Introduction
Germline genetic variants in genes including CDKN2A, MITF, POT1, and BRCA1/2 predispose to the development of melanoma and other cancers.1 Pathogenic variants in CDKN2A specifically increase the risk for melanoma and pancreatic cancer.2,3 Hereditary breast and ovarian cancer syndrome, caused by pathogenic variants in BRCA1/2, is most strongly associated with breast and ovarian cancer, but in the case of BRCA2, also increases the risk for melanoma.4 In patients with melanoma, taking a careful family history is essential to guide consideration of germline genetic testing.
Here we present a patient whose striking personal and family history of cancer prompted sequential testing in multiple cancer predisposition genes, leading to an unusual diagnosis of pathogenic germline variants in both CDKN2A and BRCA1.
Case report
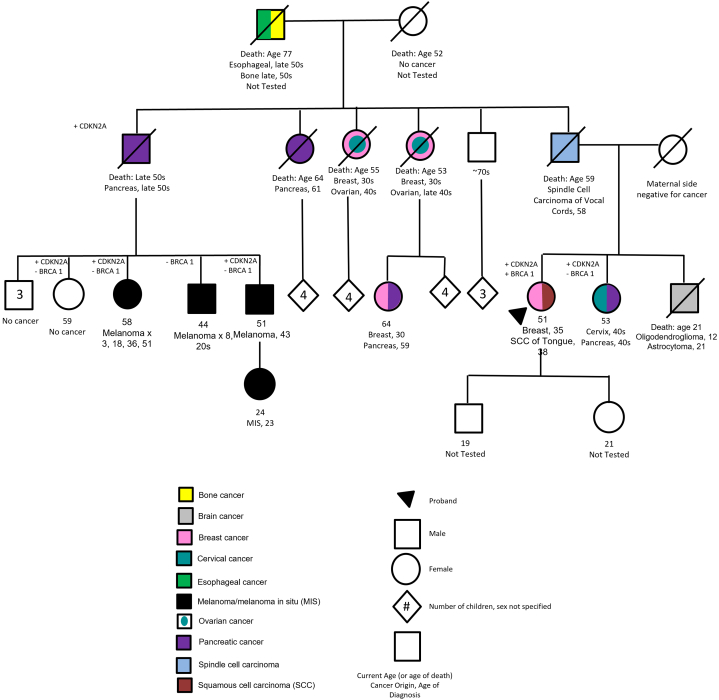
After being diagnosed at age 34 with infiltrating ductal carcinoma of the breast (ER/PR negative, HER2/neu negative), a woman presented in 2005 to an academic institution’s cancer genetics clinic. Her paternal family history (Fig 1) included first-degree relatives with brain and vocal cord cancers, as well as second-degree relatives with esophageal cancer, breast cancer, pancreatic cancer, ovarian cancer, and melanoma. Many were diagnosed at relatively young ages. Her maternal family history did not include cancer.
Fig 1.
Family pedigree.
Initial full sequencing of BRCA1/2 did not reveal a pathogenic variant in 2005. No other genes were tested at that time. The patient underwent bilateral mastectomy and adjuvant chemotherapy, and remained cancer-free until age 38 when she developed a squamous cell carcinoma (SCC) of the right lateral tongue, subsequently excised with clear margins. She returned to cancer genetics in 2009 for follow-up and evaluation by a genetic counselor.
Updated BRCA1/2 testing, including large rearrangement testing, a newer technology at the time, revealed a large deletion within BRCA1 (deletion of exon 12) associated with cancer risk. TP53 testing was pursued given personal and extensive family history beyond classical BRCA-associated neoplasms; this was negative. At a subsequent visit, she reported additional family cancer history including several paternal cousins with melanoma and was tested for CDKN2A given the family history of melanoma and pancreatic cancer, as well as cancers of the head and neck (including oral cancers) and brain tumors. CDKN2A testing revealed a pathogenic variant [c.148C>T (p.Q50X)]. Based on pathogenic germline findings in BRCA1 and CDKN2A and her personal history of oral SCC, the proband underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy to mitigate risk of ovarian cancer and was recommended the following surveillance plan: full skin exam every 6-12 months; pancreatic cancer screening via alternating magnetic resonance cholangiopancreatography and endoscopic ultrasound every 2-3 years, yearly brain magnetic resonance imaging, and oral exams through otolaryngology every 6 months.
Dermatology examination revealed approximately 20-30 evenly pigmented brown macules with slightly irregular borders, favored to represent slightly atypical nevi, scattered on patient's trunk and extremities. She had 2 skin biopsies consistent with atypical nevi. At age 40, she began treatment with raloxifene for osteopenia, which has the additional benefit of reducing breast cancer risk. She has remained in remission with no further cancer diagnoses to date.
Cascade testing for the BRCA1 and CDKN2A pathogenic variants was recommended to the patient’s family. Six family members pursued testing. Testing was completed for BRCA1 in 5, all negative. Insurance refused coverage of CDKN2A testing in one cousin despite personal history of multiple primary melanomas. Four individuals, including her sister with pancreatic and cervical cancer and 2 first cousins with histories of multiple melanomas, were diagnosed with the pathogenic CDKN2A variant. Numerous individuals, including her healthy adult children, deferred testing.
Discussion
Identification of pathogenic germline variants in cancer predisposition genes can influence patient risk behavior and allow implementation of screening protocols aimed at early detection. Features that increase concern for inherited cancer predisposition include early age of cancer diagnosis, multiple primary cancer diagnoses, rare cancer diagnoses, and personal history of cancer within the context of first- or second-degree family members with these risk factors.5 Oncologists routinely screen patients with specific diagnoses, including breast cancer, for risk factors for inherited cancer predisposition syndromes such as BRCA1/2.4 For example, the National Comprehensive Cancer Network recommends further evaluation for those with a personal history of breast cancer that is diagnosed younger than age 46, accompanied by a personal or family history of ovarian or pancreatic cancer, or triple-negative and diagnosed under age 60, among other criteria.6
Guidelines also exist for screening for CDKN2A pathogenic variants, which are thought to account for 40% of the approximately 5%-12% of melanoma cases deemed familial.1,2 Patients with pathogenic CDKN2A variants are often younger when diagnosed with melanoma, have multiple primary melanomas, or have a family history of multiple primary melanomas and/or pancreatic cancer. Specifically, National Comprehensive Cancer Network recommends a genetic counseling referral for the presence of 3 or more invasive cutaneous melanomas, or a mix of invasive melanoma, pancreatic cancer, and/or astrocytoma diagnoses in an individual or family.7 Head and neck SCC, as identified in this patient, has also been reported to be increased with CDKN2A variants and is typically diagnosed at younger ages in this population.8,9 A thorough screening algorithm for other cancer predisposition syndromes that may include melanoma has also been proposed but not evaluated in clinical practice.10
Dermatologists often diagnose and treat melanoma and are well-positioned to inquire regarding patient personal and family histories of melanoma. Though it may not be practical to implement comprehensive personal and family cancer history screening in a typical dermatologic encounter, performing relatively straightforward screening for CDKN2A risk may invite patients to share additional information about their personal and family cancer history that may influence clinicians’ decision to recommend referral to cancer genetics. For example, referral for BRCA1/2 testing should be considered for patients with an early personal or family diagnosis of breast cancer, personal history of breast and other cancers, or triple negative breast cancer diagnosed below age 60.4 Geneticists and genetic counselors can construct a gene test list that encompasses testing for variants that would include all features in a patient. While a pathogenic variant in BRCA1 explains this patient’s breast cancer, it does not explain a strong family history of melanoma. Conversely, a CDKN2A pathogenic variant explains the family history of melanoma but does not increase the risk for breast cancer. Therefore, even if a patient’s family history does not seem fully consistent with one particular pathogenic variant, a referral to genetics may still be warranted given the potential for 2 pathogenic variants coexisting in one family.
Genetic testing has advanced in recent years, and testing technologies have significantly improved over time, now allowing large panels with numerous genetic variants.11 Those with previous negative testing should have a detailed review of prior genetic testing results (eg, genes evaluated, methods used) and may benefit from updated testing.12 Insurance has also increased coverage of genetic testing in recent years, potentially increasing availability to patients who were previously denied coverage.13 As testing options and guidelines change, it may also become more common for a patient to be diagnosed with multiple pathogenic variants.14
Dermatologists can play a crucial role in identifying patients at risk for an inherited predisposition to melanoma and other cancers, and attention to evolving standards in genetic testing can help patients obtain appropriate testing to inform proper education and surveillance.
Conflicts of interest
None disclosed.
Footnotes
Drs Else and Cha contributed equally to this study & manuscript.
Funding sources: None.
IRB approval status: Approved (HUM0004340).
Financial disclosure: None.
References
- 1.Swetter S.M., Tsao H., Bichakjian C.K., et al. Guidelines of care for the management of primary cutaneous melanoma. J Am Acad Dermatol. 2019;80(1):208–250. doi: 10.1016/j.jaad.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 2.Leachman S.A., Carucci J., Kohlmann W., et al. Selection criteria for genetic assessment of patients with familial melanoma. J Am Acad Dermatol. 2009;61(4):677.e1-e14. doi: 10.1016/j.jaad.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eckerle Mize D., Bishop M., Resse E., et al. In: Cancer syndromes [internet] Riegert-Johnson D.L., Boardman L.A., Hefferon T., et al., editors. National Center for Biotechnology Information (US); 2009. Familial atypical multiple mole melanoma syndrome. [PubMed] [Google Scholar]
- 4.Petrucelli N., Daly M.B., Pal T. In: GeneReviews® [internet] Adam M.P., Mirzaa G.M., Pagon R.A., et al., editors. University of Washington, Seattle; 1998. BRCA1-and BRCA2-associated hereditary breast and ovarian cancer; pp. 1993–2022. [Google Scholar]
- 5.Lu K.H., Wood M.E., Daniels M., et al. American Society of Clinical Oncology expert statement: collection and use of a cancer family history for oncology providers. J Clin Oncol. 2014;32(8):833. doi: 10.1200/JCO.2013.50.9257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NCCN Guidelines for Genetics/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. National Comprehensive Cancer Network. 2022. https://www.nccn.org/professionals/physician_gls/pdf/genetics_bop.pdf
- 7.NCCN Guidelines for Genetics/Familial High-Risk Assessment: Breast and Ovarian. National Comprehensive Cancer Network. 2019. Accessed September 27, 2022. https://www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf
- 8.Cabanillas R., Astudillo A., Valle M., et al. Novel germline CDKN2A mutation associated with head and neck squamous cell carcinomas and melanomas. Head Neck. 2013;35(3):E80–E84. doi: 10.1002/hed.21911. [DOI] [PubMed] [Google Scholar]
- 9.Cury S.S., de Miranda P.M., Marchi F.A., et al. Germline variants in DNA Repair genes are associated with young-onset head and neck cancer. Oral Oncol. 2021;122 doi: 10.1016/j.oraloncology.2021.105545. [DOI] [PubMed] [Google Scholar]
- 10.Leachman S.A., Lucero O.M., Sampson J.E., et al. Identification, genetic testing, and management of hereditary melanoma. Cancer Metastasis Rev. 2017;36(1):77–90. doi: 10.1007/s10555-017-9661-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall M.J., Forman A.D., Pilarski R., Wiesner G., Giri V.N. Gene panel testing for inherited cancer risk. J Natl Compr Cancer Netw. 2014;12(9):1339–1346. doi: 10.6004/jnccn.2014.0128. [DOI] [PubMed] [Google Scholar]
- 12.Yadav S., Reeves A., Campian S., Paine A., Zakalik D. Outcomes of retesting BRCA negative patients using multigene panels. Fam Cancer. 2017;16(3):319–328. doi: 10.1007/s10689-016-9956-7. [DOI] [PubMed] [Google Scholar]
- 13.Grant P., Langlois S., Lynd L.D., GenCOUNSEL Study. Austin J.C., Elliott A.M. Out-of-pocket and private pay in clinical genetic testing: a scoping review. Clin Genet. 2021;100(5):504–521. doi: 10.1111/cge.14006. [DOI] [PubMed] [Google Scholar]
- 14.Slaught C., Berry E.G., Bacik L., et al. Clinical challenges in interpreting multiple pathogenic mutations in single patients. Hereditary Cancer Clin Pract. 2021;19(1):1–11. doi: 10.1186/s13053-021-00172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]