Highlights
-
•
Executive function is primarily associated with free water in early old age.
-
•
Associations were driven by individuals with mild cognitive impairment.
-
•
No interaction with cognitive reserve assessed about 45 years earlier.
-
•
Working memory related to fractional anisotropy in cognitively normal subjects only.
Keywords: Executive control, Cognitive control, Working memory, Abnormal white matter
Abstract
Background
Studies have investigated white matter microstructure in relation to late-life cognitive impairments, with fractional anisotropy (FA) and mean diffusivity (MD) measures thought to capture demyelination and axonal degradation. However, new post-processing methods allow isolation of free water (FW), which captures extracellular fluid contributions such as atrophy and neuroinflammation, from tissue components. FW also appears to be highly relevant to late-life cognitive impairment. Here, we evaluated whether executive functions are associated with FW, and FA and MD corrected for FW (FAFWcorr and MDFWcorr).
Method
We examined 489 non-demented men in the Vietnam Era Twin Study of Aging (VETSA) at mean age 68. Two latent factors capturing ‘common executive function’ and ‘working-memory specific’ processes were estimated based on 6 tasks. Analyses focused on 11 cortical white matter tracts across three metrics: FW, FAFWcorr, and MDFWcorr.
Results
Better ‘common executive function’ was associated with lower FW across 9 of the 11 tracts. There were no significant associations with intracellular metrics after false discovery rate correction. Effects also appeared driven by individuals with MCI (13.7% of the sample). Working memory-specific tasks showed some associations with FAFWcorr, including the triangularis portion of the inferior frontal gyrus. There was no evidence that cognitive reserve (i.e., general cognitive ability assessed in early adulthood) moderated these associations between executive function and FW or FA.
Discussion
Executive function abilities in early old age are associated primarily with extracellular fluid (FW) as opposed to white matter (FAFWcorr or MDFWcorr). Moderation analyses suggested cognitive reserve does not play a strong role in these associations, at least in this sample of non-demented men.
1. Introduction
Executive functions (EFs) are cognitive control abilities that regulate thought and action (Friedman and Miyake, 2017, Miyake and Friedman, 2012). EFs are some of the first cognitive abilities to decline in aging, with cortical thinning occurring in their associated brain regions (Bakkour et al., 2013, Buckner, 2004, Fjell et al., 2009, Huizinga et al., 2006). EF deficits are also prominent in the early stages of Alzheimer’s disease and mild cognitive impairment (MCI; Baudic et al., 2006, Junquera et al., 2020, Kirova et al., 2015, Ramanan et al., 2017), making their study critically important with respect to cognitive aging. However, the multi-faceted nature of EFs has hampered our understanding of their associations with brain structure. The goal of this study was to shed light onto the neural substrates of EF in early old age (mean age 68) by examining associations with white matter microstructure while also accounting for free water (FW), extracellular fluid that can be isolated from tissue comments in measures of fractional anisotropy (FA) and mean diffusivity (MD) using post-processing techniques (Pasternak et al., 2009). Moreover, to advance our understanding of the possible role of cognitive reserve in these associations, we evaluated whether these associations are moderated by general cognitive ability measured in young adulthood (mean age 20).
2. Framework of executive function
EFs capture a heterogeneous set of processes, with measures typically including tests of prepotent response inhibition, working memory updating, and/or task-set shifting (Miyake and Friedman, 2012, Miyake et al., 2000). These EF processes are moderately to highly correlated with one another, especially at the latent construct level (Friedman et al., 2008, Gustavson et al., 2018b, Miyake et al., 2000, Vaughan and Giovanello, 2010). A highly influential model – the unity/diversity model – has highlighted this common variance across multiple EF sub-domains, and models it as a “Common EF” latent factor (Friedman et al., 2008, Gustavson et al., 2018b, Miyake and Friedman, 2012). The general variance reflected in Common EF represents the goal management abilities needed to initiate a task and pursue goal-directed actions in the face of other distractions (Friedman and Miyake, 2017, Miyake and Friedman, 2012), and this model has demonstrated good fit in a range of samples across the lifespan (Engelhardt et al., 2015, Freis et al., 2021, Friedman et al., 2016, Gustavson et al., 2018a).
Depending on the availability of data, other factors are fit to capture variance specific to one EF subdomain and not the others (e.g., working memory-specific variance). Working memory-specific variance is of particular interest because it has been proposed to reflect gating in the basal ganglia (Friedman and Miyake, 2017), but is also strongly genetically correlated with intelligence (Friedman et al., 2008, Gustavson et al., 2022a), suggesting its neural correlates could be distributed across the brain. In the Vietnam Era Twin Study of Aging (VETSA) sample analyzed here, our model of EF includes Common EF and Working Memory-Specific factors, both of which demonstrate strong stability across middle age (Gustavson et al., 2018a). Longitudinal decline in the Common EF factor across the first three waves of VETSA (mean ages 56 to 68) is also associated with higher Alzheimer’s disease genetic risk scores (Gustavson et al., 2022b) and greater self-reported subjective cognitive decline across the same window (Gustavson et al., 2021). These findings underscore the importance of studying the neural correlates of EF during the transition from late middle age to early old age.
3. Measurement of white matter microstructure
The most common metrics for assessing white matter microstructure in studies of cognitive aging are FA and MD. FA quantifies directional diffusion within a given voxel (with higher FA corresponding to stronger directionality) and is thought to represent density and coherence of white matter regions. MD represents the average diffusivity of water molecules within a voxel regardless of direction and may reflect reduction in neuropil or increases in cerebrospinal fluid (Alexander et al., 2007, Clark et al., 2011, Selemon and Goldman-Rakic, 1999). Typically, better cognitive function is associated with higher FA and lower MD (Bennett and Madden, 2014, Charlton et al., 2006, Mabbott et al., 2006). Age-related trends indicate that FA increases through early adulthood before decreasing throughout the rest of the lifespan (Westlye et al., 2010) with MD displaying the opposite pattern (decreasing through early adulthood before increasing later in life). Thus, reduced FA in older populations is thought to be associated with axonal degradation and demyelination (Beaulieu, 2002) and with cognitive decline in normal aging and AD (Bozzali et al., 2012, Cremers et al., 2016, Kennedy and Raz, 2009, Mielke et al., 2012). By contrast, increased MD in normal aging and AD may reflect reduction in neuropil or increases in cerebrospinal fluid (Alexander et al., 2007, Clark et al., 2011, Selemon and Goldman-Rakic, 1999).
Importantly, new post-processing techniques now allow for isolation of extracellular fluid (FW) from tissue components of white matter microstructure (Pasternak et al., 2009). Such measures yield FA and MD measures that are corrected for FW and therefore focused on intracellular diffusion (hereafter, FAFWcorr and MDFWcorr). Beyond this, FW may be useful as it is associated with objective cognitive assessments and cognitive change (Archer et al., 2020), self-perceived cognitive decline (Archer et al., 2021), and is elevated in MCI and AD (Maier-Hein et al., 2015) even after correcting for white matter hyperintensities (Dumont et al., 2019). Moreover, such cognitive measures do not appear associated with FAFWcorr and MDFWcorr measures (Archer et al., 2020), thus raising the possibility that earlier findings for FA and MD may have been driven by FW. FW measures are thought to represent a combination of FW in extracellular space and FW contamination from cerebrospinal fluid in adjacent voxels. Increased FW may also indicate neuroinflammation, atrophy, or the breakdown of myelin cell membranes (Dumont et al., 2019, Gullett et al., 2020, Pasternak et al., 2012). Therefore, their continued study in relation to cognition across midlife and early old age will help shed light on neurocognitive changes and risk for AD.
4. Executive function and white matter microstructure
In one of the few studies of younger adults to examine white matter microstructure using a Common EF factor, individual differences in Common EF were associated with greater FA in the right superior longitudinal fasciculus (SLF) and left anterior thalamic radiation (Smolker, Friedman, Hewitt, & Banich, 2018). Working memory updating-specific ability was not associated with white matter microstructure measures and shifting-specific ability was associated with MD throughout the brain. However, as noted above, it is unclear whether findings from FA and MD measures in this earlier work may be driven by FW.
Studies of older adult samples that have examined associations between EFs and FW had relatively high proportions individuals with MCI or dementia, and focused on EF composite scores that shed light on common but not specific aspects of EF (Archer et al., 2020, Ji et al., 2017, Maillard et al., 2019). For example, in a study of 319 older adults (M = 72–73 years; 49 % MCI), EF was associated with lower FW in multiple cortical white matter tracts including the fornix, inferior longitudinal fasciculus, tapetum, uncinate fasciculus, & cingulum bundle, but not associated with FAFWcorr in these same tracts (Archer et al., 2020). Another study (M = 78 years; >50 % MCI or dementia) demonstrated that baseline levels of global FW were associated with cross-sectional and longitudinal changes in EF and episodic memory, but that FAFWcorr and MDFWcorr were not associated with EF or memory (Maillard et al., 2019). Finally, in AD patients, FW was associated with EFs across nearly all white matter regions, as well as lower FA in the bilateral frontal, parietal, and occipital fibers (Ji et al., 2017).
These findings highlight the importance of directly modelling FW in studies of EF as Common EF may be primarily associated with extracellular fluid (i.e., FW) rather than intracellular white matter microstructure (i.e., FA and MD), with associations with FA being observed only in patients with dementia. Measures of extracellular diffusion may be more sensitive to subtle differences in structural integrity compared to intracellular measures, therefore FW represents a particularly relevant measure to assess brain health in early old age. Additionally, because studies have focused on composite measures, they have not examined whether these associations with FW are observed for Common EF versus other specific EF components (e.g., working memory-specific). Moreover, existing studies have focused on samples with high proportions of subjects with MCI and/or AD, and it will be important to examine whether these associations still exist when focusing on a sample more representative of the population (i.e., MCI rates closer to 10–15 %), or when focusing solely on cognitively normal adults.
Finally, when examining the neural underpinnings of EFs, we considered the role of young adult cognitive reserve, which we defined as an individual’s total cognitive resources during early adulthood (Kremen et al., 2022). Theories of cognitive reserve suggest that some individuals do not exhibit the cognitive or functional deficits expected based on their brain pathology (Barulli and Stern, 2013, Stern, 2012, Stern, 2013, Whalley et al., 2004), which might manifest as reduced associations between EF and white matter (and FW) in individuals with high reserve. We focus on young adulthood as a time when aging effects will have had essentially no impact on cognitive capacity. While some studies suggest that cognitive decline in EFs are sensitive to cognitive reserve (McKenzie et al., 2020, O'Shea et al., 2015, Roldan-Tapia et al., 2012), little research has examined whether cognitive reserve moderates associations between EFs and brain (Krch et al., 2019). In a prior study of individuals in the same sample as the present study, the association between hippocampal volume and episodic memory was higher among individuals with lower levels of cognitive reserve; those with higher reserve were more resilient against the potentially deleterious effects of hippocampal atrophy (Vuoksimaa et al., 2013). However, whether cognitive reserve moderates associations between Common EF and white matter microstructure has not been examined. If EFs are sensitive to cognitive reserve, then EF abilities should be more strongly associated with neuroimaging measures in individuals with low reserve. Such findings would indicate that individuals with high reserve are more able to retain the same level of EF ability in the face of neurodegeneration compared to individuals with low reserve.
5. The current study
In the current study, we used data from the third wave of the Vietnam Era Twin Study of Aging (VETSA) sample (mean age 68) to examine associations between EF and white matter microstructure using 3 metrics: FW, FAFWcorr, and MDFWcorr. We predicted that EFs would be uniquely associated with FW within multiple cortical white matter tracts, consistent with earlier work (Archer et al., 2020). Less is known about the associations between working memory-specific abilities and white matter, so these analyses are considered exploratory. Finally, we examined whether associations between EFs and white matter would be moderated by cognitive reserve based on general cognitive ability assessed when subjects were about age 20 (over 45 years prior to the assessments of EFs and white matter described here) (Kremen et al., 2022).
6. Methods
6.1. Subjects
Data analyses focus on 489 male twins who participated in the third wave of the longitudinal VETSA project. VETSA participants were recruited randomly from a previous study of members of the Vietnam Era Twin Registry (Tsuang, Bar, Harley, & Lyons, 2001). All individuals served in the United States military at some time between 1965 and 1975, but nearly 80 % reported no combat exposure. Sample characteristics are displayed in Table 1, alongside descriptive statistics for executive function tests and our index of cognitive reserve. Participants are generally representative of American men in their age cohort with respect to health, education, and lifestyle characteristics (Kremen et al., 2011, Kremen et al., 2006, Schoenborn and Heyman, 2009).
Table 1.
Demographic characteristics and descriptive statistics for executive function tasks.
| N | Mean | SD | Range | Skewness | Kurtosis | |
|---|---|---|---|---|---|---|
| Demographic Characteristics | ||||||
| Age | 489 | 67.53 | 2.62 | 61.37, 71.72 | −0.44 | −1.34 |
| Years of Education | 489 | 14.01 | 2.07 | 8, 20 | 0.45 | −0.23 |
| Diabetes (% yes) | 489 | 0.22 | ||||
| Hypertension (% yes) | 489 | 0.56 | ||||
| Race/Ethnicity (% White Non-Hispanic) | 489 | 0.88 | ||||
| Executive Function Tests | ||||||
| Stroop - Color-Word Score* | 483 | 30.23 | 8.79 | 2.17, 59.17 | 0.03 | 0.28 |
| Color Score | 483 | 60.22 | 10.66 | 17.44, 88.44 | −0.29 | 0.60 |
| Word Score | 487 | 85.40 | 14.20 | 36.37, 126.37 | −0.01 | 0.19 |
| Trail Making Test - Switching* (log RT) | 485 | 4.58 | 0.38 | 3.73, 5.48 | 0.54 | −0.12 |
| Number Sequencing (log RT) | 485 | 3.54 | 0.35 | 2.65, 4.97 | 0.65 | 0.69 |
| Letter Sequencing (log RT) | 485 | 3.56 | 0.35 | 2.77, 5.01 | 0.63 | 0.36 |
| Category Switch - Switching Trial Score* | 488 | 11.87 | 2.60 | 0.17, 20.17 | −0.10 | 1.26 |
| Category Fluency Score | 488 | 36.25 | 7.50 | 15.92, 62.92 | 0.25 | 0.26 |
| Letter Number Sequencing | 488 | 8.83 | 2.30 | 1.74, 15.74 | −0.11 | 0.14 |
| Reading Span | 482 | 33.03 | 4.90 | 18.53, 44.53 | −0.10 | −0.27 |
| Digit Span | 488 | 16.27 | 3.50 | 6.89, 26.79 | 0.30 | −0.40 |
| Cognitive Reserve | ||||||
| AFQT (Age 20) | 481 | 0.34 | 0.67 | −1.18, 2.32 | 0.07 | −0.33 |
Note: Some executive function measures (indicated with a *) were first corrected for baseline conditions prior to analyses (e.g., for the Stroop task, the Color-Word Score was regressed on the Color Score and Word Score and the residuals were exported as the primary dependent measure). All executive function measures are adjusted for practice effects, leveraging data from returnees and new subjects at waves 2 and 3 to adjust for the fact that some subjects have been exposed to the task multiple times. AFQT = Armed Forces Qualifications Test percentile score (transformed based on military norms).
All wave 3 MRI data were collected at the University of California, San Diego (UCSD). All participants gave their written informed consent before participation, and the study protocol was approved by the Institutional Review Boards at all participating institutions.
6.2. Measures
6.2.1. Executive function
EF abilities were measured with 6 tasks spanning prepotent response inhibition, task-set switching, and working memory span domains. Inhibition was assessed with the Stroop task (Golden and Freshwater, 2002, Stroop, 1935). Shifting was assessed using the (a) Trail Making Test switching trial and (b) the category-switching subtest for verbal fluency from the Delis-Kaplan Executive Function System (d-KEFS) (d-KEFS; Delis, Kaplan, & Kramer, 2001). All measures of inhibition and switching were adjusted for appropriate baseline conditions. Working memory span was assessed with the letter number sequencing and digit span subtests of the Wechsler Memory Scale-III (Wechsler, 1997) and the reading span test (Daneman and Carpenter, 1980). Prior to analyses, all cognitive scores in the full VETSA wave 3 study were adjusted for practice effects, leveraging data from attrition replacement participants who completed the task battery for the first time at wave 2 or wave 3 to estimate the increase in performance expected in returnees who completed the tests two or more times (Elman et al., 2018).
Our model of EF was initially validated in waves 1 and 2 of VETSA (Gustavson et al., 2018a, Gustavson et al., 2018b) and includes 2 latent factors: a “Common EF” latent factor (based on performance across all 6 tests) and a “Working Memory-Specific” factor (based on additional variance in the 3 working memory span tests not already captured by the latent factor). Prior waves also administered an additional test (the AX-Continuous Performance Test), but this was not included in the wave 3 assessment due to time constraints. Preliminary analyses indicated the latent factor model of EF continued to fit the data well in this subsample of individuals who completed the MRI assessment at wave 3 of VETSA, so we did not fit any additional confirmatory models of EF. Additionally, our confirmatory model of EF is supported by a recent study that fit a latent growth model of Common EF and Working Memory-Specific factors across all 3 waves of VETSA in the full sample (Gustavson et al., 2022b).
6.2.2. General cognitive ability (age 20)
General cognitive ability—our index of cognitive reserve—was assessed in young adulthood when VETSA participants were first inducted into the military (mean age 20 years) with the 100-item multiple-choice Armed Forces Qualifications Test (AFQT; Bayroff and Anderson, 1963). The AFQT demonstrates a strong correlation (r = 0.84) with measures of intelligence such as the Wechsler Adult Intelligence Scale (Lyons et al., 2009) and consists of 4 subscales assessing vocabulary, arithmetic ability, tool/mechanical knowledge and reasoning, and visual-spatial ability. AFQT scores also correlate moderately with the self-reported number of years of education (r = 0.31), but here we capitalized on having a far more precise index than years of education, i.e., a direct measure of overall cognitive ability from young adulthood (Kremen et al., 2022). AFQT percentile scores were converted into z-scores. Thus, the mean of 0.34 (see Table 1) is approximately equivalent to an IQ of 105.
6.3. Image acquisition
Images were acquired with two GE 3 T Discovery 750 × scanners (GE Healthcare, Waukesha, WI, USA) with eight-channel phased array head coils. The imaging protocol included a sagittal 3D fast spoiled gradient echo (FSPGR) T1-weighted (T1w) volume optimized for maximum gray/white contrast (TE = 3.164 msec, TR = 8.084 msec, TI = 600 msec, flip angle = 8°, matrix = 256x192, in-plane resolution = 1x1 mm, slice thickness = 1.2 mm, slices = 172). Diffusion data were acquired with a multi-shell diffusion-weighted scan (54-directions, b values = [0 (x3), 666 (x6), 1333 (x15), 2666 (x15), 4000 (x15)] s/mm2, integrated with a pair of b = 0 images with opposite phase-encode polarity, TR = 6600 msec, TE = 81.1 msec, matrix = 96x96, in-plane resolution = 2.5x2.5 mm, slice thickness = 2.5 mm, 54 slices).
6.4. Image processing
Data were preprocessed using the PreQual pipeline to correct for distortions/motions and eddy currents (Cai et al., 2021, Schilling et al., 2019). The multi-shell data was then subset to a single shell (b = 1333) and inputted into DTIFIT to calculate FA and MD for each participant. The single shell data was also input into MATLAB code to calculate FW, FAFWcorr, and MDFWcorr (Jenkinson et al., 2012, Pasternak et al., 2009). In short, this code leverages a variational network framework to split the diffusion image into a bi-tensor model – one which is the FW contamination, and the other is the tissue compartment. New, FW-corrected metrics (FAFWcorr, MDFWcorr) can then be quantified. Importantly, the FW metric itself can also be leveraged in analysis. A standard space representation for the FW, FAFWcorr, and MDFWcorr maps was created by non-linearly registering the DTIFIT-derived raw FA image and applying this transform to the FW-corrected maps (Avants, Epstein, Grossman, & Gee, 2008).
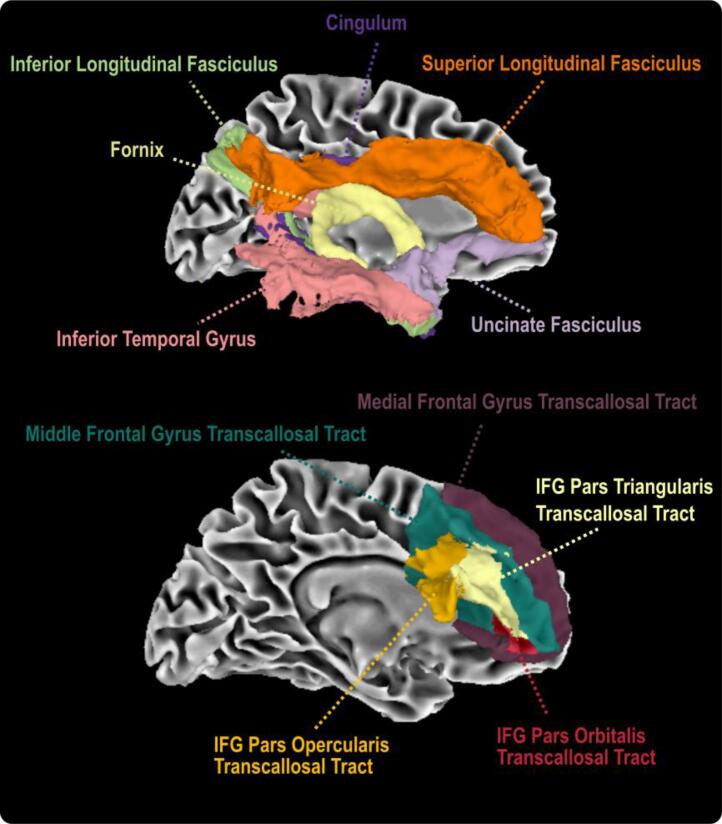
Following standardization, mean FW, FAFWcorr, and MDFWcorr values were quantified within several well-established white matter tractography templates for each imaging session (Archer et al., 2019, Archer et al., 2020, Brown et al., 2017). These templates included the cingulum bundle, fornix, superior longitudinal fasciculus (SLF), inferior longitudinal fasciculus (ILF), and uncinate fasciculus as well as homologous transcallosal connections of the inferior frontal gyrus (IFG) pars opercularis, IFG pars orbitalis, IFG pars triangularis, inferior temporal gyrus, medial frontal gyrus, and middle frontal gyrus (see Fig. 1 for a visual representation of all 11 tracts). We focused on this set of 11 cortical white matter tracts which have previously been linked to executive function and subjective cognitive decline in recent studies (Archer et al., 2021, Archer et al., 2020). Additionally, we included a final set of measures capturing FW, FAFWcorr, and MDFWcorr across all white matter tracts in the brain (including both cortical and subcortical tracts) to capture global FW and white matter microstructure as our recent work has highlighted strong genetic influences shared across all white matter tracts (Gustavson et al., 2019).
Fig. 1.
Tractography templates used in the study. All tract templates were previously developed and are freely .
available at https://github.com/VUMC-VMAC/Tractography_Templates
6.5. Data analysis
Phenotypic correlational and regression analyses were conducted in Mplus version 8.3 (Muthén and Muthén, 1998–2017), which accounts for missing observations using full-information maximum likelihood. Significance of individual parameter estimates were established with standard error-based 95 % confidence intervals and confirmed with χ2 difference tests by fixing that parameter to zero. Standard errors and chi-squares were adjusted for clustering within families (twin pairs), and the χ2 difference tests were appropriately scaled based on scaling factors provided in the Mplus output (Satorra & Bentler, 2001).
To examine associations between EFs and white matter microstructure, we fit a series of regression models in which the latent EF factors were regressed on the candidate white matter measures (one model per white matter measure). The following covariates were included in all analyses: age (M = 67.53, SD = 2.63, Range = 61.37 to 71.71), diabetes status (22.3 % yes), hypertension status (55.6 % yes), a variable capturing whether individuals were Hispanic and/or nonwhite (11.7 % yes), and two variables capturing scanner differences (one of the two scanners’ software was upgraded during the study, so orthogonal contrasts were created to account for potential differences across the three scanner/software groups). Diabetes and hypertension status were based on whether the participant (1) reported being diagnosed by a doctor, (2) reported that they were currently taking medication for diabetes or high blood pressure, and/or (3) reported whether they had high blood pressure on the day of testing (hypertension only).
After identifying which FW and white matter measures were associated with EF factors, we fit additional regression models (one for each measure) in which age 20 general cognitive ability (AFQT) was added to the model. Both EF factors were regressed on AFQT scores, and an interaction term was added (AFQT * diffusion measure) for whichever EF factor was associated with that measure in prior analyses.
6.5.1. Additional statistical considerations
The comprehensive battery of cognitive tasks in VETSA enables diagnosis of mild cognitive impairment (MCI) using the Jak-Bondi approach (Bondi et al., 2014, Jak et al., 2009, Kremen et al., 2014). Due to the relatively young age of this community-dwelling sample, MCI prevalence in this subsample of wave 3 participants who completed MRI measures was 13.7 % (2.2 % missing diagnoses). With 84 % cognitively unimpaired, the primary analyses focused on all participants (see Supplemental Method for detailed description of MCI diagnoses). However, we also report analyses after removing all participants with MCI (or missing MCI diagnosis) from analyses (new N = 411). The latter analyses inform whether individuals with lower cognitive ability were primarily driving associations with white matter microstructure. No participants were diagnosed with dementia.
7. Results
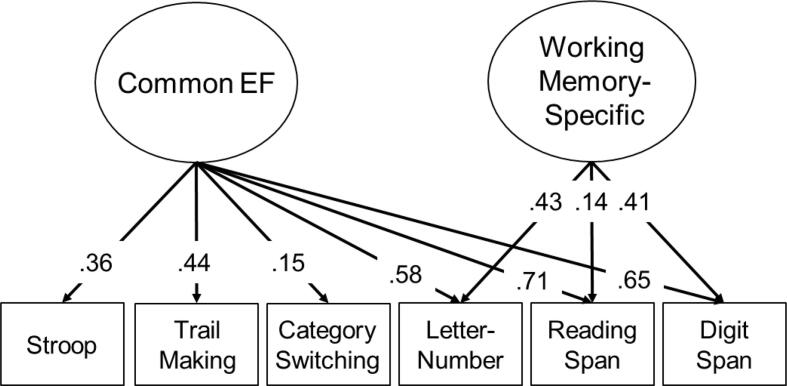
We first fit a correlational model of the 2 latent EF factors, age 20 general cognitive ability (AFQT), and covariates. Correlations among these measures are displayed in supplemental Table S1 and factor loadings on the EF factors from this model are displayed in Fig. 2. This basic model fit the data well, χ2(30) = 36.84, p =.182, RMSEA = 0.022, CFI = 0.991. The supplement also displays comparisons between FW and white matter measures across cognitively normal and MCI groups (Table S2).
Fig. 2.
Latent variable model of executive function (EF) from the current study. Ovals represent latent variables and rectangles represent measured variables. This model also includes all covariates (i.e., it is the same as that described in Table S1). All paths are signficant (p <.05).
7.1. Associations between executive function and white matter microstructure
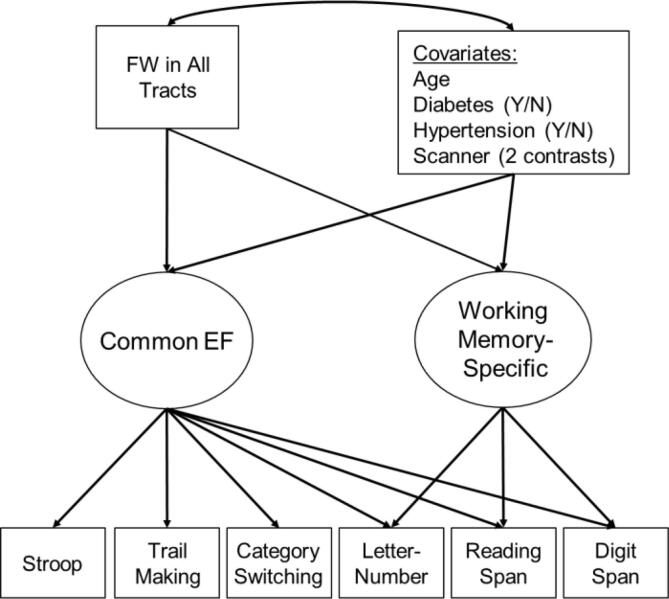
Associations between the EF factors and the FW and white matter microstructure measures are displayed in Table 2. The corresponding associations between all covariates and the FW and white matter microstructure measures from these models are displayed in Table 3. Analyses were conducted separately for each tract and for each metric (FW, FAFWcorr, and MDFWcorr), but associations between any given metric and the Common EF and Working Memory-Specific factors were estimated within the same model. FDR corrections in Table 2 were based on all p-values from that column only (e.g., across all associations between the Common EF factor and FW metrics, with separate FDR corrections for FAFWcorr and MDFWcorr; 3 additional sets of FDR corrections were examined for associations between Working Memory-Specific and FW, FAFWcorr, and MDFWcorr). An example of these analyses is displayed in Fig. 3 (for FW across all tracts).
Table 2.
Associations between executive function and white matter microstructure (N = 489).
| Independent Variable | Free Water | FAFWcorr | MDFWcorr | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | p (FDR) | β | p | p (FDR) | β | p | p (FDR) | |
| Common EF | |||||||||
| All Tracts | −0.23 | < 0.001 | < 0.001 | 0.15 | 0.252 | 0.432 | 0.04 | 0.355 | 0.429 |
| Cingulum | −0.26 | < 0.001 | < 0.001 | 0.06 | 0.446 | 0.670 | 0.06 | 0.486 | 0.530 |
| Fornix | −0.17 | 0.013 | 0.020 | 0.09 | 0.231 | 0.432 | 0.14 | 0.093 | 0.429 |
| IFG Opercularis | −0.24 | 0.005 | 0.012 | 0.17 | 0.097 | 0.388 | 0.11 | 0.329 | 0.429 |
| IFG Orbitalis | −0.22 | 0.016 | 0.021 | 0.04 | 0.594 | 0.713 | 0.09 | 0.193 | 0.429 |
| IFG Triangularis | −0.24 | 0.019 | 0.023 | 0.15 | 0.025 | 0.153 | 0.11 | 0.317 | 0.429 |
| Inferior Longitudinal Fasciculus | −0.15 | 0.002 | 0.007 | 0.01 | 0.872 | 0.872 | −0.07 | 0.348 | 0.429 |
| Inferior Temporal Gyrus | −0.15 | 0.078 | 0.078 | 0.03 | 0.724 | 0.790 | −0.05 | 0.311 | 0.429 |
| Medial Frontal Gyrus | −0.17 | 0.049 | 0.054 | 0.11 | 0.249 | 0.432 | −0.07 | 0.350 | 0.429 |
| Middle Frontal Gyrus | −0.25 | 0.007 | 0.014 | 0.14 | 0.138 | 0.414 | 0.10 | 0.327 | 0.429 |
| Superior Longitudinal Fasciculus | −0.22 | 0.001 | 0.005 | 0.17 | 0.516 | 0.688 | −0.02 | 0.768 | 0.768 |
| Uncinate Fasciculus | −0.18 | 0.008 | 0.014 | 0.13 | 0.021 | 0.153 | 0.93 | 0.357 | 0.429 |
| Working Memory-Specific | |||||||||
| All Tracts | −0.01 | 0.893 | 0.973 | 0.07 | 0.619 | 0.852 | 0.00 | 0.956 | 0.997 |
| Cingulum | 0.05 | 0.569 | 0.973 | 0.05 | 0.680 | 0.852 | 0.08 | 0.230 | 0.997 |
| Fornix | 0.03 | 0.694 | 0.973 | 0.02 | 0.820 | 0.895 | 0.03 | 0.707 | 0.997 |
| IFG Opercularis | −0.06 | 0.570 | 0.973 | 0.00 | 0.996 | 0.996 | 0.04 | 0.772 | 0.997 |
| IFG Orbitalis | 0.05 | 0.694 | 0.973 | 0.17 | 0.148 | 0.852 | −0.06 | 0.439 | 0.997 |
| IFG Triangularis | −0.02 | 0.907 | 0.973 | 0.21 | < 0.001 | < 0.001 | 0.00 | 0.985 | 0.997 |
| Inferior Longitudinal Fasciculus | 0.02 | 0.827 | 0.973 | 0.14 | 0.646 | 0.852 | 0.04 | 0.675 | 0.997 |
| Inferior Temporal Gyrus | −0.05 | 0.631 | 0.973 | 0.06 | 0.492 | 0.852 | 0.04 | 0.704 | 0.997 |
| Medial Frontal Gyrus | 0.02 | 0.829 | 0.973 | 0.07 | 0.527 | 0.852 | 0.05 | 0.587 | 0.997 |
| Middle Frontal Gyrus | 0.00 | 0.973 | 0.973 | 0.04 | 0.705 | 0.852 | 0.00 | 0.997 | 0.997 |
| Superior Longitudinal Fasciculus | −0.04 | 0.661 | 0.973 | 0.14 | 0.241 | 0.852 | 0.02 | 0.782 | 0.997 |
| Uncinate Fasciculus | 0.04 | 0.645 | 0.973 | −0.03 | 0.710 | 0.852 | −0.14 | 0.117 | 0.997 |
Note: Each value represents a separate model where Common EF and Working Memory-Specific latent factors were regressed on that white matter microstructure measure, controlling for covariates (age, diabetes & hypertension status, and scanner). Uncorrected p values (middle columns) and false-discovery-rate-corrected (FDR-corrected) p values (right columns) are also displayed. FDR correction was conducted separately within each set of white matter measures (i.e., within FW measures, within FAFWcorr, and within MDFWcorr) and separately for Common EF and Working Memory-Specific factors. Bold indicates values that are statistically significant after FDR correction (p <.05). IFG = Inferior Frontal Gyrus.
Table 3.
Associations between covariates and free water and white matter microstructure measures.
| Age | Diabetes | Hyper-tension | Scanner Contrast 1 | Scanner Contrast 2 | |
|---|---|---|---|---|---|
| Free Water (FW) | |||||
| All Tracts | 0.25 | 0.01 | 0.15 | 0.15 | 0.03 |
| Cingulum | 0.17 | 0.06 | 0.14 | 0.10 | 0.06 |
| Fornix | 0.26 | −0.04 | 0.06 | 0.06 | −0.03 |
| IFG Opercularis | 0.24 | 0.03 | 0.17 | 0.06 | 0.00 |
| IFG Orbitalis | 0.22 | 0.00 | 0.14 | 0.00 | −0.01 |
| IFG Triangularis | 0.23 | 0.01 | 0.18 | 0.11 | 0.06 |
| Inferior Longitudinal Fasciculus | 0.15 | −0.01 | 0.12 | 0.14 | 0.04 |
| Inferior Temporal Gyrus | 0.16 | 0.06 | 0.14 | 0.12 | 0.05 |
| Medial Frontal Gyrus | 0.23 | 0.00 | 0.14 | 0.13 | 0.00 |
| Middle Frontal Gyrus | 0.23 | 0.02 | 0.16 | 0.12 | 0.05 |
| Superior Longitudinal Fasciculus | 0.22 | 0.00 | 0.17 | 0.14 | 0.05 |
| Uncinate Fasciculus | 0.23 | 0.01 | 0.15 | 0.13 | 0.00 |
| FW-Corrected FA | |||||
| All Tracts | −0.10 | 0.04 | −0.10 | −0.23 | −0.12 |
| Cingulum | −0.09 | −0.01 | −0.09 | 0.02 | 0.02 |
| Fornix | −0.14 | 0.01 | −0.03 | −0.06 | 0.02 |
| IFG Opercularis | −0.10 | −0.02 | −0.16 | −0.21 | −0.12 |
| IFG Orbitalis | −0.11 | 0.07 | −0.05 | −0.24 | −0.20 |
| IFG Triangularis | −0.13 | 0.01 | −0.12 | −0.17 | −0.10 |
| Inferior Longitudinal Fasciculus | −0.02 | 0.04 | 0.00 | −0.20 | −0.14 |
| Inferior Temporal Gyrus | 0.06 | 0.02 | −0.05 | −0.22 | −0.23 |
| Medial Frontal Gyrus | −0.15 | 0.07 | −0.12 | −0.17 | −0.02 |
| Middle Frontal Gyrus | −0.15 | 0.02 | −0.12 | −0.11 | −0.02 |
| Superior Longitudinal Fasciculus | −0.07 | 0.04 | −0.06 | −0.15 | −0.10 |
| Uncinate Fasciculus | −0.08 | 0.03 | −0.05 | −0.10 | −0.02 |
| FW-Corrected MD | |||||
| All Tracts | −0.16 | 0.04 | −0.05 | −0.01 | 0.07 |
| Cingulum | −0.07 | −0.03 | −0.07 | −0.03 | 0.00 |
| Fornix | −0.18 | 0.04 | −0.05 | 0.01 | 0.07 |
| IFG Opercularis | −0.09 | 0.03 | −0.07 | −0.05 | −0.05 |
| IFG Orbitalis | −0.10 | 0.04 | −0.06 | 0.15 | 0.10 |
| IFG Triangularis | −0.10 | 0.04 | −0.05 | −0.05 | −0.09 |
| Inferior Longitudinal Fasciculus | −0.06 | 0.00 | 0.01 | −0.03 | 0.03 |
| Inferior Temporal Gyrus | 0.03 | 0.05 | 0.00 | 0.03 | −0.01 |
| Medial Frontal Gyrus | −0.13 | 0.05 | −0.03 | 0.03 | 0.08 |
| Middle Frontal Gyrus | −0.15 | 0.04 | −0.09 | 0.02 | 0.02 |
| Superior Longitudinal Fasciculus | −0.06 | 0.04 | 0.02 | −0.05 | −0.02 |
| Uncinate Fasciculus | −0.04 | 0.02 | −0.06 | 0.12 | 0.08 |
Note: Each row represents a separate regression model (corresponding to Table 2) where the latent executive function factors are regressed on that white matter microstructure measure and the covariates. Dichotomous variables were used for diabetes and hypertension (0 = no, 1 = yes). The orthogonal ‘Scanner’ contrasts capture differences among scanners (Contrast 1) and software within one of the scanners (Contrast 2). Significant associations are indicated in bold (p <.05; no multiple test correction).
Fig. 3.
Example of the primary regression analyses where executive function (EF) latent factors are regressed on free water (FW) or white matter microstructure measures (FW across all white matter tracts in this example) and covariates (displayed in a single box for simplicity). All covariates except age were dichotomous or orthogonal contrasts.
The Common EF factor was associated with FW across 9 of the 11 white matter tracts (and the ‘all tracts’ measure). In all cases, more FW corresponded to lower Common EF ability (range in β = -0.15 to -0.26). Common EF was not associated with FAFWcorr or MDFWcorr in any of the tracts. The Working Memory-Specific factor was associated with greater FAFWcorr in the IFG Triangularis (β = 0.21), but not with any other FW or white matter microstructure metrics after FDR correction.
Analyses after excluding individuals with MCI are displayed in Table 4. For Common EF and FW, the negative associations were nonsignificant after FDR correction, though significant associations were observed for 2 of the 11 white matter tracts based on uncorrected p-values (cingulum and unicinate fasciculus). Associations with the remaining tracts were attenuated by approximately half (range β = -0.09 to -0.15). For the Working Memory-Specific factor, the association with FA in the IFG Triangularis remained significant even after FDR correction (β = 0.25). Additionally, significant associations were observed for FA in the inferior longitudinal fasciculus and the superior longitudinal fasciculus (β = 0.26 and 0.28, respectively).
Table 4.
Associations between executive function and white matter microstructure in cognitively normal subjects (N = 411).
| Independent Variable | Free Water | FAFWcorr | MDFWcorr | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | p | p (FDR) | β | p | p (FDR) | β | p | p (FDR) | |
| Common EF | |||||||||
| All Tracts | −0.15 | 0.113 | 0.247 | 0.05 | 0.793 | 0.891 | −0.06 | 0.432 | 0.740 |
| Cingulum | −0.27 | 0.005 | 0.062 | −0.06 | 0.797 | 0.891 | 0.05 | 0.670 | 0.869 |
| Fornix | −0.09 | 0.428 | 0.467 | −0.01 | 0.891 | 0.891 | 0.03 | 0.797 | 0.869 |
| IFG Opercularis | −0.14 | 0.071 | 0.213 | 0.07 | 0.233 | 0.792 | −0.07 | 0.428 | 0.740 |
| IFG Orbitalis | −0.14 | 0.065 | 0.213 | −0.05 | 0.658 | 0.891 | 0.02 | 0.894 | 0.894 |
| IFG Triangularis | −0.12 | 0.268 | 0.325 | −0.02 | 0.264 | 0.792 | −0.03 | 0.722 | 0.869 |
| Inferior Longitudinal Fasciculus | −0.09 | 0.210 | 0.315 | −0.10 | 0.260 | 0.792 | −0.13 | 0.111 | 0.454 |
| Inferior Temporal Gyrus | −0.11 | 0.643 | 0.643 | −0.06 | 0.154 | 0.792 | −0.15 | 0.151 | 0.454 |
| Medial Frontal Gyrus | −0.11 | 0.271 | 0.325 | 0.02 | 0.870 | 0.891 | −0.13 | 0.087 | 0.454 |
| Middle Frontal Gyrus | −0.15 | 0.137 | 0.247 | 0.02 | 0.481 | 0.891 | −0.06 | 0.744 | 0.869 |
| Superior Longitudinal Fasciculus | −0.11 | 0.144 | 0.247 | 0.04 | 0.776 | 0.891 | −0.09 | 0.138 | 0.454 |
| Uncinate Fasciculus | −0.19 | 0.023 | 0.141 | 0.10 | 0.403 | 0.891 | 0.10 | 0.383 | 0.740 |
| Working Memory-Specific | |||||||||
| All Tracts | −0.08 | 0.486 | 0.839 | 0.22 | 0.018 | 0.054 | 0.06 | 0.547 | 0.772 |
| Cingulum | 0.00 | 0.986 | 0.986 | 0.22 | 0.231 | 0.323 | 0.08 | 0.420 | 0.772 |
| Fornix | −0.04 | 0.629 | 0.839 | 0.06 | 0.541 | 0.591 | 0.08 | 0.152 | 0.772 |
| IFG Opercularis | −0.11 | 0.340 | 0.839 | 0.16 | 0.252 | 0.323 | 0.09 | 0.388 | 0.772 |
| IFG Orbitalis | 0.00 | 0.981 | 0.986 | 0.25 | 0.149 | 0.255 | −0.02 | 0.871 | 0.871 |
| IFG Triangularis | −0.08 | 0.560 | 0.839 | 0.25 | 0.011 | 0.042 | 0.08 | 0.528 | 0.772 |
| Inferior Longitudinal Fasciculus | −0.06 | 0.575 | 0.839 | 0.26 | 0.002 | 0.027 | 0.05 | 0.665 | 0.772 |
| Inferior Temporal Gyrus | −0.11 | 0.550 | 0.839 | 0.24 | 0.064 | 0.127 | 0.05 | 0.702 | 0.772 |
| Medial Frontal Gyrus | −0.03 | 0.796 | 0.955 | 0.16 | 0.269 | 0.323 | 0.10 | 0.397 | 0.772 |
| Middle Frontal Gyrus | −0.06 | 0.615 | 0.839 | 0.19 | 0.056 | 0.127 | 0.10 | 0.708 | 0.772 |
| Superior Longitudinal Fasciculus | −0.10 | 0.323 | 0.839 | 0.28 | 0.008 | 0.042 | 0.04 | 0.661 | 0.772 |
| Uncinate Fasciculus | 0.07 | 0.551 | 0.839 | −0.02 | 0.862 | 0.862 | −0.07 | 0.570 | 0.772 |
Note: Each value represents a separate model where Common EF and Working Memory-Specific latent factors were regressed on that white matter microstructure measure, controlling for covariates (age, diabetes & hypertension status, and scanner). Bold indicates values that are statistically significant (uncorrected p-values < 0.05). IFG = Inferior Frontal Gyrus.
7.2. Interactions between cognitive reserve and white matter microstructure
Next, in the full sample (i.e., including individuals with MCI and those missing MCI diagnoses), we repeated the same set of analyses after adding age 20 general cognitive ability and its interaction with the relevant white matter measure to the regression model. We only conducted moderation analyses for the white matter measures significantly associated with EF factors in the primary analyses (i.e., Common EF and FW in 9 tracts and the ‘all tracts’ measure, Working Memory-Specific and FAFWcorr in the IFG Triangularis tract only). Results are displayed in Table 5. Although AFQT scores were strongly predictive of Common EF (βs = 0.41 to 0.64), they did not moderate any of the associations between the EF factors and FW described above (all uncorrected ps > 0.243, FDR-corrected ps > 0.782). Finally, we conducted sensitivity analyses using a dichotomous score for cognitive reserve (i.e., grouping subjects as above or below the mean), which also revealed no evidence that cognitive reserve moderated associations between EF factors and FW or FA (see supplemental Table S3).
Table 5.
Results of moderation analyses involving cognitive reserve.
| Association | Main Effect: Free Water | Main Effect: Cognitive Reserve | Interaction Term: (Free Water * Cognitive Reserve) | ||||
|---|---|---|---|---|---|---|---|
| β | p | β | p | β | p | p (FDR) | |
| Common EF and Free Water | |||||||
| All Tracts | −0.15 | 0.208 | 0.62 | <0.001 | 0.03 | 0.661 | 0.782 |
| Cingulum | −0.23 | 0.024 | 0.58 | <0.001 | 0.05 | 0.608 | 0.782 |
| Fornix | −0.17 | 0.017 | 0.62 | <0.001 | −0.05 | 0.243 | 0.782 |
| IFG_Opercularis | −0.14 | 0.265 | 0.59 | <0.001 | 0.02 | 0.602 | 0.782 |
| IFG_Orbitalis | −0.18 | 0.003 | 0.58 | <0.001 | 0.07 | 0.361 | 0.782 |
| IFG_Triangularis | −0.11 | 0.586 | 0.41 | <0.001 | 0.16 | 0.518 | 0.782 |
| Medial_Frontal_Gyrus | −0.15 | 0.560 | 0.60 | <0.001 | 0.06 | 0.828 | 0.828 |
| Middle_Frontal_Gyrus | −0.15 | 0.307 | 0.61 | <0.001 | 0.03 | 0.623 | 0.782 |
| SLF | −0.15 | 0.174 | 0.53 | <0.001 | 0.02 | 0.711 | 0.782 |
| Uncinate Fasciculus | −0.19 | 0.024 | 0.64 | <0.001 | 0.05 | 0.352 | 0.782 |
| Working Memory-Specific and FAFWcorr | |||||||
| IFG Triangularis | 0.36 | 0.000 | −0.36 | 0.121 | −0.09 | 0.274 | 0.782 |
Note: Each row represents a separate model where Common EF and Working Memory-Specific Latent factors were regressed on that white matter microstructure measure, controlling for covariates (age, diabetes & hypertension status, and scanner). Additionally, EF factors were regressed on the indicator of cognitive reserve (age 20 general cognitive ability) and an interaction term was included (AFQT * FW). IFG = Inferior Frontal Gyrus.
8. Discussion
The goal of the study was to better understand the associations between EF abilities and white matter microstructure in older adults. Results indicated that EF abilities (specifically a Common EF factor comprising performance across 6 tasks) were associated with FW across almost all cortical tracts examined here, but not with FAFWcorr or MDFWcorr. Greater Common EF ability was associated with less FW. These findings mirror the associations with age, which was associated with free water across all cortical tracts, but only associated with FAFWcorr or MDFWcorr in some tracts.
These results are consistent with two recent studies that have suggested EFs are associated with FW (but not FAFWcorr or MDFWcorr) in older adult samples (Archer et al., 2020, Maillard et al., 2019). These prior studies focused on samples with high rates of MCI (∼50 %) and our study extends these findings to a slightly younger sample with a substantially lower prevalence of MCI. Importantly, after excluding individuals with MCI (13.7 % of the sample), the associations between Common EF and FW described above were nonsignificant, suggesting individuals with MCI may be driving many of the observed FW associations, though there was some evidence that Common EF remained associated with FW in the cingulum and uncinate (based on raw p-values). Thus, it is possible that widespread associations between Common EF and FW are primarily observed in samples with some MCI/AD cases.
Another explanation for the lack of significance of many tracts after excluding MCI cases is reduced power in the smaller cognitively normal sample (with restricted range in scores). However, associations between Common EF and FW were about half the magnitude as they were when including participants with MCI (Table 2 vs Table 4), suggesting that the differences are not simply due to reduction in sample size. Thus, EF may be modestly related to FW in cognitively normal individuals, with associations observed potentially for frontally-connected tracts (e.g., the uncinate and cingulum) in normal aging. This is consistent with the notion that FW metrics primarily capture neurodegeneration (Pasternak et al., 2012), with individual differences in FW not being strongly associated with cognitive abilities until after some neurodegeneration has taken place. Another recent study of cognitively normal older adults revealed similar negative associations between FW and fluid cognition (which included multiple EF tasks) in some, but not all, white matter tracts (including the cingulum and SLF) and no associations with FAFWcorr in any of the candidate tracts (Gullett et al., 2020). Therefore, associations between Common EF and FW may be relatively restricted to certain brain regions in adulthood and normal aging, but expand to others with age and/or early AD pathology. In either case, associations appear unique to FW rather than FAFWcorr or MDFWcorr metrics.
Because this was one of the first studies to examine these associations at the level of latent factors, we were also able to examine associations between variance unique to working memory tasks not already captured by Common EF (i.e., the Working Memory-Specific factor). Results in the full sample (including individuals with MCI) were consistent with an earlier study of younger adults in which working memory updating-specific ability was not associated with white matter microstructure (Smolker et al., 2018), though the FA and MD metrics were not corrected for FW in this prior study. However, the Working Memory-Specific factor was associated with FAFWcorr in the IFG-Triangularis in the primary analysis (including participants with MCI) and with FAFWcorr in three tracts (IFG-Triangularis, inferior and superior longitudinal fasciculus) after excluding participants with MCI.
It will be important to further examine these novel associations with working memory-specific to understand these positive associations with FAFWcorr, including why some associations were only observed in cognitively normal subjects. Importantly, there was little evidence for group-level differences in FAFWcorr across cognitively normal and MCI subjects (see Table S3), suggesting the lack of association in the full sample was not driven by MCI subjects having lower FAFWcorr. Rather, the individual differences captured by microstructural measures may be more subtle and shed light into individual variability in normal aging. That is, while FW metrics capture neurodegeneration or axonal degradation (and therefore relate to cognitive ability after some atrophy has taken place), microstructural measures may tell us more about normal function and/or may possibly be of use in predictive studies. This would be consistent with prior work showing FW was strongly associated with neurodegeneration (e.g., hippocampal volume) but FAFWcorr in the fornix interacted with hippocampal volume to predict future executive function decline (Archer et al., 2020). Regardless, these findings highlight the importance of considering the role of MCI in these associations and separately evaluating cognitive correlates of white matter microstructure within cognitively normal individuals. Furthermore, because earlier work has suggested that working memory-specific operations may be more associated with subcortical brain regions such as gating in the basal ganglia (Friedman and Miyake, 2017), it will be interesting to further probe these associations with FW and white matter in other tracts beyond those cortical tracts examined here.
Another goal of the study was to examine whether an indicator of cognitive reserve (age 20 general cognitive ability) moderates associations between EFs and white matter microstructure. For example, earlier work in this VETSA sample has demonstrated that individuals with low general cognitive ability at age 20 demonstrated stronger associations between hippocampal volume and memory at mean age 56 (Vuoksimaa et al., 2013). The present results indicated no evidence for such interactions for Common EF. It is possible that moderating effects of cognitive reserve are only apparent in the context of disease- or age-related pathology. Although FW in all tracts was significantly associated with age, the cross-sectional nature of this study makes it difficult to determine whether this association reflects age-related neuropathology or instead reflects pre-existing individual differences or other factors beyond age-related neuropathology. Indeed, white matter abnormalities have been observed in wave 2 of VETSA (mean age 62) (Fennema-Notestine et al., 2016, Sanderson-Cimino et al., 2021), suggesting some pathology was present in at least some subjects, yet a significant interaction with age 20 cognitive ability was not observed. Alternatively, or additionally, moderation of FAFWcorr and MDFWcorr by cognitive reserve may be possible in samples with greater rates of impairments (MCI or AD), as EFs appear more related to these measures in AD patients (Ji et al., 2017).
While associations between EF factors and FW differed after removing MCI subjects from these analyses (i.e., Table 2 vs Table 4), there were no group differences between MCI and cognitively normal subjects on our index of young adult cognitive reserve (p =.263). The fact that our MCI subjects had comparable levels of young adult cognitive reserve to cognitively normal subjects, but appeared to show much stronger associations between Common EF and FW, adds further support to the idea that associations between EF and FW differ in normal versus pathological aging. It may therefore be interesting to examine moderating effects of cognitive reserve within samples of MCI cases only, though it will be necessary to do so in a sample with a greater number of MCI subjects.
8.1. Strengths and weaknesses
The comprehensive assessment of EFs in VETSA allowed for examination of associations between EF and white matter microstructure using a latent variable approach that isolated Common EF variance from Working Memory-Specific variance at a time in early old age where most individuals were cognitively normal. This study also represents one of the first examinations into the role of cognitive reserve in these associations. Some weaknesses of the study include the fact that all participants are men, and the vast majority are non-Hispanic and White. It will be important to evaluate these associations in more diverse samples.
Additionally, we used established white matter tract templates (Archer, Vaillancourt, & Coombes, 2018) and a recently-available white matter tract atlas that provides strong coverage of the brain, replicating prior associations between EF and FW in older adults (Archer et al., 2020). While our results showed consistent associations between the Common EF factor and extracellular (FW) but not intracellular metrics (FAFWcorr and MDFWcorr), it is still unclear what specific cellular processes contribute to each variable. Better understanding the cognitive correlates of FW, FAFWcorr, and MDFWcorr will help shed light on the nature of these measures, and their role as predictors and indicators of aging, but additional studies are also needed to quantify what gives rise to individual differences in these measures.
Finally, this study is cross-sectional and cannot speak to whether these associations reflect pre-existing associations between Common EF and FW, or if these effects are specific to aging. Findings from one existing study suggest that baseline levels of FW are also associated with longitudinal changes in EF, though this sample was at about 10 years older than the present sample and most individuals were diagnosed with MCI or dementia (Maillard et al., 2019). In our study, age was consistently associated with all free water measures (and some white matter measures), suggesting that the free water measures examined here are sensitive to age. The correlations with age may actually be relatively low estimates given the narrow age range of our sample (∼10 years). In any case, it will be necessary to examine these associations in early adulthood and middle age to inform whether associations between FW and EF reflect age-related changes in EF or perhaps whether they account for individual differences in EF across the lifespan.
8.2. Concluding remarks
EF are complex cognitive control abilities that are highly relevant to aging. This study sheds light on the neural underpinnings of common and specific components of EF by demonstrating that FW measures across many cortical white matter tracts are associated with individual differences in Common EF abilities. By contrast, working memory-specific abilities were associated with FAFWcorr, but only after excluding individuals with MCI from the analyses. Associations between Common EF ability and FW were not moderated by cognitive reserve in the current investigation, but it will be important to consider whether these factors may contribute more strongly to associations between EFs and white matter in later stages of aging or progression toward cognitive decline or dementia.
CRediT authorship contribution statement
Daniel E. Gustavson: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. Derek B. Archer: Data curation, Visualization, Writing – original draft, Writing – review & editing. Jeremy A. Elman: Data curation, Writing – review & editing. Olivia K. Puckett: Data curation, Writing – review & editing. Christine Fennema-Notestine: Data curation, Methodology, Writing – review & editing. Matthew S. Panizzon: Data curation, Writing – review & editing. Niranjana Shashikumar: Data curation, Writing – review & editing. Timothy J. Hohman: Methodology, Writing – review & editing. Angela L. Jefferson: Writing – review & editing. Lisa T. Eyler: Methodology, Writing – review & editing. Linda K. McEvoy: Methodology, Writing – review & editing. Michael J. Lyons: Funding acquisition, Writing – review & editing. Carol E. Franz: Funding acquisition, Methodology, Supervision, Project administration, Writing – review & editing. William S. Kremen: Funding acquisition, Methodology, Supervision, Project administration, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This research was supported by Grants R03 AG065643, R01 AG050595, R01 AG076838, R01 AG022381, P01 AG055367, K01 AG073584, and K08 AG047903 from the National Institutes of Health. Publication of this article was funded by the University of Colorado Boulder Libraries Open Access Fund.
The content of this manuscript is the responsibility of the authors and does not represent official views of NIA/NIH, or the Veterans’ Administration. Numerous organizations provided invaluable assistance in the conduct of the VET Registry, including: U.S. Department of Veterans Affairs, Department of Defense; National Personnel Records Center, National Archives and Records Administration; Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. The authors gratefully acknowledge the continued cooperation of the twins and the efforts of many staff members.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2022.103279.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alexander A.L., Lee J.E., Lazar M., Field A.S. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer D.B., Vaillancourt D.E., Coombes S.A. A template and probabilistic atlas of the human sensorimotor tracts using diffusion MRI. Cereb. Cortex. 2018;28:1685–1699. doi: 10.1093/cercor/bhx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer D.B., Coombes S.A., McFarland N.R., DeKosky S.T., Vaillancourt D.E. Development of a transcallosal tractography template and its application to dementia. Neuroimage. 2019;200:302–312. doi: 10.1016/j.neuroimage.2019.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer D.B., Moore E.E., Shashikumar N., Dumitrescu L., Pechman K.R., Landman B.A., Hohman T.J. Free-water metrics in medial temporal lobe white matter tract projections relate to longitudinal cognitive decline. Neurobiol. Aging. 2020;94:15–23. doi: 10.1016/j.neurobiolaging.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer D.B., Moore E.E., Pamidimukkala U., Shashikumar N., Pechman K.R., Blennow K., Gifford K.A. The relationship between white matter microstructure and self-perceived cognitive decline. Neuroimage: Clinical. 2021;32 doi: 10.1016/j.nicl.2021.102794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants B.B., Epstein C.L., Grossman M., Gee J.C. Symmetric diffeomorphic image registration with cross-correlation: Evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 2008;12:26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkour A., Morris J.C., Wolk D.A., Dickerson B.C. The effects of aging and Alzheimer's disease on cerebral cortical anatomy: Specificity and differential relationships with cognition. Neuroimage. 2013;76:332–344. doi: 10.1016/j.neuroimage.2013.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D., Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013;17:502–509. doi: 10.1016/j.tics.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudic S., Dalla Barba G., Thibaudet M.C., Smagghe A., Remy P., Traykov L. Executive function deficits in early Alzheimer's disease and their relations with episodic memory. Arch. Clin. Neuropsychol. 2006;21:15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Bayroff, A. G., & Anderson, A. A. (1963). Development of Literacy Screening Scales for AFQT 7 and 8 Failures. Washington DC.
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine: An International Journal Devoted to the Development and Application of Magnetic Resonance In Vivo. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Bennett I.J., Madden D.J. Disconnected aging: Cerebral white matter integrity and age-related differences in cognition. Neuroscience. 2014;276:187–205. doi: 10.1016/j.neuroscience.2013.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi M.W., Edmonds E.C., Jak A.J., Clark L.R., Delano-Wood L., McDonald C.R., Salmon D.P. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J. Alzheimers Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzali M., Giulietti G., Basile B., Serra L., Spano B., Perri R., Cercignani M. Damage to the cingulum contributes to Alzheimer's disease pathophysiology by deafferentation mechanism. Hum. Brain Mapp. 2012;33:1295–1308. doi: 10.1002/hbm.21287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C.A., Johnson N.F., Anderson-Mooney A.J., Jicha G.A., Shaw L.M., Trojanowski J.Q., Gold B.T. Development, validation and application of a new fornix template for studies of aging and preclinical Alzheimer's disease. Neuroimage: Clinical. 2017;13:106–115. doi: 10.1016/j.nicl.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L. Memory and executive function in aging and AD: Multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44:195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Cai L.Y., Yang Q., Hansen C.B., Nath V., Ramadass K., Johnson G.W., Landman B.A. PreQual: An automated pipeline for integrated preprocessing and quality assurance of diffusion weighted MRI images. Magn. Reson. Med. 2021;86:456–470. doi: 10.1002/mrm.28678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton R.A., Barrick T.R., McIntyre D.J., Shen Y., O'Sullivan M., Howe F.A., Markus H.S. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006;66:217–222. doi: 10.1212/01.wnl.0000194256.15247.83. [DOI] [PubMed] [Google Scholar]
- Clark K.A., Nuechterlein K.H., Asarnow R.F., Hamilton L.S., Phillips O.R., Hageman N.S., Narr K.L. Mean diffusivity and fractional anisotropy as indicators of disease and genetic liability to schizophrenia. J. Psychiatr. Res. 2011;45:980–988. doi: 10.1016/j.jpsychires.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers L.G., de Groot M., Hofman A., Krestin G.P., van der Lugt A., Niessen W.J., Ikram M.A. Altered tract-specific white matter microstructure is related to poorer cognitive performance: The Rotterdam Study. Neurobiol. Aging. 2016;39:108–117. doi: 10.1016/j.neurobiolaging.2015.11.021. [DOI] [PubMed] [Google Scholar]
- Daneman M., Carpenter P.A. Individual differences in working memory and reading. J. Verbal Learn. Verbal Behav. 1980;19:450–466. doi: 10.1016/S0022-5371(80)90312-6. [DOI] [Google Scholar]
- Delis D.C., Kaplan E., Kramer J.H. Psychological Corporation; (D-KEFS): 2001. Delis-Kaplan executive function system. [Google Scholar]
- Dumont M., Roy M., Jodoin P.M., Morency F.C., Houde J.C., Xie Z. Alzheimer's Disease Neuroimaging, I. Free water in white matter differentiates MCI and AD from control subjects. Front. Aging Neurosci. 2019;11:270. doi: 10.3389/fnagi.2019.00270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elman J.A., Jak A.J., Panizzon M.S., Tu X.M., Chen T., Reynolds C.A., Kremen W.S. Underdiagnosis of mild cognitive impairment: A consequence of ignoring practice effects. Alzheimer's & Dementia: Diagnosis, Assessment & Disease Monitoring. 2018;10:372–381. doi: 10.1016/j.dadm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt L.E., Briley D.A., Mann F.D., Harden K.P., Tucker-Drob E.M. Genes unite executive functions in childhood. Psychol. Sci. 2015;26:1151–1163. doi: 10.1177/0956797615577209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C., McEvoy L.K., Notestine R., Panizzon M.S., Yau W.Y.W., Franz C.E., Kremen W.S. White matter disease in midlife is heritable, related to hypertension, and shares some genetic influence with systolic blood pressure. Neuroimage-Clinical. 2016;12:737–745. doi: 10.1016/j.nicl.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell A.M., Westlye L.T., Amlien I., Espeseth T., Reinvang I., Raz N., Walhovd K.B. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex. 2009;19:2001–2012. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freis S.M., Morrison C.L., Lessem J.M., Hewitt J.K., Friedman N.P. Genetic and environmental influences on executive functions and intelligence in middle childhood. Dev. Sci. 2021;e13150 doi: 10.1111/desc.13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N.P., Miyake A., Young S.E., Defries J.C., Corley R.P., Hewitt J.K. Individual differences in executive functions are almost entirely genetic in origin. J. Exp. Psychol. Gen. 2008;137:201–225. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N.P., Miyake A., Altamirano L.J., Corley R.P., Young S.E., Rhea S.A., Hewitt J.K. Stability and change in executive function abilities from late adolescence to early adulthood: A longitudinal twin study. Dev. Psychol. 2016;52:326–340. doi: 10.1037/dev0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman N.P., Miyake A. Unity and diversity of executive functions: Individual differences as a window on cognitive structure. Cortex. 2017;86:186–204. doi: 10.1016/j.cortex.2016.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden, C. J., & Freshwater, S. M. (2002). The Stroop color and word test: A manual for clinical and experimental uses[adult version]: Stoelting.
- Gullett J.M., O'Shea A., Lamb D.G., Porges E.C., O'Shea D.M., Pasternak O., Woods A.J. The association of white matter free water with cognition in older adults. Neuroimage. 2020;219 doi: 10.1016/j.neuroimage.2020.117040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson D.E., Panizzon M.S., Elman J.A., Franz C.E., Reynolds C.A., Jacobson K.C., Kremen W.S. Stability of genetic and environmental influences on executive functions in midlife. Psychol. Aging. 2018;33:219–231. doi: 10.1037/pag0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson D.E., Panizzon M.S., Franz C.E., Friedman N.P., Reynolds C.A., Jacobson K.C., Kremen W.S. Genetic and environmental architecture of executive functions in midlife. Neuropsychology. 2018;32:18–30. doi: 10.1037/neu0000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson D.E., Hatton S.N., Elman J.A., Panizzon M.S., Franz C.E., Hagler D.J., Jr., Kremen W.S. Predominantly global genetic influences on individual white matter tract microstructure. Neuroimage. 2019;184:871–880. doi: 10.1016/j.neuroimage.2018.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson D.E., Jak A.J., Elman J.A., Panizzon M.S., Franz C.E., Gifford K.A., Kremen W.S. How well does subjective cognitive decline correspond to objectively measured cognitive decline? Assessment of 10–12 year change. J. Alzheimers Dis. 2021;83:291–304. doi: 10.3233/JAD-210123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson D.E., Reynolds C.A., Corley R.P., Wadsworth S.J., Hewitt J.K., Friedman N.P. Genetic associations between executive functions and intelligence: A combined twin and adoption study. J. Exp. Psychol. Gen. 2022 doi: 10.1037/xge0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavson D.E., Reynolds C.A., Hohman T.J., Jefferson A.L., Elman J.A., Panizzon M.S., Kremen W.S. Alzheimer’s disease polygenic scores predict changes in episodic memory and executive function across 12 years in late middle age. Journal of the International Neuropsychological Society, in press. 2022 doi: 10.1017/S1355617722000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga M., Dolan C.V., van der Molen M.W. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Jak A.J., Bondi M.W., Delano-Wood L., Wierenga C., Corey-Bloom J., Salmon D.P., Delis D.C. Quantification of five neuropsychological approaches to defining mild cognitive impairment. Am. J. Geriatr. Psychiatry. 2009;17:368–375. doi: 10.1097/JGP.0b013e31819431d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Beckmann C.F., Behrens T.E., Woolrich M.W., Smith S.M. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Ji F., Pasternak O., Liu S., Loke Y.M., Choo B.L., Hilal S., Zhou J. Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimers Res. Ther. 2017;9:63. doi: 10.1186/s13195-017-0292-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junquera A., Garcia-Zamora E., Olazaran J., Parra M.A., Fernandez-Guinea S. Role of executive functions in the conversion from mild cognitive impairment to dementia. J. Alzheimers Dis. 2020;77:641–653. doi: 10.3233/JAD-200586. [DOI] [PubMed] [Google Scholar]
- Kennedy K.M., Raz N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive functions, and speed. Neuropsychologia. 2009;47:916–927. doi: 10.1016/j.neuropsychologia.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirova A.M., Bays R.B., Lagalwar S. Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer's disease. Biomed Res. Int. 2015;2015 doi: 10.1155/2015/748212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krch D., Frank L.E., Chiaravalloti N.D., Vakil E., DeLuca J. Cognitive reserve protects against memory decrements associated with neuropathology in traumatic brain injury. J. Head Trauma Rehabil. 2019;34:E57–E65. doi: 10.1097/HTR.0000000000000472. [DOI] [PubMed] [Google Scholar]
- Kremen, W. S., Elman, J. A., Panizzon, M. S., Eglit, G. M. L., Sanderson-Cimino, M., Williams, M. E., . . . Franz, C. E. (2022). Cognitive reserve and related constructs: A unified framework across cognitive and brain dimensions of aging. Frontiers in Aging Neuroscience, in press. [DOI] [PMC free article] [PubMed]
- Kremen W.S., Thompson-Brenner H., Leung Y.M., Grant M.D., Franz C.E., Eisen S.A., Lyons M.J. Genes, environment, and time: The Vietnam Era Twin Study of Aging (VETSA) Twin Res. Hum. Genet. 2006;9:1009–1022. doi: 10.1375/183242706779462750. [DOI] [PubMed] [Google Scholar]
- Kremen W.S., Panizzon M.S., Xian H., Barch D.M., Franz C.E., Grant M.D., Lyons M.J. Genetic architecture of context processing in late middle age: More than one underlying mechanism. Psychol. Aging. 2011;26:852–863. doi: 10.1037/a0025098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremen W.S., Jak A.J., Panizzon M.S., Spoon K.M., Franz C.E., Thompson W.K., Lyons M.J. Early identification and heritability of mild cognitive impairment. Int. J. Epidemiol. 2014;43:600–610. doi: 10.1093/ije/dyt242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons M.J., York T.P., Franz C.E., Grant M.D., Eaves L.J., Jacobson K.C., Kremen W.S. Genes determine stability and the environment determines change in cognitive ability during 35 years of adulthood. Psychol. Sci. 2009;20:1146–1152. doi: 10.1111/j.1467-9280.2009.02425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabbott D.J., Noseworthy M.D., Bouffet E., Rockel C., Laughlin S. Diffusion tensor imaging of white matter after cranial radiation in children for medulloblastoma: Correlation with IQ. Neuro Oncol. 2006;8:244–252. doi: 10.1215/15228517-2006-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier-Hein K.H., Westin C.F., Shenton M.E., Weiner M.W., Raj A., Thomann P., Pasternak O. Widespread white matter degeneration preceding the onset of dementia. Alzheimers & Dementia. 2015;11(485–493):e482. doi: 10.1016/j.jalz.2014.04.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillard, P., Fletcher, E., Singh, B., Martinez, O., Johnson, D. K., Olichney, J. M.,..., DeCarli, C. (2019). Cerebral white matter free water: A sensitive biomarker of cognition and function. Neurology, 92, e2221-e2231. doi:10.1212/WNL.0000000000007449. [DOI] [PMC free article] [PubMed]
- McKenzie C., Bucks R.S., Weinborn M., Bourgeat P., Salvado O., Gavett B.E., Alzheimer's Disease Neuroimaging, I Cognitive reserve predicts future executive function decline in older adults with Alzheimer's disease pathology but not age-associated pathology. Neurobiol. Aging. 2020;88:119–127. doi: 10.1016/j.neurobiolaging.2019.12.022. [DOI] [PubMed] [Google Scholar]
- Mielke M.M., Okonkwo O.C., Oishi K., Mori S., Tighe S., Miller M.I., Lyketsos C.G. Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer's disease. Alzheimers Dement. 2012;8:105–113. doi: 10.1016/j.jalz.2011.05.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P. The nature and organization of individual differences in executive functions: Four general conclusions. Curr. Dir. Psychol. Sci. 2012;21:8–14. doi: 10.1177/0963721411429458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Friedman N.P., Emerson M.J., Witzki A.H., Howerter A., Wager T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cogn. Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Muthén, L. K., & Muthén, B. O. (1998-2017). Mplus User's Guide: Eighth Edition. Los Angeles, CA: Muthén & Muthén.
- O'Shea D.M., Fieo R.A., Hamilton J.L., Zahodne L.B., Manly J.J., Stern Y. Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. Int. J. Geriatr. Psychiatry. 2015;30:614–622. doi: 10.1002/gps.4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O., Sochen N., Gur Y., Intrator N., Assaf Y. Free water elimination and mapping from diffusion MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2009;62:717–730. doi: 10.1002/mrm.22055. [DOI] [PubMed] [Google Scholar]
- Pasternak O., Shenton M.E., Westin C. In: Ayache N., Delingette H., Golland P., Mori K., editors. Vol. 7511. Springer; Berlin, Heidelberg: 2012. Estimation of extracellular volume from regularized multi-shell diffusion MRI. (Medical Image Computing and Computer-Assisted Intervention). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan S., Bertoux M., Flanagan E., Irish M., Piguet O., Hodges J.R., Hornberger M. Longitudinal executive function and episodic memory profiles in behavioral-variant frontotemporal dementia and Alzheimer's Disease. J. Int. Neuropsychol. Soc. 2017;23:34–43. doi: 10.1017/S1355617716000837. [DOI] [PubMed] [Google Scholar]
- Roldan-Tapia L., Garcia J., Canovas R., Leon I. Cognitive reserve, age, and their relation to attentional and executive functions. Appl. Neuropsychol. Adult. 2012;19:2–8. doi: 10.1080/09084282.2011.595458. [DOI] [PubMed] [Google Scholar]
- Sanderson-Cimino M., Panizzon M.S., Elman J.A., Tu X., Gustavson D.E., Puckett O., Kremen W.S. Periventricular and deep abnormal white matter differ in associations with cognitive performance at midlife. Neuropsychology. 2021;35:252–264. doi: 10.1037/neu0000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satorra A., Bentler P.M. A scaled difference chi-square test statistic for moment structure analysis. Psychometrika. 2001;66:507–514. doi: 10.1007/Bf02296192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K.G., Blaber J., Huo Y., Newton A., Hansen C., Nath V., Landman B.A. Synthesized b0 for diffusion distortion correction (Synb0-DisCo) Magn. Reson. Imaging. 2019;64:62–70. doi: 10.1016/j.mri.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenborn C.A., Heyman K.M. Health characteristics of adults aged 55 years and over: United States, 2004–2007. National Health Statistics Report. 2009;16:1–31. [PubMed] [Google Scholar]
- Selemon L.D., Goldman-Rakic P.S. The reduced neuropil hypothesis: A circuit based model of schizophrenia. Biol. Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Smolker H.R., Friedman N.P., Hewitt J.K., Banich M.T. Neuroanatomical correlates of the unity and diversity model of executive function in young adults. Frontiers in Human Neurocience. 2018;12:283. doi: 10.3389/fnhum.2018.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve: Implications for assessment and intervention. Folia Phoniatr. Logop. 2013;65:49–54. doi: 10.1159/000353443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroop J.R. Studies of interference in serial verbal reactions. J. Exp. Psychol. 1935;18:643–662. doi: 10.1037/0096-3445.121.1.15. [DOI] [Google Scholar]
- Tsuang M.T., Bar J.L., Harley R.M., Lyons M.J. The Harvard Twin Study of Substance Abuse: What we have learned. Harv. Rev. Psychiatry. 2001;9:267–279. doi: 10.1093/hrp/9.6.267. [DOI] [PubMed] [Google Scholar]
- Vaughan L., Giovanello K. Executive function in daily life: Age-related influences of executive processes on instrumental activities of daily living. Psychol. Aging. 2010;25:343–355. doi: 10.1037/a0017729. [DOI] [PubMed] [Google Scholar]
- Vuoksimaa E., Panizzon M.S., Chen C.H., Eyler L.T., Fennema-Notestine C., Fiecas M.J.A., Kremen W.S. Cognitive reserve moderates the association between hippocampal volume and episodic memory in middle age. Neuropsychologia. 2013;51:1124–1131. doi: 10.1016/j.neuropsychologia.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1997. Wechsler Memory Scale (WMS-III) [Google Scholar]
- Westlye L.T., Walhovd K.B., Dale A.M., Bjornerud A., Due-Tonnessen P., Engvig A., Fjell A.M. Life-span changes of the human brain white matter: Diffusion tensor imaging (DTI) and volumetry. Cereb. Cortex. 2010;20:2055–2068. doi: 10.1093/cercor/bhp280. [DOI] [PubMed] [Google Scholar]
- Whalley L.J., Deary I.J., Appleton C.L., Starr J.M. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res. Rev. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.