Abstract
Progresses in the medicinal application of nanocompounds were accepted for the treatment of cancer. Nanoparticles-based therapy is of benefit for effective biodistribution and specific targeting. The current study investigated the anticancer effect of green synthesized platinum nanoparticles (PtNPs) against colon cancer cells (HCT-116). Flow cytometry and ELISA techniques were employed for detecting apoptotic and oxidative stress markers. Furthermore, PtNPs-lycopene (PtNPs-LP) on cell migration and invasion of HCT-116 cells was also examined. The PtNPs-LP was capable of diminishing cell proliferation and viability of HCT-116 cells in a dose-dependent mode. After treatment with PtNPs-LP, a significant increase in pro-apoptotic Bax and caspase-3 and a decrease in anti-apoptotic Bcl-2 was observed in treated cells that subsequently released cytochrome C into its cytoplasm, initiating cell death. Moreover, PtNPs-LP induced excessive generation of reactive oxygen species (ROS) and oxidative stress in cancer cells. In conclusion, PtNPs-LP exerts an antitumor effect against colon cancer cells via mediating important mechanisms such as cytotoxicity, apoptosis, and oxidative stress.
Keywords: Platinum nanoparticles, HCT-116 cells, Apoptosis, Lycopene and cytotoxicity, ROS
Platinum nanoparticles; HCT-116 cells; Apoptosis; Lycopene and cytotoxicity; ROS.
1. Introduction
Colorectal cancer (CRC) is the third leading cause of cancer-related deaths worldwide, and its burden is expected to increase further in the coming years. CRC resulted from mutations in oncogenes, tumor suppressor genes, and DNA repair genes that cause the transition of the normal epithelium of the colon to adenoma [1]. In terms of clinical practice, chemotherapy, surgery, and radiation therapy are frequently used in combination to treat CRC. However, the strategy of administering chemotherapy for CRC has a number of drawbacks, particularly the side-effects of medication after repeated administrations. In order for chemotherapy to be effective, tumor cells must get high enough quantities of a therapeutic agent without the patient experiencing unbearable side effects [2].
Cisplatin (CP) is a powerful anticancer drug commonly used in the chemotherapy of colorectal cancer. The effectiveness of cisplatin depends on interfering with nucleotides [3] and induction of DNA adducts that stimulate apoptosis via various signaling pathways [4]. Moreover, cisplatin can interact with the cell membrane and inactivating many vital membranous components such as membrane channels, transporter proteins, and enzymes [5].
Nanoparticles (NPs) act as unique functional biometals due to their specific electronic, chemical, and physical features that make them appropriate agents in diagnosis, drug delivery procedures, and treatment of cancer [6]. The size of NPs is the same as that of human proteins, which are frequently in the 1 to 100 nm range [7]. Numerous in vitro studies indicated that platinum NPs (PtNPs) exert anticancer effects through modulation of important pathways such as apoptosis, oxidative stress, inflammatory reaction, genotoxicity, and inappropriate gene transcription [4]. Green biosynthesis procedures are safe, cheap, rapid, and sustainable. In these methods, chemicals extracted from plants or microorganisms are utilized as reducing agents [8]. PtNPs resulting from these methods are more safe and stable than those produced from physical or chemical methods [7]. PtNPs are effectively used in biomedical fields because of their thermo-stability and photothermal efficacy [9]. Furthermore, plants are chosen over all other biological processes for synthetic methods because they do not require maintaining cell cultures. Plant extracts were also discovered to be more successful and efficient at regulating the size, shape, and dispersion of the nanoparticles [10].
Lycopene (LP) is one of the biological stabilizing agents utilized in the green synthesis of PtNPs that presents in many vegetables and protects against diseases like cancer [11, 12, 13]. Furthermore, amassing evidence proposed that the efficacy of LP for treating cancer makes it a powerful anticancer agent. In this regard, Teodoro et al. [14] discovered that lycopene may reduce cell proliferation, arrest the cell cycle at various stages, and promote apoptosis, suggesting that the change of cell cycle-regulatory proteins may be a potential mechanism. Additionally, Cui et al. [15] found that by reducing the protein expressions of NF-κB (nuclear factor-κB) and COX-2 (cyclooxygenase-2) in the esophageal tissue, LP may limit the carcinogenesis of esophageal in F344 rats by inhibiting inflammation and drastically reducing inflammatory cytokines. According to Aktepe et al. [16] study's LP increases cervical cancer cells' susceptibility to cisplatin by suppressing NF-κB-mediated inflammatory responses and regulating Nrf2 (nuclear factor erythroid 2–related factor 2)-mediated oxidative stress.
Therefore, this study aimed to assess the anticancer capabilities of green biosynthesized PtNPs based on the suggestion that biological agents can stabilize nanoparticles' synthesis when they are utilized in a hybridized model.
2. Material and methods
2.1. Chemicals
Cisplatin was bought from Sigma-Aldrich. Deionized distilled water was used for dissolving cisplatin. First, however, LP was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, USA) to make a stock solution of LP. Formazan powder was obtained from Sigma-Aldrich.
2.2. Preparation and characterization of PtNPs-LP
PtNPs-LP were biosynthesized via reduction of PtCl6− ions to PtNPs by mixing of 2 mL of lycopene (0.1 mM) and 10 mL of H2PtCl6·6H2O (0.1 mM). This Mixture was incubated for 1 h at 50 °C in tightly closed flasks to prevent dehydration, where high temperatures catalyze the reduction mechanism. The solution of reduced platinum was centrifuged at 4000 x g for 10 min to isolate platinum nanoparticles from other biomolecules. The purification of platinum was performed by repeating centrifugation until getting pellets that were washed away with deionized distilled water (ddH2O) to eliminate impurities [17]. X-ray diffraction (XRD) and Fourier-transform infrared spectroscopy (FTIR) characterize PtNPs-LP. The average size of PtNPs-LP was measured by Zetasizer (Nano series, ZEN 3600, Malvern, UK).
2.3. Culture of HCT-116 cells
The human colon cancer cell line (HCT-116) was purchased from American Type Culture Collection (ATCC). HCT-116 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM; Gibco Life Technologies) sustained with 10% fetal bovine serum and 100 μg/mL penicillin-streptomycin. Then tissue culture flasks were kept in a 5% CO2 atmosphere at room temperature and appropriate humidity.
2.4. MTT assay
The MTT (dimethyl thiazolyl tetrazolium bromide) test was utilized to detect PtNPs-LP and cisplatin's cytotoxic effect. Previously cultured HCT-116 cells were separately incubated in 96-well plates with various concentrations (from 0.0 μM/mL to 100 μM/mL) of cisplatin and PtNPs-LP for 24 h at 37 °C in a humidified 5% CO2 incubator [17]. The wells containing cells were swept away with 1X PBS, followed by 20 μl of MTT (5 mg/mL) at 37 °C for 30 min. For dissolving the purple formazan crystals, 200 μl of DMSO was added to all wells and incubated at 37 °C for 30 min [7]. The absorbance related to resultant color was measured at 570 nm using a microplate ELISA reader.
The cell viability was evaluated using the following equation (7):
Cell viability (%) = Treated/control × 100.
2.5. Detection of LDH leakage
The integrity of the HCT-116 cell's membrane was assessed by using cytotoxicity detection kits. Cells were incubated with cisplatin and PtNPs-LP for 24 h. Cell lines were incubated with two different concentrations of PtNPs-LP; 5 and 10 μM/mL or with CP at 0.2 μM/mL. Consequently, 100 μL of supernatant was incubated with 100 μl of LDH mix (11644793001; Sigma-Aldrich; USA) for 30 min then absorbance was read at 490 nm using a microplate reader.
2.6. Measurement of apoptotic biomarkers
Protein levels of apoptotic markers; Bcl-2, Bax, cytochrome c, and cleaved caspase-3 were investigated following the methodology of ELISA kits purchased from Abcam (Cambridge, UK). HCT-116 cells were independently incubated with PtNPs-LP (5 and 10 μM/mL) and cisplatin at IC30 concentration (0.2 μM/mL as obtained in the current study, Figure 2) for 24 h, whereas the control was incubated with vehicle. For collection, the cells were centrifuged at 1800 × g for 5 min; then, they washed away twice with PBS for removing the media and forming pellet. The resulting pellet was lysed with 50 μl of cold buffer yielding lysate centrifuged at 12,000 × g at 4 °C for 1 min resulting in the supernatant. The levels of protein expression were quantified in the supernatant using the Bradford method. If the expression level is more than 4 μg/μl, the sample is diluted in a PTR buffer. The intensity of resultant color was measured at 405 nm using a microplate reader (Biotech, Inc., USA).
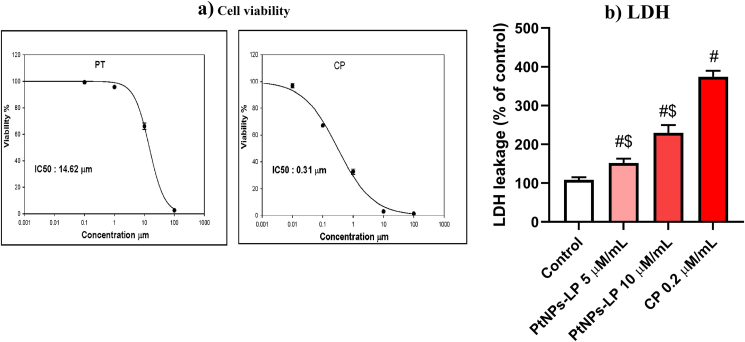
Figure 2.
Cytotoxic effects evidenced by (a) decrease of cell viability and (b) LDH release effect of different concentrations of biosynthesized PtNPs using lycopene (PtNPs-LP) and ciplatin (CP) on HCT-116 cells. # Indicates the significance compared to the control (P < 0.05) whereas, $ Indicates the significance compared to the cisplatin (P < 0.05).
2.7. Transwell migration and invasion assay
Invasion assay of HCT-116 cells was performed in Transwell 8.0-μm pore membranes supplied by Corning [18]. 100 μl of 1:8 DMEM-diluted Matrigel (BD, USA) coated the upper chamber of the Transwell, then 1 × 105 cells were added. The inferior chamber contained a chemo-attractant medium with the presence or absence of PtNPs-LP. After incubation at room temperature for 48 h, non-invading cells on the superior membranes were eliminated with a cotton pad; however, those invaded cells to the lower membrane chamber were treated with 4% paraformaldehyde fixative and 0.1% crystal violet staining solution [19]. The counting and photographing of the invading cells were carried out by a light microscope of 200x magnification.
2.8. Investigation of the cell cycle phases by flow-cytometry
Cultured HCT-116 cells (2 × 105 cell/mL) were treated with cisplatin (0.2 μM/mL and PtNPs-LP (5 μM/mL) for 24 h. HCT-116 cells were harvested and treated with binding buffer followed by incubation with fluorescein isothiocyanate (FITC)-conjugated annexin V (Cat. No: ab14085, Abcam, UK) for 10 min in a dark area at 30 °C). Cooling centrifugation of the cells at 4,000 × g followed by suspension in the binding buffer and labeling by propidium iodide [20]. The percentage of labeled cells in different cell cycle stages was investigated by flow cytometer (BD FACSCalibur flow cytometer, USA).
2.9. Assessment of oxidative and anti-oxidative markers
Cultured HCT-116 cells were incubated with cisplatin (0.2 μM/mL) and PtNPs -LP (5 and 10 μM/mL) for 24 h; then, cells were harvested and lysed. The supernatant was formed after cooling centrifugation of cell lysate at 12,000 × g for 1 min. The protein expression levels in cell lysate were investigated using the Bradford method. The resulting cell lysate was used for localizing ROS levels by means of the green fluorescence stain 2,7-dichlorofluorescein diacetate (DCFH-DA) previously described [21].
The levels of ROS-related markers such as lipid peroxide (LPO) and Nitric oxide (NO) were detected according to the methods of Ohkawa et al. [22] and Green et al. [23], respectively. Expression levels of anti-oxidant parameters such as reduced glutathione (GSH) and proteins carbonyl were assessed as described by Ellman [24], Levine et al. [25], respectively. Whereas 8-hydroxy-20-deoxyguanosine (8-OHdG) was detected using an ELISA kit obtained from MyBioSource (San Diego, CA, USA) according to the supplier's instructions.
2.10. Statistical analysis
All statistics were carried out using SPSS software (20.0), and all data were presented as mean ± SD. One-way ANOVA and post hoc Duncan's test were used to compare different groups. Differences between groups were considered significant if P values <0.05.
3. Results
3.1. Characterization of PtNPs-LP
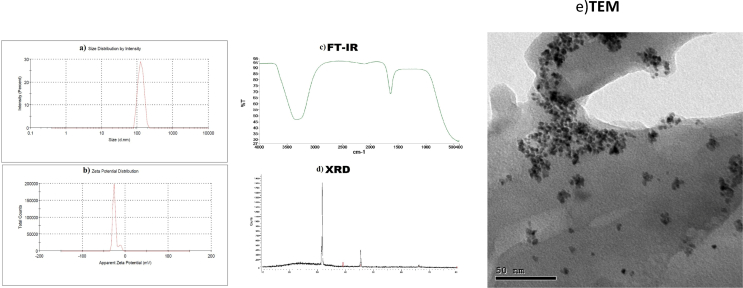
a) Zeta sizer and potential of PtNPs-LP: In this study, the mean zeta potential of PtNPs-LP was -28.7 mV, and the mean diameter was 124.3 nm (Figures 1a and 1b). b) FTIR of PtNPs-LP: The result of FTIR of synthesized PtNPs-LP was represented in Figure 1c. PtNPs interacted with lycopene and produced a wide peak at 3326.20 cm−1, which refers to O–H group. C–H stretch alkynes produce an absorption peak of 2115.02 cm−1. The C–O asymmetric stretch carbon complexes give a band at 1636.00 cm−1. In alkyl halides, C–X extending produces bands at 458.88, 435.21, and 424.18 cm−1. These results predict the presence of several functional groups that might be a good indicator for reduced and well-stabilized lycopene PtNPs. c) XRD of PtNPs-LP: XRD detected the crystal nature and purity of PtNPs patterns at 2θ (deg) = 31.8°, 39.1°, and 36.7°. The XRD bands of PtNPs wide but lacked definite Braggs peaks. These observations ensure that PtNPs-LP reflects the synthesized sample, indicating the particles' nanocrystalline nature (Figure 1d). d) TEM of PtNPs-LP: The mean particle size of PtNPs-LP as measured by transmission electron microscopy (TEM) was <50 nm with narrow distributed sphere-shaped particles and scattered particles (Figure 1e).
Figure 1.
Characters of biosynthesized PtNPs using lycopene. a) Zeta sizer b)Zeta potential c)FTIR d)XRD e)TEM.
3.2. PtNPs-LP induce cytotoxicity and growth inhibition in HCT-116 cells
In the current study, the anticancer effect of PtNPs-LP against HCT-116 cells was investigated via MTT assay. Cells incubated separately with different concentrations (0.001–1000 μM/mL) of PtNPs-LP and cisplatin for 24 h. PtNPs-LP remarkably suppressed the growth of HCT-116 cells with rising concentrations in a dose-dependent mode (Figure 2a). The growth inhibitory effect of PtNPs-LP against HCT-116 cells was not as much as 10% at the concentration of 100 μM/mL, and the half-maximal inhibitory concentration (IC50) value was 14.62 μM/mL. Nevertheless, the cytotoxic effect of PtNPs-LP against cancer cells was lesser than that of cisplatin, for whom IC50 was 0.31 μM/mL. In the current results, the levels of LDH were significantly increased (P < 0.05) in the medium of cells incubated with PtNPs-LP than in controls (Figure 2b). LDH concentration was found to be 151.8 and 229.7 at 5 and 10 μm/mL PtNPs-LP, respectively.
3.3. Effect of PtNPs-LP treatment on the cell death signaling of HCT-116 cells
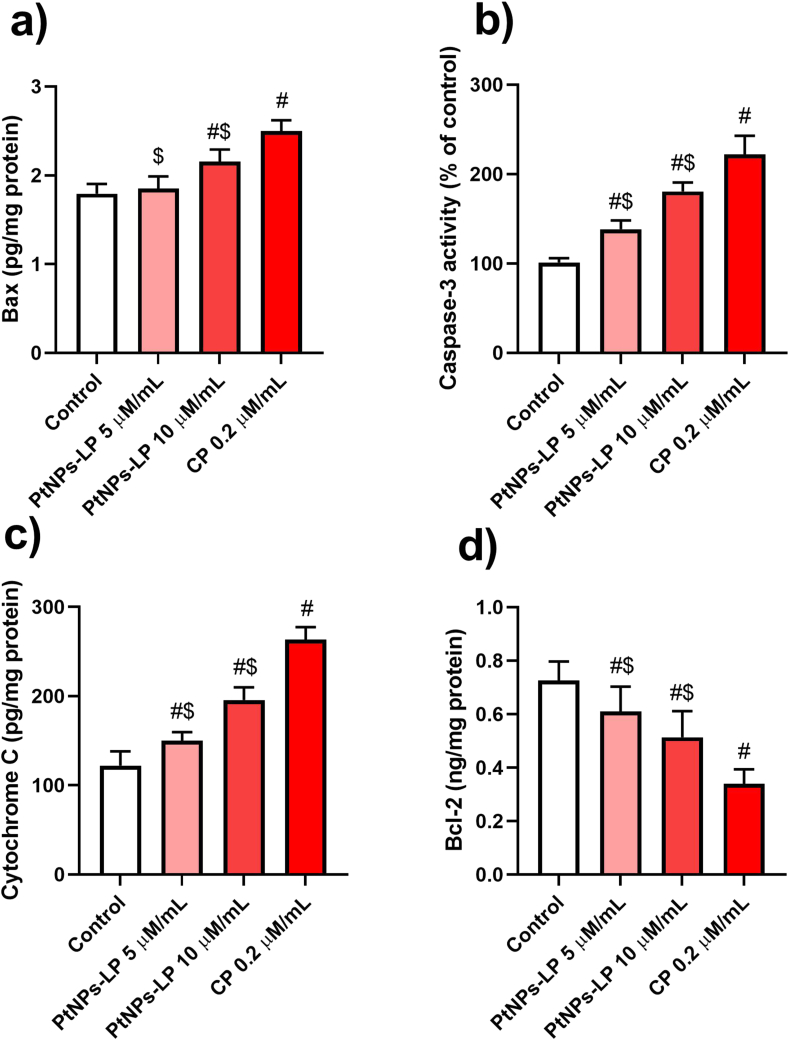
The balance between anti- and pro-apoptotic proteins is central for cell survival. Therefore, we evaluated level of the important pro-apoptotic proteins Bax, caspase-3, and cytochrome c, in addition to, the anti-apoptotic protein Bcl2 in HCT-116 cells incubated with various concentrations of PtNPs-LP (5 and 10 μm/mL) and cisplatin (0.2 μm/mL) for 24 h. The results revealed that PtNPs-LP and cisplatin significantly increased (P < 0.05) the pro-apoptotic proteins (Figure 3a, b, and c), and significantly reduced the anti-apoptotic protein Bcl-2 (Figure 3d) compared to the untreated HCT-116 control cells. The obtained results revealed that PtNPs-LP treatment can enhance cell death in HCT-116 cells.
Figure 3.
Cell death signaling [)a) Bax)b) caspase-3)c) cytochrome c)d) Bcl2] effects of different concentrations of biosynthesized PtNPs using lycopene (PtNPs-LP) and ciplatin (CP) on HCT-116 cells. # Indicates the significance compared to the control (P < 0.05) whereas, $ Indicates the significance compared to the cisplatin (P < 0.05).
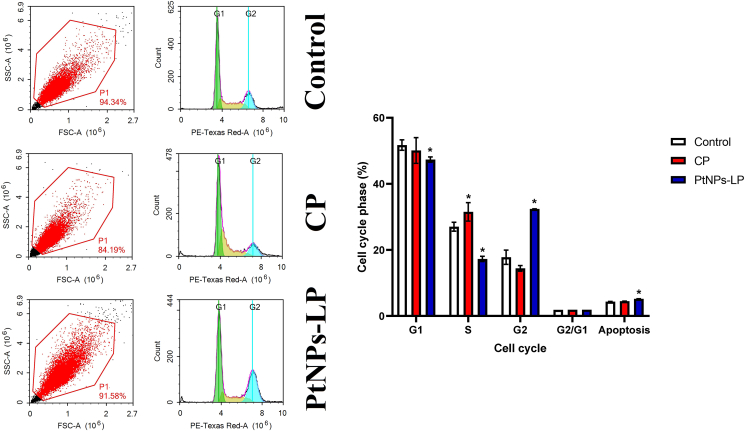
3.4. Suppression of cell cycle of HCT-116 cells by PtNPs-LP
In comparison to control cells, PtNPs-LP at 5 M/mL caused considerable cell cycle arrest in HCT-116 cells at the G2 phase (Figure 4), and the distribution of cells in the G1 and S phases was significantly reduced (P < 0.05). This demonstrates that platinum nanoparticles successfully confine cells in the G2 phase of the cell cycle and prohibit cells from progressing to the S phase of the cell cycle, preventing cell division and, as a result, cell proliferation in colon cancer cells. Interestingly, the obtained data revealed that lycopene conjugated with PtNPs modified its properties as evidenced by the difference between cisplatin and PtNPs-LP in accumulating cells in G2 instead of S phase.
Figure 4.
Cell cycle suppression effects of biosynthesized PtNPs using (PtNPs-LP) and ciplatin (CP) on HCT-116 cells. # Indicates the significance compared to the control (P < 0.05) whereas, $ Indicates the significance compared to the cisplatin (P < 0.05).
3.5. Suppression of migration and invasion of HCT-116 cells by PtNPs-LP
HCT-116 cells are characterized by their capacity for migration and invasiveness that help them metastasize to the secondary site and colonize there. In the current work, we examined if PtNPs-LP could suppress the migration and invasion of HCT-116 cells to prevent their outgrowth to a distant location. Unfortunately, the invasion procedure revealed that PtNPs-LP has no observable effect on diminishing the invasive and migratory ability of HCT-116 cells (Figure 5).
Figure 5.
HCT-116 cells migration and invasion after the treatments by biosynthesized PtNPs using lycopene (PtNPs-LP) and ciplatin (CP) at different points of time compared to the control untreated cells.
3.6. Effect of PtNPs-lycopene on oxidative stress
Oxidative stress is involved in NPs-mediated cytotoxicity and cell death. Hence, we aimed to investigate the role of PtNPs-LP induced oxidative stress in HCT-116 cells, the cells were treated with PtNPs-LP (5 and 10 μM/mL) or cisplatin (0.2 μM/mL) for 24 h, and subsequently LPO, PC, NO, and GSH were measured. The obtained results revealed that significant increases in the pro-oxidants markers such as LPO, PC, and NO (Figure 6a, b, and c) and an apparent reduction in anti-oxidants such as GSH level were noticed in PtNPs-LP and cisplatin-treated cells compared to control cells. Remarkably, GSH was greater in HCT-116 cells that treated with PtNPs-lycopene than cisplatin (Figure 6d).
Figure 6.
Oxidant/anti-oxidant [)a) LPO)b) PC)c) NO)d) GSH] imbalance effects of different concentrations of biosynthesized PtNPs using lycopene (PtNPs-LP) and ciplatin (CP) on HCT-116 cells. # Indicates the significance compared to the control (P < 0.05) whereas, $ Indicates the significance compared to the cisplatin (P < 0.05).
3.7. PtNPs induce oxidative DNA damage
The level of 8-OHdG was measured in HCT-116 cells treated with PtNPs and cisplatin for 24 h. Remarkably, the two doses (5 and 10 μM/mL) of PtNPs resulted in significant upregulation of accumulated 8-OHdG (315.6 ng/mg protein and 411.9 ng/mg protein) in treated cells compared with that of control (100 ng/mg protein) as shown in Figure (7). Nevertheless, the accumulation of OHdG with two doses of PtNPs was still significantly lower than that of the cisplatin-treated rats (524.6 ng/mg protein).
Figure 7.
8-hydroxy-20-deoxyguanosine concentration after treatment with different concentrations of biosynthesized PtNPs using lycopene (PtNPs-LP) and ciplatin (CP) on HCT-116 cells. # Indicates the significance compared to the control (P < 0.05) whereas, $ Indicates the significance compared to the cisplatin (P < 0.05).
4. Discussion
With the progress of NPs applications, the diagnosis and treatment of colon cancer are possible with diverse types, forms, and sizes of NPs [26]. Nanomaterials such as platinum NPs have been either used or combined with other nanometals or drugs for colon cancer therapy. Cisplatin was one of the most effective platinum derivatives used to treat many types of cancer [27]. However, cisplatin triggers genotoxicity through intercalating with DNA nucleotides, consequently leading to cancer cell apoptosis. Despite its success, cisplatin possesses some disadvantages, such as low specific interaction, inadequate biodissemination rate, severe side effects, and development of resistances [28]. Therefore, biosynthesizing of PtNPs using lycopene act as new platinum-based combinations that may diminish toxic side effects on cancer cells by targeting proteins expressed in cancer cells. The stable PtNPs biosynthesis with antioxidant molecules or plant extracts were achieved by other researchers using apigenin [29] and Punica granatum (pomegranate) [30].
The cytotoxic effect of cisplatin and PtNPs-LP was inspected in HCT-116 cells, where the values of IC50 were found to be 0.31 μM/mL and 14.62 μM/mL, respectively. HCT-116 cells exhibited remarkable cytotoxicity following treatment with PtNPs-LP. Compared to control, HCT-116 cells incubated with PtNPs-LP showed increased release of the cytoplasmic LDH in their extracellular space. Similar to our results, cytotoxicity of PtNPs-LP nanocompounds was detected in THP-1 cells that showed a reduction in their viability growth and a significant increase in levels of the cytotoxic LDH [31]. Moreover, the authors indicated that LDH leakage referred to cell lysis and membrane damage due to PtNPs cytotoxicity. In the same line, PtNPs enhanced cytotoxicity in lung, prostate, and OS epithelium cancer cell lines via releasing of LDH [32, 33].
ROS impairs the intracellular balance between oxidative stress and anti-oxidant mechanisms, leading to lasting cellular damage [17]. Therefore, the intracellular accumulation of ROS interrupts the functions of cellular organelles and causes cytotoxicity. We measured the expression of oxidative stress markers such as LPO, NO, and PC in HCT-116 cells incubated with two different concentrations of PtNPs. After treatment, a significant increase in ROS was observed in treated cells compared to the untreated control. This observation was similar to that recorded in embryonic renal cells [34], lung cancer cells [35], and HepG2 cells [36] treated with PtNPs that exerted cytotoxic and anticancer effects via the generation of ROS. Furthermore, a significant increase of LPO was observed in PtNPs-LP -treated cells concerning the control. Formation of lipid peroxides may be due to liberation of ROS and disability of anti-oxidants [20] following PtNPs treatment. Excessive ROS formation can oxidize cellular macromolecules like proteins, and fatty acids generate highly reactive radicals, consequently increasing LPO concentration [37]. In the current study, the level of NO was significantly increased in HCT-116 cells after incubation with PtNP. Similarly, NO formation is recorded in different cell lines of cancer cells treated with PtNPs [29, 38]. NO regulates signaling and homeostasis of redox pathways; nevertheless, atypical overproduction of NO can modulate a cascade of events within the cells, comprising ROS formation, disturbance of mitochondrial membrane integrity, and finally apoptosis [6]. Proteins can be oxidized in carbonylation that can be stimulated by ROS [39]. Compared to the control cells, the levels of carbonylated proteins increased significantly in HCT-116 cells treated with PtNPs. This finding was also noticed in macrophages, neurons, and human colon cancer cells treated with silver NPs [40]. In the same way, in a previous study, a combination of PtNPs and doxorubicin-induced protein carbonylation in treated osteosarcoma cells [33].
ROS causes DNA damage via oxidizing nitrogenous bases, causing DNA adducts. Guanosine is the furthermost sensitive base to oxidation and modification by ROS, leading to the production of 8-oxoguanine [41]. Therefore, we measured the concentration of 8-hydroxy-2-deoxyguanosine (8-OHdG) in HCT-116 cells incubated with PtNPs and cisplatin for 24 h using ELISA. Compared to the control cells, the accumulated 8-OHdG was increased significantly in HCT-116 cells treated with PtNPs (P < 0.05). Similar to our findings, PtNPs induced substantial oxidative DNA damage and accumulation of 8-OHdG in human monocytic THP-1 cells [29] and osteosarcoma cancer cell lines [33]. Many previous studies performed on other metallic NPs indicated that the excessive release of ROS and RNS leads to DNA irreversible changes due to the accumulation of 8-OHdG [29, 42].
GSH is an important thiol-dependent enzymatic anti-oxidant involved in a defense mechanism against oxidative stress [18]. GSH is considered a guardian of cellular homeostasis, DNA replication, and repair [43]. In the current study, GSH content was significantly decreased in PtNPs-LP treated cells compared to the untreated control. These results were parallel to those observed in many human cancer cell lines treated with PtNPs [29, 44]. A decline in GSH level may be due to oxidation to glutathione as a result of oxidative stress. Collectively, our results indicate that oxidative stress, which is characterized by an increase in intracellular LPO, NO, and protein carbonylation and a decrease in GSH, is a common mechanism of PtNP-induced toxicity in HCT-116 cells. However, PtNPs have recently demonstrated antioxidant activity by scavenging free radicals caused by hydrogen peroxide in the A549 cell line, and their antioxidant capacity is comparatively lower than vitamin C. This was reported by Ismail et al. in 2022 [45]. In reality, PtNPs with smaller sizes (<100 nm) can enter cells directly; in contrast, PtNPs with bigger sizes (>100 nm) must enter cells through traditional endocytosis, which may be more harmful to cells.
The mitochondria are key regulators of proteins involved in apoptotic signaling pathways [46]. Bcl-2 and Bax are considered the main proteins targeted by mitochondria. Keeping the balance between pro-apoptotic and anti-apoptotic protein families is essential for avoiding mitochondrial dysfunction. The abnormal function of mitochondria is responsible for initiating ER stress [47] that activates Bax and Bcl-2 genes involved in deciding if cell die or survive [48]. The current results revealed a significant increase in caspase-3, cytochrome C, and Bax expressions after PtNPs-LP treatment, while a Bcl-2 expression was reduced. Similarly, Gurunathan et al. [17, 31] indicated that PtNPs significantly upregulated Bax and caspase-3 and downregulated Bcl-2 in THP-1 and SH-SY5Y cancer cells compared to non-treated cells. Caspase is one of the apoptosis-interrelated proteins where its activation transduces a signaling cascade that results in apoptosis [49]. Similar to our results, it was previously proposed that the nanoemulsion carrying gold nanoparticles and lycopene can hasten apoptosis through activating caspases 8, 9, and 3 [50]. It was reported that NO production leads to apoptosis characterized by discharge of cytochrome c into the cytosol, activation of pro-apoptotic caspases, and breakup of DNA [51]. Moreover, atypical NO production can also cause apoptosis through overexpression of p53 [52].
It is generally known that cancer cells can proliferate indefinitely, primarily linked to the disruption of cell cycle progression and the stimulation of invasion [53]. In the G2 phase of the cell cycle, HCT-116 cells treated with PtNPs-LP showed a high buildup of proliferative cells. This is parallel to the finding suggesting that PtNPs caused an accumulation of cervical cancer cell line (SiHa) cells in the G2/M phase, which reflects the DNA damage effect of PtNPs, which can lead to a variety of fates, including apoptosis, prolonged permanent arrest, and recovery after repair of DNA damage or adaptation to the damage, allowing progression through the cell cycle with the DNA damage that initially induced the arrest, as reported by Alshatwi et al. [54]. Cells that emerge from G2 arrest may proceed through the cell cycle or die by apoptosis [55].
Metastasis is an important stage of cancer progression and aggressiveness. The acquisition of migratory and invasive properties contributes to the formation of micro and macrometastasis that worsens prognosis [56]. The migratory and invasive characteristics of treated HCT-cells were examined. Unexpectedly, PtNPs treatment cannot inhibit migration and invasion of treated cells. Misappropriate size, concentration, or shape of PtNP may negatively affect the combination efficiency of PtNPs and lycopene and the delivery process. Additional in vivo experiments are required to confirm and support the current finding.
4.1. Study limitations
The present study had two limitations: it did not assess the dose-dependent effect with more than two doses, and it validated its findings using an in vivo model, which might have precisely limited the results. Despite these limitations, this experimental investigation used defects in the oxidant/antioxidant balance, cell cycle, and programmed cell death pathway to demonstrate the anticancer effect of PtNPs-LP. The results of this study, however, are probably applicable to other malignancies.
5. Conclusion
The present study showed that PtNPs-LP could arrest cell cycle at the G2 phase and inhibit proliferation, and induce cytotoxicity against HCT-116 cells via triggering oxidative stress that mediates DNA damage and related apoptotic mechanisms. Caspase-3 and Bax overexpression and Bcl-2 downregulation may be the underlying mechanisms of PtNPs-LP, which may further result in the suppression of HCT-116 cell growth and proliferation and the encouragement of apoptosis. This suggests that the antitumor effect exerted by PtNPs-LP could be useful in treating colon cancer. However, further studies should be conducted in the future to confirm the anticancer effect PtNPs-LP in an in vivo model.
Declarations
Author contribution statement
Nouf M. Alyami, Rafa Almeer and Hanadi M. Alyami: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Researchers Supporting Project number (RSP2022R177), King Saud University, Riyadh, Saudi Arabia.
Data availability statement
Data will be made available on request.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgments
The writer would like to thank the Researchers Supporting Project number (RSP2022R177), King Saud University, Riyadh, Saudi Arabia.
References
- 1.Afrin S., Giampieri F., Gasparrini M., Forbes-Hernández T.Y., Cianciosi D., Reboredo-Rodriguez P., et al. Dietary phytochemicals in colorectal cancer prevention and treatment: a focus on the molecular mechanisms involved. Biotechnol. Adv. 2020;38 doi: 10.1016/j.biotechadv.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 2.El-Garawani I.M., Elkhateeb W.A., Zaghlol G.M., Almeer R.S., Ahmed E.F., Rateb M.E., et al. Candelariella vitellina extract triggers in vitro and in vivo cell death through induction of apoptosis: a novel anticancer agent. Food Chem. Toxicol. 2019;127:110–119. doi: 10.1016/j.fct.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Fernandez-Gil B.I., Guerra-Librero A., Shen Y.Q., Florido J., Martinez-Ruiz L., Garcia-Lopez S., et al. Melatonin enhances cisplatin and radiation cytotoxicity in head and neck squamous cell carcinoma by stimulating mitochondrial ROS generation, apoptosis, and autophagy. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/7187128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Almeer R.S., Abdel Moneim A.E. Evaluation of the protective effect of olive leaf extract on cisplatin-induced testicular damage in rats. Oxid. Med. Cell. Longev. 2018;2018:11. doi: 10.1155/2018/8487248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dkhil M.A., Al-Quraishy S., Aref A., Othman M.S., El-Deib K., Abdel Moneim A.E. The potential role of azadirachta indica treatment on cisplatin-induced hepatotoxicity and oxidative stress in female rats. Oxid. Med. Cell. Longev. 2013;2013 doi: 10.1155/2013/741817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Othman M.S., Obeidat S.T., Al-Bagawi A.H., Fareid M.A., Fehaid A., Abdel Moneim A.E. Green-synthetized selenium nanoparticles using berberine as a promising anticancer agent. J. Integr. Med. 2021 doi: 10.1016/j.joim.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Mohammadi H., Abedi A., Akbarzadeh A., Mokhtari M.J., Shahmabadi H.E., Mehrabi M.R., et al. Evaluation of synthesized platinum nanoparticles on the MCF-7 and HepG-2 cancer cell lines. Int. Nano Lett. 2013;3(1):28. [Google Scholar]
- 8.Parlinska-Wojtan M., Depciuch J., Fryc B., Kus-Liskiewicz M. Green synthesis and antibacterial effects of aqueous colloidal solutions of silver nanoparticles using clove eugenol. Appl. Organomet. Chem. 2018;32(4) doi: 10.1007/s00449-016-1599-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samadi A., Klingberg H., Jauffred L., Kjær A., Bendix P.M., Oddershede L.B. Platinum nanoparticles: a non-toxic, effective and thermally stable alternative plasmonic material for cancer therapy and bioengineering. Nanoscale. 2018;10(19):9097–9107. doi: 10.1039/c8nr02275e. [DOI] [PubMed] [Google Scholar]
- 10.Al-Radadi N.S. Green synthesis of platinum nanoparticles using Saudi’s Dates extract and their usage on the cancer cell treatment. Arab. J. Chem. 2019;12(3):330–349. [Google Scholar]
- 11.Torchilin V.P. Passive and active drug targeting: drug delivery to tumors as an example. Drug Deliv. 2010:3–53. doi: 10.1007/978-3-642-00477-3_1. [DOI] [PubMed] [Google Scholar]
- 12.Al-Brakati A., Alsharif K.F., Alzahrani K.J., Kabrah S., Al-Amer O., Oyouni A.A., et al. Using green biosynthesized lycopene-coated selenium nanoparticles to rescue renal damage in glycerol-induced acute kidney injury in rats. Int. J. Nanomed. 2021;16:4335–4349. doi: 10.2147/IJN.S306186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albrahim T., Alonazi M.A. Lycopene corrects metabolic syndrome and liver injury induced by high fat diet in obese rats through antioxidant, anti-inflammatory, antifibrotic pathways. Biomed. Pharmacother. 2021;141 doi: 10.1016/j.biopha.2021.111831. [DOI] [PubMed] [Google Scholar]
- 14.Teodoro A.J., Oliveira F.L., Martins N.B., Maia Gde A., Martucci R.B., Borojevic R. Effect of lycopene on cell viability and cell cycle progression in human cancer cell lines. Cancer Cell Int. 2012;12(1):1475–2867. doi: 10.1186/1475-2867-12-36. 36. 12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui L., Xu F., Wu K., Li L., Qiao T., Li Z., et al. Anticancer effects and possible mechanisms of lycopene intervention on N-methylbenzylnitrosamine induced esophageal cancer in F344 rats based on PPARgamma(1) Eur. J. Pharmacol. 2020;881 doi: 10.1016/j.ejphar.2020.173230. [DOI] [PubMed] [Google Scholar]
- 16.Aktepe O.H., Sahin T.K., Guner G., Arik Z., Yalcin S. Lycopene sensitizes the cervical cancer cells to cisplatin via targeting nuclear factor- kappa B (NF-kappaB) pathway. Turk. J. Med. Sci. 2021;51(1):368–374. doi: 10.3906/sag-2005-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gurunathan S., Jeyaraj M., Kang M.-H., Kim J.-H. Anticancer properties of platinum nanoparticles and retinoic acid: combination therapy for the treatment of human neuroblastoma cancer. Int. J. Mol. Sci. 2020;21(18):6792. doi: 10.3390/ijms21186792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe J., Yamada M., Niibe K., Zhang M., Kondo T., Ishibashi M., et al. Preconditioning of bone marrow-derived mesenchymal stem cells with N-acetyl-L-cysteine enhances bone regeneration via reinforced resistance to oxidative stress. Biomaterials. 2018;185:25–38. doi: 10.1016/j.biomaterials.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 19.Justus C.R., Leffler N., Ruiz-Echevarria M., Yang L.V. In vitro cell migration and invasion assays. JoVE : JoVE. 2014;(88) doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentile F., Arcaro A., Pizzimenti S., Daga M., Cetrangolo G.P., Dianzani C., et al. DNA damage by lipid peroxidation products: implications in cancer, inflammation and autoimmunity. AIMS Gen. 2017;4(2):103–137. doi: 10.3934/genet.2017.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Othman M.S., Al-Bagawi A.H., Obeidat S.T., Fareid M.A., Habotta O.A., Moneim A.E.A. Antitumor activity of zinc nanoparticles synthesized with berberine on human epithelial colorectal adenocarcinoma (Caco-2) cells through acting on Cox-2/NF-kB and p53 pathways. Anti Cancer Agents Med. Chem. 2021 doi: 10.2174/1871520621666211004115839. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 23.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 24.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 25.Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., et al. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 26.Khan F.A., Albalawi R., Pottoo F.H. Trends in targeted delivery of nanomaterials in colon cancer diagnosis and treatment. Med. Res. Rev. 2022;42(1):227–258. doi: 10.1002/med.21809. [DOI] [PubMed] [Google Scholar]
- 27.Dasari S., Tchounwou P.B. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 2014;740:364–378. doi: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg. Chem. 2019;88 doi: 10.1016/j.bioorg.2019.102925. [DOI] [PubMed] [Google Scholar]
- 29.Gurunathan S., Jeyaraj M., Kang M.-H., Kim J.-H. The effects of apigenin-biosynthesized ultra-small platinum nanoparticles on the human monocytic THP-1 cell line. Cells. 2019;8(5):444. doi: 10.3390/cells8050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sahin B., Aygun A., Gunduz H., Sahin K., Demir E., Akocak S., et al. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf. B Biointerfaces. 2018;163:119–124. doi: 10.1016/j.colsurfb.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 31.Gurunathan S., Jeyaraj M., La H., Yoo H., Choi Y., Do J.T., et al. Anisotropic platinum nanoparticle-induced cytotoxicity, apoptosis, inflammatory response, and transcriptomic and molecular pathways in human acute monocytic leukemia cells. Int. J. Mol. Sci. 2020;21(2):440. doi: 10.3390/ijms21020440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gurunathan S., Jeyaraj M., Kang M.-H., Kim J.-H. Graphene oxide–platinum nanoparticle nanocomposites: a suitable biocompatible therapeutic agent for prostate cancer. Polymers. 2019;11(4):733. doi: 10.3390/polym11040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurunathan S., Jeyaraj M., Kang M.-H., Kim J.-H. Tangeretin-assisted platinum nanoparticles enhance the apoptotic properties of doxorubicin: combination therapy for osteosarcoma treatment. Nanomaterials. 2019;9(8):1089. doi: 10.3390/nano9081089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Almeer R.S., Ali D., Alarifi S., Alkahtani S., Almansour M. Green platinum nanoparticles interaction with HEK293 cells: cellular toxicity, apoptosis, and genetic damage. Dose-Response. 2018;16(4) doi: 10.1177/1559325818807382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiny P., Mukherjee A., Chandrasekaran N. DNA damage and mitochondria-mediated apoptosis of A549 lung carcinoma cells induced by biosynthesised silver and platinum nanoparticles. RSC Adv. 2016;6(33):27775–27787. [Google Scholar]
- 36.Labrador-Rached C.J., Browning R.T., Braydich-Stolle L.K., Comfort K.K. Toxicological implications of platinum nanoparticle exposure: stimulation of intracellular stress, inflammatory response, and akt signaling in vitro. J. Toxicol. 2018;2018 doi: 10.1155/2018/1367801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valdiglesias V., Fernández-Bertólez N., Kiliç G., Costa C., Costa S., Fraga S., et al. Are iron oxide nanoparticles safe? Current knowledge and future perspectives. J. Trace Elem. Med. Biol. 2016;38:53–63. doi: 10.1016/j.jtemb.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Gurunathan S., Kim J.-H. Graphene oxide–silver nanoparticles nanocomposite stimulates differentiation in human neuroblastoma cancer cells (SH-SY5Y) Int. J. Mol. Sci. 2017;18(12):2549. doi: 10.3390/ijms18122549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thanan R., Oikawa S., Hiraku Y., Ohnishi S., Ma N., Pinlaor S., et al. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int. J. Mol. Sci. 2015;16(1):193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verano-Braga T., Miethling-Graff R., Wojdyla K., Rogowska-Wrzesinska A., Brewer J.R., Erdmann H., et al. Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano. 2014;8(3):2161–2175. doi: 10.1021/nn4050744. [DOI] [PubMed] [Google Scholar]
- 41.Urbaniak S.K., Boguszewska K., Szewczuk M., Kaźmierczak-Barańska J., Karwowski B.T. 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine (8-oxodG) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a potential biomarker for gestational diabetes mellitus (GDM) development. Molecules. 2020;25(1):202. doi: 10.3390/molecules25010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magdolenova Z., Lorenzo Y., Collins A., Dusinska M. Can standard genotoxicity tests be applied to nanoparticles? J. Toxicol. Environ. Health, Part A. 2012;75(13-15):800–806. doi: 10.1080/15287394.2012.690326. [DOI] [PubMed] [Google Scholar]
- 43.Ren X., Zou L., Zhang X., Branco V., Wang J., Carvalho C., et al. Redox signaling mediated by thioredoxin and glutathione systems in the central nervous system. Antioxidants Redox Signal. 2017;27(13):989–1010. doi: 10.1089/ars.2016.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shiny P., Mukherjee A., Chandrasekaran N. Haemocompatibility assessment of synthesised platinum nanoparticles and its implication in biology. Bioproc. Biosyst. Eng. 2014;37(6):991–997. doi: 10.1007/s00449-013-1069-1. [DOI] [PubMed] [Google Scholar]
- 45.Ismail N.A.S., Lee J.X., Yusof F. Platinum nanoparticles: the potential antioxidant in the human lung cancer cells. Antioxidants. 2022;11(5) doi: 10.3390/antiox11050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kluck R.M., Bossy-Wetzel E., Green D.R., Newmeyer D.D. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 47.Wei X., Qi Y., Jia N., Zhou Q., Zhang S., Wang Y. Hyperbaric oxygen treatment sensitizes gastric cancer cells to melatonin-induced apoptosis through multiple pathways. J. Cell. Biochem. 2018;119(8):6723–6731. doi: 10.1002/jcb.26864. [DOI] [PubMed] [Google Scholar]
- 48.Galehdar Z., Swan P., Fuerth B., Callaghan S.M., Park D.S., Cregan S.P. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4–CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J. Neurosci. 2010;30(50):16938–16948. doi: 10.1523/JNEUROSCI.1598-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bevers E., Comfurius P., Dekkers D., Harmsma M., Zwaal R. Regulatory mechanisms of transmembrane phospholipid distributions and pathophysiological implications of transbilayer lipid scrambling. Lupus. 1998;7(2_suppl):126–131. doi: 10.1177/096120339800700228. [DOI] [PubMed] [Google Scholar]
- 50.Huang R.-F.S., Wei Y.-J., Inbaraj B.S., Chen B.-H. Inhibition of colon cancer cell growth by nanoemulsion carrying gold nanoparticles and lycopene. Int. J. Nanomed. 2015;10:2823–2846. doi: 10.2147/IJN.S79107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Othman M., Obeidat S., Al-Bagawi A., Fareid M., El-Borady O., Kassab R., et al. Evaluation of the potential role of silver nanoparticles loaded with berberine in improving anti-tumor efficiency. Pharmaceut. Sci. 2022;28(1):86–93. [Google Scholar]
- 52.Bedoya F.J., Salguero-Aranda C., Cahuana G.M., Tapia-Limonchi R., Soria B., Tejedo J.R. Regulation of pancreatic β-cell survival by nitric oxide. Islets. 2012;4(2):108–118. doi: 10.4161/isl.19822. [DOI] [PubMed] [Google Scholar]
- 53.Al-Otaibi A.M., Al-Gebaly A.S., Almeer R., Albasher G., Al-Qahtani W.S., Abdel Moneim A.E. Potential of green-synthesized selenium nanoparticles using apigenin in human breast cancer MCF-7 cells. Environ. Sci. Pollut. Res. Int. 2022 doi: 10.1007/s11356-022-19166-2. [DOI] [PubMed] [Google Scholar]
- 54.Alshatwi A.A., Athinarayanan J., Vaiyapuri Subbarayan P. Green synthesis of platinum nanoparticles that induce cell death and G2/M-phase cell cycle arrest in human cervical cancer cells. J. Mater. Sci. Mater. Med. 2015;26(1):5330. doi: 10.1007/s10856-014-5330-1. [DOI] [PubMed] [Google Scholar]
- 55.Sorenson C.M., Barry M.A., Eastman A. Analysis of events associated with cell cycle arrest at G2 phase and cell death induced by cisplatin. J. Natl. Cancer Inst. 1990;82(9):749–755. doi: 10.1093/jnci/82.9.749. [DOI] [PubMed] [Google Scholar]
- 56.Tian J., Wei X., Zhang W., Xu A. Effects of selenium nanoparticles combined with radiotherapy on lung cancer cells. Front. Bioeng. Biotechnol. 2020;8 doi: 10.3389/fbioe.2020.598997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.