Introduction
Talquetamab is a first-in-class humanized antibody that targets both the G protein-coupled receptor class 5 member D (GPRC5D), a novel orphan receptor on malignant plasma cells, and CD3, a receptor present in T cells.1 This bispecific T-cell engager is currently being used in phase II clinical trials for the treatment of relapsed or refractory (R/R) multiple myeloma (MM). A preliminary report from a phase I clinical trial reported that 75% of treated patients had some skin-related adverse effect (all grade 3/4) with 18% being nail disorders, though there were no treatment discontinuations.2 Although talquetamab has shown excellent treatment efficacy in patients with R/R MM, there have been no published studies, to the best of our knowledge, of any dermatologic reactions related to talquetamab. Here, we report a case of a patient undergoing treatment for R/R MM with talquetamab who presented with acquired onychomadesis and palmoplantar keratoderma, which is the first report of its kind.
Case report
A 78-year-old man with a previous medical history of colorectal carcinoma status after a hemicolectomy and R/R IgG kappa MM for 8 years presented to our outpatient dermatology clinic for skin peeling of the hands and feet with nail changes. The patient had worsening progression of R/R MM 2 years before presentation at our clinic after autologous hematopoietic stem cell transplant along with maintenance therapies using bortezomib, carfilzomib, and selinexor. Overall, the patient had failed 7 lines of therapy, so the decision to start experimental treatment with talquetamab was made. Physical examination revealed desquamating, hyperkeratotic plaques on bilateral palmoplantar surfaces (Figs 1 and 2), proximal separation of the nail plate from the nail matrix on all 20 nails (Fig 3), and diffuse xerosis of the back. These symptoms along with associated intense pruritus started 2 weeks after initiating treatment with talquetamab. Laboratory findings were unremarkable, rendering alternative nutritional or systemic causes of palmoplantar keratoderma and pruritus unlikely. On account of the temporal relationship with the initiation of talquetamab, the diagnoses of acquired (drug-induced) palmoplantar keratoderma, onychomadesis of all digits, and xerosis cutis were made. Treatment was initiated with clobetasol 0.05% ointment for the hands and feet and fluocinonide 0.05% mixed with a ceramide-containing moisturizer for the xerosis and pruritus on the body. This treatment regimen resolved the pruritus and xerosis, although all other symptoms remained. The patient was able to continue receiving the treatment with talquetamab.
Fig 1.
Palmar keratoderma. Clinical image of distal digital peeling and hyperkeratosis of the bilateral palms attributed to talquetamab treatment.
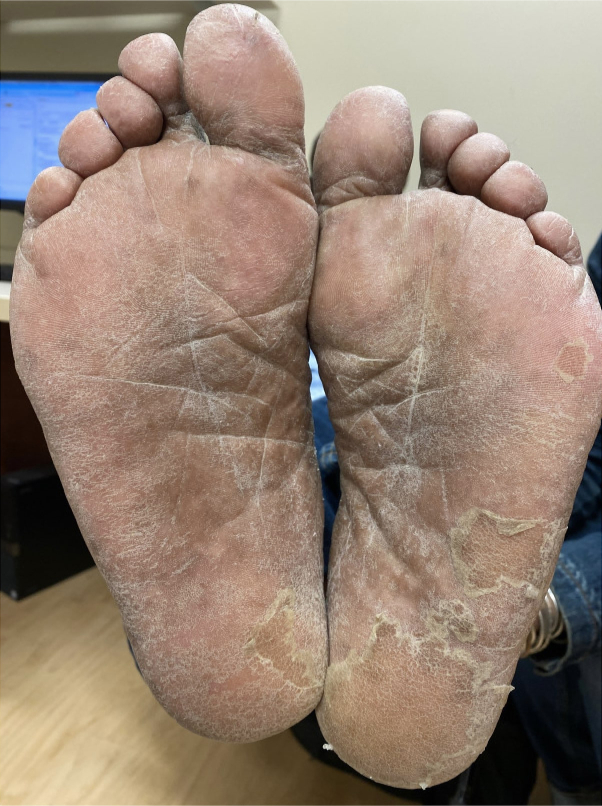
Fig 2.
Plantar keratoderma. Clinical image of bilateral thickened soles and peeling due to talquetamab treatment.
Fig 3.
Onychomadesis. Clinical images of the hands demonstrating onychomadesis of all digits and diffuse xerosis of the dorsal surfaces of the hands after starting treatment with talquetamab.
Discussion
There have been reports of targeted therapies for cancer associated with palmoplantar keratoderma, with the most common agents being targeted kinase inhibitors because of the increase in mitogen-activated protein pathway signaling, which results in increased keratinocyte proliferation.3 Another mechanism of chemotherapy-induced palmoplantar keratoderma is evidenced by capecitabine, which induces eccrine secretion.4 A target of talquetamab, GPRC5D is upregulated in MM cells and expressed at normal levels by cells that produce hard keratin.5 These cells include the cortical cells of the hair shaft, keratogenous zone in the nail matrix, and the central area of the filiform papillae of the tongue.5 GPRC5D is expressed at low levels by fibroblasts and medium levels by Langerhans cells of palmoplantar skin.6 Therefore, we propose that our case of palmoplantar keratoderma could be caused by the targeting of GPRC5D by talquetamab or by eccrine secretion induced by the drug. Although our patient did not show signs or symptoms of alopecia or glossitis given the expression of GPRC5D on the hair shaft and filiform papillae of the tongue, respectively, these may be associated adverse effects to look for in patients receiving talquetamab therapy.
The pathogenesis of onychomadesis is unclear, although we posit that the talquetamab target of highly proliferating cells, such as those in the nail matrix, leads to nail matrix arrest. Moreover, the onychomadesis could be explained by the targeting of GPRC5D in the nail matrix.5 Interestingly, nail changes are a common adverse effect of chemotherapeutics and targeted agents, although a review of the literature found only a few reports of drugs associated with onychomadesis.7
Overall, the dermatologic treatments started, in this case, were largely unsuccessful in changing the appearance of the nails and hyperkeratotic lesions; however, the pruritus was better controlled. Therefore, the decision to continue talquetamab despite the dermatologic disease was made. Since talquetamab is still in phase II clinical trials, the details of the nail and skin changes that were reported initially are unknown. To our knowledge, our case is the first to report the adverse dermatologic effects of onychomadesis and palmoplantar keratoderma associated with a novel treatment for R/R MM, the GPRC5D bispecific antibody talquetamab.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Pillarisetti K., Edavettal S., Mendonça M., et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135(15):1232–1243. doi: 10.1182/blood.2019003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berdeja J.G., Krishnan A.Y., Oriol A., et al. Updated results of a phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) × CD3 bispecific antibody, in relapsed/refractory multiple myeloma (MM) J Clin Oncol. 2021;39(15 suppl) doi: 10.1200/JCO.2021.39.15_suppl.8008. 8008-8008. [DOI] [Google Scholar]
- 3.Mirali S., Abduelmula A., Mufti A., Sachdeva M., Yeung J. Drugs associated with the development of palmoplantar keratoderma: a systematic review. J Cutan Med Surg. 2021;25(5):553–554. doi: 10.1177/12034754211004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do J.E., Kim Y.C. Capecitabine-induced diffuse palmoplantar keratoderma: is it a sequential event of hand–foot syndrome? Clin Exp Dermatol. 2007;32(5):519–521. doi: 10.1111/j.1365-2230.2007.02451.x. [DOI] [PubMed] [Google Scholar]
- 5.Inoue S., Nambu T., Shimomura T. The RAIG family member, GPRC5D, is associated with hard-keratinized structures. J Invest Dermatol. 2004;122(3):565–573. doi: 10.1046/j.0022-202X.2004.12628.x. [DOI] [PubMed] [Google Scholar]
- 6.Uhlén M., Fagerberg L., Hallström B.M., et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- 7.Mittal S., Khunger N., Kataria S.P. Nail changes with chemotherapeutic agents and targeted therapies. Indian Dermatol Online J. 2022;13(1):13–22. doi: 10.4103/idoj.IDOJ_801_20. [DOI] [PMC free article] [PubMed] [Google Scholar]