Abstract
Streptococcus pneumoniae and Moraxella catarrhalis are two common respiratory pathogens, colonizing as many as 54 and 72% of children, respectively, by 1 year of age. The immune responses to surface protein A of S. pneumoniae (PspA) and the high-molecular-weight outer membrane protein of M. catarrhalis (UspA) in the sera of various age groups in the general population and in the nasopharynges of 30 children monitored from birth through 1 year of age were evaluated. Immunoglobulin G (IgG) was the dominant serum antibody to PspA and UspA. Whereas the serum antibody response to PspA peaked in childhood, the antibody response to UspA peaked in adulthood. In the first 2 years of life, comparable amounts of IgM and IgG antibodies to both proteins were observed. In older persons, IgG antibodies to both antigens predominated over IgM antibodies. The levels of IgA antibody to these antigens in serum remained low during the first 2 years of life. The levels of IgM antibody to the two antigens in serum exceeded the levels of IgA antibody to the same two antigens throughout life. Although IgA was the dominant antibody to PspA and UspA in airway secretions, it was detected in a minority of the children (3 of 15 for PspA and 0 of 15 for UspA). Even the majority of the children previously colonized with these pathogens lacked antibody to them in their secretions.
The upper airway rapidly becomes colonized with bacteria after birth. Although the majority of members of the normal flora are nonpathogenic, potential pathogens such as Streptococcus pneumoniae and Moraxella catarrhalis initiate colonization early in life in a manner similar to nonpathogens and continue to colonize the host throughout childhood. By 6 months of age, more than one-third of children will have been colonized with S. pneumoniae and more than one-half will have been colonized with M. catarrhalis (10). Between 6 and 12 months of life, approximately one-fourth of children will be colonized monthly with one or both of these pathogens (10). Typically, the pathogens remain in the airway for several months before disappearing or are replaced by a different strain of the same pathogen (1, 13, 18, 22). The host factors responsible for elimination of the pathogen are not well understood. However, experience with another airway pathogen, nontypeable Haemophilus influenzae, suggests that the local immune response may play a role in controlling the trafficking of the organisms (9, 14).
Pneumococcal surface protein A (PspA) and the high-molecular-weight protein of the outer membrane of M. catarrhalis (UspA) were selected as target antigens for immunologic study because they are both potential vaccine candidates. The plan of this study was to determine whether these antigens were recognized as immunogens in young children and whether the magnitude or presence of the response correlated with known information about the colonization status of the children.
MATERIALS AND METHODS
Study populations.
In 1990, we initiated a prospective study of nasopharyngeal colonization and otitis media. Children were enrolled at birth and monitored through the age of 2 years. They were examined monthly from 1 to 6 months of age and then at 8, 10, 12, 15, 18, 21, and 24 months. At each of these visits, a nasopharyngeal culture was obtained. Middle ear pathogens such as S. pneumoniae, M. catarrhalis and nontypeable H. influenzae were identified in the culture (10). Because the original study was designed to focus on the local immune response to nontypeable H. influenzae, nasopharyngeal secretions (NPS) were collected 1 month after the initial colonization with nontypeable H. influenzae for antibody determinations (14). Thus, the NPS may have been collected before, during, or after colonization with S. pneumoniae and M. catarrhalis. Thirty children represent the study group in this report. Seventeen of the children were colonized with either S. pneumoniae or M. catarrhalis prior to the collection of NPS; 13 children were not colonized.
Three adults with chronic lung disease were also selected for study. They were part of a large prospective study being conducted by T. Murphy and associates at the Veterans Administration Hospital in Buffalo, N.Y. The adults were examined monthly after entry into the study. At each visit, sputum samples were collected. Potential pathogens such as S. pneumoniae, M. catarrhalis, and nontypeable H. influenzae were identified in culture.
A total of 80 children and 10 adults provided blood samples for serum antibody determinations. The children had been monitored prospectively from the age of 2 months through 5 years as part of a poliovirus vaccine trial (8). A pool of sera from 10 children was prepared at 2, 4, and 5 months and 1, 2, 3, 4, and 5 years. A pool of sera from 10 normal adults was also prepared.
Preparation of NPS, sputa, and sera for antibody determinations.
NPS were aspirated via a soft plastic catheter into a trap. A 1-ml volume of saline was aspirated through the catheter to remove residual secretions. The secretions were delivered to the laboratory on ice. The secretions were centrifuged at 2,000 rpm for 10 min to remove large particles. The supernatants were filtered through a 0.45-μm-pore-size filter. The filtrates were frozen at −70°C until assayed for antibody. Sputa were processed in a similar manner except for the use of saline. Sera were separated from whole blood by centrifuging for 10 minutes at 2,000 rpm. The sera was frozen at −70°C until assayed for antibody. Ten sera were pooled for antibody determinations for each age group.
Antigens for the enzyme-linked immunosorbent assay (ELISA).
The PspA from S. pneumoniae strain Rx1 was used for this study. Recombinant Rx1 PspA was purified by nickel affinity chromotography from a cloned gene product which includes amino acids 1 to 303 of the mature PspA protein and is 37 kDa long (29). Rx1 PspA is a representative member of one of two major families of PspA molecules. UspA of M. catarrhalis came from strain 25240 (17). The UspA preparation produced a band with a molecular mass of approximately 400 kDa on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with Coomassie blue staining.
Procedure for ELISA.
The wells in a 96-well microtiter plate (Immulon 1B; Dynex Technologies, Chantilly, Va.) were coated overnight at room temperature with 100 μl of M. catarrhalis UspA containing 0.25 μg of protein per ml in carbonate-bicarbonate coating buffer (pH 9.6) or 100 μl of S. pneumoniae PspA containing 1 μg of protein per ml. The wells were washed three times with phosphate-buffered saline (PBS) (pH 7.2)–0.05% Tween 20. NPS, sputa, and sera were similarly diluted in PBS–0.05% Tween 20–1% bovine serum albumin and added to the wells in 100-μl volumes. The plates were incubated at room temperature for 2 h. Preliminary studies demonstrated that 2 h of incubation was optimal. At the same time, a microtiter plate for immunoglobulin standards was prepared. The wells were coated with rabbit anti-human immunoglobulin G (IgG) (1:10,000), rabbit anti-human IgM (1:5,000), or rabbit anti-human IgA (1:20,000) (Dako Corp., Santa Barbara, Calif.) in carbonate-bicarbonate coating buffer and incubated overnight at room temperature. The wells were washed three times with PBS–0.05% Tween 20. Portions (100 μl) of decreasing concentrations of pure human IgG, IgM, or IgA, starting with 0.85, 0.29, and 0.25 μg/ml, respectively, were added, and the wells were incubated at room temperature for 2 h. The wells were washed three times in PBS–0.05% Tween 20. Horseradish peroxidase-conjugated rabbit anti-human IgG (1:10,000), IgM (1:5,000), or IgA (1:20,000) were added to the wells (100 μl per well), and the wells were incubated for 1 h at 37°C. The wells were washed three times in PBS–0.05% Tween 20 before 100 μl of o-phenylenediamine dihydrochloride (OPD; Sigma Chemical Co., St. Louis, Mo.), prepared in citrate buffer (pH 5.0), was added at 1 μg/ml. The OPD was incubated for 30 min in the dark. The reaction was stopped by adding 75 μl of 1 N H2SO4 to each well. Antibody concentrations were calculated from the standard isotypic immunoglobulin curve and expressed as micrograms or nanograms of antibody per milliliter.
The optimal concentrations of UspA and PspA needed to coat the wells were determined in premilinary studies by conducting dose-response experiments. The optimal concentrations of anti-human IgG, IgM, and IgA reagents were determined by checkerboard analysis. Preliminary studies with hypogammaglobulinemic sera demonstrated the specificity of the ELISA reactions. Two normal adults provided NPS, prepared in the same manner as for the samples from the children, as positive controls for the NPS assays with young children. A pool of NPS from 50 children younger than 1 year in the original study group was also used as a positive control for subsequent NPS assays to ensure the stability of IgA stored in our freezer for >8 years. These controls demonstrated detectable levels of total and specific IgA but low or no detectable levels of total and specific IgG or IgM. The sensitivity of the IgG, IgM, and IgA assays were 1, 5, and 5 ng/ml, respectively.
Statistics.
All comparasions between two groups were assessed by the Mann-Whitney U test for independent samples. Less than 5 ng of IgM and IgA per ml and less than 1 ng of IgG per ml were assigned the value 0. The results are presented as P values. All statistical tests were based on a significant value of P < 0.05.
RESULTS
Serum antibody.
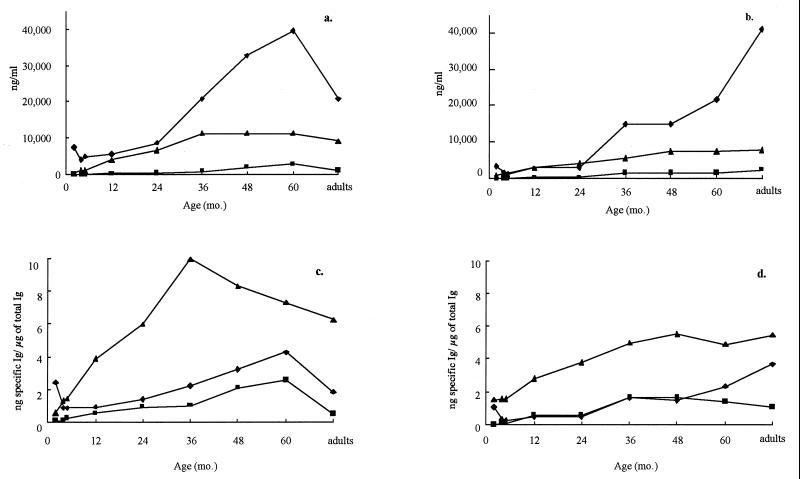
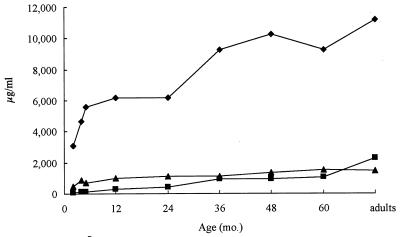
The age groups selected for study were children of 2, 4, and 5 months and 1, 2, 3, 4, and 5 years and adults between 20 and 60 years. Total levels of IgG, IgM, and IgA in serum as well as specific antibody concentrations of PspA of S. pneumoniae and UspA of M. catarrhalis in serum were determined. As seen in Fig. 1a and b, IgG was the dominant antibody class of specific antibody detected, followed by IgM. Interestingly, there was little if any IgG antibody response until 2 years of age, even though the total IgG concentrations rose rapidly in the first 2 years (Fig. 2). The pattern of specific IgG antibody differed between the two pathogens. The response to PspA was more robust and peaked during childhood. Levels of PspA IgG antibody in adults declined to levels seen at 36 months. In contrast, the UspA IgG antibody level rose more gradually and peaked in adulthood. These patterns were corroborated when the specific antibody was represented as a proportion of total IgG (Fig. 1c and d). The IgM response to both pathogens in serum represented a higher proportion of the total corresponding immunoglobulin than either IgG or IgA specific antibody, and the proportion peaked between 36 and 48 months. IgA antibody levels to both antigens in serum remained relatively low throughout life, varying between undetectable at 2 months for M. catarrhalis to a high of 2,735 ng/ml for PspA at 5 years.
FIG. 1.
Antibody to PspA of S. pneumoniae and UspA of M. catarrhalis in serum according to age. (a) PspA-specific antibody; (b) UspA-specific antibody; (c) PspA-specific antibody/total antibody; (d) UspA specific antibody/total antibody. ⧫, IgG; ▴, IgM; ■, IgA.
FIG. 2.
Total IgG (⧫), IgM (▴), and IgA (■) antibody levels in serum according to age.
Mucosal antibody.
IgG, IgM, and IgA antibody responses to PspA and UspA were first measured in the NPS of two normal adults. Only IgA-specific antibody was detected. Based on this observation, as well as on our previous experience with mucosal immunity to nontypeable H. influenzae (14), we measured the IgA response exclusively in NPS from 30 infants. These children had been monitored prospectively from birth through the first year of life. Each child was examined at nine monthly scheduled visits. At each visit a nasopharyngeal culture was obtained. Colonization with either S. pneumoniae or M. catarrhalis was documented and recorded according to the age of the child. As seen in Table 1, seven children were colonized with S. pneumoniae before NPS were collected for antibody determinations. The mean age at the time of antibody determination was 6.1 months. NPS were collected 1 month after S. pneumoniae colonization in three children, 2 months after colonization in three children, and 4 months after colonization in one child. Even though total IgA was detected in the NPS of all children, only two NPS samples had any measurable antigen specific antibody (9 and 18 ng/ml). Each of the seven children were colonized on more than one occasion. Six of the seven children were colonized after the NPS collection, and these included the two children with antigen-specific mucosal IgA antibody. Eight children who had not been colonized prior to NPS collection were also studied. They were similar in age to the colonized group. Four of the noncolonized children became colonized after the NPS collection. One of these children had 5 μg of antigen-specific mucosal antibody per ml detected at 4.5 months of age even though NPS cultures at 1 to 4 months of age were negative for S. pneumoniae, suggesting prior undetected colonization.
TABLE 1.
IgA antibody to PspA of S. pneumoniae in NPS of children
| Subject | Age (mo) at time ofa:
|
IgA titera
|
|||
|---|---|---|---|---|---|
| NPS collection | Colonization | Total (μg/ml) | Specific (ng/ml) | Specific/ total (ng/μg) | |
| Noncolonized | |||||
| 1 | 2.5 | 3, 4, 5, 10 | <0.005 | <5 | |
| 2 | 4.0 | 12 | 0.2 | <5 | |
| 3 | 4.1 | 5, 8 | 7.1 | <5 | |
| 4 | 4.2 | 0 | 9.5 | <5 | |
| 5 | 4.4 | 0 | 1.9 | <5 | |
| 6 | 4.5 | 0 | 7.6 | 5.0 | 0.658 |
| 7 | 6.0 | 8, 10 | 91.2 | <5 | |
| 8 | 9.0 | 0 | 0.8 | <5 | |
| Mean ± SD | 4.8 ± 1.9a | 16.9 ± 33.0c | |||
| Colonized | |||||
| 1 | 3.9 | 3, 4, 6, 10 | 76.0 | <5 | |
| 2 | 5.0 | 4, 6 | 38.0 | 17.8 | 0.469 |
| 3 | 5.2 | 4, 10 | 4.7 | <5 | |
| 4 | 6.0 | 4, 5, 8, 12 | 22.8 | 8.9 | 0.390 |
| 5 | 7.5 | 3, 6, 8, 10, 12 | 7.1 | <5 | |
| 6 | 7.5 | 5, 6 | 19.0 | <5 | |
| 7 | 7.6 | 5, 8, 10, 12 | 7.6 | <5 | |
| Mean ± SD | 6.1 ± 1.5b | 25.0 ± 25.3d | |||
a versus b, P = 0.148; c versus d, P = 0.105.
Ten children were colonized with M. catarrhalis before collection of NPS for antibody determinations (Table 2). The mean age at the time of antibody determination was 6.1 months. NPS were collected 1 month after colonization in one child, 2 months after colonization in three children, 4 months after colonization in three children, and 5 months after colonization in one child. Three children were colonized at the time of NPS collection, but all of them had been colonized previously. Even though total mucosal IgA was detected in the secretions of all children, none of the samples had measurable antigen-specific antibody. Of the 10 children, 8 were colonized after the NPS collection. Five children who had not been colonized prior to NPS collection were studied also. Their mean age was 4.4 months, significantly younger than the control group. The concentration of total mucosal IgA in the NPS of noncolonized children was significantly lower than that in the colonized group (P = 0.008). This may reflect the age difference in the two groups. None of these children had detectable antigen-specific antibody.
TABLE 2.
IgA antibody to UspA of M. catarrhalis in NPS of children
| Subject | Age (mo.) at time ofa:
|
IgA titerab
|
||
|---|---|---|---|---|
| NPS collection | Colonization | Total (μg/ml) | Specific (ng/ml) | |
| Noncolonized | ||||
| 1 | 3.1 | 5, 8 | 0.5 | <5 |
| 2 | 3.9 | 5, 6, 12 | 0.7 | <5 |
| 3 | 4.1 | 10, 12 | 0.9 | <5 |
| 4 | 5.2 | 0 | 4.7 | <5 |
| 5 | 5.8 | 10, 12 | 1.2 | <5 |
| Mean ± SD | 4.4 ± 1.1a | 1.6 ± 1.8c | ||
| Colonized | ||||
| 1 | 4.1 | 3, 12 | 136.8 | <5 |
| 2 | 5.0 | 4, 5, 6, 8 | 136.8 | <5 |
| 3 | 5.2 | 3 | 4.7 | <5 |
| 4 | 5.9 | 3, 4, 12 | 6.6 | <5 |
| 5 | 6.1 | 2 | 2.4 | <5 |
| 6 | 6.3 | 1, 8, 10 | 9.5 | <5 |
| 7 | 6.4 | 3, 4, 5, 8 | 4.3 | <5 |
| 8 | 6.6 | 2, 3, 4, 6, 8 | 3.3 | <5 |
| 9 | 7.0 | 3, 4, 8, 10 | 19.0 | <5 |
| 10 | 8.3 | 1, 3, 5, 6, 8, 10 | 53.2 | <5 |
| Mean ± SD | 6.1 ± 1.2b | 37.7 ± 54.4d | ||
a versus b, P = 0.02; c versus d, P = 0.008.
The ratio of specific to total IgA titer is zero for all cases because the specific titer is below the detection level.
Because of the failure to detect significant numbers of colonized children with antigen-specific mucosal antibody, we next examined sputa from three adults with chronic lung disease. One of them had been colonized with S. pneumoniae 1, 7, and 8 months before sputum collection (Table 3). Each of them had been colonized with M. catarrhalis; one had been colonized 2 months before sputum collection, one had been colonized 10 months before sputum collection, and one had been colonized 3 months before sputum collection. IgA-specific mucosal antibody to PspA was detected in all three specimens. The highest concentration was detected in subject 3, who had been colonized prior to sputum collection. IgA-specific mucosal antibody to the UspA of M. catarrhalis was detected in all three specimens.
TABLE 3.
IgA antibody to PspA of S. pneumoniae and UspA of M. catarrhalis in sputa of adults with chronic obstructive lung disease
| Subject | Time (mo) of study
|
IgA titer
|
|||
|---|---|---|---|---|---|
| Sputum collection | Colonization | Total (μg/ml) | Specific (ng/ml) | Specific/total (ng/μg) | |
| S. pneumoniae | |||||
| 1 | 5 | 0 | 202 | 284.9 | 1.407 |
| 2 | 20 | 0 | 53 | 83.1 | 1.573 |
| 3 | 12 | 4, 5, 11, 14 | 405 | 22,794.2 | 56.264 |
| M. catarrhalis | |||||
| 1 | 5 | 3, 8 | 202 | 163.2 | 0.806 |
| 2 | 20 | 10 | 53 | 6.7 | 0.127 |
| 3 | 12 | 9 | 405 | 1,899.5 | 468.860 |
DISCUSSION
Although both S. pneumoniae and M. catarrhalis are currently considered airway pathogens, S. pneumoniae has been recognized as a major respiratory pathogen for 100 years whereas M. catarrhalis has been recognized as a major respiratory pathogen only during the past 30 years (7). The amount of information about the pneumococcus, associated disease, and immunity has increased substantially. A number of recent publications described the importance of S. pneumoniae PspA in both disease production and immunity. For example, PspA is attached to the surface of the pneumococcus by the C-terminal end of the molecule, and much of the immune response elicited by immunization in animals is directed against the N-terminal α-helical portion of the molecule (20). The pspA gene is expressed in all strains of pneumococci, regardless of their capsular serotype (5). Antibody responses to PspA in animals protect against sepsis and nasopharyngeal colonization (25, 26). Although PspA is a heterogenous protein, there is a high degree of serologic cross-reactivity among different PspA molecules from the two major families of PspA (2, 23). A single recombinant PspA protein is capable of inducing protection against pneumococcal strains of diverse capsular serotypes and different PspA serotypes in animal models (2, 19). Thus, it is hypothesized that a single PspA protein may be able to provide protection against multiple diverse strains of S. pneumoniae (2, 25).
In 1988, Bartos and Murphy (1) first demonstrated the homogeneity of the outer membrane proteins of a diverse group of M. catarrhalis strains. A high-molecular-weight protein, UspA, in the outer membrane was subsequently identified, purified, and found to be a target for protective antibodies (15, 17). The protein varies between 300 and 720 kDa (15, 17) and is found in all strains of M. catarrhalis. UspA contains highly conserved as well as variable surface-exposed epitopes (3). The conserved epitopes are immunogenic and elicit functional antibodies. Convalescent-phase sera from adults with M. catarrhalis pneumonia contain antibody to the protein (15). Antibody to the protein enhances pulmonary clearance of the organism in an animal model. We chose to study the immune response to the UspA in young children and secondarily in the general population.
The serological survey of sera in the general population in the present study clearly demonstrated a predominance of IgG antibodies to S. pneumoniae and M. catarrhalis. Although the concentration of PspA IgG antibody was slightly higher than that of UspA IgG antibody early in life, the concentrations of both types of antibody remained low till after the age of 2 years. A decline in PspA IgG antibody concentration was noted in sera from adults, and this was reflected in a similar decline in the proportion of total IgG represented by PspA-specific IgG. This pattern was observed previously with nontypeable H. influenzae but to a lesser degree (28). In contrast, the level of M. catarrhalis UspA IgG antibody peaked during adulthood. This pattern has been observed recently in another study of M. catarrhalis (4). The reason for the age-related difference between the organisms is unclear, since colonization with any of the pathogens is relatively uncommon among adults (6, 16, 24).
The slow induction of an IgG antibody response to the antigens, especially UspA, in serum early in life may relate to the type of IgG subclass specific antibodies stimulated. For example, Goldblatt et al. (12) demonstrated that IgG3 antibodies recognized all of the outer membrane proteins displayed by M. catarrhalis while IgG1, IgG2, and IgG4 recognized only the 82- and 60-kDa proteins. Children younger than 4 years exhibited no detectable IgG3 specific antibody to M. catarrhalis in their study, and thus their immune systems would not have detected antibody to UspA. Chen et al. (4) confirmed the relatively poor IgG3 specific antibody response to M. catarrhalis UspA in children younger than 4 years. In contrast to the importance of an IgG3 response to M. catarrhalis, IgG1 proved to be the major antibody subclass response to nontypeable H. influenzae in children younger than 4 years, thus explaining the frequent detection of anti-H. influenzae antibody in young children (27). Little or no information is available about the IgG subclass responses to PspA because immunologic studies of the pneumococcus have focused on the responses to capsular polysaccharide antigens (21).
Only 2 of 7 children colonized with S. pneumoniae and 0 of 10 children colonized with M. catarrhalis in the present study had detectable specific IgA antibodies in NPS, even though NPS were collected at the time of colonization as well as from 1 to 5 months after colonization. These results differ from those observed with nontypeable H. influenzae, since every young child colonized with nontypeable H. influenzae developed antigen-specific IgA antibody in NPS (14). Children who generated a good local antibody response to H. influenzae tended to decrease or eliminate subsequent colonization with nontypeable H. influenzae (9, 14). NPS had been processed similarly in each of these studies; thus, dilution with saline at the time of collection does not explain the differences observed in detectable specific antibody. In addition, the levels of total IgA antibody detected in the NPS were consistent with the levels expected in young children, affirming the stability of the immunoglobulin during storage at −70°C. The less robust response to the two immunogens in the present study could be interpreted in several ways. First, for PspA, some of the response might have been missed due to the diversity of the PspA molecule and the nature of our ELISA screening procedure. Only a recombinant PspA was used to coat the ELISA plates; this was a family 1 PspA protein. Some colonizations may have occurred with strains containing a heterogeneous PspA protein from family 2. Although cross-reactivity occurs between all PspA molecules in high-titer sera, the low titers of the response in children coupled with the diversity of coating antigen and eliciting antigen may have lowered the detection limits of our assay. The detection of specific mucosal IgA antibodies in one of the “control” samples in which no colonization had been detected suggests that undetected colonization may have occurred in that child. Thus, in at least some children, local responses to PspA might follow initial colonization and the colonization itself may be of short duration. It is also possible that capsular antibody plays a role in limiting colonization of S. pneumoniae. For example, in mice, immunization with capsular polysaccharide-tetanus toxoid conjugate reduces but does not eliminate colonization (25). In humans, immunization with a polysaccharide-protein conjugate vaccine reduces but does not eliminate colonization even with homologous types (29). In a similar manner, antibody to PspA probably aids in preventing or reducing colonization but is not the only factor.
Epidemiologic studies with both S. pneumoniae and M. catarrhalis indicate that acquisition of the strain and length of colonization decrease with increasing age, suggesting that maturation of the immune system in some way plays a role in controlling colonization patterns (11, 13). The results of this study suggest that both PspA and UspA are recognized as immunogens in an age-dependent manner. The data also suggest that a single episode of colonization does not induce a strong mucosal response to these two antigens.
ACKNOWLEDGMENTS
We thank Timothy Murphy for providing adult sputa as well as clinical information about the subjects in his prospective study of chronic obstructive lung disease. We thank Judith Wolf for technical assistance.
REFERENCES
- 1.Bartos L C, Murphy T F. Comparison of the outer membrane proteins of 50 strains of Branhamella catarrhalis. J Infect Dis. 1988;158:761–766. doi: 10.1093/infdis/158.4.761. [DOI] [PubMed] [Google Scholar]
- 2.Briles D E, Tart R C, Swiatlo E, Dillard J P, Smith P, Benton K A, Ralph B A, Brooks-Walter A, Crain M J, Hollingshead S K, McDaniel L S. Pneumococcal diversity: considerations for new vaccine strategies with emphasis on pneumococcal surface protein A (PspA) Clin Microbiol Rev. 1998;11:645–657. doi: 10.1128/cmr.11.4.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen D, McMichael J C, VanDerMeid K R, Hahn D, Mininni T, Cowell J, Eldridge J. Evaluation of purified UspA from Moraxella catarrhalis as a vaccine in a murine model after active immunization. Infect Immun. 1996;64:1900–1905. doi: 10.1128/iai.64.6.1900-1905.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen D, Barniak V, VanDerMeid K R, McMichael J C. The levels and bactericidal capacity of antibodies directed against the UspA1 and UspA2 outer membrane proteins of Moraxella (Branhamella) catarrhalis in adults and children. Infect Immun. 1999;67:1310–1316. doi: 10.1128/iai.67.3.1310-1316.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crain M J, Waltman II W D, Turner J S, Yother J, Talkington D F, Mcdaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ejlertsen T. Pharyngeal carriage of Moraxella (Branhamella) catarrhalis in healthy adults. Eur J Clin Microbiol Infect Dis. 1990;10:89. doi: 10.1007/BF01964414. [DOI] [PubMed] [Google Scholar]
- 7.Faden H. Otitis media. In: Long S, Pickering L, Prober C, editors. Principles and practice of pediatric infectious diseases. New York, N.Y: Churchill Livingstone, Inc.; 1997. pp. 211–227. [Google Scholar]
- 8.Faden H, Modlin J F, Thomas M L, McBean A M, Ferdon M B, Ogra P L. Comparative evaluation of immunization with live attenuated and enhanced-potency inactivated trivalent poliovirus vaccines in childhood: systemic and local immune responses. J Infect Dis. 1990;162:1291–1297. doi: 10.1093/infdis/162.6.1291. [DOI] [PubMed] [Google Scholar]
- 9.Faden H, Duffy L, Williams A, Krystofik D A, Wolf J. Epidemiology of nasopharyngeal colonization with nontypeable Haemophilus influenzae in the first 2 years of life. J Infect Dis. 1995;172:132–135. doi: 10.1093/infdis/172.1.132. [DOI] [PubMed] [Google Scholar]
- 10.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. J Infect Dis. 1997;175:1440–1445. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 11.Faden H, Harabuchi Y, Hong J J. Epidemiology of Moraxella catarrhalis in children during the first 2 years of life: relationship to otitis media. J Infect Dis. 1994;169:1307–1312. doi: 10.1093/infdis/169.6.1312. [DOI] [PubMed] [Google Scholar]
- 12.Goldblatt D, Turner M W, Levinsky R J. Branhamella catarrhalis: antigenic determinants and the development of the IgG subclass response in childhood. J Infect Dis. 1990;162:1128–1135. doi: 10.1093/infdis/162.5.1128. [DOI] [PubMed] [Google Scholar]
- 13.Gray B M, Concerse III G M, Dillon H C., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–933. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 14.Harabuchi Y, Faden H, Yamanaka N, Duffy L, Wolf J, Krystofik D, Pediatrics T W. Nasopharyngeal colonization with nontypeable Haemophilus influenzae and recurrent otitis media. J Infect Dis. 1994;170:862–866. doi: 10.1093/infdis/170.4.862. [DOI] [PubMed] [Google Scholar]
- 15.Helminen M F, Maciver I, Latimer J L, Klesney-Tait J, Cope L D, Paris M, McCracken G H, Jr, Hansen E. A large, antigenically conserved protein on the surface of Moraxella catarrhalis is a target for protective antibodies. J Infect Dis. 1994;170:867–872. doi: 10.1093/infdis/170.4.867. [DOI] [PubMed] [Google Scholar]
- 16.Hendley J O, Sande M A, Stewart P M, Gwaltney J M., Jr Spread of Streptococcus pneumoniae in families. I. Carriage rates and distribution of types. J Infect Dis. 1975;132:55–62. doi: 10.1093/infdis/132.1.55. [DOI] [PubMed] [Google Scholar]
- 17.Klingman K L, Murphy T F. Purification and characterization of a high-molecular-weight outer membrane protein of Moraxella (Branhamella) catarrhalis. Infect Immun. 1994;62:1150–1155. doi: 10.1128/iai.62.4.1150-1155.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loda F A, Collier A M, Glezen W P, Strangert K, Clyde W A, Jr, Denny F W. Occurrence of Diplococcus pneumoniae in the upper respiratory tract of children. J Pediatr. 1975;87:1087–1093. doi: 10.1016/s0022-3476(75)80120-x. [DOI] [PubMed] [Google Scholar]
- 19.McDaniel L S, McDaniel D O, Hollingshead S K, Briles D E. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998;66:4748–4754. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDaniel L S, Sheffield J S, Swiatlo E, Yother J, Crain M J, Briles D E. Molecular localization of variable and conserved regions of PspA and identification of additional PspA homologous sequences in Streptococcus pneumoniae. Microb Pathog. 1992;13:261–269. doi: 10.1016/0882-4010(92)90036-n. [DOI] [PubMed] [Google Scholar]
- 21.Musher D M, Luchi M J, Watson D A, Hamilton R, Baughn R E. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–735. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 22.Sluijter M, Faden H, Groot R D, Lemmens N, Goessens W H F, Belkum A V, Hermans P W M. Molecular characterization of pneumococcal nasopharynx isolates collected from children during their first 2 years of life. J Clin Microbiol. 1998;36:2248–2253. doi: 10.1128/jcm.36.8.2248-2253.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Talkington D F, Voellinger D C, McDaniels L S, Briles D E. Analysis of pneumococcal PspA microheterogeneity is SDS polyacrylamide gels and the association of PspA with the cell membrane. Microb Pathog. 1992;13:343–355. doi: 10.1016/0882-4010(92)90078-3. [DOI] [PubMed] [Google Scholar]
- 24.Vaneechoutte M, Verschraegen G, Claeys G, Weise B, Van Den Abeele A M. Respiratory tract carrier rates of Moraxella (Branhamella) catarrhalis in adult and children and interpretation of the isolation of M. catarrhalis from sputum. J Clin Microbiol. 1990;28:2674–2680. doi: 10.1128/jcm.28.12.2674-2680.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu H-Y, Nahm M H, Guo Y, Russel M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M, McDaniel L S, Kawabata K, Briles D E, Jackson R J, McGhee J R, Kiyono H. Oral immunization with PspA elicits protective humoral immunity against Streptococcus pneumoniae infection. Infect Immun. 1977;65:640–644. doi: 10.1128/iai.65.2.640-644.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamanaka N, Faden H. Immune response to P6 of nontypeable Haemophilus influenzae in otitis-prone children with special reference to IgG subclass. In: Honjo I, editor. The auditory system. Proceedings of the Satellite Symposium of the Second Extraordinary International Symposium on Recent Advances in Otitis Media. Amsterdam, The Netherlands: Kugler Publications; 1994. pp. 27–32. [Google Scholar]
- 28.Yamanaka N, Faden H. Antibody response to outer membrane protein of nontypeable Haemophilus influenzae in otitis-prone children. J Pediatr. 1993;93:212–218. doi: 10.1016/s0022-3476(06)80115-0. [DOI] [PubMed] [Google Scholar]
- 29.Yother J, Handsome G L, Briles D. Truncated forms of PspA that are secreted from Streptococcus pneumoniae and their use in functional studies and cloning the pspA gene. J Bacteriol. 1992;174:610–618. doi: 10.1128/jb.174.2.610-618.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]