Abstract
We examined associations between prenatal oxidative stress (OS) and child autism-related outcomes. Women with an autistic child were followed through a subsequent pregnancy and that younger sibling’s childhood. Associations between glutathione (GSH), glutathione thiol disulfide (GSSG), 8-oxo-deoxyguanine (8-OHdG), and nitrotyrosine and younger sibling Social Responsiveness Scale (SRS) scores were examined using quantile regression. Increasing GSH:GSSG (suggesting decreasing OS) was associated with minor increases in SRS scores (50th percentile β: 1.78, 95% CI: 0.67, 3.06); no other associations were observed. Results from this cohort with increased risk for autism do not support a strong relationship between OS in late pregnancy and autism-related outcomes. Results may be specific to those with enriched autism risk; future work should consider other timepoints and biomarkers.
Keywords: autism spectrum disorder, oxidative stress, risk factors, cohort, epidemiology, neurodevelopment
Autism spectrum disorder (ASD) is defined by impairments in social communication and presence of restricted or repetitive behaviors and is a serious neurodevelopmental condition with significant public health impact (Maenner et al., 2020). ASD is frequently accompanied by cognitive impairment; the prevalence of intellectual disability defined by intelligence quotient (IQ) of <70 is at least 3–5 times greater in ASD cases than overall population prevalence (Boyle et al., 2011; Postorino et al., 2016; Van Naarden Braun et al., 2015). Individuals diagnosed with ASD have a range of symptoms and level of severity and this heterogeneity of phenotype has plagued research into its etiologic origins (London, 2014). Research suggests that genetic as well as environmental factors play a role in the development of ASD (Hallmayer et al., 2011; Risch et al., 2014) and that gestation, as a critical window of neurodevelopmental susceptibility, is a period where genetic and environmental hits may take place, leading to altered neurodevelopment (Rice & Barone Jr, 2000).
Oxidative stress (OS), which arises when the generation of reactive oxygen species (ROS) exceeds the antioxidant defense mechanisms, is a normal physiological process; however, left unregulated, OS can induce DNA damage, protein oxidation, and lipid peroxidation (Birben, Sahiner, Sackesen, Erzurum, & Kalayci, 2012; Thompson & Al-Hasan, 2012). The brain is particularly susceptible to OS due to its demand for oxygen and high lipid cell content, particularly during fetal development (Kim, Kim, Rhie, & Yoon, 2015; Rose et al., 2012). OS has been hypothesized as a mechanism in ASD etiology, given the susceptibility of the fetus to ROS (Dennery, 2010; Thompson & Al-Hasan, 2012), and the links between multiple risk factors for ASD and OS, including low birth weight, maternal conditions such as preeclampsia, as well as environmental exposures such as air pollution and pesticides (Shelton, Hertz-Picciotto, & Pessah, 2012; Lopez-Tinoco et al., 2013; Matsubasa et al., 2002; Olsson et al., 2010; Atamer et al., 2005). Thus, OS in mothers may directly impact neurodevelopment and OS in fetuses:, or may have indirect impacts on neurodevelopment via relationships with other mechanisms, such as placental functioning and development or inflammation.
Some prior work has suggested associations between maternal OS and broader neurodevelopmental outcomes ( Rommel et al., 2020; Wells, Bhatia, Drake, & Miller-Pinsler, 2016; Wells et al., 2009). Direct links between OS and ASD specifically have primarily come from studies examining levels of ROS in children diagnosed with ASD, suggesting increased OS in cases vs controls in plasma and post-mortem brain samples (James et al., 2004; James et al., 2006; Melnyk et al., 2012; Rose et al., 2012). However, retrospective studies in children already diagnosed with ASD cannot provide evidence as to whether OS differences are a cause or a consequence of ASD. Only one prospective cohort study, to our knowledge, has examined the OS-neurodevelopmental disorder relationship during the potential critical window of gestation; this study found evidence of an increase in ASD-related traits for an interquartile range increase in maternal gestational urine OS biomarkers (Rommel et al., 2020).
Given limited prior work addressing the role of prenatal OS in ASD, the objective of this study was to examine the associations between maternal biomarkers of OS, measured during pregnancy, and child ASD-related outcomes. We hypothesized that increased OS during pregnancy may increase ASD-related traits in the child, given the multiple potential pathways noted above by which OS may relate to neurodevelopment.
Methods
Study Population
Participants for this study were drawn from the Early Autism Risk Longitudinal Investigation (EARLI), a multi-site enriched risk cohort following pregnancies among women who already had an autistic child. These younger siblings were then followed until they were 36 months old. Eligibility criteria for enrollment of mothers in the study included: (1) ability to communicate in English or Spanish; (2) 18 years of age or older; (3) residence within 2 hours of a study site; and (4) gestational age at enrollment less than 29 weeks. Participants had two to four clinical visits during the prenatal period where biological samples, including plasma, were collected. Additionally, clinicians completed ASD and behavioral assessments on the younger siblings at 36 months of age. Participants also completed standardized interviews and questionnaires periodically which covered health behaviors, reproductive history, demographics, and more. Additional details on the study design can be found elsewhere (Newschaffer et al., 2012).
Selection for our study is shown in Online Resource 1. In total, 273 women were enrolled in EARLI. For these analyses, participants were excluded if they did not have prenatal plasma samples available (n = 15) and if 36-month child outcome assessments of interest to this study (listed below) were not obtained (n = 47). We also excluded twin births (n=8 pairs), and those with spoiled or unusable biosamples as determined at the time of measurement (n = 3). The total number of participants included in final analyses varied by outcome of interest (see below), from 149–164 participants.
Oxidative Stress Biomarkers
Blood samples were collected from mothers during the prenatal period following accepted and published protocols for collection, processing, temperature control, and storage (Melnyk et al., 2012). For analyses, we included plasma and DNA samples from mid- to late-pregnancy (plasma: median 33 weeks, IQR 30–35 weeks; DNA: median 26 weeks, IQR 21–32 weeks) to reduce both variability in gestational age as well variability in OS across the course of pregnancy (see Online Resource 2 for full distribution). High performance liquid chromatography (HPLC) with electrochemical detection and liquid chromatography-mass spectrometry (LC-MS) were utilized to detect the primary biomarkers in each of the OS pathways.
The biomarkers of interest included: 1) 8-oxo-deoxyguanine (8-OHdG), an oxidative DNA adduct (Valavanidis, Vlachogianni, & Fiotakis, 2009); 2) Glutathione (GSH), the primary intracellular ROS antioxidant and detoxification mechanism in the body (Kim et al., 2015); 3) Glutathione disulfide (GSSG), the oxidized counterpart to GSH and a marker of extra-cellular oxidative stress; 4) the ratio of GSH:GSSG, which acts as an indicator of redox balance (Jones, 2002); and 5) 3-nitrotyrosine (nitrotyrosine), an amino acid modified by nitrosylation that is formed under oxidative/nitrosative stress (Svatikova et al., 2004). 8-OHdG was measured in maternal DNA samples, while all other biomarkers were measured in maternal plasma samples. Levels of 8-OHdG, were measured to quantify DNA oxidative damage; increased levels of 8-OHdG are indicative of increased OS. Levels of GSH and GSSG, as well as the ratio of the two, were measured to serve as markers of antioxidant balance in the body; increasing levels of the ratio of GSH:GSSG demonstrate increased antioxidant balance (less OS). Finally, nitrotyrosine levels were measured as markers of protein oxidation/nitrosylation; increasing levels of nitrotyrosine indicate increased OS. Previous research has suggested increased levels of plasma GSSG as well as decreased levels of GSH and the GSH:GSSG ratio, consistent with reduced antioxidant status, in autistic children, while 8-OHdG and nitrotyrosine levels have been shown to accurately distinguish autistic from neurotypical children (Howsmon, Kruger, Melnyk, James, & Hahn, 2017; James et al., 2004; James et al., 2006).
ASD-related outcomes
ASD-related outcomes were assessed via the Social Responsiveness Scale (SRS) (Constantino et al., 2003; Constantino & Gruber, 2012), Mullen Scales of Early Learning (MSEL-ELC) (Mullen, 1995), and ASD clinical diagnosis at 36 months. The SRS is a 65-item questionnaire designed to assess a child’s reciprocal social behaviors in social interactions. Parents respond to questions about their child’s social behaviors on a scale from 1 (“Never true”) to 4 (“Almost always true”). A single score is generated, with higher scores indicative of more autism-related traits. SRS scores present a continuous approach to measuring autism-related traits, and have shown strong psychometric properties and validity as compared to gold standard autism diagnostic measures like the Autism Diagnostic Interview-Revised (ADI-R) (Constantino et al., 2003; Constantino & Gruber, 2012). Total raw SRS scores were used here in primary analyses.
The MSEL assesses early intellectual development and school readiness that is strongly correlated with intellectual quotient (IQ) scores. The assessment yields 5 sub-scales (assessing motor skills, language, and visual reception), each derived as T-scores, that can be combined into a composite score (the early learning composite, ELC; mean of 100, standard deviation 15). Lower MSEL-ELC scores indicate poorer intellectual functioning (Bishop, Guthrie, Coffing, & Lord, 2011).
ASD diagnosis at 36 months was determined by clinical evaluation, which included the MSEL and the Autism Diagnostic Observation Schedule (ADOS) (Lord et al., 1989). Following previously outlined criteria incorporating scores on these measures, subjects were then classified into three categories: ASD; non-typical development (but not ASD; non-TD); and typically developing (TD) (Ozonoff et al., 2014).
Covariates
Covariates included in analyses were selected based on a priori knowledge, as well as statistical relevance (≥10% change in Beta estimate from model with vs without the covariate). Our primary models were adjusted for maternal age, race/ethnicity (non-Hispanic white, Hispanic, and Other and missing race –as a proxy to address potential disparities that may relate to OS and ASD), maternal pre-pregnancy body mass index (BMI), child’s sex, and prenatal vitamin use in the first month of pregnancy. Data for all covariates, excluding sex, were collected from maternal interviews about the first 20 weeks of pregnancy. Additional covariates considered in sensitivity analyses included pregnancy complications (defined as any of gestational diabetes, pre-eclampsia, or medication use for hypertension in the current or previous pregnancy yes/no, collected based on maternal report during the 2nd trimester), smoking status (defined as any smoking or exposure to smoking during pregnancy yes/no), gestational age at DNA/plasma sampling (as a continuous variable), antioxidant intake (as a continuous variable based on dietary data from a dietary questionnaire), and use of folic acid supplementation in the first month of pregnancy (yes/no) (see Online Resource 3).
Statistical analyses
The distribution of each biomarker in our analytic sample was examined, and biomarkers that were skewed, including GSH, GSSG, and 3-nitrotyrosine, were log transformed prior to analyses examining associations with child outcomes. In primary analyses, we used quantile regression to examine associations between OS biomarker levels and ASD-related outcomes as captured by continuous outcomes, the SRS and MSEL scores. Briefly, quantile regression examines associations at quantiles, or percentiles, of continuous outcome scores, rather than as conditioned on the mean as in traditional linear regression. This method is particularly useful for analyses conducted on outcomes that are non-normally distributed or when the upper or lower outcome values are of particular interest for the research question (Koenker & Hallock, 2001; Patti et al., 2021). Crude and adjusted models are presented. In secondary analyses, we also examined associations between an interquartile range increase in biomarker levels and SRS scores (as conducted in prior work (Rommel et al., 2020), to assess associations with a more meaningful increase in biomarker levels), using quantile regression. As an additional secondary analysis, given our small number of ASD cases, we explored associations between the biomarkers of OS and the categorically-defined ASD outcomes (ASD diagnosis and non-TD, each compared to TD) using logistic regression. In these analyses, OS biomarkers were parameterized to represent an interquartile range increase in biomarker level. Analyses were conducted using SAS 9.4 (SAS Institute, Cary NC) as well as R Studio 4.0.3 with the Quantreg package for quantile regression analyses (Koenker, 2021).
Sensitivity analyses were conducted to assess the robustness of results obtained in primary analyses. First, we tested adjustment of quantile regression models for additional covariates as outlined above. Second, individuals with any indication of hemolysis in plasma samples were removed from the analytic sample. Third, analyses of SRS scores were also conducted using sex standardized T scores to provide clinical context.
Results
Characteristics of the analytic sample are described in Table 1 and compared to those of the full EARLI cohort. Among the 173 women eligible for inclusion in these analyses, the median age at pregnancy was 34 (interquartile range 31,37) and pre-pregnancy BMI was 25.8 (23.2, 30.8). The majority of participants had a college degree or higher and identified as non-Hispanic White, and about 40% had household incomes ≥ $100,000. Overall, characteristics of the analytic sample were comparable to those of the full EARLI study, with the exception of higher rates of missingness across several covariates in the total study population. The distribution of each biomarker for our study sample as well as the interquartile range (IQR) are provided in Online Resource 4. Distributions of biomarkers of antioxidant balance (GSH, GSSG) and nitrotyrosine were similar to those from available studies on pregnant women who already had an autistic child (Hollowood et al., 2018) as well as pregnant women in general (Moore et al., 2019).
Table 1.
Characteristics of the Study Population by inclusion in the Analytic Sample and Enrollment in EARLI
| Analytic sample (n = 173) | EARLI n = (273) | |
|---|---|---|
|
| ||
| Child Sex, n(%) | ||
| Male | 89 (51.5%) | 131 (48.0%) |
| Female | 84 (48.6%) | 116 (42.5%) |
| Unknown | -- | 26 (9.5%) |
|
| ||
| Pre-pregnancy BMI, median(IQR) | 25.8 (23.3, 30.9) | 25.7 (23.0, 31.0) |
|
| ||
| Maternal age, median(IQR) | 34 (31, 37) | 34 (31, 37) |
|
| ||
| Maternal education level, n(%) | ||
| High school or less | 21 (12.1%) | 32 (11.7%) |
| Some college | 51 (29.5%) | 72 (26.4%) |
| College degree | 52 (30.1%) | 72 (26.4%) |
| Graduate school or above | 48 (27.8%) | 70 (25.6%) |
| Unknown | 1 (0.6%) | 27 (9.9%) |
|
| ||
| Maternal race/ethnicity, n(%) | ||
| Non-Hispanic White | 94 (54.3%) | 137 (50.2%) |
| Hispanic | 31 (17.9%) | 42 (15.4%) |
| Other race | 48 (27.8%) | 75 (27.5%) |
| Unknown race | -- | 19 (7.0%) |
|
| ||
| Income, n(%) | ||
| Less than $50,000 | 43 (24.9%) | 66 (24.2%) |
| $50,000 to $99,999 | 62 (35.8%) | 88 (32.2%) |
| $100,000+ | 68 (39.3%) | 100 (36.6%) |
| Unknown | -- | 19 (7.0%) |
|
| ||
| Pregnancy complicationsa, n(%) | ||
| None | 147 (85.0%) | 232 (85.0%) |
| At least 1 | 26 (15.0%) | 41 (15.0%) |
|
| ||
| Smoking during pregnancy, n(%) | ||
| Smoker or household exposure | 10 (5.8%) | 10 (3.7%) |
| Non-smoker, no household exposure | 131 (75.7%) | 176 (64.5%) |
| Unknown | 32 (18.5%) | 87 (31.9%) |
|
| ||
| Prenatal vitamin during 1st month | ||
| Yes | 99 (57.2%) | 126 (46.2%) |
| No | 73 (42.2%) | 84 (30.8%) |
| Unknown | 1 (0.6%) | 63 (23.1%) |
|
| ||
| Folic acid supplementation during 1st month | ||
| Yes | 10 (5.8%) | 19 (7.0%) |
| No | 162 (93.6%) | 191 (70.0%) |
| Unknown | 1 (0.6%) | 63 (23.1%) |
|
| ||
| Alcohol consumptionc, median (IQR) | 0.01 (0.01 – 0.03) | 0.01 (0.01–0.03) |
|
| ||
| Total SRS raw score, mean (SD)c | 36.3 (29.0) | 37.7 (28.4) |
|
| ||
| MSEL-ELC score, mean (SD)d | 99.0 (20.6) | 98.1 (21.1) |
|
| ||
| ASD diagnosis, n (%) | ||
| Typically developing | 80 (46.2%) | 91 (33.3%) |
| Non-typically developing | 52 (30.1%) | 61 (22.3%) |
| ASD | 32 (18.5%) | 43 (15.8%) |
| Unknown | 9 (5.2%) | 78 (28.6%) |
Included women who reported pre-eclampsia, gestational diabetes or medication use for high blood pressure (indicative of hypertension) in either this or the prior pregnancy.
Average number of drinks per day in pregnancy weeks 1–20
24 participants in the analytic sample and 77 participants from the full sample (EARLI) did not have SRS scores
10 participants in the analytic sample and 62 participants from the full sample (EARLI) did not have MSEL-ELC scores
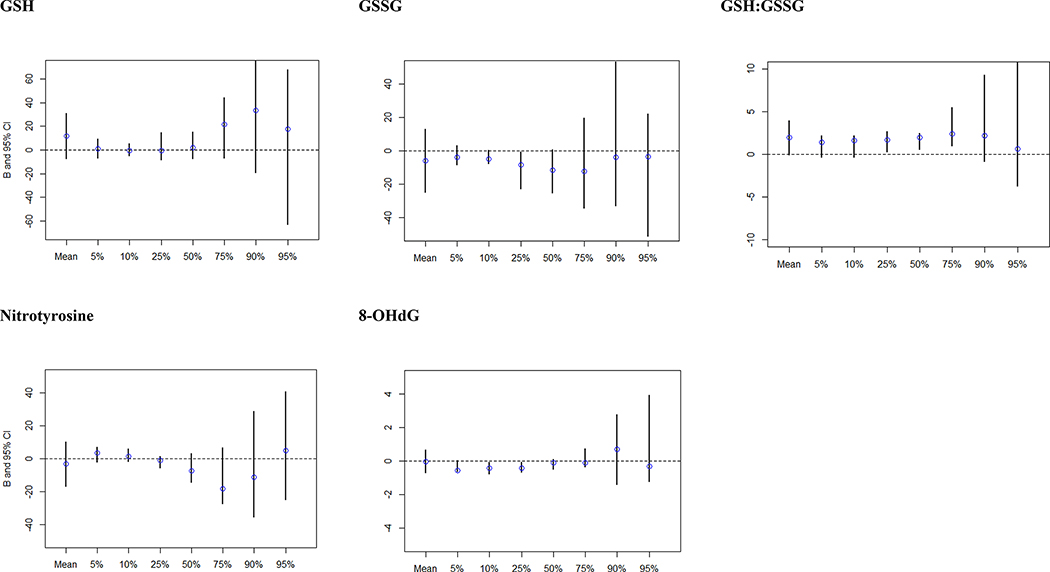
Sample size and total SRS raw scores at each percentile of interest for quantile regression analysis are shown in Online Resource 5. Results of crude quantile regression analyses suggested stronger associations for all biomarkers within the highest quantile of SRS scores (Online Resource 6). In adjusted models, this trend was not evident, and confidence intervals for upper quantiles were wide (Figure 1). However, there was indication of increases in SRS scores with increasing GSH:GSSG ratio (suggesting less OS/ increasing antioxidant balance) for those in mid-quantiles of SRS scores, as evidenced by elevations for the 25th-75th percentiles. This result was also observed in analyses examining an IQR increase in biomarker levels (IQR increase in GSH:GSSG β for SRS at the 50th percentile: 5.52, 95% CI: 0.98, 7.46; Online Resource 7). No associations were seen with other biomarkers.
Figure 1.
Adjusted Beta estimates and confidence intervals for quantile regression analysis examining the relationship between OS biomarkers and autistic traits measured by total SRS raw scores
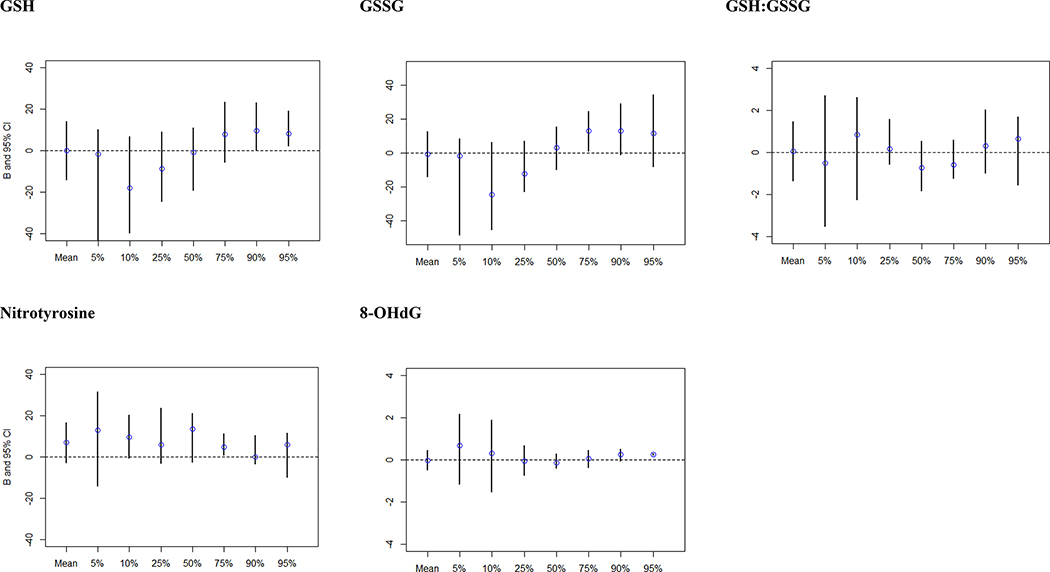
Sample size and MSEL-ELC scores at each percentile of interest for quantile regression analysis are shown in Online Resource 8. In analyses examining associations with MSEL-ELC scores, overall, no associations were observed. Crude analyses suggested somewhat stronger associations with the highest quantile of MSEL-ELC scores for GSH:GSSG, and with the lowest quantiles for 8-OHdG (Online Resource 9), though these were attenuated in adjusted models (Figure 2). A slight J-shaped relationship was observed for GSH and GSSG and MSEL-ELC scores, with an inverse relationship in the 5th and 10th percentiles of MSEL-ELC, and positive associations in the 75th-95th quantiles, but confidence intervals crossed the null. No significant associations were seen between IQR increases of the biomarkers and MSEL scores (Online Resource 10).
Figure 2.
Adjusted Beta estimates and confidence intervals for quantile regression analysis examining the relationship between OS biomarkers and cognitive function measured by MSEL-ELC scores
Secondary analyses
Analyses examining associations with categorical outcomes of ASD diagnosis are provided in Table 2. Models suggested a non-significant inverse association for antioxidant balance and ASD diagnosis with an IQR increase in the GSH:GSSG ratio (adjOR: 0.57, 95% CI: 0.30, 1.09), with a similar relationship suggested when non-TD and ASD were combined and compared to TD children (adjOR: 0.67, 95% CI: 0.43, 1.04). Similarly, increased GSSG levels, suggesting increasing OS, were positively associated with an ASD diagnosis (adjOR: 2.03, 95% CI: 0.89, 4.62) as well as in the models where children with ASD & non-TD diagnoses were combined (adjOR: 1.70, 95% CI: 0.99, 2.92). No strong relationships were observed between GSH, nitrotyrosine and 8-OHdG with either ASD and non-TD diagnosis or when we combined the ASD & non-TD outcomes.
Table 2.
Crude and adjusted logistic regression models examining association of an interquartile (IQR) increase in biomarker levels on probability of ASD and/or non-typically developing diagnosis
| ASD vs TD (N = 30, 74) | Non-TD vs TD (N = 49, 74) | ASD/Non-TD vs TD (N = 79, 74) | ||||
| Crude OR (95% CI) | Adjusted ORa (95% CI) | Crude OR (95% CI) | Adjusted ORa (95% CI) | Crude OR (95% CI) | Adjusted ORa (95% CI) | |
| GSH | 0.91 (0.44, 1.90) | 0.86 (0.40, 1.85) | 1.15 (0.61, 2.18) | 1.20 (0.62, 2.35) | 1.04 (0.61, 1.77) | 1.02 (0.58, 1.80) |
| GSSG | 2.27 (1.07, 4.81) | 2.03 (0.89, 4.62) | 1.67 (0.94, 2.98) | 1.55 (0.85, 2.83) | 1.82 (1.08, 3.06) | 1.70 (0.99, 2.92) |
| GSH:GSSG | 0.56 (0.32, 1.00) | 0.57 (0.30, 1.09) | 0.70 (0.43, 1.13) | 0.76 (0.46, 1.26) | 0.64 (0.43, 0.98) | 0.67 (0.43, 1.04) |
| Nitrotyrosine | 1.34 (0.67, 2.70) | 1.68 (0.53, 2.59) | 1.11 (0.60, 2.05) | 0.96 (0.50, 1.83) | 1.20 (0.70, 2.05) | 1.06 (0.60, 1.87) |
| ASD vs TD (N = 27, 60) | Non-TD vs TD (N = 40, 60) | ASD/Non-TD vs TD (N = 67, 60) | ||||
| 8-OHdG | 0.83 (0.40, 1.73) | 0.78 (0.35, 1.74) | 0.86 (0.47, 1.57) | 0.74 (0.38, 1.44) | 0.84 (0.48, 1.46) | 0.72 (0.39, 1.34) |
Adjusted for pre-pregnancy BMI, maternal age, maternal race/ethnicity, child sex, and prenatal vitamin use in the first month of pregnancy.
Sensitivity analyses
In sensitivity analyses examining adjustment for other covariates, further adjustment for smoking, pregnancy complications, gestational age at sampling, antioxidant nutrient intake based on dietary data, and total folic acid intake in the first month of pregnancy according to reported supplement use did not change conclusions (Online Resource 11). In analyses restricted to participants with no evidence of hemolysis in their plasma samples, results were also similar to those from primary analyses (Online Resource 12), however, the association between GSH:GSSG and SRS total raw scores was not limited to mid-quantiles and extended into the 90th and 95th percentiles. Finally, analyses using SRS T-scores did not yield differing interpretations of associations (Online Resource 13).
Discussion
Overall, results from this high familial risk cohort did not support a strong relationship between these biomarkers of OS measured in maternal samples from mid- to late-pregnancy and child ASD-related outcomes measured at 36 months. We did observe evidence of modest increases in ASD-related traits with increasing GSH:GSSG ratio, a marker of antioxidant balance, for those in the middle percentiles of SRS scores typically not consistent with clinical severity. There was also some evidence that greater antioxidant balance (higher GSH:GSSG ratio) was inversely associated with risk of ASD and non-typical development according to diagnostic assessments. Thus, results across our analyses suggest that differences in the association between oxidative balance and ASD-related outcomes may exist across the spectrum of severity and functioning, but further work is needed to address these associations.
The goal of this study was to examine whether gestational exposure to OS influences developmental outcomes in the offspring. We assessed behavioral outcomes in children, and did not measure OS levels in children, as may be useful for addressing potentially related questions such as whether OS in the mother influences OS in the child. Studies examining OS in children with an existing ASD diagnosis have suggested decreased OS balance, as measured by GSH:GSSG and other biomarkers, in children with ASD (James et al., 2004; James et al., 2006; Melnyk et al., 2012). However, these studies have not addressed the relationship between OS prior to ASD diagnosis, and its potential role in the development or etiology of ASD. While it is well established that maternal OS in gestation is associated with pregnancy complications (e.g., preeclampsia) and adverse pregnancy outcomes (e.g., low birth weight), and that these complications and outcomes are associated with ASD,(Jenabi, Karami, Khazaei, & Bashirian, 2019; Lyall et al., 2017; Lyall, Pauls, Spiegelman, Ascherio, & Santangelo, 2012; Toboła-Wróbel et al., 2020), studies directly addressing mediation by these factors and clarifying mechanisms are needed.
Few prospective studies have examined associations between biomarkers of OS, in particular GSH:GSSG, in association with ASD-related outcomes. Some work in animal models has reported an association between decreased maternal levels of GSH:GSSG, indicative of greater OS, and adverse neurodevelopment in offspring (Lanté et al., 2007). One study found that pregnant women from the general population had significantly higher GSH:GSSG during the second trimester of pregnancy compared to high risk women who already had an autistic child (Hollowood et al., 2018). However, results concerning antioxidant balance according to GSH:GSSG in our study were mixed – our findings suggested that the effect of OS on ASD-related traits may not be the same across the distribution of those traits, and that patterns of associations may differ in score ranges consistent with subclinical deficits. The direction of this association with subclinical ASD-related traits was not in accord with our hypothesis, in suggesting modest increases in ASD-related traits with increased oxidative balance. Findings for clinically assessed ASD diagnosis, though, suggested instead an inverse association, with reductions in ASD or non-TD with increased oxidative balance. Though we conducted several sensitivity analyses, we cannot rule out chance findings, unmeasured confounding, measurement error in traits assessed at this age, or mediating factors that may be involved in complex relationships not yet elucidated. It is also possible that the familial background risk could change the nature of associations observed here; therefore, future work should address the generalizability of these findings by examining these relationships in other study populations. Our study did not find significant associations between biomarkers of OS and MSEL-ELC scores. There was a subtle J-shaped relationship for GSH and GSSG and MSEL-ELC scores across MSEL-ELC quantiles, with a slight dip in the relationship at the 10th percentiles, and indications of positive associations in the 75th-95th quantiles, but confidence intervals crossed the null, and no clear pattern was observed with the ratio of these markers. We also found some evidence for reductions in risk of categorically-defined and clinically-determined non-TD, or non-TD combined with ASD, with increasing GSH:GSSG ratio. To our knowledge, no studies have examined prenatal levels of OS and IQ scores; however, a few studies have suggested OS as a predictor of cognitive decline. One study following older adults for four years found nearly 2-fold increased odds of cognitive decline (OR: 2.25; 95% CI 1.26–4.02) among those who were most antioxidant deficient (Berr et al., 2000). Further, a study examining the role of GSH and cognitive decline found that lower levels of GSH at baseline was associated with a longitudinal decline in the executive domain of cognition over four years (RR 1.70; 95% CI: 1.02–2.85) and longitudinal decline in GSH was associated with an even faster decline in executive functioning (Hajjar et al., 2018). Thus, research examining the role of OS in cognitive functioning in gestation, a period of critical neurodevelopment, is warranted.
Additional studies have examined other biomarkers related to OS during pregnancy and ASD. Work by Rommel and colleagues supported an association between OS and autistic traits as measured by SRS scores (Rommel et al., 2020). Specifically, they found a 2.58% (95% CI: 0.08, 5.16) increase in child SRS T-scores as measured at age 4–5 years with an IQR increase in levels of free 8-isoprostane-prostaglandin-F2α (8-iso-PGF2α) measured in third trimester urine samples in a general population prospective cohort. Isoprostanes are formed from free-radical induced peroxidation of essential fatty acids, and as such are markers of redox imbalance and lipid peroxidation specifically (Lawson, FitzGerald, & Rokach, 1999). Further, their work suggested potential effect modification by maternal education such that the positive, significant association between isoprostane levels and child SRS scores was only present in more educated mothers. We did not have the ability to examine effect modification here due to sample size considerations. It is possible that among those with higher familial risk, the effects of environmental factors that lead to increased OS may be dwarfed.
Biomarkers examined here were selected given involvement in OS pathways of potential relevance to neurodevelopment; 8-OHdG, as an indicator of oxidative damage to DNA; nitrotyrosine, as an indicative of oxidative damage to proteins; and GSH and GSSG as measures of global oxidative stress. As noted, the most suggestive findings observed in our work were with GSH:GSSG. A high ratio of the antioxidant GSH to its oxidized counterpart GSSG is important for cellular protection from OS (Birben et al., 2012). GSH is a product of the transsulfuration pathway, which converts homocysteine into the amino acid cysteine, a building block for GSH. Disruption of this pathway, and subsequently, GSH metabolism, has been linked to a lack of redox homeostasis and neurodegenerative diseases such as Huntington’s disease (Sbodio, Snyder, & Paul, 2019). The transsulfuration pathway is also linked to the transmethylation pathway through homocysteine, which can either be remethylated to generate methionine or converted to cysteine, which is necessary for creating GSH. Alterations to the methylation pathway through the folate-methionine cycle has also been suggested as a potential etiologic pathway for ASD; the link between maternal folic acid supplementation and ASD is supported by multiple studies (DeVilbiss, Gardner, Newschaffer, & Lee, 2015; Goodrich et al., 2018; Neggers, 2014; Schmidt et al., 2011).While our results did not differ when adjusted for prenatal supplement use, or folic acid or antioxidant intake, nor did we find evidence for modification of results by folic acid intake in exploratory analyses, future work should examine this possibility given our small sample may have limited ability to conduct formal mediation analyses.
Strengths of this study include utilization of prospectively collected biomarkers, during a biologically relevant time period of potential susceptibility to maternal OS. We also had the ability to examine multiple ASD-related outcomes, and included consideration of quantitative traits, which enables assessment of associations across an underlying distribution relevant to the whole population and not just above a clinical threshold (Sagiv, Kalkbrenner, & Bellinger, 2015). However, several limitations should also be noted. First, while the biomarkers of OS assessed here are reported to represent OS levels during a key window of neurodevelopmental susceptibility, we only measured them at a single timepoint in maternal samples in mid to late pregnancy, and it is possible associations may differ for OS in early pregnancy. Additionally, while we captured OS according to several pathways (e.g., protein oxidation, DNA oxidation, and global oxidation), we did not measure all potential biomarkers of OS and it is possible other markers (such as isoprostanes), or OS in other matrices (such as in the placenta), may relate more strongly to child outcomes like ASD. Future analyses examining the association between isoprostanes and ASD-related outcomes in this study are planned. In addition, our study did not directly address by which of the potential mechanistic pathways gestational OS may influence neurodevelopment (e.g., via hypothesized direct effects via influences on fetal neurodevelopment, fetal OS, or via various indirect pathways). Given our small sample size, we did not have the ability to examine potential modification by other factors, such as maternal education, which has been suggested as a modifier in prior work (Rommel et al., 2020), or differences by those with and without pregnancy complications. Our study also lacked information on medically confirmed pregnancy complications, and was not able to address mediation or modification by these factors, though adjustment using available information did not alter conclusions. Future work may address these limitations, as well as determine generalizability of our findings given potential differences in this enriched likelihood of ASD population.
Conclusions
Overall, we did not observe strong associations between maternal markers of OS in the late second and third trimester and ASD-related traits as measured by the SRS, or cognitive scores as measured by the MSEL in this high familial risk cohort. Examination of OS at other time points, and in other study populations, is needed to further clarify findings here. In addition, suggestive evidence for reductions in odds of ASD and non-TD diagnosis with greater oxidative balance according to the ratio of GSH:GSSG should be further examined in future work.
Supplementary Material
Acknowledgements:
The authors would like to thank Marisa Patti and Dr. Loni Tabb for their guidance in statistical modeling.
Funding:
This research was supported by the Eunice Shriver Kennedy National Institute of Child Health and Human Development (NICHD) at the National Institutes of Health (NIH) (R21HD096356). The EARLI Study was funded by the National Institute of Environmental Health Sciences, the National Institute of Mental Health, the National Institute of Child Health and Human Development, and the National Institute of Neurologic Disease and Stroke (R01 ES016443; R24 ES030893), with additional funding from Autism Speaks (AS 5938).
Footnotes
Competing interests: The authors have no relevant financial or non-financial interests to disclose.
Ethics approval: This study was approved by the Drexel University Institutional Review Board (IRB). The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent: Informed consent was obtained from all individual participants included in the study.
References
- Atamer Y, Koçyigit Y, Yokus B, Atamer A, Erden AC (2005). Lipid peroxidation, antioxidant defense, status of trace metals and leptin levels in preeclampsia. European Journal of Obstetrics & Gynecology and Reproductive Biology, 119(1), 60–66. [DOI] [PubMed] [Google Scholar]
- Berr C, Balansard B, Arnaud J, Roussel AM, Alpérovitch A, & Group ES (2000). Cognitive decline is associated with systemic oxidative stress: the EVA study. Journal of the American Geriatrics Society, 48(10), 1285–1291. [DOI] [PubMed] [Google Scholar]
- Birben E, Sahiner UM, Sackesen C, Erzurum S, & Kalayci O (2012). Oxidative stress and antioxidant defense. World Allergy Organization Journal, 5(1), 9–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, & Lord C (2011). Convergent validity of the Mullen Scales of Early Learning and the differential ability scales in children with autism spectrum disorders. American journal on intellectual and developmental disabilities, 116(5), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, . . . Kogan MD (2011). Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics, 127(6), 1034–1042. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Davis SA, Todd RD, Schindler MK, Gross MM, Brophy SL, . . . Reich W (2003). Validation of a brief quantitative measure of autistic traits: comparison of the social responsiveness scale with the autism diagnostic interview-revised. Journal of autism and developmental disorders, 33(4), 427–433. [DOI] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). The social responsiveness scale (2nd ed.): Western Psychological Services. [Google Scholar]
- Dennery PA (2010). Oxidative stress in development: nature or nurture?. Free Radical Biology and Medicine, 49(7), 1147–1151. [DOI] [PubMed] [Google Scholar]
- DeVilbiss EA, Gardner RM, Newschaffer CJ, & Lee BK (2015). Maternal folate status as a risk factor for autism spectrum disorders: a review of existing evidence. British Journal of Nutrition, 114(5), 663–672. [DOI] [PubMed] [Google Scholar]
- Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, & Schmidt RJ (2018). Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Research, 11(1), 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajjar I, Hayek SS, Goldstein FC, Martin G, Jones DP, & Quyyumi A (2018). Oxidative stress predicts cognitive decline with aging in healthy adults: an observational study. Journal of Neuroinflammation, 15(1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, . . . Smith K (2011). Genetic heritability and shared environmental factors among twin pairs with autism. Archives of general psychiatry, 68(11), 1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollowood K, Melnyk S, Pavliv O, Evans T, Sides A, Schmidt RJ, . . . Kruger U (2018). Maternal metabolic profile predicts high or low risk of an autism pregnancy outcome. Research in autism spectrum disorders, 56, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howsmon DP, Kruger U, Melnyk S, James SJ, & Hahn J (2017). Classification and adaptive behavior prediction of children with autism spectrum disorder based upon multivariate data analysis of markers of oxidative stress and DNA methylation. PLoS computational biology, 13(3), e1005385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James SJ, Cutler P, Melnyk S, Jernigan S, Janak L, Gaylor DW, & Neubrander JA (2004). Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. The American journal of clinical nutrition, 80(6), 1611–1617. [DOI] [PubMed] [Google Scholar]
- James SJ, Melnyk S, Jernigan S, Cleves MA, Halsted CH, Wong DH, . . . Bradstreet JJ (2006). Metabolic endophenotype and related genotypes are associated with oxidative stress in children with autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 141(8), 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenabi E, Karami M, Khazaei S, & Bashirian S (2019). The association between preeclampsia and autism spectrum disorders among children: a meta-analysis. Korean journal of pediatrics, 62(4), 126–130. doi: 10.3345/kjp.2018.07010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DP (2002). [11] Redox potential of GSH/GSSG couple: assay and biological significance. Methods in enzymology, 348, 93–112. [DOI] [PubMed] [Google Scholar]
- Kim GH, Kim JE, Rhie SJ, & Yoon S (2015). The role of oxidative stress in neurodegenerative diseases. Experimental neurobiology, 24(4), 325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- quantreg: Quantile Regression. R package version 5.85. (2021). Koenker, R. [Mobile application software] [Google Scholar]
- Koenker R, & Hallock KF (2001). Quantile regression. Journal of economic perspectives, 15(4), 143–156. [Google Scholar]
- Lanté F, Meunier J, Guiramand J, Maurice T, Cavalier M, de Jesus Ferreira M-C, . . . Barbanel G (2007). Neurodevelopmental damage after prenatal infection: Role of oxidative stress in the fetal brain. Free Radical Biology and Medicine, 42(8), 1231–1245. doi: 10.1016/j.freeradbiomed.2007.01.027 [DOI] [PubMed] [Google Scholar]
- Lawson JA, FitzGerald GA, & Rokach J (1999). Isoprostanes: formation, analysis and use as indices of lipid peroxidation in vivo. Journal of Biological Chemistry, 274(35), 24441–24444. [DOI] [PubMed] [Google Scholar]
- London EB (2014). Categorical diagnosis: a fatal flaw for autism research? Trends in neurosciences, 37(12), 683–686. [DOI] [PubMed] [Google Scholar]
- López-Tinoco C, Roca M, García-Valero A, Murri M, Tinahones FJ, Segundo C, . . . Aguilar-Diosdado M (2013). Oxidative stress and antioxidant status in patients with late-onset gestational diabetes mellitus. Acta Diabetologica, 50(2), 201–208. doi: 10.1007/s00592-011-0264-2 [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Goode S, Heemsbergen J, Jordan H, Mawhood L, & Schopler E (1989). Austism diagnostic observation schedule: A standardized observation of communicative and social behavior. Journal of autism and developmental disorders, 19(2), 185–212. [DOI] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, . . . Volk H (2017). The changing epidemiology of autism spectrum disorders. Annual review of public health, 38, 81–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall K, Pauls DL, Spiegelman D, Ascherio A, & Santangelo SL (2012). Pregnancy complications and obstetric suboptimality in association with autism spectrum disorders in children of the Nurses’ Health Study II. Autism Research, 5(1), 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Shaw KA, Baio J, EdS, Washington A, Patrick M, . . . Dietz PM (2020). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. Morbidity and mortality weekly report. Surveillance summaries (Washington, D.C. : 2002), 69(4), 1–12. doi: 10.15585/mmwr.ss6904a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubasa T, Uchino T, Karashima S, Kondo Y, Maruyama K, Tanimura M, Endo F (2002). Oxidative stress in very low birth weight infants as measured by urinary 8-OHdG. Free Radical Biology and Medicine, 36(2), 189–193. [DOI] [PubMed] [Google Scholar]
- Melnyk S, Fuchs GJ, Schulz E, Lopez M, Kahler SG, Fussell JJ, . . . Seidel L (2012). Metabolic imbalance associated with methylation dysregulation and oxidative damage in children with autism. Journal of autism and developmental disorders, 42(3), 367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TA, Ahmad IM, Schmid KK, Berger AM, Ruiz RJ, Pickler RH, & Zimmerman MC (2019). Oxidative stress levels throughout pregnancy, at birth, and in the neonate. Biological research for nursing, 21(5), 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM (1995). Mullen scales of early learning manual: American Guidance Service. [Google Scholar]
- Neggers Y (2014). The Relationship between Folic Acid and Risk of Autism Spectrum Disorders. Healthcare (Basel, Switzerland), 2(4), 429–444. doi: 10.3390/healthcare2040429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newschaffer CJ, Croen LA, Fallin MD, Hertz-Picciotto I, Nguyen DV, Lee NL, . . . Shedd-Wise KM (2012). Infant siblings and the investigation of autism risk factors. Journal of Neurodevelopmental Disorders, 4(1), 7. doi: 10.1186/1866-1955-4-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozonoff S, Young GS, Belding A, Hill M, Hill A, Hutman T, . . . Schwichtenberg A (2014). The broader autism phenotype in infancy: when does it emerge? Journal of the American Academy of Child & Adolescent Psychiatry, 53(4), 398–407. e392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson MG, Centlow M, Rutardóttir S, Stenfors I, Larsson J, Hosseini-Maaf B,… Akerström B (2010). Increased levels of cell-free hemoglobin, oxidation markers, and the antioxidative heme scavenger alpha(1)-microglobulin in preeclampsia. Free Radical Biology and Medicine, 48(2), 284–291. [DOI] [PubMed] [Google Scholar]
- Patti MA, Newschaffer C, Eliot M, Hamra GB, Chen A, Croen LA, . . . Khoury JC (2021). Gestational exposure to phthalates and social responsiveness scores in children using quantile regression: The EARLI and HOME studies. International journal of environmental research and public health, 18(3), 1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postorino V, Fatta L, Sanges V, Giovagnoli G, De Peppo L, Vicari S, & Mazzone L (2016). Intellectual disability in autism spectrum disorder: investigation of prevalence in an Italian sample of children and adolescents. [DOI] [PubMed]
- Rice D, & Barone S Jr (2000). Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environmental health perspectives, 108(suppl 3), 511–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risch N, Hoffmann TJ, Anderson M, Croen LA, Grether JK, & Windham GC (2014). Familial recurrence of autism spectrum disorder: evaluating genetic and environmental contributions. American Journal of Psychiatry, 171(11), 1206–1213. [DOI] [PubMed] [Google Scholar]
- Rommel A-S, Milne GL, Barrett ES, Bush NR, Nguyen R, Sathyanarayana S, . . . Ferguson KK (2020). Associations between urinary biomarkers of oxidative stress in the third trimester of pregnancy and behavioral outcomes in the child at 4 years of age. Brain, Behavior, and Immunity, 90, 272–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose S, Melnyk S, Pavliv O, Bai S, Nick T, Frye R, & James S (2012). Evidence of oxidative damage and inflammation associated with low glutathione redox status in the autism brain. Translational psychiatry, 2(7), e134–e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Kalkbrenner AE, & Bellinger DC (2015). Of decrements and disorders: assessing impairments in neurodevelopment in prospective studies of environmental toxicant exposures. Environmental Health, 14(1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbodio JI, Snyder SH, & Paul BD (2019). Regulators of the transsulfuration pathway. British journal of pharmacology, 176(4), 583–593. doi: 10.1111/bph.14446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RJ, Hansen RL, Hartiala J, Allayee H, Schmidt LC, Tancredi DJ, . . . Hertz-Picciotto I (2011). Prenatal vitamins, one-carbon metabolism gene variants, and risk for autism. Epidemiology (Cambridge, Mass.), 22(4), 476–485. doi: 10.1097/EDE.0b013e31821d0e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Hertz-Picciotto I, & Pessah IN (2012). Tipping the balance of autism risk: potential mechanisms linking pesticides and autism. Environmental health perspectives, 120(7), 944–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaga I, Niedzielska E, Gawlik M, Moniczewski A, Krzek J, Przegaliński E, . . . Filip M (2015). Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism. Pharmacological Reports, 67(3), 569–580. [DOI] [PubMed] [Google Scholar]
- Svatikova A, Wolk R, Wang HH, Otto ME, Bybee KA, Singh RJ, & Somers VK (2004). Circulating free nitrotyrosine in obstructive sleep apnea. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 287(2), R284–R287. [DOI] [PubMed] [Google Scholar]
- Thompson LP, & Al-Hasan Y (2012). Impact of oxidative stress in fetal programming. Journal of pregnancy, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toboła-Wróbel K, Pietryga M, Dydowicz P, Napierała M, Brązert J, & Florek E (2020). Association of Oxidative Stress on Pregnancy. Oxidative Medicine and Cellular Longevity, 2020, 6398520. doi: 10.1155/2020/6398520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, & Fiotakis C (2009). 8-hydroxy-2′-deoxyguanosine (8-OHdG): a critical biomarker of oxidative stress and carcinogenesis. Journal of environmental science and health Part C, 27(2), 120–139. [DOI] [PubMed] [Google Scholar]
- Van Naarden Braun K, Christensen D, Doernberg N, Schieve L, Rice C, Wiggins L, . . . Yeargin-Allsopp M (2015). Trends in the prevalence of autism spectrum disorder, cerebral palsy, hearing loss, intellectual disability, and vision impairment, metropolitan Atlanta, 1991–2010. PloS one, 10(4), e0124120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PG, Bhatia S, Drake DM, & Miller-Pinsler L (2016). Fetal oxidative stress mechanisms of neurodevelopmental deficits and exacerbation by ethanol and methamphetamine. Birth Defects Research Part C: Embryo Today: Reviews, 108(2), 108–130. [DOI] [PubMed] [Google Scholar]
- Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJ, Perstin J, . . . Wong AW (2009). Oxidative stress in developmental origins of disease: teratogenesis, neurodevelopmental deficits, and cancer. Toxicological sciences, 108(1), 4–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.