Abstract
Maternal trauma has intergenerational implications, including worse birth outcomes, altered brain morphology, and poorer mental health. Research investigating intergenerational effects of maternal trauma on infant stress reactivity and regulation is limited. Maternal mental health during pregnancy may be a contributor: psychopathology is a sequela of trauma exposure and predictor of altered self-regulatory capacity in offspring of affected mothers. We assessed associations among maternal lifetime trauma and infant stress responsivity, mediated by psychological symptoms in pregnancy. Mothers reported lifetime trauma history and anxiety, depressive, and posttraumatic stress symptoms during pregnancy. At infant age 6 months, stress reactivity and regulation were assessed via maternal behavior ratings (IBQ-R) and behavioral (negative mood) and physiological (RSA) markers during a laboratory stressor (Still-Face Paradigm). Maternal trauma was directly associated with lower infant physiological regulation, and indirectly associated with lower levels of both infant behavioral and physiological regulation via higher maternal anxiety during pregnancy. Maternal trauma was also indirectly associated with higher infant reactivity via higher maternal anxiety during pregnancy. Post hoc analyses indicated differential contributions of maternal prenatal versus postnatal anxiety to infant outcomes. Findings highlight potential contributory mechanisms toward maladaptive child stress response, which has been associated with poor behavioral, cognitive, and academic outcomes.
Keywords: Intergenerational trauma, anxiety, pregnancy, regulation, infant
Self-regulation—the ability to manage emotions, behavior, and cognitive processes—is considered a fundamental component of early childhood development. Regulatory capabilities influence a range of outcomes across the life course, including physical and mental health, social-emotional competence, academic achievement, and employment (Wagner et al., 2020). Further, self-regulation skills are critical to ongoing learning behaviors, achievement, adjustment, and well-being. Poor self-regulation is characterized by the inability to harness cognitive, emotional, and motivational resources to achieve goals and is hypothesized to contribute to unhealthy behaviors across the lifespan (Miller et al., 2018). In children, poorer self-regulation is associated with increased risk for ADHD, conduct disorder, and anxiety disorders (Woodward, Lu, Morris, & Healey, 2017). Given the importance of self-regulatory skills for health and wellbeing, there have been increased efforts to identify early markers of and predictors for poor self-regulation. Evidence suggests that maternal experiences, including adversity and psychopathology, are contributing influences.
Infant stress reactivity and regulation
Over the first year of life, infants develop both reactive and regulatory mechanisms related to stress, which are shaped by the social norms and environment they experience in interaction with their temperament characteristics (Posner & Rothbart, 2000). These systems are complex and can be observed in physiological, attentional, emotional, behavioral, cognitive, interpersonal, and social processes (Calkins & Fox, 2002). Infant reactivity can be understood as the ways in which an infant responds emotionally, physiologically, and attentionally to changes in their internal and external environment; more specifically, negative emotionality is conceptualized as the tendency to react to stimuli with negative affect (e.g., fussing, crying, frustration), whereas positive emotionality refers to responses such as positive affect, activity level, approach behaviors, and vocal activity (Rothbart & Bates, 2006). Infant regulation refers to the physiological, behavioral, and psychological ways in which an infant attempts to regulate these responses, through both internal and external (e.g., parental or dyadic) means (Geeraerts, Backer, & Stifter, 2020). Developing an adaptively reactive and regulated stress system is imperative for exhibiting appropriate behaviors and functioning (Calkins & Fox, 2002; Posner & Rothbart, 2000), with data suggesting that early differences in regulation of attention (Colombo & Mitchell, 2009; Rose, Feldman, & Jankowski, 2002; Rose, Feldman, & Jankowski, 2012; Wass, Scerif, & Johnson, 2012) and emotionality (Nigg, 2017) impact a range of cognitive and psychological outcomes. While children gradually develop internalized self-regulatory mechanisms throughout childhood and adolescence, parent-infant dyadic regulation processes are critical predecessors of self-regulatory capacities (Lobo & Lunkenheimer, 2020; Lunkenheimer, Kemp, Lucas-Thompson, Cole, & Albrecht, 2017).
Behavioral and psychological regulation rely heavily on maturation of biophysiological regulatory systems for stress reactivity, with significant involvement of the autonomic nervous system (ANS) (Gardner et al., 2006; Geva, Zivan, Warsha, & Olchik, 2013). Development of the ANS and other physiological stress response systems (e.g., hypothalamic-pituitary-adrenal [HPA] axis) in early life is affected by both environmental (Alexander et al., 2009) and endophenotypic (Boyce & Ellis, 2005) influences. As bioindicators of stress reactivity and regulation, various metrics of ANS functioning have been studied for the past several decades, both as predictors of later outcomes and as concurrent biomarkers of behavioral expression (Kreibig, 2010; Levenson, 2014). Thus, identifying specific early exposures that shape ANS functioning may expand our understanding of risk processes involved in the development of maladaptive infant stress reactivity and regulation systems.
The Still-Face Paradigm (SFP) (Weinberg & Tronick, 1996) is a well-established protocol for assessing infant behavioral and physiological stress responses. In this task, the mother is asked to interact normally with her infant, interspersed with set periods of withholding interaction (the “still-face” episodes), which serves as a stressor for the infant. During the SFP, infants reliably demonstrate both behavioral and physiological stress responses (Bosquet Enlow et al., 2014; Conradt & Ablow, 2010; Moore et al., 2009; Ritz, Schulz, Rosenfield, Wright, & Bosquet Enlow, 2020). Affective and behavioral responses during the still-face episodes typically include increases in negative affect (frustration, anger, sadness, fear, general distress), decreases in positive affect, and increases in behaviors that indicate attempts at self-soothing or regulation of distress (Mesman, IJezdoorn, & Bakermans-Kranenburg, 2009). Standardized behavioral coding systems have been developed to objectively assess infant affective and behavioral responses during the SFP. Respiratory sinus arrhythmia (RSA), a specific index of the ANS system, is also commonly assessed during the SFP. RSA is a measure of the beat-to-beat variation in heart rate, in synchrony with respiration, and is interpreted as an indicator of cardiac parasympathetic activity, a commonly used measure of stress management (Grossman & Taylor, 2007). The expected response to a physical or psychological stressor, including in infants, is inhibition of the parasympathetic nervous system (PNS), as well as activation of the sympathetic nervous system (SNS) (Ritz et al., 2020; Ziegler, 2012). Inhibition of the PNS allows for an increase in heart rate and blood flow throughout the body to support a stress response (Contrada & Baum, 2010; Ziegler, 2012). In general, inhibition of the PNS in response to stressors or emotional triggers is associated with more optimal development and behavior (Blair & Peters, 2003; Marcovitch et al., 2010; Scrimin et al., 2019). In contrast, overactivation of the PNS has been associated with poorer child developmental outcomes (Beauchaine, 2015; Ginty, Phillips, Der, Deary, & Carroll, 2011; Marcovitch et al., 2010; Scrimin et al., 2019); some evidence suggests that underactivation of the PNS may be linked with less optimal outcomes as well (Beauchaine, 2015).
As a complement to laboratory-based methods of assessing infant stress functioning in response to a structured task, parent rating scales have been used to assess infants’ characteristic behaviors across a variety of settings. A commonly used measure of these behaviors is the Infant Behavior Questionnaire-Revised (IBQ-R) (Gartstein & Rothbart, 2003), a parent rating scale of infant temperament centered around behavioral indicators of reactivity and regulation. Despite the wealth of literature assessing markers of infant reactivity to and regulation of stress, only a few studies offer examination of parent ratings of infant stress reactivity and regulation in conjunction with the SFP. Braungart-Rieker et al. (1998) found associations between infant negative temperament as measured via the IBQ-R and lower levels of infant self-comforting behaviors during the SFP at age 4 months. In contrast, Tarabulsy et al. (2003) did not find links between maternal ratings of infant negative temperament and infant affect or self-soothing behaviors during the SFP. More recent evidence suggests that markers of positive temperament from the IBQ-R may be associated with the use of self-soothing strategies during the SFP (Planalp & Braungart-Rieker, 2015). These studies provide evidence that parent ratings may correspond modestly with observed behaviors during the SFP but also may diverge, with each method potentially offering different information regarding infant regulatory development. Moreover, there remains a significant gap in the literature, with virtually no research investigating the association between temperament-related reactivity and regulatory behaviors in everyday life and behavioral and physiological markers of the stress response to an acute stressor in infants. Incorporating both behavioral and physiological measures of stress functioning in infants is a key component to better understanding these processes, as both physiological and behavioral functioning during infancy have implications for later mental health outcomes (e.g., Feldman, 2015).
Maternal trauma and child regulation
Studies indicate that infants and children of mothers with a history of exposure to trauma or adversity are at increased vulnerability for a range of stress management and regulation difficulties, including behavioral dysregulation (Chemtob et al., 2010; Pat-Horenczyk et al., 2020), poor social-emotional functioning (McDonnell & Valentino, 2016), and alterations in stress physiology (Bosquet Enlow et al., 2009; Brand et al., 2010). Notably, these associations are observed even when the children have not experienced maltreatment or trauma themselves. Various strands of research implicate several potential mechanisms for these intergenerational effects. For example, a large body of research suggests that parenting styles and behaviors can differ between families with and without a maternal history of trauma or adversity (Banyard, Williams, & Siegel, 2003; Cohen, Hien, & Batchelder, 2008; MacMillan, Lewis, Watson, Jansen, & Galbally, 2020) and that more negative parenting behaviors may mediate associations between maternal trauma history and child outcomes, including self-regulation behaviors (Bailey, Deoliveira, Wolfe, Evans, & Hartwick, 2012; Collishaw et al., 2007; Delker, Noll, Kim, & Fisher, 2014).
More recently, studies have begun to accumulate that implicate a role for processes in the prenatal period. For example, a prospective study demonstrated that maternal exposure to childhood maltreatment is associated with reduced neonatal cortical gray matter volume (Moog et al., 2018), suggesting that maternal traumatic events experienced prior to pregnancy confer intergenerational effects during pregnancy and do not operate solely via differences in the postnatal environment or parenting practices. Studies have specifically linked prenatal stress exposure to accelerated ANS development during gestation (DiPietro et al., 2010; Bosquet Enlow et al., 2009). Infant RSA has been shown to be sensitive to maternal adversity, trauma, and stress experienced during, as well as prior to, pregnancy (DiPietro, Novak, Costigan, Atella, & Reusing, 2006; Gray, Jones, Theall, Glackin, & Drury, 2017). Further, individuals who experience traumatic events, particularly early in life, have been found to be at increased risk for exposure to additional stressful or negative events in later life, potentially creating cyclical patterns whereby repeated stressors or trauma increase risk for further exposures, including during pregnancy (Bandoli et al., 2017; McLaughlin, Conron, Koenen, & Gilman, 2010).
Maternal psychological symptoms during pregnancy and infant reactivity/regulation
One specific prenatal mechanism through which maternal trauma history may negatively influence offspring development is maternal psychological symptoms. Psychological difficulties are well-established sequalae of traumatic experiences both in the general population (Brown et al., 2014; McLaughlin et al., 2019) and in pregnant women (Meltzer-Brody et al., 2013; Robertson-Blackmore et al., 2013). Importantly, data suggest that women are at increased risk for developing or experiencing increased severity of psychological symptoms in pregnancy, and women with a history of trauma may be particularly vulnerable to such mental health challenges (Flynn, Blow, & Marcus, 2006; Marcus, Flynn, Blow, & Barry, 2003; Meltzer-Brody et al., 2013; Powers et al., 2020). Moreover, maternal psychological symptoms in pregnancy appear to influence fetal neurobehavioral maturation (Monk et al., 2000), and there is evidence that children of mothers with a history of psychological symptoms in the pre- and perinatal phases are at increased risk for difficulties with self-regulation later in life (Choe, Olson, & Sameroff, 2013; Davis et al., 2007; Davis et al., 2004; Gustafsson et al., 2018; McGrath, Records, & Rice, 2008). Although much of this literature has focused on the effects of perinatal mood and anxiety disorders, the presence of other psychopathology in this period, including posttraumatic stress disorder (PTSD) symptoms, has also been associated with increased infant behavioral regulation difficulties (e.g., Bosquet Enlow et al., 2011; Bosquet Enlow et al., 2009).
Sex differences
Research in the field of developmental origins of health and prenatal stress has increasingly suggested that there may be differential effects of maternal stress and psychopathology depending on fetal or infant sex assignment. Prior literature has suggested that female offspring are more likely than males to show anxiety, depressive, or stress behavioral responses following prenatal stress or maternal psychopathology exposures, whereas males are more likely to demonstrate exposure effects on cognitive abilities (e.g., learning, memory) (Glover & Hill, 2012; Van den Bergh et al., 2020). Infant sex is also relevant for understanding the development of physiological stress systems. Tibu et al. (2014) demonstrated that associations between prenatal anxiety exposure and infant stress reactivity during the SFP vary by sex, with girls showing increased reactivity and boys showing decreased reactivity. That said, there is limited evidence demonstrating consistent differences by sex in baseline ANS/RSA functioning or in response to specific tasks such as the SFP (Jones-Mason, Alkon, Coccia, & Bush, 2018), necessitating further exploration of these patterns by sex assigned at birth.
Current Study
Although there is literature supporting associations between maternal adversity/trauma and impaired child self-regulation, limited research has examined the intergenerational effects of maternal history of trauma/adversity on the development of infant stress reactivity and regulation. The goal of the present study was to assess associations among maternal lifetime trauma history, psychological symptoms in pregnancy, and behavioral and physiological markers of infant stress reactivity and regulation.
Based on extant research and theory, we first hypothesized that greater maternal lifetime trauma exposure is associated with increased infant stress reactivity and poorer infant stress regulation. We used a comprehensive approach to characterizing infant stress reactivity and regulation, including observed physiological (RSA) and behavioral (negative mood) responses during an acute laboratory stressor (i.e., the Still-Face Paradigm), as well as maternal ratings of infant behavioral and affective reactivity and regulation (IBQ-R) in daily life. Second, we hypothesized that maternal lifetime trauma history is associated with increased maternal psychological symptoms (anxiety, depression, PTSD) in pregnancy. Finally, we hypothesized that maternal psychological symptoms in pregnancy mediate associations between maternal lifetime trauma exposures and infant behavioral and physiological stress reactivity and regulation. Analyses included tests for differential effects by infant sex assigned at birth. Given that prior exposure to adversity increases likelihood of later exposure, we considered the role of negative life events during pregnancy in analyses. We further considered the contributions of postnatal maternal psychological symptoms in post hoc analyses to assess for specificity of any observed prenatal effects.
Methods
Participants
Participants were mother-infant pairs, drawn from the ongoing PRISM study, a prospective pregnancy cohort designed to assess the role of maternal and child stress on child development. Pregnant women were recruited from prenatal clinics in New York, NY and Boston, MA. Eligibility criteria included: 1) English- or Spanish-speaking; 2) age ≥18 years at enrollment; 3) single gestation birth. Exclusion criteria included 1) maternal endorsement of drinking ≥ 7 alcoholic drinks/week prior to pregnancy recognition or any alcohol following pregnancy recognition; 2) HIV positive status, which would influence/confound biomarkers of interest; 3) pregnancy loss, or 4) the presence of a major congenital or genetic disorder identified during pregnancy or at birth. Based on screening data, there were no differences in race/ethnicity, education, or income between women who enrolled in PRISM and those who declined. The current analyses include 1,077 mother-infant dyads who provided data from the pregnancy assessment, 677 of whom also completed infant behavior rating assessments. Mothers and infants were also invited to attend a laboratory visit where they participated in an acute stressor task, which resulted in usable infant behavioral data for n=412 and physiology data for n=248 at the time of the current analyses. Reasons for lack of available behavioral and/or RSA data varied, including families unwilling or unable to participate in the postnatal assessment and/or laboratory visit (e.g., COVID restrictions); infant refusal to wear the equipment necessary for collecting RSA data; infant unable to complete at least the first still-face test (i.e., first three episodes) due to fussiness, sleepiness, or maternal request to end the visit early; technological issues (e.g., physiology data too noisy to be considered sufficiently reliable and valid for inclusion in analyses); and data not fully processed at the time of manuscript preparation for inclusion. To minimize sampling bias, all cohort participants with any available data were included in analyses.
Procedures
Maternal sociodemographics were assessed shortly following recruitment in mid-pregnancy (23.8 ± 9.1 weeks gestational age). Within two weeks of enrollment, trained research assistants administered interviews inquiring about maternal lifetime stress and trauma exposures; current anxiety, depression, and PTSD symptoms; and exposure to negative life events during pregnancy. When the infants were 6 months of age, mothers completed a parent rating assessment of infant temperament and reports of current anxiety, depression, and PTSD symptoms during a home visit. Approximately one week later, mother-infant dyads were invited to complete standardized laboratory protocols, including the Repeated Still-Face Paradigm, during which behavioral and physiological data were gathered. Study procedures were approved by the relevant institutions’ human studies ethics committees. Mothers provided informed consent in their preferred language prior to initiation of study activities.
Measures
Maternal lifetime stress and trauma exposures.
Maternal exposure to potentially stressful and traumatic events was measured using the Life Stressor Checklist-Revised (LSC-R) (Wolfe, Kimerling, Brown, Chrestman, & Levin, 1997). The LSC-R assesses exposure to 30 events (e.g., experiencing a serious accident, natural disaster), including experiences particularly relevant to women (e.g., sexual assault, interpersonal violence). The LSC-R has established test-retest reliability and validity in diverse populations (McHugo et al., 2005; Wolfe et al., 1997). During pregnancy, mothers were asked to report on their lifetime exposure to each event up until the point of the interview. A continuous exposure score was derived by summing the number of endorsed events, with higher scores indicating exposure to more events (possible range 0–30).
Maternal psychological symptoms during pregnancy.
Maternal anxiety, depressive, and posttraumatic stress symptoms were assessed during pregnancy and again at the 6-month infant assessment.
Anxiety.
Maternal anxiety symptoms were measured using the Trait Scale of the Spielberger State-Trait Anxiety Inventory (STAI; Spielberger, Gorsuch, & Lushene, 1970). The STAI Trait Scale is a 10-item measure of the respondent’s general feelings of worry and anxiety. Each item was scored for severity from 1 to 4 and then summed to provide a total score (possible range 10–40). The STAI has demonstrated high internal consistency (α=0.92), stability, and validity in the peripartum period (e.g., Grant, McMahon, & Austin, 2008; Adhikari et al., 2021). The Trait Scale was chosen as an estimate of the respondent’s general dispositional anxiety around the time of assessment (e.g., during pregnancy for the prenatal assessment).
Depression.
Maternal depressive symptoms were measured using the Edinburgh Postnatal Depression Scale (EPDS; Cox, Holden, & Sagovsky, 1987). The EPDS is a 10-item self-report questionnaire designed to measure the presence of depressive symptoms in the past seven days in women during the perinatal period. Each EPDS item was scored for severity from 0 to 3 and then summed to provide a total score (possible range 0–30). The EPDS has demonstrated high internal consistency (α=0.87) and validity for detecting major depression in the perinatal period (Cox et al., 1987; Gibson, McKenzie‐McHarg, Shakespeare, Price, & Gray, 2009).
PTSD.
Maternal PTSD symptoms were assessed using the PTSD Checklist for DSM-IV, Civilian version (PCL-C; Weathers, Litz, Herman, Huska, & Keane, 1993). The PCL-C is a standardized self-report rating scale for PTSD comprising 17 items that correspond to the key symptoms of PTSD. The participant was asked to rate on a scale from 1–5 how much she was bothered by each symptom in the prior month. The item scores were summed to create a total symptom severity score (possible range 17–85). The PCL-C has high internal consistency for the total symptom severity scale and good test-retest reliability and convergent validity with a number of other PTSD scales and with the Clinician-Administered PTSD Scale, a structured clinical interview for PTSD (Weathers, Keane, & Davidson, 2001); it has also been found to have good consistency in pregnant populations (α=0.90) (Gelaye, Zheng, Medina-Mora, Rondon, Sánchez, & Williams, 2017).
Infant stress reactivity and regulation.
We assessed several dimensions of infant reactivity and regulation using three different types of measurement: maternal-completed inventories of infant negative emotionality and regulating behaviors in everyday life; observation ratings of infant negative mood in response to an acute stressor task in a laboratory setting, coded by independent raters; and physiological responses to an acute stressor task in a laboratory setting. Specifically, infant stress reactivity was assessed through 1) maternal ratings of negative emotionality indexed by the Negative Affectivity factor score of the IBQ-R; 2) observed levels of negative mood during the SFP-R stressor episodes; and 3) changes in RSA from pre-task baseline or preceding recovery episodes in response to SFP-R stressor episodes. Infant stress regulation was assessed through 1) maternal ratings of infant and dyadic regulation, indexed by the Orienting/Regulation factor score of the IBQ-R; 2) observed levels of negative mood during the SFP-R recovery episodes; and 3) changes in RSA from SFP-R stressor episodes to the subsequent SFP-R recovery episodes.
Infant behavioral stress reactivity and regulation in daily life (maternal report).
Two factor scores from the Infant Behavior Questionnaire-Revised (IBQ-R; Gartstein & Rothbart, 2003) were used as indicators of infant stress reactivity and regulation. The 191-item IBQ-R is a reliable and validated measure of infant temperament, rationally derived based on a definition of temperament as constitutionally based individual differences in reactivity and self-regulation (Gartstein & Rothbart, 2003; Parade & Leerkes, 2008). The IBQ-R has demonstrated good internal consistency (Parade & Leerkes, 2008) and has been found to provide complementary information to laboratory observations regarding infant reactivity and regulation (Forman et al., 2003). Mothers rated the frequency that their infant engaged in specific day-to-day behaviors in the prior week using a 7-point scale, with responses ranging from 1 (never) to 7 (always). Scores were summed across items according to IBQ-R scoring criteria to create 14 scales assessing a variety of behavioral domains. These 14 scales load onto three factors: labeled by the developers as (1) Negative Affectivity, consisting of the scales Sadness, Distress to Limitations, Fear, and Falling Reactivity/Rate of Recovery from Distress (reverse-scored); (2) Orienting/Regulation, consisting of the scales Low Intensity Pleasure, Cuddliness, Duration of Orienting, and Soothability; and (3) Surgency/Extraversion, consisting of the scales Approach, Vocal Reactivity, High Intensity Pleasure, Smiling and Laughter, Activity Level, and Perceptual Sensitivity (Gartstein & Rothbart, 2003). Internal consistency ratings of the factors were high, with Cronbach’s alpha values ranging from 0.91 to 0.92 (Gartstein & Rothbart, 2003). The current analyses used the Negative Affectivity and Orienting/Regulation factors as indicators of infant stress reactivity and regulation, respectively. The Negative Affectivity factor reflects stress reactivity, in that it estimates levels of negative emotions (intensity, frequency), as well as how the child responds to limitations (physical or otherwise) and their tendencies to remain distressed following an incident; higher Negative Affectivity score reflect more negative emotionality. The Orienting/Regulation factor estimates how much the child is able to respond to soothing and physical closeness with a parent, demonstrate pleasure or contentedness without becoming overly aroused or excited, and orient to a stimulus, all of which reflect regulatory capacity; higher Orienting/Regulation scores reflect greater orienting and/or regulation.
Infant negative mood and behavior during acute stressor (observed).
Independent trained coders scored maternal, infant, and dyadic behaviors during the SFP-R using a modified version (Bosquet Enlow, Carter, Hails, King, & Cabrera, 2014) of the Parent-Child Interaction Rating Scales (PCIRS; Sosinsky, Marakovitz, & Carter, 2004), a detailed scoring scheme created for the Connecticut Early Development Project. PCIRS scales were adapted from several well-validated behavioral coding schemes, including the NICHD Study of Early Child Care’s 24-month and 36-month Mother-Child Interaction Rating Scales (MCIRS) for the Three-Boxes Procedure (NICHD Early Child Care Research Network, 1999); the Caregiver-Child Affect, Responsiveness, and Engagement Scales (C-Cares; Tamis-LeMonda et al., 2002); the Parent-Child Early Relational Assessment (PCERA; Clark, 1999); and the Emotional Availability Scales (Biringen, Robinson, & Emde, 1994). The PCIRS was developed for use in a mother-toddler sample and adapted in consultation with the PCIRS developers for use with mother-infant dyads during the SFP-R.
For this study, the PCIRS Infant Negative Mood scale was used as an index of stress reactivity and regulation during the SFP-R. The Infant Negative Mood scale assesses the extent to which (i.e., frequency and intensity) the infant cried, whimpered, fussed, frowned, screamed, tensed body while crying, or otherwise expressed discontentment, anger, or hostility and/or displayed signs of anxiety, fear, wariness, and/or hypervigilance. For the current analyses, Negative Mood levels during the still-face episodes were used to index stress reactivity, with higher levels indicating greater negative emotional reactivity to the stress of the still-face episode; Negative Mood levels during the Recovery episodes were used to index stress regulation, with lower levels indicating better regulation following cessation of the preceding stressor still-face episode.
Infant physiological and behavioral stress reactivity and regulation in response to an acute stressor (laboratory observation).
Repeated Still-Face Paradigm (SFP-R).
Mother-infant dyads participated in the Repeated Still-Face Paradigm (SFP-R) (Haley & Stansbury, 2003; Tronick, Als, Adamson, Wise, & Brazelton, 1978). The original SFP is a videorecorded observational procedure that has been well-validated in sociodemographically diverse samples to assess infant affective, behavioral, and physiological reactivity to brief, moderate levels of stress (Adamson & Frick, 2003; Mesman et al., 2009). The standard SFP involves three 2-minute episodes during which the infant is seated in an infant seat across from the mother. The mother is instructed to play with her infant for 2 minutes (“play,” or baseline; P) such that they are in a neutral, content state at the time of the stressor onset. The play episode is followed by a still-face episode, during which the mother is instructed to maintain a neutral facial expression and avoid touching or vocalizing at the infant (“still-face,” or stressor; SF1). The still-face episode is hypothesized to be a stressor for the infant because the mother is no longer providing behavioral cues needed for the infant to maintain an organized social and affective state (Weinberg & Tronick, 1996). The mother is then instructed to resume playing with the infant during a reunion episode (“reunion,” or recovery; R1), which provides an opportunity for the infant to recover, with the mother’s assistance, from stress elicited by the still-face episode. The repeated version (SFP-R) includes a second still-face episode (still-face 2; SF2) and reunion episode (reunion 2; R2), administered immediately after cessation of the first reunion episode, to introduce a more sustained level of challenge. The still-face episodes were curtailed if the infant displayed one minute of continuous fussing or 30 seconds of continuous crying to prevent the infant from becoming excessively distressed. The SF2 and R2 episodes were only administered if the infant had returned to a non-distressed state by the end of R1. Previous research has demonstrated that the SFP-R effectively activates the infant ANS (Bosquet Enlow et al., 2014; Ritz et al., 2012; Ritz et al., 2020).
Infant RSA.
Before beginning the SFP-R, the infant was fitted with an ambulatory respiratory inductance plethysmography device (BioRadio, Great Lakes NeuroTechnologies), which continuously measured respiration and cardiac activity throughout the procedure via two inductance bands and a 3-lead ECG (Ritz et al., 2002). ECG recordings were visually inspected for ectopic beats and sequences with excessive movement artifacts that visibly masked the R-wave and thus led to its misspecification or omission. Video recordings of the infant were used simultaneously when necessary (e.g., to confirm excessive motion), and affected sequences were deleted using the VivoSense biosignal analysis software (Vivonoetics, Newport Coast, CA). Mean distances between adjacent R-waves were calculated for each episode. RSA was extracted to estimate cardiac vagal activity as an indicator of parasympathetic influences on the heart. Inductance band output was volume calibrated using the Qualitative Diagnostic Calibration procedure (Sackner et al., 1989). As previously described (Bosquet Enlow et al., 2014; Ritz et al., 2012; Ritz et al., 2020), for each SFP-R episode, we calculated the time-domain peak-valley index of RSA (Grossman, Karemaker, & Wieling, 1991) within each breathing cycle as the difference between the longest and shortest cardiac interbeat interval (IBI) using dedicated MATLAB-based software (rsaToolox v2.0.1) (Schulz, Ayala, Dahme, & Ritz, 2009). The software excludes breaths that are too short for at least two full IBIs and sets RSA to 0 for breaths that do not meet peak-valley criterion (which is IBIminimum preceding IBImaximum) (Grossman, 1992; Grossman & Taylor, 2007; Rother, Witte, Zwiener, Eiselt, & Fischer, 1989). High correlations (r ≥ .92) between the time-domain peak-valley index and frequency-domain high-frequency heart-rate variability (HRV) index have been reported (Grossman, Van Beek, & Wientjes, 1990). We averaged RSA across the breathing cycles within each episode, resulting in up to five RSA data points per infant (one for each episode). RSA amplitude is strongly associated with tidal volume (VT) and total respiratory cycle time (TTOT) in both adults and infants (Grossman & Taylor, 2007; Ritz et al., 2012). To adjust for these respiration-related variables, we calculated the transfer function of RSA per liter VT and performed a within infant regression of this index, after natural log transformation to normalize the distribution on TTOT. We then added each infant’s resulting residual value for a given episode to the grand mean across episodes to obtain respiration-adjusted natural-log transformed RSA values for each episode (P, SF1, R1, SF2, R2). Adjustment for respiration has been shown to improve the expected prediction of stress-related changes in vagal tone among infants (Ritz et al., 2012). Greater reductions in RSA in response to stressful stimuli indicate greater parasympathetic withdrawal. Thus, during SF1 and SF2, which were designed to elicit moderate infant stress in response to maternal disengagement, we expected reductions in RSA relative to P as well as to R1 and R2.
Changes in RSA during the SFP-R were calculated to reflect infant physiological stress reactivity and regulation; because RSA values at baseline are variable between individuals, it is the difference scores between episodes that indicate physiological responses to the different episode conditions (i.e., stressor or recovery), rather than the absolute RSA values in each episode. Specifically, infant reactivity to stress was indexed by calculating intraindividual differences between RSA values during the neutral baseline (P) episode and the first stressor task (SF1) and between the first recovery period (R1) and subsequent stressor task (SF2). Infant physiological stress regulation was indexed by calculating intraindividual differences between RSA values during the recovery periods and the preceding stressor period (R1-SF1, R2-SF2).
Infant activity level.
Independent trained coders scored infant activity level during the SFP-R in 10-second intervals from videos using a 4-point scale: 0=quiet motor (e.g., no movement other than slow moving of fingers), 1=slow/mild movements (e.g., slow bending but not lifting of limbs), 2=moderate movements (e.g., slow lifting of limbs), 3=pronounced movements (e.g., forceful lifting of limbs) (Bosquet Enlow et al., 2014); scale modified from Bazhenova, Plonskaia, & Porges, 2001. Interval-specific scores were averaged and multiplied by 100, resulting in a possible score range of 0 to 300. Inter-rater reliability was high (ICC=0.92). We a priori selected infant activity level during the SFP-R for adjustment in analyses involving RSA to minimize the influence of motor activity, which often accompanies distress, on RSA measures.
Covariates
We considered as covariates variables commonly associated with maternal and child outcomes, including maternal age, education, race/ethnicity, and smoking during pregnancy, and infant gestational age at birth, age at 6-month assessment, and sex assigned at birth. Maternal, gestational, and child ages were treated as continuous variables. Maternal education was scored as less than high school diploma, high school diploma/GED, some college/Associate’s degree, college degree, or graduate degree. Maternal race/ethnicity was scored as White, Black, Hispanic/Latina, and other racial group and was treated as a categorical variable. Child sex assigned at birth was a dichotomous variable (male/female). Maternal exposure to negative life events during pregnancy, assessed via the Crisis in Family Systems-Revised (CRISYS-R; (Shalowitz, Berry, Rasinski, & Dannhausen-Brun, 1998), was also included as a covariate in analyses. The 63-item CRISYS-R inquires about exposure to negative life events during the prior six months (Berry et al., 2001) and was used to assess exposures over the course of pregnancy. It is suitable for sociodemographically diverse populations, has good test/retest reliability, has been validated in English and Spanish in samples of parents, and has been utilized as a measure of prenatal stressor exposures (Bosquet Enlow et al., 2017). The items encompass 11 domains (financial, legal, career, stability in relationships, medical issues pertaining to self, medical issues pertaining to others, safety in the community, safety in the home, housing problems, difficulty with authority, discrimination), with multiple items assessing each domain. Research suggests increased vulnerability when exposed to negative events across multiple domains (Myers, 2009). Thus, the number of domains with one or more negative events endorsed was summed to create a negative life events domain score (possible range 0–11), as done in prior research (Bosquet Enlow et al., 2017; Cowell et al., 2015). For analyses including infant RSA scores, infant length and weight at the time of assessment and activity level during the SFP-R episodes also were included as covariates. Finally, given evidence that maternal psychological symptoms may vary in intensity over the course of pregnancy, we considered gestational age at the time of the prenatal assessment as a potential covariate.
Data Analytic Plan
Statistical analyses were performed using STATA 16.1 (StataCorp, 2019). Full information maximum likelihood (FIML) was used to account for missing data; accordingly, all cohort participants who had data for at least one measure were included in analytic models (see Table 1 for ns for primary variables). First, descriptive and correlational analyses were run. Bivariate correlational analyses tested associations among the primary variables. Correlation analyses also tested for associations between gestational age at the prenatal assessment and maternal prenatal symptom scores to determine if analyses should control for gestational age at the prenatal assessment. Next, primary analyses were run, adjusted for a priori determined covariates, including maternal age, race/ethnicity, educational status, and smoking during pregnancy and infant sex, gestational age at birth, and age at the time of the infant assessment. Analyses utilizing infant RSA scores additionally included infant length, weight, and activity level during the SFP-R as covariates. Linear regressions tested for associations between maternal lifetime trauma exposure and infant behavioral and physiological markers of stress reactivity and regulation. Pathway analysis was used to examine indirect effects of maternal lifetime trauma exposure effects on infant behavioral and physiological markers of stress reactivity and regulation through maternal psychological symptoms during pregnancy. Separate models were run for each psychological symptom type (i.e., anxiety, depression, PTSD) and for each of the infant outcomes. We ran post hoc analyses on statistically significant pathway models to determine the specificity of prenatal psychological symptoms as mediators. Specifically, we added scores reflecting postnatal psychological symptoms along the pathways between maternal trauma and our infant outcomes. We describe a decomposition of the indirect effect of maternal trauma on infant outcomes as the contribution stemming from prenatal psychological symptoms alone, postnatal psychological symptoms alone, and the combined prenatal to postnatal pathway. Finally, sex differences in pathway analyses were tested with infant sex (male/female) as a dichotomous moderator. Confidence intervals (95%) for indirect effects were calculated by bootstrap (1,000 replications). Standardized betas are reported throughout. All analyses were two-tailed (α = 0.05).
Table 1.
Descriptive statistics for overall sample (N = 1077) and participants with RSA data (n = 248).
| Overall Sample | Participants with RSA Data | |||
|---|---|---|---|---|
| Mean or % | SD | Mean or % | SD | |
| Maternal age (years) | 29.3 | 5.9 | 30.9 | 5.4 |
| Child sex (% male) | 52.0 | - | 55.5 | - |
| Gestational age at birth (weeks) | 38.7 | 2.1 | 38.9 | 1.7 |
| Birthweight (grams) | 3229.3 | 579.1 | 3321.9 | 532.6 |
| Birthweight adjusted by gestational age (z) | −0.2 | 0.9 | −0.1 | 1.0 |
| Maternal race/ethnicity (%) | ||||
| White | 16.8 | - | 33.6 | - |
| Black | 43.9 | - | 25.5 | - |
| Hispanic/Latina | 34.5 | - | 31.2 | - |
| Other race/ethnicity identity | 4.8 | - | 9.7 | - |
| Maternal education (%) | ||||
| < HS education | 18.5 | - | 22.3 | - |
| HS/GED | 17.6 | - | 8.1 | - |
| Some college/Associate’s degree | 28.5 | - | 21.9 | - |
| Four-year college degree | 14.3 | - | 22.3 | - |
| Graduate degree | 11.7 | - | 24.3 | - |
| Did not report | 9.5 | - | 1.2 | - |
| Annual household income (%) | ||||
| <$10,000 | 13.8 | - | 8.9 | - |
| $10,000–24,999 | 26.1 | - | 18.2 | - |
| $25,000–49,999 | 22.6 | - | 18.2 | - |
| $50,000–69,999 | 5.8 | - | 6.9 | - |
| $70,000–99,999 | 5.9 | - | 11.3 | - |
| >=$100,000 | 11.9 | - | 27.5 | - |
| Did not report | 13.8 | - | 8.9 | - |
| Maternal smoking during pregnancy (% yes) | 10.8 | - | 11.3 | - |
| Infant age at lab session (months) | - | - | 6.7 | 0.6 |
| Infant weight at lab session (kg) | - | - | 8.2 | 1.0 |
| Infant length at lab session (cm) | - | - | 68.2 | 3.4 |
| LSC-R (n = 916) | 6.4 | 4.4 | 5.3 | 3.7 |
| STAI (n = 879) | 16.4 | 5.0 | 16.4 | 4.9 |
| EPDS (n = 879) | 6.2 | 6.0 | 5.6 | 5.8 |
| PCL-C (n = 785) | 25.1 | 13.3 | 23.2 | 12.1 |
| CRISYS-R (n = 735) | 3.0 | 1.9 | 2.9 | 1.9 |
| IBQ-R Negative Affect (n = 677) | 3.1 | 0.7 | 3.0 | 0.7 |
| IBQ-R Orienting/Regulation (n = 677) | 5.3 | 0.6 | 5.3 | 0.5 |
| Infant Negative Mood during SFP-R (n = 412) | ||||
| Play | 1.8 | 1.2 | 1.6 | 0.9 |
| Still-Face 1 | 3.9 | 2.1 | 3.8 | 2.0 |
| Recovery 1 | 3.3 | 2.0 | 3.3 | 2.0 |
| Still-Face 2 | 4.7 | 2.0 | 4.6 | 2.0 |
| Recovery 2 | 3.8 | 2.0 | 3.7 | 2.0 |
| RSA during SFP-R (n = 248) | ||||
| Play | - | - | 2.6 | 0.2 |
| Still-Face 1 | - | - | 2.2 | 0.4 |
| Recovery 1 | - | - | 2.6 | 0.3 |
| Still-Face 2 | - | - | 2.2 | 0.5 |
| Recovery 2 | - | - | 2.5 | 0.4 |
Note: LSC-R = Life Stressor Checklist-Revised; STAI = Spielberger State-Trait Anxiety Inventory; EPDS = Edinburgh Postnatal Depression Scale; PCL-C = PTSD Checklist for DSM-IV, Civilian version; CRISYS-R = Crisis in the Family Systems-Revised; IBQ-R = Infant Behavior Questionnaire-Revised; SFP-R = Revised Still-Face Paradigm; Infant Negative Mood during SFP-R = Negative Mood scale of the Parent-Child Interaction Rating Scales (PCIRS); RSA = respiratory sinus arrhythmia
Results
Descriptive Statistics and Preliminary Analyses
Table 1 presents sample characteristics and descriptive data for the main study variables. As shown in Table 1, the sample was racially/ethnically and socioeconomically diverse, and infant birthweight and gestational age at birth were within typical ranges for healthy pregnancies. At the group level, the sample displayed the expected negative mood and RSA patterns of responding to the SFP-R, specifically minimal negative mood during the baseline play episode, relative increases in negative mood and decreases in RSA during the still-face episodes, and relative decreases in negative mood and increases in RSA during the subsequent reunion episodes, with RSA returning to near “baseline” (i.e., play) levels during the reunion episodes.
Participants with and without PCIRS data and with and without RSA data were compared on the main study variables of interest. Compared to participants without PCIRS data, participants with PCIRS data had lower scores on maternal trauma history (LSC-R), maternal PTSD symptoms during pregnancy (PCL-C), maternal depression symptoms during pregnancy (EPDS), and maternal ratings of infant negative affect (IBQ-R NA); there were no group differences on maternal trait anxiety symptoms during pregnancy (STAI), maternal exposure to negative life events during pregnancy (CRISYS-R), or maternal ratings of orienting/regulation (IBQ-R O/R). Compared to participants without RSA data, participants with RSA data had lower scores on maternal trauma history (LSC-R) and maternal PTSD symptoms during pregnancy (PCL-C), as well as lower maternal ratings of negative affect (IBQ-R NA); there were no group differences on maternal trait anxiety symptoms during pregnancy (STAI), maternal depression symptoms during pregnancy (EPDS), maternal exposure to negative life events during pregnancy (CRISYS-R), maternal ratings of infant orienting/regulation (IBQ-R O/R), or infant negative mood during the SFP-R.
Table 2 presents the correlation coefficients among the primary variables. As shown in Table 2, greater maternal lifetime trauma exposure, maternal negative life events during pregnancy, and maternal trait anxiety, depressive, and PTSD symptoms in pregnancy were positively associated with one another (all ps < .01). Maternal lifetime trauma and greater maternal exposure to negative life events during pregnancy were associated with higher maternal ratings of infant negative affectivity on the IBQ-R. Maternal lifetime trauma was associated with larger changes in infant RSA between the second stressor and the subsequent recovery (R2-SF2). Maternal ratings of orienting/regulation were negatively associated with infant negative mood during the recovery period following the second stressor episode during the SFP-R task: higher levels of behavioral orienting/regulation in daily life were associated with lower levels of negative mood during the second recovery period. There were no other associations between maternal characteristics or ratings of infant behavior with observed infant behavior during the SFP-R. Correlations between RSA markers of stress reactivity (SF1-P and SF2-R1) and negative mood during the respective SFP-R stressor episodes indicated associations between greater RSA responses to the stressor and higher levels of negative mood. RSA markers of stress reactivity (SF1-P and SF2-R1) were also associated with negative mood during recovery periods of the SFP-R, with greater RSA responses to the stressor associated with higher levels of negative mood during the recovery episodes. Correlations between RSA markers of stress regulation (R1-SF1 and R2-SF2) and negative mood during the SFP-R still-face stressor episodes indicated that higher levels of RSA regulation during the recovery periods were associated with higher levels of negative mood during the preceding stressor periods. Correlations between RSA markers of stress regulation/recovery (R1-SF1 and R2-SF2) and negative mood during the respective SFP-R recovery episodes were not statistically significant. None of the other RSA measures were associated with maternal characteristics or maternal ratings of infant reactivity or regulation (all ps > .05). Gestational age at the time of the prenatal assessment was not associated with scores on any of the maternal rating scales completed during pregnancy (LSC-R, STAI, PCL-C, EPDS; all ps > .05), and thus was not considered as a covariate in the primary analyses.
Table 2.
Correlation coefficients among main study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. LSC-R | -- | ||||||||||||||
| 2. STAI | 0.29** | -- | |||||||||||||
| 3. EPDS | 0.34** | 0.66** | -- | ||||||||||||
| 4. PCL-C | 0.46** | 0.37** | 0.37** | -- | |||||||||||
| 5. CRISYS-R | 0.46** | 0.34** | 0.38** | 0.30** | -- | ||||||||||
| 6. IBQ-R NA | 0.14** | 0.15** | 0.13** | 0.10* | 0.12** | -- | |||||||||
| 7. IBQ-R O/R | 0.07 | −0.11** | −0.03 | 0.08 | 0.04 | −0.22** | -- | ||||||||
| 8. NM SF1 | 0.07 | 0.03 | −0.03 | −0.06 | −0.04 | 0.08 | −0.08 | -- | |||||||
| 9. NM SF2 | 0.11 | 0.00 | 0.00 | 0.04 | −0.07 | 0.04 | −0.11 | 0.66** | -- | ||||||
| 10. NM R1 | 0.10 | 0.00 | −0.04 | 0.03 | 0.01 | 0.05 | −0.10 | 0.69** | 0.66** | -- | |||||
| 11. NM R2 | 0.00 | 0.01 | −0.01 | 0.03 | −0.04 | 0.06 | −0.18** | 0.45** | 0.68** | 0.71** | -- | ||||
| 12. RSA SF1-P | −0.03 | 0.03 | −0.05 | 0.03 | 0.01 | 0.06 | 0.05 | −0.34** | −0.22** | −0.21** | −0.10 | -- | |||
| 13. RSA SF2-R1 | −0.10 | 0.07 | −0.01 | 0.03 | −0.05 | −0.01 | −0.07 | −0.35** | −0.35** | −0.13 | −0.16* | 0.25** | -- | ||
| 14. RSA R1-SF1 | −0.10 | −0.10 | −0.05 | −0.10 | 0.03 | −0.10 | −0.07 | 0.20** | 0.25** | −0.07 | 0.13 | −0.63** | −0.59** | -- | |
| 15. RSA R2-SF2 | 0.17* | 0.06 | 0.04 | −0.01 | 0.13 | −0.03 | 0.09 | 0.14 | 0.16* | −0.12 | −0.14 | −0.15* | −0.67** | 0.31** | -- |
| 16. RSA P | 0.11 | 0.03 | 0.10 | 0.00 | −0.09 | −0.02 | −0.06 | 0.14* | 0.09 | 0.14* | 0.14 | −0.66** | −0.05 | 0.07 | −0.18** |
p<.05;
p<.01
Note: LSC-R = Life Stressor Checklist-Revised; EPDS = Edinburgh Postnatal Depression Scale; STAI = Spielberger State-Trait Anxiety Inventory; PCL-C = PTSD Checklist for DSM-IV, Civilian version; CRISYS-R = Crisis in the Family Systems-Revised; IBQ-R = Infant Behavior Questionnaire-Revised; NA = Negative Affect factor score; O/R = Orienting/Regulation factor score; NM = negative mood during the Repeated Still-Face Paradigm (SFP-R), as rated by the Negative Mood scale of the Parent-Child Interaction Rating Scales (PCIRS); SF1 = still-face 1 episode; SF2 = still-face 2 episode; R1 = reunion 1 episode; R2 = reunion 2 episode; RSA = respiratory sinus arrhythmia; P = play episode.
Primary Analyses
All models tested in primary analyses included covariates as described above.
Linear regression.
Although preliminary correlational analyses indicated a number of significant associations among the main study variables, when covariates were added to the linear regression models, maternal lifetime trauma exposure was no longer directly associated with maternal ratings of infant negative affectivity on the IBQ-R. However, with the inclusion of covariates, maternal lifetime trauma history was associated with decreased infant physiological regulation in response to the still-face stressor during the first phase of the SFP-R (R1-SF1), with higher levels of trauma exposure predicting lower infant regulation of their RSA response to the first SFP-R stressor (β = −0.19, p = 0.02). There were no other associations between maternal trauma history and infant RSA variables. Maternal IBQ-R ratings were not associated with infant RSA changes during the SFP-R (all ps > .05). There were no significant associations between maternal trauma history and observed infant negative mood during either the stressor or recovery episodes of the SFP-R (all ps > .05).
Pathway analysis.
In pathway analyses adjusted for covariates, maternal trauma history was again directly associated with lower infant physiological regulation after the first stressor task (R1-SF1) (β = −0.19, p = 0.02), such that greater maternal trauma was associated with less infant RSA recovery following the first still-face episode. This effect was partially mediated via an indirect effect of maternal trait anxiety symptoms during pregnancy (β = −0.03, p < .05; Figure 1), indicating that greater maternal lifetime trauma exposure was associated with increased trait anxiety during pregnancy, which, in turn, was associated with less infant RSA recovery following the stressor of the first phase of the SFP-R. Indirect effects of maternal lifetime trauma via trait anxiety symptoms in pregnancy on other infant RSA measures during the SFP-R were not significant. There also were no significant indirect effects of maternal lifetime trauma via trait anxiety symptoms in pregnancy on infant negative mood during the SFP-R.
Figure 1.
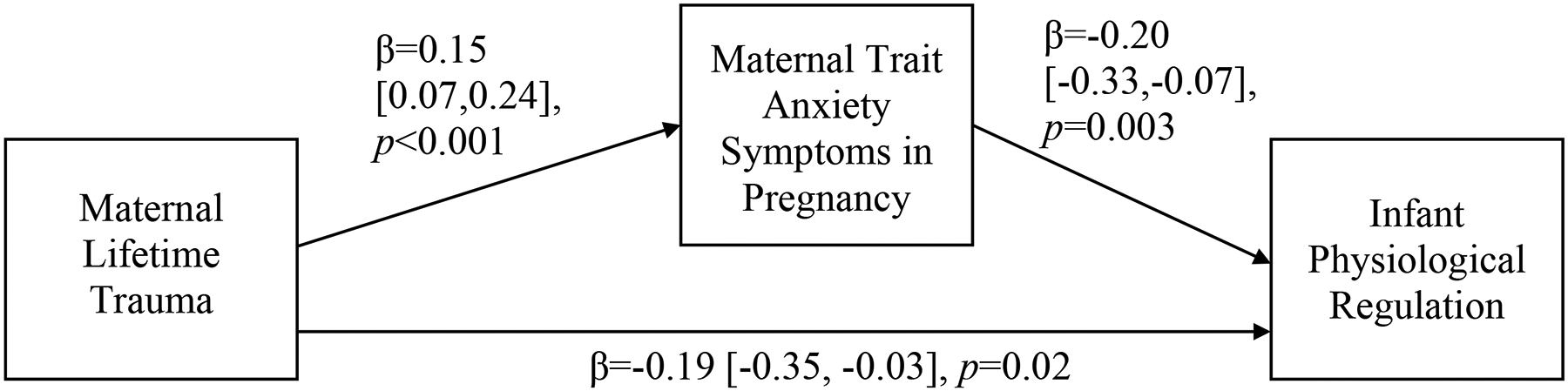
Mediation model relating maternal lifetime trauma exposure, maternal trait anxiety symptoms during pregnancy, and infant physiological regulation.
Note: Indirect effect of maternal lifetime trauma exposures on infant physiological regulation via maternal anxiety symptoms in pregnancy: β=−0.03, p<0.05, 95% CI=−0.07, −0.01. Solid black lines indicate significant paths. All parameter estimates presented are standardized. Maternal lifetime trauma indexed by the Life Stressor Checklist-Revised; maternal trait anxiety symptoms in pregnancy indexed by the Spielberger State-Trait Anxiety Inventory, Trait Scale; infant physiological regulation assessed as respiration-adjusted respiratory sinus arrhythmia (RSA) values measured during the repeated Still-Face Paradigm (SFP-R), specifically indexed as the difference in values between the first stressor period (SF1) and subsequent recovery period (R1). This model included the following covariates: maternal age, race/ethnicity, educational status, and smoking during pregnancy and infant sex, gestational age, activity level during the SFP-R, and length, weight, and age at the time of the SFP-R.
Although maternal trauma history was not directly associated with maternal IBQ-R ratings of infant reactivity or regulation when all relevant covariates were included in the models (all ps > .05), there were significant indirect effects of maternal trauma history on maternal IBQ-R ratings of infant negative affectivity (β = .02, p < .05; Figure 2), and orienting/regulation (β = −.02, p < .05; Figure 3) via maternal symptoms of trait anxiety during pregnancy, such that maternal trauma exposure was associated with increased trait anxiety during pregnancy, which, in turn, was associated with higher maternal ratings of infant negative affectivity and lower maternal ratings of infant orienting/regulation.
Figure 2.
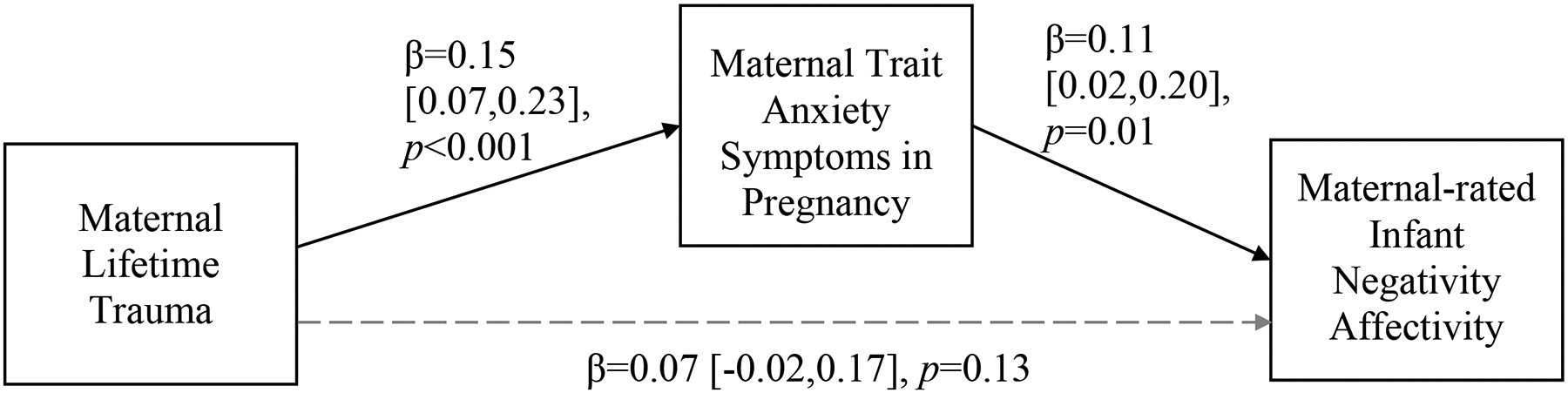
Mediation model relating maternal lifetime trauma exposure, maternal trait anxiety symptoms during pregnancy, and maternal ratings of infant negative affectivity.
Note: Indirect effect of maternal lifetime trauma exposures on maternal-rated infant negative affectivity via maternal anxiety symptoms in pregnancy: β=0.02, p<0.05, 95% CI=0.004, 0.04. Solid black lines indicate significant paths; gray dashed lines indicate non-significant paths. All parameter estimates presented are standardized. Maternal lifetime trauma indexed by the Life Stressor Checklist-Revised; maternal trait anxiety symptoms in pregnancy indexed by the Spielberger State-Trait Anxiety Inventory, Trait Scale; maternal-rated infant negative affectivity indexed by the Infant Behavior Questionnaire-Revised Negativity Affectivity factor score. This model included the following covariates: maternal age, race/ethnicity, educational status, and smoking during pregnancy and infant sex, gestational age, and age at the time of assessment.
Figure 3.
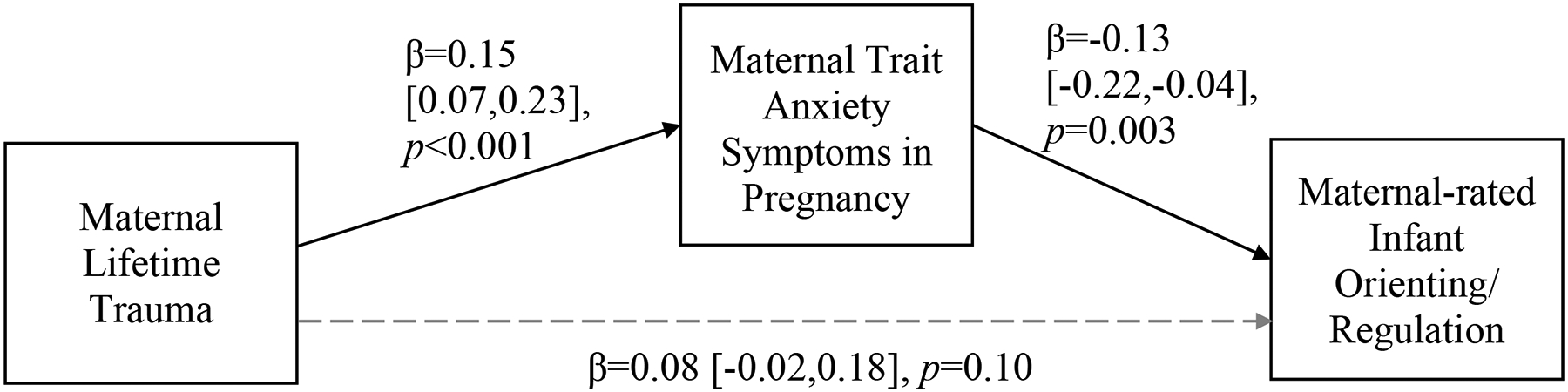
Mediation model relating maternal lifetime trauma exposure, maternal trait anxiety symptoms during pregnancy, and maternal ratings of infant orienting/regulation.
Note: Indirect effect of maternal lifetime trauma exposures on maternal-rated infant orienting/regulation via maternal anxiety symptoms in pregnancy: β=−0.02, p<0.05, 95% CI=−0.04, −0.01. Solid black lines indicate significant paths; gray dashed lines indicate non-significant paths. All parameter estimates presented are standardized. Maternal lifetime trauma indexed by the Life Stressor Checklist-Revised; maternal trait anxiety symptoms in pregnancy indexed by the Spielberger State-Trait Anxiety Inventory, Trait Scale; maternal-rated infant orienting/regulation indexed by the Infant Behavior Questionnaire-Revised Orienting/Regulation factor score. This model included the following covariates: maternal age, race/ethnicity, educational status, and smoking during pregnancy and infant sex, gestational age, and age at the time of assessment.
There were no significant indirect effects of maternal lifetime trauma on IBQ-R, PCIRS, or infant RSA scores via maternal depressive or PTSD symptoms during pregnancy.
In secondary analyses, we explored the effects of maternal postnatal trait anxiety, reported when infants were 6 months of age using the Trait Scale of the STAI, on the indirect effects of maternal trauma on both RSA recovery and IBQ-R scores via maternal trait anxiety symptoms in pregnancy. Our decomposition of the indirect effects showed that the negative association between maternal trauma and RSA recovery was entirely explained by the prenatal anxiety alone pathway (β = −0.04, 95% CI [−0.09, −0.01], p < 0.05; postnatal alone and prenatal to postnatal pathways both βs = 0.006, ps > 0.05). The decomposition of the indirect effects for the IBQ-R ratings was more mixed. For infant negative affectivity, 44% (β = 0.012) of the indirect effect was explained through the postnatal anxiety only pathway, 41% (β = 0.011) was explained through the prenatal to postnatal anxiety pathway, and 15% (β = 0.004) was explained through the prenatal anxiety only pathway. For infant orienting/regulation, 50% (β = −0.013) of the indirect effect was explained by prenatal anxiety alone, 27% (β = −0.007) by postnatal anxiety alone, and 23% (β = −0.006) through the prenatal to postnatal anxiety pathway.
Moderation by infant sex.
All statistically significant pathway models were tested for sex differences via interaction terms for paths predicting infant outcomes (i.e., IBQ-R ratings, RSA scores). Because maternal trait anxiety emerged as the only significant mediator, only the anxiety models were tested for sex differences. There were no significant differences by infant sex assigned at birth on direct or indirect effects (via trait anxiety during pregnancy) of maternal trauma history on IBQ-R ratings or on infant RSA scores (all ps > .05).
Discussion
Adversity and trauma are well-established risks to public health, impacting physical and mental wellbeing not only in the individual, but across generations. However, the specific mechanisms through which these effects are transmitted intergenerationally are not well understood. Stress reactivity and regulation in infancy have emerged as particular outcomes of interest in this cascade, given that they are associated with physical and mental health, social-emotional competence, academic achievement, and employment later in life. Obtaining a better understanding of factors that contribute to these critical foundations of development is an important component of identifying both the children most vulnerable to deleterious effects of maternal trauma and the mechanisms through which these effects are transmitted.
The goals of this study were to test 1) whether maternal trauma history confers negative effects on infant reactivity to and regulation of stress during an acute stressor task or in daily life, and 2) whether maternal psychological symptoms in pregnancy serve as a mechanism through which these effects are conferred. To index different components of infant stress reactivity, we used markers of infant negative affectivity in daily life, as rated by mothers; of infant negative mood during an acute stressor, the still-face episodes of the Repeated Still-Face Paradigm (SFP-R), as coded by independent raters; and of infant physiological stress reactivity, i.e., changes in respiratory sinus arrhythmia (RSA) in response to the still-face episodes of the SFP-R. To index different components of infant stress regulation, we used maternal ratings of infant orienting/regulation in daily life; of infant negative mood during the recovery periods of the SFP-R; and of RSA recovery following the stressor of the still-face episodes of the SFP-R. When testing our first hypothesis, we found that higher maternal lifetime trauma was associated with greater infant negative affect as indexed by maternal ratings; however, the magnitude of this associations was attenuated when a measure of negative life events during pregnancy was included in the model. These findings suggest that the intergenerational effects of maternal trauma history on the infant stress response may be tied to negative life events occurring during pregnancy, as exposure to more negative life events during pregnancy was associated with both increased maternal lifetime trauma and higher infant negative affect ratings. Early history of adversity has been associated with higher levels of stressful events in adulthood and has been theorized to lower one’s tolerance to stress and trauma exposures later in life (Bandoli et al., 2017; McLaughlin et al., 2010). Thus, women who have experienced greater lifetime adversity may be at particular risk for elevated stressors and negative life event exposures during pregnancy. Moreover, maternal stress during pregnancy has long been associated with negative infant outcomes, as both a primary predictor and as a mechanism through which maternal experiences transmit effects (Graignic-Philippe, Dayan, Chokron, Jacquet, & Tordjman, 2014; Huizink & de Rooij, 2018).
When testing our second hypothesis, there were several key findings. There was a direct effect of maternal lifetime trauma on infant physiological regulation (i.e., RSA) in response to an acute stressor, such that maternal trauma exposure was associated with reduced infant physiological recovery during the first recovery period of the SFP-R. There was also a partial mediation of this direct effect of maternal trauma exposure on reduced infant physiological regulation via higher maternal trait anxiety during pregnancy (see Figure 1). These findings suggest that, in addition to maternal anxiety and stress exposures, there are likely other mechanisms implicated in the programming and development of biological stress regulatory systems during pregnancy and the early infancy period; putative mechanisms include disruptions in maternal stress physiology (e.g., cortisol and the HPA axis) (Cowell et al., 2021), the maternal immune system (Hantsoo, Kornfield, Anguera, & Epperson, 2019), and placental cellular aging (Jones et al., 2019).
Regarding infant affective and behavioral outcomes, there was no direct effect of maternal lifetime trauma history on maternal ratings of infant negative affectivity or orienting/regulation when covariates were included in the models. However, there were significant indirect effects of maternal trauma history on both types of maternal ratings via maternal trait anxiety symptoms during pregnancy, even when controlling for negative life events during pregnancy. Specifically, greater maternal trauma was associated with greater infant negative affectivity and lower infant orienting/regulation, both via elevated maternal trait anxiety symptoms during pregnancy. Moreover, for both observed infant physiological regulation (RSA) during the SFP-P first recovery episode and maternal ratings of infant orienting/regulation in everyday life, the indirect effect of maternal trauma history via elevated maternal trait anxiety in pregnancy predicted poorer infant regulation abilities. There were no significant direct or indirect associations among maternal trauma, psychological symptoms during pregnancy, and observed infant negative mood during the SFP-R.
Following our main hypothesis testing, we conducted additional post hoc analyses to explore the effects of postnatal maternal trait anxiety at the time of the laboratory assessment on the indirect effects described above. We found that the negative association between maternal trauma and RSA recovery/regulation was entirely explained by the prenatal trait anxiety alone pathway, suggesting that prenatal trait anxiety confers a specific vulnerability to decreased physiological recovery from an acute stressor. Similarly for maternal ratings of orienting/regulation, prenatal anxiety was the primary driver, with postnatal trait anxiety alone and the prenatal to postnatal trait anxiety path both accounting for much smaller proportions. In contrast, for maternal ratings of infant negative affectivity, postnatal maternal trait anxiety appeared to account for the largest contribution, with the direct prenatal to postnatal trait anxiety path also a significant contributor; prenatal anxiety accounted for a small proportion of the variance.
Taken together, these findings suggest that prenatal trait anxiety confers specific vulnerabilities for the development of infant stress regulation competencies, as reflected in both decreased physiological recovery following an acute stressor task and lower orienting and affective regulation behaviors in everyday life. While maternal postnatal trait anxiety was associated with concurrent maternal ratings of infant orienting and affective regulation behaviors, it was not the primary driver of these findings. In contrast, maternal postnatal trait anxiety was the largest predictor of infant negative affectivity, with maternal prenatal trait anxiety conferring additional vulnerability. These findings highlight potential differential risk from exposure to maternal trait anxiety in the pre- versus postnatal periods on specific components of the infant stress response, with implications for the identification of at-risk or vulnerable groups and the design of targeted interventions to potentially mitigate these effects.
Another set of findings to emerge from this study is the relations between “parallel” measures of infant stress reactivity and regulation. We found that higher maternal ratings of infant orienting/regulation behaviors in everyday life were associated with lower levels of infant negative mood in the second recovery episode of the SFP-R, suggesting associations in the expected direction between these different measures of infant stress regulation. Because of the importance of dyadic regulation, rather than explicit self-regulation, for young infants who are managing their response to a stressor, improved orienting abilities may allow the infant to seek out and connect with their mother more easily or efficiently during the recovery periods (Braungart-Rieker et al., 1998; Conradt & Ablow, 2010). In turn, this may promote dyadic regulation of the infant’s stress response, subsequently yielding lower levels of negative affect once the stressor has remitted. There was limited correspondence between maternal ratings of infant negative affectivity or orienting/regulation in daily life and observed infant negative mood during the still-face episodes of the SFP-R. This is somewhat consistent with prior research examining these different dimensions of behavioral reactivity and regulation (Braungart-Rieker et al., 1998; Planalp & Braungart-Rieker, 2015; Tarabulsy et al., 2003), suggesting that these types of assessments may be capturing distinct yet related components of the stress response system in infants (e.g., responses to everyday events versus an acute stressful task).
The findings from this study suggest both direct and indirect effects of maternal lifetime trauma exposures on biological aspects of offspring regulatory capabilities in response to acute stressors or arousal and behavioral aspects of orienting and regulation in daily life, with a substantive contribution from maternal trait anxiety symptoms during pregnancy. Maternal trait anxiety symptoms in the postpartum period, in contrast, appeared to confer vulnerability for greater infant negative affectivity in daily life, a component of stress reactivity. These findings bring together separate bodies of literature that have shown increased psychopathology in response to trauma exposure and associations between maternal psychopathology during pregnancy and negative infant stress and behavior regulation outcomes (Choe et al., 2013; Davis et al., 2007; Davis et al., 2004; Gustafsson et al., 2018; McGrath et al., 2008). While much of the extant literature has assessed the effects of perinatal depression on infant outcomes, our findings suggest that prenatal trait anxiety may have particular effects in the context of infant distress/arousal regulation, as it was a mediator of effects of maternal lifetime trauma on both maternal ratings of infant behavioral regulation of distress or arousal in everyday life and objectively-measured infant physiological regulation during the recovery phase of an acute stressor task. Our post hoc analyses exploring potential contributions from postnatal anxiety to this model further suggested that prenatal trait anxiety confers unique risks for infant regulation of attention (i.e., orienting) and affective and physiological stress responses. In contrast, postnatal anxiety appeared to be a primary contributor to infant negative affectivity, a key aspect of stress reactivity. These findings point to the importance of isolating and specifying maternal factors that influence infant outcomes so that interventions can be appropriately targeted. The current findings suggest that maternal negative life event exposures and trait anxiety symptoms are mechanisms by which maternal lifetime trauma history is intergenerationally transmitted, with differential effects of prenatal versus postnatal anxiety on infant stress regulation versus reactivity components. These findings highlight maternal psychological functioning in the perinatal period, and more specifically trait anxiety, as a potential primary focus for treatment, especially in women who have experienced trauma and stress exposures.
Other prenatal and early life factors likely mediate maternal trauma history effects on infant ANS development. Some prior literature examining differential effects of maternal childhood adversities and prenatal stress on infant RSA during the SFP identified separate effects of the two types of maternal exposures (i.e., pre-conception traumatic events and prenatal stress) on infant RSA outcomes (Gray et al., 2017). Specifically, in Gray et al.’s study, prenatal stress was associated with a suppressed infant RSA response following the stressor, whereas maternal childhood adversities were associated with a lower overall RSA, interpreted as a lower “physiologic start point.” Different maternal exposures were used in our study (i.e., lifetime trauma exposures, negative life events in pregnancy, and trait anxiety in pregnancy versus childhood adversity and stress in pregnancy). We did not find associations between our maternal trauma exposure or psychopathology measures and infant baseline RSA during the Play episode (see Table 2); thus, we did not find evidence for lower overall RSA in relation to maternal exposures or psychological symptoms. In combination, these studies indicate the importance of carefully operationalizing the type and timing of exposures to understand offspring effects and point to the possibility of multiple pathways through which maternal lifetime trauma experiences and psychological symptoms during pregnancy may impact fetal and infant development. Determining the specific effects and mechanisms of each type of risk factor is particularly important when designing and implementing interventions.
Some non-significant results are important to mention. When exposure to negative life events during pregnancy was considered, there were no significant direct associations of maternal lifetime trauma history or psychological symptoms in pregnancy with any measure of infant stress reactivity, either physiological or behavioral, assessed during the acute stressor phase of the SFP-R or via maternal ratings of infant negativity. These findings, in combination with the significant direct and indirect effects observed when examining infant physiological recovery from reaction to an acute stressor task, suggest that infants of mothers with elevated lifetime trauma histories may not necessarily demonstrate greater RSA responses to acute stressors, but may have more difficulty regulating their stress responses once activated. This pattern of findings is consistent with other studies that have demonstrated specific challenges with behavioral and physiological stress regulation but not stress reactivity in infants of trauma-affected mothers (Bosquet Enlow et al., 2011; Bosquet Enlow et al., 2009). Thus, children of mothers with a trauma history and/or psychopathology in pregnancy may benefit from programs that specifically promote the development of dyadic and self-regulatory skills. Also noteworthy is that approximately 24% of infants who participated in the SFP-R were unable to participate in the second still-face and reunion episodes due to failure to return to a non-distressed state during the first reunion. We have previously shown that infants unable to complete the second SFP-R trial show more pronounced ANS reactivity to and poorer recovery from the first trial (Ritz et al., 2020). Thus, the infants included in analyses focused on reactivity to and recovery from the second still-face episode likely did not include the infants in the sample with the most difficulty with stress regulation. This fact may explain why the main findings were observed when using the first but not the second still-face trial.
In contrast to one of our secondary hypotheses, there was no evidence of sex differences in the effects of maternal trauma or anxiety on infant behavioral or physiological stress responses. These findings are similar with the majority of research using the SFP-R, which, to date, has found that sex effects in the SFP-R are inconsistent at best. While a few select studies have demonstrated sex differences in response to the task (Haley & Stansbury, 2003; Tibu et al., 2014), both the majority of individual studies and meta-analyses or systematic reviews of ANS functioning during the SFP-R have indicated that sex differences are rare (Conradt & Ablow, 2010; Jones-Mason et al., 2018; Mesman et al., 2009; Moore et al., 2009). Looking more broadly, there is more evidence documenting sex differences in the effects of maternal stress across other infant behavioral and physiological outcomes (e.g., Glover & Hill, 2012; Van den Bergh et al., 2020); however, as is the case with SFP-R findings, these sex differences are not consistently found, even when identical measures are used. Notably, a prior study in this cohort found that maternal hair cortisol in pregnancy, a potential indicator of maternal physiological stress, was associated with sex differences in infant RSA over the course of the SFP-R (Cowell et al., 2021). Thus, sex differences in the associations between maternal trauma and stress history and the developing infant stress regulatory system may depend on numerous factors, including the timing of maternal exposures, the timing of maternal and infant assessments, and the specific maternal and infant measures involved (e.g., maternal report of infant behavior, physiology of various maternal and infant stress systems [e.g., ANS, HPA], observed infant behavior) involved. Our lack of sex-differential findings in the current study suggests that the specific processes assessed here may function similarly across sexes. Future research should continue to assess for sex differences in intergenerational effects of maternal trauma and psychological symptoms during pregnancy on the development of the infant stress response to specify the pathways that exhibit sex-specific effects.
We did not find associations between maternal ratings of infant negative affectivity or orienting/regulation and the related physiological metrics, with the exception of the association between higher maternal ratings of infant orienting/regulation and lower observed levels of infant negative mood during the final recovery period of the SFP-R. There are several possible reasons for this relative lack of associations. First, these measures are designed to assess related but separate constructs: parental reports of behaviors that are considered indicators of negative emotionality (i.e., frustration, sadness, fear) and efforts to manage stress/arousal (using both dyadic regulation and emerging self-directed abilities) in everyday life across multiple contexts, versus singular point-in-time physiological or behavioral markers of an acute stress reaction and subsequent regulatory capacity to a standardized laboratory stressor. The extant literature examining associations between the IBQ-R or other parent ratings of infant behavior and objective assessments during the SFP is inconsistent in demonstrating associations even at the behavior-behavior level. For example, positive and negative infant affect as rated by parents have been associated with higher and lower (respectively) use of self-soothing or self-comforting behaviors during the SFP in some studies (Braungart-Rieker et al., 1998; Planalp & Braungart-Rieker, 2015), but not in others (Tarabulsy et al., 2003). Further, there is a paucity of published research examining parent ratings of behavior in association with infant physiological stress metrics, suggesting that these constructs are not frequently studied together. Our findings suggest that, rather than being directly correlated, physiological stress response systems and externally-visible stress management behaviors may be complementary, working in concert to adapt to changing circumstances, possibly with other components (e.g., psychological and emotional manifestations) (Campbell & Ehlert, 2012). For example, physiological components may occur first, with the behavioral manifestation following shortly behind, as is the case in Porges’ polyvagal theory: decreases in RSA indicate a decrease of parasympathetic activity in preparation to mount a sympathetic and behavioral/emotional stress response (Porges, 2003). Alternatively, infants may mount a strong physiological response only when they do not anticipate or expect the stressor, based on their history of caregiver interactions, or are not experiencing success in engaging their caregiver via behavioral signals (e.g., crying) to assist in dyadic regulation of their distress.
Some of our other correlational findings may lend insight into the complex and nuanced relations between physiological and emotional or behavioral components of the stress response system. We found that higher levels of infant negative mood during the stressor periods of the SFP-R were associated with greater physiological stress responses during these stressor periods as well as during the recovery periods. Possibly, infants who exhibit greater emotional responses to acute stressors need to exert greater physiological regulatory responses to manage their reaction. We also found that greater physiological reactivity during each of the stressor episodes was associated with greater negative mood in the subsequent recovery episode (e.g., a greater RSA response during SF1 was associated with higher negative mood during R1). This pattern suggests possible diminished recovery/regulation of the affective stress response when high levels of physiological reactivity are activated. Additional research that deeply interrogates the joint contributions of physiological, emotional, cognitive/attentional, and behavioral processes to stress reactivity and regulation across development is necessary.
Strengths of the study are worth noting. We used three different approaches (i.e., physiological metrics, standardized behavior coding, parent ratings) to capture components of the infant stress response. Utilizing multiple methods is key to furthering our understanding of the different dimensions of stress regulation, particularly as they relate to prediction of future outcomes. Another strength is that, while this study focused on measures of maternal psychological symptoms in pregnancy to test potential prenatal mechanisms by which maternal trauma affects infant outcomes, we also incorporated assessment of postnatal symptoms in post hoc analyses. Further consideration of both pre- and postnatal maternal psychological symptoms in future studies is encouraged, particularly given that our findings suggest that symptoms during each period may have differential or possibly interactive effects on infant outcomes through multiple pathways. Explicating the nature of such effects may help guide the development of more effective and targeted screening and intervention efforts.
Another strength is our measurement of RSA. We adjusted RSA for respiration, which has been shown to result in a more sensitive index of vagal activity that better predicts vagal modulation in response to stress (Ritz et al., 2012). In adults, a range of paced breathing frequencies is typically used as baseline calibration for respiratory adjustments. Such a technique cannot be applied with infants; however, our approach of using a within-individual correction based on the transfer function is aligned with adjustment approaches suggested for adults (Ritz et al., 2012). We also controlled for infant activity level during the SFP-R to increase the likelihood that observed changes in RSA were due to the stress of the protocol rather than to physical activity. Additionally, the study sample included a racially, ethnically, and socioeconomically diverse sample of pregnant women, an understudied demographic despite their increased risk for experiencing adversity and psychological sequelae. The sample was a community multi-site sample, and thus the findings may have wide generalizability; however, the findings may not generalize to clinical samples (e.g., mothers who meet criteria for a psychological disorder). Future research should explore how the hypothesized associations operate in clinical groups. Other strengths include the prospective collection of data and the availability of a rich covariate dataset.
This study had some limitations. First, because we relied on maternal self-report for lifetime trauma exposures and psychological symptoms in pregnancy and the postnatal period and for ratings of infant temperament, there is a risk of reporter bias inflating associations between maternal and infant ratings (Brewin, Andrews, & Gotlib, 1993; Davis et al., 2007). Encouragingly, retrospective reports of trauma exposure are generally found to be fairly accurate, and inaccuracies tend to involve underreporting rather than overreporting (Hardt & Rutter, 2004; McHugo et al., 2005; Wolfe, Kimerling, Wilson, & Keane, 1997), which would lead to smaller observed effect sizes than the true effect sizes. Additionally, recent evidence suggests that maternal psychopathology minimally biases maternal reports of child emotional and behavioral problems (Olino, Michelini, Mennies, Kotov, & Klein, 2021). Further, the IBQ-R was designed to avoid asking parents to provide subjective judgments about their child, but rather inquires about multiple examples of concrete, observable infant behaviors (Gartstein & Rothbart, 2003) to reduce biased responding. Moreover, the inclusion of infant negative mood and RSA during the SFP-R provided standardized observational measures of infant affective, behavioral, and physiological reactivity and regulation not subject to maternal bias.
Another potential limitation was this study’s use of the Trait subscale of the STAI. Notably, this measure has been used frequently in perinatal research (Meades & Ayers, 2011). This subscale asks respondents to indicate how often they experience various markers of anxiety based on how they “generally feel,” rather than giving a specific recent timeframe in which to report symptoms (as do the EPDS and PCL-C, our other symptom reports). Studies of reliability and validity of the STAI in non-pregnant populations tend to find fairly stable test-retest reliability for the Trait subscale across time (Barnes, Harp, & Jung, 2002). In contrast, the Trait subscale has been found to fluctuate somewhat over pregnancy and tends to decrease in the postpartum period, thus appearing to capture general anxiety symptoms that do not appear to be unchanged over long periods and instead may be uniquely experienced during the prenatal period (Giakoumaki et al., 2009; Gimbel et al., 2021; Gunning et al., 2010; Haddad, Morris, & Spielberger, 1985; Hundley et al., 1998; Meades & Ayers, 2011). However, using a measure designed to capture trait anxiety, rather than a reporting of anxiety symptoms in a specific timeframe, may limit our ability to conclude that the symptoms were reflective of anxiety symptoms specific to pregnancy. Future studies could mitigate this limitation by using an anxiety symptom inventory to assess anxiety levels in a recent delimited period of time. Another potential limitation is the number of models that were needed to fully evaluate our research questions. In order to mitigate the risk of type II error, our statistical approach did not include use of multiple comparison correction. However, it should be noted that the increased risk for type I error means that these findings should be reproduced in future research.
Finally, as noted in the Introduction, both hypo- and hyperreactivity of the PNS to stress have been associated with poorer child developmental outcomes; clinical cutoffs for “healthy” moderate levels of RSA are not established to guide analyses. Moreover, studies that have examined associations of maternal stress and psychopathology with infant ANS responsivity have reported mixed findings. For example, Bush and colleagues (Bush et al., 2017) found that infants born to mothers reporting both more stressful life events and moderate-to-high levels of perceived stress during pregnancy showed heightened RSA reactivity to and weaker recovery from the SFP. However, Field and colleagues (Field et al., 2003) found that elevated anxiety during pregnancy was associated with lower infant vagal tone and reduced vagal withdrawal following stress. Thus, more research is needed to establish the nature of the potential impact of maternal traumatic experiences and psychopathology in pregnancy on the developing fetal stress regulatory system. The current analyses employed a linear approach to test associations between the maternal predictors and infant outcomes. Given that early adverse environmental exposures may increase risk of both hypo- and hyperreactive infant stress response profiles, future research should consider other statistical approaches that can address various directional associations. Such studies should also carefully consider the timing of the maternal exposures, the infant age at assessment, the specific ANS indicators measured (e.g., RSA, HRV, SNS measures), the stress-response procedure employed (e.g., Still-Face Paradigm, frustration procedure), and the sociodemographic characteristics of the study samples, as these characteristics may influence the magnitude and nature of observed associations (Cowell et al., 2021).
In conclusion, the current findings highlight potential contributory mechanisms in the development of maladaptive infant stress response and regulation abilities, which have been associated with increased risk for ADHD and internalizing, externalizing, and conduct problems, and with poorer cognitive and academic outcomes in childhood. Post hoc analyses further indicated the potential for differential vulnerabilities in the development of the stress response following exposure to prenatal versus postnatal maternal trait anxiety, which may be helpful in both the identification of children at risk for difficulties with stress reactivity versus regulation as well as the targeting of appropriate intervention services for mothers during pregnancy and/or the postpartum period. This study adds to the small but growing literature assessing early life predictors of later self-regulatory capacity. Given the high rates of trauma and adversity in the general population, these findings are important in helping to elucidate intergenerational effects of these experiences. Additional research is needed to explicate further the multiple underlying mechanisms involved in the associations identified here so that appropriate interventions to address these intergenerational effects may be developed. Prenatal exposure to biophysiological processes associated with maternal stress and trauma, including maternal-fetal HPA axis dysregulation, inflammatory cytokines, dysregulated endocrine processes, oxidative stress, epigenetic processes, and/or the maternal-fetal microbiome, may serve as key mediating factors in the cascade of effects of maternal trauma on infant neurodevelopment and stress regulation (Coussons-Read, 2013; Cowell et al., 2021; Dowell, Elser, Schroeder, & Stevens, 2019; Glover, O’Donnell, O’Connor, & Fisher, 2018; McGowan & Matthews, 2018). By identifying connections between maternal experiences and infant stress response capacity, this study highlights important risk factors for maladaptive child outcomes that can be identified during pregnancy, potentially offering earlier identification of families at risk. In concert with future research that can further identify putative mechanisms in this cascade, targeted interventions to reduce the likelihood of maladaptive outcomes can be developed and implemented.
Acknowledgements:
This research was supported by grants from the National Heart, Lung, & Blood Institute (R01HL095606; R01HL114396), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21HD080359), the National Institute of Environmental Health Sciences (P30ES023515), the Office of the Director, National Institutes of Health (UG3OD023337), and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital. None of the funding agencies had any role in the study design, the collection, analysis or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of any granting agency. The authors wish to thank research study staff and participants. There are no conflicts of interest to report.
References
- Adamson LB, & Frick JE (2003). The still face: A history of a shared experimental paradigm. Infancy, 4(4), 451–473. [Google Scholar]
- Adhikari K, Patten SB, Williamson T, Patel AB, Premji S, Tough S, Letourneau N, Giesbrecht G, & Metcalfe A (2021). Assessment of anxiety during pregnancy: are existing multiple anxiety scales suitable and comparable in measuring anxiety during pregnancy?. Journal of Psychosomatic Obstetrics & Gynecology, 42(2), 140–146. [DOI] [PubMed] [Google Scholar]
- Alexander N, Kuepper Y, Schmitz A, Osinsky R, Kozyra E, & Hennig J (2009). Gene-environment interactions predict cortisol responses after acute stress: implications for the etiology of depression. Psychoneuroendocrinology, 34(9), 1294–1303. doi: 10.1016/j.psyneuen.2009.03.017 [DOI] [PubMed] [Google Scholar]
- Bailey HN, Deoliveira CA, Wolfe VV, Evans EM, & Hartwick C (2012). The impact of childhood maltreatment history on parenting: A comparison of maltreatment types and assessment methods. Child Abuse & Neglect, 36(3), 236–246. doi: 10.1016/j.chiabu.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Bandoli G, Campbell-Sills L, Kessler RC, Heeringa SG, Nock MK, Rosellini AJ, Sampson NA, Schoenbaum M, Ursano RJ, & Stein MB (2017). Childhood adversity, adult stress, and the risk of major depression or generalized anxiety disorder in US soldiers: a test of the stress sensitization hypothesis. Psychological Medicine, 47(13), 2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banyard VL, Williams LM, & Siegel JA (2003). The impact of complex trauma and depression on parenting: an exploration of mediating risk and protective factors. Child Maltreatment, 8(4), 334–349. doi: 10.1177/1077559503257106 [DOI] [PubMed] [Google Scholar]
- Barnes LL, Harp D, & Jung WS (2002). Reliability generalization of scores on the Spielberger state-trait anxiety inventory. Educational and Psychological Measurement, 62(4), 603–618. [Google Scholar]
- Bazhenova OV, Plonskaia O, & Porges SW (2001). Vagal reactivity and affective adjustment in infants during interaction challenges. Child Development, 72(5), 1314–1326. doi: 10.1111/1467-8624.00350 [DOI] [PubMed] [Google Scholar]
- Beauchaine TP (2015). Respiratory Sinus Arrhythmia: A Transdiagnostic Biomarker of Emotion Dysregulation and Psychopathology. Current Opinion in Psychology, 3, 43–47. doi: 10.1016/j.copsyc.2015.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, & Peters R (2003). Physiological and neurocognitive correlates of adaptive behavior in preschool among children in Head Start. Developmental Neuropsychology, 24(1), 479–497. doi: 10.1207/S15326942DN2401_04 [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, Bollati V, Sideridis G, Flom JD, Hoxha M, Hacker MR, & Wright RJ (2018). Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology, 95, 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Carter A, Hails K, King L, & Cabrera I (2014). Parent-Child Interaction Rating Scales (PCIRS)–Infant Adaptation. Unpublished manual, Boston Children’s Hospital. [Google Scholar]
- Bosquet Enlow M, Devick KL, Brunst KJ, Lipton LR, Coull BA, & Wright RJ (2017). Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy, 22(4), 492–513. doi: 10.1111/infa.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, & Wright RJ (2014). Maternal sensitivity and infant autonomic and endocrine stress responses. Early Human Development, 90(7), 377–385. doi: 10.1016/j.earlhumdev.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Kitts RL, Blood E, Bizarro A, Hofmeister M, & Wright RJ (2011). Maternal posttraumatic stress symptoms and infant emotional reactivity and emotion regulation. Infant Behavior and Development, 34(4), 487–503. doi: 10.1016/j.infbeh.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosquet Enlow M, Kullowatz A, Staudenmayer J, Spasojevic J, Ritz T, & Wright RJ (2009). Associations of maternal lifetime trauma and perinatal traumatic stress symptoms with infant cardiorespiratory reactivity to psychological challenge. Psychosomatic Medicine, 71(6), 607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce WT, & Ellis BJ (2005). Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Development and Psychopathology, 17(2), 271–301. doi: 10.1017/s0954579405050145 [DOI] [PubMed] [Google Scholar]
- Brand SR, Brennan PA, Newport DJ, Smith AK, Weiss T, & Stowe ZN (2010). The impact of maternal childhood abuse on maternal and infant HPA axis function in the postpartum period. Psychoneuroendocrinology, 35(5), 686–693. doi: 10.1016/j.psyneuen.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braungart-Rieker J, Garwood MM, Powers BP, & Notaro PC (1998). Infant affect and affect regulation during the still-face paradigm with mothers and fathers: the role of infant characteristics and parental sensitivity. Developmental Psychology, 34(6), 1428–1437. doi: 10.1037//0012-1649.34.6.1428 [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, & Gotlib IH (1993). Psychopathology and early experience: a reappraisal of retrospective reports. Psychological Bulletin, 113(1), 82–98. doi: 10.1037/0033-2909.113.1.82 [DOI] [PubMed] [Google Scholar]
- Brown RC, Berenz EC, Aggen SH, Gardner CO, Knudsen GP, Reichborn-Kjennerud T, Kendler KS, & Amstadter AB (2014). Trauma exposure and Axis I psychopathology: A cotwin control analysis in Norwegian young adults. Psychological Trauma: Theory, Research, Practice, and Policy, 6(6), 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush NR, Jones-Mason K, Coccia M, Caron Z, Alkon A, Thomas M, Coleman-Phox K, Wadhwa PD, Laraia BA, Adler NE, & Adler NE (2017). Effects of pre-and postnatal maternal stress on infant temperament and autonomic nervous system reactivity and regulation in a diverse, low-income population. Development and Psychopathology, 29(5), 1553–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, & Fox NA (2002). Self-regulatory processes in early personality development: a multilevel approach to the study of childhood social withdrawal and aggression. Development and Psychopathology, 14(3), 477–498. doi: 10.1017/s095457940200305x [DOI] [PubMed] [Google Scholar]
- Campbell J, & Ehlert U (2012). Acute psychosocial stress: does the emotional stress response correspond with physiological responses? Psychoneuroendocrinology, 37(8), 1111–1134. doi: 10.1016/j.psyneuen.2011.12.010 [DOI] [PubMed] [Google Scholar]
- Chemtob CM, Nomura Y, Rajendran K, Yehuda R, Schwartz D, & Abramovitz R (2010). Impact of Maternal Posttraumatic Stress Disorder and Depression Following Exposure to the September 11 Attacks on Preschool Children’s Behavior. Child Development, 81(4), 1129–1141. doi: 10.1111/j.1467-8624.2010.01458.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe DE, Olson SL, & Sameroff AJ (2013). Effects of early maternal distress and parenting on the development of children’s self-regulation and externalizing behavior. Development and Psychopathology, 25(2), 437–453. doi: 10.1017/S0954579412001162 [DOI] [PubMed] [Google Scholar]
- Cohen LR, Hien DA, & Batchelder S (2008). The impact of cumulative maternal trauma and diagnosis on parenting behavior. Child Maltreatment, 13(1), 27–38. doi: 10.1177/1077559507310045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collishaw S, Dunn J, O’Connor TG, Golding J, Avon Longitudinal Study of, P., & Children Study, T. (2007). Maternal childhood abuse and offspring adjustment over time. Development and Psychopathology, 19(2), 367–383. doi: 10.1017/S0954579407070186 [DOI] [PubMed] [Google Scholar]
- Colombo J, & Mitchell DW (2009). Infant visual habituation. Neurobiology of Learning and Memory, 92(2), 225–234. doi: 10.1016/j.nlm.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt E, & Ablow J (2010). Infant physiological response to the still-face paradigm: contributions of maternal sensitivity and infants’ early regulatory behavior. Infant Behavior and Development, 33(3), 251–265. doi: 10.1016/j.infbeh.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Contrada R, & Baum A (2010). The handbook of stress science: Biology, psychology, and health: Springer Publishing Company. [Google Scholar]
- Coussons-Read ME (2013). Effects of prenatal stress on pregnancy and human development: mechanisms and pathways. Obstetric Medicine, 6(2), 52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell W, Khoury JE, Petty CR, Day HE, Benitez BE, Cunningham MK, Schulz SM, Ritz T, Wright RJ, & Enlow MB (2021). Integrated and diurnal indices of maternal pregnancy cortisol in relation to sex-specific parasympathetic responsivity to stress in infants. Developmental Psychobiology, 63(2), 350–363. doi: 10.1002/dev.22015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell WJ, Bellinger DC, Coull BA, Gennings C, Wright RO, & Wright RJ (2015). Associations between prenatal exposure to black carbon and memory domains in urban children: modification by sex and prenatal stress. PLOS ONE, 10(11), e0142492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Holden J, & Sagovsky R (1987). Edinburgh postnatal depression scale (EPDS). British Journal of Psychiatry, 150, 782–786. [DOI] [PubMed] [Google Scholar]
- Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, & Sandman CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. Journal of the American Academy of Child & Adolescent Psychiatry, 46(6), 737–746. [DOI] [PubMed] [Google Scholar]
- Davis EP, Snidman N, Wadhwa PD, Glynn LM, Schetter CD, & Sandman CA (2004). Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy, 6(3), 319–331. [Google Scholar]
- Delker BC, Noll LK, Kim HK, & Fisher PA (2014). Maternal abuse history and self-regulation difficulties in preadolescence. Child Abuse & Neglect, 38(12), 2033–2043. doi: 10.1016/j.chiabu.2014.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Kivlighan KT, Costigan KA, Rubin SE, Shiffler DE, Henderson JL, & Pillion JP (2010). Prenatal antecedents of newborn neurological maturation. Child Development, 81(1), 115–130. doi: 10.1111/j.1467-8624.2009.01384.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Novak MF, Costigan KA, Atella LD, & Reusing SP (2006). Maternal psychological distress during pregnancy in relation to child development at age two. Child Development, 77(3), 573–587. doi: 10.1111/j.1467-8624.2006.00891.x [DOI] [PubMed] [Google Scholar]
- Dowell J, Elser BA, Schroeder RE, & Stevens HE (2019). Cellular stress mechanisms of prenatal maternal stress: heat shock factors and oxidative stress. Neuroscience Letters, 709, 134368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R (2015). Mutual influences between child emotion regulation and parent–child reciprocity support development across the first 10 years of life: Implications for developmental psychopathology. Development and Psychopathology, 27(4pt1), 1007–1023. [DOI] [PubMed] [Google Scholar]
- Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, & Bendell D (2003). Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depression and Anxiety, 17(3), 140–151. doi: 10.1002/da.10071 [DOI] [PubMed] [Google Scholar]
- Flynn HA, Blow FC, & Marcus SM (2006). Rates and predictors of depression treatment among pregnant women in hospital-affiliated obstetrics practices. General Hospital Psychiatry, 28(4), 289–295. doi: 10.1016/j.genhosppsych.2006.04.002 [DOI] [PubMed] [Google Scholar]
- Forman DR, O’Hara MW, Larsen K, Coy KC, Gorman LL, & Stuart S (2003). Infant emotionality: Observational methods and the validity of maternal reports. Infancy, 4(4), 541–565. [Google Scholar]
- Gardner JM, Karmel BZ, Freedland RL, Lennon EM, Flory MJ, Miroshnichenko I, Phan HT, Barone A, & Harin A (2006). Arousal, attention, and neurobehavioral assessment in the neonatal period: implications for intervention and policy. Journal of Policy and Practice in Intellectual Disabilities, 3(1), 22–32. doi: 10.1111/j.1741-1130.2006.00049.x [DOI] [Google Scholar]
- Gartstein MA, & Rothbart MK (2003). Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior and Development, 26(1), 64–86. doi: 10.1016/s0163-6383(02)00169-8 [DOI] [Google Scholar]
- Geeraerts SB, Backer PM, & Stifter CA (2020). It takes two: Infants’ moderate negative reactivity and maternal sensitivity predict self-regulation in the preschool years. Developmental Psychology, 56(5), 869–879. doi: 10.1037/dev0000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelaye B, Zheng Y, Medina-Mora ME, Rondon MB, Sánchez SE, & Williams MA (2017). Validity of the posttraumatic stress disorders (PTSD) checklist in pregnant women. BMC psychiatry, 17(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva R, Zivan M, Warsha A, & Olchik D (2013). Alerting, orienting or executive attention networks: differential patters of pupil dilations. Frontiers in Behavioral Neuroscience, 7. doi: 10.3389/fnbeh.2013.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giakoumaki O, Vasilaki K, Lili L, Skouroliakou M, & Liosis G (2009). The role of maternal anxiety in the early postpartum period: screening for anxiety and depressive symptomatology in Greece. Journal of Psychosomatic Obstetrics & Gynecology, 30(1), 21–28. [DOI] [PubMed] [Google Scholar]
- Gibson J, McKenzie‐McHarg K, Shakespeare J, Price J, & Gray R (2009). A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatrica Scandinavica, 119(5), 350–364. [DOI] [PubMed] [Google Scholar]
- Gimbel LA, Blue NR, Allshouse AA, Silver RM, Gimbel B, Grobman WA, Haas DM, Simhan HN, Mercer BM, & Hatfield T (2021). Pregnancy outcomes and anxiety in nulliparous women. The Journal of Maternal-Fetal & Neonatal Medicine, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginty AT, Phillips AC, Der G, Deary IJ, & Carroll D (2011). Heart rate reactivity is associated with future cognitive ability and cognitive change in a large community sample. International Journal of Psychophysiology, 82(2), 167–174. doi: 10.1016/j.ijpsycho.2011.08.004 [DOI] [PubMed] [Google Scholar]
- Glover V, & Hill J (2012). Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: an evolutionary perspective. Physiology & Behavior, 106(5), 736–740. [DOI] [PubMed] [Google Scholar]
- Glover V, O’Donnell KJ, O’Connor TG, & Fisher J (2018). Prenatal maternal stress, fetal programming, and mechanisms underlying later psychopathology—a global perspective. Development and Psychopathology, 30(3), 843–854. [DOI] [PubMed] [Google Scholar]
- Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, & Tordjman S (2014). Effects of prenatal stress on fetal and child development: a critical literature review. Neuroscience and Biobehavioral Reviews, 43, 137–162. doi: 10.1016/j.neubiorev.2014.03.022 [DOI] [PubMed] [Google Scholar]
- Grant KA, McMahon C, & Austin MP (2008). Maternal anxiety during the transition to parenthood: a prospective study. Journal of Affective Disorders, 108(1–2), 101–111. doi: 10.1016/j.jad.2007.10.002 [DOI] [PubMed] [Google Scholar]
- Gray SAO, Jones CW, Theall KP, Glackin E, & Drury SS (2017). Thinking across generations: Unique contributions of maternal early life and prenatal stress to infant physiology. Journal of the American Academy of Child & Adolescent Psychiatry, 56(11), 922–929. doi: 10.1016/j.jaac.2017.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman P (1992). Respiratory and cardiac rhythms as windows to central and autonomic biobehavioral regulation: selection of window frames, keeping the panes clean and viewing the neural topography. Biological Psychology, 34(2–3), 131–161. [DOI] [PubMed] [Google Scholar]
- Grossman P, Karemaker J, & Wieling W (1991). Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology, 28(2), 201–216. [DOI] [PubMed] [Google Scholar]
- Grossman P, & Taylor EW (2007). Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology, 74(2), 263–285. doi: 10.1016/j.biopsycho.2005.11.014 [DOI] [PubMed] [Google Scholar]
- Grossman P, Van Beek J, & Wientjes C (1990). A comparison of three quantification methods for estimation of respiratory sinus arrhythmia. Psychophysiology, 27(6), 702–714. [DOI] [PubMed] [Google Scholar]
- Gunning MD, Denison FC, Stockley CJ, Ho SP, Sandhu HK, & Reynolds RM (2010). Assessing maternal anxiety in pregnancy with the State‐Trait Anxiety Inventory (STAI): issues of validity, location and participation. Journal of Reproductive and Infant Psychology, 28(3), 266–273. [Google Scholar]
- Gustafsson HC, Sullivan EL, Nousen EK, Sullivan CA, Huang E, Rincon M, Nigg JT, & Loftis JM (2018). Maternal prenatal depression predicts infant negative affect via maternal inflammatory cytokine levels. Brain, Behavior, and Immunity, 73, 470–481. doi: 10.1016/j.bbi.2018.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad PF, Morris NF, & Spielberger CD (1985). Anxiety in pregnancy and its relation to use of oxytocin and analgesia in labour. Journal of Obstetrics and Gynaecology, 6(2), 77–81. [Google Scholar]
- Haley DW, & Stansbury K (2003). Infant stress and parent responsiveness: regulation of physiology and behavior during still-face and reunion. Child Development, 74(5), 1534–1546. doi: 10.1111/1467-8624.00621 [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Kornfield S, Anguera MC, & Epperson CN (2019). Inflammation: a proposed intermediary between maternal stress and offspring neuropsychiatric risk. Biological Psychiatry, 85(2), 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, & Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. Journal of Child Psychology and Psychiatry, 45(2), 260–273. doi: 10.1111/j.1469-7610.2004.00218.x [DOI] [PubMed] [Google Scholar]
- Heron J, O’Connor TG, Evans J, Golding J, Glover V, & Team AS (2004). The course of anxiety and depression through pregnancy and the postpartum in a community sample. Journal of Affective Disorders, 80(1), 65–73. doi: 10.1016/j.jad.2003.08.004 [DOI] [PubMed] [Google Scholar]
- Huizink AC, & de Rooij SR (2018). Prenatal stress and models explaining risk for psychopathology revisited: Generic vulnerability and divergent pathways. Development and Psychopathology, 30(3), 1041–1062. doi: 10.1017/S0954579418000354 [DOI] [PubMed] [Google Scholar]
- Hundley V, Gurney E, Graham W, & Rennie AM (1998). Can anxiety in pregnant women be measured using the State-Trait Anxiety Inventory. Midwifery, 14(2), 118–121. [DOI] [PubMed] [Google Scholar]
- Jones CW, Esteves KC, Gray SAO, Clarke TN, Callerame K, Theall KP, & Drury SS (2019). The transgenerational transmission of maternal adverse childhood experiences (ACEs): Insights from placental aging and infant autonomic nervous system reactivity. Psychoneuroendocrinology, 106, 20–27. doi: 10.1016/j.psyneuen.2019.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Mason K, Alkon A, Coccia M, & Bush NR (2018). Autonomic nervous system functioning assessed during the Still-Face Paradigm: A meta-analysis and systematic review of methods, approach and findings. Developmental Review, 50(Pt B), 113–139. doi: 10.1016/j.dr.2018.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibig SD (2010). Autonomic nervous system activity in emotion: a review. Biological Psychology, 84(3), 394–421. doi: 10.1016/j.biopsycho.2010.03.010 [DOI] [PubMed] [Google Scholar]
- Levenson RW (2014). The autonomic nervous system and emotion. Emotion Review, 6(2), 100–112. doi: 10.1177/1754073913512003 [DOI] [Google Scholar]
- Lobo FM, & Lunkenheimer E (2020). Understanding the parent-child coregulation patterns shaping child self-regulation. Developmental Psychology, 56(6), 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunkenheimer E, Kemp CJ, Lucas-Thompson RG, Cole PM, & Albrecht EC (2017). Assessing biobehavioural self-regulation and coregulation in early childhood: The parent-child challenge task. Infant and Child Development, 26(1), e1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan KK, Lewis AJ, Watson SJ, Jansen B, & Galbally M (2020). Maternal trauma and emotional availability in early mother-infant interaction: findings from the Mercy Pregnancy and Emotional Well-being Study (MPEWS) cohort. Attachment & Human Development, 23(6), 853–875. doi: 10.1080/14616734.2020.1790116 [DOI] [PubMed] [Google Scholar]
- Marcovitch S, Leigh J, Calkins SD, Leerks EM, O’Brien M, & Blankson AN (2010). Moderate vagal withdrawal in 3.5-year-old children is associated with optimal performance on executive function tasks. Developmental Psychobiology, 52(6), 603–608. doi: 10.1002/dev.20462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus SM, Flynn HA, Blow FC, & Barry KL (2003). Depressive symptoms among pregnant women screened in obstetrics settings. Journal of Women’s Health, 12(4), 373–380. doi: 10.1089/154099903765448880 [DOI] [PubMed] [Google Scholar]
- McDonnell CG, & Valentino K (2016). Intergenerational effects of childhood trauma: Evaluating pathways among maternal ACEs, perinatal depressive symptoms, and infant outcomes. Child Maltreatment, 21(4), 317–326. doi: 10.1177/1077559516659556 [DOI] [PubMed] [Google Scholar]
- McGowan PO, & Matthews SG (2018). Prenatal stress, glucocorticoids, and developmental programming of the stress response. Endocrinology, 159(1), 69–82. doi: 10.1210/en.2017-00896 [DOI] [PubMed] [Google Scholar]
- McGrath JM, Records K, & Rice M (2008). Maternal depression and infant temperament characteristics. Infant Behavior and Development, 31(1), 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugo GJ, Caspi Y, Kammerer N, Mazelis R, Jackson E, Russell L, Clark C, Liebschutz J, & Kimerling R (2005). The assessment of trauma history in women with co-occurring substance abuse and mental disorders and a history of interpersonal violence. The Journal of Behavioral Health Services & Research, 32(2), 113–127. doi: 10.1007/bf02287261 [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Alvarez K, Fillbrunn M, Green JG, Jackson JS, Kessler RC, Sadikova E, Sampson NA, Vilsaint CL, Williams DR, & Williams DR (2019). Racial/ethnic variation in trauma-related psychopathology in the United States: a population-based study. Psychological Medicine, 49(13), 2215–2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Conron KJ, Koenen KC, & Gilman SE (2010). Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychological Medicine, 40(10), 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meades R, & Ayers S (2011). Anxiety measures validated in perinatal populations: a systematic review. Journal of Affective Disorders, 133(1–2), 1–15. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Bledsoe-Mansori SE, Johnson N, Killian C, Hamer RM, Jackson C, Wessel J, & Thorp J (2013). A prospective study of perinatal depression and trauma history in pregnant minority adolescents. American Journal of Obstetrics and Gynecology, 208(3), 211–217. doi: 10.1016/j.ajog.2012.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesman J, van IJzendoorn MH, & Bakermans-Kranenburg MJ (2009). The many faces of the Still-Face Paradigm: A review and meta-analysis. Developmental Review, 29(2), 120–162. [Google Scholar]
- Miller AL, Gearhardt AN, Fredericks EM, Katz B, Shapiro LF, Holden K, Kaciroti N, Gonzalez R, Hunter C, & Lumeng JC (2018). Targeting self-regulation to promote health behaviors in children. Behaviour Research and Therapy, 101, 71–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, & Hurtado A (2000). Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate. Developmental Psychobiology, 36(1), 67–77. [PubMed] [Google Scholar]
- Moog NK, Entringer S, Rasmussen JM, Styner M, Gilmore JH, Kathmann N, Heim CM, Wadhwa PD, & Buss C (2018). Intergenerational effect of maternal exposure to childhood maltreatment on newborn brain anatomy. Biological Psychiatry, 83(2), 120–127. doi: 10.1016/j.biopsych.2017.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore GA, Hill-Soderlund AL, Propper CB, Calkins SD, Mills-Koonce WR, & Cox MJ (2009). Mother-infant vagal regulation in the face-to-face still-face paradigm is moderated by maternal sensitivity. Child Development, 80(1), 209–223. doi: 10.1111/j.1467-8624.2008.01255.x [DOI] [PubMed] [Google Scholar]
- Myers HF (2009). Ethnicity- and socio-economic status-related stresses in context: an integrative review and conceptual model. Journal of Behavioral Medicine, 32(1), 9–19. doi: 10.1007/s10865-008-9181-4 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58(4), 361–383. doi: 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolvi S, Bridgett DJ, Korja R, Kataja EL, Junttila N, Karlsson H, & Karlsson L (2019). Trajectories of maternal pre- and postnatal anxiety and depressive symptoms and infant fear: Moderation by infant sex. Journal of Affective Disorders, 257, 589–597. doi: 10.1016/j.jad.2019.07.055 [DOI] [PubMed] [Google Scholar]
- Olino TM, Michelini G, Mennies RJ, Kotov R, & Klein DN (2021). Does maternal psychopathology bias reports of offspring symptoms? A study using moderated non-linear factor analysis. Journal of Child Psychology and Psychiatry. 62(10), 1195–1201. doi: 10.1111/jcpp.13394 [DOI] [PubMed] [Google Scholar]
- Parade SH, & Leerkes EM (2008). The reliability and validity of the Infant Behavior Questionnaire-Revised. Infant Behavior and Development, 31(4), 637–646. doi: 10.1016/j.infbeh.2008.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pat-Horenczyk R, Zamir O, Yochman A, Schiff M, Brickman S, Lerner M, & Brom D (2020). Long-term impact of maternal posttraumatic symptoms on children’s regulatory functioning: A four-year follow-up study. Psychological Trauma: Theory, Research, Practice, and Policy, 12(2), 131–137. [DOI] [PubMed] [Google Scholar]
- Planalp EM, & Braungart-Rieker JM (2015). Trajectories of regulatory behaviors in early infancy: Determinants of infant self-distraction and self-comforting. Infancy, 20(2), 129–159. doi: 10.1111/infa.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW (2003). The polyvagal theory: Phylogenetic contributions to social behavior. Physiology & Behavior, 79(3), 503–513. [DOI] [PubMed] [Google Scholar]
- Posner MI, & Rothbart MK (2000). Developing mechanisms of self-regulation. Developmental Psychopathology, 12(3), 427–441. doi: 10.1017/s0954579400003096 [DOI] [PubMed] [Google Scholar]
- Powers A, Woods-Jaeger B, Stevens JS, Bradley B, Patel MB, Joyner A, Smith AK, Jamieson DJ, Kaslow N, & Michopoulos V (2020). Trauma, psychiatric disorders, and treatment history among pregnant African American women. Psychological Trauma: Theory, Research, Practice, and Policy, 12(2), 138–146. doi: 10.1037/tra0000507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Bosquet Enlow M, Schulz SM, Kitts R, Staudenmayer J, & Wright RJ (2012). Respiratory sinus arrhythmia as an index of vagal activity during stress in infants: respiratory influences and their control. PLOS ONE, 7(12), e52729. doi: 10.1371/journal.pone.0052729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz T, Dahme B, Dubois AB, Folgering H, Fritz GK, Harver A, Kotses H, Lehrer PM, Ring C, Steptoe A, & Van de Woestijne KP (2002). Guidelines for mechanical lung function measurements in psychophysiology. Psychophysiology, 39(5), 546–567. doi: 10.1017/S0048577202010715 [DOI] [PubMed] [Google Scholar]
- Ritz T, Schulz SM, Rosenfield D, Wright RJ, & Bosquet Enlow M (2020). Cardiac sympathetic activation and parasympathetic withdrawal during psychosocial stress exposure in 6-month-old infants. Psychophysiology, 57(12), e13673. doi: 10.1111/psyp.13673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson-Blackmore E, Putnam FW, Rubinow DR, Matthieu M, Hunn JE, Putnam KT, Moynihan JA, O’Connor TG (2013). Antecedent trauma exposure and risk of depression in the perinatal period. The Journal of Clinical Psychiatry, 74(10), 942–948. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2002). Processing speed in the 1st year of life: a longitudinal study of preterm and full-term infants. Developmental Psychology, 38(6), 895–902. doi: 10.1037//0012-1649.38.6.895 [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2012). Implications of infant cognition for executive functions at age 11. Psychological Science, 23(11), 1345–1355. doi: 10.1177/0956797612444902 [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament. In Eisenberg N & Damon W (Eds.) Handbook of child psychology: Vol 3. Social, emotional, and personality development (pp. 99–166). Hoboken, NJ: John Wiley & Sons. [Google Scholar]
- Rother M, Witte H, Zwiener U, Eiselt M, & Fischer P (1989). Cardiac aliasing—a possible cause for the misinterpretation of cardiorespirographic data in neonates. Early Human Development, 20(1), 1–12. [DOI] [PubMed] [Google Scholar]
- Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, Bizousky FR, & Krieger B (1989). Calibration of respiratory inductive plethysmograph during natural breathing. Journal of Applied Physiology, 66(1), 410–420. doi: 10.1152/jappl.1989.66.1.410 [DOI] [PubMed] [Google Scholar]
- Savory K, Garay S, Sumption L, Kelleher J, Daughters K, Janssen A, Van Goozen S, & John R (2020). Prenatal symptoms of anxiety and depression associated with sex differences in both maternal perceptions of one year old infant temperament and researcher observed infant characteristics. Journal of Affective Disorders, 264, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz SM, Ayala E, Dahme B, & Ritz T (2009). A MATLAB toolbox for correcting within-individual effects of respiration rate and tidal volume on respiratory sinus arrhythmia during variable breathing. Behavior Research Methods, 41(4), 1121–1126. doi: 10.3758/BRM.41.4.1121 [DOI] [PubMed] [Google Scholar]
- Scrimin S, Patron E, Lanfranchi S, Moscardino U, Palomba D, & Mason L (2019). Profiles of vagal withdrawal to challenging interactions: Links with preschoolers’ conceptual shifting ability. Developmental Psychobiology, 61(1), 116–124. doi: 10.1002/dev.21787 [DOI] [PubMed] [Google Scholar]
- Shalowitz MU, Berry CA, Rasinski KA, & Dannhausen-Brun CA (1998). A new measure of contemporary life stress: development, validation, and reliability of the CRISYS. Health Services Research, 33(5 Pt 1), 1381–1402. [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch R, & Lushene R (1970). STAI. Manual for the State-Trait Anxiety Inventory (Self Evaluation Questionnaire). Palo Alto California: Consulting Psychologist, 22, 1–24. [Google Scholar]
- Tarabulsy GM, Provost MA, Deslandes J, St-Laurent D, Moss E, Lemelin J-P, Bernier A, & Dassylva JF (2003). Individual differences in infant still-face response at 6 months. Infant Behavior and Development, 26(3), 421–438. [Google Scholar]
- Tibu F, Hill J, Sharp H, Marshall K, Glover V, & Pickles A (2014). Evidence for sex differences in fetal programming of physiological stress reactivity in infancy. Development and Psychopathology, 26(4 Pt 1), 879–888. doi: 10.1017/S0954579414000194 [DOI] [PubMed] [Google Scholar]
- Tronick E, Als H, Adamson L, Wise S, & Brazelton TB (1978). The infant’s response to entrapment between contradictory messages in face-to-face interaction. Journal of the American Academy of Child Psychiatry, 17(1), 1–13. doi: 10.1016/s0002-7138(09)62273-1 [DOI] [PubMed] [Google Scholar]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, Hoyer D, Roseboom T, Räikkönen K, King S, & Schwab M (2020). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neuroscience and Biobehavioral Reviews, 117, 26–64. doi: 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Wagner B, Latimer J, Adams E, Carmichael Olson H, Symons M, Mazzucchelli TG, Jirikowic T, Watkins R, Cross D, Carapetis J, & Carapetis J (2020). School-based intervention to address self-regulation and executive functioning in children attending primary schools in remote Australian Aboriginal communities. PLOS ONE, 15(6), e0234895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass SV, Scerif G, & Johnson MH (2012). Training attentional control and working memory – Is younger, better? Developmental Review, 32(4), 360–387. doi: 10.1016/j.dr.2012.07.001 [DOI] [Google Scholar]
- Weathers FW, Keane TM, & Davidson JR (2001). Clinician-administered PTSD scale: a review of the first ten years of research. Depression and Anxiety, 13(3), 132–156. doi: 10.1002/da.1029 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, & Keane TM (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at the annual convention of the International Society for Traumatic Stress Studies, San Antonio, TX. [Google Scholar]
- Weinberg MK, & Tronick EZ (1996). Infant affective reactions to the resumption of maternal interaction after the still-face. Child Development, 67(3), 905–914. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8706534 [PubMed] [Google Scholar]
- Wolfe J, Kimerling R, Brown P, Chrestman K, & Levin K (1997). Life Stressor Checklist-Revised (LSC-R). Measurement instrument]. http://www.ptsd.gov. [Google Scholar]
- Wolfe J, Kimerling R, Wilson J, & Keane T (1997). Assessing psychological trauma and PTSD. Gender issues in the assessment of Posttraumatic Stress Disorder, IJWTM Keane, Editor. New York: Guilford, 192–238. [Google Scholar]
- Woodward LJ, Lu Z, Morris AR, & Healey DM (2017). Preschool self regulation predicts later mental health and educational achievement in very preterm and typically developing children. The Clinical Neuropsychologist, 31(2), 404–422. [DOI] [PubMed] [Google Scholar]
- Ziegler MG (2012). Psychological stress and the autonomic nervous system. In Primer on the autonomic nervous system (pp. 291–293): Elsevier. [Google Scholar]


