with 267 (0.048%) patients in the general pediatric cohort (1.4 per 10,000 person-years). Adjusted analyses indicated that children with glomerular disease are 3.4 times more likely (95% CI, 1.9 to 5.9) to experience SCFE (Supplemental Table 12). Obesity was associated with a 10.5-fold (95% CI, 8.4 to 13.2) increased risk. The model that compared glomerular disease CKD and non-CKD person-time to the general pediatric cohort revealed that this effect was driven by CKD. The IRR for glomerular CKD was 10 (95% CI, 5.6 to 18.5), whereas the IRR for glomerular disease without CKD did not reach significance (Supplemental Table 13).
Corticosteroid Exposure Analyses
Contrary to expectations, time-to-event analyses examining time between first glomerular disease diagnosis and first fracture within the glomerular cohort indicated that patients with corticosteroid exposure had a reduced hazard of fracture (hazard ratio=0.6; 95% CI, 0.5 to 0.8; Supplemental Table 14). Within the nephrotic subcohort, steroid exposure did not reach significance for hazard of fracture (Supplemental Table 15). For AVN, as expected, patients with steroid exposure had an increased risk (hazard ratio=2.8; 95% CI, 1.2 to 6.6; Supplemental Table 16).
Sensitivity Analyses
Given the established increased risks of bone loss and fracture in patients with systemic lupus erythematosus (SLE),41 a sensitivity analysis for fracture that excluded patients with one or more diagnosis codes for systemic lupus erythematosus did not produce a different overall pattern of results (Supplemental Table 17). Of the general pediatric and glomerular disease cohorts, 18.8% and 20.8%, respectively, had more than one fracture. Sensitivity analyses (Supplemental Tables 18–20), which restricted analyses of incidence to each patient’s first fracture event, did not produce a different pattern of results. Similar analyses restricted to the first event of AVN or SCFE also yielded similar results (Supplemental Tables 21 and 22). Finally, a sensitivity analysis for fracture restricting the glomerular disease cohort to the three sites with large regional primary care networks (CHOP, Nemours, and Nationwide) produced similar findings to the main analysis (Supplemental Table 23).
Discussion
Within the population of nearly seven million children in the PEDSnet network, this retrospective cohort study of skeletal outcomes in glomerular disease demonstrated that rates of hip/femur and vertebral fractures, AVN, and SCFE were all at least two-fold higher in the 4598 children with glomerular disease compared with the general pediatric population of 553,624 individuals. There was also a higher risk of all fractures in girls with glomerular disease compared with the reference cohort.
This study leveraged a previously published algorithm that reliably identified children and young adults with glomerular disease and a subcohort of those with nephrotic conditions, with positive predictive values of 89% and 92%, respectively, in our prior study.28 Defining a cohort of children and young adults representative of the general pediatric population from a network of hospital-based health systems poses a challenge due to an overrepresentation of children seeking specialty care. Our approach was to select patients receiving primary care and to exclude patients for whom there was evidence in the EHR of chronic disease. The most frequent visit diagnoses for the general pediatric cohort support the validity of this approach (Supplemental Table 24). Furthermore, the age- and sex-specific patterns of fracture incidence observed in this study among the general pediatric cohort align closely with prior findings for other pediatric studies in the literature across several countries. These include the curvilinear relationship between age and fracture whereby risk is heightened around the time of peak linear growth, an earlier peak in girls than boys, and an increased incidence in boys versus girls particularly accentuated between 10 and 19 years of age.35,42–47 Consistent with these prior studies, fractures of the forearm/wrist and hand (fingers and metacarpals) were most common, representing 29.8% and 14.6% of all fracture diagnoses, respectively. Obesity was associated with an increased risk of fracture in the reference cohort, also consistent with several previous pediatric studies.36–38
In comparisons of overall fracture incidence (at any body site) in the glomerular disease and general pediatric cohorts, we found an interaction between sex and cohort. Sex-stratified models indicated that among girls, patients with glomerular disease have an increased risk of fracture compared with the reference cohort—an effect that was not observed in boys. This interaction between sex and glomerular disease was maintained when analyses were restricted to patients who met criteria for the nephrotic subcohort. This sex difference warrants further investigation and could be due to differential effects of glomerular disease on bone quality in girls versus boys. Sex differences in growth-related indices of bone quality, such as greater bone size and strength across growth in boys, may render girls more vulnerable to the effects of glomerular disease.48 The sex difference in fracture risk may also be mediated by the effect of glomerular disease on the complex interplay of body composition, pubertal timing, and physical activity on bone accrual.49–52 Mechanistic studies are needed to determine the deficits in bone structure and strength associated with glomerular disease.
Children with glomerular disease were at increased risk of vertebral and hip/femur fractures. We focused on these fracture sites in particular because they are rare but associated with significant morbidity. In the absence of high energy trauma or falls from a significant height, these fracture sites also indicate underlying bone fragility.53,54 Furthermore, vertebral fractures may be asymptomatic, and their incidence is likely underestimated. Two prior studies have examined vertebral fractures in children with nephrotic syndrome. One study performed lateral spine radiography in 78 children with nephrotic syndrome within 37 days of corticosteroid initiation and found that 8% had asymptomatic vertebral fractures,55 and a prospective cohort study of 54 children with nephrotic syndrome found that 6% experienced vertebral fractures within their first year of corticosteroid therapy.18
For AVN, which is extremely rare in the general pediatric population,56 our analyses indicated that patients with glomerular disease may be at more than a 50-fold increased risk. This is of particular concern because this risk only captures diagnosed AVN. In its early stages, AVN may be associated with only mild symptoms; magnetic resonance imaging is required for adequate detection. For SCFE, our analyses indicate that patients with glomerular disease are at an approximately three-fold greater risk, independent of obesity as a major risk factor for SCFE.
Given the established increased risk of fracture in children with CKD,13,14 we sought to evaluate how CKD affected the association of glomerular disease with skeletal outcomes. Our analyses suggest that although moderate to advanced CKD increases fracture risk among girls with glomerular disease, the higher fracture risk associated with glomerular disease was still observed in the absence of CKD. Similarly, the higher risks of vertebral and hip/femur fractures and AVN were also independent of CKD. In contrast, the increased SCFE risk among the glomerular disease cohort was driven by those with CKD.
In time-to-event analyses within the cohort of children with glomerular disease and in the subcohort with nephrotic conditions, we did not find the expected association of corticosteroid exposure with fracture risk.57,58 This unexpected result is likely due to confounding effects by other associated patient characteristics (e.g., an inverse relationship between corticosteroid exposure and physical activity or pattern of steroid responsiveness). Albeit unexpected, this finding highlights that the skeletal risk in this patient population is not attributable to corticosteroid exposure alone and underscores the importance of addressing other modifiable risk factors. Time-to-event analyses demonstrating that corticosteroid exposure was associated with increased hazard of AVN were consistent with previous literature.56
This study has several limitations, some of which are inherent in the use of multi-institutional EHR data resources. The data are limited to care received within the PEDSnet network. If, for example, a patient received care for a fracture at an urgent care facility outside the network, this fracture would not necessarily be captured by this approach. This limitation poses a particular concern if patients in one of our cohorts of interest were more likely to seek care within the network for reasons of geography or increased clinical concern. We attempted to mitigate this issue by requiring evidence of consistent follow-up in our cohort definitions. If a single patient received care at multiple institutions, they would be represented as a separate individual at each site. For children with a chronic health condition, such assignment may occur due to “second opinion” visits. We attempted to mitigate against this issue by requiring 12 months of clinical follow-up. This frequency of required follow-up may have selected for more “sick” visits and less representativeness of the healthy pediatric population but would likely have biased our results toward the null. Because our intention was to identify a cohort broadly representative of the general pediatric population in the network, our application of the PMCA algorithm did not exclude all chronic diseases from this cohort. Although infrequent, the inclusion of patients with chronic conditions, such as inflammatory bowel disease or diabetes, would have biased our results toward the null. Although the classification accuracy of the computable phenotype used to identify glomerular disease was previously evaluated in PEDSnet with a positive predictive value >85%, we assume there was misclassification of exposure likely by other forms of kidney disease. Given the inherent limitations of secondary use of EHR data, we were unable to categorize the patients with nephrotic conditions according to their steroid responsiveness.
Our approach to outcome ascertainment could be sensitive to timing of the outcome relative to a patient’s birthday, creating some misclassification with respect to age or double counting of an event proximate to a birthday, although we expect this would be nondifferential between the cohorts. Exposure to corticosteroids was based on number of prescriptions and not cumulative dose or duration. Some covariates of interest were either unavailable or incompletely captured for analysis, such as 25-hydroxyvitamin D levels, which are not routinely measured in the healthy population, and pubertal stage, measures of physical activity, and sports participation, which are not well recorded in the EHR. Although the cohort size of more than 4500 children and young adults with glomerular disease is large, given the rarity of these conditions, we still had limited power to investigate multiplicative interactions for rare outcome events such as hip and femur fractures and SCFE. Our analytical approach to fracture, AVN, and SCFE incidence did not account for potential correlation at the patient level if a patient had more than one event during their follow-up period. Sensitivity analyses examining incidence of first events for fracture, AVN, and SCFE were, however, consistent with the primary analyses.
In summary, these data show that children with glomerular disease are at increased risk for fractures of the vertebra and hip/femur, AVN, and SCFE compared with a general pediatric reference cohort. For fractures at any body site, girls with glomerular disease are at increased risk compared with girls in the general pediatric population. Our findings also add to a literature that has demonstrated an association between nephrotic syndrome and reduced bone mineral density.15–27 This large retrospective cohort study of skeletal outcomes in children and young adults with glomerular disease highlights the strength of multi-institutional EHR data for studying outcomes in pediatric rare diseases. Studies are needed to address mechanisms and prevention of skeletal complications in this population.
Disclosures
L.C. Bailey reports research funding from Jazz Pharmaceuticals, Takeda Pharmaceuticals, and UCB Pharmaceuticals. C. Gluck reports honoraria from Retrophin and Sanofi Genzyme, and an advisory or leadership role for Retrophin and Sanofi Genzyme. B.P. Dixon reports consultancy for Alexion Pharmaceuticals and Apellis Pharmaceuticals, and honoraria from Alexion Pharmaceuticals and Apellis Pharmaceuticals. J.T. Flynn reports honoraria from Springer (royalties) and UpToDate (royalties); an advisory or leadership role for Blood Pressure Monitoring (editorial board member), Hypertension (editorial board member), Journal of Pediatrics (editorial board member), Pediatric Nephrology (editor-in-chief), and the Renal Physicians Association (board member); and other interests or relationships with the American Society of Pediatric Nephrology, International Pediatric Nephrology Association, and Renal Physicians Association. D. Ranade reports an advisory or leadership role for PEDSnet (research subcommittee chair). W.E. Smoyer reports consultancy for Otsuka, Vertex, and Visterra; ownership interest in NephKey Therapeutics; research funding from Aurinia; honoraria from UCLA–CTSA External Advisory Committee and USC–CTSA External Advisory Committee; patents or royalties from UpToDate; and an advisory or leadership role for the Institute for the Advancement of Clinical Trials in Children (member of the coordinating committee), Kidney International, NephCure (member of the board of directors), and the Pediatric Nephrology Research Consortium (member of the board of directors). V.R. Dharnidharka reports consultancy for Atara Biotherapeutics and Medincell; research funding from CareDx; honoraria from CareDx; an advisory or leadership role for the North American Pediatric Renal Trials and Collaborative Studies; and other interests or relationships with Akebia/MedPace and the Independent Data Safety Monitoring Committee. B. Magnusen reports being an employee of AT&T, and consultancy for Bayer, Daugherty Business Solutions, and The Climate Corporation. M.M. Mitsnefes reports consultancy for KBP Biosciences. M. Somers reports consultancy for Alnylam, Dicerna, Horizon, and Orfan Biotech, and an advisory or leadership role for ASPN (president) and NAPRTCS (board of directors). E.K. Burrows reports being an employee of Helix, and ownership interest in Helix. C.B. Forrest reports research funding to institution from Bayer, Lily, Sanofi, and UCB, and patents or royalties from Johns Hopkins University. M.R. Denburg reports research funding from Mallinckrodt; an advisory or leadership role for Kidney International Reports (editorial board member) and the NKF Delaware Valley Medical Advisory Board; and other interests or relationships with the American Society of Pediatric Nephrology Research and Program Committees and the National Kidney Foundation Pediatric Education Planning Committee. All remaining authors have nothing to disclose.
Funding
This study was funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R21DK116151 (principal investigator: M.R. Denburg). This work was also supported in part by The Patient-Centered Outcomes Research Institute grant award RI-CHOP-01-PS1 and NIDDK grant P50DK114786.
Supplementary Material
Acknowledgments
The research presented includes data from the following PEDSnet institutions: Children’s Hospital of Philadelphia, Children’s Hospital Colorado, Cincinnati Children’s Hospital Medical Center, Nationwide Children’s Hospital, Nemours Children’s Health System (a Delaware and Florida health system), St. Louis Children’s Hospital, and Seattle Children’s Hospital. We would like to thank the patients and families at the PEDSnet member institutions. We would also like to thank Shweta Chavan, Kimberley Dickinson, and Nicole Marchesani for assistance with project management, data operations, and technical support. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK, the National Institutes of Health, the Department of Health and Human Services, or the government of the United States.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
L.C. Bailey, E.K. Burrows, S.J. Deakyne Davies, D. Eckrich, C.B. Forrest, A.J. Goodwin Davies, M. Kitzmiller, B. Magnusen, D. Ranade, H. Razzaghi, and L. Utidjian were responsible for data curation; L.C. Bailey, D.J. Claes, M.R. Denburg, V.R. Dharnidharka, B.P. Dixon, J.T. Flynn, C.B. Forrest, S.L. Furth, C. Gluck, M.M. Mitsnefes, W.E. Smoyer, and M. Somers were responsible for the investigation; L.C. Bailey, M.R. Denburg, C. Forrest, A.J. Goodwin Davies, H. Razzaghi, and R. Xiao were responsible for the formal analysis; L.C. Bailey, M.R. Denburg, C.B. Forrest, A.J. Goodwin Davies, H. Razzaghi, R. Xiao, and L. Utidjian were responsible for the methodology; L.C. Bailey, M.R. Denburg, H. Razzaghi, and R. Xiao were responsible for supervision; M.R. Denburg and C.B. Forrest were responsible for conceptualization and funding acquisition; M.R. Denburg, C.B. Forrest, and I.Y. Luna were responsible for project administration; M.R. Denburg, A.J. Goodwin Davies, H. Razzaghi, and R. Xiao wrote the original draft of the manuscript; A.J. Goodwin Davies was responsible for visualization; and all authors reviewed and edited the manuscript.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021101372/-/DCSupplemental.
Supplemental Table 1. Summary of all analyses performed.
Supplemental Table 2. Poisson regression model for incident fracture in the general pediatric cohort.
Supplemental Table 3. Overall Poisson regression model for incident fracture in the glomerular disease and general pediatric cohorts (not stratified by sex).
Supplemental Table 4. Poisson regression model for incident vertebral fractures.
Supplemental Table 5. Poisson regression model for incident hip and femur fractures.
Supplemental Table 6. Poisson regression model for incident fracture in the nephrotic subcohort and general pediatric cohort.
Supplemental Table 7. Poisson regression model for incident fracture in the glomerular and general pediatric cohorts, evaluating person-time exposed to chronic kidney disease (CKD) in the glomerular cohort.
Supplemental Table 8. Poisson regression model for vertebral fracture, evaluating person-time exposed to chronic kidney disease (CKD) in the glomerular cohort.
Supplemental Table 9. Poisson regression model for hip and femur fractures, evaluating person-time exposed to chronic kidney disease (CKD) in the glomerular cohort.
Supplemental Table 10. Poisson regression model for avascular necrosis/osteonecrosis (AVN).
Supplemental Table 11. Poisson regression model for avascular necrosis/osteonecrosis (AVN), evaluating person-time exposed to chronic kidney disease (CKD) in the glomerular cohort.
Supplemental Table 12. Poisson regression model for slipped capital femoral epiphysis (SCFE).
Supplemental Table 13. Poisson regression model for slipped capital femoral epiphysis (SCFE), evaluating person-time exposed to chronic kidney disease (CKD) in the glomerular cohort.
Supplemental Table 14. Cox proportional hazards model evaluating the association between corticosteroid exposure and time to first fracture within the glomerular disease cohort.
Supplemental Table 15. Cox proportional hazards model evaluating the association between corticosteroid exposure and time to first fracture within the nephrotic subcohort.
Supplemental Table 16. Cox proportional hazards model evaluating the association between corticosteroid exposure and time to avascular necrosis/osteonecrosis (AVN) within the glomerular disease cohort.
Supplemental Table 17. Poisson regression model for incident fracture in the glomerular disease (excluding the 448 patients with one or more lupus diagnosis codes) and general pediatric cohorts.
Supplemental Table 18. Poisson regression model for first fracture in the glomerular disease and general pediatric cohorts.
Supplemental Table 19. Poisson regression model for first vertebral fracture in the glomerular disease and general pediatric cohorts.
Supplemental Table 20. Poisson regression model for first hip/femur fracture in the glomerular disease and general pediatric cohorts.
Supplemental Table 21. Poisson regression model for first diagnosis of avascular necrosis/osteonecrosis (AVN) in the glomerular disease and general pediatric cohorts.
Supplemental Table 22. Poisson regression model for first diagnosis of slipped capital femoral epiphysis (SCFE) in the glomerular disease and general pediatric cohorts.
Supplemental Table 23. Overall Poisson regression model for incident fracture in the glomerular disease and general pediatric cohorts (not stratified by sex), limiting the glomerular disease cohort to patients at sites with a primary care network.
Supplemental Table 24. The most frequent visit diagnoses for the general pediatric cohort (ranked by number of patients).
References
- 1.Alem AM, Sherrard DJ, Gillen DL, Weiss NS, Beresford SA, Heckbert SR, et al. : Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 58: 396–399, 2000 [DOI] [PubMed] [Google Scholar]
- 2.Ball AM, Gillen DL, Sherrard D, Weiss NS, Emerson SS, Seliger SL, et al. : Risk of hip fracture among dialysis and renal transplant recipients. JAMA 288: 3014–3018, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Beaubrun AC, Kilpatrick RD, Freburger JK, Bradbury BD, Wang L, Brookhart MA: Temporal trends in fracture rates and postdischarge outcomes among hemodialysis patients. J Am Soc Nephrol 24: 1461–1469, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jadoul M, Albert JM, Akiba T, Akizawa T, Arab L, Bragg-Gresham JL, et al. : Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 70: 1358–1366, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Mittalhenkle A, Gillen DL, Stehman-Breen CO: Increased risk of mortality associated with hip fracture in the dialysis population. Am J Kidney Dis 44: 672–679, 2004 [PubMed] [Google Scholar]
- 6.Tentori F, McCullough K, Kilpatrick RD, Bradbury BD, Robinson BM, Kerr PG, et al. : High rates of death and hospitalization follow bone fracture among hemodialysis patients. Kidney Int 85: 166–173, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dooley AC, Weiss NS, Kestenbaum B: Increased risk of hip fracture among men with CKD. Am J Kidney Dis 51: 38–44, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Ensrud KE, Barbour K, Canales MT, Danielson ME, Boudreau RM, Bauer DC, et al. : Renal function and nonvertebral fracture risk in multiethnic women: The Women’s Health Initiative (WHI). Osteoporos Int 23: 887–899, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried LF, Biggs ML, Shlipak MG, Seliger S, Kestenbaum B, Stehman-Breen C, et al. : Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol 18: 282–286, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Jassal SK, von Muhlen D, Barrett-Connor E: Measures of renal function, BMD, bone loss, and osteoporotic fracture in older adults: The Rancho Bernardo study. J Bone Miner Res 22: 203–210, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naylor KL, McArthur E, Leslie WD, Fraser LA, Jamal SA, Cadarette SM, et al. : The three-year incidence of fracture in chronic kidney disease. Kidney Int 86: 810–818, 2014 [DOI] [PubMed] [Google Scholar]
- 12.Nickolas TL, McMahon DJ, Shane E: Relationship between moderate to severe kidney disease and hip fracture in the United States. J Am Soc Nephrol 17: 3223–3232, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Denburg MR, Kumar J, Jemielita T, Brooks ER, Skversky A, Portale AA, et al. : Fracture burden and risk factors in childhood CKD: Results from the CKiD Cohort Study. J Am Soc Nephrol 27: 543–550, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Denburg MR, Tsampalieros AK, de Boer IH, Shults J, Kalkwarf HJ, Zemel BS, et al. : Mineral metabolism and cortical volumetric bone mineral density in childhood chronic kidney disease. J Clin Endocrinol Metab 98: 1930–1938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aceto G, D’Addato O, Messina G, Carbone V, Cavallo L, Brunetti G, et al. : Bone health in children and adolescents with steroid-sensitive nephrotic syndrome assessed by DXA and QUS. Pediatr Nephrol 29: 2147–2155, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Koşan C, Ayar G, Orbak Z: Effects of steroid treatment on bone mineral metabolism in children with glucocorticoid-sensitive nephrotic syndrome. West Indian Med J 61: 627–630, 2012 [PubMed] [Google Scholar]
- 17.Pańczyk-Tomaszewska M, Adamczuk D, Kisiel A, Skrzypczyk P, Przedlacki J, Górska E, et al. : Markers of bone metabolism in children with nephrotic syndrome treated with corticosteroids. Adv Exp Med Biol 840: 21–28, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Phan V, Blydt-Hansen T, Feber J, Alos N, Arora S, Atkinson S, et al. ; Canadian STOPP Consortium : Skeletal findings in the first 12 months following initiation of glucocorticoid therapy for pediatric nephrotic syndrome. Osteoporos Int 25: 627–637, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ribeiro D, Zawadynski S, Pittet LF, Chevalley T, Girardin E, Parvex P: Effect of glucocorticoids on growth and bone mineral density in children with nephrotic syndrome. Eur J Pediatr 174: 911–917, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Gulati S, Godbole M, Singh U, Gulati K, Srivastava A: Are children with idiopathic nephrotic syndrome at risk for metabolic bone disease? Am J Kidney Dis 41: 1163–1169, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Kano K, Yamada Y, Nishikura K, Kojima E, Arisaka O: Low bone mineral density in nephrotic children with steroid dependence and/or frequent relapsers. Clin Nephrol 64: 323–324, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Broyer M, Terzi F, Lehnert A, Gagnadoux MF, Guest G, Niaudet P: A controlled study of deflazacort in the treatment of idiopathic nephrotic syndrome. Pediatr Nephrol 11: 418–422, 1997 [DOI] [PubMed] [Google Scholar]
- 23.Lettgen B, Jeken C, Reiners C: Influence of steroid medication on bone mineral density in children with nephrotic syndrome. Pediatr Nephrol 8: 667–670, 1994 [DOI] [PubMed] [Google Scholar]
- 24.Takeda Y: Evaluation of bone mineral turnover in children with nephrotic syndrome—The implications of original disease and the effects of corticosteroids on bone metabolism. Nihon Jinzo Gakkai Shi 35: 705–713, 1993 [PubMed] [Google Scholar]
- 25.Wetzsteon RJ, Shults J, Zemel BS, Gupta PU, Burnham JM, Herskovitz RM, et al. : Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res 24: 503–513, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hegarty J, Mughal MZ, Adams J, Webb NJ: Reduced bone mineral density in adults treated with high-dose corticosteroids for childhood nephrotic syndrome. Kidney Int 68: 2304–2309, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Kyrieleis HA, Löwik MM, Pronk I, Cruysberg HR, Kremer JA, Oyen WJ, et al. : Long-term outcome of biopsy-proven, frequently relapsing minimal-change nephrotic syndrome in children. Clin J Am Soc Nephrol 4: 1593–1600, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denburg MR, Razzaghi H, Bailey LC, Soranno DE, Pollack AH, Dharnidharka VR, et al. : Using electronic health record data to rapidly identify children with glomerular disease for clinical research. J Am Soc Nephrol 30: 2427–2435, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Forrest CB, Margolis P, Seid M, Colletti RB: PEDSnet: How a prototype pediatric learning health system is being expanded into a national network. Health Aff (Millwood) 33: 1171–1177, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Forrest CB, Margolis PA, Bailey LC, Marsolo K, Del Beccaro MA, Finkelstein JA, et al. : PEDSnet: A national pediatric learning health system. J Am Med Inform Assoc 21: 602–606, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R: Development and Validation of the Pediatric Medical Complexity Algorithm (PMCA) version 3.0. Acad Pediatr 18: 577–580, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oskarsson T, Duun-Henriksen AK, Bautz A, Montgomery S, Harila-Saari A, Petersen C, et al. : Skeletal adverse events in childhood cancer survivors: An Adult Life after Childhood Cancer in Scandinavia cohort study. Int J Cancer 149: 1863–1876, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Fassier A, Gaucherand P, Kohler R: Fractures in children younger than 18 months. Orthop Traumatol Surg Res 99: S160–S170, 2013 [DOI] [PubMed] [Google Scholar]
- 34.Rosendahl K, Myklebust R, Ulriksen KF, Nøttveit A, Eide P, Djuve Å, et al. : Incidence, pattern and mechanisms of injuries and fractures in children under two years of age. BMC Musculoskelet Disord 22: 555, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper C, Dennison EM, Leufkens HG, Bishop N, van Staa TP: Epidemiology of childhood fractures in Britain: A study using the general practice research database. J Bone Miner Res 19: 1976–1981, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Lane JC, Butler KL, Poveda-Marina JL, Martinez-Laguna D, Reyes C, de Bont J, et al. : Preschool obesity is associated with an increased risk of childhood fracture: A longitudinal cohort study of 466,997 children and up to 11 years of follow-up in Catalonia, Spain. J Bone Miner Res 35: 1022–1030, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nhan DT, Leet AI, Lee RJ: Associations of childhood overweight and obesity with upper-extremity fracture characteristics. Medicine (Baltimore) 100: e25302, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kessler J, Koebnick C, Smith N, Adams A: Childhood obesity is associated with increased risk of most lower extremity fractures. Clin Orthop Relat Res 471: 1199–1207, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laster M, Denburg M, Okuda Y, Kumar J, Furth S, Warady B, et al. : Race and ethnicity predict bone markers and fracture in pediatric patients with chronic kidney disease. J Bone Miner Res 36: 298–304, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. : New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang X, Yan S, Liu C, Xu Y, Wan L, Wang Y, et al. : Fracture risk and bone mineral density levels in patients with systemic lupus erythematosus: A systematic review and meta-analysis. Osteoporos Int 27: 1413–1423, 2016 [DOI] [PubMed] [Google Scholar]
- 42.Hedström EM, Svensson O, Bergström U, Michno P: Epidemiology of fractures in children and adolescents. Acta Orthop 81: 148–153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mäyränpää MK, Mäkitie O, Kallio PE: Decreasing incidence and changing pattern of childhood fractures: A population-based study. J Bone Miner Res 25: 2752–2759, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Naranje SM, Erali RA, Warner WC Jr, Sawyer JR, Kelly DM: Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop 36: e45–e48, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Randsborg PH, Gulbrandsen P, Saltytė Benth J, Sivertsen EA, Hammer OL, Fuglesang HF, et al. : Fractures in children: Epidemiology and activity-specific fracture rates. J Bone Joint Surg Am 95: e42, 2013 [DOI] [PubMed] [Google Scholar]
- 46.Rennie L, Court-Brown CM, Mok JY, Beattie TF: The epidemiology of fractures in children. Injury 38: 913–922, 2007 [DOI] [PubMed] [Google Scholar]
- 47.Moon RJ, Harvey NC, Curtis EM, de Vries F, van Staa T, Cooper C: Ethnic and geographic variations in the epidemiology of childhood fractures in the United Kingdom. Bone 85: 9–14, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gabel L, Macdonald HM, McKay HA: Sex differences and growth-related adaptations in bone microarchitecture, geometry, density, and strength from childhood to early adulthood: A mixed longitudinal HR-pQCT study. J Bone Miner Res 32: 250–263, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Medina-Gomez C, Heppe DHM, Yin JL, Trajanoska K, Uitterlinden AG, Beck TJ, et al. : Bone mass and strength in school-age children exhibit sexual dimorphism related to differences in lean mass: The Generation R Study. J Bone Miner Res 31: 1099–1106, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Christoffersen T, Emaus N, Dennison E, Furberg AS, Gracia-Marco L, Grimnes G, et al. : The association between childhood fractures and adolescence bone outcomes: A population-based study, the Tromsø Study, Fit Futures. Osteoporos Int 29: 441–450, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuh D, Muthuri SG, Moore A, Cole TJ, Adams JE, Cooper C, et al. : Pubertal timing and bone phenotype in early old age: Findings from a British birth cohort study. Int J Epidemiol 45: 1113–1124, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burrows M, Liu D, Moore S, McKay H: Bone microstructure at the distal tibia provides a strength advantage to males in late puberty: An HR-pQCT study. J Bone Miner Res 25: 1423–1432, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Ma J, Siminoski K, Wang P, Jaremko JL, Koujok K, Matzinger MA, et al. ; Canadian STOPP Consortium : The accuracy of incident vertebral fracture detection in children using targeted case-finding approaches. J Bone Miner Res 36: 1255–1268, 2021 [DOI] [PubMed] [Google Scholar]
- 54.Papalia R, Torre G, Maffulli N, Denaro V: Hip fractures in children and adolescents. Br Med Bull 129: 117–128, 2019 [DOI] [PubMed] [Google Scholar]
- 55.Feber J, Gaboury I, Ni A, Alos N, Arora S, Bell L, et al. ; Canadian STOPP Consortium : Skeletal findings in children recently initiating glucocorticoids for the treatment of nephrotic syndrome. Osteoporos Int 23: 751–760, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cooper C, Steinbuch M, Stevenson R, Miday R, Watts NB: The epidemiology of osteonecrosis: Findings from the GPRD and THIN databases in the UK. Osteoporos Int 21: 569–577, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Staa TP, Cooper C, Leufkens HG, Bishop N: Children and the risk of fractures caused by oral corticosteroids. J Bone Miner Res 18: 913–918, 2003 [DOI] [PubMed] [Google Scholar]
- 58.van Staa TP, Leufkens HG, Cooper C: The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos Int 13: 777–787, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.