Highlights
-
•
MDR-TB is difficult to manage; updated clinical guidance of new drug use is needed
-
•
No recent systematic review/meta-analysis on delamanid (DLM) is available
-
•
In observational studies including DLM (591 patients) the success rate was 80.9%
-
•
In experimental studies including DLM (391 patients) the success rate was 72.5%
-
•
Few adverse events attributable to DLM were reported
Keywords: TB, MDR-TB, delamanid, bedaquiline, effectiveness, safety
Abstract
Introduction
Multidrug-resistant tuberculosis (MDR-TB) is a life-threatening condition needing long poly-chemotherapy regimens. As no systematic reviews/meta-analysis is available to comprehensively evaluate the role of delamanid (DLM), we evaluated its effectiveness and safety.
Methods
We reviewed the relevant scientific literature published up to January 20, 2022. The pooled success treatment rate with 95% confidence intervals (CI) was assessed using a random-effect model. We assessed studies for quality and bias, and considered P<0.05 to be statistically significant.
Results
After reviewing 626 records, we identified 25 studies that met the inclusion criteria, 22 observational and 3 experimental, with 1276 and 411 patients, respectively. In observational studies the overall pooled treatment success rate of DLM-containing regimens was 80.9% (95% CI 72.6-87.2) with no evidence of publication bias (Begg's test; P >0.05). The overall pooled treatment success rate in DLM and bedaquiline-containing regimens was 75.2% (95% CI 68.1-81.1) with no evidence of publication bias (Begg's test; P >0.05). In experimental studies the pooled treatment success rate of DLM-containing regimens was 72.5 (95% CI 44.2-89.8, P <0.001, I2: 95.1%) with no evidence of publication bias (Begg's test; P >0.05).
Conclusions
In MDR-TB patients receiving DLM, culture conversion and treatment success rates were high despite extensive resistance with limited adverse events.
Introduction
Tuberculosis (TB) continues to be a global emergency, with 10 million incident TB cases, 1.3 million HIV-negative and 0.214 million HIV-positive TB deaths and only 157,903 cases of rifampicin-resistant (RR)-TB cases detected and reported in 2020 (about one third of estimated cases) of which 150,359 were enrolled on treatment as reported by the World Health Organization (WHO) (World Health Organization, 2021).
The emergence and spread of multidrug-resistant (MDR) and extensively drug-resistant (XDR)-TB has further complicated the clinical and public health management of the disease (World Health Organization, 2021). This is especially alarming in this temporal phase when subsequent waves of COVID-19 pandemic are affecting the whole world, causing pressure on (TB) health services (Migliori et al., 2021; Motta et al., 2020; Tadolini et al., 2020; TB/COVID-19 Global Study Group, 2021; Visca et al., 2021)
MDR-TB is caused by strains of Mycobacterium tuberculosis resistant to at least the two core anti-TB drugs, isoniazid (INH) and rifampicin (RIF). XDR-TB was previously defined as TB caused by MDR Mycobacterium tuberculosis with further resistance to any fluoroquinolone (FLQs) and at least one of the three injectable second-line drugs (kanamycin, amikacin, and/or capreomycin) (Borisov et al., 2019; Viney et al., 2021; World Health Organization, 2009). The WHO definition of XDR was recently modified focusing on resistance to Group A MDR-TB drugs: now defined as MDR plus resistance to FLQs and either linezolid (LZD) or bedaquiline (BDQ), the drugs which proved to be effective and reasonably safe (Ahmad et al., 2018; Borisov et al., 2019; Viney et al., 2021; World Health Organization, 2020).
The treatment success of MDR-TB treatment is still sub-optimal, with a point estimate of 59% in the 2018 global cohort (World Health Organization, 2021), owing to difficulties to provide rapid and quality diagnosis, to design effective regimens (particularly for XDR-TB, as few drugs are still effective), and to manage frequent (and severe) adverse events. Last, but not least, the high cost of these drugs and, therefore, the difficulty for resource-limited countries to prescribe them, is still limiting the effective management of MDR- and XDR-TB at the global level (Migliori et al., 2020).
In this scenario the availability of new safe and effective drugs is of paramount importance. Among the few new anti-TB drugs, while much has been published on BDQ (Borisov et al., 2017; Hatami et al., 2022, World Health Organization, 2020), much less evidence is available on delamanid (DLM), which, for the relative paucity of available information, is presently classified among WHO Group C drugs (World Health Organization, 2020).
DLM is a promising nitro-dihydro-imidazooxazole derivative administered to treat MDR-TB. DLM inhibits the synthesis of methoxy- and keto-mycolic acid (which are components of Mycobacterium tuberculosis cell wall) through the F420 coenzyme mycobacteria system, while generating nitrous oxide.
Three systematic reviews investigated preliminary data on the combination of BDQ and DLM (D'Ambrosio et al., 2017; Migliori et al., 2017, Pontali et al., 2018) (one of them in children (D'Ambrosio et al., 2017)), and one systematic review described outcomes of children with MDR-TB (Harausz et al., 2018).
More recently, two systematic reviews evaluated mutations conferring resistance to BDQ and DLM (Kadura et al., 2020, Nieto Ramirez et al., 2020). A better understanding of genetic and phenotypic resistance is urgently needed to guide clinical management of DLM (Nguyen et al., 2020).
So far, no comprehensive systematic review on the efficacy/effectiveness and safety of DLM-containing regimens is available.
The aim of the present systematic review and meta-analysis is to evaluate effectiveness (bacteriological conversion and outcomes) and safety of DLM-containing regimens to manage MDR/RR-TB patients.
Methods
Search strategy
We searched Pubmed/MEDLINE, EMBASE, and Cochrane Library for studies reporting on the efficacy and effectiveness of individualized regimens containing DLM in patients with drug susceptibility testing (DST)-confirmed MDR/RR-TB, published up to January 20, 2022. The search terms were as follow: [(tuberculosis [Title/Abstract]) AND (delamanid [Title/Abstract]) OR (bedaquiline[Title/Abstract]) AND (efficacy[Title/Abstract] OR effectiveness[Title/Abstract]) OR safety[Title/Abstract]] (Appendix). Only studies written in English were selected. This study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (PRISMA) (Moher et al., 2009).
Study Selection
The records found through database searching were merged, and the duplicates were removed using EndNote X7 (Thomson Reuters, Toronto, ON, Canada). Two reviewers (MZ and EA) independently screened the records by title/abstract and full text to exclude those unrelated to the study objectives. Included studies met the following criteria: (1) patients diagnosed with MDR-TB according to the WHO criteria (World Health Organization, 2021); (2) patients treated with DLM-containing regimens; (3) treatment success (sputum and culture conversion), and (4) safety of the investigated drug/regimen. Conference abstracts, editorials, reviews, study protocols, molecular or experimental studies on animal models, and articles describing TB patients recruited without a confirmed bacteriological diagnosis, or administering DLM for other diseases like leishmaniosis were excluded.
Both the old and new definition of XDR-TB were used, as defined by the authors of the articles selected (Viney et al., 2021). Pre-XDR-TB was defined according to the new definition (TB caused by M. tuberculosis strains that fulfill the definition of MDR/RR-TB and that are also resistant to any FLQ) as this definition did not officially exist before (Viney et al., 2021).
Treatment outcomes were recorded in accordance with those used by the authors of the original studies selected, which were in agreement with the WHO definitions (treatment success, defined as the combination of patients who were cured and those who completed treatment; death, defined as death from any cause while on treatment; and treatment failure, defined as unsuccessful treatment, as determined by positive cultures at the end of the treatment regimen) (World Health Organization, 2011).
The regimens were considered DLM- and DLM/BDQ-containing based on what appeared in the methods of the original studies selected. The analysis was performed separately for experimental and observational studies and pooling the results together.
Optimized background regimens (OBR) were concomitantly prescribed with DLM. Basically, their characteristics were decided by the attending physician based on the DST results, WHO or national guidelines in force at the time of the diagnosis, and drugs' availability. In the studies selected the best regimen was tailored on the patient's characteristics and was not standardized. However, not all selected papers disclosed in detail the therapeutic approach.
Data extraction
Two reviewers (MZ and EA) designed a data extraction form and extracted data from all eligible studies, with differences being resolved by consensus. The following data were extracted: first author's name; year of publication; study duration; type of study; country or countries where the study was conducted; number of patients with MDR-TB; patient age; treatment protocols (treatment regimens and duration of treatment); HIV history; demographics (i.e., age, sex, nationality); type of adverse events; drug resistance status; culture conversion, and treatment outcomes.
Quality assessment
Two reviewers (MZ and EA) assessed the quality of the studies using two different assessment tools. A third reviewer (MJN) was involved in case of inconsistencies.
The Newcastle-Ottawa Scale (NOS) for observational studies and the Cochrane tool for experimental studies (Higgins et al., 2011; Wells GA et al., 2012) were adopted to assess the study quality. The NOS scale evaluates the risk of bias of observational studies with three domains: (1) selection of participants, (2) comparability, and (3) outcomes. A study can be awarded a maximum of one point for each numbered item within the selection and outcome categories, and a maximum of two points can be given for comparability. Scores of 0–3, 4–6, and 7–9 were assigned for the low, moderate, and high quality of studies, respectively.
The Cochrane tool is based on; use of random sequence generation; concealment of allocation to conditions; blinding of participant and personnel; blinding of outcome assessors; completeness of outcome data and other; selective reporting and other biases. Each study was rated as at low risk of bias when there was no concern regarding bias; as high risk of bias when there was concern regarding bias; or unclear risk of bias if the information was absent.
Data analysis
Statistical analyses were performed with Comprehensive Meta-Analysis software, version 2.0 (Biostat Inc., Englewood, NJ, USA). Point estimates and 95% CIs for the proportion of patients achieving treatment outcomes were calculated. The random-effects model was used because of the estimated heterogeneity of the true effect sizes. The between-study heterogeneity was assessed by Cochran's Q test and the I2 statistic. Publication bias was assessed statistically by using Begg's test (P value <0.05 was considered indicative of statistically significant publication bias) (Begg and Mazumdar, 1994).
Results
A total of 626 records were found in the initial search; after removing duplicate articles, the titles and abstracts of 351 references were screened (Figure 1). Of these, 42 articles were selected for a full-text review. After the full-text review, 25 articles met the inclusion criteria (Auchynka et al., 2021; Chang et al., 2018; Das et al., 2020; Das et al., 2021; Dooley et al., 2021; Ferlazzo et al., 2018; Ghosh et al., 2021; Gler et al., 2012; Häcker et al., 2020; Hafkin et al., 2017, Hafkin et al., 2019; Hewison et al., 2017; Kang et al., 2020; Kim et al., 2018; Kuksa et al., 2017; Kwon et al., 2021; Lee et al., 2020; Madzgharashvili et al., 2021; Mohr-Holland et al., 2020; Mok et al., 2019; Olayanju et al., 2020; Pirmahmadzoda et al., 2021; Sarin et al., 2019; Solodovnikova et al., 2021; von Groote-Bidlingmaier et al., 2019) of which 22 were observational (with 1,276 patients) (Auchynka et al., 2021; Chang et al., 2018; Das et al., 2020; Das et al., 2021; Ferlazzo et al., 2018; Ghosh et al., 2021; Häcker et al., 2020; Hafkin et al., 2017; Hafkin et al., 2019; Hewison et al., 2017; Kang et al., 2020; Kim et al., 2018; Kuksa et al., 2017; Kwon et al., 2021; Lee et al., 2020; Madzgharashvili et al., 2021; Mohr-Holland et al., 2020; Mok et al., 2019; Olayanju et al., 2020; Pirmahmadzoda et al., 2021; Sarin et al., 2019; Solodovnikova et al., 2021;) and three experimental studies (with 411 patients) (Dooley et al., 2021; Gler et al., 2012; von Groote-Bidlingmaier et al., 2019) (Tables 1 and 2). The study period ranged from 2012 to 2021. The mean age of the patients was 36.1 years.
Figure 1.
Flow chart of study selection for inclusion in the systematic review and meta-analysis.
Table 1.
Observational and experimental studies included in the meta-analysis (DLM-containing regimens group)
| Author | Year | Country | Type of study | Meanage | HIVN (%) | Pre-treated for TB | TB disease | No. of patients receiving DLM | Other drugsincludedin regimen | Length of treatment (months) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment successN (%) | FailureN (%) | DeathN (%) | |||||||||||
| Auchynka et al., 2021 | 2021 | Belarus | RC | NR | NR | NR | MDR/Pre-XDR/XDR | 105 | FLQs;LZD; CFZ;CYC; IMP;PZA; AMGs | 6 | 94(89.5%) | NR | NR |
| Chang et al., 2018 | 2018 | Hong Kong | PC | 48 | 0 | 6 | Pre-XDR/XDR | 11 | FLQ;LZD | 11.5 | 9(81.8%) | 1(9%) | 0 |
| Dooley et al., 2021 | 2021 | South Africa & Peru | RCT | 32 | 11(39) | 6 | RR | 24 | CFZ;FLQs | 6 | 22(91.6%) | 2(8.3%) | 0 |
| Gler et al., 2012 | 2012 | 9 countries | RCT | 36 | NR | 141 | MDR | 141 | FLQs;AMGs;PZA; CYC;ETM;ETH |
2 | 64(45.3%) | NR | NR |
| Häcker et al., 2020 | 2020 | Germany | RC | 30 | 1 | NR | MDR/Pre-XDR/XDR | 25 | LZD;FLQs;TRD;CFZ | 18.3 | 18(72%) | 1(4%) | 1(4%) |
| Hafkin et al., 2017 | 2017 | USA | PC | 32 | 12(15) | 64 | MDR | 8 | LZD;FLQs;CFZ;AMGs | 6 | 53(67.9%) | 11(14.1%) | 8(10.2%) |
| Pre-XDR | 26 | ||||||||||||
| XDR | 44 | ||||||||||||
| Hewison et al., 2017 | 2017 | 7 countries1 | RC | 29.5 | 8(15) | 49 | MDR | 10 | FLQs;CFZ;LZD | 6 | 39(76.4%) | 4(7.8%) | 7(13.7%) |
| Pre-XDR | 14 | ||||||||||||
| XDR | 27 | ||||||||||||
| Kuksa et al., 2017 | 2017 | Latvia | RC | 41.5 | 1(5.3) | 13 | MDR | 2 | TRD;LZD;PZA;FLQs | 7.8 | 16(84.2%) | 0 | 0 |
| Pre-XDR | 8 | ||||||||||||
| XDR | 9 | ||||||||||||
| Madzgharashvili et al., 2021 | 2021 | USA | RC | 15.1 | 0 | 0 | MDR/Pre-XDR/XDR | 8 | PZA;ETM;FLQs;CYC | 19.6 | 7(87.5%) | 0 | 0 |
| Mohr-Holland et al., 2020 | 2020 | South Africa | RC | NR | 78(78.8) | 58 | RR | 64 | PZA;FLQs;TRD; ETM;hINH;LZD |
6.3 | 57(57.5%) | 6(6%) | 14(14.1%) |
| Pre-XDR | 35 | ||||||||||||
| Mok et al., 2019 | 2019 | South Korea | RC | 47 | 0 | 27 | MDR | 14 | FLQs;AMGs;LZD;CFZ | 24 | 40(81.6%) | 3(6.1%) | 3(6.1%) |
| Pre-XDR | 27 | ||||||||||||
| XDR | 8 | ||||||||||||
| von Groote-Bidlingmaier et al., 2019 | 2019 | 7 countries2 | RCT | 32 | 12(5.3) | NR | MDR | 177 | Optimized background regimen (NR) | 6 | 173(76.5%) | NR | 18(7.9%) |
| Pre-XDR | 39 | ||||||||||||
| XDR | 10 | ||||||||||||
| Solodovnikova et al., 2021 | 2021 | Belarus | RC | NR | NR | NR | RR/MDR/XDR | 19 | LZD;CFZ;CYC;FLQs | 6 | 19(100%) | 0 | 0 |
| Kim et al., 2018 | 2018 | South Korea | RC | 48 | NR | 10 | MDR/Pre-XDR/XDR | 8 | WHO-recommended regimen | 5.6 | 8(100%) | NR | 0 |
| Kang et al., 2020 | 2020 | South Korea | RC | 47.8 | 1(0.9) | 55 | MDR | 50 | AMG;FLQs;LZD;CYC | 6 | 95(87.9%) | 1(0.9%) | 8(7.4%) |
| Pre-XDR | 49 | ||||||||||||
| XDR | 9 | ||||||||||||
| Das et al., 2020 | 2020 | India | RC | 15.5 | 0 | NR | Pre-XDR /XDR | 11 | LZD;CFZ | 22 | 10(90.9%) | NR | NR |
PC: prospective cohort; RC: retrospective cohort; RCT: randomized clinical trial; BDQ: bedaquiline; DLM: delamanid; FLQs: fluoroquinolones; LZD: linezolid; CFZ: clofazimine; CYC: cycloserine; AMGs: aminoglycosides; MEM/CLV: meropenem-clavulanate; TRD: terizidone; IMP: imipenem; ETH: ethionamide; hINH: high-dose isoniazid; ETM: ethambutol; PZA: pyrazinamide; PMD: pretomanid; MDR: multidrug-resistant; XDR: extensively drug-resistant; RR: rifampin-resistant; and NR: not reported. 1: the Philippines, Peru, Latvia, Estonia, China, Japan, Korea, Egypt, the United States; 2: Armenia, Belarus, Georgia, India, Russia, South Africa, Swaziland; 3: Estonia, Latvia, Lithuania, Moldova, Peru, the Philippines, and South Africa
Table 2.
Observational and experimental studies included in the meta-analysis (DLM and BDQ-containing regimens group)
| Author | Year | Country | Type of study | Meanage | HIVN (%) | Pre-treated for TB | TB disease | No. of patients receiving DLM+ BDQ | Other drugsincludedin regimen | Length of treatment (months) | Outcomes |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment success | Failure | Death | |||||||||||
| Auchynka et al., 2021 | 2021 | Belarus | RC | NR | NR | NR | MDR/Pre-XDR/XDR | 20 | FLQs;LZD; CFZ;CYC; IMP;PZA; AMGs | 6 | 16(80%) | NR | NR |
| Das et al., 2021 | 2021 | India | RC | 25 | 1(1.4) | 70 | Pre-XDR | 28 | LZD;CFZ | 19 | 49(70%) | 5(7.1%) | 13(18.5%) |
| XDR | 42 | ||||||||||||
| Dooley et al., 2021 | 2021 | South Africa & Peru | RCT | 34 | 10(36) | 8 | RR | 20 | CFZ;FLQs | 6 | 19(95%) | 1(5%) | 0 |
| Hafkin et al., 2019 | 2019 | USA | RC | 37 | 46(54.8) | 74 | MDR | 4 | LZD;PZA; CFZ;FLQs | 6 | 51(60.7%) | 4(4.7%) | 10(11.9%) |
| Pre-XDR | 18 | ||||||||||||
| XDR | 62 | ||||||||||||
| Madzgharashvili et al., 2021 | 2021 | USA | RC | 15.5 | 0 | 0 | MDR/Pre-XDR/XDR | 2 | LZD;PZA; CYC;CFZ | 22 | 2(100%) | 0 | 0 |
| Kwon et al., 2021 | 2021 | South Korea | RC | 49 | 0 | 19 | Pre-XDR/XDR | 28 | LZD;CFZ; MEM/CLV;CYC | 6 | 23(82.1%) | 2(7.1%) | 1(3.5%) |
| Das et al., 2020 | 2020 | India | RC | 15.5 | 0 | NR | Pre-XDR /XDR | 12 | LZD;CFZ | 22 | 11(91.6%) | NR | NR |
| Lee et al., 2020 | 2020 | South Korea | RC | 49.8 | 1 (1.4) | 49 | MDR | 13 | FLQs; LZD;CFZ;CYC | 5.5 | 42(56.7%) | 1(1.3%) | 4(5.4%) |
| Pre-XDR | 41 | ||||||||||||
| XDR | 20 | ||||||||||||
| Olayanju et al., 2020 | 2020 | South Africa | PC | 34 | 22 (55) | 29 | MDR | 6 | AMGs; FLQs;LZD;CFZ;TRD | 6 | 27(67.5%) | NR | NR |
| Pre-XDR | 15 | ||||||||||||
| XDR | 19 | ||||||||||||
| Kang et al., 2020 | 2020 | South Korea | RC | 47.7 | 1 (1.5) | 47 | MDR | 8 | AMG;FLQs;LZD;CYC | 6 | 58(86.5%) | 3(4.4%) | 3(4.4%) |
| Pre-XDR | 37 | ||||||||||||
| XDR | 22 | ||||||||||||
| Sarin et al., 2019 | 2019 | India | PC | 24 | 0 | NR | MDR/Pre-XDR/XDR | 42 | FLQs;LZD;CFZ;IMP | 6 | 25(59.5%) | NR | 10(23.8%) |
| Kim et al., 2018 | 2018 | South Korea | RC | 50 | NR | 11 | MDR/Pre-XDR/XDR | 11 | WHO-recommended regimen | 11/3 | 7(63.6%) | NR | 0 |
| Ferlazzo et al., 2018 | 2018 | Armenia, India, South Africa | RC | 32.5 | 11 (39) | 4 | MDR | 2 | FLQs;LZD;CFZ;IMP | 6 | 22(78.5%) | NR | 1(3.5%) |
| Pre-XDR | 12 | ||||||||||||
| XDR | 14 | ||||||||||||
| Pirmahmadzoda et al., 2021 | 2021 | Tajikistan | RC | NR | NR | NR | XDR | 11 | WHO-recommended regimen | 20-36 | 11(100%) | 0 | 0 |
| Ghosh et al., 2021 | 2021 | Germany | RC | 35 | 66 (33) | 169 | MDR/Pre-XDR/XDR | 147 | WHO-recommended regimen | 6 | 116(78.9%) | NR | NR |
PC: Prospective cohort; RC: retrospective cohort; RCT: randomized clinical trial; BDQ: bedaquiline; DLM: delamanid; FLQs: fluoroquinolones; LZD: linezolid; CFZ: clofazimine; CYC: cycloserine; AMGs: aminoglycosides; MEM/CLV: meropenem-clavulanate; TRD: terizidone; IMP: imipenem; ETH: ethionamide; hINH: high-dose isoniazid; ETM: ethambutol; PZA: pyrazinamide; PMD: pretomanid; MDR: multidrug-resistant; XDR: extensively drug-resistant; RR: rifampin-resistant; NR: not reported.
Overall, 591 patients were included in DLM-containing regimen group and 685 patients in the DLM/BDQ-containing regimen group.
Quality of included studies
Based on the Newcastle-Ottawa Scale, which was used to evaluate the quality of the observational studies, the mean (standard deviation [SD]) NOS score was 8.0 (0.6), which is suggestive for a high methodological quality and a low risk of bias of the included studies (Table 3).
Table 3.
Quality assessment of the observational studies included in the meta-analysis (The NOS tool)
| Author | Selection |
Comparability |
Outcome |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed cohort | Selection of non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Adjust for the most important risk factors | Adjust for other risk factors | Assessment of outcome | Follow-up length | Lossto follow-uprate | Total quality score | |
| Auchynka et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Chang et al., 2018 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Das et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Häcker et al., 2020 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Hafkin et al., 2017 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Hafkin et al., 2019 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Hewison et al., 2017 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Kuksa et al., 2017 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Madzgharashvili et al., 2021 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 9 |
| Mohr-Holland et al., 2020 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Mok et al., 2019 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Solodovnikova et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Kwon et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Das et al., 2020 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Lee et al., 2020 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Olayanju et al., 2020 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Kang et al., 2020 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Sarin et al., 2019 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Kim et al., 2018 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Ferlazzo et al., 2018 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Pirmahmadzoda et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Ghosh et al., 2021 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
NOS: The Newcastle-Ottawa Scale
Only one experimental study (Dooley et al., 2021) has a high risk of bias in the cases of allocation concealment, blinding of participants, and blinding of outcome (Table 4).
Table 4.
Quality assessment of the experimental studies included in the meta-analysis (the Cochrane tool)
| Author | Random sequencegeneration | Allocation concealment | Blinding of participants andpersonnel | Blinding of outcomeassessment | Incomplete outcomedata | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Gler et al., 2012 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Dooley et al., 2021 | Low risk | High risk | High risk | High risk | Low risk | Low risk | Low risk |
| von Groote-Bidlingmaier et al., 2019 | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
Outcomes in observational studies
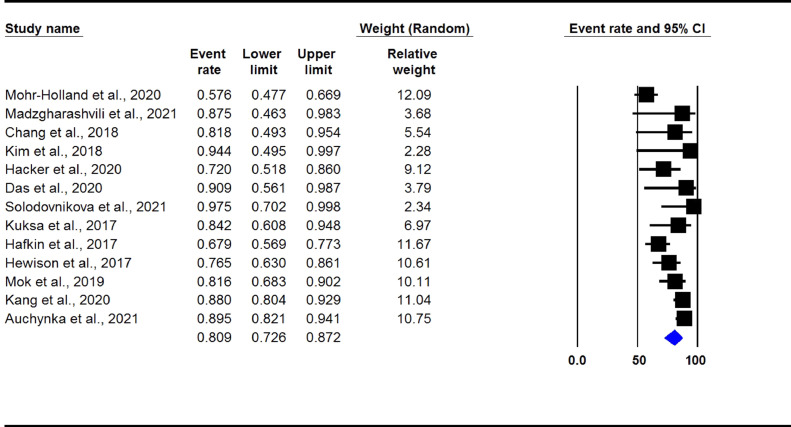
The overall pooled treatment success rate in DLM-containing regimens group was found to be 80.9% (95% CI 72.6-87.2, I2: 73%) (Figure 2). There was no evidence of publication bias (Begg's test P >0.05).
Figure 2.
Treatment success rate in observational studies. (DLM-containing regimens group)
Legend: DLM: delamanid.
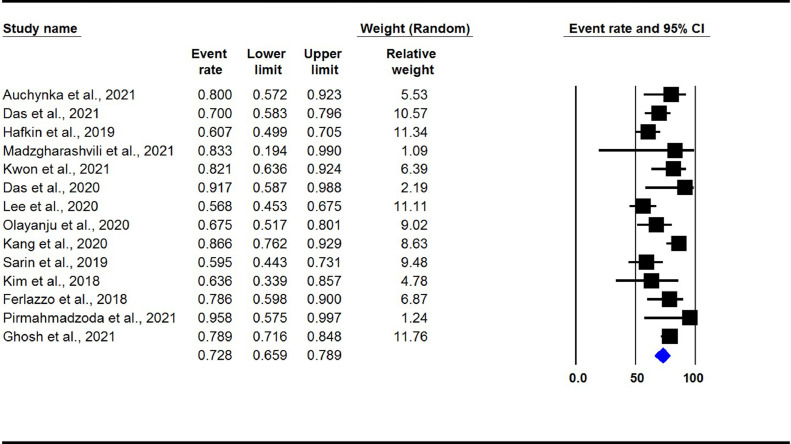
The overall pooled treatment success rate in DLM- and BDQ-containing regimens group was found to be 72.8% (95% CI 65.9-78.9, I2: 62%) (Figure 3). There was no evidence of publication bias (Begg's test P >0.05).
Figure 3.
Treatment success rate in observational studies. (DLM and BDQ-containing regimens group)
DLM: delamanid; BDQ: bedaquiline.
Outcomes in experimental studies
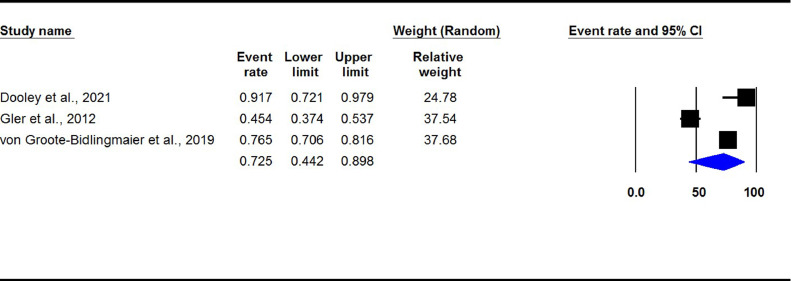
The pooled treatment success rate in in DLM-containing regimens group was 72.5% (95% CI 44.2-89.8, I2: 95%) (Figure 4). The result of the Begg's test showed no evidence of publication bias (P >0.05).
Figure 4.
Treatment success rate in experimental studies. (DLM-containing regimens group)
DLM: delamanid.
Time to sputum culture conversion
The median time to sputum culture conversion ranged from 1.1 to 1.7 months in the DLM-containing regimens group (Auchynka et al., 2021; Chang et al. 2018; Das et al., 2020; Mok et al., 2019; Solodovnikova et al., 2021; von Groote-Bidlingmaier et al., 2019). In an additional study by Kim et al., 2018, reporting the information separately, the median time to culture conversion for DLM-containing regimens was 4.1 months and for DLM plus BDQ-containing regimens it was 10.3 months.
The pooled death rate and treatment failure in DLM-containing regimens group was found to be 7.8% (95% CI 5.5-11.0, I2: 13.0%) and 9.2% (95% CI 7.2-11.6, I2: 0.0%), respectively.
Adverse events
In the DLM-containing regimens group only 4/165 (2.4%) patients had QTcF prolongation (Fridericia correction, as reported by the original studies selected) definitely attributed to DLM. Also 2/127 (1.5%) patients with gastrointestinal symptoms and 1/27 (3.7%) patient with dermatologic symptoms were reported in this group (Table 5). Most of the adverse events potentially attributed to DLM and BDQ-containing regimens group (Table 6) were QTcF prolongation (12.8%, 55/427), psychiatric disorders (7.1%, 2/28), gastrointestinal symptoms (4.5%, 12/267), peripheral neuropathy (3.5%, 1/28), renal failure/ increased creatinine (2%, 2/102), and hepatic disorders/elevated liver enzymes (1.4%, 1/70).
Table 5.
Adverse effects in included studies (DLM-containing regimens group)
| Author | Number of patients | QTcF prolongation | Hepatic disorder/ Elevated liver enzyme | Renal failure/ Increased creatinine | Optic neuropathy/ Blurred vision | Ototoxicity/Hearing loss | Hematological disorders (Anemia, thrombocytopenia, eosinophilia) | Gastrointestinal symptoms (Diarrhea, vomiting, nausea, abdominal pain) | Peripheral neuropathy | Electrolyte disturbance | Arthralgia | Psychiatric disorder | Dermatologic symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auchynka et al., 2021 | 105 | - | - | - | - | - | - | - | - | - | - | - | - |
| Chang et al., 2018 | 11 | 0 | - | - | - | - | - | - | - | - | - | - | - |
| Dooley et al., 2021 | 24 | - | - | - | - | - | - | - | - | - | - | - | - |
| Gler et al., 2012 | 141 | - | - | - | - | - | - | - | - | - | - | - | - |
| Häcker et al., 2020 | 25 | - | - | - | - | - | - | - | - | - | - | - | - |
| Hafkin et al., 2017 | 78 | - | - | - | - | - | - | - | - | - | - | - | - |
| Hewison et al., 2017 | 51 | - | - | - | - | - | - | - | - | - | - | - | - |
| Kuksa et al., 2017 | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Madzgharashvili et al., 2021 | 8 | 0 | - | - | - | - | - | - | - | - | - | - | - |
| Mohr-Holland et al., 2020 | 99 | - | - | - | - | - | - | - | - | - | - | - | - |
| Mok et al., 2019 | 49 | - | - | - | - | - | - | - | - | - | - | - | - |
| von Groote-Bidlingmaier et al., 2019 | 226 | - | - | - | - | - | - | - | - | - | - | - | - |
| Solodovnikova et al., 2021 | 19 | - | - | - | - | - | - | - | - | - | - | - | - |
| Kim et al., 2018 | 8 | 1 | - | - | - | - | - | - | - | - | - | - | 1 |
| Kang et al., 2020 | 108 | 2 | - | - | - | - | - | 2 | - | - | - | - | - |
| Das et al., 2020 | 11 | 1 | - | - | - | - | - | - | - | - | - | - | - |
QTcF: corrected QT with the Fredericia formula; DLM: delamanid
Table 6.
Adverse effects in included studies (DLM and BDQ-containing regimens group)
| Author | Number of patients | QTcF prolongation | Hepatic disorder/ Elevated liver enzyme | Renal failure/ Increased creatinine | Optic neuropathy/ Blurred vision | Ototoxicity/Hearing loss | Hematological disorders (Anemia, thrombocytopenia, eosinophilia) | Gastrointestinal symptoms (Diarrhoea, vomiting, nausea, abdominal pain) | Peripheral neuropathy | Electrolyte disturbance | Arthralgia | Psychiatric disorder | Dermatologic symptoms |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Auchynka et al., 2021 | 20 | - | - | - | - | - | - | - | - | - | - | - | - |
| Das et al., 2021 | 70 | 5 | 1 | - | - | - | - | 3 | - | - | - | - | - |
| Hafkin et al., 2019 | 84 | - | - | - | - | - | - | - | - | - | - | - | - |
| Madzgharashvili et al., 2021 | 2 | 0 | - | - | - | - | - | - | - | - | - | - | - |
| Dooley et al., 2021 | 20 | - | - | - | - | - | - | - | - | - | - | - | - |
| Kwon et al., 2021 | 28 | 17 | - | - | - | - | - | 1 | - | - | - | - | - |
| Das et al., 2020 | 12 | 1 | - | - | - | - | - | - | - | - | - | - | - |
| Lee et al., 2020 | 74 | 23 | - | 1 | - | - | - | 4 | - | - | - | - | - |
| Olayanju et al., 2020 | 40 | - | - | - | - | - | - | - | - | - | - | - | - |
| Kim et al., 2018 | 11 | 2 | - | - | - | - | - | - | - | - | - | - | - |
| Ferlazzo et al., 2018 | 28 | 4 | - | 1 | - | - | - | 1 | 1 | - | - | 2 | - |
| Kang et al., 2020 | 67 | - | - | - | - | - | - | 3 | - | - | - | - | - |
| Sarin et al., 2019 | 42 | - | - | - | - | - | - | - | - | - | - | - | - |
| Pirmahmadzoda et al., 2021 | 11 | - | - | - | - | - | - | - | - | - | - | - | - |
| Ghosh et al., 2021 | 147 | 3 | - | - | - | - | - | - | - | - | - | - | - |
QTcF: corrected QT with the Fredericia formula; DLM: delamanid; BDQ: bedaquiline
Subgroup analysis
The treatment success rate in patients aged ≤40 and >40 in DLM containing regimens was 74.2% and 85.6%, respectively (Table 7), whereas in males and females was found to be 80.7% and 83.6%, respectively. The treatment success rate in children and adults was found to be 89.4% and 78.4%, respectively.
Table 7.
Pooled treatment success rate among subgroups of studies in DLM group
| Subgroups | No. of study | No. of patients | Treatment success %(95 % CI) | HeterogeneityI2 (%) | Begg's testP-value |
|---|---|---|---|---|---|
| Type of study: | |||||
| Observational studiesExperimental studies | 13 studies3 studies | 591391 | 80.9 (72.6-87.2)72.5 (44.2-89.8) | 7395 | 0.160.90 |
| Age: | |||||
| ≤40>40 | 8 studies5 studies | 564195 | 74.2 (61.3-84)85.6 (79.9-89.9) | 85.40.0 | 0.711.00 |
| Sex: | |||||
| MaleFemale | 3 studies3 studies | 2315 | 80.7 (59.7-92.1)83.6 (56.5-95.2) | 0.00.0 | 1.001.00 |
| Children/adult: | |||||
| Children/adolescentAdult | 2 studies14 studies | 19963 | 89.4 (66.0-97.0)78.4 (69.3-85.4) | 0.086.0 | NA*0.45 |
There must be at least three studies to run publication bias.
DLM: delamanid; CI: confidence interval
Discussion
Our study was aimed at evaluating efficacy/effectiveness and safety of DLM-containing regimens to manage MDR/RR-TB. The results of our study show that culture conversion and treatment success rates were high despite extensive drug resistance patterns. Overall, DLM-containing regimens achieved a treatment success exceeding 80%, being lower when both DLM and BDQ were prescribed. Unfortunately, the details provided in the different studies on the resistance profile and, specifically, on BDQ resistance did not allow to perform additional analyses to determine why outcomes in the latter group was worse.
In observational studies on BDQ the results of 3,536 patients were analyzed (Hatami et al., 2022), with a success rate of 74.7%, while 591 patients undergoing treatment with DLM achieved a success rate of 80.9%. In experimental studies on BDQ, 441 patients achieved a success rate of 86.1%, whereas the 391 patients treated with DLM had a success rate of 72.5%.
The success rate on the 292 patients undergoing combined treatment with BDQ and DLM was 73.9%, with patients likely to harbor strains of Mycobacterium tuberculosis with a more challenging drug resistance pattern (Pontali et al., 2018).
Few adverse events were reported overall, especially in the studies containing DLM without BDQ. Although under-reporting of adverse events is likely, they seemed to be rare.
A parallel recent systematic review and meta-analysis conducted on BDQ (Hatami et al., 2022) allows to compare effectiveness and safety with those found for DLM.
Overall, more studies were available on BDQ (1,946 identified and 29 selected) (Hatami et al., 2022) than for DLM (351 and 25, respectively). DLM-containing regimens achieved higher success rate than BDQ-containing ones in observational studies and lower in experimental studies.
In terms of adverse events, in DLM-containing regimen a lower proportion of QTcF prolongation was observed (2.4%) than in BDQ-containing regimens (10.4%), as well as a lower frequency of gastro-intestinal adverse effects (1.8% vs. 15.3%). In BDQ-containing regimens peripheral neuropathy (13.8%) and hematological disorders (13.6%) were also noted (Pontali et al., 2018), but there were no such reports for DLM-containing regimens.
More adverse events were identified among the 225 patients undergoing combined treatment with BDQ and DLM: QTcF prolongation 12.8%, psychiatric disorders 7.1%, gastrointestinal effects 4.5%, peripheral neuropathy 3.5%, renal failure/increased creatinine 2%, and hepatic disorders/elevated liver enzyme 1.4%. The authors of the different studies reporting combined BDQ and DLM regimens were unable to assign the adverse events to a specific drug.
No evidence of publications bias was identified in our study as well as in the BDQ study (Pontali et al., 2018).
Several studies not reporting both effectiveness and safety of DLM have been published, supporting the results of our systematic review and meta-analysis. An early bactericidal activity (EBA) trial demonstrated that DLM in monotherapy was able to lower Colony Forming Units from baseline over 14 days of daily treatment (Diacon et al., 2011). DLM added to an optimized background regimen (OBR) in adult MDR-TB patients increased sputum culture conversion rates after 2 months (phase IIb, randomized, placebo-controlled, multinational clinical trial) (Gler et al., 2012). Other phase IIb trials demonstrated that DLM-containing regimens improved treatment outcomes and reduced mortality (Skripconoka et al., 2013; Wells et al., 2015). Conversely, the results of another trial included in our analysis, (von Groote-Bidlingmaier et al., 2019) in which the reduction in median time to sputum culture conversion over 6 months was not significant in the DLM arm, although the strong OBR with placebo was highly effective; possibly the study was not powered sufficiently to see a discernible difference with a very effective OBR and placebo arm.
A large prospective study by Global Tuberculosis Network (GTN) (Koirala et al., 2021), not included in this meta-analysis (no separate outcomes for patients treated with DLM only), reported interesting results on regimens including BDQ and/or DLM. It included 883 consecutive patients treated with BDQ and/or DLM from 52 centres in 29 countries. Of the 477 patients treated with BDQ and/or DLM and completing treatment, 344 (72.1%) achieved treatment success. Of 383 patients treated with BDQ but not DLM, 284 (74.2%) achieved treatment success, while 25 (6.5%) died, 11 (2.9%) failed and 63 (16.5%) were lost to follow-up. In this cohort the drug-resistance pattern of the patients was severe (>30% with XDR-TB; median number of resistant drugs and 6 (4−8) among patients with a final outcome). The small number of paediatric patients involved prevented the authors to conduct specific analyses.
In terms of safety, the proportion of serious adverse events was low in the first trials (Diacon et al., 2011; Skripconoka et al., 2013), and the few patients with prolonged QTcF interval had no clinical cardiac events (Skripconoka et al., 2013).
Evidence in children is modest. In a study that enrolled 16 children treated with DLM on compassionate basis, no adverse event was reported in fifteen children, while one child treated with a combination of DLM, capreomycin, ethionamide, cycloserine, clofazimine, imipenem, amoxicillin/clavulanate, and pyrazinamide experienced vomiting, renal impairment, electrolyte disturbances, and prolonged QTcF (Tadolini et al., 2016). In a recent study (Sasaki et al., 2021) the cardiac safety of DLM administered according to the recommended dosing was further emphasized. Other case series confirmed the safety of DLM in the pediatric age group (Esposito et al., 2014; Hewison et al., 2017; Kuksa et al., 2017; Mohr et al., 2018; Shah et al., 2020).
Our systematic review and meta-analysis updates the available evidence on DLM efficacy/effectiveness and safety, showing the drug has a promising profile. DLM is presently included among WHO Group C drugs, mainly because of previous lack of data and its non-inclusion in the large individual data meta-analysis which informed the new WHO classification of the drugs to manage MDR-TB (Ahmad et al., 2018). Similarly, the priority of DLM is rather low in the ATS/CDC/ERS/IDSA guidelines (Nahid et al., 2019).
Our study has some limitations as it does not evaluate adherence to treatment regimens containing DLM (an important outcome determinant) and different patient characteristics exist across studies.
Although a population pharmacokinetic analysis of available trial data suggests that DLM exposure is not affected by age, mild or moderate renal impairment, HIV, or CYP3A4 inhibitors or inducers (Wang et al., 2020) subgroup analyses are needed to better understand the role played by some confounders (e.g., levels of drug resistance, setting and adherence)
Furthermore, another confounding factor could be the OBR prescribed to the recruited patients. Its characteristics are based on patient's needs (e.g., DST results, available drugs) and could have varied following updates of international (e.g., WHO and international scientific societies) and national guidelines. Missing information on the details on which the OBRs were designed can hinder the between-study comparison, increasing the risk of over- or under-estimation of the efficacy/effectiveness and safety profiles of the regimens.
In conclusion, the results of this study and the direct comparison with a recent study focused on BDQ (Hatami et al, 2022) suggest that DLM-containing regimens are effective and safe to treat MDR-TB patients.
Transparency declaration
This article is part of a supplement entitled Commemorating World Tuberculosis Day March 24th, 2022: “Invest to End TB. Save Lives” published with support from an unrestricted educational grant from QIAGEN Sciences Inc.
Acknowledgments
Conflict of interest
The authors declare no conflicts of interest.
Funding source
No funding source was used in the development of this manuscript
Ethical approval
Not applicable.
Acknowledgments
The article is part of the scientific activities of the Global Tuberculosis Network (GTN and of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, Tradate, ITA-80, 2020-2024- GBM/RC/LDA).
Sir Ali Zumla acknowledges support from Pan-African Network on Emerging and Re-Emerging Infections (PANDORA-ID-NET – https://www.pandora-id.net/), CANTAM-3 and EACCR-3 funded by the European and Developing Countries Clinical Trials Partnership the EU Horizon 2020 Framework Programme. Sir Ali Zumla is a Mahathir Science Award and EU-EDCTP Pascoal Mocumbi Prize Laureate.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.02.043.
Contributor Information
Mohammad Javad Nasiri, Email: mj.nasiri@hotmail.com.
Moein Zangiabadian, Email: zangiabadian1998@gmail.com.
Erfan Arabpour, Email: erfanarabpour1999@gmail.com.
Sirus Amini, Email: sirusamini@gmail.com.
Farima Khalili, Email: farimakhalili@yahoo.com.
Rosella Centis, Email: rosella.centis@icsmaugeri.it.
Lia D'Ambrosio, Email: liadambrosio59@gmail.com.
Justin T. Denholm, Email: justin.denholm@mh.org.au.
H. Simon Schaaf, Email: hss@sun.ac.za.
Martin van den Boom, Email: vandenboomm@who.int.
Xhevat Kurhasani, Email: xhevat.kurhasani@gmail.com.
Margareth Pretti Dalcolmo, Email: margarethdalcolmo@gmail.com.
Seif Al-Abri, Email: salabri@gmail.com.
Jeremiah Chakaya, Email: chakaya.jm@gmail.com.
Jan-Willem Alffenaar, Email: johannes.alffenaar@sydney.edu.au.
Onno Akkerman, Email: o.w.akkerman@umcg.nl.
Denise Rossato Silva, Email: denise.rossato@terra.com.br.
Marcela Muňoz-Torrico, Email: dra_munoz@hotmail.com.
Barbara Seaworth, Email: Barbara.Seaworth@dshs.texas.gov.
Emanuele Pontali, Email: pontals@yahoo.com.
Laura Saderi, Email: lsaderi@uniss.it.
Simon Tiberi, Email: s.tiberi@qmul.ac.uk.
Alimuddin Zumla, Email: a.zumla@ucl.ac.uk.
Giovanni Battista Migliori, Email: giovannibattista.migliori@icsmaugeri.it.
Giovanni Sotgiu, Email: gsotgiu@uniss.it.
Appendix. Supplementary materials
References
- Ahmad N, Ahuja SD, Akkerman OW, Alffenaar JC, Anderson LF, Baghaei P, et al. Treatment correlates of successful outcomes in pulmonary multidrug-resistant tuberculosis: an individual patient data meta-analysis. Lancet. 2018;392(10150):821–834. doi: 10.1016/S0140-6736(18)31644-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchynka V, Kumar AMV, Hurevich H, Sereda Y, Solodovnikova V, Katovich D, et al. Effectiveness and cardiovascular safety of delamanid-containing regimens in adults with multidrug-resistant or extensively drug-resistant tuberculosis: A nationwide cohort study from Belarus, 2016-18. Monaldi Arch Chest Dis. 2021;91(1) doi: 10.4081/monaldi.2021.1647. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994:1088–1101. [PubMed] [Google Scholar]
- Borisov SE, Dheda K, Enwerem M, Romero Leyet R, D'Ambrosio L, Centis R, et al. Effectiveness and safety of bedaquiline-containing regimens in the treatment of MDR- and XDR-TB: a multicentre study. Eur Respir J. 2017;49(5) doi: 10.1183/13993003.00387-2017. [DOI] [PubMed] [Google Scholar]
- Borisov S, Danila E, Maryandyshev A, Dalcolmo M, Miliauskas S, Kuksa L, et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J. 2019;54(6) doi: 10.1183/13993003.01522-2019. [DOI] [PubMed] [Google Scholar]
- Chang KC, Leung EC, Law WS, Leung WM, Tai LB, Lee SN, et al. Early experience with delamanid-containing regimens in the treatment of complicated multidrug-resistant tuberculosis in Hong Kong. Eur Respir J. 2018 Jun 14;51(6) doi: 10.1183/13993003.00159-2018. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio L, Centis R, Tiberi S, Tadolini M, Dalcolmo M, Rendon A, et al. Delamanid and bedaquiline to treat multidrug-resistant and extensively drug-resistant tuberculosis in children: a systematic review. J Thorac Dis. 2017;9(7):2093–2101. doi: 10.21037/jtd.2017.06.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Mamnoon F, Mansoor H, Meneguim AC, Singh P, Shah I, et al. New TB drugs for the treatment of children and adolescents with rifampicin-resistant TB in Mumbai, India. Int J Tuberc Lung Dis. 2020;24(12):1265–1271. doi: 10.5588/ijtld.20.0165. [DOI] [PubMed] [Google Scholar]
- Das M, Dalal A, Laxmeshwar C, Ravi S, Mamnoon F, Meneguim AC, et al. One Step Forward: Successful End-of-Treatment Outcomes of Patients With Drug-Resistant Tuberculosis Who Received Concomitant Bedaquiline and Delamanid in Mumbai, India. Clin Infect Dis. 2021;73(9):e3496–e3504. doi: 10.1093/cid/ciaa1577. [DOI] [PubMed] [Google Scholar]
- Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, et al. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis. 2011;15(7):949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- Dooley KE, Rosenkranz SL, Conradie F, Moran L, Hafner R, von Groote-Bidlingmaier F, et al. QT effects of bedaquiline, delamanid, or both in patients with rifampicin-resistant tuberculosis: a phase 2, open-label, randomised, controlled trial. Lancet Infect Dis. 2021;21(7):975–983. doi: 10.1016/S1473-3099(20)30770-2. Jul. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito S, D'Ambrosio L, Tadolini M, Schaaf HS, Caminero Luna J, Marais B, et al. ERS/WHO Tuberculosis Consilium assistance with extensively drug-resistant tuberculosis management in a child: case study of compassionate delamanid use. Eur Respir J. 2014;44(3):811–815. doi: 10.1183/09031936.00060414. [DOI] [PubMed] [Google Scholar]
- Ferlazzo G, Mohr E, Laxmeshwar C, Hewison C, Hughes J, Jonckheere S, et al. Early safety and efficacy of the combination of bedaquiline and delamanid for the treatment of patients with drug-resistant tuberculosis in Armenia, India, and South Africa: a retrospective cohort study. Lancet Infect Dis. 2018;18(5):536–544. doi: 10.1016/S1473-3099(18)30100-2. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Breitscheidel L, Lazarevic N, Martin A, Hafkin J, Hittel N. Compassionate use of delamanid in adults and children for drug-resistant tuberculosis: 5-year update. Eur Respir J. 2021;57(5) doi: 10.1183/13993003.02483-2020. May 20. [DOI] [PubMed] [Google Scholar]
- Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, Gao M, Awad M, Park S.K, Shim T.S, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N. Engl. J. Med. 2012;366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- Häcker B, Schönfeld N, Krieger D, Otto-Knapp R, Hittel N, Pflugmacher P, et al. Long-term safety and tolerability of delamanid-containing regimens in MDR- and XDR-TB patients in a specialised tuberculosis treatment center in Berlin, Germany. Eur Respir J. 2020 doi: 10.1183/13993003.00009-2020. Jul 6. [DOI] [PubMed] [Google Scholar]
- Hafkin J, Hittel N, Martin A, Gupta R. Early outcomes in MDR-TB and XDR-TB patients treated with delamanid under compassionate use. Eur Respir J. 2017;50(1) doi: 10.1183/13993003.00311-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafkin J, Hittel N, Martin A, Gupta R. Compassionate use of delamanid in combination with bedaquiline for the treatment of multidrug-resistant tuberculosis. Eur Respir J. 2019;53(1) doi: 10.1183/13993003.01154-2018. [DOI] [PubMed] [Google Scholar]
- Harausz EP, Garcia-Prats AJ, Law S, Schaaf HS, Kredo T, Seddon JA, et al. Collaborative Group for Meta-Analysis of Paediatric Individual Patient Data in MDR-TB. Treatment and outcomes in children with multidrug-resistant tuberculosis: A systematic review and individual patient data meta-analysis. PLoS Med. 2018;15(7) doi: 10.1371/journal.pmed.1002591. Jul 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatami H, Sotgiu G, Bostanghadiri N, Dolat Abadi SS, Mesgarpour B, Goudarzi H, et al. Bedaquiline-containing regimens and multidrug-resistant tuberculosis: a systematic review and meta-analysis. J Bras Pneumol. 2022;48(2) doi: 10.36416/1806-3756/e20210384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewison C, Ferlazzo G, Avaliani Z, Hayrapetyan A, Jonckheere S, Khaidarkhanova Z, et al. Six-Month Response to Delamanid Treatment in MDR TB Patients. Emerg Infect Dis. 2017;23(10):1746–1748. doi: 10.3201/eid2310.170468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadura S, King N, Nakhoul M, Zhu H, Theron G, Köser CU, Farhat M., et al. Systematic review of mutations associated with resistance to the new and repurposed Mycobacterium tuberculosis drugs bedaquiline, clofazimine, linezolid, delamanid and pretomanid. J Antimicrob Chemother. 2020;75(8):2031–2043. doi: 10.1093/jac/dkaa136. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H, Jo KW, Jeon D, Yim JJ, Shim TS. Interim treatment outcomes in multidrug-resistant tuberculosis using bedaquiline and/or delamanid in South Korea. Respir Med. 2020;167 doi: 10.1016/j.rmed.2020.105956. [DOI] [PubMed] [Google Scholar]
- Kim CT, Kim TO, Shin HJ, Ko YC, Hun Choe Y, Kim HR, et al. Bedaquiline and delamanid for the treatment of multidrug-resistant tuberculosis: a multicentre cohort study in Korea. Eur Respir J. 2018;51(3) doi: 10.1183/13993003.02467-2017. [DOI] [PubMed] [Google Scholar]
- Koirala S, Borisov S, Danila E, Mariandyshev A, Shrestha B, Lukhele N, et al. Outcome of treatment of MDR-TB or drug-resistant patients treated with bedaquiline and delamanid: Results from a large global cohort. Pulmonology. 2021;27(5):403–412. doi: 10.1016/j.pulmoe.2021.02.006. [DOI] [PubMed] [Google Scholar]
- Kuksa L, Barkane L, Hittel N, Gupta R. Final treatment outcomes of multidrug- and extensively drug-resistant tuberculosis patients in Latvia receiving delamanid-containing regimens. Eur Respir J. 2017;50(5) doi: 10.1183/13993003.01105-2017. [DOI] [PubMed] [Google Scholar]
- Kwon YS, Jeon D, Kang H, Yim JJ, Shim TS. Concurrent use of bedaquiline and delamanid for the treatment of fluoroquinolone-resistant multidrug-resistant tuberculosis: a nationwide cohort study in South Korea. Eur Respir J. 2021;57(3) doi: 10.1183/13993003.03026-2020. [DOI] [PubMed] [Google Scholar]
- Lee HH, Jo KW, Yim JJ, Jeon D, Kang H, Shim TS. Interim treatment outcomes in multidrug-resistant tuberculosis patients treated sequentially with bedaquiline and delamanid. Int J Infect Dis. 2020;98:478–485. doi: 10.1016/j.ijid.2020.07.001. [DOI] [PubMed] [Google Scholar]
- Madzgharashvili T, Salindri AD, Magee MJ, Tukvadze N, Avaliani Z, Blumberg HM, et al. Treatment Outcomes Among Pediatric Patients With Highly Drug-Resistant Tuberculosis: The Role of New and Repurposed Second-Line Tuberculosis Drugs. J Pediatric Infect Dis Soc. 2021;10(4):457–467. doi: 10.1093/jpids/piaa139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliori GB, Pontali E, Sotgiu G, Centis R, D'Ambrosio L, Tiberi S, et al. Combined Use of Delamanid and Bedaquiline to Treat Multidrug-Resistant and Extensively Drug-Resistant Tuberculosis: A Systematic Review. Int J Mol Sci. 2017;18(2):341. doi: 10.3390/ijms18020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliori GB, Tiberi S, Zumla A, Petersen E, Chakaya JM, Wejse C, et al. MDR/XDR-TB management of patients and contacts: Challenges facing the new decade. The 2020 clinical update by the Global Tuberculosis Network. Int J Infect Dis. 2020;92S:S15–S25. doi: 10.1016/j.ijid.2020.01.042. [DOI] [PubMed] [Google Scholar]
- Migliori GB, Thong PM, Alffenaar JW, Denholm J, Tadolini M, Alyaquobi F, et al. Global Tuberculosis Network. Gauging the impact of the COVID-19 pandemic on tuberculosis services: a global study. Eur Respir J. 2021;58(5) doi: 10.1183/13993003.01786-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr-Holland E, Reuter A, Hughes J, Daniels J, Beko B, Makhanda G, et al. Correspondence regarding "Delamanid for rifampicin-resistant tuberculosis: a retrospective study from South Africa". Eur Respir J. 2020;56 doi: 10.1183/13993003.00837-2020. [DOI] [PubMed] [Google Scholar]
- Mohr E, Hughes J, Reuter A, Trivino Duran L, Ferlazzo G, Daniels J, et al. Delamanid for rifampicin-resistant tuberculosis: a retrospective study from South Africa. Eur Respir J. 2018;51(6) doi: 10.1183/13993003.00017-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok J, Kang H, Koh WJ, Jhun BW, Yim JJ, Kwak N, et al. Final treatment outcomes of delamanid-containing regimens in patients with MDR-/XDR-TB in South Korea. Eur Respir J. 2019;54(5) doi: 10.1183/13993003.00811-2019. [DOI] [PubMed] [Google Scholar]
- Motta I, Centis R, D'Ambrosio L, García-García JM, Goletti D, Gualano G, et al. Tuberculosis, COVID-19 and migrants: Preliminary analysis of deaths occurring in 69 patients from two cohorts. Pulmonology. 2020;26(4):233–240. doi: 10.1016/j.pulmoe.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahid P, Mase SR, Migliori GB, Sotgiu G, Bothamley GH, Brozek JL, et al. Treatment of Drug-Resistant Tuberculosis. An Official ATS/CDC/ERS/IDSA Clinical Practice Guideline. Am J Respir Crit Care Med. 2019;200(10):e93–e142. doi: 10.1164/rccm.201909-1874ST. Nov 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TVA, Anthony RM, Cao TTH, Bañuls AL, Nguyen VAT, Vu DH, et al. Delamanid Resistance: Update and Clinical Management. Clin Infect Dis. 2020;71(12):3252–3259. doi: 10.1093/cid/ciaa755. [DOI] [PubMed] [Google Scholar]
- Nieto Ramirez LM, Quintero Vargas K, Diaz G. Whole Genome Sequencing for the Analysis of Drug Resistant Strains of Mycobacterium tuberculosis: A Systematic Review for Bedaquiline and Delamanid. Antibiotics (Basel) 2020;9(3):133. doi: 10.3390/antibiotics9030133. Mar 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayanju O, Esmail A, Limberis J, Dheda K. A regimen containing bedaquiline and delamanid compared to bedaquiline in patients with drug-resistant tuberculosis. Eur Respir J. 2020;55(1) doi: 10.1183/13993003.01181-2019. [DOI] [PubMed] [Google Scholar]
- Pirmahmadzoda B, Hann K, Akopyan K, Grigoryan R, Geliukh E, Hushvaht S, et al. Treatment success using novel and adapted treatment regimens in registered DR-TB children in Dushanbe, Tajikistan, 2013-2019. J Infect Dev Ctries. 2021;15(9.1):7S–16S. doi: 10.3855/jidc.14798. [DOI] [PubMed] [Google Scholar]
- Pontali E, Sotgiu G, Tiberi S, Tadolini M, Visca D, D'Ambrosio L, et al. Combined treatment of drug-resistant tuberculosis with bedaquiline and delamanid: a systematic review. Eur Respir J. 2018;52(1) doi: 10.1183/13993003.00934-2018. pii: 1800934. [DOI] [PubMed] [Google Scholar]
- Sarin R, Vohra V, Singla N, Singla R, Puri MM, Munjal SK, et al. Early efficacy and safety of Bedaquiline and Delamanid given together in a "Salvage Regimen" for treatment of drug-resistant tuberculosis. Indian J Tuberc. 2019;66(1):184–188. doi: 10.1016/j.ijtb.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Svensson EM, Wang X, Wang Y, Hafkin J, Karlsson MO, et al. Population Pharmacokinetic and Concentration-QTc Analysis of Delamanid in Pediatric Participants with Multidrug-Resistant Tuberculosis. Antimicrob Agents Chemother. 2021 doi: 10.1128/AAC.01608-21. Nov 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah I, Gandhi S, Shetty NS. Bedaquiline and Delamanid in Children With XDR Tuberculosis: What is prolonged QTc? Pediatr Infect Dis J. 2020;39(6):512–513. doi: 10.1097/INF.0000000000002601. [DOI] [PubMed] [Google Scholar]
- Skripconoka V, Danilovits M, Pehme L, Tomson T, Skenders G, Kummik T, et al. Delamanid improves outcomes and reduces mortality in multidrug-resistant tuberculosis. Eur Respir J. 2013;41(6):1393–1400. doi: 10.1183/09031936.00125812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solodovnikova V, Kumar AMV, Hurevich H, Sereda Y, Auchynka V, Katovich D, et al. Effectiveness and safety of delamanid- or bedaquiline-containing regimens among children and adolescents with multidrug resistant or extensively drug resistant tuberculosis: A nationwide study from Belarus, 2015-19. Monaldi Arch Chest Dis. 2021;91(1) doi: 10.4081/monaldi.2021.1646. Jan 14. [DOI] [PubMed] [Google Scholar]
- Tadolini M, Garcia-Prats AJ, D'Ambrosio L, Hewison C, Centis R, Schaaf HS, et al. Compassionate use of new drugs in children and adolescents with multidrug-resistant and extensively drug-resistant tuberculosis: early experiences and challenges. Eur Respir J. 2016;48(3):938–943. doi: 10.1183/13993003.00705-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadolini M, Codecasa LR, García-García JM, Blanc FX, Borisov S, Alffenaar JW, et al. Active tuberculosis, sequelae and COVID-19 co-infection: first cohort of 49 cases. Eur Respir J. 2020;56(1) doi: 10.1183/13993003.01398-2020.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TB/COVID-19 Global Study Group Tuberculosis and COVID-19 co-infection: description of the global cohort. Eur Respir J. 2021 doi: 10.1183/13993003.02538-2021. Nov 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viney K, Linh NN, Gegia M, Zignol M, Glaziou P, Ismail N, et al. New definitions of pre-extensively and extensively drug-resistant tuberculosis: update from the World Health Organization. Eur Respir J. 2021;57(4) doi: 10.1183/13993003.00361-2021. [DOI] [PubMed] [Google Scholar]
- Visca D, Ong CWM, Tiberi S, Centis R, D'Ambrosio L, Chen B, et al. Tuberculosis and COVID-19 interaction: A review of biological, clinical and public health effects. Pulmonology. 2021;27(2):151–165. doi: 10.1016/j.pulmoe.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Groote-Bidlingmaier F, Patientia R, Sanchez E, Balanag V, Jr, Ticona E, Segura P, et al. Efficacy and safety of delamanid in combination with an optimised background regimen for treatment of multidrug-resistant tuberculosis: a multicentre, randomised, double-blind, placebo-controlled, parallel group phase 3 trial. Lancet Respir Med. 2019;7(3):249–259. doi: 10.1016/S2213-2600(18)30426-0. [DOI] [PubMed] [Google Scholar]
- Wang X, Mallikaarjun S, Gibiansky E. Population Pharmacokinetic Analysis of Delamanid in Patients with Pulmonary Multidrug-Resistant Tuberculosis. Antimicrob Agents Chemother. 2020;65(1):e01202–e01220. doi: 10.1128/AAC.01202-20. Dec 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells CD, Gupta R, Hittel N, Geiter LJ. Long-term mortality assessment of multidrug-resistant tuberculosis patients treated with delamanid. Eur Respir J. 2015;45(5):1498–1501. doi: 10.1183/09031936.00176314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2012. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (accessed 31 January 2022).
- World Health Organization . 4th ed. World Health Organization; Geneva: 2009. Guidelines for surveillance of drug resistance in tuberculosis. [Google Scholar]
- World Health Organization . World Health Organization; 2011. Guidelines for the programmatic management of drug-resistant tuberculosis-2011 update. [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2020. WHO consolidated guidelines on tuberculosis. Module 4: treatment - drug-resistant tuberculosis treatment. [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; Geneva: 2021. Global tuberculosis report 2021. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.