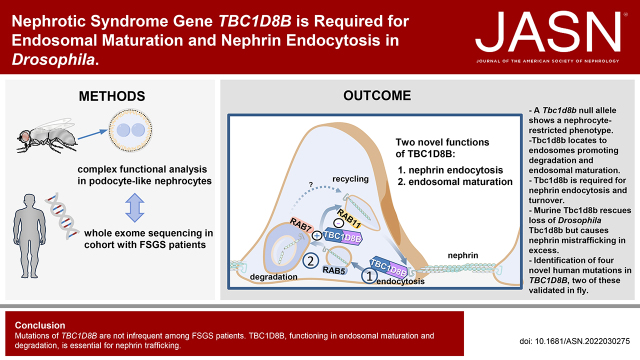
Significance Statement
Variants in TBC1D8B cause isolated nephrotic syndrome. TBC1D8B protein interacts with the slit diaphragm protein nephrin, but the pathogenesis remains unclear. We used Drosophila to elucidate the functional role of the recently discovered disease-causing gene. A null allele of Tbc1d8b in Drosophila exhibits a nephrocyte-restricted phenotype similar to patient presentation. Tbc1d8b protein localizes to mature early and late endosomes and promotes endosomal maturation and degradation, and is further required for nephrin transport. Expression of the murine ortholog rescues loss-of-function of the Drosophila TBC1D8B, which indicates evolutionary conservation. Discovery of two novel variants in TBC1D8B in a cohort of 363 patients with FSGS and functional validation in Drosophila suggest that TBC1D8B variants significantly underlie hereditary FSGS.
Keywords: podocyte, nephrotic syndrome, genetic kidney disease, endocytosis, focal segmental glomerulosclerosis, glomerular filtration barrier, human genetics, nephrin, Drosophila, nephrocyte
Visual Abstract
Abstract
Background
Variants in TBC1D8B cause nephrotic syndrome. TBC1D8B is a GTPase-activating protein for Rab11 (RAB11-GAP) that interacts with nephrin, but how it controls nephrin trafficking or other podocyte functions remains unclear.
Methods
We generated a stable deletion in Tbc1d8b and used microhomology-mediated end-joining for genome editing. Ex vivo functional assays utilized slit diaphragms in podocyte-like Drosophila nephrocytes. Manipulation of endocytic regulators and transgenesis of murine Tbc1d8b provided a comprehensive functional analysis of Tbc1d8b.
Results
A null allele of Drosophila TBC1D8B exhibited a nephrocyte-restricted phenotype of nephrin mislocalization, similar to patients with isolated nephrotic syndrome who have variants in the gene. The protein was required for rapid nephrin turnover in nephrocytes and for endocytosis of nephrin induced by excessive Rab5 activity. The protein expressed from the Tbc1d8b locus bearing the edited tag predominantly localized to mature early and late endosomes. Tbc1d8b was required for endocytic cargo processing and degradation. Silencing Hrs, a regulator of endosomal maturation, phenocopied loss of Tbc1d8b. Low-level expression of murine TBC1D8B rescued loss of the Drosophila gene, indicating evolutionary conservation. Excessive murine TBC1D8B selectively disturbed nephrin dynamics. Finally, we discovered four novel TBC1D8B variants within a cohort of 363 patients with FSGS and validated a functional effect of two variants in Drosophila, suggesting a personalized platform for TBC1D8B-associated FSGS.
Conclusions
Variants in TBC1D8B are not infrequent among patients with FSGS. TBC1D8B, functioning in endosomal maturation and degradation, is essential for nephrin trafficking.
Filtration of blood plasma across the glomerular filtration barrier is the basis of renal function. The filtrate is generated by passing through endothelial pores, the glomerular basement membrane and the slit diaphragm formed by podocytes. Dysfunction of the filtration barrier manifests with severe proteinuria and protracted disturbance entails loss of renal function. There is increasing recognition that genetic alterations underlie CKD. Recent studies indicate that a Mendelian disorder underlies approximately 10% of CKD with unexplained etiology.1–4 Variants in about 60 different genes have been identified as monogenic causes of filter dysfunction.5,6 We and others recently described variants in the gene TBC1 Domain Family Member 8B (TBC1D8B) as a monogenic nephrotic syndrome gene.7,8 TBC1D8B, a member of the TBC protein family,9 was previously uncharacterized. We identified a functional role as a GTPase-activating protein (GAP) for the Rab GTPase RAB11 that directs endocytic recycling and physical interaction with nephrin.7 Importantly, discovery of variants in TBC1D8B and the endosomal regulator GAPVD1 as monogenic causes of nephrotic syndrome offered the first support in human for a role of endocytosis for the glomerular filtration barrier.7,10 Such a role had been suggested by previous studies.11–15 Surprisingly, disease manifestations of affected patients were limited to the kidney, although endocytosis is a fundamental cellular function that occurs incessantly in all cells. Endocytosis originates at the cell membrane and endocytic vesicles fuse to form the early endosome that undergoes a complex maturation process while sorting the internalized cargo either for degradation via late endosomes or recycling back to the plasma membrane in recycling endosomes.16 Endocytosis is orchestrated by Rab proteins that facilitate transport and compartmentalization.17
In this study, we used the Drosophila model to explore the function of TBC1D8B. Generating a stable genetic deletion, we observed a phenotype restricted to nephrocytes, an established podocyte model,18,19 reflecting the phenotype of patients with TBC1D8B variants. We discovered a broader role for endocytosis concerning endosomal maturation and showed that Tbc1d8b is required for nephrin endocytosis. Finally, we identified novel TBC1D8B variants in an FSGS cohort and used the nephrocyte as a personalized platform for functional assessment of patient-derived variants.
Methods
Fly Strains and Husbandry
Flies were raised on standard food at 25°C unless indicated otherwise. We utilized the UAS/GAL4 system for overexpression and transgenic RNAi studies (RNAi crosses grown at 31°C). The term nephrocyte refers to the subtype of garland cell nephrocytes throughout the manuscript. The following stocks were obtained from the Bloomington Drosophila Stock Center (BDSC, Bloomington, IN): vas-Cas9 (#66554), UAS-Cas9.P2 (#58985), UAS-YFP-Rab5Q88L (constitutively active, #9774), UAS-Hrs-RNAi (#33900), UAS-FYVE-GFP (#42712), UAS-Tbc1d8b-RNAi (#32929), UAS-Rab5-RNAi (#34832), UAS-Rab7-RNAi (#27051), UAS-Rab11-RNAi (#42709), deficiency spanning the Tbc1d8b locus (#24923), and UAS-lacZ (#1776). GAL80ts (BDSC #7019) was used to modify GAL4-dependent expression. To direct expression in nephrocytes, prospero-GAL418 and Dorothy-GAL4 (#6903; BDSC) were used. UAS-EGFP-RNAi (#41553; BDSC) or wild type (yw1118) were crossed to GAL4-drivers for control conditions. Sns-GFP has been described elsewhere.20 UAS-LAMP1-GFP was a gift from Helmut Krämer (UT Southwestern Medical Center, Dallas, TX).
To generate various mTbc1d8b-expressing transgenic Drosophila, we transferred mouse Tbc1d8b full-length cDNA7 (GenBANK BC147581.1) as wild type, or the respective variant (details for DNA cloning see below), into the pUASg-attB vector (from K. Basler, University of Zurich, Zurich, Switzerland). The plasmids were injected into animals expressing phiC31 integrase under control of a vas promoter, with an attP landing site (51C1 on chromosome 2, BDSC #24482) by BestGene (Chino Hills, CA) or the Fly Facility of the University of Cambridge (Cambridge, UK). Flies expressing the human RAB11B were injected in an analogous manner (BDSC #24749, attP at 86F on chromosome 3). The CRISPR gRNA B construct targeting Tbc1d8b (target sequences TTTGTCCTGCAGAAGAGACG and GTGGGCGCCCAAATCGATCA) was generated by introducing two gRNAs via PCR and Gibson assembly (NEBuilder HiFi DNA assembly, New England Biolabs, Frankfurt, Germany) into the pCFD5_w plasmid (Addgene #112645, Watertown, MA), and inserted at chromosome 2 (BDSC #24482). A second tandem gRNA A was inserted into the X chromosome using pCFD5_w (gRNA target sequences CTACTACAAGGATAAAGTGG and CGTGGCCATGGGGAATCACG, BDSC #24480). For microhomology-mediated end-joining (MMEJ)–mediated genome editing, nos-Cas9 flies (BDSC #54591) were coinjected with a repair template (pDONR221 backbone and a tandem gRNA plasmid (pCFD4, locus specific gRNA target TATGATTATTCAGTAATTGG and generic gRNA GTACTGATCTTGCGTTAATG). The MMEJ repair template concurrently introduced red fluorescence in the eye to allow selection of flies for successful genome editing. An Hsp27 terminator was introduced to stabilize expression from the edited locus. The desired genomic insert in the template was placed between homologies of 20 bp, corresponding to the sequences flanking the cutting site at the Tbc1d8b locus. The entire cassette was positioned between two generic gRNA target sites to release linearized double-strand DNA for efficient MMEJ. This template was coinjected with a tandem gRNA plasmid (pCFD421) in nos-Cas9 animals that express Cas9 within the germline. The gRNA plasmid supplied both the generic gRNA and the gRNA directed against the Tbc1d8b locus. Flies were selected for successful editing on the basis of red fluorescence in the eye. The selection cassette was removed by crossing to Cre-expressing flies (BDSC #1501), leaving only tag and terminator at the edited locus.
Primer sequences are listed in Supplemental Table 1. HA tags are present in all mTbc1d8b transgenes. New lines generated for this study are mTbc1d8b (wild type), mTbc1d8b-T779S, mTbc1d8b-G39R, mTbc1d8b-L191S, mTbc1d8b-W815*, mTbc1d8b-R764C, mTbc1d8b-R537K, UAS-hRAB11B, Tbc1d8b-gRNA B, Tbc1d8b-gRNA A on X, Tbc1d8b-HA (MMEJ–mediated genome edit), and Tbc1d8bΔ1 (null allele). Genetic combinations were achieved by standard crosses.
Immunofluorescence Studies using Drosophila Tissue
For immunofluorescence, nephrocytes were dissected, followed by fixation for 20 minutes in PBS containing 4% paraformaldehyde. Staining with primary and secondary antibodies were performed according to the standard procedures. We used the following primary antibodies: rabbit anti-nephrin (Sns)22 (1:100, gift from S. Abmayr), or guinea pig anti-nephrin (Sns) (1:100, this work) and guinea pig anti-Kirre23 (1:100, gift from S. Abmayr). Other antibodies used were rat anti-HA (1:100, 1186742300, Merck/Roche, Taufkirchen, Germany), rabbit anti-Rab5 (1:100, ab18211, abcam, Cambridge, UK), mouse anti-Rab7 (1:100, Developmental Studies Hybridoma Bank [DSHB], Iowa City, IA), mouse anti-Hrs (1:100, Hrs 8–2, DSHB), mouse anti-pyd (1:100, PYD2, DSHB), and rabbit anti-RAB11 (1:100, #5589S; Cell Signaling Technology, Leiden, Netherlands). For imaging, a Zeiss (Oberkochen, Germany) LSM 880 laser scanning microscope was used applying Airyscan technology for super-resolution microscopy. Image processing was done by ImageJ and GIMP software. In nonquantitative studies, laser power and brightness were adjusted for each image to ensure optimal image quality. In quantitative studies, all conditions were imaged and processed equally. Scale bars represent 5 µm if not stated otherwise.
Mounting of Drosophila Wings and Legs
Wings and legs were incubated in isopropanol for 15 minutes, mounted in Euparal (Roth, Karlsruhe, Germany), and imaged using a Zeiss Axiovert 200M microscope. Images were stitched using ImageJ’s Stitching plugin.24
Drosophila Survival Studies
To study the life span of adult Drosophila, we collected 20 newly hatched animals, with an equal proportion of males and females for each indicated genotype. Flies were maintained at 25°C and moved to vials containing fresh food every 2 days. Dead animals were counted daily.
Generation of Guinea Pig Antinephrin (Sns) Polyclonal Antibody
A single guinea pig was immunized by Eurogentech (Seraing, BE) with the following two peptides derived from the cytosolic domain of nephrin (Sns): QPHGILKDPNRNKQQ and EGSDMPPPRYQKDGT. Crude antisera were affinity purified against the original peptides. The specificity for nephrin (Sns) was confirmed by immunofluorescence.
Live Antibody Labeling and Internalization
Nephrocyte live antibody labeling was performed as described elsewhere.20 Briefly, nephrocytes carrying a genomic tag within the extracellular domain of fly nephrin were dissected in PBS and immediately exposed to the primary antibody (mouse anti-Myc, 9E10; DSHB, or sc-40; Santa Cruz Biotechnology, Dallas, TX, both 1:100) for 25 minutes at 4°C. Several brief washing steps with cold PBS followed, to remove the unbound antibody. The living cells now labeled with antibody were incubated at 29°C for the indicated time. Now, the tissue was fixed, permeabilized, and washed further before the first Alexa488-coupled antimouse secondary antibody was used to visualize the live labeled antibody. To obtain total nephrin staining anti-Myc primary antibody was applied in turn. The preceding permeabilization had rendered the entire nephrin protein of the cell accessible to the anti-Myc antibody for the second exposure. Finally, for visualization of total nephrin staining an Alexa568-coupled anti-mouse secondary was used. For imaging, a Zeiss LSM 880 laser scanning microscope was utilized, applying Airyscan technology. Images were processed by ImageJ and GIMP software.
Electron Microscopy
In preparation for transmission electron microscopy, nephrocytes were dissected, followed by fixation in 4% formaldehyde and 0.5% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4. Transmission electron microscopy was conducted using standard techniques.
For scanning electron microscopy, whole flies were fixed in Bouin solution. Scanning electron microscopy was carried out using standard techniques.
Fluorescent Tracer Uptake
Fluorescent tracer uptake in nephrocytes to evaluate nephrocyte function was performed as previously described.19 Briefly, after dissection in PBS, nephrocytes were incubated with FITC-albumin (Merck/Sigma, Taufkirchen, Germany) for 30 seconds. After a fixation step of 5 minutes in 8% paraformaldehyde containing Hoechst 33342 (1:1000, Thermo Fisher Scientific, Waltham, MA) cells were rinsed in PBS and mounted in Roti-Mount (Carl Roth, Karlsruhe, Germany). Cells were imaged using a Zeiss LSM 880 laser scanning microscope. Quantitation of fluorescent tracer uptake was performed with ImageJ software for the brightest three cells per animal. The results are expressed as a ratio to a control experiment.
For the sequential fluorescent tracer uptake to study the processing of endocytic cargo, nephrocytes were dissected in PBS and incubated with FITC-albumin for 3 minutes. Four washing steps with cold PBS were followed by a 30-minute chase period in Schneider’s medium at 25°C. Afterward, a second tracer (Texas Red–albumin, Thermo Fisher Scientific) was applied and incubated for 3 minutes. Immediately after rinsing with cold PBS, nephrocytes were fixed for 5 minutes in 8% paraformaldehyde containing Hoechst 33342 (1:1000, Thermo Fisher Scientific). After fixation, cells were rinsed in PBS and mounted in Roti-Mount (Carl Roth), and imaged using a Zeiss LSM 880 laser scanning microscope with Airyscan technology. Due to the different amount of tracer uptake in diverging genotypes laser intensity was adjusted for each animal individually to ensure sufficient detection of subcellular tracer localization. Image quantitation was performed for two to three cells per animal using ImageJ software (Coloc2-tool).
LysoTracker Assay
To label cells with lysotracker, we dissected larvae, incubated the nephrocytes in PBS containing 0.25 µM lysotracker (LysoTracker Red DND-99; Thermo Fisher Scientific; L7528) for 5 minutes, before immediate live cell imaging on a Zeiss LSM 880 laser-scanning microscope. Image processing was performed by ImageJ and GIMP software.
Channel Diffusion Assay
To detect passive passage of a tracer into the membrane invaginations of nephrocytes, we dissected and fixed the cells briefly for 5 minutes in PBS containing 4% paraformaldehyde (#15,700, Electron Microscopy Sciences, Hatfield, PA). The unusually short fixation preserves slit diaphragm permeability.20 Cells were then incubated for 10 minutes in FITC-albumin (Merck/Sigma) to allow tracer diffusion into the channels and to visualize the network of labyrinthine channels. The regular staining protocol was performed according to our standard procedure after a second fixation step in paraformaldehyde for another 15 minutes.
Immunoblotting of Drosophila Tissue
For immunoblotting from Drosophila, we used five whole third-instar larvae per genotype. Larvae were ground and incubated for 15 minutes in ice-cold immunoprecipitation lysis buffer containing protease inhibitor (Merck/Roche) before sonication. The extracts were centrifuged for 15 minutes at 14,000 rpm and protein concentration was determined using a colorimetric assay (Bio-Rad, Feldkirchen, Germany). Samples were heated to 72°C for 10 minutes, and loaded on a 4%–12% SDS–polyacrylamide gel for electrophoresis. Protein was transferred to polyvinylidene difluoride membranes (Millipore/Thermo Fisher Scientific). Expression of HA-tagged proteins was detected using rat anti-HA (1:1000, 1186742300, Merck/Roche); mouse anti-actin (1:1000, JLA20, DSHB) served as loading control.
Plasmids, Cell Culture, Transfection, and Immunoblotting
Murine full-length Tbc1d8b cDNA was subcloned by PCR as described previously7 (GenBANK BC147581.1). The variants reflecting patient-derived variants or the R537K mutant were generated by PCR followed by Gibson Assembly (NEBuilder HiFi DNA assembly, New England Biolabs, Frankfurt, Germany) and LR reactions (LR clonase II Enzyme Mix, Thermo Fisher Scientific) were used for transfer of wild-type Tbc1d8b and its variants into pCDNA6.2-N-GFP for expression in cells and into pUASg-attB (from K. Basler, University of Zurich, Zurich, Switzerland) for transgenic flies. Primers are shown in Supplemental Table 1.
Immortalized human podocytes were a gift from Dr. Moin Saleem (University of Bristol, Bristol, UK). Podocytes were maintained in RPMI plus GlutaMAX-I (Gibco/Thermo Fisher Scientific) supplemented with 10% FBS, 50 IU/ml penicillin, 50 μg/ml streptomycin, and insulin–transferrin–selenium-X.
Cultured podocytes were grown at the permissive temperature of 33°C and transfected using Lipofectamine 2000 (Invitrogen/Thermo Fisher Scientific). Fluorescent images of transfected cells were obtained after fixation and mounting with an Axio Observer microscope (Zeiss).
Overexpression and immunoblotting was performed in HEK293T cells. HEK293T cells were maintained in DMEM (Thermo Fisher Scientific), supplemented with 10% FBS, 50 IU/ml penicillin, and 50 μg/ml streptomycin. Polyethylenimine (Merck/Sigma) was used for transfection into HEK293T cells at 37°C. Immunoblotting from cellular lysates was performed analogously to the procedure described above for whole Drosophila larvae. For detection of GFP-tagged constructs, a mouse anti-GFP antibody was applied (1:1000, Santa Cruz, sc-9996).
Study Approval
Approval for human subject research was obtained from the institutional review board at Beth Israel Deaconess Medical Center. All participants or their guardians provided written informed consent.
Study Participants
After informed consent, clinical data and blood samples were obtained from individuals with nephrotic syndrome, or who were given the diagnosis of FSGS. Renal biopsies were evaluated by renal pathologists. For further information, please refer to Wang et al.25 Ethnicity was included to illustrate ancestry for the human genetic data on the basis of the information provided by primary care providers.
Whole-Exome Resequencing and Mutation Calling
Whole-exome resequencing, and mutation calling were performed as described previously.25
Statistics
Paired t test was used to test for statistical significance between two groups. One-way ANOVA followed by Dunnett’s correction for multiple testing (unless otherwise indicated) was used for multiple comparisons. Chi-squared test was used to test for statistical significance in ordinal data. Measurements were from distinct samples and were tested for Gaussian distribution. All statistic tests were done using GraphPad Prism software. Asterisks indicate significance as follows: *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. A statistically significant difference was defined as P<0.05. Error bars indicate standard deviation (SD).
Results
Tbc1d8b Null Flies Exhibit a Nephrocyte-Restricted Phenotype
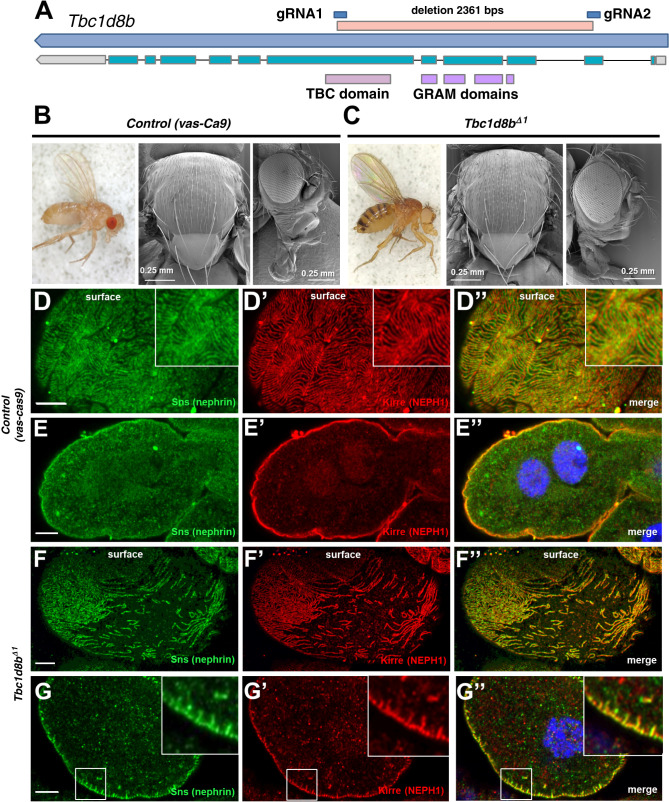
Previous studies established a role of TBC1D8B as a RAB11-GAP.7,8 However, the phenotypic analysis in Drosophila revealed only a partial overlap of Tbc1d8b-RNAi with overexpression of Rab11, suggesting additional functions.7 To examine the role of Tbc1d8b further, we established a stable genetic deletion. Germline expression of Cas9 together with tandem Tbc1d8b-guide RNAs resulted in a deletion of 2361 base pairs within the Tbc1d8b locus causing a frameshift after residue Lys28 (Tbc1d8bΔ1). The deletion comprised 1692 base pairs that are coding, while affecting all functional domains (Figure 1A, Supplemental Figure 1A). Homozygous mutant animals were viable without an overt phenotype (Figure 1, B and C, Supplemental Figure 1, B–E) and showed an undiminished lifespan (Supplemental Figure 1F). However, the podocyte-like nephrocytes of these flies revealed mislocalization and focal loss of slit-diaphragm proteins (control Figure 1, D–E″, allele Figure 1, F–G″). The phenotype was confirmed compound heterozygously using an independent deletion (Supplemental Figure 1, G–H″). The nephrocyte-restricted phenotype recapitulates the clinical picture of human patients carrying TBC1D8B variants that presented with isolated nephrotic syndrome.7,8 The same tandem guide RNA used in a conditional CRISPR/Cas9 approach with nephrocyte-restricted Cas9 expression resulted in a severer phenotype (Supplemental Figure 1, I–J″). The phenotype thus was more pronounced despite disrupting gene function later in development. This ostensible discrepancy is probably explained by genetic compensation.26 An independent set of gRNAs previously resulted in an almost identical phenotype,7 making a CRISPR off-target highly unlikely.
Figure 1.
A stable genetic deletion of Tbc1d8b in Drosophila shows a nephrocyte-restricted phenotype. (A) Schematic shows the Drosophila Tbc1d8b locus (CG7324) with the longest transcript and the protein’s functional domains corresponding to the respective exons. The deletion of the Tbc1d8bΔ1 allele is denoted between the respective gRNA target sites. (B) and (C) Stereomicroscopy of the whole fly (left) and scanning EM of thorax (middle) and head (right) are shown for control animals (vas-Cas9, B) and adult Tbc1d8bΔ1 allele flies (C). Tbc1d8b null animals show no overt phenotypic difference. (D)–(G″) Confocal microscopy of nephrocytes stained for nephrin (Sns) and Kirre (NEPH1) in tangential (D)–(D″) and (F)–(F″) or cross sections (E)–(E″) and (G)–(G″) are shown. Control cells (vas-Cas9, D–E″) show a regular slit diaphragm staining pattern, whereas Tbc1d8bΔ1 nephrocytes reveal a localized loss of slit diaphragm on the surface (F)–(F″) and small protrusions of slit diaphragm protein toward the interior of the cell (insets in G–G″). Nuclei are marked by Hoechst 33342 in blue. EM, electron microscopy.
Tbc1d8b Is Specifically Required for Endocytosis of Drosophila Nephrin
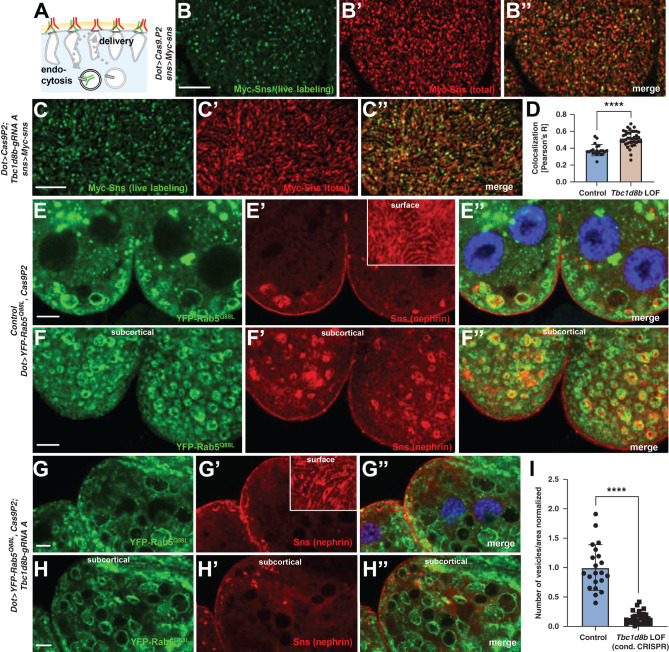
To explore how lack of Tbc1d8b causes mislocalization of fly nephrin, we studied a role for nephrin endocytosis. For simplicity, we will use the human name (nephrin) for the Drosophila protein (Sns). We first correlated the ectopic nephrin induced by CRISPR/Cas9-mediated loss of Tbc1d8b with endocytic tracer FITC-albumin19 after extended exposure. Mislocalized nephrin did not overlap with FITC-albumin, suggesting the protein does not reside in (early) endosomes (insets Supplemental Figure 2, A–A″) but potentially diffused into the membrane invaginations, which is a consequence of defective endocytosis.20 Similarly, passive diffusion of FITC-albumin into the labyrinthine channels was reduced (Supplemental Figure 2, B–C″). This is compatible with incipient filter clogging that follows defective endocytic turnover of the slit diaphragms.20 To examine the effect of Tbc1d8b loss-of-function on nephrin turnover directly, we used a knock-in of Myc into the locus of fly nephrin and performed live antibody labeling as previously described.20 In this assay, living nephrocytes are labeled with Myc-antibody ex vivo followed by tracking of the labeled nephrin20 (schematic Figure 2A). In CRISPR/Cas9-mediated loss of Tbc1d8b we observed retention of live labeled nephrin on the surface after 2 hours, compared with control conditions (Figure 2, B–D). This indicates decelerated nephrin endocytosis without Tbc1d8b. Constitutively active Rab5 triggers accumulation of fly nephrin (Sns) within enlarged Rab5-positive endosomes as we previously showed elsewhere20 (Figure 2, E–F″). Thus, we examined Rab5 gain-of-function together with loss-of-function of Tbc1d8b. The nephrin-containing vesicles were missing after CRISPR/Cas9-mediated loss of Tbc1d8b (Figure 2, G–I). Tbc1d8b seems required for endocytic transport of nephrin into the enlarged endosomes. Endosomal hyperfusion is a result of excessive function of Rab5 (schematic Supplemental Figure 2D), and the YFP-RAB5Q88L–positive vesicles were still enlarged after Tbc1d8b loss-of-function. Thus, a general disruption of Rab5 function appears unlikely. Although the effects of constitutively active Rab5 on the endocytic machinery are complex, combining aspects of gain- and loss-of-function, these data do suggest that Tbc1d8b is required for nephrin endocytosis at the early endosomal level.
Figure 2.
Tbc1d8b is required for nephrin endocytosis. (A) The schematic illustrates live antibody labeling. Living nephrocytes are labeled with anti-Myc antibody (green) that may undergo endocytosis during the chase period. Total nephrin stain follows fixation and permeabilization (red) including newly delivered nephrin. (B)–(C″) Confocal microscopy images of nephrocytes with CRISPR/Cas9-mediated loss of Tbc1d8b (C)–(C″) are shown in comparison to control cells without Tbc1d8b loss-of-function (lacking the gRNA, B–B″). Both groups carry one copy of a genomic Myc-nephrin and are subject to live antibody labeling of fly nephrin followed by a chase period of 2 hours. Whereas control cells show a widespread removal of live labeled fly nephrin from the surface through nephrin turnover (B)–(B″), removal is decreased upon loss of Tbc1d8b (C)–(C″). The total nephrin stain in red confirms that slit diaphragms are still present. (D) Quantitation of results analogous to (B)–(C″) expressed as Pearson correlation coefficient after thresholding for individual cells (mean±SD, n=7–12 animals per genotype, P<0.0001) confirms stronger colocalization of live labeling and total stain upon loss of Tbc1d8b indicating slower nephrin turnover. (E)–(H″) Shown are nephrocytes expressing a constitutively active variant of Rab5 that carries an N-terminal YFP-tag with CRISPR/Cas9-mediated loss of Tbc1d8b or Cas9 alone as control after nephrin staining. The YFP-reporter visualizes the enlarged early endosomes that are induced by endosomal hyperfusion. These atypical endosomes, likely reflecting excessive nephrin endocytosis with improper sorting, are filled with extensive amounts of endocytosed nephrin protein in control cells (central section E–E″, subcortical section F–F″), whereas the slit diaphragm pattern in tangential sections remains intact (inset E’). In contrast, upon loss-of-function of Tbc1d8b, the endocytosed nephrin is largely lacking (central section G–G″, sucbcortical section H–H″). (I) Quantitation of data analogous to (E)–(H″) expressed as number of nephrin containing YFP-Rab5Q88L vesicles per surface area and cell (mean±SD, n=8–15 animals per genotype, P<0.0001) confirms that translocation of nephrin to these endosomes is lacking on loss of Tbc1d8b.
MMEJ–Mediated Knock-In of a Hemagglutinin-Tag into the Tbc1d8b Locus Reveals Localization to Mature Early Endosomes and Late Endosomes
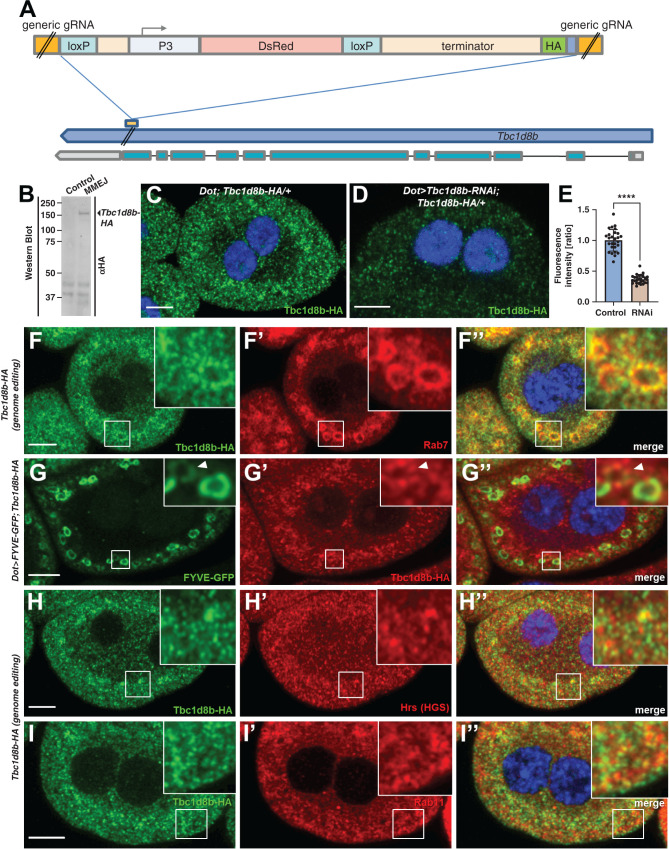
To explore the subcellular localization of Tbc1d8b protein we used a genome editing strategy using MMEJ. This secondary DNA repair mechanism (Supplemental Figure 3A) employs homologies for repair of double strand breaks while causing deletions of the interjacent sequence. However, when harnessed for genome editing, MMEJ allows for seamless repair requiring much shorter homology domains than homology directed repair.27,28 We modified the approach to introduce a C-terminal hemagglutinin (HA) tag into the Tbc1d8b locus (Figure 3A, Supplemental Figure 3B). Knock-in at the C terminus was confirmed by sequencing (Supplemental Figure 3C). Immunoblotting and HA-staining of larval protein extracts revealed an additional band reflecting the expected size of full length Tbc1d8b (Figure 3B), suggesting that Tbc1d8b-HA was expressed from the edited locus. To our knowledge this represents the first successful MMEJ-based genome editing in Drosophila. HA-staining of flies carrying Tbc1d8b-HA (heterozygously) revealed a fine vesicular pattern (Figure 3C), which was strongly diminished with coexpression of Tbc1d8b-RNAi (Figure 3, D and E). Nephrocytes dissected from homozygous Tbc1d8b-HA animals showed a regular slit diaphragm staining, suggesting the tagged protein was functional (Supplemental Figure 3, D–D″). We costained Tbc1d8b-HA with several endosomal compartment markers and noticed the strongest colocalization with the late endosomal protein Rab7 (Figure 3, F–F″). Colocalization was most pronounced in punctate vesicular subdomains (insets Figure 3, F–F″). We also studied colocalization with FYVE-GFP (insets Figure 3, G–G″). This transgenic reporter detects phosphatidylinositol(3)-phosphate (PI3P) on mature early endosomes, a compartment intermediate of Rab5- and Rab7-positive vesicles in nephrocytes (Supplemental Figure 3, E–E″). We observed Tbc1d8b on weakly positive FYVE-GFP vesicles, potentially indicating very mature early endosomes at the intersection to late endosomes. We further detected partial overlap with Hrs (ortholog of HGS), a member of the ESCRT-0 complex that promotes maturation of early endosomes29 (Figure 3, H–H″). Consistent with Rab11-GAP function we also observed colocalization with Rab11 (Figure 3, I–I″). Weak colocalization was finally detectable for Rab5 (Supplemental Figure 3, F–F″). We confirmed the colocalization with all of these endosomal markers by quantitative analysis (Supplemental Figure 3G). We conclude that Tbc1d8b-HA is found within several endosomal compartments, and is strongest on very mature early endosomes and late endosomes. The subcellular localization of Tbc1d8b suggests a broader functional role.
Figure 3.
MMEJ–mediated genome editing of Tbc1d8b suggests a broad localization in mature early endosomes, late endosomes, and recycling endosomes. (A) Schematic illustrates the strategy to insert an HA-tag into the C terminus of Tbc1d8b via MMEJ. The insert is placed between target sites for the generic gRNA and contains short homologies. Inserted is a terminator sequence and red fluorescent DsRed under control of P3 promoter. LoxP sites flanking the DsRed cassette allow removal of the marker, whereas the HA tag and terminator will be retained. (B) Immunoblotting of protein derived from whole third-instar Drosophila larvae reveals an additional band using an anti-HA antibody that is present after the knock-in but not in control animals. (C) and (D) Nephrocytes carrying the HA knock-in heterozygously reveal a diffuse vesicular pattern upon staining with anti-HA and confocal microscopy (C). The signal is strongly diminished on coexpression of Tbc1d8b-RNAi (D). Nuclei are marked by Hoechst 33342 in blue throughout the figure. (E) Quantitation of data analogous to (D) and (E) is shown as ratio of the fluorescence intensity for individual cells between control and knockdown animals (mean±SD, n=9 animals per genotype, P<0.0001). (F)–(I″) Nephrocytes costained for Tbc1d8b-HA and various endosomal marker proteins. Late endosomal Rab7 shows considerable colocalization with Tbc1d8b-HA (F)–(F″). Overlap is most pronounced in small domains on the outer membrane of the late endosomes (insets F–F″). FYVE-GFP expressed under control of Dot-GAL4 marks mature early endosomes. The reporter shows partial colocalization with Tbc1d8b-HA (G)–(G″) in particular on vesicles that are weakly positive for FYVE-GFP (insets G–G″). Colocalization of Tbc1d8b is further observed with Hrs (H)–(H″), a member of the ESCRT-0 complex, and the recycling regulator Rab11 (I)–(I″).
Tbc1d8b Is Required for Rapid Transport from Early to Late Endosomes
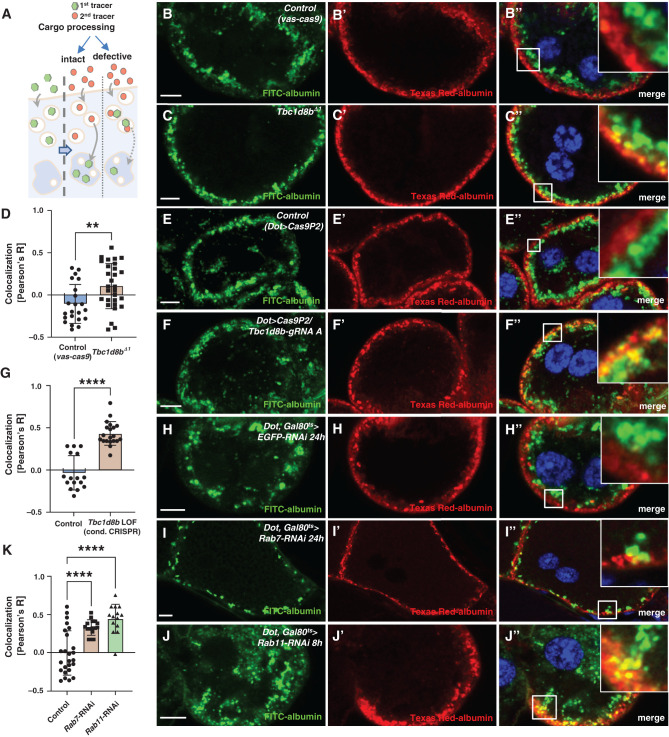
To investigate a role for endosomal cargo processing, we performed a sequential tracer assay that visualizes the transport from early to late endosomes.30 Early and late endosomes are spatially separated in nephrocytes. Thus, it is possible to monitor cargo processing by the sequential exposure of nephrocytes to endocytic tracers. The tracer applied first (green) is transported to late endosomes during a chase period. The second tracer (red) applied immediately before fixation remains in the early endosomal-sorting compartment. Consequently, slower cargo processing is indicated by colocalization (Figure 4A). In control cells, the tracers were effectively separated (Figure 4, B–B″). However, in Tbc1d8bΔ1 nephrocytes, colocalization was mildly but significantly increased (Figure 4, C–D). This suggested that loss of Tbc1d8b caused defective cargo processing. This defect was more pronounced after conditional CRISPR/Cas9-mediated loss-of-function of Tbc1d8b (Figure 4, E–G). Tbc1d8b-HA colocalizes strongest with Rab7. Thus, we analyzed the effect of Rab7-RNAi. Tracer overlap after silencing this late endosomal regulator was comparable to Tbc1d8b loss-of-function (Figure 4, H–K). Surprisingly, expression of Rab11-RNAi also delayed cargo processing suggesting a compensatory effect within the endocytic pathway (Figure 4, J–K). The result of Rab5-RNAi differed, likely due to early mis-sorting (Supplemental Figure 4, A and B). We concluded that loss-of-function of Tbc1d8b slowed cargo processing, causing a defect similar to that observed with Rab7- and Rab11-RNAi.
Figure 4.
Sequential tracer endocytosis indicates a role of Tbc1d8b for cargo processing. (A) Schematic illustrates the principle of the sequential tracer assay. The first tracer (green) is applied and endocytosed (left). Endocytic processing moves the first tracer to the deeper late endosomes during a chase period (middle). If endocytosis is defective both tracers will overlap in the endosomal sorting compartment (right). (B) and (C″) Sequential tracer endocytosis indicates a mild increase of colocalization between the first and second tracers for Tbc1d8bΔ1 nephrocytes (C)–(C″) compared with control cells (B)–(B″). Nuclei are marked by Hoechst 33342 in blue. (D) Quantitation of data analogous to (B) and (C) expressed as Pearson correlation coefficient for individual cells (mean±SD, n=8–12 animals per genotype, P<0.01) confirms increased colocalization of sequential tracers, suggesting delayed cargo processing. (E)–(F″) Sequential tracer endocytosis reveals enhanced colocalization of sequential tracers for CRISPR/Cas9-mediated loss-of-function of Tbc1d8b (F)–(F″) compared with control nephrocytes (E)–(E″). (G) Quantitation of data analogous to (E) and (F) expressed as Pearson correlation coefficient for individual cells (mean±SD, n=6–7 animals per genotype, P<0.0001) confirms increased tracer overlap indicating decelerated processing of endocytic cargo. (H)–(J″) Sequential tracer endocytosis in nephrocytes expressing Rab7-RNAi (I)–(I″) and Rab11-RNAi (J)–(J″) also show an elevated colocalization of first and second tracers compared with control cells (H)–(H″). Late endosomes appear smaller upon Rab7 silencing (I)–(I″) and are more diffuse on expression of Rab11-RNAi. (K) Quantitation of data analogous to (H)–(J) expressed as Pearson correlation coefficient for individual cells (mean±SD, n=8–12 animals per genotype, P<0.0001) supports enhanced colocalization by defective degradation and loss of Rab11.
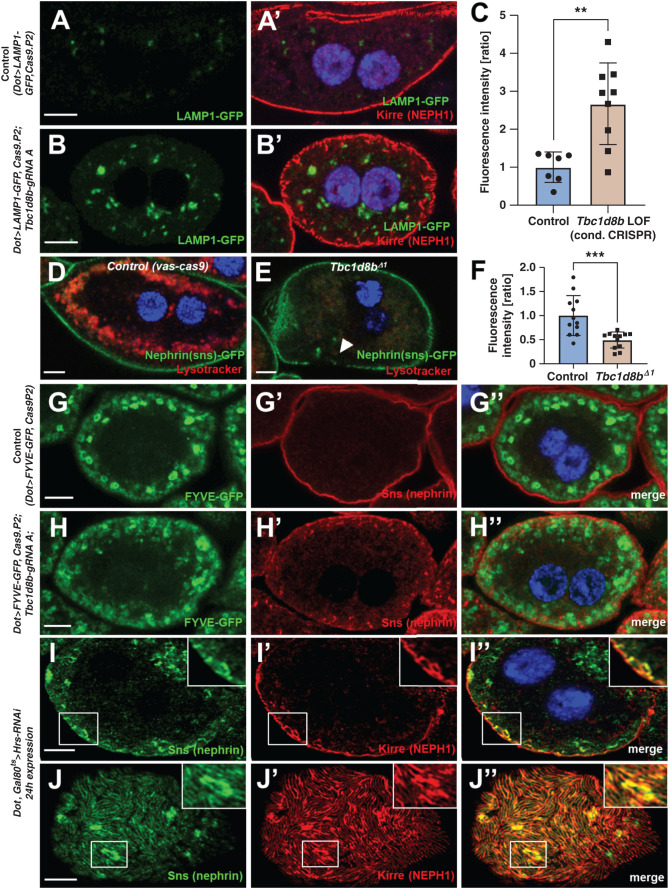
Loss of Tbc1d8b Slows Endolysosomal Degradation
To assess endolysosomal degradation, we used GFP-LAMP1. This truncated fusion protein is directed to lysosomes and defective degradation results in accumulation and consequently increased GFP-derived fluorescence.31,32 Coexpression of GFP-LAMP1 with CRISPR/Cas9-mediated loss of Tbc1d8b significantly enhanced fluorescence compared with control (Figure 5, A–C). Endosomes acidify during maturation to promote degradation.16 To examine this, we exposed living nephrocytes to lysotracker, a dye that accumulates in acidic endosomes. Compatible with defective endosomal maturation, we observed a strong reduction of lysotracker-derived fluorescence on loss of Tbc1d8b (Figure 5, D–F). We costained Rab5 and Rab7 after CRISPR/Cas9-mediated loss of Tbc1d8b and observed largely undisturbed staining patterns (Supplemental Figure 5, A–B″). Coexpression of GFP-FYVE, whose double FYVE domains facilitate sensitive detection of PI3P-containing early endosomes,33 revealed a more diffuse distribution on loss of Tbc1d8b, losing the largest and brightest vesicles (compare Figure 5G and Figure 5H). This suggests defective PI3P recruitment to early endosomes, consistent with defective maturation of advanced early to late endosomes. Taken together, our findings support a putative functional role of Tbc1d8b in endosomal maturation at the intersection between early and late endosomes. To test the hypothesis that this novel functional role is critical for nephrin trafficking, we silenced Hrs, a member of the ESCRT complex that promotes sorting and early endosomal maturation.29 Acute silencing of Hrs in nephrocytes phenocopied loss of Tbc1d8b (Figure 5, I–J″, validation of knockdown/antibody Supplemental Figure 5, C–D″). The phenotypic overlap of Tbc1d8b loss-of-function with Hrs-RNAi appeared much more pronounced than with overexpression of Rab11,7 suggesting that defective maturation may be more central for nephrin mistrafficking.
Figure 5.
Loss-of-function of Tbc1d8b results in delayed endosomal degradation. (A)–(B’) Nephrocytes expressing a GFP-LAMP1 fusion protein under control of UAS are costained for Kirre (NEPH1). The vesicles carrying the (unstained) reporter become brighter and expand on CRISPR/Cas9-mediated loss of Tbc1d8b (compare B to A). Nuclei are marked by Hoechst 33342 in blue. (C) Quantitation of data analogous to (A)–(B’) expressed as ratio of mean fluorescence intensity per animal (mean±SD, n=7–9 animals per genotype, P<0.01) supports accumulation of GFP-LAMP1 suggesting defective degradation. (D)–(E″) Shown are confocal images of living, unfixed nephrocytes exposed to lysotracker dye ex vivo. The cells express nephrin-GFP from a genome edited locus. Lysotracker-derived fluorescence intensity is diminished in Tbc1d8bΔ1 animals (E) compared with control (D). (F) Quantitation of lysotracker intensity in cells analogous to (D)–(E’) (gray value, mean±SD, n=12 animals per genotype, P<0.001) supports decreased acidification, in turn suggesting defective endosomal maturation. (G)–(H″) Nephrocytes expressing the reporter FYVE-GFP costained for nephrin show a more diffuse pattern of the vesicles positive for the (unstained) FYVE-GFP reporter comparing loss-of-function of Tbc1d8b (H)–(H″) with control cells (G)–(G″). The brightest and largest vesicles are missing, suggesting a reduced formation of PI3P on mature endosomes. (I)–(J″) Confocal microscopy images of a nephrocyte stained for slit diaphragm proteins are shown. A cell expressing Hrs-RNAi acutely for 24 hours shows accumulation of subcortical slit diaphragm protein in cross sections (I)–(I″), see also inset. Tangential sections reveal brighter sections within the lines of slit diaphragm protein that are blurry and partially confluent (J)–(J″).
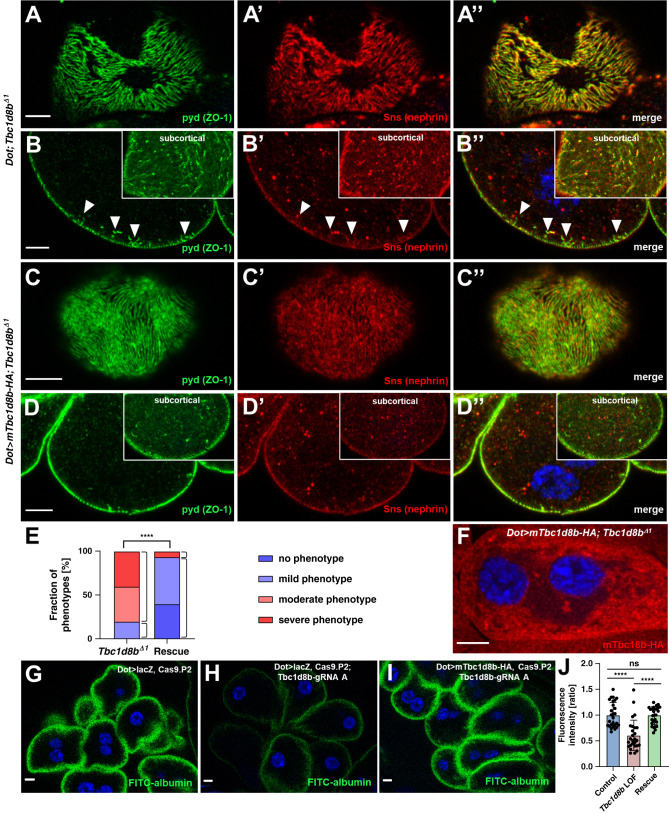
Transgenic Expression of Murine Tbc1d8b Rescues Loss-of-Function of Drosophila Tbc1d8b
To explore the evolutionary conservation of Tbc1d8b function, we analyzed compensation of a mammalian Tbc1d8b for lack of the Drosophila ortholog. We generated transgenic flies that allow expression of murine full length Tbc1d8b with a C-terminal HA-tag (mTbc1d8b) (protein alignment Supplemental Figure 6A). We coexpressed mTbc1d8b in Tbc1d8bΔ1 nephrocytes to rescue the null allele and observed a strongly restored staining pattern of the slit diaphragm proteins nephrin (Sns) and polychaetoid (Pyd/ZO-1) compared with Tbc1d8bΔ1 nephrocytes with the GAL4 transgene alone (Figure 6, A–E). The mTbc1d8b rescue suggests functional correspondence between mammalian and Drosophila Tbc1d8b and further confirms the specificity of Tbc1d8bΔ1. The murine protein in rescue animals localized in small vesicles (Figure 6F). In line with the proposed role as Rab11-GAP,7 we further observed partial colocalization of human RAB11B and mTbc1d8b on coexpression in nephrocytes (Supplemental Figure 6B).
Figure 6.
Mild overexpression of murine Tbc1d8b rescues loss of Drosophila Tbc1d8b. (A)–(D″) Confocal microscopy of nephrocytes stained for nephrin (Sns) and Kirre (NEPH1) in tangential (A)–(A″) and (C)–(C″) or cross sections (B)–(B″) and (D)–(D″) are shown. Tbc1d8bΔ1 nephrocytes carrying the GAL4 driver (Dot-GAL4) alone show the regular phenotype of the Tbc1d8b null allele with a localized loss of slit diaphragms on the surface (A)–(A″) and small protrusions of slit diaphragm protein toward the interior of the cell in cross sections (arrow heads in B–B″). In contrast, when mTbc1d8b is mildly overexpressed at 25°C, the severity of the phenotype is strongly diminished (C)–(D″). This indicates that the mammalian Tbc1d8b rescues loss of the Drosophila protein. Nuclei are marked by Hoechst 33342 in blue, far red fluorescence (Alexa647) is shown as red. (E) Quantitation of data analogous to (A)–(D″) expressed as percentage of animals categorized for phenotypic severity. The categories shown are wild-type appearance (dark blue), mild phenotype (light blue), moderate phenotype (light red), and severe phenotype (dark red). Categorization was performed in a blinded fashion. For the statistical analysis using chi-squared test, the categories are simplified to mild versus severe phenotype and the results indicate a highly significant difference (n=15 per genotype, P<0.0001) This confirms a successful rescue by mTbc1d8b. (F) Confocal microscopy image of a Tbc1d8bΔ1 nephrocyte expressing the HA-tagged mTbc1d8b under control of Dot-GAL4 stained for the protein tag indicates a localization that is reminiscent of the Drosophila Tbc1d8b-HA protein. (G)–(I) The FITC-albumin endocytosis assay as read-out for nephrocyte function shows reduced uptake for CRISPR/Cas9-mediated loss of Tbc1d8b (H) compared with control overexpression (UAS lacZ alone, G), which is restored on concomitant rescue by the mTbc1d8b at 18°C (I). (J) Quantitation of results from (G)–(I) for individual cells in ratio to a control experiment performed in parallel (mean±SD, n=9 animals per genotype, P<0.0001 for control overexpression (lacZ) and P<0.0001 for mTbc1d8b rescue compared with loss-of-function. The rescue does not differ significantly from the control group (P=0.98).
To assess whether mTbc1d8b restores nephrocyte function after CRISPR/Cas9-mediated loss of Tbc1d8b, we studied FITC-albumin endocytosis. Uptake was comparable to control animals after coexpression of mTbc1d8b but not of a control transgene (Figure 6, G–J), indicating that nephrocyte function largely returned to endogenous levels. This underlines the ability of mTbc1d8b to compensate for loss of the Drosophila ortholog.
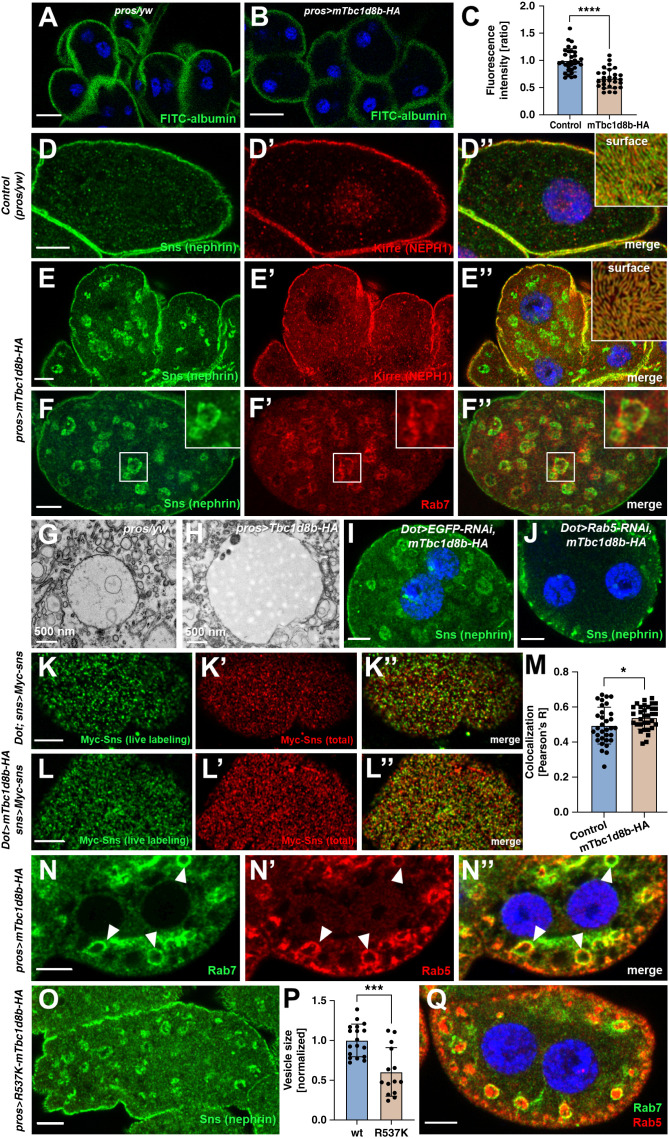
Murine Tbc1d8b Triggers the Appearance of Nephrin in Late Endosomes, which May Occur Independently of Tbc1d8b GAP Function
Having established evolutionary conservation, we studied more aggressive overexpression of mTbc1d8b at higher temperatures (31°C) in a wild-type background. This resulted in slightly reduced nephrocyte function compared with physiologic levels (Figure 7, A–C) suggesting that excessive amounts of the mTbc1d8b show a dose-dependent detrimental effect. We stained nephrin with excessive overexpression of mTbc1d8b and detected translocation toward large intracellular vesicles (Figure 7, D–E″). The pattern in tangential sections was undisturbed, suggesting that regular slit diaphragms were still being formed (inset Figure 7E″). Fly nephrin and the NEPH1 ortholog Kirre commonly share their subcellular localization. However, Kirre was unaffected by mTbc1d8b overexpression, implying a specific interaction with nephrin (Figure 7E’). Nephrin colocalizes with Rab7 in this setting, indicating a transfer of nephrin toward late endosomes by an unknown mechanism (Figure 7, F–F″, electron microscopy of late endosomes Figure 7, G and H). Coexpression of Rab5-RNAi with mTbc1d8b abrogated formation of nephrin-containing vesicles (Figure 7, I and J), confirming that nephrin is translocated by excessive or misguided endocytosis. The murine protein further was detectable on these late endosomes (Supplemental Figure 7 A–A″). To assess nephrin turnover on excess of mTbc1d8b, we performed the live labeling assay and observed a mild decelaration (Figure 7, K–M). The slower turnover suggests that mTbc1d8b induced nephrin missorting and delayed degradation, not excessive nephrin endocytosis. To investigate potential endocytic mistrafficking on mTbc1d8b expression, we studied distribution of early and late endosomes in nephrocytes. Overexpression of mTbc1d8b caused enlarged endosomes that showed the unusual simultaneous presence of early and late endosomal compartment markers Rab5 and Rab7 (arrow heads Figure 7, N–N″). Such vesicles were absent in control nephrocytes (Supplemental Figure 7, B–B″). Overexpression of mTbc1d8b thus triggers premature recruitment of Rab7 or delayed dissociation of Rab5 making disturbed endosomal maturation a plausible cause of nephrin mistrafficking associated with mTbc1d8b overexpression. To examine whether this effect is linked to TBC/GAP function, we generated an mTbc1d8b variant with a dysfunctional TBC domain by introducing a R537K mutation. Residue R537 is likely critical for mTbc1d8b GAP function being the catalytic arginine finger within the canonical IxxDxxR motif34 (Supplemental Figure 7C). Introducing R537K and overexpression of the mutant transgene in nephrocytes still resulted in formation of vesicles with Rab5/Rab7 colocalization and appearance of nephrin vesicles, albeit with a reduced size (Figure 7, O–Q). We concluded that excessive overexpression of mTbc1d8b promotes mistrafficking of fly nephrin and affects endosomal maturation for which GAP function may be partially dispensable.
Figure 7.
Elevated overexpression of murine Tbc1d8b entails nephrin mistrafficking. (A) and (B) The FITC-albumin endocytosis assay as read-out for nephrocyte function shows reduced uptake for excessive overexpression of mTbc1d8b using prospero-GAL4 (31°C, without loss-of-function of the Drosophila protein), compared with a wild-type control with the GAL4-driver alone. Nuclei are marked by Hoechst 33342 in blue here and throughout the figure. Scale bars represent 15 µm here. (C) Quantitation of results for individual cells from (A) and (B) in ratio to a control experiment performed in parallel (mean±SD, n=10–12 animals per genotype, P<0.0001 for strong overexpression of mTbc1d8b). (D)–(E″) Cross-sectional confocal microscopy images of control nephrocytes (D)–(D″) and nephrocytes expressing murine mTbc1d8b-HA at high levels (E)–(E″) show appearance of enlarged vesicles containing endogenous fly nephrin that is not accompanied by its binding partner Kirre (NEPH1). Insets show tangential sections of the same cells revealing a regular slit diaphragm pattern. (F)–(F″) Subcortical sections of Tbc1d8b-HA expressing nephrocyte costained for HA and Rab7 show localization within the same vesicles, indicating that ectopic fly nephrin resides within a late endosomal compartment. (G) and (H) Electron microscopy images show enlarged late endosome of a Tbc1d8b-HA expressing nephrocyte (H) compared with control cell (G). (I) and (J) Confocal microscopy images of a nephrocyte expressing mTbc1d8b-HA together with either a control knockdown (I) or Rab5-RNAi (J) show that the prominent nephrin (Sns) vesicles are lost on silencing of Rab5. This indicates that endocytic uptake is required for nephrin translocation by overexpression of mTbc1d8b. (K-L) Live antibody labeling of Myc-nephrin nephrocytes co-expressing mTbc1d8b (L-L″) or the GAL4 driver alone (control, K-K″) show that excessive overexpression of mammalian Tbc1d8b slows nephrin turnover. Chase time was 1 hour. (M) Quantitation of results analogous to (K) and (L) expressed as Pearson correlation coefficient after thresholding for individual cells (mean±SD, n=10–12 animals per genotype, P<0.05) indicates mildly stronger colocalization of live labeling and total stain on overexpression of mTbc1d8b indicating slower nephrin turnover. (N)–(N″) Confocal microscopy image of a nephrocyte expressing mTbc1d8b costained for Rab5 and Rab7 reveals large vesicles carry both the early and the late endosomal marker. This indicates altered endosomal maturation. (O) Shown is a confocal microscopy image of a nephrocyte expressing mTbc1d8b with an R537K mutation. This mutation targets the catalytic residue for the TBC domain’s function as GAP protein. Fly nephrin vesicles remain detectable albeit with a reduced size. (P) Quantitation of results analogous to (O) and (E) expressed as two-dimensional vesicle size normalized to the average of wild type murine mTbc1d8b for individual cells (mean of area of the five largest vesicles±SD, n=6 animals per genotype, P<0.001) indicates that nephrin (Sns) vesicles become smaller if the TBC domain is blocked. (Q) Nephrocytes expressing Tbc1d8bR537K show enhanced colocalization of Rab5 and Rab7 on the same vesicles. This indicates that altered endosomal maturation is not (entirely) dependent on the TBC/GAP function of Tbc1d8b.
Functional Assessment of Patient-Derived Variants of TBC1D8B in Drosophila
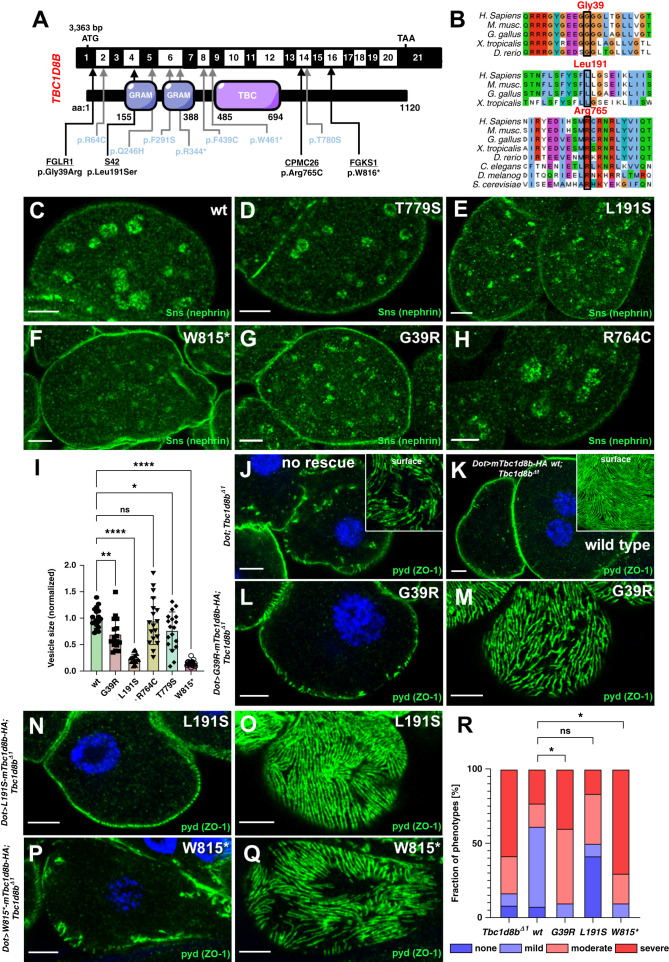
Finally, we wanted to examine TBC1D8B variants in patients with FSGS and explored the frequency of rare variants in TBC1D8B among the patients with FSGS and controls recently described in an exome-wide sequencing study.25 Out of 363 unrelated patients or families, we identified six TBC1D8B variants that passed our initial filters, compared with only one similar variant among 363 matched control samples (Table 1). This increased frequency in patients yields a P value of 0.0005 by the analysis outlined in Wang et al.25 This finding prompted us to review these variants in more detail. The single variant in the control cohort (c.922T>C, p.W308R) was heterozygous in the proband and turned up 29 times hemi- or homozygously in the gnomAD database that contains control populations (Table 1). Consequently, this variant appears benign and the only variant in the control group was excluded from the analysis. Two variants in the FSGS cohort similarly were excluded after inspection due to low evolutionary conservation (c.55A>G, p.M18V and c.1494G>A, p.M498I, Table 1). Four rare and conserved variants (c.2448G>A, p.W816*, c.572T>C, p.L191S, c.2293C>T p.R765C, and c.115G>A, p.G39R) passed inspection suggesting novel, putatively disease causing variants of TBC1D8B (Figure 8, A–B, Table 1). Of note, the age of onset of the disease (Table 1) ranged from childhood (3 and 9 years) to late adulthood (55 and 59 years), suggesting a potential late onset for some variants that had not been described previously. The occurrence of c.2293C>T p.R765C homozygously in a female patient without the father being affected, combined with hemizygous occurrence in three individuals in gnomAD, suggested a very low pretest probability for this variant. However, considering the advanced age at which the disease manifested in the affected carrier, the disease might appear later in life or with incomplete penetrance in the other, apparently unaffected carriers. Thus, the variant was included for further analysis.
Table 1.
Novel mutations in TBC1D8B
| Family | Nucleotide Change | Amino Acid Change | Zygosity | Exon | PPH2 | SIFT | MT | Amino Acid Conserved to Species | ExAC (het/hemi) | gnomAD (hom/het /hemi) | Sex | Ethnic Origin | Parental Consan- guinity | Response to Steroids | Age of Onset (Proteinuria), yr | ESKD | Number of Affecteds | Renal Biopsy | Verdict |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FGLR1 | c.115 G>A | p.Gly39Arg | Hemi | 1 | 1.0 | Del | DC | D.r. | 0/0/0 | M | Non-Hispanic White | No | No steroid treatment | 59 | Yes (age 59) | 1 | ND | Pass | |
| S42 | c.572 T>C | p.Leu191Ser | Hemi | 4 | 0.95 | Del | DC | X.t. | 0/0/0 | M | Non-Hispanic White | No | Yes | 3 | Not until age 8 | 1 | ND | Pass | |
| CPMC26 | c.2293 C>T | p.Arg765Cys | Hom | 14 | 1.0 | Del | DC | S.c. | 0/5/3 | F | Non-Hispanic White | No | ND | 55 | Not until age 55 | 1 | FSGS | Pass | |
| FGKS1 | c.2448G>A | p.Trp816* | Hemi | 16 | — | — | — | — | 0/7/1 | M | Hispanic | No | ND | 9 | Not until age 12 | 2 | FGGS | Pass | |
| FGKA1 | c.1494 G>A | p.Met498Ile | Het | 9 | 0.0 | T | DC | M.m. | 0/18/3 | F | ND | ND | ND | 45 | ND | 3 | FSGS | Reject | |
| FGAO1 | c.55 A>G | p.Met19Val | Hemi | 1 | 0.0 | T | PM | M.m. | 0/0/0 | M | ND | ND | ND | 24 | ND | 2 | FSGS | Less likely | |
| CTRL_G47551 | c.922 T>C | Trp308Arg | Het | 6 | 0.12 | T | DC | D.r. | 1/53/87 | N/A | ND | ND | — | — | — | — | control | Reject |
PPH2, PolyPhen-2 prediction score (http://genetics.bwh.harvard.edu/pph2/); SIFT, Sorting Tolerant From Intolerant prediction score (http://sift.jcvi.org/); MT, mutation taster (http://www.mutationtaster.org/); hom, homozygous; het, heterozygous; hemi, hemizygous; gnomAD, The Genome Aggregation Database (https://gnomad.broadinstitute.org/); del, deleterious; DC, disease causing; D.r., Danio rerio; M, male; X.t., Xenopus tropicalis; S.c., Saccharomyces cerevisiae; F, female; ND, no data; FGGS, focal global glomerulosclerosis; M.m., mus musculus; PM, polymorphism.
Figure 8.
Identification of novel variants in TBC1D8B in patients with FSGS and functional validation in Drosophila. (A) Schematic of TBC1D8B cDNA with the corresponding protein, including its functional domains. Arrows indicate the position of four novel variants of TBC1D8B discovered by whole exome sequencing within an FSGS cohort (black letters) together with the known variants (blue letters). (B) Alignment of amino-acid sequence of TBC1D8B protein for the indicated species illustrates level of evolutionary conservation for residues Gly39, Leu191, and Arg765. (C)–(H) Confocal microscopy images of nephrocytes stained for the ortholog of nephrin are shown. Animals express wild-type (C) or several mutant variants of mTbc1d8b carrying a C-terminal HA-tag reflecting patient-derived variants under pros-GAL4 at 31°C (D)–(H). Nephrin vesicles appear smaller for all mutant transgene except R764C. Variant T779S was previously validated (Kampf et al.).7 Note that variants are numbered according to mTbc1d8b (longest isoform) and thus may deviate from the corresponding residue in human TBC1D8B. (I) Quantitation of results analogous to (C)–(H) expressed as two-dimensional vesicle size normalized to the average of wild-type mTbc1b8 for individual cells are shown for the indicated genotypes (mean of area of the five largest vesicles±SD, n=7–10 animals per genotype, P<0.05 for T779S, P<0.01 for G39R, P<0.0001 for L191S, P>0.05 for R764C, and P<0.0001 for W815*). This indicates that nephrin (Sns) vesicles become smaller for mutants of TBC1D8B associated with FSGS with the exception of R765C. Analysis was performed in a blinded fashion. (J)–(Q) Shown are confocal microscopy images of nephrocytes stained for slit diaphragm protein pyd (ZO1) that carry the Tbc1d8b null allele together with Dorothy-GAL4 for expression of UAS transgenes. Without a UAS construct to express murine Tbc1d8b, the typical Tbc1d8b-associated phenotype with mislocalization of pyd can be observed (J), whereas expression of wild-type murine Tbc1d8b rescues this phenotypic defect (K). Tangential (M), (O), and (Q) or cross-sectional images (L), (N), and (P) of nephrocytes expressing murine Tbc1d8b variants reflecting the indicated patient-derived variants show only a partial rescue for variant G39R (L) and (M), and W815* (P) and (Q). In contrast, the expression of L191S (N) and (O) retains a substantial ability to rescue. (R) Quantitation of results analogous to (J)–(Q) expressed as percentage of animals categorized for phenotypic severity are shown for the indicated genotypes (n=10–13 animals per genotype, P<0.05 for G39R, P>0.05 for L191S, P>0.05 for R764C, and P<0.05 for W815*). Categorization was performed in a blinded fashion. For the statistical analysis using the chi-squared test, the categories were simplified to mild versus severe phenotype. This indicates that two variants (G39R and W815*) significantly lack the ability to rescue.
We introduced patient-derived variants into murine cDNA constructs and observed mislocalization and reduced expression exclusively for the truncating variant in cultured cells (Supplemental Figure 8, A–F). We used the Drosophila model to expand our analysis and generated transgenic Drosophila lines for overexpression of the patient-derived variants. Equal expression of the transgenes, all inserted into the same locus, was confirmed except for the truncation (Supplemental Figure 8, G–H). We chose a stepwise analysis of the variants beginning with overexpression in nephrocytes. We examined formation of nephrin vesicles and observed slightly smaller vesicles for the known variant T779S whose functional effect had previously been shown7 (Figure 8, C and D). Applying the same strategy to examine the novel variants, we observed significantly decreased vesicle size for overexpression of Tbc1d8b variants G39R, L191S, and W815* (the latter corresponding to human residue W816, Figure 8, E–I). The variant R764C (corresponding human residue R765, Figure 8, H and I) showed no reduction in the size of nephrin vesicles in line with lower pretest probability due to homozygosity. We further observed a staining pattern with slightly coarser-grained vesicles for L191S and W815* compared with the other variants (Supplemental Figure 8, I–N). The three variants passing the first analysis step were tested for their ability to rescue. We generated flies combining the UAS-transgenes with Tbc1d8bΔ1 and Dot-GAL4 and assessed the phenotype in nephrocytes in a blinded fashion (Figure 8, J–R). Variants W815* and G39R exhibited a significantly more disrupted slit diaphragm architecture compared with rescue using wild-type murine Tbc1d8b. This demonstrates a severe functional effect of these variants. Variant L191S retained a significant ability to rescue. This does not formally rule out this variant, but indicates that at least partial function is retained. The mutant protein might retain the ability to rescue under the higher expression levels of an overexpression rescue. The effect of this variant remains unclear. The confirmed variant G39R was derived from an individual with an age of onset of proteinuria of 59 years, suggesting FSGS associated with variants of TBC1D8B may manifest much later in life. Taken together, our Drosophila data suggest a hypomorphic loss-of-function for two of the variants.
Discussion
In this study, we investigated the functional role of the recently discovered disease gene TBC1D8B in Drosophila. A stable genetic deletion exhibited a nephrocyte-restricted phenotype consistent with the clinical picture of patients. We successfully applied MMEJ–mediated genome editing for the first time in flies, and observed that Tbc1d8b protein localizes most prominently in subdomains of mature early and late endosomes. Utilizing functional ex vivo assays to study endocytic dynamics, we showed delayed endosomal transport and acidification upon loss of Tbc1d8b. Exploring the connection to nephrin trafficking, we found that Tbc1d8b is required for rapid turnover and Rab5-mediated nephrin endocytosis. Mammalian Tbc1d8b rescued loss of the Drosophila ortholog, but resulted in selective mistrafficking of nephrin when overexpressed in excess. Finally, we positively evaluated two novel variants of TBC1D8B proposing a personalized platform for TBC1D8B-associated FSGS.
Our findings shift the focus of the emergent understanding of TBC1D8B-associated FSGS from recycling toward endosomal maturation. This disease gene is not exclusively a RAB11-GAP, but plays a much broader role within the endocytic pathway. Given the complexity and interconnectedness of the endolysosomal system, the impaired degradation observed with loss of Tbc1d8b could also be due to defects in earlier steps of endocytosis, likely in the transition from early to late endosome.
TBC1D8B possibly elicits a functional role independently of the TBC/GAP function and its target RAB11. GAP-independent roles were also described for other TBC proteins.9,35 The GRAM domains in particular might bind proteins or lipids,36 because nephrin resides in lipid rafts.37 Nevertheless, a contribution of TBC-mediated RAB11 regulation appears likely. Disease-causing variants are distributed across the entire protein and the catalytically inactive R537K-mutant entails smaller vesicles, similar to patient-derived variants. Lacking inhibition of Rab11 could decrease the vesicle size through nephrin recycling. To elucidate the mechanism of the novel functional aspect of TBC1D8B on the molecular level, more investigation is necessary.
TBC1D8B is expressed in various tissues in both flies and humans. However, the endocytic function seems largely dispensable in both, which is likely achieved through upregulation of compensatory endosomal regulators. However, genetic compensation fails for trafficking of nephrin. Interaction of TBC1D8B with nephrin may be needed to prevent premature recycling. This may help to expose nephrin to an acidic environment in endosomes, which promotes shedding of attached particles to prevent filter clogging.20 We discover novel variants of TBC1D8B within a small FSGS cohort. Although not being representative, this suggests variants in TBC1D8B may underlie FSGS not that infrequently. In conclusion, our findings identify a novel function of Tbc1d8b for endosomal maturation and underline its essential role for nephrin endocytosis.
Disclosures
M. Pollak reports having consultancy agreements with Vertex; reports having an ownership interest in Apolo1bio; reports receiving research funding from Vertex; reports receiving honoraria from various academic talks; reports having patents or royalties with Athena Diagnostics; reports having an advisory or leadership role NephCure Foundation scientific advisory board; and reports having other interests or relationships with the Scientific Advisory Board, NephCure Foundation. T. Hermle reports receiving research funding from Atomwise. All remaining authors have nothing to disclose.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (grants HE 7456/3-1, HE 7456/4-1 to T. Hermle), project-ID 431984000–SFB 1453 (to T. Hermle and G. Walz), the National Institutes of Health (grant RC2-DK122397 to M. R. Pollak); the China Scholarship Council (Research Fellowship to M. Chen), the Deutsche Gesellschaft für Innere Medizin, (Clinician Scientist Fellowship to T. Hermle and doctoral fellowship to K. Lang), and the Berta-Ottenstein-Programme of the Faculty of Medicine, University of Freiburg (Advanced Clinician Scientist Fellowship to T. Hermle). C. Leroy, H. Heinkele, J. Milosavljevic, and L. L. Kampf acknowledge support by the MOTI-VATE program of the Medical Faculty of the University of Freiburg (MOTI-VATE Fellowship).
Supplementary Material
Acknowledgments
We thank Charlotte Meyer for excellent technical support and Roland Nitschke, Life Imaging Centre, University of Freiburg, for help with confocal microscopy. We thank the DSHB for antibodies and the Bloomington Drosophila Stock Center for providing fly stocks. Gerd Walz acknowledges support by Germany's Excellence Strategy: CIBSS– EXC-2189– project-ID 390939984. Because Martin Pollak is an associate editor of the JASN, he was not involved in the peer review process for this manuscript. A guest editor oversaw the peer review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Slit Diaphragms: Junctions That Never Sleep,” on pages 2127–2128.
Author Contributions
T. Hermle conceptualized the study; J. Milosavljevic was responsible for the formal analysis; T. Hermle and M. Pollak were responsible for the funding acquisition; M. Chen, H. Heinkele, M. Helmstädter, T. Hermle, L. Gerstner, L.L. Kampf, S. Kayser, A. Knob, J. Milosavljevic, K. Lang, C. Lempicki, C. Leroy, D. Spitz, and M. Wang were responsible for the investigation; T. Hermle and J. Milosavljevic were responsible for the methodology; T. Hermle was responsible for the project administration; T. Hermle was responsible for the resources; T. Hermle and M. Pollak provided supervision; T. Hermle and J. Milosavljevic were responsible for the validation; T. Hermle and J. Milosavljevic were responsible for the visualization; and T. Hermle, J. Milosavljevic, M. Pollak, and G. Walz reviewed and edited the manuscript.
Data Sharing Statement
The datasets generated during this study are available from the corresponding author on reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022030275/-/DCSupplemental
Supplemental Figure 1. Validation of Tbc1d8bΔ1.
Supplemental Figure 2. Loss of Tbc1d8b affects nephrin localization and barrier permeability.
Supplemental Figure 3. Microhomology-mediated end joining and localization of Tbc1d8b.
Supplemental Figure 4. Effects of Rab5-RNAi for sequential tracer uptake.
Supplemental Figure 5. Rab5- and Rab7-staining in Tbc1d8b-LOF and validation of Hrs knockdown and antibody.
Supplemental Figure 6. Alignment of human, murine and Drosophila Tbc1d8b and coexpression with human Rab11.
Supplemental Figure 7. Overexpression studies of murine Tbc1d8b.
Supplemental Figure 8. Patient-derived mutations in TBC1D8B.
Supplemental Table 1. Primer sequences.
References
- 1.Hays T, Groopman EE, Gharavi AG: Genetic testing for kidney disease of unknown etiology. Kidney Int 98: 590–600, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, et al. : Diagnostic utility of exome sequencing for kidney disease. N Engl J Med 380: 142–151, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vivante A, Hildebrandt F: Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12: 133–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devuyst O, Knoers NV, Remuzzi G, Schaefer F; Board of the Working Group for Inherited Kidney Diseases of the European Renal Association and European Dialysis and Transplant Association : Rare inherited kidney diseases: Challenges, opportunities, and perspectives. Lancet 383: 1844–1859, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Preston R, Stuart HM, Lennon R: Genetic testing in steroid-resistant nephrotic syndrome: Why, who, when and how? Pediatr Nephrol 34: 195–210, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lovric S, Ashraf S, Tan W, Hildebrandt F: Genetic testing in steroid-resistant nephrotic syndrome: When and how? Nephrol Dial Transplant 31: 1802–1813, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kampf LL, Schneider R, Gerstner L, Thünauer R, Chen M, Helmstädter M, et al. : TBC1D8B mutations implicate RAB11-dependent vesicular trafficking in the pathogenesis of nephrotic syndrome. J Am Soc Nephrol 30: 2338–2353, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorval G, Kuzmuk V, Gribouval O, Welsh GI, Bierzynska A, Schmitt A, et al. : TBC1D8B loss-of-function mutations lead to x-linked nephrotic syndrome via defective trafficking pathways. Am J Hum Genet 104: 348–355, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fukuda M: TBC proteins: GAPs for mammalian small GTPase Rab? Biosci Rep 31: 159–168, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Hermle T, Schneider R, Schapiro D, Braun DA, van der Ven AT, Warejko JK, et al. : GAPVD1 and ANKFY1 mutations implicate RAB5 regulation in nephrotic syndrome. J Am Soc Nephrol 29: 2123–2138, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue K, Ishibe S: Podocyte endocytosis in the regulation of the glomerular filtration barrier. Am J Physiol Renal Physiol 309: F398–F405, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, et al. : beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T: Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol 20: 2534–2545, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soda K, Balkin DM, Ferguson SM, Paradise S, Milosevic I, Giovedi S, et al. : Role of dynamin, synaptojanin, and endophilin in podocyte foot processes. J Clin Invest 122: 4401–4411, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bechtel W, Helmstädter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, et al. : Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J Am Soc Nephrol 24: 727–743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huotari J, Helenius A: Endosome maturation. EMBO J 30: 3481–3500, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wandinger-Ness A, Zerial M: Rab proteins and the compartmentalization of the endosomal system. Cold Spring Harb Perspect Biol 6: a022616, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al. : The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hermle T, Braun DA, Helmstädter M, Huber TB, Hildebrandt F: Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J Am Soc Nephrol 28: 1521–1533, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang K, Milosavljevic J, Heinkele H, Chen M, Gerstner L, Spitz D, et al. : Selective endocytosis controls slit diaphragm maintenance and dynamics in Drosophila nephrocytes. eLife 11: e79037, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Port F, Chen HM, Lee T, Bullock SL: Optimized CRISPR/Cas tools for efficient germline and somatic genome engineering in Drosophila. Proc Natl Acad Sci U S A 111: E2967–E2976, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bour BA, Chakravarti M, West JM, Abmayr SM: Drosophila SNS, a member of the immunoglobulin superfamily that is essential for myoblast fusion. Genes Dev 14: 1498–1511, 2000 [PMC free article] [PubMed] [Google Scholar]
- 23.Galletta BJ, Chakravarti M, Banerjee R, Abmayr SM: SNS: Adhesive properties, localization requirements and ectodomain dependence in S2 cells and embryonic myoblasts. Mech Dev 121: 1455–1468, 2004 [DOI] [PubMed] [Google Scholar]
- 24.Preibisch S, Saalfeld S, Tomancak P: Globally optimal stitching of tiled 3D microscopic image acquisitions. Bioinformatics 25: 1463–1465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang M, Chun J, Genovese G, Knob AU, Benjamin A, Wilkins MS, et al. : Contributions of rare gene variants to familial and sporadic FSGS. J Am Soc Nephrol 30: 1625–1640, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Brolosy MA, Kontarakis Z, Rossi A, Kuenne C, Günther S, Fukuda N, et al. : Genetic compensation triggered by mutant mRNA degradation. Nature 568: 193–197, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakuma T, Nakade S, Sakane Y, Suzuki KT, Yamamoto T: MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat Protoc 11: 118–133, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Lin DW, Chung BP, Huang JW, Wang X, Huang L, Kaiser P: Microhomology-based CRISPR tagging tools for protein tracking, purification, and depletion. J Biol Chem 294: 10877–10885, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lloyd TE, Atkinson R, Wu MN, Zhou Y, Pennetta G, Bellen HJ: Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108: 261–269, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Gerstner L, Chen M, Kampf LL, Milosavljevic J, Lang K, Schneider R, et al. : Inhibition of endoplasmic reticulum stress signaling rescues cytotoxicity of human apolipoprotein-L1 risk variants in Drosophila. Kidney Int 101: 1216–1231, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, et al. : Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci 118: 3663–3673, 2005 [DOI] [PubMed] [Google Scholar]
- 32.Hermle T, Guida MC, Beck S, Helmstädter S, Simons M: Drosophila ATP6AP2/VhaPRR functions both as a novel planar cell polarity core protein and a regulator of endosomal trafficking. EMBO J 32: 245–259, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillooly DJ, Morrow IC, Lindsay M, Gould R, Bryant NJ, Gaullier JM, et al. : Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J 19: 4577–4588, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan X, Eathiraj S, Munson M, Lambright DG: TBC-domain GAPs for Rab GTPases accelerate GTP hydrolysis by a dual-finger mechanism. Nature 442: 303–306, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Martinu L, Masuda-Robens JM, Robertson SE, Santy LC, Casanova JE, Chou MM: The TBC (Tre-2/Bub2/Cdc16) domain protein TRE17 regulates plasma membrane-endosomal trafficking through activation of Arf6. Mol Cell Biol 24: 9752–9762, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ercan B, Naito T, Koh DHZ, Dharmawan D, Saheki Y: Molecular basis of accessible plasma membrane cholesterol recognition by the GRAM domain of GRAMD1b. EMBO J 40: e106524, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, et al. : Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am J Pathol 159: 1069–1077, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.