Abstract
The objective of this study was to investigate the effect of phytobiotics combination of Origanum vulgare and Andrographis paniculata water extracts (FOA) mixed into the feed of broiler and specific-pathogen-free chickens as an alternative to Antibiotics Growth Promoter (AGP). Performance, intestinal bacteria characteristic, and oocysts of Eimeria spp. in feces were measured and compared with the AGP-added group. The first experiment in broiler chickens compared FOA, Zinc Bacitracin (ZB, as an AGP group), and negative control. On day 28, FOA group and ZB group showed significantly higher body weight than the control group (P < 0.05). The FCR of ZB group was better than FOA group. However, FOA group displayed better microbiota profile than ZB group and negative control, with more Lactobacillus spp. and Bacillus spp., and less Escherichia coli and Salmonella spp. isolated from intestines. The second experiment in specific-pathogen-free chickens showed the anticoccidial effect of FOA addition to reduce the number of oocysts per gram (OPG) from live coccidia vaccine. FOA group and Amprolium group showed OPG reduction (82.53% and 92.02%, respectively) after 7 days of treatment. In conclusion, the combination of Origanum vulgare and Andrographis paniculata extract can function as an AGP replacement in feed.
Keywords: AGPs, Phytobiotic, Andrographis paniculata, Origanum vulgare, Poultry performance, Anticoccidia
1. Introduction
The ban on the use of Antibiotics Growth Promoters (AGP) leads to decreasing poultry performance and increasing incidence of pathogenic infections that cause a burden on production cost and economic loss (Cardinal et al., 2019). Zinc bacitracin and Amprolium are commonly used before AGP and coccidiostat were banned (Gadde et al., 2018; Martin et al., 2022). The development of alternatives to AGPs must be carried out to maintain poultry performance and keep production cost-efficient. One approach on developing AGP alternatives is using empirical observational strategies. Probiotics, prebiotics, antimicrobial peptide, polyphenol, and natural extract are several examples of this empirical approach (Brown et al., 2017). Natural extract or phytobiotic feed additives (PFA) have demonstrated favorable impacts on poultry output among potential substitutes that are natural, non-toxic, and residue free. (Gheisar et al., 2015; Yitbarek, 2015; Abudabos et al., 2018). Due to its pharmacological properties, PFA has long been used. It has been hypothesized that herbs, spices, and extracted oils can promote endogenous enzyme secretion, boost antioxidant status, encourage feed intake, and exhibit antimicrobial effects (Lee et al., 2015; Kim et al., 2016; Gheisar & Kim. 2018).
Feed additives with advantageous bioactive components that can protect against microbes and parasites have been developed to improve the performance of broilers. Oregano (Origanum vulgare), an aromatic-medicinal plant found in Mediterranean nations, is one potential source of bioactive chemicals acting as a natural growth promoter with strong anticoccidial and antibacterial capabilities (Akrayi et al., 2015; Bozkurt et al., 2016; Pop et al., 2019). It has been demonstrated that oregano and its primary bioactive components (carvacrol and thymol) have synergistic/additive effects such as the antifungal, antiparasitic, antioxidant, positive impact on intestinal microbiota, and improve intestinal cell activity (Zhang et al., 2014; Tzora et al, 2017; Bauer et al, 2019).
Experimentally, Andrographis paniculata has antiviral, antimicrobial, antioxidant, immunomodulatory, anti-inflammatory, anti-tumour, chemo preventive, spasmolytic, uterorelaxant, antithrombotic, and antimalarial effect (EMA, 2014). Andrographolide, the main constituent of A. paniculata is believed to play important roles in antioxidant, antimicrobial, and antiparasite activity (Dai et al, 2019). In some trials, A. paniculata has shown to have potent impacts against Eimeria spp., the causative agent of coccidiosis (Setyorini et al, 2016; Indrati & Titisari, 2020) and inhibit pathogenic bacteria growth resulting improve immune status in poultry (Hertamawati et al, 2019).
The potential interactions between different combinations of bioactive ingredients have not been studied in depth. This experiment aims to determine the effect of the dietary use of A. paniculata and O. vulgare water extracts combination (FOA) on the performance and intestinal microbiota profile as an alternative to AGP addition in feed. Additionally, a preliminary experiment was conducted to see how the FOA affect the oocysts of Eimeria spp.
2. Material and methods
2.1. Animals and management
In Experiment I, one-day-old (DOC) Cobb broiler chickens of mixed sex weighing 42–52 gram were used. On day 1, all chickens were vaccinated against Infectious Bronchitis (live), Avian Influenza (killed), and New Castle Disease (live and killed). On day 10, all chickens were vaccinated against Infectious Bursal Disease (live). All chickens were raised in a commercial closed house with a stocking density of 8–10 chickens / m2 and husks spread on the floor as pen litter under 24 h lighting. Temperature, humidity, and wind speed in the pen were monitored and controlled through digital panels per the requirements of Cobb 2021. Prior to the experiment, the pen and equipment were disinfected using liquid disinfectant and fumigated with powder formaldehyde.
In Experiment II, 14-day-old male Hy-Line W-36 specific-pathogen-free chickens were raised in battery cages with a Bio-safety level 2 environment. The battery cage was made of stainless steel with the dimension of 45 × 45 × 50 cm and had fecal collection trays beneath the cages. Lighting was provided for 24 h everyday. Feed and drinking water were given individually to each chicken. Prior to the experiment, the pen and equipment were disinfected and passed the sterility test.
2.2. Extracts and medicine
The extracts used in this experiment were a commercial 10:1 aqueous extract of O. vulgare from Hunan Nutramax, China and a 25% aqueous extract of A. paniculata from Phytochemindo Reksa Indonesia. Both powder extracts were formulated into a final product containing 0.5% O. vulgare extract and 0.75% A. paniculata extract (Optigrin, Medion Farma Jaya, Indonesia). Zinc bacitracin was used as the positive control in the first experiment, while amprolium was used in the second experiment.
2.3. Experimental design and dietary treatments
All experiments were conducted according to WAAVP (World Association for the Advancement of Veterinary Parasitology) guidelines for evaluating the efficacy of anticoccidial drugs in chickens and turkeys.
In experiment I chickens were randomly divided into 3 groups each consisting of 250 birds. Each treatment was replicated 5 times containing 50 birds per replicate. FOA group was supplemented with basal diet plus 10 mg of Origanum vulgare and 15 mg of Andrographis paniculata extract per kg of feed, ZB group was supplemented with basal diet plus 6.3 mg/kg feed of Zinc bacitracin as positive control. Control group was given basal diet without AGP addition as negative control. All groups were kept in the same pen and separated by a partition. Feed supplementation was given ad libitum for 28 days. Concomitant treatments were prohibited except where deemed necessary and would not influence the performance of any product. Body weight (BW) was recorded weekly with 10% samples of the total population according to Cobb standard (2021) while Feed Intake (FI) was recorded daily. Quantification of intestinal microbiota was conducted at the end of the experiment on 5 chickens per group.
Experiment II was conducted to evaluate the anticoccidial activity of FOA compared with Amprolium (AMP). SPF chickens were randomly divided into 3 groups each consisting of 9 birds (3 birds per replicate) according to the sample size in an animal study by Ko & Lim (2021). Fourteen-days old chickens were infected with approximately 35,000 oocysts from the live coccidia vaccine. All chickens showed clinical signs after 6 days of infection. On the next day, FOA group was treated with 10 mg of Origanum vulgare extract and 15 mg of Andrographis paniculata per kg of feed, AMP group was treated with 10 mg per kg feed of Amprolium, the remaining group was left untreated to serve as control. Oocyst per gram feces (OPG) from each group was quantified 7 days after treatment with FOA or AMP.
The non-medicated commercial feed for both experiments was based on the nutritional value of National Research Council (NRC). Samples were coded and not disclosed to the researchers during the data analysis. No other treatments were given in both experiments.
2.4. Poultry performance and intestinal microbiota examination
Average daily weight (ADG) and feed conversion ratio (FCR) were calculated using BW and FI data (n = 50). At the end of the experiment, on day 28, five chickens from each group were randomly sacrificed. Duodenum, jejunum, and ileum were separated using a cord on the border of the intestine's part, then all samples were frozen immediately (n = 5) (sample size was calculated based on the ANOVA method). For the analysis, the intestine was defrosted and then cut into separate parts of the duodenum, jejunum, and ileum. The content of the intestine parts was collected and mashed. Around 2 grams of intestinal content was suspended in 18 mL of NaCl 0.9% and diluted with 10-fold serial dilution. Every 0.1 mL of the dilution was inoculated into HiChrome Bacillus Agar (HBA) for Bacillus spp. quantification, De Man, Rogosa and Sharpe Agar (MRSA) for Lactobacillus spp. quantification; and MacConkey Agar (MCA) for Eschericia coli quantification. HBA was incubated for 18–24 h, MRSA and MCA for 24–72 h. Incubation temperature was set at 35 – 37°C. Bacteria quantification was performed according to Sajitha et al. (2014), Devi et al. (2013) and Jin LZ et al. (1996).
2.5. . Coccidial oocyst count
In experiment II, a live coccidia vaccine (Coccivac D, MSD Animal Health, NJ, USA) containing 3.5 × 10^4 oocysts of mixed Eimeria spp. was inoculated to each chicken at 14-days old. Before treatment, three chickens were euthanized to see the preliminary anatomical pathology, intestinal score, and OPG. FOA and AMP were given for seven days post-infection (21-days old). Lesion score and OPG were examined on day 0, day 3, day 5 and day 7 of treatment. OPG was calculated using the McMaster technique according to RCV/FAO guidelines. Intestinal lesions was scored based on Johnson & Reid Method (1970), then OPG based on (Jankiewicz et al., 1972)
2.6. Statistical analysis
The data were statistically analyzed using SPSS version 18.0. Differences between other groups were tested by the ANOVA-DUNCAN test, with P < 0.05 as the significant level.
3. Results
3.1. Growth performance of broiler fed with dietary additives
Experiment I using broiler chickens was conducted to observe the effect of FOA on performance and microbiota profile compare with ZB as positive control and negative control. The parameter of performance observed in this study are BW, FI, and FCR (Table 1). Groups fed with dietary additives showed superior body weight and FCR throughout the experiment. The body weight and average daily gain of the FOA group and ZB group were not significantly different. Both FOA and ZB groups had lower feed intake than the control group. The FCR of the FOA and ZB group was identical but significantly different from the control group (P < 0.05). This indicates that the administration of a herbal combination exhibits performance on equivalent with AGP.
Table 1.
Effect of feed dietary additives on growth performance in broiler.
| Performance parameter | Control | FOA | ZB | SEM |
|---|---|---|---|---|
| BW (g) | ||||
| 7 days | 185.4 a | 197.04 b | 195 ab | 2.05 |
| 14 days | 485.8 a | 494.12 a | 499.36 a | 4.67 |
| 21 days | 983.76 a | 1022.72 b | 1025.44 b | 7.46 |
| 28 days | 1579.52 a | 1663.92 b | 1682.84 b | 14.61 |
| ADG (g) | ||||
| 7 days | 19.82 a | 21.49 b | 21.19 ab | 0.29 |
| 14 days | 42.91 a | 42.44 a | 43.48 a | 0.62 |
| 21 days | 71.14 a | 75.51 b | 75.15 b | 0.64 |
| 28 days | 85.11 a | 91.60 ab | 93.91 b | 1.4 |
| 1 to 28 days | 54.75 a | 57.76 b | 58.44 b | 0.52 |
| FI (g/week) | ||||
| 7 days | 180 | 168 | 172 | |
| 14 days | 620.33 | 547.59 | 591.75 | |
| 21 days | 1285.58 | 1232.47 | 1217.29 | |
| 28 days | 2205.94 | 2150.92 | 2116.85 | |
| FCR | ||||
| 7 days | 0.97 b | 0.85 a | 0.88 a | 0.01 |
| 14 days | 1.28 c | 1.11 a | 1.19 b | 0.01 |
| 21 days | 1.31 b | 1.21 a | 1.19 a | 0.01 |
| 28 days | 1.40 b | 1.29 a | 1.26 a | 0.01 |
Different superscripts at the same row are significantly different (P < 0.05).
3.2. Intestinal microbiota profile of broiler fed with dietary additives
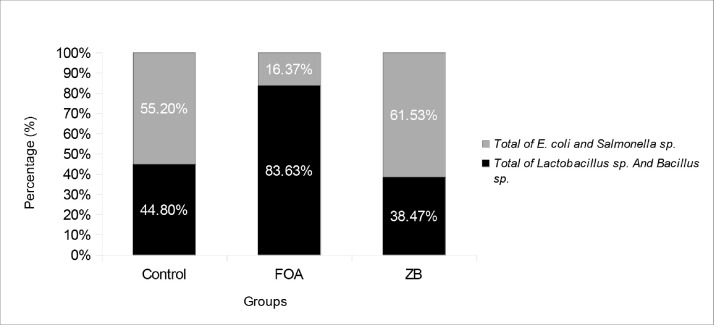
This study examined the effects of feed additives on the profile of the intestinal microbiota. The comparison between beneficial bacteria (Lactobacillus spp. and Bacillus spp.) and pathogenic bacteria (E.coli and Salmonella spp.) was displayed in Fig. 1. The percentage of beneficial bacteria in the FOA group was higher than pathogenic bacteria, while it was lower in both ZB and control groups. The ZB group showed the lowest percentage of beneficial bacteria. This data revealed that the use of antibiotics could affect the composition of beneficial bacteria residing in the intestines.
Fig. 1.
Effect of feed dietary additives on microbiota profile.
The microbiota profile from each segment of the small intestine can be seen in Table 2. FOA group showed the highest amount of beneficial bacteria in all segments of small intestine (duodenum, jejunum, and ileum) among the other group, while control and ZB group showed higher amount of pathogenic bacteria in all segment of the small intestine.
Table 2.
Effect of feed dietary additives on intestinal bacteria in broiler chicken intestinal population.
| Bacterial Load (Log CFU/g) | Control | FOA | ZB |
|---|---|---|---|
| Total of Lactobacillus sp. and Bacillus sp. | |||
| Duodenum | 4.61 | 8.43 | 4.08 |
| Jejunum | 5.87 | 9.26 | 5.90 |
| Ileum | 8.80 | 12.56 | 6.43 |
| Total of E. coli and Salmonella sp. | |||
| Duodenum | 7.91 | 2.12 | 8.77 |
| Jejunum | 8.54 | 2.28 | 10.37 |
| Ileum | 7.46 | 1.52 | 7.11 |
3.3. Anticoccidial activity of SPF chickens fed with dietary additives
The anticoccidial activity was analyzed through OPG quantification. The initial number of OPG in each group was not significantly different as shown in Table 3. After treatments, the OPG count of FOA and AMP groups were reduced significantly (P < 0.05) compared to the Control group. At day-7, in FOA and AMP groups were significantly lower than Control group.
Table 3.
Effect of feed dietary additives on fecal oocyst.
| Day of Treatments | Control | FOA | AMP | SEM |
|---|---|---|---|---|
| Before treatments | 28300a | 15550a | 10233a | 3777.02 |
| Day – 3 | 85400b | 1883a | 217a | 18429.78 |
| Day – 5 | 22588b | 1233a | 33a | 3864.27 |
| Day – 7 | 207500b | 2717a | 817a | 42828.52 |
Different superscripts at the same row are significantly different (P < 0.05).
3.4. Intestinal lesion of SPF chickens fed with dietary additives
There was no significant difference in intestinal lesion in all groups. Even so, the control group had worsening intestinal lesion day by day, while the FOA and AMP groups were slowly improving in 16 days (Table 4).
4. Discussion
In veterinary medicine, antibiotics are frequently used to treat bacterial animal diseases and to protect the health of farm animals (Hockenhull et al., 2017). Due to the low ability of chicken intestine's to absorp many antimicrobials, a huge amount of the drug is excreted in feces unaltered. The widespread use of animal excrement as fertilizer in many nations raises concerns about the negative environmental impacts of antibiotic residues that might contaminate over wide geographical areas (Hao et al., 2014). WHO states that there are currently serious worries regarding the spread of antibiotic resistance genes in bacteria detected in human patients, probably as a result of veterinary usage of antibiotics (WHO, 2014). Additionally, the general public is now demanding reduced use of drugs in livestock production, including ionophores or chemical coccidiostats, as well as antibiotic growth promoters. Due to increased research and exploration of safe and effective solutions to improve performance and decrease coccidiosis, herbal extracts are being utilised (Giannenas et al., 2020; Franz et al., 2020).
Herbal extracts are increasingly used as a safe and effective solution to improve performance and decrease coccidiosis cases. O. vulgare and A. paniculata have been separately reported to improve performance when added in the feed. However, there were no studies of the use of both extracts combined. The purpose of this study is to determine whether combining O. vulgare and A. paniculata extract (FOA) as a dietary additive can provide anticoccidial activity comparable to AGP without the need for withdrawal period or the potential adverse effects of anticoccidial drugs.
In the first experiment in broiler, the performance of broiler chickens fed with either FOA or ZB was better than control without dietary additive. The effect of FOA addition was not significantly different to ZB addition. FOA is as effective as ZB (p > 0.05) for optimizing broiler performance. In accordance with previous study by Scocco et al., 2017, feed supplemented with 2 g/kg feed O. vulgare aquoeus extract showed the highest body weight at 21 days of treatment compared to vitamin E and control groups. Ri et al., (2017) also stated that use 150 mg/kg of O.vulgare powder in the broiler chicks diet significantly increased the body weight and improved FCR as effective as antibiotic virginiamycin. Feed supplemented with 30% dry A. paniculata leaves could increase ADG and reduce FCR (Hidanah et al., 2020). Our study showed that lower dosage of both extracts, 15 mg of A. paniculata and 10 mg of O. vulgare extract per kg of feed, when used in combination can provide similar results.
Table 4.
The average of intestinal (duodenum, jejunum, ileum, caecum) lesion score.
| Days of Treatments | Treatments | SEM | ||
|---|---|---|---|---|
| Control | FOA | AMP | ||
| Before treatments | 1.25a | 1.25a | 1.25a | 0 |
| After treatments | ||||
| Day – 2 | 1.83a | 1.42a | 1.58a | 0.09 |
| Day – 10 | 2.00a | 2.08a | 1.92a | 0.09 |
| Day – 16 | 2.08a | 1.75a | 1.75a | 0.13 |
Different superscripts at the same row are significantly different (P < 0.05).
Lesion Scoring: 0 = no lesions; 1 = mild lesions; 2 = moderate lesions; 3 = severe lesions;
4 = extremely severe lesions and death (Johnson & Reid Method,1970)
The dietary addition of FOA in broiler can shift the intestinal microbiota profile towards beneficial bacteria. FOA group showed highest amount of beneficial bacteria in all segment of small intestine (duodenum, jejunum, and ileum) among the other groups. As the number of pathogenic bacteria decreases, beneficial bacteria are able to increase. Previous studies using O. vulgare aqueous extract to reduce E.coli and the other pathogen bacteria in poultry intestines. Hidanah et al. (2020) also reported that feed supplemented with aqueous A. paniculata extract could reduce E.coli bacterial infection. This is in accordance with the statement of Aruwa et al. (2021), that beneficial bacteria such as Lactobacillus spp. was predominant in small intestine, especially in the ileum. One interesting result in this study is that ZB addition results in the least amount of beneficial bacteria. It confirms with the study by Johnson et al. (2015) that antibiotics can have an adverse effect on gut communities by reducing the abundance and diversity of commensal microbes. Higher number of pathogenic bacteria could be caused by ZB resistancy in E. coli (Boulianne et al., 2016).
It is well known that the bacterial cell wall is the primary target for the antibacterial effects of phenols. The aqueous extract of O. vulgare contained a high concentration of phenolic compounds and was a powerful antioxidant (Teixeira et al., 2013). The mechanism of O. vulgare was likely due to its ability to penetrate cell membranes and disrupt their integrity. Gholami et al. (2022) stated that if bacteria cells come into contact with oregano, fluids would leak out from the bacteria due to one of its active ingredients, phenolic compound (carvacrol or thymol). The internal pH turned acidic, impairing metabolism and replication. These finding was consistent with electron microscope observations that revealed the damage to bacteria cell membranes exposed to oregano (Gholami et al., 2022). A. paniculata primary bioactive compound, andrographolide, had been reported in several studies to have antibacterial activity. Andrographolide worked to inhibit bacterial growth by inhibiting DNA synthesis, almost as effectively as the fluoroquinolone class of antibiotics (Banerjee et al., 2017). O. vulgare and A. paniculata exhibited antimicrobial action that targets two different bacterial pathways to synergistically reduce pathogenic bacteria, causing a balance of microbiota in the intestines of chicken. This balance of microbiota resulted in more optimal nutritional absorption in the intestine, hence improves poultry performance (Gholami et al., 2022).
In this experiment, treatment with FOA can control the number of Eimeria spp. as effective as AMP (P > 0.05). Studies conducted by Setyorini (2016) and Indrati & Titisari (2020), showed that A. paniculata extract supplemented in broiler feed could reduce the number of Eimeria tenella oocysts. According to Bozkurt et al. (2016), dietary oregano essential oil supported the intestinal absorptive capacity and antioxidant defense system during Eimeria infection; however, it displayed minimal direct activity on the reproductive capacity of Eimeria. The commercial herbal formula of O. vulgare, Satureja horetensis, and Chelidonium majus could reduce the cecal lesions but had no efficacy on Eimeria spp. infection shown by anticoccidial index (Pop et al., 2019). This study proves that the combination of O. vulgare and A. paniculata has anticoccidial activity to reduce oocyst in FOA-treated groups. Although the mechanism of action has not been clear, a reasonable explanation for this anticoccidial activity is the hydrophobic character and low molecular weight of the main phenolic compounds present in those that allow them to disintegrate outer cell membrane (Jitviriyanon, 2016). Furthermore, the high lipid solubility of O. vulgare and A. paniculata is likely to permit rapid diffusion through parasite and host cell membranes. Other possible mechanism is the interference of calcium-mediated signaling necessary for invasion by E. Tenella sporozoites (Jitviriyanon, 2016). The content of andrographolid in A. paniculata acts to increase immunity. Andrographolide found in A. paniculata can improve immune function. Interferon produced by lymphocytes increased the response of phagocytic activity by macrophage cells to inhibit the replication of E. tenella (Dai et al., 2019).
Decreased lession score in both FOA and AMP-treated groups is observed in this study. O. vulgare and A. paniculata have anti-inflammatory activity that can inhibit inflammatory mediators caused by bacterial and parasitic infections such as prostaglandins, interleukins (IL), interferons (IFN) and tumor necrosis factor (TNF), etc (Gholami et al., 2022; Dai et al., 2019).
From this experiments, there were no adverse effects reported in all treatment groups. More extensive large scale studies with in-vitro and in-vivo challenge are required to confirm this.
5. Conclusion
The supplementation of 10 mg of Origanum vulgare and 15 mg of Andrographis paniculata extract (FOA) can increase broiler performance and reduce pathogenic intestinal bacteria as good as Zinc Bacitracin. FOA reduces pathogenic intestinal bacteria better than Zinc Bacitracin. In addition, FOA has anticoccidial activity to reduce oocyst per gram feces of Eimeria spp. as effective as Amprolium. Based on this study, FOA can be used as an alternative to antibiotic growth promoters. Further research is required to identify and quantify the chemical content of both extracts that improve the poultry performance.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abudabos A.M., Alyemni A.H., Dafalla Y.M., Khan R.U. The effect of phytogenics on growth traits, blood biochemical and intestinal histology in broiler chickens exposed to Clostridium perfringens challenge. Journal of Applied Animal Research. 2018;46:691–695. [Google Scholar]
- Akrayi H.F., Salih R.M., Hamad P.A. In vitro screening of antibacterial properties of Rhus coriaria and Origanum vulgare against some pathogenic bacteria. Aro The Scientific Journal of Koya University. 2015;3(2):35–41. [Google Scholar]
- Aruwa C.E., Pillay C., Nyaga M.M., Sabiu S. Poultry gut health–microbiome functions, environmental impacts, microbiome engineering and advancements in characterization technologies. Journal of Animal Science and Biotechnology. 2021;12(1):1–15. doi: 10.1186/s40104-021-00640-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee M., Parai D., Chattopadhyay S., Mukherjee S.K. Andrographolide: Antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiologica. 2017;62(3):237–244. doi: 10.1007/s12223-017-0496-9. [DOI] [PubMed] [Google Scholar]
- Bauer B.W., Radovanovic A., Willson N.L., Bajagai Y.S., Van T.T.H., Moore R.J., Stanley D. Oregano: A potential prophylactic treatment for the intestinal microbiota. Heliyon. 2019;5(10):e02625. doi: 10.1016/j.heliyon.2019.e02625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozkurt M., Ege G., Aysul N., Akşit H., Tüzün A.E., Küçükyılmaz K., Borum A.E., Uygun M., Akşit D., Aypak S., Şimşek E.M.R.A.H. Effect of anticoccidial monensin with oregano essential oil on broilers experimentally challenged with mixed Eimeria spp. Poultry Science. 2016;95(8):1858–1868. doi: 10.3382/ps/pew077. [DOI] [PubMed] [Google Scholar]
- Boulianne M., Arsenault J., Daignault D., Archambault M., Letellier A., Dutil L. Drug use and antimicrobial resistance among Escherichia coli and Enterococcus spp. isolates from chicken and turkey flocks slaughtered in Quebec. Canadian Journal of Veterinary Research. 2016;80(1):49–59. [PMC free article] [PubMed] [Google Scholar]
- Brown K., Uwiera R.R., Kalmokoff M.L., Brooks S.P., Inglis G.D. Antimicrobial growth promoter use in livestock: A requirement to understand their modes of action to develop effective alternatives. International Journal of Antimicrobial Agents. 2017;49(1):12–24. doi: 10.1016/j.ijantimicag.2016.08.006. [DOI] [PubMed] [Google Scholar]
- Cardinal K.M., Kipper M., Andretta I., Ribeiro A.M.L. Withdrawal of antibiotic growth promoters from broiler diets: Performance indexes and economic impact. Poultry Science. 2019;98(12):6659–6667. doi: 10.3382/ps/pez536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb . Cobb-Vantress; Siloam Springs (AR): 2021. Cobb broiler management guide. [Google Scholar]
- Dai Y., Chen S.R., Chai L., Zhao J., Wang Y., Wang Y. Overview of pharmacological activities of Andrographis paniculata and its major compound andrographolide. Critical Reviews in Food Science and Nutrition. 2019;59(sup1):S17–S29. doi: 10.1080/10408398.2018.1501657. [DOI] [PubMed] [Google Scholar]
- Devi M., Rebecca L.J., Sumathy S. Bactericidal activity of the lactic acid bacteria Lactobacillus delbreukii. Journal of Chemical and Pharmaceutical Research. 2013;5(2):176–180. [Google Scholar]
- EMA. (2014). Assesment report on Andrographis paniculata Nees, folium, EMA, 8–14.
- Franz C.M., Baser K.H.C., Hahn-Ramssl I. Feed additives. Academic Press; 2020. Herbs and aromatic plants as feed additives: Aspects of composition, safety, and registration rules; pp. 35–56. [Google Scholar]
- Gadde U.D., Oh S., Lillehoj H.S., Lillehoj E.P. Antibiotic growth promoters virginiamycin and bacitracin methylene disalicylate alter the chicken intestinal metabolome. Scientific Reports. 2018;8:3592. doi: 10.1038/s41598-018-22004-6. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gheisar M., Hosseindoust M.A., Kim I.H. Evaluating the effect of microencapsulated blends of organic acids and essential oils in broiler chickens diet. Journal of Applied Animal Research. 2015;24:511–519. [Google Scholar]
- Gheisar M.M., Kim I.H. Phytobiotics in poultry and swine nutrition. Italian Journal of Animal Science. 2018;17:92–99. [Google Scholar]
- Gholami-Ahangaran M., Ahmadi-Dastgerdi A., Azizi S., Basiratpour A., Zokaei M., Derakhshan M. Thymol and carvacrol supplementation in poultry health and performance. Veterinary Medicine and Science. 2022;8(1):267–288. doi: 10.1002/vms3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannenas I., Sidiropoulou E., Bonos E., Christaki E., Florou-Paneri P. Feed additives. Academic Press; 2020. The history of herbs, medicinal and aromatic plants, and their extracts: Past, current situation and future perspectives; pp. 1–18. [Google Scholar]
- Hao H., Cheng G., Iqbal Z., Ai X., Hussain H.I., Huang L., Dai M., Wang Y., Liu Z., Yuan Z. Benefits and risks of antimicrobial use in food-producing animals. Frontiers in Microbiology. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertamawati R.T., Hasanah N., Sabrina A.P. Efektivitas tepung daun sambiloto (Andrographis paniculata nees) sebagai antibakteri terhadap performans broiler yang terinfeksi escherichia coli. Jurnal Ilmiah Peternakan Terpadu. 2019;7(2):247–250. [Google Scholar]
- Hidanah S., Sabdoningrum E.K., Al Arif M.A., Ansori A.N.M., Hasanah T.P, Widaya L.V.A. Sambiloto (Andrographis paniculata) extract improves the performance of animal model infected with Escherichia coli. Indian Journal of Forensic Medicine & Toxicology. 2020;14(4):3491–3496. [Google Scholar]
- Hockenhull J., Turner A.E., Reyher K.K., Barrett D.C., Jones L., Hinchliffe S., Buller H.J. Antimicrobial use in food-producing animals: A rapid evidence assessment of stakeholder practices and beliefs. Veterinary Record. 2017;181(19):510. doi: 10.1136/vr.104304. -510. [DOI] [PubMed] [Google Scholar]
- Indrati R., Titisari N. IOP conference series: Earth and environmental science. Vol. 478. IOP Publishing; 2020. The effectiveness of phytopharmaca of ethanol extract sambiloto leaf as an alternative of control to coccidiosis. [Google Scholar]
- Jankiewicz, Scofield, Hofstad M.S., Calnek B.W., Helmbolt C.F., Reid W.M., Yoder H.W. The Iowa State University Press; 1972. Disease of poultry. 1934. [Google Scholar]
- Jin L.Z., Ho Y.W., Abdullah N., Jalaudin S. Influence of dried Bacillus substillis and Lactobacilli cultures on intestinal microflora and performance in broilers. Asian Australasian Journal of Animal Sciences. 1996;9(4):397–404. [Google Scholar]
- Jitviriyanon S., Phanthong P., Lomarat P., Bunyapraphatsara N., Porntrakulpipat S., Paraksa N. In vitro study of anti-coccidial activity of essential oils from indigenous plants against Eimeria tenella. Veterinary Parasitology. 2016;228:96–102. doi: 10.1016/j.vetpar.2016.08.020. [DOI] [PubMed] [Google Scholar]
- Johnson J., Reid W.M. Anticoccidial drugs: lesion scoring techniques in battery and floor-pen experiments with chickens. Experimental Parasitology. 1970;28:30–36. doi: 10.1016/0014-4894(70)90063-9. [DOI] [PubMed] [Google Scholar]
- Johnson L.P., Walton G.E., Psichas A., Frost G.S., Gibson G.R., Barraclough T.G. Prebiotics modulate the effects of antibiotics on gut microbial diversity and functioning in vitro. Nutrients. 2015;7(6):4480–4497. doi: 10.3390/nu7064480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Lee K., Kang C., An B. Growth performance; relative meat and organ weights; cecal microflora and blood characteristics in broiler chickens fed diets containing different nutrient density with or without essential oils. Asian Australasian Journal of Animal Sciences. 2016;29:549–554. doi: 10.5713/ajas.15.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko M.J., Lim C.Y. General considerations for sample size estimation in animal study. Korean Journal of Anesthesiology. 2021;74(1):23–29. doi: 10.4097/kja.20662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Kim J., Oh S., Kang C., An B. Effects of dietary sanguinarine on growth performance; relative organ weight; cecal microflora; serum cholesterol level and meat quality in broiler chickens. The Journal of Poultry Science. 2015;52:15–22. [Google Scholar]
- Martins R.R., Silva L.J., Pereira A.M., Esteves A., Duarte S.C., Pena A. Coccidiostats and poultry: A comprehensive review and current legislation. Foods. 2022;11(18):2738. doi: 10.3390/foods11182738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pop L.M., Varga E., Coroian M., Nedișan M.E., Mircean V., Dumitrache M.O., Farczádi L., Fülöp I., Croitoru M.D., Fazakas M., Gyӧrke A. Efficacy of a commercial herbal formula in chicken experimental coccidiosis. Parasites & Vectors. 2019;12(1):343. doi: 10.1186/s13071-019-3595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ri C.S., Jiang X.R., Kim M.H., Wang J., Zhang H.J., Wu S.G., Bontempo V., Qi G.H. Effects of dietary oregano powder supplementation on the growth performance, antioxidant status and meat quality of broiler chicks. Italian Journal of Animal Science. 2017;16(2):246–252. [Google Scholar]
- Sajitha K.L., Florence E.M., Dev S.A. Screening of bacterial biocontrols against sapstain fungus (Lasiodiplodia theobromae Pat.) of rubberwood (Hevea brasiliensis Muell. Arg.) Research in Microbiology. 2014;165(7):541–548. doi: 10.1016/j.resmic.2014.07.002. [DOI] [PubMed] [Google Scholar]
- Setyorini E., Suprihati E., Supranianondo K. Pengaruh ekstrak sambiloto (Andrographis paniculata) terhadap produksi ookista dan skor lesi sekum ayam yang diinfeksi Eimeria tenella. Veterinaria Medika. 2016;9(1):83–90. [Google Scholar]
- Scocco P., Forte C., Franciosini M.P., Mercati F., Casagrande-Proietti P., Dall'Aglio C., Acuti G., Tardella F.M., Trabalza-Marinucci M. Gut complex carbohydrates and intestinal microflora in broiler chickens fed with oregano (Origanum vulgare L.) aqueous extract and vitamin E. Journal of Animal Physiology and Animal Nutrition. 2017;101(4):676–684. doi: 10.1111/jpn.12588. [DOI] [PubMed] [Google Scholar]
- Teixeira B., Marques A., Ramos C., Serrano C., Matos O., Neng N.R., Nogueira J.M., Saraiva J.A., Nunes M.L. Chemical composition and bioactivity of different oregano (Origanum vulgare) extracts and essential oil. Journal of the Science of Food and Agriculture. 2013;93(11):2707–2714. doi: 10.1002/jsfa.6089. [DOI] [PubMed] [Google Scholar]
- Tzora A., Giannenas I., Karamoutsios A., Papaioannou N., Papanastasiou D., Bonos E., Skoufos S., Bartzanas T., Skoufos I. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. The Journal of Poultry Science. 2017;54(3):218–227. doi: 10.2141/jpsa.0160071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2014. Antimicrobial resistance: Global report on surveillance. [Google Scholar]
- Yitbarek M.B. Phytogenics as feed additives in poultry production: A review. International Journal of Extensive Research. 2015;3:49–60. [Google Scholar]
- Zhang X.L., Guo Y.S., Wang C.H., Li G.Q., Xu J.J., Chung H.Y., Ye W.C., Li Y.L., Wang G.C. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chemistry. 2014;152:300–306. doi: 10.1016/j.foodchem.2013.11.153. [DOI] [PubMed] [Google Scholar]