Significance Statement
Gut dysbiosis contributes to dysfunctional mucosal immunity, which may lead to production of nephrotoxic immune complexes specific to IgA nephropathy (IgAN). However, the key bacterial taxa closely associated with IgAN onset and treatment response have not been determined. We conducted a comprehensive observational study and found that, compared with healthy controls, patients with IgAN have a distinct gut microbial composition characterized by excessive expansion of the taxonomic chain Proteobacteria–Gammaproteobacteria–Enterobacteriales–Enterobacteriaceae–Escherichia-Shigella. Escherichia-Shigella contributed the most to the abundant taxonomic chain and performed best in the bacterial diagnosis model for distinguishing patients with IgAN from healthy controls. Strikingly, immunosuppressive therapy reversed the expansion of genus Escherichia-Shigella and increased bacterial diversity, but only in patients who achieved clinical remission. These results identify a crucial role of Escherichia-Shigella expansion in IgAN.
Keywords: IgA nephropathy, gut microbiota, Escherichia-Shigella, 16S ribosomal RNA, treatment response
Visual Abstract
Abstract
Background
Gut dysbiosis is postulated to participate in the pathogenesis of IgA nephropathy (IgAN). However, the key bacterial taxa closely associated with IgAN onset and treatment response have not been identified.
Methods
We recruited 127 patients with IgAN who were treatment naive and 127 matched healthy controls (HCs) who were randomly divided into discovery and validation cohorts to investigate the characteristics of their gut microbiota and establish a bacterial diagnosis model for IgAN. A separate cohort of 56 patients and HCs was investigated to assess crossregional validation. A further 40 patients with primary membranous nephropathy (MN) were enrolled to probe disease-specific validation. A subgroup of 77 patients was prospectively followed to further dissect the association between alterations in gut microbiota and treatment response after 6 months of immunosuppressive therapy. Fecal microbiota samples were collected from all participants and analyzed using 16S ribosomal RNA sequencing.
Results
Decreased α-diversity (Shannon, P=0.03), altered microbial composition (Adonis, P=0.0001), and a striking expansion of the taxonomic chain Proteobacteria–Gammaproteobacteria–Enterobacteriales–Enterobacteriaceae–Escherichia-Shigella (all P<0.001) were observed in patients with IgAN who were treatment naive, which reversed only in patients who achieved clinical remission after 6 months of immunosuppressive therapy. Importantly, seven operational taxa units, of which Escherichia-Shigella contributed the most, were determined to be the optimal bacterial classifier of IgAN (AUC=0.8635, 0.8551, 0.8026 in discovery, validation, and cross-regional validation sets, respectively), but did not effectively distinguish patients with IgAN versus those with MN (AUC=0.6183). Bacterial function prediction further verified enrichment of the shigellosis infection pathway in IgAN.
Conclusion
Gut dysbiosis, characterized by a striking expansion of genus Escherichia-Shigella, is a hallmark of patients with IgAN and may serve as a promising diagnostic biomarker and therapeutic target for IgAN. Further studies are warranted to investigate the potential contribution of Escherichia-Shigella in IgAN pathogenesis.
There have been numerous attempts to define the characteristics of gut microbiota in patients with CKD, with the aim of finding the specific bacterial taxon or metabolites tightly associated with the occurrence and/or progression of CKD that could provide promising treatment options.1 IgA nephropathy (IgAN), the most common GN of CKD, especially in the Asian population,2 is characterized by glomerular mesangial deposition of immune complexes specific for galactose-deficient IgA1 (gd-IgA1),3 which is well known for the “four-hit” theory.4 However, the origin of gd-IgA1 is unclear, creating challenges for pathogenesis exploration and dissatisfaction in clinical practice due to nonetiologic treatment.
Gut dysbiosis may contribute to the dysfunction of intestinal and systemic mucosal immunity and mediate the production of gd-IgA1, resulting in the onset of IgAN.5–8 Depletion of gut microbiota by antibiotics in an IgAN model (α1KI-CD89Tg mice) reduced IgA1 mesangial deposition, urinary protein, and glomerular inflammation.9,10 Fecal microbiota transplantation (FMT) from patients with IgAN to α1KI-CD89Tg mice induced an increase in serum B cell–activating factor and gd-IgA1, and heavy deposition of soluble CD89 and IgA1 in mesangial cells,11 which together implied a causal link between gut dysbiosis and IgAN. We previously showed that administering fresh FMT from healthy donors to two patients with refractory IgAN led to a marked reduction in urinary protein, further suggesting a contributing role of gut dysbiosis to IgAN.12 Discovery of the key bacterial taxa in the gut microbiota of patients with IgAN is a critical next step. To date, eight cross-sectional studies13–20 have revealed some characteristics of the gut microbiota in patients with IgAN. However, due to differences in disease severity, treatment history, geographic location, ethnicity, and relatively small sample sizes, these studies neither produce a consistent profile of gut microbiota nor identify the key bacterial taxa most closely related to IgAN.
Therefore, we conducted a large-scale observational study that included an IgAN cohort with discovery and validation sets, an independent crossregion validation cohort, and a non-IgAN disease cohort to investigate the characteristics of gut microbiota in patients with IgAN and to establish a bacterial classifier as a novel, noninvasive diagnostic tool to distinguish patients with IgAN from healthy individuals. Currently, 30%–70%21 of patients with IgAN do not achieve remission in clinical practice, despite receiving several rounds of supportive or/and immunosuppressive therapies as recommended by current guidelines.22 This response rate is clinically unacceptable and we hypothesized that differences in the baseline microbiota, or differences in how the gut microbiota change during treatment, may affect patients’ response to therapy. Thus, we prospectively examined interactions between alterations in gut microbiota and the clinical therapeutic response after 6 months of immunosuppressive therapy and established a promising therapeutic target for IgAN. To our knowledge, this is the first study to combine horizontal and longitudinal perspectives in investigating the gut microbiota and its influence on therapeutic outcomes in a large cohort of patients with IgAN.
Methods
Study Design and Subjects
We consecutively recruited 127 patients with IgAN who were treatment naive and 86 healthy controls (HCs) matched for age, sex, and body mass index (BMI) between September 2017 and February 2021 in Xijing hospital (Xi’an China). Additionally, 41 HCs were acquired from published data23 in the Chinese population. In total, 254 subjects were randomly divided into discovery and validation sets. The discovery set included 84 patients and 84 HCs; this cohort was established with the aim of establishing a bacterial classifier as a noninvasive diagnostic tool for IgAN via machine learning. The validation cohort comprised 43 patients and 43 HCs; for this cohort, we applied the receiver operating characteristic (ROC) curve to assess the diagnostic efficiency of the bacterial classifier. An independent validation cohort, including 56 patients with IgAN and 56 HCs (with no age limitation) from The First Affiliated Hospital of Zhengzhou University in Henan province of China, was established to further corroborate the findings from the discovery and validation sets. Finally, we used a cohort of 40 patients with primary membranous nephropathy (MN), reported in our previously published study,14 to determine the disease specificity of the bacterial classifier. Inclusion and exclusion criteria are provided in Supplemental Appendix 1. Patients who received immunosuppressive therapy were followed prospectively, and 77 donated fecal samples after 6 months to further investigate associations between alterations of gut microbiota and therapeutic outcomes (Figure 1, Supplemental Appendix 1).
Figure 1.
Study design and flow diagram. A total of 331 samples were collected prospectively from 127 patients with IgAN who were treatment naive and 127 matched HCs. Of these, 77 patients with IgAN were assessed after 6 months of immunosuppressive treatment. A separate cohort, including 56 patients with IgAN and 56 HCs, was established for cross-regional validation, and a cohort of 40 patients with primary MN was used for disease-specific validation. CTX, cyclophosphamide; FK506, tacrolimus; IT, immunosuppressive therapy.
Fecal Sample Collection and Bacterial Taxon Identification
Fasting fresh fecal samples were collected from HCs, patients with IgAN who were treatment naive, and from the subgroup of patients followed up after 6 months of immunosuppressive therapy. Fecal samples were quickly placed into sterile specimen tubes and transferred to a −80°C cryogenic refrigerator. The 16S ribosomal RNA (rRNA) gene sequence24 targeting the V3-V4 region was applied to identify bacterial taxa using an Illumina Miseq instrument (Illumina, San Diego, CA) as previously described.14
Bioinformatic Statistical Analysis
α-Diversity, β-diversity, and taxonomic differences of gut microbiota were analyzed as described previously.14 α-Diversity represents the richness (number of taxonomic groups or species, indicated by Chao, Ace, and operational taxonomic unit [OTU] indices) and evenness (distribution of abundances of the groups, indicated by Shannon and Simpson indices) of gut microbiota.25 β-Diversity assesses the change in the diversity of species from one environment to another (structural differences).26 We constructed the Kyoto Encyclopedia of Genes and Genomes (KEGG) orthology and KEGG pathway/module profiles27 using PICRUSt2 (version 2.4.1; https://github.com/picrust/picrust2/wiki), which is software used to predict functional abundances on the basis of 16S rRNA marker gene sequences. A random forest 4.6-12 package was used to establish a disease diagnostic model on the basis of five-fold cross-validation. We calculated the probability of disease (POD) index to predict whether samples were from patients with IgAN or HCs, and the area under curve (AUC), on the basis of the ROC curve, was determined to evaluate the diagnostic efficiency of the established model (R version 3.4.1, pROC package).
We described quantitative data with normal distribution using the mean and SD, and presented data with non-normal distribution as median with 25th and 75th quartiles. We used a t test to compare the two groups of normally distributed quantitative data, and a nonparametric test (Mann–Whitney U test) for non-normally distributed quantitative data. Qualitative data were described using percentages and analysis using the chi-squared test. We adjusted multiple hypothesis tests using false discovery rate (FDR), and considered differences significant below a FDR threshold (Pfdr) of 0.05.
Ethics Approval and Consent to Participate
The study was approved by the ethics committee for clinical trials of the Xijing Hospital of the Fourth Military Medical University in China (KY20192070), and written informed consent was obtained from each study participant.
Results
Clinical Characteristics of Patients with IgAN Who Are Treatment Naive
Patients with IgAN did not differ significantly in terms of sex, BMI, or age, but they had a higher level of serum creatinine and lower levels of serum albumin and eGFR, compared with the HC group (Table 1, Supplemental Table 1). The 254 subjects were randomly divided into discovery and validation sets (Supplemental Table 2). Of 127 patients with IgAN, 93 patients with a 24-hour proteinuria >0.75 g/d after receiving the optimal supportive treatment for at least 3 months commenced immunosuppressive therapy. Of these, 77 patients donated fecal samples after 6 months of immunosuppressive therapy; 31 received treatment with corticosteroids (CS) and cyclophosphamide (CTX), 28 received CS and mycophenolate mofetil (MMF), nine received MMF monotherapy, eight received tacrolimus monotherapy, and one received multiple immunosuppressive agents. Clinical remission (defined in Supplemental Appendix 1) was achieved in 51 patients (66.2%). Demographic and clinical characteristics were comparable between patients who did/did not achieve clinical remission, except for a lower proteinuria in the former group (Figure 1, Table 2).
Table 1.
Baseline characteristics of patients with IgAN and HCs
| Parameters | IgAN Group (n=127) | HC Group (n=127) | P Value | |
|---|---|---|---|---|
| Age (yr), mean±SD | 35.6±10.3 | 34.8±10.9 | 0.59 | |
| Male, n (%) | 76 (59.8) | 68 (53.3) | 0.31 | |
| BMI (kg/m2), mean±SD | 24.3±3.9 | 23.5±2.0 | 0.06 | |
| WBC (109/L), mean±SD | 6.7±1.8 | 6.1±1.4 | 0.001 | |
| Platelets (109/L), mean±SD | 235.1±75.6 | 229.9±50.1 | 0.52 | |
| Anemia, n (%) | 16 (12.6) | 2 (1.6) | 0.001 | |
| Albumin (g/L), mean±SD | 40.3±5.4 | 47.8±3.6 | 0.00 | |
| SCr (μmol/L), mean±SD | 104.0±39.7 | 72.1±15.1 | 0.001 | |
| eGFR (ml/min per 1.73 m2), mean±SD | 76.8±30.0 | 106.3±17.0 | 0.00 | |
| eGFR <60 ml/min per 1.73 m2, n (%) | 38 (29.9) | 0 (0.0) | 0.00 | |
| Hypertension, n (%) | 34 (26.8) | 0 (0.0) | 0.00 | |
| Proteinuria (mg/d), median (IQR) | 1070.0 (518.0–1760.0) | NA | ||
| Proteinuria >1.0 g/d, n (%) | 68 (53.8) | NA | — | |
| Cystatin C (mg/L), mean±SD | 1.23±0.46 | NA | ||
| Uric acid (μmol/L), mean±SD | 356.8±114.7 | NA | ||
| Serum urea (mmol/L), median (IQR) | 5.6 (4.5–7.9) | NA | ||
| Histologic lesion scoring, n (%) | ||||
| M1 | 75 (59.1) | |||
| E1 | 17 (13.4) | |||
| S1 | 98 (77.2) | |||
| T lesion scoring, n (%) | ||||
| T0 | 73 (57.5) | |||
| T1 | 35 (27.6) | |||
| T2 | 19 (15.0) | |||
| C lesion scoring, n (%) | ||||
| C0 | 84 (66.1) | |||
| C1 | 38 (29.9) | |||
| C2 | 5 (3.9) | |||
WBC, white blood cell; SCr, serum creatinine; IQR, interquartile range; NA, not available; M1, mesangial hypercellularity; E1, endocapillary hypercellularity; S1, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis; T0, T ≤25%; T1, T 26%–50%; T2, T >50%; C0, no crescents; C1, crescents in less than one fourth of glomeruli; C2, crescents in over one fourth of glomeruli.
Table 2.
Clinical characteristics of patients with IgAN who did/did not achieve clinical remission
| Parameters | Clinical Remission (n=51) | No Clinical Remission (n=26) | P Value |
|---|---|---|---|
| Age (yr), mean±SD | 36.2±10.3 | 39.4±11.1 | 0.21 |
| Male, n (%) | 35 (68.6) | 13 (50.0) | 0.11 |
| BMI (kg/m2), mean±SD | 24.2±3.5 | 26.1±5.1 | 0.06 |
| WBC (109/L), mean±SD | 6.6±1.8 | 7.5±2.4 | 0.11 |
| Platelets (109/L), mean±SD | 224.1±63.0 | 236.5±71.6 | 0.44 |
| Anemia, n (%) | 6 (11.8) | 7 (26.9) | 0.09 |
| Albumin (g/L), mean±SD | 39.2±4.3 | 37.5±6.2 | 0.17 |
| SCr (μmol/L), mean±SD | 109.5±40.0 | 106.4±42.9 | 0.75 |
| eGFR (ml/min per 1.73 m2), mean±SD | 73.7±28.1 | 70.8±35.0 | 0.70 |
| eGFR <60 ml/min per 1.73 m2, n (%) | 18 (35.3) | 12 (46.2) | 0.36 |
| Hypertension, n (%) | 11 (21.6) | 10 (38.5) | 0.12 |
| Proteinuria (mg/d), median (IQR) | 1349.0 (818.0–1669.5) | 1541.5 (1083.8–3720.0) | 0.04 |
| Proteinuria >1.0 g/d, n (%) | 34 (66.7) | 20 (76.9) | 0.35 |
| Cystatin C (mg/L), median (IQR) | 1.19 (0.97–1.59) | 1.33 (1.12–1.71) | 0.13 |
| Uric acid (μmol/L), median (IQR) | 368.0 (278.5–430.5) | 380.0 (300.5–467.5) | 0.37 |
| Serum urea (mmol/L), median (IQR) | 5.6 (6.3–7.9) | 6.1 (4.7–9.3) | 0.71 |
| Histologic lesion scoring, n (%) | |||
| M1 | 34 (66.7) | 20 (76.9) | 0.35 |
| E1 | 10 (19.6) | 4 (15.4) | 0.65 |
| S1 | 42 (82.4) | 25 (96.2) | 0.09 |
| T lesion scoring | |||
| T0 | 23 (45.1) | 12 (46.2) | 0.79 |
| T1 | 19 (37.3) | 8 (30.8) | |
| T2 | 9 (17.6) | 6 (23.1) | |
| C lesion scoring | |||
| C0 | 30 (58.8) | 13 (66.1) | 0.76 |
| C1 | 18 (35.3) | 11 (29.9) | |
| C2 | 3 (5.9) | 2 (3.9) | |
WBC, white blood cell; SCr, serum creatinine; IQR, interquartile range; NA, not available; M1, mesangial hypercellularity; E1, endocapillary hypercellularity; S1, segmental glomerulosclerosis; T, tubular atrophy/interstitial fibrosis; T0, T ≤25%; T1, T 26%–50%; T2, T >50%; C0, no crescents; C1, crescents in less than one fourth of glomeruli; C2, crescents in over one fourth of glomeruli.
Hallmark of Gut Dysbiosis in Patients with IgAN Who Are Treatment Naive
In the discovery cohort, Shannon–Wiener analysis showed that the bacterial diversity nearly approached saturation in each sample as the sequence depth increased, suggesting a vast majority of taxa had been detected (Figure 2A). A total of 1068 and 1351 OTUs were identified in the IgAN and HC groups, respectively, of which 1018 were shared OTUs. Compared with HCs, the α-diversity of gut microbiota was statistically lower in patients with IgAN who were treatment naive, as evaluated by the Shannon index (P=0.03) and number of observed OTUs (P=0.02; Figure 2B, Supplemental Table 2). Additionally, the Chao, Ace, and Simpson indices also showed a tendency toward a lower α-diversity of gut microbiota in patients with IgAN (Supplemental Table 3). The Bray–Curtis, unweighted UniFrac, and weighted UniFrac-based principal coordinate analysis and nonmetric multidimensional scaling (NMDS) demonstrated the composition of gut microbiota in patients with IgAN was significantly distinct from HCs (Adonis test, P=0.0001, P=0.0001, smfP=0.006, respectively; Figure 2, C and D).
Figure 2.
Gut dysbiosis was determined in patients with IgAN who were treatment naive compared with HCs (discovery set; 84 patients with IgAN, 84 HCs). (A) Shannon–Wiener curve shows microbial diversity in each sample at different sequencing depths. The curve tends to be flat, indicating the amount of sequencing data are large enough to reflect the vast majority of microbial information in the sample. (B) Lower α-diversity in patients with IgAN than in HCs, as estimated by observed_otus index. The vertical bar within each box represents the median, and the left and right of each box represent the 25th and 75th percentiles, respectively. (C) Scatter diagram representing the PCoA showed the gut taxonomic composition (β-diversity) was significantly different between patients with IgAN and HCs. The ovals represent the different clustering tendencies of the two groups. The boxes on the left and below present the median and upper and lower quartile values of a set of samples on the horizontal and vertical coordinates, respectively. (D) Scatter diagram representing the NMDS analysis showed the gut taxonomic composition (β-diversity) was significantly different between patients with IgAN and HCs. The ovals represent the different clustering tendencies of the two groups. The boxes on left and below present the median and upper and lower quartile values of a set of samples on the horizontal and vertical coordinates, respectively. PCoA, principal coordinate analysis; NMDS, nonmetric multidimensional scaling; PC, principal component.
We further evaluated the abundance difference of gut microbiota at the different taxonomic levels between the patients with IgAN who were treatment naive and HCs. At the phylum level, Proteobacteria (Pfdr<0.001) was significantly enriched in patients with IgAN relative to HCs (Figure 3A, Supplemental Figure 1A, Supplemental Table 4). Accordingly, the relative abundance at the class, order, and family levels showed alterations in gut microbiota between patients and HCs (Figure 3, B–D, Supplemental Figure 1, B–D, Supplemental Tables 5–7). At the genus level, five genera, including Escherichia-Shigella (Pfdr<0.001), Pseudomonas (Pfdr<0.001), Erysipelatoclostridium (Pfdr<0.001), Ruminococcaceae_UBA1819 (Pfdr=0.03), and Ruminococcaceae_CAG-352 (Pfdr=0.009) were over-represented in patients with IgAN; whereas 16 genera, mainly belonging to family Lachnospiraceae and phylum Firmicutes, including Lachnospira (Pfdr=0.0004), Lachnospiraceae_ND3007_group (Pfdr<0.001), Fusicatenibacter (Pfdr<0.001), Lachnospiraceae_NC2004_group (Pfdr<0.001), Lachnospiraceae_UCG-001 (Pfdr<0.0001), Lachnospiraceae_UCG-004 (Pfdr=0.001), Lachnospiraceae_UCG-010 (Pfdr= 0.0039), Lachnospiraceae_unclassified (Pfdr=0.02), Agathobacter (Pfdr=0.02), and Romboutsia (Pfdr=0.004), were less abundant relative to HCs (Figure 3E, Supplemental Figure 1E, Supplemental Table 8).
Figure 3.
Relative abundance of the bacterial community in patients with IgAN compared with HCs (discovery set; 84 patients with IgAN, 84 HCs). (A) Relative abundance of the bacterial community in both groups at the phylum level. Proteobacteria was significantly enriched in patients with IgAN relative to HCs. (B) Relative abundance of the bacterial community in both groups at the class level. Gammaproteobacteria were more abundant in patients with IgAN compared with HCs. (C) Relative abundance of the bacterial community in both groups at the order level. Enterobacterales and Pseudomonadales were more abundant, whereas Lachnospirales, Clostridiales, Clostridia_unclassified, Pasteurellales, and Peptostreptococcales-Tissierellales were less abundant in patients with IgAN compared with HCs. (D) Relative abundance of the bacterial community in both groups at the family level. Enterobacteriaceae and Pseudomonadaceae were more abundant, whereas Clostridiaceae, Peptostreptococcaceae, Pasteurellaceae, Butyricicoccaceae, and Clostridia_unclassified were less abundant in patients with IgAN compared with HCs. (E) Relative abundance of the bacterial community in both groups at the genus level. Five genera, including Escherichia-Shigella and Pseudomonas, were more abundant, whereas 16 genera, including Lachnospira, Lachnospiraceae_UCG-001, and Fusicatenibacter, were less abundant in patients with IgAN compared with HCs. The box plot shows the prominent taxa that differ significantly in abundance between patients with IgAN and HCs. The horizontal bar within each box represents the median. The bottom and top of each box represent the 25th and 75th percentiles, respectively. The upper and lower whiskers extend to data no more than 1.5× the interquartile range from the upper and lower edges of the box, respectively, and black dots represent outliers beyond the whiskers. *P<0.05, **P<0.01, ***P<0.001.
Notably, we found that two taxonomic chains, Proteobacteria–Gammaproteobacteria–Enterobacteriales–Enterobacteriaceae–Escherichia-Shigella and Proteobacteria–Gammaproteobacteria–Pseudomonadales–Pseudomonadaceae–Pseudomonas, were significantly over-represented in gut microbiota of patients with IgAN. Genus Escherichia-Shigella (constituent ratio, 10.0% versus 1.0%) and Pseudomonas (0.3% versus 0.001%) showed an impressive increase in gut microbiota of patients with IgAN compared with that of HCs, which together contributed to the robust enrichment of the phylum Proteobacteria in patients with IgAN.
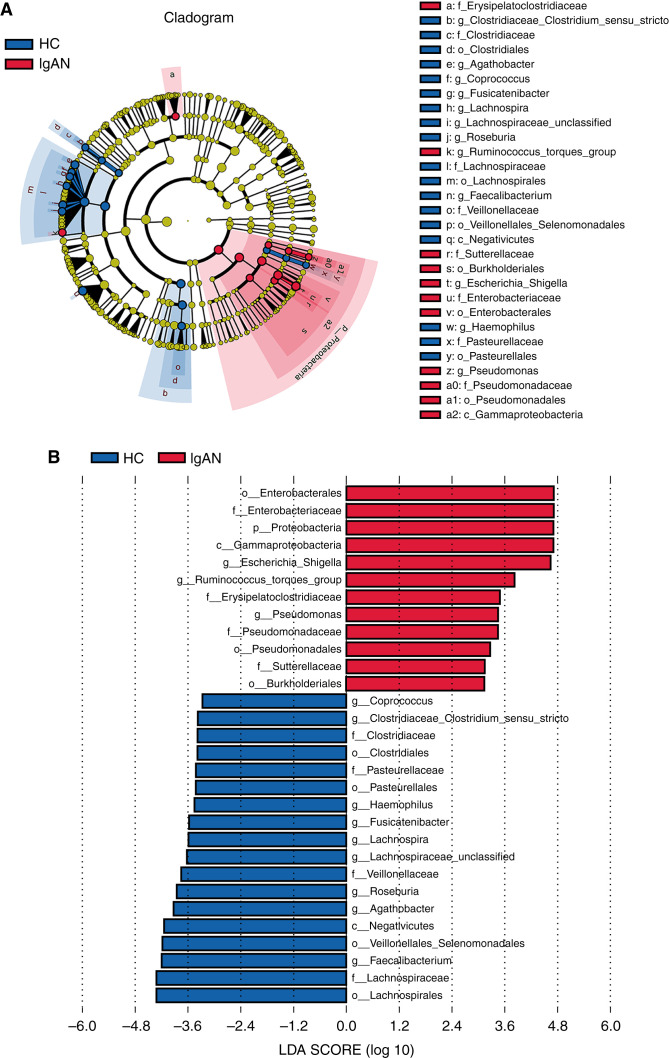
A supervised comparison of the phylogenetic distribution of gut microbiota between patients and HCs was conducted by applying the linear discriminant analysis (LDA) effect size. We selected important taxonomic differences with LDA scores greater than three. The phylogenetic profile of the specific bacterial taxa revealed that the taxonomic chains Proteobacteria–Gammaproteobacteria–Enterobacteriales–Enterobacteriaceae–Escherichia-Shigella and Proteobacteria–Gammaproteobacteria–Pseudomonadales–Pseudomonadaceae–Pseudomonas, contributed the most to the difference in gut microbiota between patients with IgAN and HCs (Figure 4, A and B; Supplemental Table 9).
Figure 4.
Crucial taxa that contribute to the difference between patients with IgAN and HCs on the basis of LDA effect size analysis (discovery set; 84 patients with IgAN, 84 HCs). (A) Cladogram shows crucial bacteria with an evolutionary relationship associated with IgAN on the basis of the LDA selection (LDA>3). Each circle represents a classification level from phylum to species, from the inner to outer circles. The size of each circle is proportional to relative abundance. Microbes with no significant difference in abundance are shown in brown. Taxa with an LDA value greater than three in the IgAN and HC groups are marked with red and green, respectively. (B) Bar chart shows crucial bacteria associated with IgAN on the basis of the LDA selection (LDA>3). Red bars represent taxa enriched in those with IgAN, and green bars represent taxa enriched in HCs. p, phylum; c, class; o, order; f, family; g, genus.
Spearman Correlation between Key Clinical Parameters and IgAN-Associated OTU
We analyzed the correlation between 29 differentially abundant OTUs and five clinical parameters to further verify the key OTUs were specific to IgAN severity. The abundance of Escherichia-Shigella (OTU1) was positively correlated with serum creatinine (Rho=0.329, P<0.001), proteinuria (Rho=0.427, P<0.001), and the severity of Oxford pathologic classification (M, mesangial hypercellularity, Rho=0.353, P<0.001; S, segmental glomerulosclerosis, Rho=0.329, P<0.001; T, tubular atrophy/interstitial fibrosis, Rho=0.345, P<0.001; C, Rho=0.304, P<0.001) and was negatively correlated with albumin (Rho=−0.336, P<0.001) and eGFR (Rho=−0.337, P<0.001). Similar findings were reproduced in Escherichia-Shigella (OTU1201; Figure 5, Supplemental Table 10).
Figure 5.
Oblique triangle heatmap presents the Spearman correlation between five key clinical parameters and 29 IgAN-associated OTUs (discovery set; 84 patients with IgAN, 84 HCs). The red lines show a positive correlation, and green lines show negative correlation. Solid lines, P≤0.01; dotted lines, P<0.05. ALB, albumin; C, crescents; E, endocapillary hypercellularity; M, mesangial hypercellularity; S, segmental glomerulosclerosis; S-Cre, serum creatinine; T, tubular atrophy/interstitial fibrosis.
Escherichia-Shigella (OTU1) were negatively correlated with Lachnospiraceae_unclassified (OTU441, Rho=−0.335, P<0.05), Lachnospira (OTU87, Rho=−0.407, P<0.001), Lachnospiraceae_UCG.001 (OTU186, Rho=−0.335, P< 0.001), and Fusicatenibacter (OTU26, Rho=−0.220, P=0.004) and were positively correlated with Pseudomonas (OTU191, Rho=0.394, P<0.001) and Escherichia-Shigella (OTU1201, Rho=0.582, P<0.001) (Figure 5; Supplemental Table 11).
Diagnostic Capacity of IgAN on the Basis of the Gut Microbial Markers
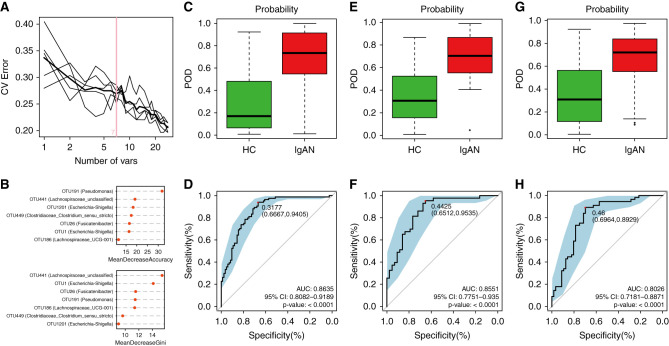
To explore the capacity of the gut microbiota to discriminate patients with IgAN from HCs, we constructed a random forest model on the basis of the IgAN-associated gut microbiota at the OTU level. Seven OTUs (Escherichia-Shigella, Pseudomonas, Fusicatenibacter, Lachnospiraceae_unclassified, Lachnospiraceae_UCG-001, and Clostridiaceae_Clostridium sensu stricto) were identified as the optimal bacterial markers of IgAN by a five-fold cross-validation of the random forest model (Figure 6A). Notably, OTU1 and OTU1201 are annotated as Escherichia-Shigella, indicating its maximal significance in the IgAN classifier (Figure 6B).
Figure 6.
Diagnostic efficiency of gut bacterial markers to distinguish patients with IgAN from HCs. (A) Seven microbial OTUs were selected as the optimal markers on the basis of the random forest model. (B) Escherichia-Shigella had the greatest contribution to the bacterial classifier, as shown by mean decrease accuracy (top) and mean decrease Gini (bottom) analyses. (C) The POD value was significantly increased in those with IgAN (n=84) versus HCs (n=84) in the discovery cohort. (D) The POD index achieved an AUC value of 0.8635 in the discovery cohort, as shown in ROC. (E) The POD value was significantly increased in those with IgAN (n=43) versus HCs (n=43) in the validation cohort. (F) The POD index achieved an AUC value of 0.8551 in the validation cohort, as shown in ROC. (G) The POD value was significantly increased in those with IgAN (n=56) versus HCs (n=56) in the independent validation cohort. (H) The POD index achieved an AUC value of 0.8026 in the independent validation cohort, as shown in ROC. POD, probability of disease.*P<0.05, **P<0.01, ***P<0.001.
In the discovery cohort, the POD of the IgAN classifier achieved an AUC under the ROC curve of 0.8635 (95% CI, 0.81 to 0.92; P<0.001; Figure 6, C and D). We then assessed the power of the classifier for discriminating patients with IgAN from HCs in the validation set, the AUC value for the POD reached 0.8551 (95% CI, 0.78 to 0.94; P<0.001; Figure 6, E and F). In the independent validation cohort (Supplemental Table 12), the bacterial classifier could also effectively distinguish patients with IgAN from HCs (AUC, 0.8026; 95% CI, 0.72 to 0.89; P<0.001; Figure 6, G and H). Together, these data suggest the bacterial classifier may have powerful diagnostic potential as a noninvasive tool in distinguishing patients with IgAN from HCs. However, the bacterial classifier may not efficiently discriminate IgAN from MN (AUC, 0.6183; 95% CI, 0.49 to 0.74; P=0.06; Supplemental Figure 2).
Microbial Function Alterations in Patients with IgAN
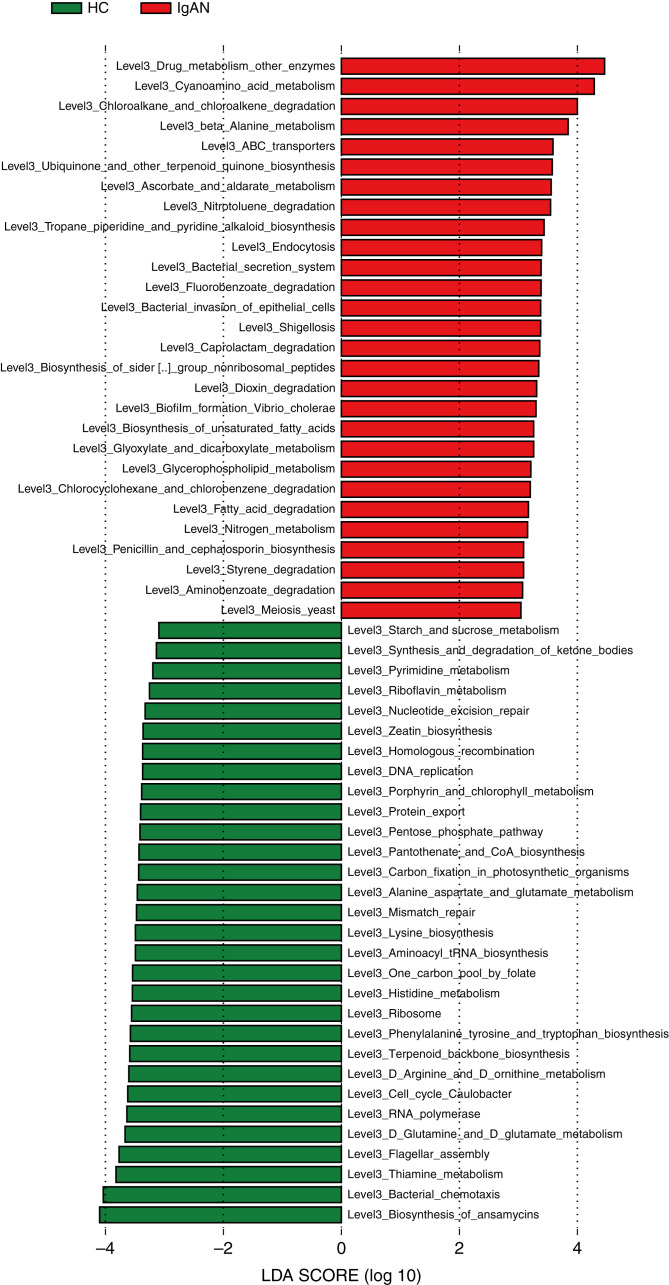
To explore the functional alterations in the gut microbiome in IgAN, we predicted the functional composition profiles on the basis of 16S rRNA sequencing data with PICRUSt2. We constructed the KEGG orthology and the KEGG pathway/module profile. On the basis of the LDA selection (LDA>3), 28 predicted microbial function pathways were significantly enriched in patients with IgAN. In particular, two kinds of Infectious_diseases_Bacterial pathways (shigellosis and invasion of epithelial cells) were enriched, which was closely related to the expansion of Escherichia-Shigella. Nine kinds of Xenobiotics_biodegradation_and_metabolism pathways were also overexpressed, mainly with respect to metabolism of hazardous chemical substances, including degradation of styrene, aminobenzoate, caprolactam, nitrotoluene, chlorocyclohexane, chlorobenzene, fluorobenzoate, and Drug_metabolism_other_enzymes. In addition, fatty acid degradation, biosynthesis of unsaturated fatty acids, and glycerophospholipid metabolism pathways were also overexpressed. Conversely, 30 predicted functions, mainly involved in normal biochemical processes, were remarkably decreased in patients with IgAN compared with HCs (all P<0.05; Figure 7, Supplemental Table 13).
Figure 7.
Alterations in microbial function in patients with IgAN according to PICRUSt2 analysis. On the basis of the LDA selection (LDA>3), 28 predicted microbial function pathways were significantly enriched, whereas 30 predicted functions, mainly involved in normal biochemical processes, were remarkably decreased in those with IgAN compared with HCs. Red bars represent metabolic pathways enriched in those with IgAN, and green bars represent metabolic pathways enriched in HCs.
Successful Treatment Reversed the Expansion of Genus Escherichia-Shigella
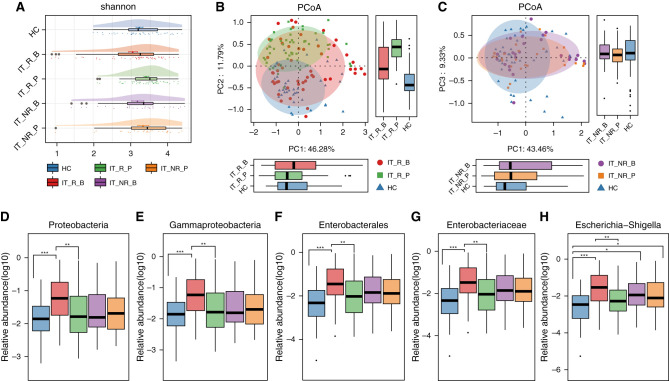
To further assess the association between gut microbiota and the response to immunosuppressive therapy, we analyzed alterations of gut microbiota in patients who achieved complete or partial clinical remission (responders, n=56) versus those who did not achieve complete or partial remission (nonresponders, n=21). At baseline, the parameters of gut microbiota were comparable between responders and nonresponders, except for a relatively higher abundance of genus Turicibacter in responders (Supplemental Table 14). Interestingly, α-diversity in responders significantly increased after treatment (Shannon index, 3.047±0.114 at baseline versus 3.397±0.066 post-treatment, P=0.05; Figure 8A, Supplemental Table 15), but remained unchanged in nonresponders (Shannon index, 3.170±0.158 at baseline versus 3.316±0.147 post-treatment, P=0.48).
Figure 8.
Immunosuppressive therapy successfully reversed the expansion of the genus Escherichia-Shigella and its associated taxonomic chain and increased bacterial diversity. (A) α-Diversity increased in patients who achieved clinical remission, but remained low in those who did not achieve clinical remission. The vertical bar within each box represents the median, and the left and right of each box represent the 25th and 75th percentiles, respectively. (B) β-Diversity changed in patients who achieved clinical remission, as revealed by principal coordinate analysis (PCoA). The ovals represent the different clustering tendencies among the three groups. The boxes on left and below represent the median and upper and lower quartile values of a set of samples on the horizontal and vertical coordinates, respectively. (C) β-Diversity remained unchanged in patients who did not achieve clinical remission, as revealed by PCoA. The ovals represent the same clustering tendencies between patients before and after treatment, and different clustering tendencies between patients with IgAN and HCs. The boxes on left and below represent the median and upper and lower quartile values of a set of samples on the horizontal and vertical coordinates, respectively. (D) Proteobacteria decreased in patients who achieved clinical remission, but remained unchanged in those who did not achieve clinical remission. (E) Gammaproteobacteria decreased in patients who achieved clinical remission, but remained unchanged in those who did not achieve clinical remission. (F) Enterobacterales decreased in patients who achieved clinical remission, but remained unchanged in those who did not achieve clinical remission. (G) Enterobacteriaceae decreased in patients who achieved clinical remission, but remained unchanged in those who did not achieve clinical remission. (H) Escherichia-Shigella decreased in patients who achieved clinical remission, but remained unchanged in those who did not achieve clinical remission. The horizontal bar within each box plot represents the median; the bottom and top of each box represent the 25th and 75th percentiles, respectively. The upper and lower whiskers extend to data no more than 1.5× the interquartile range from the upper and lower edges of the box, respectively, and black dots represent outliers beyond the whiskers. *P<0.05, **P<0.01, ***P<0.001. B, before treatment; IT, immunosuppressive therapy; NR, did not achieve clinical remission; PC, principal component; R, achieved clinical remission; P, after treatment.
In terms of β-diversity, responders experienced a striking alteration in the bacterial composition of gut microbiota (P=0.003; Figure 8B), whereas the microbiota of nonresponders remained unchanged (P=0.64; Figure 8C, Supplemental Table 16).
Notably, responders experienced a marked reduction in phylum Proteobacteria (0.155±0.032 at baseline versus 0.075±0.021 post-treatment, P=0.009), class Gammaproteobacteria (0.155±0.032 at baseline versus 0.076±0.021 post-treatment, P=0.001), order Enterobacterales (0.141±0.032 at baseline versus 0.063±0.020 post-treatment, P=0.001), family Enterobacteriaceae (0.141±0.032 at baseline versus 0.063±0.020 post-treatment, P=0.002), and genus Escherichia-Shigella (0.118±0.027 at baseline versus 0.033±0.010 post-treatment, P<0.001), which reached similar abundance to the HC group (respective P values for responders versus HCs for each category: P=0.69, P=0.65, P=0.19, P=0.19, and P=0.11, respectively; Figure 8, D–H).
Conversely, in nonresponders, there was a trend for the abundance of each of the above categories to increase post-treatment, although none of the increases was statistically significant: phylum Proteobacteria (0.101±0.034 at baseline versus 0.117±0.044 post-treatment, P=0.96), class Gammaproteobacteria (0.101±0.034 at baseline versus 0.117±0.044 post-treatment, P=0.99), order Enterobacterales (0.093±0.033 at baseline versus 0.109±0.043 post-treatment, P=0.98), family Enterobacteriaceae (0.093±0.034 at baseline versus 0.109±0.043 post-treatment, P=0.98), and genus Escherichia-Shigella (0.074±0.031 at baseline versus 0.097±0.039 post-treatment, P=0.88) (Figure 8, D–H).
In subgroup analyses by treatment received, similar patterns were observed in patients who received CS plus cyclophosphamide or CS plus MMF therapy (Supplemental Appendix 2).
We also compared the relative abundance of other genera that were differentially abundant in patients with IgAN who were treatment naïve before and after 6 months immunosuppressive therapy to further verify the unique alterations in the above taxonomic chain. No significant alterations in these genera were observed, except that the initially decreased Clostridiaceae_Clostridium sensu stricto had increased in both responders and nonresponders after treatment (Supplemental Figure 3, Supplemental Table 17).
Discussion
Gut dysbiosis is postulated to take part in the pathogenesis of IgAN through unknown mechanisms. However, the key bacterial taxa specific to IgAN have not been determined. Our findings, for the first time, establish a potential association between the expansion of genus Escherichia-Shigella in gut microbiota and IgAN onset and treatment response, which may provide a promising bacterial target for managing IgAN, and a new direction to elucidate the pathogenesis of IgAN.
We observed a distinct bacterial composition with a lower α-diversity in patients with IgAN who were treatment naive, which is consistent with four previous studies.13,15,17,19 Genera Escherichia-Shigella and Pseudomonas and their associated taxa had undergone the most remarkable expansion in the gut microbiota. Of note, Escherichia-Shigella contributed about 74% of the abundance to phylum Proteobacteria, far more than that of Pseudomonas (2%), implying the dominant expansion advantage of Escherichia-Shigella. Consistent with our finding, other studies also reported a higher abundance of genus Escherichia-Shigella in patients with IgAN13–16 and in patients with CKD.28,29 Furthermore, bacterial function prediction on the basis of the 16s rRNA sequence showed a striking enrichment of bacterial pathways involving shigellosis infection, inferring invasion of epithelial infection, which is in line with the expansion of Escherichia-Shigella in gut and implies an association between shigellosis infection and IgAN onset. In phylum Firmicutes, the abundance of genera Lachnospira, Lachnospiraceae_unclassified, Lachnospiraceae_ UCG-001, Roseburia, Agathobacter, and Fusicatenibacter, which belong to the family Lachnospiraceae, were statistically reduced in patients with IgAN. Of note, Lachnospiraceae_UCG-001, Lachnospiraceae_unclassified, and Fusicatenibacter were identified as members of the bacterial classifier for IgAN. However, the depletion of the above genera did not result in the reduction of phylum Firmicutes and even the family Lachnospiraceae, indicating the extent of their variation may be relatively less than that of Escherichia-Shigella. Even so, their reduction could be the cause or effect of expansion of Escherichia-Shigella in gut microbiota, which may have pathologic significance in the pathogenesis of IgAN and need further exploration.
The above findings were further strengthened by prospective evaluation of the gut microbiota in patients who did and did not achieve clinical remission after 6 months of immunosuppressive therapy. In contrast to nonresponders, in whom the gut microbiota remained largely unchanged post-treatment, responders experienced a striking depletion of genus Escherichia-Shigella and its associated chain post-treatment. These findings are supported by previous studies that attempted to treat IgAN by manipulation of gut microbiota. FMT from patients with IgAN to IgAN model mice promoted an expansion of phylum Proteobacteria along with a more severe renal phenotype,11 whereas a Chinese classic herbal prescription, Zhen Wu Tang, decreased the abundance of phylum Proteobacteria in gut microbiota and was accompanied by an alleviation of IgAN when administered to mice.30 In our previous study, we observed that intensive fresh FMT administered to two patients with refractory IgAN reduced proteinuria, and one patient whose gut was enriched with genus Escherichia-Shigella also experienced in a marked depletion of Escherichia-Shigella (6.0% pre-FMT versus 0.3% post–intensive treatment course) and phylum Proteobacteria (pre-FMT 20.0% versus 3.0% post–intensive treatment course).12 Taken together, our findings suggest the expansion of genus Escherichia-Shigella in gut microbiota may play a key role in the onset and response to treatment of IgAN, and warrants further verification.
On the other hand, the established bacterial classifier did not effectively distinguish patients with IgAN from those with MN, suggesting the alteration in gut microbiota of patients with IgAN or MN compared with HCs may share similar characteristics. As we have reported previously, no differences were observed in the α- and β-diversity of gut microbiota between patients with IgAN and those with MN, and phylum Proteobacteria and genus Escherichia-Shigella were also markedly abundant in patients with MN compared with HCs.14 IgAN and MN are typically recognized as autoimmune-mediated GN and may share similar etiologies or outcomes, including gut dysbiosis. Nonetheless, the taxa at the species level that are specific to IgAN or MN have not been identified and may not be identical. Therefore, the association between expansion of Escherichia-Shigella in IgAN and its distinction from MN needs further investigation.
Mucosal immune dysfunction plays a pivotal role in the pathogenesis of IgAN,31 but the mechanism involved is still unclear. Compelling evidence demonstrated the relationship between disturbed gut mucosal immunity and gut dysbiosis,32 which is typically characterized by expansion of the phylum Proteobacteria.33 Our study showed, for the first time, that a high abundance of Escherichia-Shigella may underly the expansion of phylum Proteobacteria in the gut microbiota of patients with IgAN. Previous studies linked exposure of the gut mucous membrane to outer membrane vesicles of pathogenic Escherichia coli to mitochondrial apoptosis in macrophages and activation of the Nod-like receptors protein 3 (NLRP3) inflammasome34 via autophosphorylation of double-stranded RNA-dependent protein kinase.35 This may be a mechanism by which Escherichia-Shigella excessively activates mucosal immunity. Intraperitoneal administration of E. coli was capable of inducing glomerular deposition of IgA and C3 in mice.36 Recently, the link between overgrowth of Escherichia-Shigella and activation of the intestinal NLRP3 inflammasome was put forward as a mechanism for acute pancreatitis in mouse models.37 Conversely, a decline of Escherichia-Shigella in gut occurred simultaneously with the reduction of NLRP3 in an acetate-treated bronchopulmonary dysplasia model.38 An increased proportion of Escherichia-Shigella in respiratory microbiota has also been associated with the activated NLRP3 inflammasome in high ammonia–induced lung tissue inflammation.39
In support of our findings that successful immunosuppressive therapy was associated with a significant decrease in the abundance of Escherichia-Shigella, several therapeutic approaches40–42 that block the expression of proinflammatory cytokines have also been linked to reduced abundance of Escherichia-Shigella in gut microbiota in settings of immune-mediated diseases. Therefore, we hypothesize the expansion of proinflammatory Escherichia-Shigella triggers a local or systemic mucosal immune response and results in the production of nephrotoxic gd-IgA1. However, the specific molecular mechanism by which Escherichia-Shigella influences the onset, progression, and response to treatment of IgAN awaits exploration. Moreover, the reduction of Lachnospiraceae may result in low production of the short-chain fatty acids17 that are implicated in autoimmune disorders via the aryl hydrocarbon receptor43 and may be associated with the pathogenesis of IgAN. In addition, the PICRUSt2 analysis showed the tyrosine metabolism pathway was over-represented in the gut microbiota of patients with IgAN, and spleen tyrosine kinase was demonstrated to be associated with IgAN by orchestrating inflammatory responses, cell proliferation in human mesangial cells, and tubular damage in IgAN.44 Thus, gut bacterial taxa associated with the metabolism of tyrosine are worthy of further investigation.
A poor diet may contribute to the expansion of Escherichia-Shigella in gut microbiota.45 Gluten is widely reported to be implicated in IgAN onset via a gliadin-CD89 interaction forming the IgA1-sCD89 complex,46 whereas Proteobacteria in gut microbiota, especially E. coli, may facilitate the immunopathologic effect of gluten.47 A diet low plant-based food and high in red and processed meat contributed to an enrichment of Escherichia-Shigella and depletion of Faecalibacterium in gut microbiota, which was reversed by a diversified diet.45 Therefore, a balanced diet may help prevent Escherichia-Shigella proliferation and be a potential prophylactic measure for individuals with a genetic predisposition to IgAN. In addition, hyperlipidemia is one of the most common comorbidities that increases the risk of progression to ESKD43 in patients with IgAN, whereas the reduction of cholesterol was reportedly accompanied by a marked decrease of Escherichia-Shigella.48 Essential fatty acid deficiency was also a hallmark of patients with IgAN,49 which may be attributed to the alteration of gut microbiota, with evidence of strengthened fatty acid degradation, unsaturated fatty acid biosynthesis, or glycerophospholipid metabolism. Recently, Shigella sp. peristaltic contraction-inhibiting bacterium was identified to be causally associated with intractable functional constipation via the production of docosapentaenoic acid,50 linking Escherichia-Shigella to unsaturated fatty acid biosynthesis and intractable disease.
Exposure to environmental toxins may also perpetuate the expansion of Escherichia-Shigella in gut. The addition of dishwashing detergent and cinnamaldehyde to the fecal samples of healthy individuals increased the abundance of Escherichia-Shigella, but also decreased the concentration of beneficial short-chain fatty acids.51 In our study, eight chemical product biodegradation pathways were significantly enriched in patients with IgAN compared with HCs, implying a high-level exposure of these hazardous substances, most of which are monomers of synthetic rubber and plastics, particularly microplastics. Of note, Escherichia-Shigella has been shown to be active in the biodegradation of microplastics, in accordance with its expansion in our study.52 Avoiding excessive exposure of these substances may help prevent the expansion of Escherichia-Shigella and decrease the risk of occurrence of IgAN.
Although we identified a likely role of Escherichia-Shigella expansion in IgAN, several limitations in our study should be addressed in future. The genus Escherichia-Shigella is actually made up of two genera, Escherichia and Shigella, mainly composed of opportunistic pathogens such as E. coli and Shigella. These genera are combined because they have 16S gene sequences that are indistinguishable, preventing species-level identification using 16S RNA gene sequencing methods. Metagenomics sequencing will need to be implemented to explore which species are most associated with IgAN. Our study was not designed to demonstrate a causal effect of gut microbiota on IgAN or response to treatment. Further interventional studies using animal models, or in humans, are required to understand the role of Escherichia-Shigella expansion in IgAN. The established bacterial classifier may not be readily applicable to clinical practice, because the specificity is not as high as the sensitivity, despite the strikingly good ROC curve areas. The classifier does not appear to effectively distinguish patients with IgAN from those with MN, and the association between expansion of Escherichia-Shigella with MN requires further investigation. The sample size of the follow-up subgroup was relatively small, thus observations made in responders and nonresponders should be interpreted cautiously. The HCs in the model-establishing cohort were obtained from several sources to ensure that patients and HCs were similar in terms of age, BMI, and sex. This may have led to other imbalances in other unmeasured covariates. Finally, our study was conducted in a Chinese population and should be verified in non-Asian population to assess the validity of our findings in other ethnicities.
This study demonstrated that gut dysbiosis, characterized by a striking expansion of Escherichia-Shigella, was a salient feature of patients with IgAN, and that the imbalance was reversed by successful immunosuppressive therapy. We discovered seven OTUs that were the optimal microbial classifiers for distinguishing patients with IgAN from HCs, among which Escherichia-Shigella contributed the most. Further studies are warranted to investigate the potential contribution of Escherichia-Shigella in IgAN pathogenesis.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by National Natural Science Foundation of China grants 82170722 and 81870470, Key Research and Development Plan of Shaanxi Province grant 2017ZDXM-SF-045, and Xijing Hospital, Fourth Military Medical University discipline boosting program grant XJZT18Z15.
Supplementary Material
Acknowledgments
We thank all study participants for their cooperation with our research, Prof. Zhigang Ren in The First Affiliated Hospital of Zhengzhou University for generously providing the 16S rRNA sequence data of 41 HCs, Prof. Zhanzheng Zhao in the department of nephropathy of The First Affiliated Hospital of Zhengzhou University for providing the independent cross-region validation cohort, Prof. Huaning Wang and Zhengwu Peng in the department of psychiatry of Xijing Hospital of Fourth Military Medical University for help in recruitment of HCs, and Shanghai MobioBiomedical Technology Co., Ltd. for 16S rRNA sequence and bioinformatics analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
M. Bai and X. Ning were responsible for methodology; M. Bai, S. Sun, and J. Zhao conceptualized the study; R. Dong was responsible for resources; X. Ning was responsible for data curation; X. Ning, S. Sun, Y. Wang, Z. Yu, and J. Zhao reviewed and edited the manuscript; Y. Qin was responsible for methodology; S. Sun was responsible for project administration; Y. Wang and Y. Zhang were responsible for investigation; Y. Zhang was responsible for formal analysis; and J. Zhao wrote the original draft.
Data Sharing Statement
The datasets supporting the conclusions of this article are included within the article and its additional files. The raw data for 16S rRNA gene sequences are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) with the accession numbers PRJNA574226, PRJNA801894, and PRJNA562327. Requests for the metadata from this study can be submitted via email to sunshiren@medmail.com.cn. A proposal is also required for approval.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022020189/-/DCSupplemental.
Supplemental Appendix 1. Inclusion and exclusion criteria of participants and therapeutic strategy.
Supplemental Appendix 2. Alteration of gut microbiota in patients with IgAN before and after receiving corticosteroids (CS) combined with cyclophosphamide (CTX) or mycophenolate mofetil (MMF).
Supplemental Table 1. Database of demographic characteristics and clinical parameters of the subjects in the model establishment cohort. (excel file)
Supplemental Table 2. Clinical characteristics of participants in the discovery and validation cohort.
Supplemental Table 3. Alpha diversity of gut microbiota in patients with IgAN and healthy controls.
Supplemental Table 4. The average abundance of gut microbiota at the phylum level.
Supplemental Table 5. The average abundance of gut microbiota at the class level.
Supplemental Table 6. The average abundance of gut microbiota at the order level.
Supplemental Table 7. The average abundance of gut microbiota at the family level.
Supplemental Table 8. The average abundance of gut microbiota at the genus level.
Supplemental Table 9. The crucial taxa contribute to the difference between patients with IgAN and healthy controls based on linear discriminant analysis effect size (LEfSe) analysis.
Supplemental Table 10. The spearman correlation between key clinical parameters and IgAN-associated OTUs.
Supplemental Table 11. Spearman correlation among the differentially abundant OTUs in patients with IgAN compared with healthy controls.
Supplemental Table 12. Database of demographic characteristics and clinical parameters of subjects of the cross-region validation cohort.
Supplemental Table 13. Microbial functions alteration in patients with IgAN based on PICRUSt2 analysis.
Supplemental Table 14. The parameters of baseline gut microbiota were comparable between patients who did or did not achieve clinical remission.
Supplemental Table 15. Alteration of alpha diversity of gut microbiota before and after 6 months of immunosuppressive therapy.
Supplemental Table 16. Alteration of beta diversity of gut microbiota before and after 6 months of immunosuppressive therapy.
Supplemental Table 17. Relative abundance of genera, which were differentially abundant in treatment-naïve patients with IgAN, before and after 6 months of immunosuppressive therapy.
Supplemental Figure 1. Phylogenetic profiles of the gut microbiome between IgAN and healthy controls (discovery set; IgAN=84, HC=84).
Supplemental Figure 2. The diagnostic power of the bacterial classifier for discriminate IgAN against MN (n=40).
Supplemental Figure 3. Relative abundance of genera, which were differentially abundant in treatment-naïve patients with IgAN (discovery set; IgAN=84, HC=84), before and after 6 months immunosuppressive therapy.
References
- 1.Yang T, Richards EM, Pepine CJ, Raizada MK: The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat Rev Nephrol 14: 442–456, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li LS, Liu ZH: Epidemiologic data of renal diseases from a single unit in China: Analysis based on 13,519 renal biopsies. Kidney Int 66: 920–923, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Rizk DV, Saha MK, Hall S, Novak L, Brown R, Huang ZQ, et al. : Glomerular immunodeposits of patients with IgA nephropathy are enriched for IgG autoantibodies specific for galactose-deficient IgA1. J Am Soc Nephrol 30: 2017–2026, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki H, Kiryluk K, Novak J, Moldoveanu Z, Herr AB, Renfrow MB, et al. : The pathophysiology of IgA nephropathy. J Am Soc Nephrol 22: 1795–1803, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sallustio F, Curci C, Chaoul N, Fontò G, Lauriero G, Picerno A, et al. : High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol Dial Transplant 36: 452–464, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy DD, Kujawa J, Wilson C, Papandile A, Poreci U, Porfilio EA, et al. : Mice overexpressing BAFF develop a commensal flora-dependent, IgA-associated nephropathy. J Clin Invest 121: 3991–4002, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takeshita M, Tanaka A, Nakamura T, Sato E, Node K: Effect of lubiprostone on urinary protein excretion: A report of two IgA nephropathy patients with chronic constipation. Intern Med 58: 3255–3259, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fellström BC, Barratt J, Cook H, Coppo R, Feehally J, de Fijter JW, et al. ; NEFIGAN Trial Investigators : Targeted-release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): A double-blind, randomised, placebo-controlled phase 2b trial. Lancet 389: 2117–2127, 2017 [DOI] [PubMed] [Google Scholar]
- 9.Di Leo V, Gleeson PJ, Sallustio F, Bounaix C, Da Silva J, Loreto G, et al. : Rifaximin as a potential treatment for IgA nephropathy in a humanized mice model. J Pers Med 11: 309, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chemouny JM, Gleeson PJ, Abbad L, Lauriero G, Boedec E, Le Roux K, et al. : Modulation of the microbiota by oral antibiotics treats immunoglobulin A nephropathy in humanized mice. Nephrol Dial Transplant 34: 1135–1144, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Lauriero G, Abbad L, Vacca M, Celano G, Chemouny JM, Calasso M, et al. : Fecal microbiota transplantation modulates renal phenotype in the humanized mouse model of IgA nephropathy. Front Immunol 12: 694787, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Bai M, Yang X, Wang Y, Li R, Sun S: Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren Fail 43: 928–933, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Angelis M, Montemurno E, Piccolo M, Vannini L, Lauriero G, Maranzano V, et al. : Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One 9: e99006, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong R, Bai M, Zhao J, Wang D, Ning X, Sun S: A comparative study of the gut microbiota associated with immunoglobulin a nephropathy and membranous nephropathy. Front Cell Infect Microbiol 10: 557368, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Du J, Xie Y, Huang Q, Xiao Y, Chen J, et al. : Fecal microbiota characteristics of Chinese patients with primary IgA nephropathy: A cross-sectional study. BMC Nephrol 21: 97, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong Z, Tan J, Tan L, Tang Y, Qiu Z, Pei G, et al. : Modifications of gut microbiota are associated with the severity of IgA nephropathy in the Chinese population. Int Immunopharmacol 89: 107085, 2020 [DOI] [PubMed] [Google Scholar]
- 17.Chai L, Luo Q, Cai K, Wang K, Xu B: Reduced fecal short-chain fatty acids levels and the relationship with gut microbiota in IgA nephropathy. BMC Nephrol 22: 209, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sugurmar ANK, Mohd R, Shah SA, Neoh HM, Cader RA: Gut microbiota in immunoglobulin A nephropathy: A Malaysian perspective. BMC Nephrol 22: 145, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu H, Tang D, Zheng F, Li S, Zhang X, Yin L, et al. : Identification of a novel interplay between intestinal bacteria and metabolites in Chinese patients with IgA nephropathy via integrated microbiome and metabolome approaches. Ann Transl Med 9: 32, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He JW, Zhou XJ, Li YF, Wang YN, Liu LJ, Shi SF, et al. : Associations of genetic variants contributing to gut microbiota composition in immunoglobin A nephropathy. mSystems 6: e00819–e00820, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Natale P, Palmer SC, Ruospo M, Saglimbene VM, Craig JC, Vecchio M, et al. : Immunosuppressive agents for treating IgA nephropathy. Cochrane Database Syst Rev 3: CD003965, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck L, Bomback AS, Choi MJ, Holzman LB, Langford C, Mariani LH, et al. : KDOQI US commentary on the 2012 KDIGO clinical practice guideline for glomerulonephritis. Am J Kidney Dis 62: 403–441, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Ren Z, Fan Y, Li A, Shen Q, Wu J, Ren L, et al. : Alterations of the human gut microbiome in chronic kidney disease. Adv Sci (Weinh) 7: 2001936, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. : Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75: 7537–7541, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willis AD: Rarefaction, alpha diversity, and statistics. Front Microbiol 10: 2407, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori AS, Isbell F, Seidl R: β-Diversity, community assembly, and ecosystem functioning. Trends Ecol Evol 33: 549–564, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanehisa M, Furumichi M, Tanabe M, Sato Y, Morishima K: KEGG: New perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res 45[D1]: D353–D361, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao J, Ning X, Liu B, Dong R, Bai M, Sun S: Specific alterations in gut microbiota in patients with chronic kidney disease: An updated systematic review. Ren Fail 43: 102–112, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lobel L, Cao YG, Fenn K, Glickman JN, Garrett WS: Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 369: 1518–1524, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Cao Y, Lu R, Li H, Pang Y, Fu H, et al. : Integrated fecal microbiome and serum metabolomics analysis reveals abnormal changes in rats with immunoglobulin A nephropathy and the intervention effect of Zhen Wu Tang. Front Pharmacol 11: 606689, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gesualdo L, Di Leo V, Coppo R: The mucosal immune system and IgA nephropathy. Semin Immunopathol 43: 657–668, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He JW, Zhou XJ, Lv JC, Zhang H: Perspectives on how mucosal immune responses, infections and gut microbiome shape IgA nephropathy and future therapies. Theranostics 10: 11462–11478, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin NR, Whon TW, Bae JW: Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33: 496–503, 2015 [DOI] [PubMed] [Google Scholar]
- 34.Deo P, Chow SH, Han ML, Speir M, Huang C, Schittenhelm RB, et al. : Mitochondrial dysfunction caused by outer membrane vesicles from Gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat Microbiol 5: 1418–1427, 2020 [DOI] [PubMed] [Google Scholar]
- 35.Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundbäck P, et al. : Novel role of PKR in inflammasome activation and HMGB1 release. Nature 488: 670–674, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endo Y, Kanbayashi H, Hara M: Experimental immunoglobulin A nephropathy induced by gram-negative bacteria. Nephron 65: 196–205, 1993 [DOI] [PubMed] [Google Scholar]
- 37.Li X, He C, Li N, Ding L, Chen H, Wan J, et al. : The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes 11: 1774–1789, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Q, Ran X, He Y, Ai Q, Shi Y: Acetate downregulates the activation of NLRP3 inflammasomes and attenuates lung injury in neonatal mice with bronchopulmonary dysplasia. Front Pediatr 8: 595157, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu QX, Zhou Y, Li XM, Ma DD, Xing S, Feng JH, et al. : Ammonia induce lung tissue injury in broilers by activating NLRP3 inflammasome via Escherichia/Shigella. Poult Sci 99: 3402–3410, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu B, Piao X, Niu W, Zhang Q, Ma C, Wu T, et al. : Kuijieyuan Decoction improved intestinal barrier injury of ulcerative colitis by affecting TLR4-dependent PI3K/AKT/NF-κB oxidative and inflammatory signaling and gut microbiota. Front Pharmacol 11: 1036, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng L, Gao X, Nie L, Xie J, Dai T, Shi C, et al. : Astragalin attenuates dextran sulfate sodium (DSS)-induced acute experimental colitis by alleviating gut microbiota dysbiosis and inhibiting NF-κB activation in mice. Front Pharmacol 11: 2058, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu J, Chen N, Wu Z, Song Y, Zhang Y, Wu N, et al. : 5-Aminosalicylic acid alters the gut bacterial microbiota in patients with ulcerative colitis. Front Pharmacol 9: 1274, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosser EC, Piper CJM, Matei DE, Blair PA, Rendeiro AF, Orford M, et al. : Microbiota-derived metabolites suppress arthritis by amplifying aryl-hydrocarbon receptor activation in regulatory B cells. Cell Metab 31: 837–851.e10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yiu WH, Chan KW, Chan LYY, Leung JCK, Lai KN, Tang SCW: Spleen tyrosine kinase inhibition ameliorates tubular inflammation in IgA nephropathy. Front Physiol 12: 650888, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z, Taylor L, Shommu N, Ghosh S, Reimer R, Panaccione R, et al. : A diversified dietary pattern is associated with a balanced gut microbial composition of faecalibacterium and Escherichia/Shigella in patients with Crohn’s disease in remission. J Crohn’s Colitis 14: 1547–1557, 2020 [DOI] [PubMed] [Google Scholar]
- 46.Papista C, Lechner S, Ben Mkaddem S, LeStang MB, Abbad L, Bex-Coudrat J, et al. : Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int 88: 276–285, 2015 [DOI] [PubMed] [Google Scholar]
- 47.Galipeau HJ, McCarville JL, Huebener S, Litwin O, Meisel M, Jabri B, et al. : Intestinal microbiota modulates gluten-induced immunopathology in humanized mice. Am J Pathol 185: 2969–2982, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pi X, Teng W, Fei D, Zhao G, Liu W: Effects of live combined Bacillus subtilis and Enterococcus faecium on gut microbiota composition in C57BL/6 mice and in humans. Front Cell Infect Microbiol 12: 821662, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holman RT, Johnson SB, Bibus D, Spencer DC, Donadio JV Jr: Essential fatty acid deficiency profiles in idiopathic immunoglobulin A nephropathy. Am J Kidney Dis 23: 648–654, 1994 [DOI] [PubMed] [Google Scholar]
- 50.Chen X, Qiu TT, Wang Y, Xu LY, Sun J, Jiang ZH, et al. : A Shigella species variant is causally linked to intractable functional constipation. J Clin Invest 132: e150097, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gerasimidis K, Bryden K, Chen X, Papachristou E, Verney A, Roig M, et al. : The impact of food additives, artificial sweeteners and domestic hygiene products on the human gut microbiome and its fibre fermentation capacity. Eur J Nutr 59: 3213–3230, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie H, Chen J, Feng L, He L, Zhou C, Hong P, et al. : Chemotaxis-selective colonization of mangrove rhizosphere microbes on nine different microplastics. Sci Total Environ 752: 142223, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.