Introduction
Gestational gigantomastia (GG) is a rare disorder of pregnancy, characterized by rapid, severe, painful enlargement of one or both breasts.1 While the etiology remains unclear, hormone receptor hypersensitivity, liver dysfunction, elevated prolactin, malignancy, and autoimmune conditions may contribute.1 We report a case of GG with concomitant diffuse dermal angiomatosis (DDA), characterized by dermal vascular proliferation in response to hypoxia.
Case report
A 23-year-old, primigravid female at 19 weeks gestational age presented with a several-month history of bilateral breast lumps, associated with tenderness, pruritus, decreased sensation, and skin thickening.
Previously, she was diagnosed with mastitis but did not improve with antibiotics. Given worsening pain, she saw an outside dermatologist, who performed 2 biopsies, which were non-diagnostic. She was prescribed 2 weeks of prednisone without improvement. During this time, her bra size increased from a C-cup to an immeasurable size.
Upon presentation to our clinic, she denied chronic medications or medical issues. Family history revealed a sister with lupus and mother with autoimmune thyroid disease. Review of systems was unremarkable.
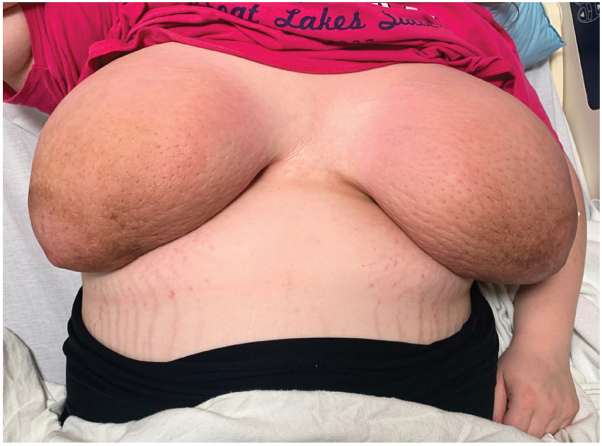
On exam, both breasts appeared massively swollen and brightly erythematous with ill-defined brownish hyperpigmentation surrounding the areolae (Fig 1). The skin surface had a peau d’orange appearance, with accentuation of follicular orifices. On palpation, both breasts exhibited diffuse woody induration and firm, subcutaneous nodules.
Fig 1.
Initial presentation of a 23-year-old, primigravid female with bilateral breast swelling, associated with bright erythema, brownish hyperpigmentation near the areolae, peau d’orange changes, and accentuation of follicular orifices. On palpation, both breasts were diffusely indurated with numerous firm, subcutaneous nodules.
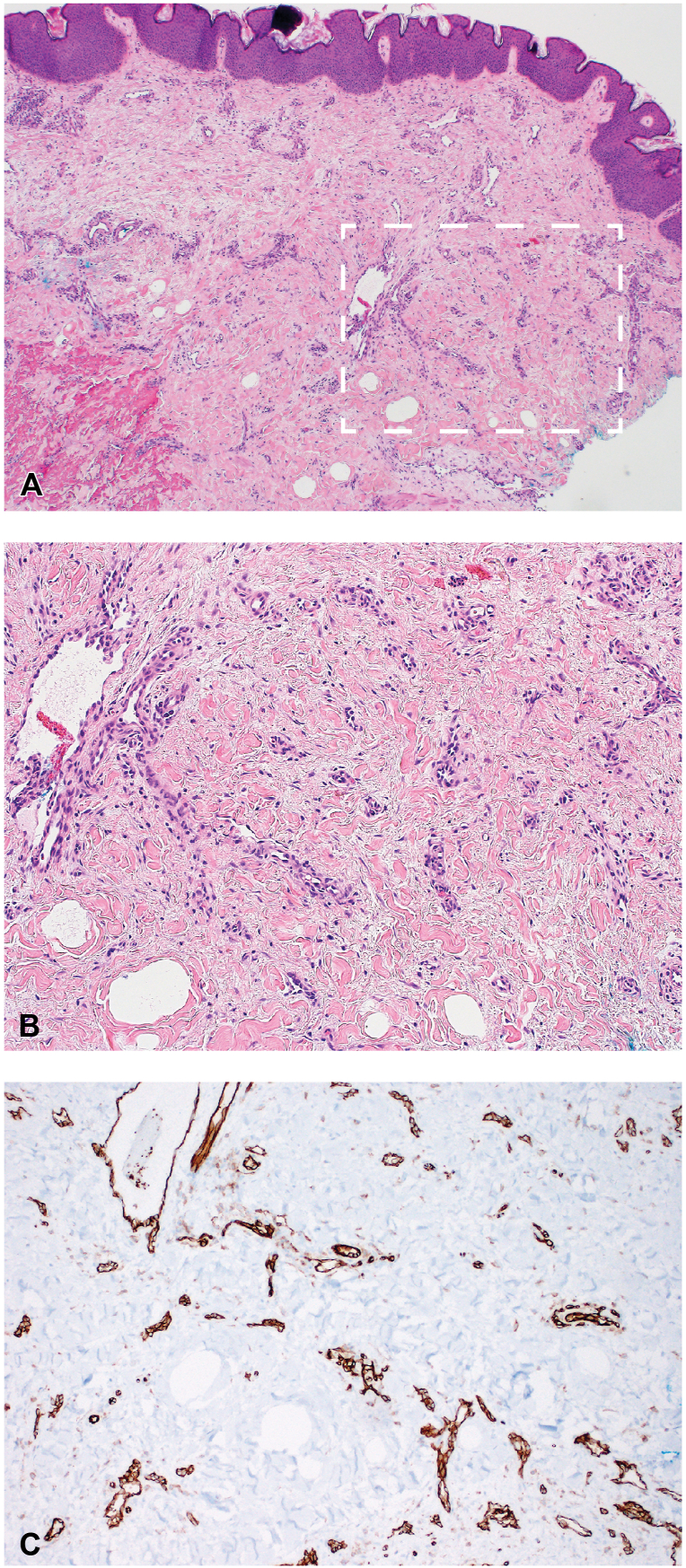
Clinically, our differential diagnosis included morphea profunda, lupus mastitis (LM), granulomatous mastitis, breast carcinoma, and diffuse DDA. A punch biopsy from a nodular area revealed diffuse angioproliferative changes of the small dermal blood vessels consistent with DDA (Fig 2) without evidence of morphea or lupus. Review of her original biopsies showed similar changes without cytologic atypia. None of the biopsies included fat or demonstrated evidence of significant lymphedema in the dermis.
Fig 2.
A, At low power magnification (40×), a punch biopsy from the right medial breast showed an increased number of small dermal blood vessels throughout the dermis. B, On higher magnification (100×), the vessels have well-formed lumens and do not demonstrate cytologic atypia. C, To highlight the vasculature, immunohistochemical staining for CD31, a vascular endothelial marker, was performed (100×).
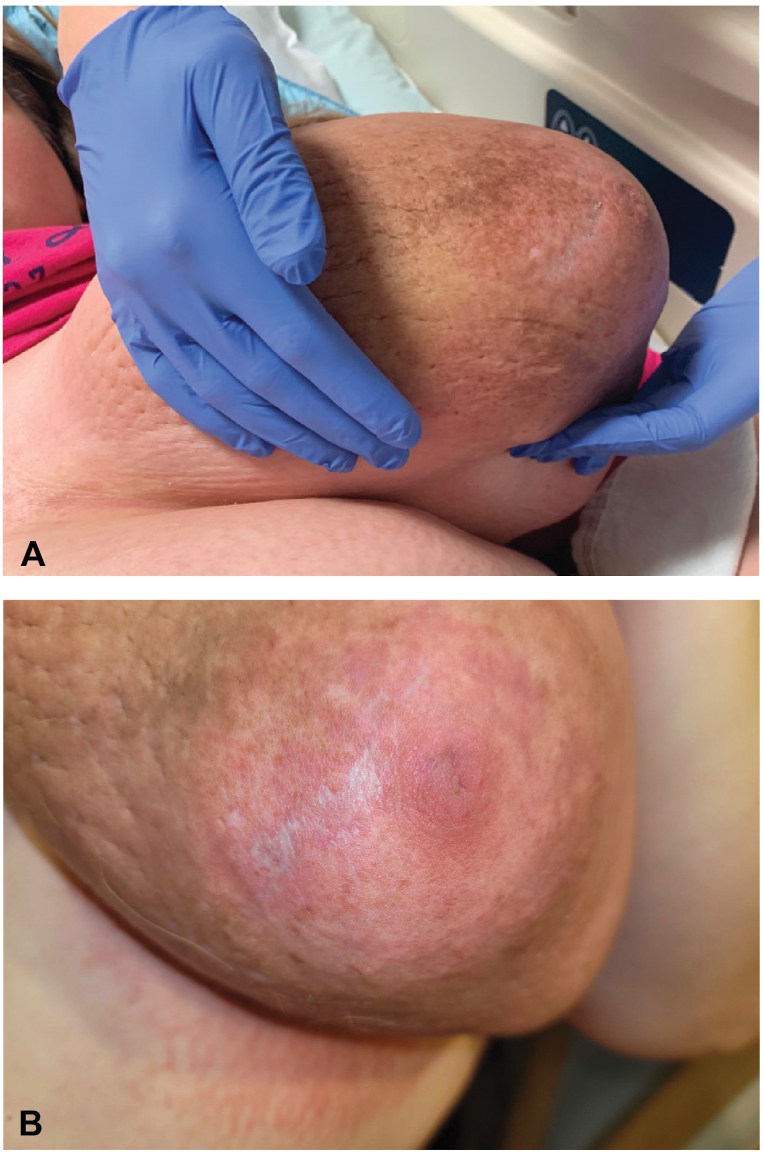
Due to worsening pain and swelling over the next 2 months, she was admitted for expedited workup. Upon admission, we noted new white, stellate plaques on the breasts, concerning for necrosis and impending ulceration (Fig 3). Ultrasound studies showed diffuse symmetric breast edema without focal masses or evidence of malignancy. Laboratory tests were positive for ANA (antinuclear antibody) (1:2560) with speckled pattern and anti-Ro autoantibodies. Prolactin level was within normal range for pregnancy (149 ng/mL). Additional punch biopsies of sun-exposed and sun-protected skin for direct immunofluorescence were negative for immune deposits. In addition to our diagnosis of DDA, our maternal fetal medicine colleagues rendered a diagnosis of GG, while our rheumatology colleagues expressed concern for LM, given her serologies.
Fig 3.
Gestational gigantomastia with concurrent diffuse dermal angiomatosis. On hospital admission, the patient continued to exhibit breast swelling (A) and was noted to have development of white, stellate, atrophic plaques (B).
The maternal fetal medicine team noted that the most effective therapy for GG would be surgical reduction postpartum to minimize risk to the mother and fetus. Until delivery, they placed the patient on cabergoline 0.5 mg twice weekly to suppress lactation and recommended supportive garments. Steroids were avoided given prior inefficacy. Given concern for LM, she was initiated on hydroxychloroquine to reduce risk of fetal heart block.
Several days later, the patient was discharged with improved erythema and pain. Despite her serologies, no evidence of autoimmune disease ultimately manifested. Her breast size did not increase further, the stellate plaques improved, and she safely delivered her baby. A year later, she underwent successful reduction mammoplasty.
Discussion
To our knowledge, this report is the first of GG with concurrent overlying DDA. GG is a rare condition seldom encountered by dermatologists. It can be associated with autoimmune diseases, including myasthenia gravis, lupus, Hashimoto thyroiditis, and psoriasis.2
Clinically, GG presents as rapid and excessive breast growth.3 As the differential diagnosis includes granulomatous mastitis,4 non-Hodgkin lymphoma, lymphoblastic lymphoma, and common breast tumors, such as phyllodes tumors and fibroadenomas,3 comprehensive workup by a breast specialist is required.5 Dermatologists can assist in making the diagnosis by ruling in/out various dermatoses, for instance, breast enlargement related to panniculitis and LM.2
Histopathologically, GG exhibits hyperplasia of glandular structures with increased connective tissue and fibrosis, consistent with normal breast changes of pregnancy.5 The findings differ from LM, which exhibits lymphocytic lobular panniculitis with hyalinized fat necrosis,6 or granulomatous mastitis, which shows lobulocentric, non-necrotizing granulomas.4 In our patient, punch biopsies were not deep enough to evaluate the fat or show features of GG, and therefore, the diagnosis was made clinically. More invasive biopsies were avoided, due to ulceration risk in the setting of overlying DDA.
Although there are reports of regression and spontaneous resolution of GG in the postpartum period, definitive treatment of persistent GG includes mastectomy.7 Alternative strategies include reduction mammoplasty or hormonal antagonists, such as cabergoline and bromocriptine.7 In our patient, cabergoline was initiated due to its reduced side-effect profile, compared to bromocriptine.8 Her response to cabergoline supported the diagnosis of GG.
DDA is a benign cutaneous disorder characterized by vascular proliferation.5 It is most common in middle-aged women with pendulous breasts, and presents as enlargement of the breasts and painful reticulated erythema, usually in dependent areas.5 A common complication is ulceration, which can present as stellate white plaques and indicates skin necrosis due to damage of newly developed superficial vessels.9 Occasionally, DDA presents with peau d’orange changes,9 which in our case, raised suspicion for DDA.
DDA is associated with macromastia, obesity, trauma, smoking, and vaso-occlusive disorders.10 Macromastia likely promotes DDA through tissue compression and increased venous hydrostatic pressure,5 leading to tissue hypoxia and upregulation of VEGF (vascular endothelial growth factor) and angioproliferative cytokines.5 Our case demonstrates that another potential cause of DDA is GG.
The differential diagnosis of DDA includes acroangiodermatitis, vasculopathies, vasculitis, and vascular tumors. A skin biopsy can distinguish these entities, and in the case of DDA, demonstrates diffuse dermal capillary and endothelial cell proliferation.10 In contrast, acroangiodermatitis displays proliferation of endothelial cells with formation of thick-walled vessels in a lobular pattern in the papillary dermis.10
Treatment of DDA is aimed at improving tissue hypoxia. Strategies include breast reduction, revascularization, and smoking cessation.5 Pentoxifylline, aspirin, oral corticosteroids, nifedipine, and isotretinoin have shown variable success.10 As our patient was pregnant, many of these therapies were contraindicated. Our case demonstrates that treating the underlying cause of DDA, in this case GG, can relieve symptoms.
In summary, we wish to inform dermatologists of the entity GG, which should be suspected in cases of massive, painful breast enlargement during pregnancy. Dermatologists can play a role in the diagnosis, particularly if clinical and histopathological findings reveal DDA. Multidisciplinary management includes the use of supportive garments, topical and systemic corticosteroids, agents to reduce lactation, and surgery.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
Consent for the publication of all patient photographs and medical information was provided by the authors at the time of article submission to the journal stating that all patients gave consent for their photographs and medical information to be published in print and online and with the understanding that this information may be publicly available.
References
- 1.Mangla M., Singla D. Gestational gigantomastia: a systematic review of case reports. J Midlife Health. 2017;8(1):40–44. doi: 10.4103/jmh.JMH_92_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinicki J.P., Gonzalez C.N., Dubinsky D., Nasswetter G., Cardinal L.H., Hojman J. Gestational gigantomastia in autoimmune diseases. J Clin Rheumatol. 2015;21(2):110–112. doi: 10.1097/RHU.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 3.Türkan H., Gökgöz M.Ş., Taşdelen İ., Dündar H.Z. Gestational gigantomastia. J Breast Health. 2016;12(2):86–87. doi: 10.5152/tjbh.2016.2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolfrum A., Kümmel S., Theuerkauf I., Pelz E., Reinisch M. Granulomatous mastitis: a therapeutic and diagnostic challenge. BRC. 2018;13(6):413–418. doi: 10.1159/000495146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galambos J., Meuli-Simmen C., Schmid R., Steinmann L.S., Kempf W. Diffuse dermal angiomatosis of the breast: a distinct entity in the spectrum of cutaneous reactive angiomatoses - clinicopathologic study of two cases and comprehensive review of the literature. Case Rep Dermatol. 2017;9(3):194–205. doi: 10.1159/000480721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Warne R.R., Taylor D., Segal A., Irish A. Lupus mastitis: a mimicker of breast carcinoma. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.11.2011.5066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin F., Si L., Zhang H., et al. Management of gestational gigantomastia with breast reconstruction after mastectomy: case report and literature review. J Int Med Res. 2020;48(6) doi: 10.1177/0300060520920463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert K., Williamson C. Review of presentation, diagnosis and management of pituitary tumours in pregnancy. Obstet Med. 2013;6(1):13–19. doi: 10.1258/OM.2012.120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reusche R., Winocour S., Degnim A., Lemaine V. Diffuse dermal angiomatosis of the breast: a series of 22 cases from a single institution. Gland Surg. 2015;4(6):554–560. doi: 10.3978/j.issn.2227-684X.2015.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang H., Ahmed I., Mathew V., Schroeter A.L. Diffuse dermal angiomatosis of the breast. Arch Dermatol. 2006;142(3):343–347. doi: 10.1001/archderm.142.3.343. [DOI] [PubMed] [Google Scholar]