Abstract
Objective:
More than 20% of the US population suffers from laryngopharyngeal reflux. While dietary/lifestyle modifications and alginates provide benefit to some, there is no gold standard medical therapy. Increasing evidence suggests that pepsin is partly, if not wholly, responsible for damage and inflammation caused by laryngopharyngeal reflux. A treatment specifically targeting pepsin would be amenable to local, inhaled delivery and could prove effective for endoscopic signs and symptoms associated with nonacid reflux. The aim herein was to identify small molecule inhibitors of pepsin and test their efficacy to prevent pepsin-mediated laryngeal damage in vivo.
Methods:
Drug and pepsin binding and inhibition were screened by high-throughput assays and crystallography. A mouse model of laryngopharyngeal reflux (mechanical laryngeal injury once weekly for 2 weeks and pH7 solvent/pepsin instillation 3days/week for 4 weeks) was provided inhibitor by gavage or aerosol (fosamprenavir or darunavir; 5days/week for 4 weeks; n=3). Larynges were collected for histopathologic analysis.
Results:
HIV protease inhibitors amprenavir, ritonavir, saquinavir and darunavir bound and inhibited pepsin with IC50 in the low micromolar range. Gavage and aerosol fosamprenavir prevented pepsin-mediated laryngeal damage (i.e. reactive epithelia, increased intraepithelial inflammatory cells, and cell apoptosis). Darunavir gavage elicited mild reactivity and no discernable protection; aerosol protected against apoptosis.
Conclusions:
Fosamprenavir and darunavir, FDA-approved therapies for HIV/AIDS, bind and inhibit pepsin, abrogating pepsin-mediated laryngeal damage in a laryngopharyngeal reflux mouse model. These drugs target a foreign virus, making them ideal to repurpose. Reformulation for local inhaled delivery could further improve outcomes and limit side effects.
Keywords: Laryngopharyngeal reflux, LPR, pepsin, fosamprenavir, Lexiva™, darunavir, Prezista™
INTRODUCTION
Laryngopharyngeal reflux (LPR), the backflow of gastric contents into the laryngopharynx, is an important health problem. LPR affects both children and adults, and the clinical spectrum is extensive. Unlike patients with gastroesophageal reflux (GER) which is limited to the esophagus, many LPR patients do not experience acid indigestion but present with symptoms due to chronic laryngeal irritation, such as chronic cough, throat-clearing, post-nasal drip, dysphonia, globus, dysphagia, and dyspnea1–3. Significant evidence supports the contribution of chronic LPR to serious and life-threatening illness including airway stenosis, reactive airway disease, and laryngeal cancer4–14. LPR is estimated to affect more than 20% of the United States population and contribute to 10% visits to otolaryngologists15–17. The economic burden of LPR is over $52 billion per year, which is 5.6-fold greater than that of GER; 52% of the burden is attributed to proton pump inhibitors (PPIs)18,19.
While PPI therapy is a mainstay in the treatment of GER disease (GERD), its efficacy for LPR is poor20–23. In clinical practice, it was believed that patients with reflux laryngitis require higher doses and longer trials of PPIs than those with typical GERD given the assumption that the upper airway is more sensitive to acid reflux than the esophagus3,24,25. However, placebo-controlled trials have failed to demonstrate therapeutic benefit of PPIs26–31. While Reichel et al. and Lam et al. reported symptom improvement in randomized, double-blind, placebo-controlled trials32,33, Vaezi argued that improvement was for heartburn rather than throat symptoms34. Where laryngeal symptom improvement has been reported it was found proportionally higher in GERD patients than in those without GERD35,36. Given the paucity of data supporting acid-suppression therapy for extraesophageal symptoms, the American Gastroenterological Association guidelines for GERD recommend against its use for acute treatment of patients with potential extraesophageal reflux (EER) syndromes (laryngitis, chronic cough) absent typical GERD symptoms37. Despite such advice, treatment for LPR frequently continues to involve empiric therapy with PPIs38,39.
While the acidity of reflux alone can damage the upper airways, combined multichannel intraluminal impedance-pH (MII-pH) monitoring has demonstrated that many episodes of LPR are nonacidic, and that weakly and nonacidic reflux is associated with persistent symptoms in acid-suppressed patients.40–43 These symptoms are alleviated by anti-reflux surgery44–50 and may be ameliorated by less invasive strategies that limit reflux occurrence or neutralize reflux constituents beyond acid (e.g. dietary and lifestyle modification and over-the-counter alginate products).51–54 Thus, one or more nonacid components of gastric refluxate must have a role in laryngeal damage. There is increasing evidence that pepsin, which is present in all refluxate55, is partly, if not wholly, responsible for damage and inflammation caused by LPR20,39,56–60.
Pepsin is a proteolytic enzyme which is synthesized and secreted as the zymogen pepsinogen by chief cells in the gastric fundus and subsequently cleaved upon introduction to the acidic stomach lumen to produce pepsin. Pepsin is maximally active at pH2 and retains activity up to pH6.5. While stable at pH8, pepsin is irreversibly inactivated at higher pH58,61. The stomach and esophagus have intrinsic defenses against pepsin (mucus, peristalsis, and bicarbonate secretion), however laryngeal tissues do not62. Pepsin is thought to play a key role in mucosal damage and inflammation during nonacidic reflux8,9,58,59,62–74. At neutral pH, pepsin is taken up by laryngeal and hypopharyngeal cells by receptor-mediated endocytosis and retained in intracellular vesicles of low pH where it is presumed to be reactivated58,67,68. The consequence is chronic inflammation, which in turn, gives rise to symptoms. Endocytosed nonacidic pepsin induces a proinflammatory cytokine gene expression profile in hypopharyngeal cells similar to that which contributes to disease severity during GERD59. Inhibition of the proteolytic activity of pepsin abrogates this damage and inflammation8,60,68,75–78. The on-surgical treatment options for nonacid reflux
With compelling evidence of nonacid proximal reflux of pepsin and its association with laryngeal and pharyngeal symptoms and endoscopic findings, the significant cost and risk of prolonged PPI therapy which continues to date despite its inefficacy in the absence of a gold standard medical therapy, and the limitations of alternative non-surgical treatment options such as the short-lived activity of over-the-counter products intended to provide temporary relief and the burden of adherence to dietary and lifestyle modifications, a new medical treatment which specifically targets pepsin would be of great value18,20,39,46,53,59,65,67,68. This new approach would be amenable to local treatment of readily accessible airways affected by LPR allowing lower dosing, the advantage of which is self-evident in that targeted delivery would simultaneously increase efficacy and limit systemic side effects.
We and others have discussed the promise of inhibitors of peptic activity and/or receptor antagonists as potential new therapeutics for LPR20,65,68,79. Given evidence that airway damage during EER is more closely associated with pepsin than acid, we hypothesize that a drug that targets pepsin will be effective for signs and symptoms associated with nonacid reflux. Local inhaled administration of such a drug would be more efficacious than oral. Herein, therapeutic compounds were screened for pepsin binding and inhibition. Specific HIV protease inhibitors that inhibited pepsin were administered orally and by inhalation in an LPR mouse model to assess their potential for the treatment of LPR.
METHODS
Binding and activity assays
To examine whether HIV protease inhibitors bound and inhibited pepsin, we developed assays based on fluorescence polarization which measures size-dependent molecular rotation thereby permitting detection of degradation, association and dissociation events80. A competitive binding assay was designed employing pepstatin, an inhibitor of sub-nanomolar affinity81. Pepstatin-Alexa647 was synthesized by dissolving 1mg pepstatin A (Sigma-Aldrich) in a 50:50 mixture of dimethylformamide (DMF) and dimethylsulfoxide (DMS) followed by the addition of N,N,N′,N′-Tetramethyl-O-(N-succinimidyl)uronium tetrafluoroborate (0.6mg) and trimethylamine (10μL) DMF. The mixture was stirred for 1 hour, after which 1mg Alexa Fluor 647 Cadaverine, Disodium Salt (ThermoFisher Scientific) was added. After 2 hours, the solvents were evaporated under high vacuum (35°C) and residue partially dissolved in 10% methanol and transferred onto a C18 cartridge (Waters Corporation, Milford, MA). Increasing percentages of methanol were used for the elution. Pepstatin-Alexa647 eluted at 45% methanol. An enzymatic inhibition assay was designed using casein substrate.82 Bovine alpha casein (Sigma-Aldrich, St. Louis, MO) was labeled with Alexa Fluor 647 Carboxylic Acid, Succinimidyl Ester (ThermoFisher Scientific, Waltham, MA) as described.82 Briefly, the two were combined at 2.5ug/mg label to protein ratio in 0.1M sodium bicarbonate for 15 minutes and labeled casein was separated from unbound label in a Sephadex G-25 (Sigma-Aldrich) column comprised of 90 × 5mm packed beads in a glass Pasteur pipette, eluted with dPBS pH7.4 (ThermoFisher Scientific). The fast-moving band (casein-bound fluorophore) was collected in ~0.4 ml volume. Concentration of resultant probe (casein-Alexa647 in PBS-azide) was estimated via spectrophotometry using Beer’s law (Implen Nanophotometer, Implen, Inc. Westlake Village, CA).
Assays were optimized using ranges of 0.3–1000μM unlabeled pepstatin, 100–500nM pepstatin-Alexa647 or casein-Alexa647 probe, 0.003–3U/μl porcine pepsin (Worthington Biochemical Corporation, Lakewood, NJ), and 5–37.5% DMSO (HIV protease inhibitor diluent) in 0.1 M HCl, pH 1 with 0.01% v/v Tween-20 in 20 μl volumes in 384-well black optical plates (Nunc, Roskilde, DK) and read on a BioTek Cytation 5 (BioTek Instruments, Winooski, VT) with far red FP filter cube (excitation/emission 620/680nm). Unlabeled pepstatin dose-response curves were used to ensure that the assays were responsive to pepsin inhibition. Conditions yielding maximal dynamic assay range were used to assess HIV protease inhibitors: 100nM probe, 0.03U/ul porcine pepsin A, 37.5% DMSO for competitive binding assay, and 200nM probe, 0.01U/μL pepsin, 5% DMSO for peptic activity assay. The HIV protease inhibitors (amprenavir, ritonavir, lopinavir, saquinavir mesylate, nelfinavir mesylate hydrate, darunavir ethanolate, indinavir sulfate salt hydrate; all Sigma-Aldrich) were dissolved in DMSO and tested under optimized assay conditions over three logs concentration. Assays were performed twice with triplicate reactions read for five minutes and mean mP plotted against probe concentration (binding assay) or read at <2minutes intervals over 30minutes with mean mP of plotted over time (activity assay). Half maximal inhibitory concentration (IC50) of inhibitors were calculated from kinetic traces analyzed using an online tool (https://icekat.herokuapp.com/icekat)83. mP was normalized to blank (absent inhibitor) to derive percent bound or activity.
Crystallization
Saturated solutions of HIV protease inhibitors (amprenavir, ritonavir and darunavir ethanolate) were prepared in DMSO and centrifuged for 10 minutes at 31,000 rcf. Supernatants were added to pepsin (200mg/ml in water) at 1.6% (v/v) f.c. Due to poor solubility, a solvent for saquinavir mesylate was selected from the CryoSol screen (Molecular Dimensions, Holland, OH). CryoSol mixture SM2 (consisting of 37.5% v/v dioxane, 25% v/v DMSO, 12.5% v/v ethylene glycol, 12.5% v/v 1,2-propanediol, and 12.5% v/v glycerol) was selected as it provided both high solubility and protein compatible conditions for the co-crystallization mixture. Supernatant of saturated saquinivar solution in SM2 was combined with pepsin at 5% f.c. (v/v). Crystallization conditions were optimized by screening 200mg/ml pepsin in the Salt RX screen (Hampton Research, Viejo, CA). Small bipyramid-shaped crystals formed in 3.5M ammonium chloride and 0.1M sodium acetate trihydrate pH 4.6 after one week at room temperature served as microseed stock for co-crystallization with amprenavir, ritonavir and darunavir ethanolate per previously described methods84. Diffraction quality crystals (triangular bi-pyramids, approximately 200 × 100 × 100μm) formed after 2–7days from hanging drops of 2ul pepsin (180–210mg/ml) and 1ul microseed solution serially diluted 10–100x above 3–4M ammonium chloride and 0.1M sodium acetate trihydrate pH4.6. Crystals were cryoprotected by 30% glucose, 5M ammonium chloride and 0.1M sodium acetate trihydrate pH4.6 and plunged in liquid nitrogen. Co-crystallization with saquinavir was performed in 0.1M acetic acid rather than sodium acetate trihydrate as this permitted large crystal formation without a microseed; crystals were cryoprotected by 30% w/v glucose, 5M ammonium chloride and 0.1M sodium acetate trihydrate pH 4.6 and plunged in liquid nitrogen.
Diffraction datasets were collected at Life Sciences Collaborative Access Team (LS-CAT) beamlines at the Advanced Photon Source (APS), Argonne National Laboratory, equipped with MAR 300 CCD or Dectris Eiger 9M detectors and data were indexed, integrated and scaled using MOSFLM85 or HKL200086.
Specifically, for pepsin:amprenavir, a 1.9Å diffraction data set was collected at LS-CAT beamline 21-ID-F with a MAR 300 CCD detector using a 50 × 50μm beam at a wavelength of 0.97872Å. A total of 262 frames were collected from φ = 0 to 130.5⁰ with an oscillation range of 0.5° and detector distance of 250mm. Exposure time was 0.5 seconds. Diffraction data were indexed, integrated and scaled using MOSFLM.
For pepsin:ritonavir, a 2.1Å diffraction data set was collected at LS-CAT beamline 21-ID-D with Dectris Eiger 9M detector using a 50 × 50 μm beam at 1.12721Å. 900 frames were collected from φ = 0 to 180⁰, while oscillating at a rate of 1 ⁰/sec and slicing of 5 images/ ⁰. Crystal-to-detector distance was 160mm. Diffraction data were indexed, integrated and scaled using MOSFLM.
For pepsin:darunavir, a 1.9Å diffraction data set was collected at LS-CAT beamline 21-ID-G with MAR 300 CCD detector and 50 × 50μm beam at 0.97856Å. 900 frames were collected from φ = 0 to 180⁰ with an oscillation range of 0.2⁰ and detector distance of 260 mm. Exposure time was 0.3 seconds. Diffraction data were indexed, integrated and scaled using HKL2000.
For pepsin:saquinavir, a 1.9 Å diffraction data set was collected at LS-CAT beamline 21-ID-F with MAR 300 CCD detector using a 50 × 50 μm beam at 0.97872Å. 400 frames were collected from φ = 20 to 100⁰ with an oscillation range of 0.2⁰ and detector distance of 200mm. Exposure time was 0.5 seconds. Diffraction data were indexed, integrated and scaled using MOSFLM.
Initial phases were obtained by molecular replacement in PHASER87. Unliganded porcine pepsin (PDB ID 4PEP) with B factors reset to 20.00 Å and solvent molecules removed was the search model. Model refinement was performed using phenix.refine (PHENIX87–89) and COOT90,91. Geometric restraints for compounds were obtained from CCP4 monomer library92. Models were validated using MolProbity93 as implemented in the PHENIX suite. Models of ritonavir and saquinavir were additionally optimized using PDB-REDO server94 prior to deposition. Electron density maps were generated via POVSCRIPT and POV-Ray and schematic representation by MarvinSketch,(http://www.ChemAxon.com) and Adobe Illustrator CC 2020.
In vivo mouse model
Experiments were approved by the University of Minnesota (UMN) Institutional Animal Care and Use Committee (1712-35415A) and performed at UMN. Three replicate animals per treatment condition were anticipated to suffice for verification of reproducibility in each experiment without excessive use of animal life. The three mice were randomly allocated to treatment groups. No data were excluded from analysis.
Six-week-old female Jackson A/J mice (Jackson Laboratory, Bar Harbor, ME) were fed D-62 powdered Wattenberg diet, 2 g/mouse/day95 and allowed to acclimate for one week upon arrival prior to experiments. In accord with previously established methods for modeling aerodigestive tract damage attributed to GERD and LPR,1,95–99 mechanical injury applied during the first two weeks of a four-week treatment course was used to predispose the laryngeal mucosa to chemical injury by pepsin/acid applied throughout the four weeks. When performed in this manner, mechanical injury increases mucosal susceptibility to subsequent chemical injury while leaving little detectable injury at the conclusion of a four-week treatment course.95 Mechanical injury was performed on all animals (including control) once weekly during the first two weeks of treatment as described (see experimental schema, Figure 1)95. Briefly, anesthetized mice were suspended by upper teeth on a slanted board under an operating microscope. Subglottis, glottis, and supraglottis were wounded under 6x magnification using a blunt, bent (135°) needle pulled distally to proximally making a mild abrasion.
Figure 1.
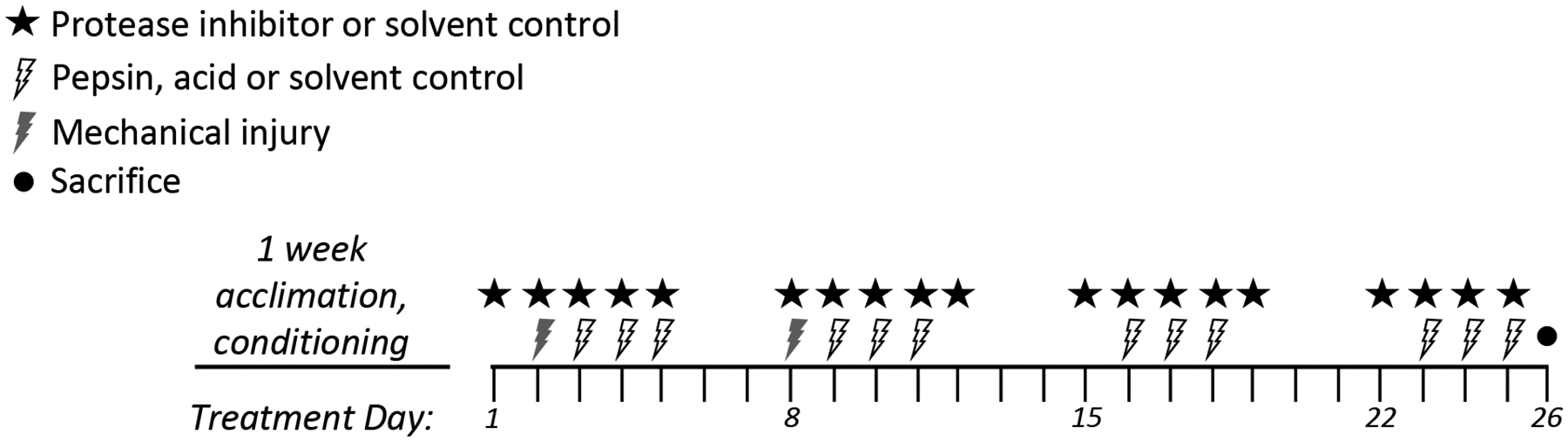
Treatment schema of in vivo mouse study.
In a preliminary experiment to validate the LPR mouse model (i.e. laryngeal damage by pepsin at neutral and acidic pH), 20μl saline (solvent control) or 0.3mg/ml pepsin at pH7.0 or 4.0 were provided to mice (n=3) by laryngeal instillation at 24, 48, and 72 hours after mechanical injury during weeks 1 and 2 (Figure 1); laryngeal instillation without wounding (3 days/week) continued during weeks 3 and 4. Mice were anesthetized with 225–240mg/kg intraperitoneal Avertin (2,2,2-Tribromoethanol) prior to each wounding and laryngeal instillation. Mice were sacrificed at conclusion of the fourth week.
To test the protective effect of HIV protease inhibitors on pepsin-mediated damage in vivo, inhibitors were delivered by aerosol or gavage concurrently with wounding (days 2, 8) and solvent/pepsin instillation (days 3–5, 9–11, 16–18 and 23–25). Aerosol or gavage was provided on days 1–5, 8–12, 15–19, and 22–25, and mice sacrificed day 26. Mice were anesthetized with isoflurane (3% in 2.5LPM, 3–5 minutes prior to procedures) as opposed to Avertin due to frequency. Lexiva and Prezista (hereafter referred to by generic: fosamprenavir and darunavir, respectively) were used for gavage, and respective pure drugs for aerosol (fosamprenavir from Anant Pharmaceuticals, Ambernath, Maharashtra India and darunavir from Ambeed, Arlington Heights, IL). Gavage dose was equivalent to that prescribed to HIV patients (20mg/kg/day fosamprenavir; 8.6mg/kg/day darunavir). Aerosol was generated as described100. Briefly, a 10ml suspension of drug in ethanol was placed in the baffle, such that the concentration would remain constant at the equilibrium solubility. Droplets of ethanol containing dissolved drug were generated by an ultrasonic atomizer (nominal frequency 1.7 MHz) and entrained by air at a flow rate of 0.5 LPM with a custom-built glass baffle (UMN Department of Chemistry Glass Shop). The aerosol cloud was then passed through a cylindrical drying column containing an annular ring of charcoal. The ethanol was removed and the emanating dry aerosol particles of pure drug were then directed into the exposure chamber. The mass deposited on filters was measured gravimetrically and total output rate (mg/min) was determined. The aerosol concentration (mass/volume of air) was calculated by dividing the total output rate by the air flow rate (0.5 LPM). The inhaled mass of drug (Minh) for each mouse was defined as Minh=[Aerosol]*RMV*t, where Aerosol is the aerosol concentration of drug, RMV is the respiratory minute volume of the mice (0.025 L/min), and t is the aerosol exposure time. Aerosol concentration was 0.09mg/L fosamprenavir or 1.2mg/L darunavir, therefore given the respiratory minute volume of mice (0.025 L/min), the inhaled mass was 0.93mg/kg/day fosamprenavir or 12mg/kg/day darunavir. Actual mass deposited was not determined but anticipated to be 10% of inhaled mass (the deposition fraction of 1μm aerosol particles in mice).
Tissues were collected, fixed in paraformaldehyde, embedded in paraffin and 4um sections stained with hematoxylin and eosin (H&E) via automated stainer. Slides were reviewed by a board-certified pathologist (JM) blinded to treatment groups.
RESULTS
Binding and Activity Assays
Four of the seven assayed HIV protease inhibitors bound and inhibited pepsin at low micromolar concentrations (Figure 2): amprenavir, darunavir, ritonavir, and saquinavir. The in vitro activity of these four HIV protease inhibitors against pepsin provided the foundational support for further study.
Figure 2.
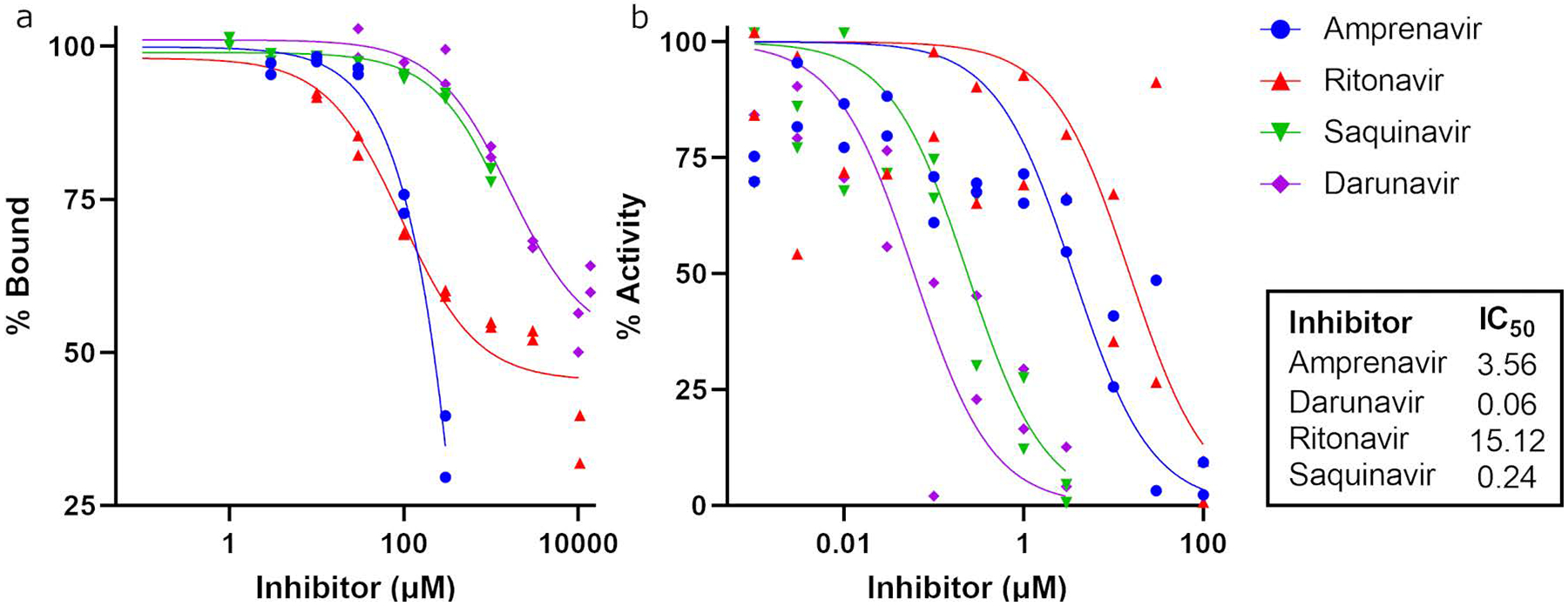
Binding (a) and activity (b) curves of pepsin with HIV protease inhibitors.
Structural data
To aid interpretation of the in vitro binding and inhibition data, commercially available porcine pepsin (EC 3.4.23.1) was used for co-crystallization experiments to obtain structural data. Crystallization of human pepsin collected from volunteers failed presumably due to sample heterogeneity. Porcine pepsin shares 86% sequence identity with the human enzyme (PDB ID 1PSN)101 and its structure is nearly identical (root mean square deviation (RMSD) for all Cα atoms=0.50Å). Minor differences in tertiary structure are localized to a loop of residues (277–282) which is not part of the binding cleft. Residues lining the active site cleft are highly conserved: of 17 making direct contact with inhibitors herein, just two differed (T12 and V291). Thus, porcine was deemed an acceptable substitute for human pepsin for assessing structural biology.
Porcine pepsin was co-crystalized amprenavir, darunavir, ritonavir, and saquinavir (Table 1 and Figure 3). All are peptidomimetics; the alcohol of the central phenylalaninol residue, which mimics the tetrahedral intermediate of peptide bond cleavage, is bound between catalytic aspartate residues, D32 and D215. Binding directionality of each (amino group of phenylalaninol on the prime side of the binding site) was the same as that for pepstatin101. Binding relied on van der Waals contacts between side chains of inhibitors and residues lining the binding site; few (5–6) hydrogen bonds were observed. For example, in the pepsin·ritonavir complex (Figure 3), the β-homophenylalanine side chain is bound in the P1 subsite, making van der Waals contacts with F111, F117, and I120. The phenylalaninol side chain is bound in the P1 subsite, contacting I213, M289, V291, and I300. The thiazole and isopropyl-thiazole groups of ritonavir do not have any stabilizing interactions with the active site. The electron density for these groups is correspondingly poorly defined, and the B-factors, which reflect the precision of the atomic positions, for these parts of the molecule are extremely high. The structure of the pepsin·saquinavir complex is similar in that the side chain of the phenylalaninol residue is interacting with the P1’ subsite, but the two ends of the molecule, the quinoline and decahydroisoquinoline moieties, also have poor density and high B-factors. The amprenavir and darunavir structures follow the same pattern. The phenylalaninol residues of both inhibitors occupy the P1’ site, interacting with I213, M289, V291, and I300. The isobutyl groups, mimicking leucine residues, occupy the P1 site, interacting with F111, F117, and I120. In both amprenavir and darunavir, one of the oxygen atoms of the sulfonamide moiety makes a hydrogen bond with the backbone amide of T77. The aniline groups make no polar contacts with the active site. At the opposite end of the molecules where the two compounds differ, the tetrahydrofuran group of amprenavir forms a hydrogen bond with the phenolic oxygen of Y189. The bis-tetrahydrofuran group of darunavir, however, cannot have this interaction with the active site and is limited to van der Waals contacts with I73, T74, I128, and Y189. The structures and binding poses of amprenavir and darunavir were similar and provided no explanation for their disparity in IC50.
Table 1.
Crystallographic data collection and model refinement statistics
| Pepsin·Amprenavir | Pepsin·Ritonavir | Pepsin·Darunavir | Pepsin·Saquinavir | |
|---|---|---|---|---|
| PDB Entry | 6XCT | 6XCY | 6XD2 | 6XCZ |
| Data collection | ||||
| Resolution (Å) (last shell)a | 72.02 – 1.99 (2.04 – 1.99) |
53.17 – 2.05 (2.11 – 2.05) |
49.34 – 1.90 (1.97 – 1.90) |
57.50 – 1.89 (1.93 – 1.89) |
| Space group | P 65 2 2 | P 65 2 2 | P 65 2 2 | P 65 2 2 |
| a, b, c (Å) | 66.1, 66.1 288.1 | 66.2, 66.2, 285.5 | 66.2, 66.2, 290.0 | 66.4, 66.4, 284.6 |
| α, β, γ (°) | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 | 90, 90, 120 |
| Rmergea | 0.057 (0.099) | 0.10 (0.56) | 0.088 (0.25) | 0.091 (0.73) |
| Rmeasa | 0.060 (0.11) | 0.13 (0.73) | 0.092 (0.26) | 0.110 (0.85) |
| Rpima | 0.021 (0.038) | 0.084 (0.46) | 0.026 (0.077) | 0.054 (0.42) |
| CC1/2a | 0.999 (0.991) | 0.989 (0.593) | 0.995 (0.981) | 0.995 (0.649) |
| No. of unique reflectionsa | 26876 (1807) | 23751 (1831) | 30916 (2998) | 30114 (1885) |
| Completeness (%)a | 99.9 (99.5) | 98.2 (99.8) | 99.85 (99.90) | 97.8 (98.0) |
| Multiplicitya | 13.4 (12.8) | 3.1 (3.2) | 12.0 (11.8) | 5.5 (5.9) |
| 〈 I/σ(I)〉a | 31.6 (18.8) | 6.3 (2.3) | 35.71 (10.34) | 7.9 (1.6) |
| Model Refinement | ||||
| Reflections used in refinementa | 26760 (2580) | 23747 (2360) | 30888 (2995) | 30072 (2948) |
| Reflections used for Rfreea | 1312 (114) | 1214 (116) | 1574 (125) | 1544 (145) |
| Rcryst (Rfree)a | 0.1907 (0.1906) | 0.2173 (0.2586) | 0.1997 (0.1887) | 0.2260 (0.2941) |
| Wilson B-factor (Å2) | 17.73 | 34.87 | 18.85 | 27.03 |
| Average B factor (Å2) | 20.66 | 45.32 | 22.66 | 38.38 |
| Protein atoms | 19.62 | 44.98 | 21.61 | 37.97 |
| Ligand atoms | 24.29 | 71.10 | 26.63 | 59.65 |
| Solvent | 27.46 | 40.81 | 29.22 | 38.11 |
| Root-mean-square (RMS) deviations | ||||
| Bond lengths (Å) | 0.009 | 0.011 | 0.013 | 0.015 |
| Bond angles (°) | 0.77 | 1.48 | 1.02 | 1.68 |
| Coordinate error (Å)b | 0.14 | 0.12 | 0.15 | 0.10 |
| Ramachandran statistics | ||||
| Favored/allowed/outliers (%) | 99.37/0.32/0.32 | 97.82/1.87/0.31 | 99.37/0.32/0.32 | 98.13/1.56/0.31 |
| Rotamer outliers (%) | 0.00 | 1.82 | 0.00 | 2.92 |
| Clashscore | 0.85 | 2.72 | 2.33 | 2.93 |
Values in parentheses apply to the high-resolution shell indicated in the resolution row
Maximum-likelihood based estimates of coordinate error
Figure 3. Pepsin and HIV protease inhibitor structural data.
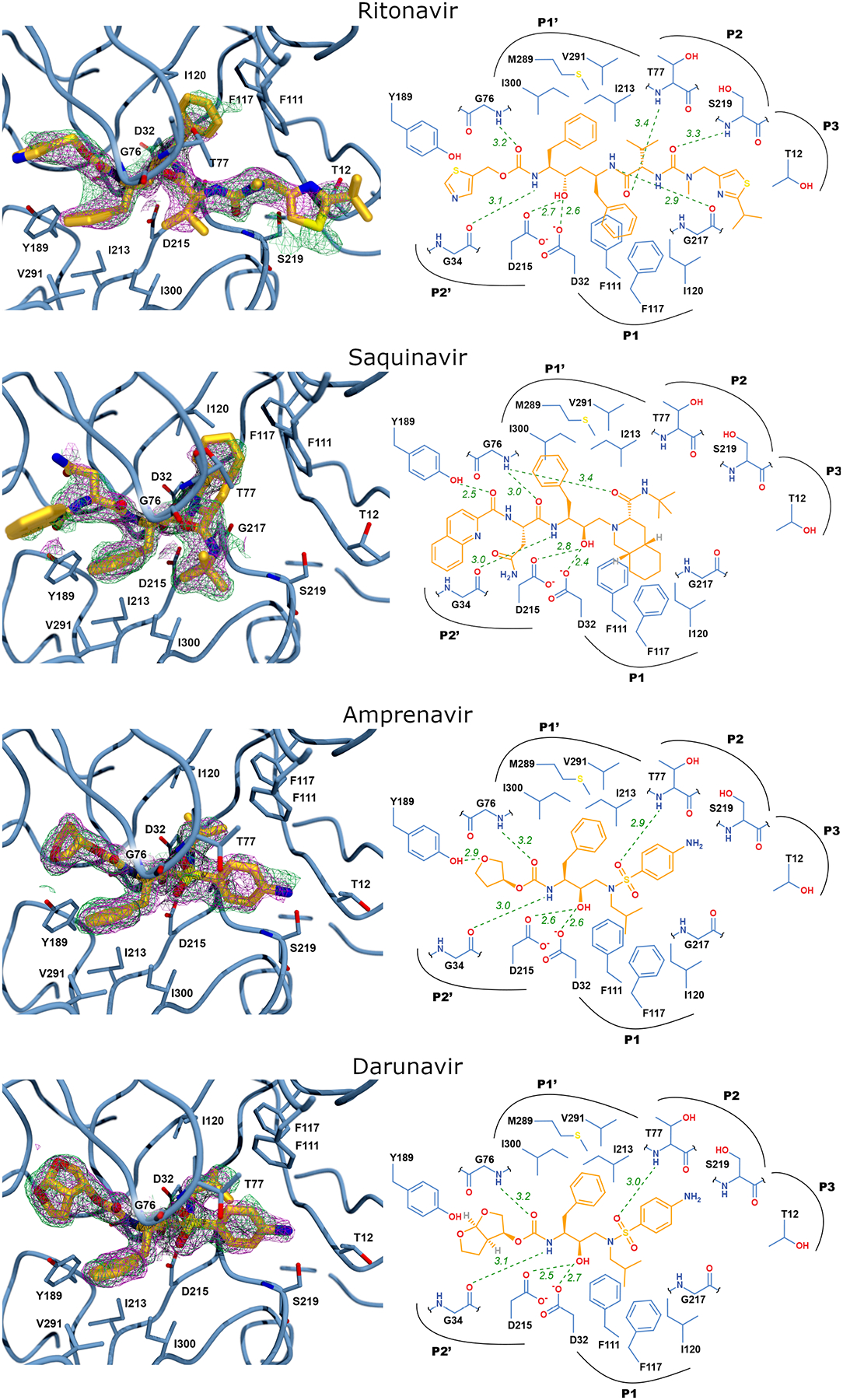
Left panels: The active site of porcine pepsin with HIV protease inhibitor bound. The 2Fo-Fc electron density map contoured at 1.0σ is shown as magenta mesh and the 2Fo-Fc simulated annealing composite omit map, also contoured at 1.0σ, is shown as green mesh. Right panels: Schematic view of the active site with ritonavir bound showing potential hydrogen bonding interactions as green, dashed lines. Electron density maps were generated via POVSCRIPT and POV-Ray and schematic representation by MarvinSketch, and Adobe Illustrator.
In vivo mouse model
Pepsin-mediated laryngeal epithelial damage was observed at pH 4 and 7 in the mouse in vivo model which employed pepsin with or without acid exposure following mechanical injury of the larynx (Figure 4). Animals in the pH7 control group had normal laryngeal epithelium of 1–2 cells thick with cilia present and no inflammation, keratinization, or necrosis; findings indicated no detectable mucosal damage in the control group due to mechanical injury during the first two weeks of treatment or pH7 solvent. Laryngeal epithelium in the pH4 group was reactive, thickened (3–4 cells thick), and keratinized with loss of cilia. That from the pepsin-pH7 group had an intermediate thickness (2–3 cells), evidence of keratinization, increased nuclear to cytoplasmic ratio and loss of polarization. That from the pepsin-pH4 group exhibited total loss of epithelium due to necrosis and inflammatory cell infiltrate.
Figure 4. Laryngeal epithelial damage by pepsin and acid in vivo.
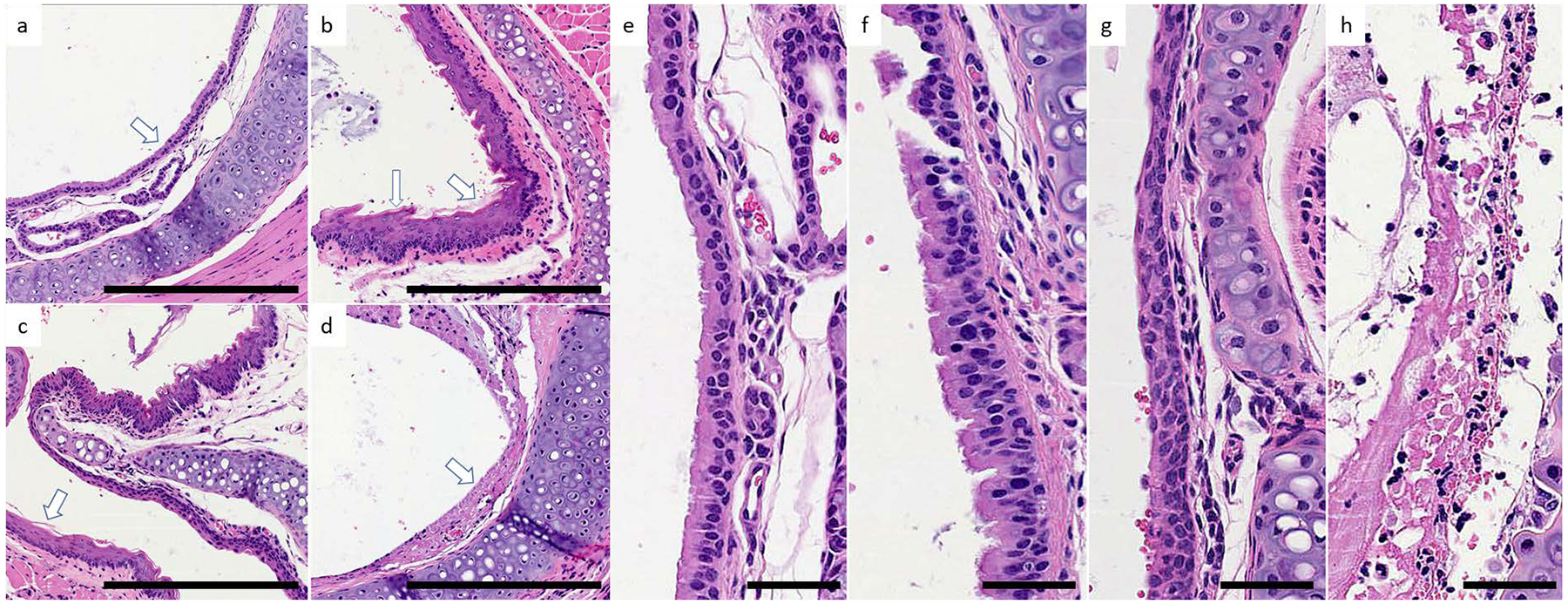
Representative specimens from treatment groups. Paired images at 40x (a-d) and 200x (e-h) magnification collected rostral to vocal folds, representing larynx: pH7 (a,e), pH4 (b,f), 0.3 mg/ml pepsin at pH7 (c,g), and 0.3 mg/ml pepsin at pH4 (d,h). (a,e) Normal respiratory columnar epithelium (arrow) about one cell layer thick with basal polarization of the nuclei and ciliated apical surfaces. (b,f) Reactive epithelium characterized by thickening (fat arrow) and focal squamous epithelia (long arrow) with loss of cilia. In other areas, relative thickening of the mucosa with moderately increased nuclear to cytoplasmic (N:C) ratio and irregular, condensed chromatin is seen. (c,g) Thickened respiratory epithelium with pseudostratification of the epithelial cells. Keratinization (arrow) is present in multiple foci. Significant increase in the N:C ratio with loss of nuclear polarization and reduction in the apical cilia is evident in several regions of this treatment group. (d,h) Respiratory epithelium is necrotic (arrow) and replaced by an inflammatory exudate. A brisk, acute inflammatory infiltrate infiltrates the submucosal area. Scale bars a-d = 100um; e-h = 50um.
Fosamprenavir gavage equivalent to the dose used to treat HIV in humans prevented pepsin-mediated laryngeal damage, defined as reactive epithelia, increased intraepithelial inflammatory cells, and apoptosis (Figure 5). Mild reactivity elicited by oral darunavir (absent in darunavir aerosol group; Figure 4) obscured the ability to detect its effect on pepsin-mediated damage. Fosamprenavir aerosol prevented pepsin-mediated laryngeal injury (Figure 4). Darunavir aerosol provided moderate protection against pepsin-mediated damage: while epithelial injury was present (mildly increased intraepithelial inflammatory cells and reactive epithelial cells), no apoptosis was observed as it was in mice treated with pepsin-pH7 and sham inhalation.
Figure 5. Fosamprenavir gavage and aerosol and darunavir aerosol prevent pepsin-mediated laryngeal damage in vivo.
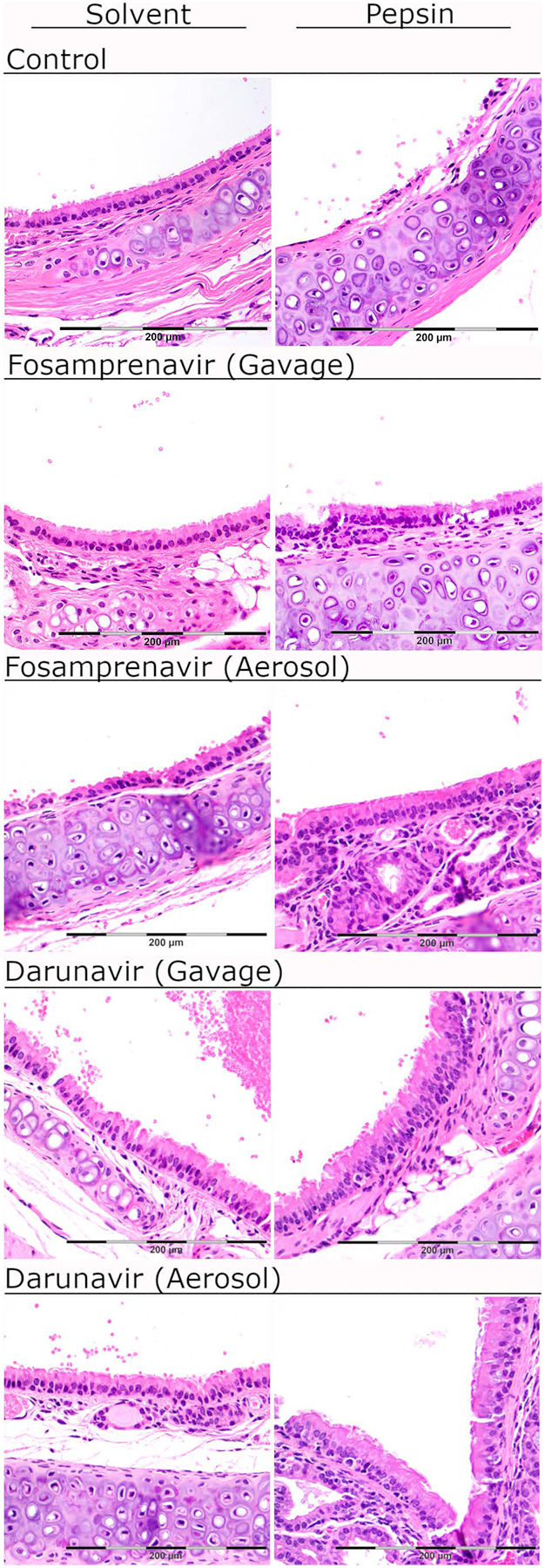
Representative specimens at 400x. Solvent control group laryngeal epithelium was characterized by a single layer of respiratory epithelium with no reactive changes. In mice treated with pepsin-pH7, the laryngeal epithelium exhibited reactive epithelial changes and apoptotic debris. Fosamprenavir gavage and aerosol protected against pepsin-mediated laryngeal damage as indicated by normal histology in mice receiving fosamprenavir gavage or aerosol with saline (solvent), or fosamprenavir gavage or aerosol with pepsin-pH7. Darunavir gavage elicited mild reactivity (rare intraepithelial lymphocytes) in the saline treatment group; the darunavir gavage group with pepsin-pH7 appeared similar. Darunavir aerosol provided mild protection against pepsin-mediated damage. Epithelial injury was still present (mildly increased intraepithelial inflammatory cells and reactive epithelial cells), however no apoptosis was observed. Scale bar=200um.
DISCUSSION
For the past two decades, the treatment of LPR has focused on suppressing gastric acid production. With the introduction of MII-pH technology, it is now understood that LPR is commonly nonacidic and that nonacid proximal events are associated with laryngeal endoscopic signs and symptoms39–46,48–50,102. These findings sparked investigations into the nonacidic components of gastric refluxate.
Although bile induces mucosal damage at weakly and non- acid pH experimentally, it has been argued that “there is no evidence that the same mechanism occurs in the human larynx”57. The clinical relevance of experimental findings has been called into question. Unconjugated bile acids, which cause damage at neutral-high pH such as that of the laryngopharynx, are rarely found in gastric refluxate.56,69 Further, concentrations of bile salts/acids found to damage the larynx and hypopharynx experimentally are 1000-fold greater than those reported in the airways of patients with LPR, GERD and asthma, or lung disease (0.3–50 mM96,103,104 versus 0.8–32uM105–109) and result in morphologic changes inconsistent with those of LPR patients such as cell membrane ‘blebbing’110.
Pepsin is present in all refluxate55. Moreover, it is frequently detected in airway tissue and secretions from LPR patients but absent in MII-pH-confirmed reflux-free subjects, and thus may be predictive of reflux-attributed symptoms and disease20,39,46,50,55,59,65,67,68,111,112. Pepsin at 1mg/ml in the stomach is diluted by saliva as it is refluxed proximally. A range of concentrations have been reported in airways: 2.5μg/ml in saliva, 61.5μg/ml in nasal secretions113,114 and 360μg/ml in middle ear fluid115. To model chronic LPR within a limited experimental timeframe, 300μg/ml was employed herein1,77,116,117. Pepsin-mediated damage and inflammatory changes reported in vitro and in vivo, including the histologic changes herein, are consistent with those observed in LPR patients62–64,66,70,118–122. Compelling evidence from multiple groups highlights a major role for pepsin, independent of gastric acid, in reflux-attributed laryngeal symptoms and findings refractory to PPI therapy.
While pepstatin is a potent pepsin inhibitor, its poor water-solubility and pharmacokinetic properties make it a suboptimal therapeutic candidate. Structural data herein indicated that inhibitor binding to the active cleft of pepsin is predominantly stabilized by van der Waals contacts, making rational design of inhibitors difficult. Testing existing inhibitors of other aspartic proteases was therefore deemed the most efficacious route for identification of a pepsin-targeting therapeutic.
There are currently ten commercially available HIV protease inhibitors.123 Seven were amenable to testing in our in vitro binding and inhibition assays and four (amprenavir, ritonavir, saquinavir and darunavir) bound and inhibited pepsin with IC50 in the low micromolar range, validating our hypothesis that existing therapeutic protease inhibitors may exhibit anti-peptic activity. Two drugs were selected for in vivo study based on anti-peptic activity from in vitro assays, cost and reported side effects. While saquinavir exhibits known side effects and interactions (QT prolongation, heart block, high blood lipids and liver problems) and has high cost, amprenavir, ritonavir, and darunavir have minimal side effects (diarrhea, nausea and vomiting).123 Darunavir is more costly than amprenavir and ritonavir, but had the lowest IC50 for pepsin. Darunavir, with the lowest IC50, and fosamprenavir, a prodrug of amprenavir with improved bioavailability and favorable tolerability were therefore selected for assessment in vivo. Given that proximal reflux is inconsistent in surgical models of GER124, we employed a model involving mechanical wounding and pepsin/acid instillation which reliably replicates epithelial alterations similar to that observed in patients with LPR1,63,70,118,119,125. Using this model, the human-equivalent dose of fosamprenavir, but not darunavir, prevented pepsin-mediated laryngeal damage. When administered locally by inhalation, treatment with either compound preserved normal laryngeal histology despite pepsin exposure.
As these data suggest, reformulation for inhaled, local delivery would be expected to improve drug efficacy and limit side effects. Preliminary computational fluid dynamics analysis (unpublished) revealed an optimal particle size of 9–12μm for deposition in the human larynx in agreement with previous studies126.
The study herein was intended to investigate whether a pepsin inhibitor may prevent laryngeal damage caused by pepsin exposure in vivo. As with any experimental observation, caution should be exercised when translating in vivo findings from a limited number of animals to the clinical situation. Potential differences between mouse and human respiratory pathobiology should be kept in mind while evaluating the clinical implications of these data. Established methods for in vivo modeling of aerodigestive tract damage attributed to GERD and LPR1,96–99 were utilized herein and demonstrated mucosal damage consistent with the clinical presentation of LPR supporting their use for assessing drug prevention of LPR-attributed injury: at the four-week conclusion of treatment, no mucosal damage was detectable given mechanical injury and neutral solvent, whereas multi-layered, reactive epithelia with apoptosis was observed in the pepsin and acid treatment groups. The mouse epiglottis occupies a transitional zone from stratified squamous epithelium of the vocal fold to ciliated pseudostratified columnar epithelium at the supraglottis and infraglottis.127 To avoid misinterpreting squamous epithelium of the vocal folds as signs of injury, representative images were collected rostral to vocal folds, exclusively from tissue with visible thyroid to serve as a guide. Additional features of reactive epithelia (darkened nuclei, variable nuclear diameter, and increased nuclear to cytoplasmic ratio, intraepithelial inflammatory cells, and apoptosis) in pepsin-treated groups, absent in control pH7.0 and those receiving fosamprenavir or darunavir, confirmed epithelial reactivity due to pepsin and the efficacy of HIV protease for prevention of pepsin-mediated injury. While these data are qualitative and would be bolstered by less subjective quantitative measures, the evidence herein provides initial proof-of-concept that a pepsin-targeting therapeutic may reduce mucosal damage akin to that observed in LPR patients and supports more in-depth investigation. Research is ongoing in our laboratory to examine fosamprenavir protection against pepsin-mediated changes in laryngeal cell viability and inflammatory and carcinogenic gene and protein expressions. Further research is also warranted to determine whether laryngeal protection by fosamprenavir aerosol in vivo was due to systemic activity or local conversion to amprenavir. The intestine is the primary site of fosamprenavir metabolism. Conversion of fosamprenavir to amprenavir by alkaline phosphatase (ALP), which is required for its transepithelial flux and subsequent metabolism by cytochrome P450 enzymes, has been shown to occur via intestinal ALP at or near the surface of Caco-2 cells.128,129 It is possible, however, that inhaled fosamprenavir is converted to amprenavir in the airways by serum ALP, just as similar phosphate ester prodrugs are converted by sera collected from healthy subjects.130 Inhaled fosamprenavir may also be converted by salivary ALP or that expressed by respiratory mucosa and immune cells recruited to tissue injury.131,132 Given that ALP is elevated during inflammation132–134 and carcinogenesis including that of the larynx to which LPR contributes,10,74,135–137 ALP may be elevated in LPR-damaged airways thereby increasing fosamprenavir conversion at the desired site of activity. Drug formulations that prolong retention in the aerodigestive tract could further improve local drug conversion and topical activity. Research is ongoing in our laboratory to examine the efficiency of fosamprenavir conversion by laryngeal epithelium, saliva and sera and a dose-response study is underway in the in vivo mouse model to compare the relative efficacies of inhaled fosamprenavir and amprenavir against pepsin-mediated damage.
While additional experimental data will aid our understanding of laryngeal protection by fosamprenavir, LPR symptom improvement will be the ultimate determinant of a successful medical therapy. A randomized placebo-controlled trial therefore represents the best test of a therapeutic compound. Such a trial of fosamprenavir is feasible given that an oral formulation is FDA-approved and an a priori responder definition of clinically meaningful symptom improvement has been established per FDA guidelines.138 Intriguingly, pilot epidemiological data (unpublished) support the therapeutic potential of HIV protease inhibitors for LPR and warrant follow-up: among 2,062 adult HIV patients prescribed an HIV protease inhibitor (Froedtert Memorial Lutheran Hospital, Milwaukee, WI, July 2014–2016; Medical College of Wisconsin Institutional Review Board, 13874) just 0.2% had documented LPR whereas the incidence in the general population is 10–34%.139,140 These data lend preliminary support for clinical investigation of fosamprenavir as a novel therapeutic approach for LPR.
CONCLUSION
Compelling evidence highlights a major role for pepsin (independent of gastric acid) in reflux-attributed laryngeal symptoms and endoscopic findings refractory to PPI therapy. Fosamprenavir and darunavir, FDA-approved retroviral therapies for HIV/AIDS, bind and inhibit pepsin, abrogating pepsin-mediated laryngeal inflammation and mucosal damage in an LPR mouse model. These drugs target a foreign virus so are ideal to repurpose, allowing a clinical trial to assess efficacy for a much-needed medical treatment for patients faster than could be achieved with novel compounds. Reformulation for local inhaled delivery could further improve outcomes and limit side effects.
Acknowledgments:
The authors would like to thank Leah Radde, Alexandra Dzubinsky, and Gino Scuncio for their preliminary work in this project.
Funding:
Work was funded by the Medical College of Wisconsin Therapeutics Accelerator Program, Department of Pharmacology, and Department of Otolaryngology and Communication Sciences. This work was also supported by the National Institutes of Health (R35GM128840 to B.C.S.).
Footnotes
Competing Interests: N.J. is an inventor on an International Patent Application: PCT/US2021/027758, Aerosolized formulations of HIV protease inhibitors for the treatment of airway reflux, filed April 16, 2021. Since completion of this study, N.J. became co-founder, Chief Scientific Officer and an investor in N-Zyme Biomedical, and T.S. an investor in N-Zyme Biomedical. The other authors have no financial relationships or conflicts of interest to disclose.
Data Availability
Structural data are available in the Worldwide Protein Databank (accession codes 6XCY, 6XCT, 6XCZ, 6XD2; http://www.wwpdb.org/). Additional information is available from the corresponding author on reasonable request.
REFERENCES
- 1.Koufman JA. The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 1991;101:1–78. [DOI] [PubMed] [Google Scholar]
- 2.Vaezi MF. Extraesophageal manifestations of gastroesophageal reflux disease. Clin Cornerstone 2003;5:32–38; discussion 39–40. [DOI] [PubMed] [Google Scholar]
- 3.Ford CN. Evaluation and management of laryngopharyngeal reflux. JAMA 2005;294:1534–1540. [DOI] [PubMed] [Google Scholar]
- 4.Bianchi ET, Guerreiro Cardoso PF, Minamoto H, et al. Impact of fundoplication for gastroesophageal reflux in the outcome of benign tracheal stenosis. J Thorac Cardiovasc Surg 2019;158:1698–1706. [DOI] [PubMed] [Google Scholar]
- 5.Esposito C, Saxena A, Irtan S, Till H, Escolino M. Laparoscopic Nissen Fundoplication: An Excellent Treatment of GERD-Related Respiratory Symptoms in Children-Results of a Multicentric Study. J Laparoendosc Adv Surg Tech A 2018;28:1023–1028. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel CE, Jones DG. The importance of chronic laryngitis. J Laryngol Otol 1960;74:349–357. [DOI] [PubMed] [Google Scholar]
- 7.Garg D, Mody M, Pal C, et al. Follicular Bronchiolitis: Two Cases with Varying Clinical and Radiological Presentation. Case Rep Pulmonol 2020;2020:4564587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston N, Yan JC, Hoekzema CR, et al. Pepsin promotes proliferation of laryngeal and pharyngeal epithelial cells. Laryngoscope 2012;122:1317–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly EA, Samuels TL, Johnston N. Chronic pepsin exposure promotes anchorage-independent growth and migration of a hypopharyngeal squamous cell line. Otolaryngol Head Neck Surg 2014;150:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim SY, Park B, Lim H, Kim M, Kong IG, Choi HG. Increased risk of larynx cancer in patients with gastroesophageal reflux disease from a national sample cohort. Clin Otolaryngol 2019;44:534–540. [DOI] [PubMed] [Google Scholar]
- 11.Parsel SM, Wu EL, Riley CA, McCoul ED. Gastroesophageal and Laryngopharyngeal Reflux Associated With Laryngeal Malignancy: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol 2019;17:1253–1264 e1255. [DOI] [PubMed] [Google Scholar]
- 12.Riley CA, Marino MJ, Hsieh MC, Wu EL, Wu XC, McCoul ED. Detection of laryngeal carcinoma in the U.S. elderly population with gastroesophageal reflux disease. Head Neck 2019;41:1434–1440. [DOI] [PubMed] [Google Scholar]
- 13.Tae K, Jin BJ, Ji YB, Jeong JH, Cho SH, Lee SH. The role of laryngopharyngeal reflux as a risk factor in laryngeal cancer: a preliminary report. Clin Exp Otorhinolaryngol 2011;4:101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wight R, Paleri V, Arullendran P. Current theories for the development of nonsmoking and nondrinking laryngeal carcinoma. Curr Opin Otolaryngol Head Neck Surg 2003;11:73–77. [DOI] [PubMed] [Google Scholar]
- 15.Altman KW, Stephens RM, Lyttle CS, Weiss KB. Changing impact of gastroesophageal reflux in medical and otolaryngology practice. Laryngoscope 2005;115:1145–1153. [DOI] [PubMed] [Google Scholar]
- 16.Koufman JA, Amin MR, Panetti M. Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg 2000;123:385–388. [DOI] [PubMed] [Google Scholar]
- 17.Reulbach TR, Belafsky PC, Blalock PD, Koufman JA, Postma GN. Occult laryngeal pathology in a community-based cohort. Otolaryngol Head Neck Surg 2001;124:448–450. [DOI] [PubMed] [Google Scholar]
- 18.Francis DO, Rymer JA, Slaughter JC, et al. High economic burden of caring for patients with suspected extraesophageal reflux. Am J Gastroenterol 2013;108:905–911. [DOI] [PubMed] [Google Scholar]
- 19.Gelardi M, Ciprandi G. Focus on gastroesophageal reflux (GER) and laryngopharyngeal reflux (LPR): new pragmatic insights in clinical practice. J Biol Regul Homeost Agents 2018;32:41–47. [PubMed] [Google Scholar]
- 20.Bardhan KD, Strugala V, Dettmar PW. Reflux revisited: advancing the role of pepsin. Int J Otolaryngol 2012;2012:646901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinucci I, de Bortoli N, Savarino E, et al. Optimal treatment of laryngopharyngeal reflux disease. Ther Adv Chronic Dis 2013;4:287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C, Wang H, Liu K. Meta-analysis of the efficacy of proton pump inhibitors for the symptoms of laryngopharyngeal reflux. Braz J Med Biol Res 2016;49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reimer C, Bytzer P. Management of laryngopharyngeal reflux with proton pump inhibitors. Ther Clin Risk Manag 2008;4:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koufman JA. Laryngopharyngeal reflux is different from classic gastroesophageal reflux disease. Ear Nose Throat J 2002;81:7–9. [PubMed] [Google Scholar]
- 25.Park W, Hicks DM, Khandwala F, et al. Laryngopharyngeal reflux: prospective cohort study evaluating optimal dose of proton-pump inhibitor therapy and pretherapy predictors of response. Laryngoscope 2005;115:1230–1238. [DOI] [PubMed] [Google Scholar]
- 26.Eherer AJ, Habermann W, Hammer HF, Kiesler K, Friedrich G, Krejs GJ. Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol 2003;38:462–467. [DOI] [PubMed] [Google Scholar]
- 27.El-Serag HB, Lee P, Buchner A, Inadomi JM, Gavin M, McCarthy DM. Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol 2001;96:979–983. [DOI] [PubMed] [Google Scholar]
- 28.Noordzij JP, Khidr A, Evans BA, et al. Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 2001;111:2147–2151. [DOI] [PubMed] [Google Scholar]
- 29.Steward DL, Wilson KM, Kelly DH, et al. Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 2004;131:342–350. [DOI] [PubMed] [Google Scholar]
- 30.Vaezi MF, Richter JE, Stasney CR, et al. Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 2006;116:254–260. [DOI] [PubMed] [Google Scholar]
- 31.Wo JM, Koopman J, Harrell SP, Parker K, Winstead W, Lentsch E. Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol 2006;101:1972–1978; quiz 2169. [DOI] [PubMed] [Google Scholar]
- 32.Lam PK, Ng ML, Cheung TK, et al. Rabeprazole is effective in treating laryngopharyngeal reflux in a randomized placebo-controlled trial. Clin Gastroenterol Hepatol 2010;8:770–776. [DOI] [PubMed] [Google Scholar]
- 33.Reichel O, Dressel H, Wiederanders K, Issing WJ. Double-blind, placebo-controlled trial with esomeprazole for symptoms and signs associated with laryngopharyngeal reflux. Otolaryngol Head Neck Surg 2008;139:414–420. [DOI] [PubMed] [Google Scholar]
- 34.Vaezi MF. Gastroesophageal reflux-related chronic laryngitis: con. Arch Otolaryngol Head Neck Surg 2010;136:908–909. [DOI] [PubMed] [Google Scholar]
- 35.Lien HC, Wang CC, Liang WM, et al. Composite pH predicts esomeprazole response in laryngopharyngeal reflux without typical reflux syndrome. Laryngoscope 2013;123:1483–1489. [DOI] [PubMed] [Google Scholar]
- 36.Masaany M, Marina MB, Sharifa Ezat WP, Sani A. Empirical treatment with pantoprazole as a diagnostic tool for symptomatic adult laryngopharyngeal reflux. J Laryngol Otol 2011;125:502–508. [DOI] [PubMed] [Google Scholar]
- 37.Kahrilas PJ. When proton pump inhibitors fail. Clin Gastroenterol Hepatol 2008;6:482–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barry DW, Vaezi MF. Laryngopharyngeal reflux: More questions than answers. Cleve Clin J Med 2010;77:327–334. [DOI] [PubMed] [Google Scholar]
- 39.Lechien JR, Bock JM, Carroll TL, Akst LM. Is empirical treatment a reasonable strategy for laryngopharyngeal reflux? A contemporary review. Clin Otolaryngol 2020. [DOI] [PubMed] [Google Scholar]
- 40.Sharma N, Castell DO. Further comment on proton pump inhibitor failures. Clin Gastroenterol Hepatol 2009;7:363. [DOI] [PubMed] [Google Scholar]
- 41.Tamhankar AP, Peters JH, Portale G, et al. Omeprazole does not reduce gastroesophageal reflux: new insights using multichannel intraluminal impedance technology. J Gastrointest Surg 2004;8:890–897; discussion 897–898. [DOI] [PubMed] [Google Scholar]
- 42.Tutuian R, Mainie I, Agrawal A, Adams D, Castell DO. Nonacid reflux in patients with chronic cough on acid-suppressive therapy. Chest 2006;130:386–391. [DOI] [PubMed] [Google Scholar]
- 43.Tutuian R, Vela MF, Hill EG, Mainie I, Agrawal A, Castell DO. Characteristics of symptomatic reflux episodes on Acid suppressive therapy. Am J Gastroenterol 2008;103:1090–1096. [DOI] [PubMed] [Google Scholar]
- 44.Falk GL, Van der Wall H, Burton L, Falk MG, O’Donnell H, Vivian SJ. Fundoplication for laryngopharyngeal reflux despite preoperative dysphagia. Ann R Coll Surg Engl 2017;99:224–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Iqbal M, Batch AJ, Spychal RT, Cooper BT. Outcome of surgical fundoplication for extraesophageal (atypical) manifestations of gastroesophageal reflux disease in adults: a systematic review. J Laparoendosc Adv Surg Tech A 2008;18:789–796. [DOI] [PubMed] [Google Scholar]
- 46.Klimara MJ, Randall DR, Allen J, Figueredo E, Johnston N. Proximal reflux: biochemical mediators, markers, therapeutic targets, and clinical correlations. Ann N Y Acad Sci 2020;1481:127–138. [DOI] [PubMed] [Google Scholar]
- 47.Lechien JR, Dapri G, Dequanter D, et al. Surgical Treatment for Laryngopharyngeal Reflux Disease: A Systematic Review. JAMA Otolaryngol Head Neck Surg 2019;145:655–666. [DOI] [PubMed] [Google Scholar]
- 48.Mainie I, Tutuian R, Shay S, et al. Acid and non-acid reflux in patients with persistent symptoms despite acid suppressive therapy: a multicentre study using combined ambulatory impedance-pH monitoring. Gut 2006;55:1398–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sidwa F, Moore AL, Alligood E, Fisichella PM. Surgical Treatment of Extraesophageal Manifestations of Gastroesophageal Reflux Disease. World J Surg 2017;41:2566–2571. [DOI] [PubMed] [Google Scholar]
- 50.Zhang C, Hu ZW, Yan C, et al. Nissen fundoplication vs proton pump inhibitors for laryngopharyngeal reflux based on pH-monitoring and symptom-scale. World J Gastroenterol 2017;23:3546–3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giacchi RJ, Sullivan D, Rothstein SG. Compliance with anti-reflux therapy in patients with otolaryngologic manifestations of gastroesophageal reflux disease. Laryngoscope 2000;110:19–22. [DOI] [PubMed] [Google Scholar]
- 52.McGlashan JA, Johnstone LM, Sykes J, Strugala V, Dettmar PW. The value of a liquid alginate suspension (Gaviscon Advance) in the management of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol 2009;266:243–251. [DOI] [PubMed] [Google Scholar]
- 53.Zalvan CH, Hu S, Greenberg B, Geliebter J. A Comparison of Alkaline Water and Mediterranean Diet vs Proton Pump Inhibition for Treatment of Laryngopharyngeal Reflux. JAMA Otolaryngol Head Neck Surg 2017;143:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koufman JA. Low-acid diet for recalcitrant laryngopharyngeal reflux: therapeutic benefits and their implications. Ann Otol Rhinol Laryngol 2011;120:281–287. [DOI] [PubMed] [Google Scholar]
- 55.Samuels TL, Johnston N. Pepsin as a marker of extraesophageal reflux. Ann Otol Rhinol Laryngol 2010;119:203–208. [DOI] [PubMed] [Google Scholar]
- 56.Ali MS, Parikh S, Chater P, Pearson JP. Bile acids in laryngopharyngeal refluxate: will they enhance or attenuate the action of pepsin? Laryngoscope 2013;123:434–439. [DOI] [PubMed] [Google Scholar]
- 57.Campagnolo AM, Priston J, Thoen RH, Medeiros T, Assuncao AR. Laryngopharyngeal reflux: diagnosis, treatment, and latest research. Int Arch Otorhinolaryngol 2014;18:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston N, Dettmar PW, Bishwokarma B, Lively MO, Koufman JA. Activity/stability of human pepsin: implications for reflux attributed laryngeal disease. Laryngoscope 2007;117:1036–1039. [DOI] [PubMed] [Google Scholar]
- 59.Samuels TL, Johnston N. Pepsin as a causal agent of inflammation during nonacidic reflux. Otolaryngol Head Neck Surg 2009;141:559–563. [DOI] [PubMed] [Google Scholar]
- 60.Tan JJ, Wang L, Mo TT, Wang J, Wang MG, Li XP. Pepsin promotes IL-8 signaling-induced epithelial-mesenchymal transition in laryngeal carcinoma. Cancer Cell Int 2019;19:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piper DW, Fenton BH. pH stability and activity curves of pepsin with special reference to their clinical importance. Gut 1965;6:506–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Axford SE, Sharp N, Ross PE, et al. Cell biology of laryngeal epithelial defenses in health and disease: preliminary studies. Ann Otol Rhinol Laryngol 2001;110:1099–1108. [DOI] [PubMed] [Google Scholar]
- 63.Gill GA, Johnston N, Buda A, et al. Laryngeal epithelial defenses against laryngopharyngeal reflux: investigations of E-cadherin, carbonic anhydrase isoenzyme III, and pepsin. Ann Otol Rhinol Laryngol 2005;114:913–921. [DOI] [PubMed] [Google Scholar]
- 64.Johnston N, Bulmer D, Gill GA, et al. Cell biology of laryngeal epithelial defenses in health and disease: further studies. Ann Otol Rhinol Laryngol 2003;112:481–491. [DOI] [PubMed] [Google Scholar]
- 65.Johnston N, Dettmar PW, Ondrey FG, Nanchal R, Lee SH, Bock JM. Pepsin: biomarker, mediator, and therapeutic target for reflux and aspiration. Ann N Y Acad Sci 2018;1434:282–289. [DOI] [PubMed] [Google Scholar]
- 66.Johnston N, Knight J, Dettmar PW, Lively MO, Koufman J. Pepsin and carbonic anhydrase isoenzyme III as diagnostic markers for laryngopharyngeal reflux disease. Laryngoscope 2004;114:2129–2134. [DOI] [PubMed] [Google Scholar]
- 67.Johnston N, Wells CW, Samuels TL, Blumin JH. Pepsin in nonacidic refluxate can damage hypopharyngeal epithelial cells. Ann Otol Rhinol Laryngol 2009;118:677–685. [DOI] [PubMed] [Google Scholar]
- 68.Johnston N, Wells CW, Samuels TL, Blumin JH. Rationale for targeting pepsin in the treatment of reflux disease. Ann Otol Rhinol Laryngol 2010;119:547–558. [DOI] [PubMed] [Google Scholar]
- 69.Pearson JP, Parikh S, Orlando RC, et al. Review article: reflux and its consequences--the laryngeal, pulmonary and oesophageal manifestations. Conference held in conjunction with the 9th International Symposium on Human Pepsin (ISHP) Kingston-upon-Hull, UK, 21–23 April 2010. Aliment Pharmacol Ther 2011;33 Suppl 1:1–71. [DOI] [PubMed] [Google Scholar]
- 70.Rees LE, Pazmany L, Gutowska-Owsiak D, et al. The mucosal immune response to laryngopharyngeal reflux. Am J Respir Crit Care Med 2008;177:1187–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Samuels TL, Altman KW, Gould JC, et al. Esophageal pepsin and proton pump synthesis in barrett’s esophagus and esophageal adenocarcinoma. Laryngoscope 2019;129:2687–2695. [DOI] [PubMed] [Google Scholar]
- 72.Sasaki CT, Toman J, Vageli D. The In Vitro Effect of Acidic-Pepsin on Nuclear Factor KappaB Activation and Its Related Oncogenic Effect on Normal Human Hypopharyngeal Cells. PLoS One 2016;11:e0168269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Samuels TL, Johnston N. Pepsin in gastroesophageal and extraesophageal reflux: molecular pathophysiology and diagnostic utility. Curr Opin Otolaryngol Head Neck Surg 2020;28:401–409. [DOI] [PubMed] [Google Scholar]
- 74.Samuels TL, Zimmermann MT, Zeighami A, et al. RNA Sequencing Reveals Cancer-Associated Changes in Laryngeal Cells Exposed to Non-Acid Pepsin. Laryngoscope 2021;131:121–129. [DOI] [PubMed] [Google Scholar]
- 75.Hurley BP, Jugo RH, Snow RF, et al. Pepsin Triggers Neutrophil Migration Across Acid Damaged Lung Epithelium. Sci Rep 2019;9:13778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim JH, Jang SJ, Yun JW, Jung MH, Woo SH. Effects of pepsin and pepstatin on reflux tonsil hypertrophy in vitro. PLoS One 2018;13:e0207090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nagahama K, Yamato M, Nishio H, Takeuchi K. Essential role of pepsin in pathogenesis of acid reflux esophagitis in rats. Dig Dis Sci 2006;51:303–309. [DOI] [PubMed] [Google Scholar]
- 78.Samuels TL, Pearson AC, Wells CW, Stoner GD, Johnston N. Curcumin and anthocyanin inhibit pepsin-mediated cell damage and carcinogenic changes in airway epithelial cells. Ann Otol Rhinol Laryngol 2013;122:632–641. [PubMed] [Google Scholar]
- 79.Niu K, Guo C, Teng S, et al. Pepsin promotes laryngopharyngeal neoplasia by modulating signaling pathways to induce cell proliferation. PLoS One 2020;15:e0227408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lea WA, Simeonov A. Fluorescence polarization assays in small molecule screening. Expert Opin Drug Discov 2011;6:17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roberts NB, Taylor WH. Comparative pepstatin inhibition studies on individual human pepsins and pepsinogens 1,3 and 5(gastricsin) and pig pepsin A. J Enzyme Inhib Med Chem 2003;18:209–217. [DOI] [PubMed] [Google Scholar]
- 82.Jolley ME. Fluorescence Polarization Assays for the Detection of Proteases and Their Inhibitors. J Biomol Screen 1996;1:33–38. [Google Scholar]
- 83.Olp MD, Kalous KS, Smith BC. ICEKAT: an interactive online tool for calculating initial rates from continuous enzyme kinetic traces. BMC Bioinformatics 2020;21:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luft JR, DeTitta GT. A method to produce microseed stock for use in the crystallization of biological macromolecules. Acta Crystallogr D Biol Crystallogr 1999;55:988–993. [DOI] [PubMed] [Google Scholar]
- 85.Battye TG, Kontogiannis L, Johnson O, Powell HR, Leslie AG. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr D Biol Crystallogr 2011;67:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 1997;276:307–326. [DOI] [PubMed] [Google Scholar]
- 87.McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, Read RJ. Phaser crystallographic software. J Appl Crystallogr 2007;40:658–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Adams PD, Afonine PV, Bunkoczi G, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 2010;66:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Afonine PV, Mustyakimov M, Grosse-Kunstleve RW, Moriarty NW, Langan P, Adams PD. Joint X-ray and neutron refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 2010;66:1153–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 2004;60:2126–2132. [DOI] [PubMed] [Google Scholar]
- 91.Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta Crystallogr D Biol Crystallogr 2010;66:486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Long F, Nicholls RA, Emsley P, et al. AceDRG: a stereochemical description generator for ligands. Acta Crystallogr D Struct Biol 2017;73:112–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen VB, Arendall WB 3rd, Headd JJ, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr D Biol Crystallogr 2010;66:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Joosten RP, Long F, Murshudov GN, Perrakis A. The PDB_REDO server for macromolecular structure model optimization. IUCrJ 2014;1:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Caicedo-Granados E, Galbraith AR, Schachern MG, et al. N-methylnitrosourea-induced carcinoma as a model for laryngeal carcinogenesis. Head Neck 2014;36:1802–1806. [DOI] [PubMed] [Google Scholar]
- 96.Adhami T, Goldblum JR, Richter JE, Vaezi MF. The role of gastric and duodenal agents in laryngeal injury: an experimental canine model. Am J Gastroenterol 2004;99:2098–2106. [DOI] [PubMed] [Google Scholar]
- 97.Little FB, Koufman JA, Kohut RI, Marshall RB. Effect of gastric acid on the pathogenesis of subglottic stenosis. Ann Otol Rhinol Laryngol 1985;94:516–519. [DOI] [PubMed] [Google Scholar]
- 98.Roh JL, Yoon YH. Effect of acid and pepsin on glottic wound healing: a simulated reflux model. Arch Otolaryngol Head Neck Surg 2006;132:995–1000. [DOI] [PubMed] [Google Scholar]
- 99.Yellon RF, Szeremeta W, Grandis JR, Diguisseppe P, Dickman PS. Subglottic injury, gastric juice, corticosteroids, and peptide growth factors in a porcine model. Laryngoscope 1998;108:854–862. [DOI] [PubMed] [Google Scholar]
- 100.Xie Y, Longest PW, Xu YH, Wang JP, Wiedmann TS. In vitro and in vivo lung deposition of coated magnetic aerosol particles. J Pharm Sci 2010;99:4658–4668. [DOI] [PubMed] [Google Scholar]
- 101.Fujinaga M, Chernaia MM, Tarasova NI, Mosimann SC, James MN. Crystal structure of human pepsin and its complex with pepstatin. Protein Sci 1995;4:960–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lechien JR, Akst LM, Hamdan AL, et al. Evaluation and Management of Laryngopharyngeal Reflux Disease: State of the Art Review. Otolaryngol Head Neck Surg 2019;160:762–782. [DOI] [PubMed] [Google Scholar]
- 103.Sasaki CT, Doukas SG, Doukas PG, Vageli DP. Weakly Acidic Bile Is a Risk Factor for Hypopharyngeal Carcinogenesis Evidenced by DNA Damage, Antiapoptotic Function, and Premalignant Dysplastic Lesions In Vivo. Cancers (Basel) 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Figueiredo AA, Sales T, Nicolau LAD, et al. Laryngeal Mucosa Alterations in Mice Model of Gastroesophageal Reflux: Effects of Topical Protection. Laryngoscope 2020;130:E889–e895. [DOI] [PubMed] [Google Scholar]
- 105.Blondeau K, Mertens V, Vanaudenaerde BA, et al. Gastro-oesophageal reflux and gastric aspiration in lung transplant patients with or without chronic rejection. Eur Respir J 2008;31:707–713. [DOI] [PubMed] [Google Scholar]
- 106.De Corso E, Baroni S, Salonna G, et al. Impact of bile acids on the severity of laryngo-pharyngeal reflux. Clin Otolaryngol 2021;46:189–195. [DOI] [PubMed] [Google Scholar]
- 107.D’Ovidio F, Mura M, Tsang M, et al. Bile acid aspiration and the development of bronchiolitis obliterans after lung transplantation. J Thorac Cardiovasc Surg 2005;129:1144–1152. [DOI] [PubMed] [Google Scholar]
- 108.McQuaid KR, Laine L, Fennerty MB, Souza R, Spechler SJ. Systematic review: the role of bile acids in the pathogenesis of gastro-oesophageal reflux disease and related neoplasia. Aliment Pharmacol Ther 2011;34:146–165. [DOI] [PubMed] [Google Scholar]
- 109.Perng DW, Chang KT, Su KC, et al. Exposure of airway epithelium to bile acids associated with gastroesophageal reflux symptoms: a relation to transforming growth factor-beta1 production and fibroblast proliferation. Chest 2007;132:1548–1556. [DOI] [PubMed] [Google Scholar]
- 110.Hopwood D, Bateson MC, Milne G, Bouchier IA. Effects of bile acids and hydrogen ion on the fine structure of oesophageal epithelium. Gut 1981;22:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Calvo-Henriquez C, Ruano-Ravina A, Vaamonde P, Martinez-Capoccioni G, Martin-Martin C. Is Pepsin a Reliable Marker of Laryngopharyngeal Reflux? A Systematic Review. Otolaryngol Head Neck Surg 2017;157:385–391. [DOI] [PubMed] [Google Scholar]
- 112.Weitzendorfer M, Antoniou SA, Schredl P, et al. Pepsin and oropharyngeal pH monitoring to diagnose patients with laryngopharyngeal reflux. Laryngoscope 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klimara MJ, Johnston N, Samuels TL, et al. Correlation of salivary and nasal lavage pepsin with MII-pH testing. Laryngoscope 2020;130:961–966. [DOI] [PubMed] [Google Scholar]
- 114.Klimara MJ, Samuels TL, Johnston N, Chun RH, McCormick ME. Detection of Pepsin in Oral Secretions of Infants with and without Laryngomalacia. Ann Otol Rhinol Laryngol 2020;129:224–229. [DOI] [PubMed] [Google Scholar]
- 115.Sone M, Yamamuro Y, Hayashi H, Niwa Y, Nakashima T. Otitis media in adults as a symptom of gastroesophageal reflux. Otolaryngol Head Neck Surg 2007;136:19–22. [DOI] [PubMed] [Google Scholar]
- 116.Durkes A, Sivasankar MP. In vivo investigation of acidified pepsin exposure to porcine vocal fold epithelia. Laryngoscope 2016;126:E12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Erickson E, Sivasankar M. Simulated reflux decreases vocal fold epithelial barrier resistance. Laryngoscope 2010;120:1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Amin SM, Abdel Maged KH, Naser AY, Aly BH. Laryngopharyngeal reflux with sore throat: an ultrastructural study of oropharyngeal epithelium. Ann Otol Rhinol Laryngol 2009;118:362–367. [DOI] [PubMed] [Google Scholar]
- 119.Andrews TM, Orobello N. Histologic versus pH probe results in pediatric laryngopharyngeal reflux. Int J Pediatr Otorhinolaryngol 2013;77:813–816. [DOI] [PubMed] [Google Scholar]
- 120.Lechien JR, Schindler A, Robotti C, Lejeune L, Finck C. Laryngopharyngeal reflux disease in singers: Pathophysiology, clinical findings and perspectives of a new patient-reported outcome instrument. Eur Ann Otorhinolaryngol Head Neck Dis 2019;136:S39–S43. [DOI] [PubMed] [Google Scholar]
- 121.Lipan MJ, Reidenberg JS, Laitman JT. Anatomy of reflux: a growing health problem affecting structures of the head and neck. Anat Rec B New Anat 2006;289:261–270. [DOI] [PubMed] [Google Scholar]
- 122.Powell J, Cocks HC. Mucosal changes in laryngopharyngeal reflux--prevalence, sensitivity, specificity and assessment. Laryngoscope 2013;123:985–991. [DOI] [PubMed] [Google Scholar]
- 123.Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl) 2015;7:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pham TH, Genta RM, Spechler SJ, Souza RF, Wang DH. Development and characterization of a surgical mouse model of reflux esophagitis and Barrett’s esophagus. J Gastrointest Surg 2014;18:234–240; discussion 240–231. [DOI] [PubMed] [Google Scholar]
- 125.Gaynor EB. Gastroesophageal reflux as an etiologic factor in laryngeal complications of intubation. Laryngoscope 1988;98:972–979. [DOI] [PubMed] [Google Scholar]
- 126.Perkins EL, Basu S, Garcia GJM, Buckmire RA, Shah RN, Kimbell JS. Ideal Particle Sizes for Inhaled Steroids Targeting Vocal Granulomas: Preliminary Study Using Computational Fluid Dynamics. Otolaryngol Head Neck Surg 2018;158:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lungova V, Verheyden JM, Herriges J, Sun X, Thibeault SL. Ontogeny of the mouse vocal fold epithelium. Dev Biol 2015;399:263–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wire MB, Shelton MJ, Studenberg S. Fosamprenavir : clinical pharmacokinetics and drug interactions of the amprenavir prodrug. Clin Pharmacokinet 2006;45:137–168. [DOI] [PubMed] [Google Scholar]
- 129.Furfine ES, Baker CT, Hale MR, et al. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother 2004;48:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dasgupta A, Schlette E. Rapid in vitro conversion of fosphenytoin into phenytoin in sera of patients with liver disease: role of alkaline phosphatase. J Clin Lab Anal 2001;15:244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bourne GH. Alkaline phosphatase in taste buds and nasal mucosa. Nature 1948;161:445. [DOI] [PubMed] [Google Scholar]
- 132.Rader BA. Alkaline Phosphatase, an Unconventional Immune Protein. Front Immunol 2017;8:897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li H, Zhao Y, Li W, Yang J, Wu H. Critical role of neutrophil alkaline phosphatase in the antimicrobial function of neutrophils. Life Sci 2016;157:152–157. [DOI] [PubMed] [Google Scholar]
- 134.Reale MF M; Grilli A; Barbacane RC; Placido F; Porreca E; Conti P Induction of alkaline phosphatase generation by il-1β and LPS on human neutrophils and macrophages and lack of inhibition by interleukin-1 receptor antagonist. Inflammopharmacology 1995;3:25–34. [Google Scholar]
- 135.Chen L, Zeng H, Yang J, et al. Survival and prognostic analysis of preoperative inflammatory markers in patients undergoing surgical resection for laryngeal squamous cell carcinoma. BMC Cancer 2018;18:816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hammond KD, Mohamed E, Gregor RT. Alkaline phosphatase and phosphoamino acid phosphatases in normal and cancerous tissues of the human larynx. Biochem Med Metab Biol 1990;43:75–79. [DOI] [PubMed] [Google Scholar]
- 137.Sharma U, Pal D, Prasad R. Alkaline phosphatase: an overview. Indian J Clin Biochem 2014;29:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lien HC, Wang CC, Lee SW, et al. Responder Definition of a Patient-Reported Outcome Instrument for Laryngopharyngeal Reflux Based on the US FDA Guidance. Value Health 2015;18:396–403. [DOI] [PubMed] [Google Scholar]
- 139.Kamani T, Penney S, Mitra I, Pothula V. The prevalence of laryngopharyngeal reflux in the English population. Eur Arch Otorhinolaryngol 2012;269:2219–2225. [DOI] [PubMed] [Google Scholar]
- 140.Lowden M, McGlashan JA, Steel A, Strugala V, Dettmar PW. Prevalence of symptoms suggestive of extra-oesophageal reflux in a general practice population in the UK. Logoped Phoniatr Vocol 2009;34:32–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Structural data are available in the Worldwide Protein Databank (accession codes 6XCY, 6XCT, 6XCZ, 6XD2; http://www.wwpdb.org/). Additional information is available from the corresponding author on reasonable request.


