Abstract
Introduction
Iron deficiency anemia (IDA) in pregnancy has a prevalence as high as 40–60% in different countries of the world. Oral iron is used to treat his commonest medical disorder in pregnancy. Ferrous sulphate is associated with considerable side effects. Ferric carboxymaltose (FCM) is a newer iron preparation which allows for single and higher dose (up to 1000 mg) of IV iron infusion. This study was conducted to compare the efficacy of FCM and FS in treating IDA during pregnancy.
Methods
A randomised control trial was done at a tertiary care centres involving 362 women (181 women each in FS and FCM group). The pregnant anemic women with IDA were enrolled between 18 and 34 weeks of pregnancy. They were given 1000 mg of FCM iv as single dose or were given FS tablets twice daily (120 mg iron daily). The data were collected for rise in the Hb and serum ferritin over a period of 6 weeks.
Results
Nine and 18 patients were lost to follow-up in the FCM and FS group, respectively. The data were analysed as per protocol analysis. FCM group women showed 2.6 gm% rise in Hb compared to 1.7 gm% of FS group. One hundred and sixty-six out of 172 women in FS group achieved anemia correction at 6 weeks. No difference was observed in the neonatal outcome. No major side effects were observed in the either group.
Conclusion
In our study, FCM was more effective than oral FS in increasing Hb in women with IDA during pregnancy. This clinical benefit with FCM was achieved without the concerns for safety and tolerability of the drug.
Keywords: Ferric carboxy maltose, Ferrous sulphate, Iron deficiency anemia, Anemia in pregnancy
Introduction
Iron deficiency anemia (IDA) in pregnancy is an important cause of morbidity and mortality. World Health Organization (WHO)/World Health Statistics data show that 40.1% of pregnant women worldwide were anemic in 2016 [1–3].
A singleton pregnancy places a demand of approximately one gram of iron on a pregnant woman. This increased demand is due to increased red cell mass, feto-placental development and expansion of blood volume in the mother [1]. This leads to IDA which is an important cause of long-term debility, many of times spilling over to subsequent conception. A number of complications, e.g. frequent infections, preterm labor, fetal growth restriction (FGR), post-partum hemorrhage, cardiac failure and puerperal sepsis have been found frequently in women who were anemic during antenatal period [4].
Anemia begins in childhood, worsens during adolescence in girls and gets aggravated during pregnancy and effect the postnatal period. In India, where multiparty is quite common, poor nutrition, malabsorption, poor compliance along with insufficient oral iron treatment also increase the risk of developing iron deficiency anemia in subsequent pregnancies in a woman’s life time [5]. Data from Indian Council of Medical Research (ICMR), National Nutrition Monitoring Bureau (NNMB) and District Level Household Survey (DLHS) surveys have shown that prevalence of anemia is extremely high (ranging between 80 and 90%) in adolescent girls, preschool children, pregnant and lactating women [5].
Majority of pregnant women are prone to develop iron deficiency anemia irrespective of their iron stores if they are not supplemented with iron in any form during antenatal period. The supplementation of the iron during pregnancy is a routine measure in India and is strongly advisable. The oral iron therapy in the therapeutic doses (120–180 mg elemental iron per day) is used in women who are detected to have IDA [6]. The oral iron therapy causes adverse effects in the form of GI upset, vomiting, loose motions, metallic taste in the mouth and causes poor compliance for its ingestion in the pregnant women [7–9]. Injectable iron in the form of Ferric carboxy maltose (FCM) newer dextran-free iron formulation with a near neutral pH, physiological osmolarity and increased bioavailability. It gives us an opportunity to supplement the needs of the pregnancy in women. FCM is with minimal side effects and a single dose infusion up to 1000 mg can be given over 15 min [7, 9–11].
Thus, we carried out the study at our centre to compare the efficacy of FCM over oral ferrous sulphate (FS) in pregnant women to treat IDA.
Materials and Methods
The study was conducted at ANC clinic of a tertiary care hospital in western Maharashtra, for a period of one and half years from Dec 2018 to May 2020. It was an interventional randomized control trial (parallel group, open label trial) with CTRI Registration no CTRI/2017/06/008884.
The study was started after the Ethics committee approval and consent was taken from each participating patient. All the antenatal women between 18 and 34 weeks POG attending ANC clinic detected to have Hb between 8.5 and < 11 gm% formed study population. IDA was diagnosed using hemoglobin (Hb), low serum ferritin and RBC indices indicating microcytic and hypochromic erythropoiesis.
All women with anemia other than IDA, or history of blood transfusion in past six months or bone marrow suppressive therapy or had received erythropoietin in last three months or were known to be hypersensitive to iron therapy were excluded from the study.
The women in showing the rise in Hb ≥ 11gm% made the primary endpoint for this study. The secondary outcomes measured during the study were to observe for the adverse effects in both the groups. The birth weight and apgar score of the newborn babies was noted to check the newborn status.
Taking a two side significance level with 95% confidence interval and 1:1 ratio of the exposed to unexposed, the calculated sample size was 173 each in both groups. However a total of 362 patients were taken for the study. Simple random sampling with computerized randomisation was done from the patients visiting the ANC clinic.
Methodology
All pregnant women with IDA and eligible as per the inclusion criteria were randomized into 2 groups. The FCM group patients were given inj FCM 1000 mg IV in 200 ml normal saline over 20 min. No additional doses of FCM were given during the pregnancy. FS group patients were given 200 mg of ferrous sulphate (60 mg elemental iron per tablet) two times a day with lemon water or fruit juice, throughout the pregnancy. Hemoglobin and serum ferritin levels were recorded after 3 and 6 weeks after starting the therapy. The rise in the Hb levels, serum ferritin levels at 3 weeks, 6 weeks was noted.
Statistical Analysis
Data were analysed according to per protocol analysis (PPA). Statistical analysis was performed using Windows Excel by using t test for baseline Hb levels serum ferritin levels of all patients before and after the therapy at 3 and 6 seeks, independent t test to compare both the groups for proportion of women achieving anemia correction, and repeated measure ANOVA test to compare the rise in the hemoglobin level in both the groups. All significance tests were two-tailed, with an α level of 0.05. Safety data and data from newborns were summarized descriptively.
Results
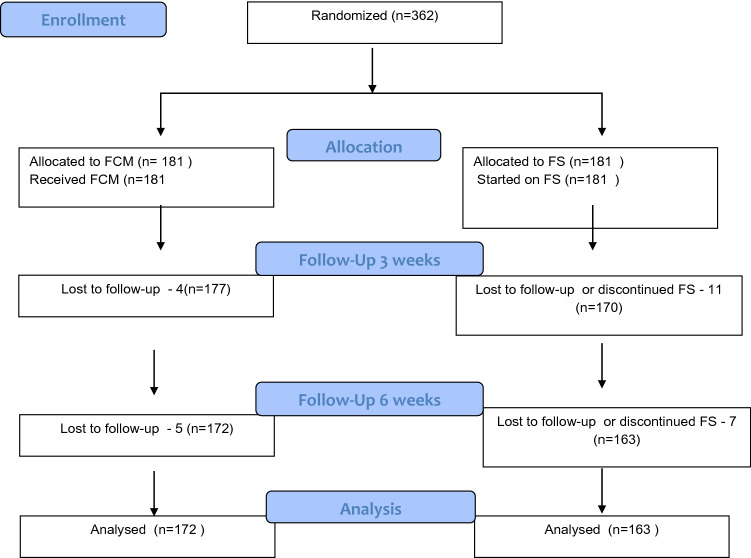
This RCT between Dec 2018 to Jun 2020 was done with the aim to compare the efficacy of injection ferriccarboxy maltose (FCM) and oral ferrous sulphate (FS) in treating iron deficiency anemia during pregnancy. A total of 362 pregnant women in the two groups, fulfilling the inclusion criteria were randomised, with 181 women in each group. Consort diagram shows the flow of the study (Fig. 1).
Fig. 1.
Consort diagram
Both the groups were matched for the age, with the mean age being 26.43 and 26.26 years in the FCM and FS groups. The youngest participant was 19 years of age and the oldest was 35 years of age. Both the groups were similar in terms of parity, pre pregnancy weight and baseline Hb and ferritin levels (Table 1).
Table 1.
Demographic characteristics of the pregnant women
| FCM | FS | p value | |
|---|---|---|---|
| Age (years) | |||
| Mean age | 26.43 ± 3.6 | 26.26 ± 3.46 | 0.647 |
| 20 | 11 | 7 | |
| 21–30 | 146 | 155 | |
| > 30 | 24 | 19 | |
| Parity | |||
| Primigravidae | 64 | 71 | |
| Multigravidae | 117 | 110 | |
| POG (weeks) | |||
| Mean | 25.8 ± 2.8 | 25.8 ± 2.9 | 0.627 |
| < 28 weeks | 105 | 109 | |
| > 28 weeks | 76 | 72 | |
| Prepregnancy wt (kg) | |||
| Mean | 51.14 ± 5.19 | 51.44 ± 5.92 | 0.634 |
| < 40 | 2 | 2 | |
| 40–50 | 137 | 141 | |
| > 50 | 42 | 38 | |
| Base line Hb (gm%) | 9.20 ± 0.44 | 9.17 ± 0.51 | 0.570 |
| Baseline serum ferritin (ng/ml) | 10.63 ± 4.9 | 10.38 ± 4.8 | 0.629 |
*Baseline comparison shows no difference in variables between FCM and FS group
The patients in both the groups showed a rise in the Hb levels during follow up assessment at 3 and 6 weeks. The mean rise in the Hb levels in FCM group was more than the FS group. The FCM group showed a mean Hb rise of 1.2 gm% compared to 0.6 gm% in the FS group (p value < 0.05). The FCM group had shown a mean rise of ferritin by 332 ng/ml compared to only 62 ng/ml rise in FS group. The difference was statistically significant (p value < 0.05). The rise in the ferritin and Hb levels at 6 weeks was also statistically significant (p value < 0.05). The mean change in the Hb levels and change in ferritin levels in the FCM and FS groups is shown in Table 2.
Table 2.
Mean rise in Hb and ferritin level after treatment at 3 and 6 weeks
| Group | N | Mean | SD | p value | |
|---|---|---|---|---|---|
| Difference in Hb (gm%) at 3 weeks | FCM | 177 | 1.2 | 0.30 | < 0.05 |
| FS | 170 | 0.6 | 0.48 | ||
| Difference in ferritin (ng/ml) at 3 weeks | FCM | 177 | 332.4 | 39.2 | < 0.05 |
| FS | 170 | 62.9 | 13.8 | ||
| Difference in Hb (gm%) at 6 weeks | FCM | 172 | 2.7 | 0.54 | < 0.05 |
| FS | 163 | 1.6 | 0.92 | ||
| Difference in ferritin (ng/ml) at 6 weeks | FCM | 172 | 448.3 | 308.73 | < 0.05 |
| FS | 163 | 221.4 | 36.01 |
p value < 0.05 therefore there is significant difference between mean hemoglobin in FCM group and FS at 3 and 6 weeks
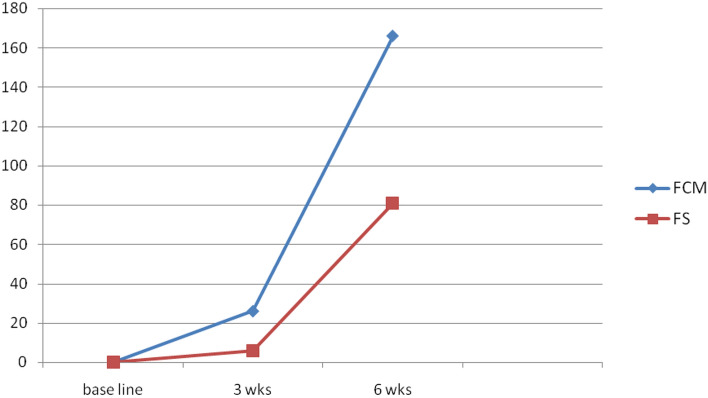
Among the subjects, 14 and 3% of pregnant women achieved anemia correction (Hb ≥ 11 gm%) within 3 weeks in the FCM group and FS groups respectively. Majority (92%) of these women in FCM group achieved Hb levels > 11gm% at 6 weeks compared to only 44% in the FS group (Fig. 2; Table 3).
Fig. 2.
Total women with Hb of ≥ 11 gm% at 3 and 6 weeks
Table 3.
Number of women achieving anemia correction at 6 weeks
| Hb at 6 weeks | Group | Total | p value | |
|---|---|---|---|---|
| FCM group (%) N = 172 | FS group (%) N = 163 | |||
| ≥ 11 gm% | 166 (96.5) | 81 (49.7) | 247 | < 0.001 |
| < 11 gm% | 6 (3.5) | 82 (50.3) | 88 | |
| Total | 172 | 163 | 335 | |
p value < 0.05 therefore there is significant difference between women achieving anemia correction at 6 weeks in FCM group and FS group
A total of 95 different side effects were observed in 61 women (16.8%). In FCM group only five (2.8%) women had nausea and vomiting as the commonest adverse effect, these same women also reported headache and dizziness. Nausea and vomiting were six times more seen in women in the FS group. The women in the FS group were really troubled by epigastric discomfort and constipation. None of the pregnant women in the FCM group had constipation or reported to the doctor with epigastric discomfort. The majority of adverse events were mild in intensity. One patient in FCM group reported discoloration of the local area due to extravasation of the compound. The side effects seen during the study are shown in Table 4.
Table 4.
Adverse effects seen with FCM and FS therapy
| Side effects | Group | Total | |
|---|---|---|---|
| FCM group (n = 181) | FS group (n = 181) | ||
| Headache | 3 | 3 | 6 |
| Dizziness | 2 | 1 | 3 |
| Nausea and vomiting | 5 | 31 | 36 |
| Constipation | 0 | 27 | 27 |
| Epigastric discomfort | 0 | 23 | 23 |
Some patients had multiple adverse effects
The mean birthweight in the FCM and FS group was 2.7 kg ± 0.2 kg, 2.67 kg ± 0.2 kg, respectively. The Apgar score at 1 min, 5 min and 10 min was similar in both the groups. Mean Apgar score of the babies at one minute after birth was 8, and increased to 9 at 5 min.
Discussion
Iron deficiency anemia during pregnancy with high prevalence all over the world is an important cause for the mortality and morbidity among the pregnant women and their newborns. This study demonstrates that injection FCM is highly effective in increasing Hb levels and in treatment of iron deficiency anemia during pregnancy as compared to oral ferrous sulphate. FCM also causes this rise in the hemoglobin without the significant adverse effects. The rise in Hb levels was seen as early as 3 weeks after the injection FCM was given and the proportion of women achieving the Hb > 11gm% at 3 and 6 weeks after the FCM (92%) were more than for the same period after starting the FS (44%) therapy. The difference was statistically significant with (p value < 0.001).
Breyman et al. in their multinational study involving 252 pregnant women with IDA compared FS to FCM and found that Hb levels improved at comparable rates across both treatments; however, significantly more women achieved anemia correction with FCM vs. FS [Hb ≥ 11.0 g/dl; 84 vs. 70%] the results were similar to this study where we found that the anemia correction at 6 weeks was 91 and 44% in FCM vs FS groups, respectively. In our study, the number of women experiencing side effects were much less in the FCM group, but the number of women having adverse effects in FS group were much more than their study. Ninety-five and 61 in this study and 11 and 19 women in Breyman’s study) [7].
Pels et al. in a retrospective study of 128 patients (FCM: 64; control: 64) between 34 weeks gestation to delivery reported no adverse events in either group, this is similar to our findings with no major adverse events in FCM group. They used FCM dose of 1000 mg and said median Hb increased from 8.4 gm% at the first FCM administration to 10.7 gm% at the time of delivery This is similar to our study where we found a Hb rise of 1.2 gm% at 3 weeks and 2.7 gm% at 6 weeks in the FCM group [12] (Table 2).
Our study included a large number of patients in the FCM and FS group with a high follow up rate thus proving the efficacy and safety of FCM in pregnancy. It is limited by being a single centre study and the use of fixed dose of FCM.
Conclusion
In our study, FCM increased Hb in all pregnant women with IDA during pregnancy. It was more effective than oral FS in increasing Hb to more than 11 gm%. This clinical benefit with FCM was achieved without the concerns for safety and tolerability of the drug. Thus, we suggest that FCM should be prescribed safely to avoid IDA related complication during second and third trimester of pregnancy.
Author Contributions
Study concept: SC and AS. Study conduct: SC, AS, AHA, and DJ. Drafting and manuscript revision: SC and AS. Statistical analysis: AS, CHA, and DJ. Study supervision: SC.
Funding
None.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the institutional ethics committee at AFMC, Pune, and I certify that the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Balarajan Y, Ramakrishnan U, Ozaltin E, et al. Anaemia in low-income and middle-income countries. Lancet. 2011;378(9809):2123–2135. doi: 10.1016/S0140-6736(10)62304-5. [DOI] [PubMed] [Google Scholar]
- 2.WHO . The global prevalence of anaemia in 2011. Geneva: World Health Organization; 2015. [Google Scholar]
- 3.Qassim A, Mol BW, Grivell RM, et al. Safety and efficacy of intravenous iron polymaltose, iron sucrose and ferric carboxymaltose in pregnancy: a systematic review. Aust N Z J Obstet Gynaecol. 2018;58(1):22–39. doi: 10.1111/ajo.12695. [DOI] [PubMed] [Google Scholar]
- 4.Nair M, Churchill D, Robinson S, et al. Association between maternal haemoglobin and stillbirth: a cohort study among a multi-ethnic population in England. Br J Haematol. 2017;179:829–837. doi: 10.1111/bjh.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toteja GS, Singh P, Dhillon BS, et al. Prevalence of anemia among pregnant women and adolescent girls in 16 districts of India. Food Nutr Bull. 2006;27(4):311–315. doi: 10.1177/156482650602700405. [DOI] [PubMed] [Google Scholar]
- 6.Rai RK, Fawzi WW, Barik A, et al. The burden of iron-deficiency anaemia among women in India: how have iron and folic acid interventions fared? WHO South East Asia J Public Health. 2018;7:18–23. doi: 10.4103/2224-3151.228423. [DOI] [PubMed] [Google Scholar]
- 7.Breymann C, Milman N, Mezzacasa A, FER-ASAP Investigators et al. Ferric carboxymaltose vs. oral iron in the treatment of pregnant women with iron deficiency anemia: an international, open-label, randomized controlled trial (FER-ASAP) J Perinat Med. 2017;45(4):443–453. doi: 10.1515/jpm-2016-0050. [DOI] [PubMed] [Google Scholar]
- 8.Mwangi MN, Mzembe G, Moya E, et al. Protocol for a multicentre, parallel-group, open-label randomised controlled trial comparing ferric carboxymaltose with the standard of care in anaemic Malawian pregnant women: the REVAMP trial. BMJ Open. 2021;11:e053288. doi: 10.1136/bmjopen-2021-053288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jose A, Mahey R, Sharma JB, et al. Comparison of ferric Carboxymaltose and iron sucrose complex for treatment of iron deficiency anemia in pregnancy-randomised controlled trial. BMC Pregnancy Childb. 2019;19:54. doi: 10.1186/s12884-019-2200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Froessler B, Gajic T, Dekker G, et al. Treatment of iron deficiency and iron deficiency anemia with intravenous ferric carboxymaltose in pregnancy. Arch Gynecol Obstet. 2018;298:75–82. doi: 10.1007/s00404-018-4782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLoughery TG. Safety of oral and intravenous iron. Acta Haematol. 2019;142:8–12. doi: 10.1159/000496966. [DOI] [PubMed] [Google Scholar]
- 12.Pels A, Ganzevoort W. Safety and efficacy of ferric carboxymaltose in anemic pregnant women: a retrospective case control study. Obstet Gynecol Int. 2015;2015:728952. doi: 10.1155/2015/728952. [DOI] [PMC free article] [PubMed] [Google Scholar]