Abstract
Tuberculosis (Tb) caused by Mycobacterium bovis is a worldwide threat to livestock and humans. One control strategy is to breed livestock that are more resistant to Mycobacterium bovis. In a 3-year heritability study 6 farmed red deer stags were selected from 39 on the basis of their differing responses to experimental challenge via the tonsillar sac with approximately 500 CFU of M. bovis. Two stags remained uninfected, two were moderately affected, and two developed serious spreading Tb. Seventy offspring, bred from these six stags by artificial insemination using stored semen, were similarly challenged with M. bovis. The offspring showed patterns of response to M. bovis challenge similar to those of their sires, providing evidence for a strong genetic basis to resistance to Tb, with an estimated heritability of 0.48 (standard error, 0.096; P < 0.01). This is the first time the heritability of Tb resistance in domestic livestock has been measured. The breeding of selection lines of resistant and susceptible deer will provide an ideal model to study the mechanisms of Tb resistance in a ruminant and could provide an additional strategy for reducing the number and severity of outbreaks of Tb in farmed deer herds. Laboratory studies to identify genetic and immunological markers for resistance to Tb are under way. Preliminary studies showed no associations between NRAMP or DRB genes and resistance to Tb in deer. Patterns of immune responses seen in resistant animals suggest that both innate and acquired pathways of immunity are necessary to produce the resistant phenotype.
Tuberculosis (Tb) is one of the most widespread diseases of mankind and animals. Although the majority of cases of human Tb are caused by Mycobacterium tuberculosis, a small proportion are caused by Mycobacterium bovis carried by cattle and other domestic animals (25). In order to reduce this zoonotic risk, most developed countries have attempted to eradicate bovine Tb from their domestic animals. The problem is compounded by the establishment of Tb in wildlife reservoirs such as badgers in the United Kingdom (31) and Ireland (8, 23), buffalo and antelope in South Africa (25), and bison and deer in North America (25). In New Zealand, traditional “test-and-slaughter” control methods (24) and the killing of wildlife vectors, such as opossums (Trichosurus vulpecula) and ferrets (Mustela furo), have nearly halved the prevalence of Tb on farms in the last 5 years. The percentage of infected herds was 1.5% of the 60,000 cattle herds and 2.3% of the 5,200 deer herds recorded at the end of June 1998 (2). However, to reach the internationally accepted level of 0.2% herd infection rate, additional strategies are needed. One strategy for reducing the incidence of Tb in domestic livestock, which has not been used previously, is to select for increased genetic resistance.
Red deer have been farmed in New Zealand for more than 25 years, and they currently number over 1.7 million on approximately 5,200 farms. At the end of June 1998 there were 118 known infected herds (2). Most herd Tb breakdowns involve a few animals; however, sporadic outbreaks of Tb affecting up to 50% of the animals in a herd continue to occur, and there is usually a range of lesions, although usually less than 10% of the affected animals have severe disease (4, 10). Similarly, when red deer are experimentally challenged by the intratonsil route with 200 to 500 CFU of M. bovis, there is a spectrum of disease outcomes (20). Over a number of trials involving 103 deer from a variety of sources this challenge model produced M. bovis infection in 87% of deer (20), with a range of lesions and typical lymphocyte transformation (LT) and antibody responses that mirrored patterns of lesions and immunological reactivity found in naturally infected animals (12, 19). The 13% that were uninfected had no visible lesions, were negative to culture, and had low, transient, or negative cell-mediated immune responses. Thus it appears that there is considerable variation in the degree of resistance and susceptibility to natural and experimental infection with M. bovis in the general red deer population. A much earlier review of experimental Tb infections in cattle also showed differences in susceptibility between and within breeds (9).
We report here the results of a 3-year study that shows that the resistance of red deer to experimental challenge with M. bovis is highly heritable.
MATERIALS AND METHODS
This study was conducted in three phases. All trials were approved by the AgResearch Invermay Ethics Committee.
Phase 1.
Forty-four 2-year-old red deer stags of wide genetic origin and average productivity, in terms of live-weight gains and antler size, were brought from eight commercial deer farms to the Invermay deer farm in late summer (February) 1994. Cell-mediated immune responses (LT) and antibody responses (enzyme-linked immunosorbent assay [ELISA] to M. bovis and Mycobacterium avium tuberculin (PPD-B and PPD-A, respectively) were measured in a blood test for Tb (BTB), which measures specific reactivity to M. bovis or nonspecific reactivity to other mycobacteria, using a subtractive (PPD-B level minus PPD-A level) assay (13). Semen was collected during the autumn mating season by electroejaculation under anesthesia (3, 7). Semen of usable quality (>25% postthaw motility) and quantity (>30 straws) was collected from only 39 of the 44 stags, and the other 5 stags were culled. The remaining 39 stags were again tested and found to be negative by the BTB and were moved to a quarantined deer farm. They were all in apparently good health; they received antihelminthic treatment and trace element supplementation, and in midwinter (July), immediately prior to Tb challenge, their live weights ranged from 101.5 to 152 kg. They were challenged via the intratonsillar route with 500 CFU of virulent M. bovis (MES/89 strain, isolated from a field case of Tb in deer). The 0.2-ml challenge inoculum was instilled into the left tonsillar sac while the animal was sedated with a mixture of xylazine hydrochloride plus fentanyl citrate (20). At 3- to 6-week intervals throughout the trial, live weights were taken to assess well-being and blood samples were taken for the BTB to monitor LT and ELISA responses (11, 13). Six months after challenge (February 1995) an intradermal skin test using PPD-B was performed, with the double skin thickness measured 72 h later, and then the 39 challenged stags were killed and necropsied. Gross, histological, and microbiological examinations were carried out to define their infection and disease status. The palatine tonsils and all lymph nodes in the head, thorax, abdomen, and carcass were excised and finely sliced. All visible lesions were described and recorded, and samples were taken for histology and culture. In addition, samples of both tonsils were subjected to histology and culture and left and right medial retropharyngeal lymph nodes and pools of the remaining head, thorax, abdominal, and carcass lymph nodes in which there were no visible lesions were also subjected to cultural examination. The severity of lesions was scored on an arbitrary scale of from 0 (least severe) to 6 (most severe) (Table 1). Using these criteria, the numbers of stags classified on the lesion severity score (LSS) scale of 0 to 6 were 5, 4, 7, 8, 7, 5, and 3, respectively (Table 1). Six stags with LSSs of 0, 0, 2, 4, 6, and 6 were selected for a laparoscopic artificial-insemination (AI) program.
TABLE 1.
Tb LSSs in 39 stags experimentally infected with M. bovis
| LSS | LSS criterion | Result of M. bovis culture | No. of stags |
|---|---|---|---|
| 0 | No visible lesions | Negative | 5 |
| 1 | No visible lesions | Positive | 4 |
| 2 | Small Tb lesion in the medial retropharyngeal lymph node or tonsil | Positive | 7 |
| 3 | Single moderate or multiple small Tb lesions in head lymph nodes | Positive | 8 |
| 4 | Moderate-to-large multiple Tb lesions in head lymph nodes | Positive | 7 |
| 5 | Multiple Tb lesions in head and thoracic or abdominal lymph nodes | Positive | 5 |
| 6 | Multiple Tb lesions in head, thoracic, and abdominal lymph nodes | Positive | 3 |
Phase 2.
In autumn (April) 1995 the frozen semen from the six selected stags was used to inseminate randomly selected Tb skin test-negative red deer hinds on a commercial deer farm (Table 2). The number of hinds inseminated was limited by the number of straws available from each stag, and although each straw contained approximately 25 million live sperm before freezing, the quality of the semen was variable, as witnessed by the postthaw motility, and this affected the success rate (Table 2). Estrus was synchronized in the hinds by using progesterone controlled intravaginal drug release (CIDR) devices for 12 days. At CIDR withdrawal an injection of 200 IU of pregnant-mare serum gonadotropin was given, and laparoscopic AI was carried out between 50 and 56 h later while the hinds were sedated with xylazine hydrochloride-fentanyl citrate (3, 7, 14).
TABLE 2.
LSSs and other data for the six selected stags and their offspring
| Sire stag | Live weight prechallenge (kg) | Sire LSS | Semen quality (% postthaw motility) | No. of inseminations | No. of offspring | No. of offspring with LSS:
|
Mean LSS of offspring | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |||||||
| 406 | 124 | 0 | 65 | 37 | 18 | 2 | 8 | 2 | 5 | 0 | 1 | 0 | 1.78 |
| 434 | 101.5 | 0 | 60 | 38 | 9 | 0 | 5 | 0 | 3 | 1 | 0 | 0 | 2.00 |
| 415 | 117 | 2 | 40 | 38 | 13 | 0 | 8 | 2 | 0 | 1 | 2 | 0 | 2.00 |
| 417 | 124 | 4 | 60 | 40 | 19 | 0 | 7 | 1 | 7 | 3 | 0 | 1 | 2.53 |
| 416 | 145.5 | 6 | 45 | 40 | 9 | 0 | 0 | 1 | 6 | 0 | 0 | 2 | 3.56 |
| 433 | 101.5 | 6 | 30 | 31 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3.00 |
One year later (autumn 1996), 70 offspring were weaned at 3 months of age and a blood sample was taken for pedigree identification. Blood samples were taken from all the hinds and the six stags used for AI. The parentage of the offspring was assigned by comparing the genotype of each progeny with the genotypes of all possible parents for 11 highly polymorphic microsatellite DNA markers (28). The process assigned each progeny to a single sire and excluded the other sires from parentage.
Phase 3.
The 70 deer calves were taken to the quarantine deer farm, were tested as Tb negative by the BTB, and, in spring 1996, were challenged with M. bovis by the intratonsillar route. They were monitored by BTB and weighing at 4- to 6-week intervals for 6 months and underwent skin testing with a comparative cervical test (CCT) using PPD-A and PPD-B 6 months after challenge. They were then slaughtered and necropsied, and their lesions were scored using the criteria applied previously to the stags.
In both phases 1 and 3 a M. bovis isolate from a typical lesion was typed by DNA restriction endonuclease analysis (5) and compared with the challenge strain.
Statistical analyses.
Heritability was calculated as twice the parent-offspring regression. Mean offspring lesion scores were regressed on sire lesion scores, weighted according to equation 17.5a of Lynch and Walsh (18).
Analysis of interactions between lesion score, sire, sex, and live weight for the offspring was carried out by parsimonious modeling using multiple linear regression.
RESULTS
Phase 1. Stags. (i) BTB results.
Around 50% of the stags challenged with M. bovis produced specific bovine reactivity within 4 weeks, and 30% produced specific bovine antibody within 8 weeks. Cellular reactivity and antibody responsiveness results showed two distinct patterns of immunity that differed markedly between the resistant stags (LSS, 0), which cleared the infectious challenge, and animals which developed tuberculous lesions (LSS, 1 to 6). Resistant stags developed cellular reactions that were cross-reactive for M. bovis and M. avium tuberculin, while all infected animals developed specific cellular reactivity to M. bovis tuberculin, between 4 and 8 weeks postchallenge. Patterns of humoral immunity (ELISA) for the resistant and susceptible animals also differed. Resistant stags did not produce antibody to tuberculin at any time postchallenge. By contrast, all susceptible stags (LSS, 6) produced M. bovis-specific ELISA reactivity 4 to 8 weeks after challenge. The level of antibody correlated directly with the severity of disease in the infected stags. A summary of lymphocyte transformation and ELISA data for the six selected stags is presented in Table 3.
TABLE 3.
Representative LT, antibody ELISA, and skin test results
| Animal no. | Sire | LSS | Results for indicated test at individual time postinfectiona
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BTB at:
|
Skin test at 23 wkb
|
|||||||||
| 0 wk
|
8 wk
|
18 wk
|
||||||||
| LT | ELISA | LT | ELISA | LT | ELISA | A (mm) | B (mm) | |||
| Stags | ||||||||||
| 406 | 0 | A2 | 0 | B7 | 0 | A7 | 0 | 7.0 | ||
| 434 | 0 | A2 | 0 | B3 | 0 | A3 | 0 | 1.3 | ||
| 415 | 2 | A1 | 0 | B7 | 0 | B8 | 25 | 11.3 | ||
| 417 | 4 | A5 | 0 | B5 | 53 | B6 | 157 | 6.6 | ||
| 433 | 6 | A2 | 0 | B3 | 55 | B2 | 36 | 13.1 | ||
| 416 | 6 | A3 | 0 | B9 | 65 | B4 | 166 | 16.6 | ||
| Offspring | ||||||||||
| 604 | 406 | 0 | A5 | 0 | A4 | 0 | B2 | 0 | 2.1 | 1.2 |
| 696 | 406 | 0 | A5 | 0 | A1 | 0 | A7 | 0 | 3.3 | 2.5 |
| 609 | 415 | 2 | A6 | 0 | A9 | 0 | A10 | 0 | 6.8 | 0.6 |
| 637 | 416 | 2 | A8 | 0 | A4 | 0 | A1 | 20 | 0.6 | 1.6 |
| 665 | 415 | 2 | A7 | 0 | A4 | 0 | Neg | 15 | 1.8 | 0.6 |
| 684 | 417 | 2 | A8 | 0 | A3 | 0 | B10 | 20 | 3.3 | 3.0 |
| 694 | 406 | 2 | A4 | 0 | A6 = B6 | 0 | B7 | 50 | 2.3 | 5.8 |
| 727 | 406 | 2 | A4 | 0 | A4 = B4 | 0 | B10 | 0 | 2.3 | 4.0 |
| 644 | 434 | 4 | A7 | 0 | A4 = B4 | 0 | A6 = B6 | 0 | 5.5 | 7.1 |
| 668 | 417 | 4 | A2 | 0 | A4 | 25 | B4 | 12 | 6.4 | 7.6 |
| 697 | 417 | 4 | A4 | 0 | A3 | 0 | B6 | 22 | 1.9 | 8.8 |
| 705 | 415 | 4 | A6 | 0 | A5 | 0 | A2 | 0 | 7.1 | 6.2 |
| 717 | 417 | 4 | A4 | 0 | A3 | 0 | A5 = B5 | 14 | 3.0 | 6.3 |
| 703 | 417 | 6 | A8 | 0 | Neg | 43 | B6 | 75 | 1.2 | 0.9 |
| 714 | 416 | 6 | A2 | 0 | B5 | 20 | B10 | 50 | + | + |
| 725 | 416 | 6 | A4 | 0 | B1 | 0 | B5 | 17 | 7.2 | 9.9 |
For the LT, relative mycobacterial reactivity is calculated using B/A. For a bovine (B) response the calculated value is >1.1, for an avian (A) response it is <0.9, and for an equivocal (e.g., A6 = B6) response it is 0.9 to 1.1; a negative response (Neg) is <2.5 × negative control. The size of the response is then expressed in counts per minute as follows: B1, up to 2,000 cpm; B2, 2,000 to 5,000 cpm; B3, 5,000 to 10,000 cpm; B4, 10,000 to 20,000 cpm; B5, 20,000 to 30,000 cpm; B6, 30,000 to 40,000 cpm; B7, 40,000 to 50,000 cpm; B8, 50,000 to 60,000 cpm; B9, 60,000 to 70,000 cpm; and B10, >70,000,000 cpm. Animals with an avian response are similarly classified from A1 to A10. ELISA results (optical density [OD] units) were obtained by subtracting the avian PPD response (OD of test serum minus the OD of negative serum) from the bovine PPD response and multiplying the result by 100. For the skin test, the results represent the differences between post- and preskin thicknesses.
+, deer was euthanized prior to skin test.
(ii) Skin test results.
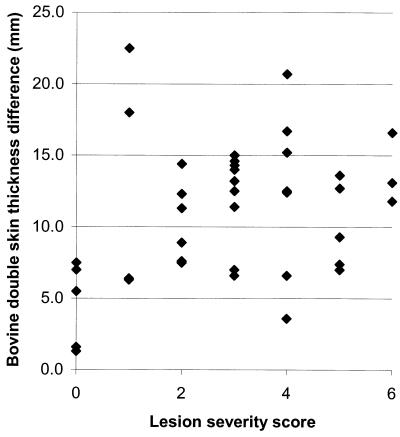
The distribution of skin test reactivity for the stags is shown in Fig. 1. Stags with an LSS of 0 had the least skin test reactivity and there was a significant relationship between skin test reactivity and lesion score (y = 8.3[+/−1.45] + 0.893[+/−0.424]; P < 0.05). There was also a good correlation between the results of the 18-week BTB and the preslaughter skin test (Table 3).
FIG. 1.
Relationship between bovine skin test reactivity and LSS for 39 stags challenged with virulent M. bovis. Regression equation, skin thickness = 8.3(+/−1.45) + 0.893(+/−0.424)LSS.
(iii) Lesion severity.
The numbers of stags classified on the LSS scale of 0 to 6 were 5, 4, 7, 8, 7, 5, and 3, respectively.
(iv) Live weight.
There were no significant associations between the live weights of the stags at the time of the challenge and the subsequent lesion score or the result of the intradermal skin test.
Phase 2. Offspring.
The parentage of each of the 70 offspring bred by AI was assigned to one of the six stags. They were all negative in a BTB conducted prior to relocation to the quarantined deer farm.
Phase 3. Offspring. (i) BTB results.
Prior to challenge almost all these deer had high levels of background avian sensitivity. Postchallenge, the patterns of immune reactivity in the offspring were similar to those seen in the stags following challenge, albeit with higher avian reactivity throughout the trial. A summary of LT and ELISA data for offspring in four of the LSS categories (0, 2, 4, and 6) is presented in Table 3. Putatively resistant offspring (LSS, 0) developed or increased their nonspecific cellular reactivity, but not ELISA reactivity, following challenge. Animals that became infected but that did not develop lesions (LSS, 1) produced specific B-cellular reactivity but no ELISA reactions. Specific cellular and ELISA reactivity to M. bovis was seen in the infected offspring with LSSs of 2 to 6, with a correlation between antibody level and disease severity. One LSS 6 animal that had negligible increases in skin thickness at the avian (1.2 mm) and bovine (0.9 mm) sites in the CCT immediately before slaughter nevertheless had high bovine cellular and ELISA reactivities. Another severely affected animal (LSS, 6), which lost weight rapidly and which was euthanized just prior to the scheduled CCT, had high B-cellular and ELISA reactivities just before it died.
(ii) Skin test results.
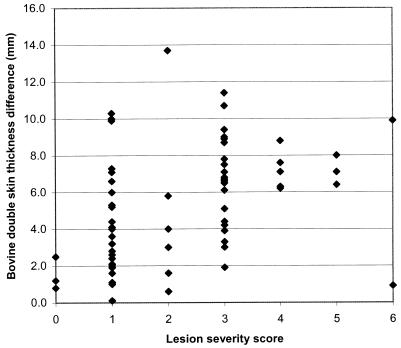
The distribution of skin test reactivity to PPD-B for the offspring is shown in Fig. 2. As with the stags, the offspring with a LSS of 0 had the least skin test reactivity (<3 mm) and there was a significant relationship between skin test reactivity and lesion score (y = 3.389[+/−0.671] + 0.868[+/−0.253]; P < 0.01). However, one of the three deer that had a lesion score of 6 had a negligible (<1-mm) increase in double skin thickness, suggesting that it had become anergic to the skin test. A comparison of skin test reactivity to PPD-A and PPD-B sites and the distribution of bovine reactivity are shown in Table 4. There was a high degree of reactivity at the avian site, and half (15 of 29) the LSS 0 and 1 animals had A > B reactivity. Animals with LSSs of 2, 3, 4, and 5 had predominantly B > A reactivity (33 of 38). By the standard New Zealand interpretation for a positive CCT (increase in skin thickness at the bovine site ≥ 2 mm and the bovine reaction ≥ the avian reaction), both the two LSS 0 animals were negative. By contrast 38% of the LSS 1 and 2 animals and 90% of the LSS 3, 4, and 5 animals were positive, and only one of the two LSS 6 animals was positive.
FIG. 2.
Relationship between bovine skin test reactivity and LSS for 69 yearling red deer challenged with virulent M. bovis. Regression equation, skin thickness =3.389(+/−0.671) + 0.868(+/−0.253)LSS.
TABLE 4.
Avian (A) and bovine (B) skin test differences, bovine site reactions, and numbers of CCT positives for the 70 offspring
| LSS | No. | No. of deer with indicated:
|
No. of deer (%) CCT positive (B ≥ A ≥ 2 mm) | ||||
|---|---|---|---|---|---|---|---|
| Difference in reactivity (%)
|
Double skin thickness at bovine site
|
||||||
| A > B | B > A | B ≥ 2 mm | B < 2 ≥ 1 mm | B < 1 mm | |||
| 0 | 2 | 2 (100) | 0 (0) | 1 | 1 | 0 | 0 (0) |
| 1 | 27 | 13 (48) | 14 (52) | 20 | 5 | 2 | 10 (37) |
| 2 | 7 | 2 (29) | 5 (71) | 4 | 1 | 2 | 3 (43) |
| 3 | 23 | 2 (9) | 21 (91) | 22 | 1 | 0 | 21 (91) |
| 4 | 5 | 1 (20) | 4 (80) | 5 | 0 | 0 | 4 (80) |
| 5 | 3 | 0 (0) | 3 (100) | 3 | 0 | 0 | 3 (100) |
| 6 | 27a | 1 (50) | 1 (50) | 1 | 0 | 0 | 1 (50) |
| Total | 69a | 21 (30) | 48 (70) | 56 | 8 | 5 | 42 (61) |
One LSS 6 animal was euthanized prior to CCT due to rapid weight loss.
(iii) Live weights.
Parsimonious modeling showed that the sire and the sex of the offspring each had a significant effect on the live weight of the offspring, whereas there was no demonstrable effect on the LSS of the offspring's live weight at the time of challenge.
(iv) LSSs.
The LSSs of the 6 stags and their 70 offspring and the mean LSSs for the offspring of each stag are presented in Table 2. The regression of mean offspring LSS on sire LSS gave a slope of 0.24 corresponding to an estimated heritability for this resistance/susceptibility score of 0.48 with a standard error of 0.096 (95% confidence interval, 0.22 to 0.75; P = 0.007). The offspring of infection-free stags had mean lesion scores of 1.78 and 2.0, the offspring of mildly to moderately affected stags had mean lesion scores of 2.0 and 2.53, and offspring of severely affected stags had mean lesion scores of 3.0 and 3.56.
M. bovis isolates from typical lesions in both trials had identical restriction endonuclease analysis typing patterns for the challenge organism.
DISCUSSION
The results of this trial indicate that there is a strongly heritable basis for the resistance and susceptibility of deer to experimental infection with M. bovis. The wide range of susceptibility scores in the outbred stags (Table 1) and in the offspring of the selected stags randomly mated with unselected outbred hinds (Table 2) suggests that the general population of deer on farms in New Zealand is likely to be quite heterogeneous for this trait. This is in keeping with the observation that where natural outbreaks of Tb involve widespread infection there is a range of disease severity, with approximately 10% of the animals severely affected. The spread of responses to Tb challenge in the offspring is likely to be due to the fact that the hinds were unselected, and this “diluted” the resistance or susceptibility of the sires and suggests that high Tb resistance or susceptibility may require the homozygous state in a number of genes.
Patterns of immune reactivity seen in animals challenged with virulent M. bovis were similar for the stags and their offspring, although there were significantly higher levels of nonspecific sensitization evident in the offspring prior to challenge. This was associated with lower levels of conversion to M. bovis-specific cellular and antibody reactivities in the offspring, at 8 and 18 weeks postinfection, than in the stags. Although postchallenge cellular sensitization to mycobacterial antigens was seen in all animals within 2 months of challenge, the specificities of these reactions differed markedly. Putatively resistant offspring (LSS, 0), which effectively cleared infection, produced increased levels of nonspecific avian cellular sensitization but no ELISA reactivity following challenge. In stark contrast, susceptible animals, which developed tuberculous lesions, produced specific bovine cellular and antibody reactions. Patterns of reactivity seen in animals challenged experimentally with virulent M. bovis were similar to responses seen in naturally infected deer in the field (11). These data highlight the fact that all animals develop acquired immune reactivity following experimental challenge with virulent M. bovis, though at qualitatively and quantitatively different levels. This indicates that the immune response in resistant animals involves acquired as well as innate immunity. The likelihood that protection against certain types of infection appears to require a direct link between innate resistance and acquired immunity has been alluded to by other workers (22). Studies using two doses of live M. bovis BCG vaccine in red deer (12) showed patterns of immunity similar to that seen in resistant animals in the present study. Vaccinated deer that were resistant to challenge with virulent M. bovis also showed nonspecific sensitization without any antibody. Thus it appears that protective immunity, resulting from either vaccination with live BCG vaccine or exposure of genetically resistant animals to virulent M. bovis, produces nonspecific sensitization without any antibody, consistent with type 1 pathways of immunity. By contrast, genetically susceptible animals and those not protected by vaccination show an early B-cell response to challenge characteristic of a type 2 pathway of immunity that is not protective against Tb. Taken together, these data suggest that resistance to Tb, whether acquired artificially following vaccination or naturally following infection, involves both the innate and acquired pathways of immunity. They also suggest that genetically susceptible deer may be incapable of developing a protective immune response to M. bovis BCG vaccine.
There was a significant correlation between the skin test reactivity and lesion severity for both the stags and the offspring of the six selected stags. The regression lines had very similar slopes (0.893 and 0.868, respectively), while the y intercept of the line for the young-adult stags (8.3 ± 1.45) was greater than that for the thinner-skinned yearling offspring (3.389 ± 0.671). Thus it appears from these data that delayed-type hypersensitivity responses are better correlated with disease than protection. The exception to this is the animal that appears to have become anergic to the skin test (Fig. 2). It was one of the three deer that had an LSS of 6, and it had a negligible (<1-mm) increase in double skin thickness at the bovine site. Such anergic animals are not unusual in severe outbreaks of Tb in farmed deer and almost invariably test positive for antibody and have severe generalized Tb lesions (11).
The low overall breeding success rate of the AI procedure was primarily due to the light condition of the hinds after a late-summer drought. The actual number of fawns born is not known because the hinds and their offspring were not yarded, identified, and blood sampled for pedigree identification until the fawns were 10 weeks old. Therefore breeding success in this context is a summation of AI success, delivery of full-term fawns, and weaning of the fawns to 3 months of age. The variability in the number of offspring from each stag appears to be largely a feature of semen quality rather than the Tb genotype of the sire. Five of the sires had breeding success rates of 23 to 49%. Only one sire had a particularly poor result (6%), and this animal's semen had the lowest postthaw motility. There are a number of factors affecting the ability of semen to achieve fertilization, and the only one that is easily assessed is sperm motility (3). The collection of semen by electroejaculation from sedated stags is highly variable, and not all stags give semen of usable quality, especially young stags. It is unfortunate that there were only a total of 11 offspring of susceptible sires because this and the “blunting” of the genetic effect due to random breeding to hinds may have been responsible for the nonparametric distribution of lesion severity (0, 0, 1, 8, 0, 0, and 2 offspring with LSSs of 0 to 6, respectively) in their offspring (Table 2).
This present study is the first time that the heritability of resistance to Tb has been measured in a domestic farm animal. Foundation studies carried out over 60 years ago established that selective breeding produced guinea pigs (32) and rabbits (17) that had increased resistance to Tb. Francis (9) reviewed a number of trials reporting that different strains of rabbits and breeds of cattle showed various degrees of resistance to experimental challenge with virulent M. bovis. Stead et al. (27) and Houk et al. (15) provided evidence for innate resistance to M. tuberculosis in humans. Natural resistance to infection with M. bovis BCG has been shown to be partially controlled by a dominant gene in mice, designated formerly as Bcg and now as Nramp (30). The human homologue of mouse Nramp, denoted NRAMP1, is believed to be associated with susceptibility to leprosy in humans (1). Cattle selected for resistance to another intracellular parasite, Brucella abortus, also display a degree of resistance to M. bovis BCG as demonstrated by the enhanced ability of their macrophages to kill organisms in vitro (26). The association between the bovine NRAMP1 gene and resistance to B. abortus and M. bovis in cattle is currently being investigated (29). A highly informative microsatellite marker derived from the 3′-untranslated region of the cervine NRAMP1 gene (21) showed no allelic associations with either the resistant or susceptible phenotype in the present study (G. Matthews, personal communication). Similarly we have found no association between the DRB genes of the cervine major histocompatibility complex and resistance to Tb (16).
We are currently investigating immunological (12) and genetic (6) markers of resistance and susceptibility to M. bovis that could be used to identify resistant and susceptible animals without the need to challenge them with virulent M. bovis. Such markers could be used to select for resistant animals and to cull susceptible animals. The results of the study reported here suggest that highly susceptible animals are most likely to become infected with M. bovis, quickly develop serious Tb, become highly infectious, and possibly become anergic to tuberculin skin tests. It is hypothesized that culling highly susceptible animals and using highly resistant breeding stags will increase the overall resistance of deer herds, reduce the number of new cases, and minimize the risk of serious outbreaks of Tb. This could provide an additional strategy to complement the existing Tb control measures aimed at reducing the number of infected herds.
Genetic and immunological markers for resistance are likely to be similar in all domestic ruminants, and their discovery in deer should provide an additional means of controlling the spread of Tb in all such domestic species. Such markers could be used to screen bulls at artificial breeding centers. The widespread use of these resistant bulls could quickly disseminate increased resistance to Tb throughout cattle herds, especially in the dairy industry, where a single bull may sire 200,000 calves in a year by AI. The identification of highly resistant or susceptible animals should also provide us with a new tool to investigate the immune response of deer and other animals to M. bovis infection.
ACKNOWLEDGMENTS
We thank the workers at AgResearch Invermay, Deer Research Laboratory, AgResearch Molecular Biology Unit, AgResearch Wallaceville, Invermay Animal Health Laboratory, Clayton Farms, Colin and Simon Batchelor, and Otago Venison.
The study was funded by the Foundation for Research, Science and Technology.
REFERENCES
- 1.Abel L, Dessein A J. The impact of host genetics on susceptibility to human infectious diseases. Curr Opin Immunol. 1997;9:509–516. doi: 10.1016/s0952-7915(97)80103-3. [DOI] [PubMed] [Google Scholar]
- 2.Animal Health Board. Annual report for the year ending 30 June 1998. Wellington, New Zealand: Animal Health Board; 1998. [Google Scholar]
- 3.Asher G W, Fisher M W, Fennessy P F, Mackintosh C G, Jabbour H N, Morrow C J. Oestrus synchronization, semen collection and artificial insemination of farmed red deer (Cervus elaphus) and fallow deer (Dama dama) Anim Reprod Sci. 1993;33:241–265. [Google Scholar]
- 4.Beatson N S, Hutton J B, De Lisle G W. Tuberculosis—test and slaughter. Proc Deer Branch NZVA. 1984;1:18–27. [Google Scholar]
- 5.Collins D M, De Lisle G W. DNA restriction endonuclease analysis of Mycobacterium bovis and other members of the tuberculosis complex. J Clin Microbiol. 1985;21:562–564. doi: 10.1128/jcm.21.4.562-564.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crawford A, Swarbrick P, Matthews G. Cloning and sequencing of candidate genes for host resistance to tuberculosis in deer. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Otago Conference Series no. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 33–34. [Google Scholar]
- 7.Fennessy P, Beatson N, Mackintosh C. Artificial insemination. Proc Deer Branch NZVA. 1987;4:33–37. [Google Scholar]
- 8.Feore M. Tb eradication update. Agric North Ireland. 1993;7:10. [Google Scholar]
- 9.Francis J. Tuberculosis in animals and man. A study in comparative pathology. London, United Kingdom: Cassell and Co. Ltd.; 1958. [Google Scholar]
- 10.Griffin F, Bisset B, Rodgers C, Mackintosh C. Uncontrollable spread of Tb within a deer herd. Proc Deer Branch NZVA. 1998;15:227–231. [Google Scholar]
- 11.Griffin J F T, Buchan G S. Aetiology, pathogenesis and diagnosis of Mycobacterium bovis in deer. Vet Microbiol. 1994;40:193–205. doi: 10.1016/0378-1135(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 12.Griffin J F T, Mackintosh C G, Buchan G S. Animal models of protective immunity in tuberculosis to evaluate candidate vaccines. Trends Microbiol. 1995;3:418–424. doi: 10.1016/s0966-842x(00)88994-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffin J F T, Chinn D N, Rodgers C R, Buchan G S. Diagnosis of tuberculosis due to Mycobacterium bovis in New Zealand red deer (Cervus elaphus) using a composite blood test and antibody assays. N Z Vet J. 1994;42:173–179. doi: 10.1080/00480169.1994.35815. [DOI] [PubMed] [Google Scholar]
- 14.Haigh J C, Hudson R J. Artificial breeding. In: Reinhardt R W, editor. Farming Wapiti and red deer. St. Louis, Mo: Mosby-Year Book, Inc.; 1993. pp. 124–134. [Google Scholar]
- 15.Houk V N, Baker J H, Sorenson K, Kent D C. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16:26–35. doi: 10.1080/00039896.1968.10665011. [DOI] [PubMed] [Google Scholar]
- 16.Linscott E M, Mackintosh C G, Griffin J F T, Crawford A M A. A simplified method for typing DRB alleles in deer. Proc N Z Soc Anim Prod. 1998;58:13–15. [Google Scholar]
- 17.Lurie M R. Heredity, constitution and tuberculosis, an experimental study. Am Rev Tuberc. 1941;44(Suppl. 3):1–125. [Google Scholar]
- 18.Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sunderland, Mass: Sinauer Associates Inc.; 1998. [Google Scholar]
- 19.Mackintosh C G, Waldrup K, Griffin J F T. A new perspective on the epidemiology of tuberculosis in farmed deer. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Otago Conference Series No. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 270–272. [Google Scholar]
- 20.Mackintosh C G, Waldrup K, Labes R, Buchan G, Griffin F. Intra-tonsil inoculation: an experimental model for tuberculosis in deer. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Otago Conference Series no. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 121–122. [Google Scholar]
- 21.Matthews G D, Crawford A M. Cloning, sequencing and linkage mapping of the NRAMP1 genes of sheep and deer. Anim Genet. 1998;29:1–6. doi: 10.1046/j.1365-2052.1998.00224.x. [DOI] [PubMed] [Google Scholar]
- 22.Medzhitov R, Janeway C A., Jr Innate immunity: impact on the adaptive immune response. Curr Opin Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- 23.Neill S. Mycobacterium bovis infection in cattle and its control in developed countries. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Otago Conference Series no. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 183–186. [Google Scholar]
- 24.O'Neil B D, Pharo J J. The control of bovine tuberculosis in New Zealand. N Z Vet J. 1995;43:249–255. doi: 10.1080/00480169./1995.35903. [DOI] [PubMed] [Google Scholar]
- 25.O'Reilly L M, Daborn C J. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tuber Lung Dis. 1995;76(Suppl. 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 26.Qureshi T, Templeton J W, Adams L F. Intracellular survival of Brucella abortus, Mycobacterium bovis BCG, Salmonella dublin and Salmonella typhimurium in macrophages from cattle genetically resistant to Brucella abortus. Vet Immunol Immunopathol. 1996;50:55–65. doi: 10.1016/0165-2427(95)05492-8. [DOI] [PubMed] [Google Scholar]
- 27.Stead W W, Senner J W, Reddick W T, Lofgren J P. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322:422–427. doi: 10.1056/NEJM199002153220702. [DOI] [PubMed] [Google Scholar]
- 28.Tate M, McDonald R, Ward J, Dodds K. DNA-matching in deer. Proc Deer Branch NZVA. 1998;15:119–125. [Google Scholar]
- 29.Templeton J, Adams G. Genetics of natural resistance to tuberculosis. In: Griffin F, de Lisle G, editors. Tuberculosis in wildlife and domestic animals. Otago Conference Series no. 3. Dunedin, New Zealand: University of Otago Press; 1995. pp. 29–32. [Google Scholar]
- 30.Vidal S M, Malo D, Vogan K, Shamene E, Gros P. Natural resistance to infection with intracellular parasites and isolation of a candidate for Bcg. Cell. 1993;73:469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- 31.Wilesmith J W. Epidemiological features of bovine tuberculosis in cattle herds in Great Britain. J Hyg (Cambridge) 1983;90:159–176. doi: 10.1017/s0022172400028837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright S, Lewis P A. Factors in the resistance of guinea pigs to tuberculosis, with especial regard to inbreeding and heredity. Am Nat. 1921;55:20. [Google Scholar]