Abstract
Tendinopathy refers to a type of tendon disease with a multifactorial spectrum. Recent research has begun to reveal the effects of inflammation on the tendinopathic process, especially in the first stage of tendinopathy. Radial extracorporeal shock wave therapy (rESWT) has been successfully used to treat orthopedic diseases. However, the molecular mechanisms underlying the anti-inflammatory effects of rESWT on tumor necrosis factor-α treated tenocytes have not been fully elucidated. In this study, we applied total protein tandem mass tag-labeled quantitative proteomics with liquid chromatography-mass spectrometer/mass spectrometer technology to identify differentially expressed proteins (DEPs) among inflammatory tenocytes, rESWT inflammatory tenocytes, and controls using three biological replicates. Human tenocytes were used and they were cultured in vitro. In total, 1028 and 40 DEPs were detected for control versus inflammatory tenocytes and for inflammatory tenocytes versus rESWT inflammatory tenocytes, respectively. Further, we identified integrin α2, selenoprotein S, and NLR family CARD domain-containing protein 4 as pivotal molecular targets of the anti-inflammatory effects of rESWT. This is the first study to provide a reference proteomic map for inflammatory tenocytes and rESWT inflammatory tenocytes. Our findings provide crucial insight into the molecular mechanisms underscoring the anti-inflammatory effects of rESWT in tendinopathy.
Keywords: Inflammation, Proteomic, Shockwave, Tendinopathy, Tenocyte
Highlights
-
•
rESWT is first reported to relieve acute inflammation of tenocytes.
-
•
First proteomic map depicts inflammatory tenocytes and rESWT inflammatory tenocytes.
-
•
ITGA2, SELS, and NLRC4 might be targets of the anti-inflammatory effects of rESWT.
Inflammation; Proteomic; Shockwave; Tendinopathy; Tenocyte.
1. Introduction
Tendinopathy is a type of tendon disorder characterized by pain that worsens with movement, impaired function, and a decline in exercise tolerance [1]. This disorder accounts for 30% of musculoskeletal counseling in general practice [2]. Modern molecular techniques have demonstrated an inflammatory phenotype throughout the spectrum of tendinopathy despite the absence of obvious clinical signs of inflammation. Inflammation may serve as an early initiation factor for tendinopathy [3]. Various inflammatory mediators are endogenously expressed by tenocytes, including pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-1β [4].
The ideal tendinopathy target should regulate the pro-inflammatory response by maintaining the healing effects induced by inflammation and supporting robust and rapid matrix repair [5]. Most of the current treatments for tendinopathy focus on the mechanisms of promoting tendon healing by improving matrix repair, while a limited number of treatments promote tendon repair by anti-inflammation in the initial stage of tendinopathy. Accordingly, treatments for acute tendinopathy that target the regulation of its initial inflammatory response to ameliorate tendinopathy at the subacute or even acute stage warrant further investigation.
There are two types of extracorporeal shock wave therapy (ESWT) differentiated by their wave patterns, namely, focused extracorporeal shock wave therapy (fESWT) and radial extracorporeal shock wave therapy (rESWT). According to their definitions, fESWT creates a pressure field with a focal zone in the treatment region, while rESWT generates radially expanding pressure. According to the physical characteristics, fESWT is characterized by powerful energy and deep penetration, and is frequently used for the treatment of osteogenic disorders, such as fracture nonunion [6] and osteonecrosis of the femoral head [7], while rESWT has less energy and shallow penetration, and is used for soft tissue injuries in clinic settings. Radial extracorporeal shock wave therapy has been proven to be beneficial in treating tendinopathy [8]. However, the molecular mechanisms underlying the anti-inflammatory effects of rESWT in tendinopathy have yet to be fully elucidated. Focused extracorporeal shock wave therapy is frequently used as an intervention in tenocyte models, whereas rESWT is seldom used. In this regard, the dose-dependent response of cells to shockwaves is closely related to the type of generator and different energy flow schemes [9].
In the present study, we explored the molecular mechanisms of rESWT in the treatment of TNF-α-induced inflammatory tenocytes from the perspective of anti-inflammatory effects by providing a reference proteomic map for inflammatory tenocytes and rESWT inflammatory tenocytes. We identified differentially expressed proteins (DEPs) among inflammatory tenocytes, rESWT inflammatory tenocytes, and controls using total protein tandem mass tag-labeled quantitative proteomics with liquid chromatography-mass spectrometer/mass spectrometer technology.
2. Materials and methods
2.1. Reagents and antibodies
Recombinant TNF-α was purchased from Bioworld (MN, USA). 0.4% trypan blue was purchased from Solarbio (Beijing, China). Cell Counting Kit-8 assay (CCK-8) was purchased from Biosharp (Hefei, China). The multiplex bead-based flow fluorescent immunoassay kit was purchased from Raisecare (Qingdao, China).
Anti-COL1 (code: bsm-33400M, lot: BJ06239206), anti-COL3 (code: bs-0948R, lot: AH10092472), anti-vimentin (code: bs-0756R, lot: AI07165538), and anti-SCX (code: bs-12364R, lot: AC08264578) antibodies were all purchased from Bioss (Beijing, China). Anti-CD34 (code: BS6481, lot: CC02181) and anti-OCT4 (code: BS70993, lot: CC02181) were both purchased from Bioworld. Primary antibodies such as anti-ITGA2 (code: BS70832, lot: CC02181), anti-SELS (code: 15591-1-AP, lot: 00091576), and anti-NLRC4 (code: ab201792, lot: GR3375757-1) were purchased from Bioworld, Proteintech (IL, USA), and Abcam (MA, USA), respectively. The secondary antibody peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H + L) (code: S8002, lot: 0528) was purchased from Jackson ImmunoResearch Inc. (West Grove, PA). The other reagents were mainly from Solarbio or Biosharp.
2.2. Tissue samples and primary culture of human tenocytes
Three male patients (aged 16, 33, and 36 years) underwent arthroscopic anterior cruciate ligament reconstruction, and three primary cultures of human tenocytes were established using semitendinosus tendon grafts after obtaining written informed consent. The study was approved by the Ethics Committee of Sports Science Experiment of Beijing Sport University (2020075H). The tendon tissue was isolated and cultured following the method proposed by Schulze-Tanzil et al. [10]. In this study, the tenocytes were successfully cultured (Figure 1A).
Figure 1.
Primary culture of human tenocytes and rESWT intervention. (A) Tendon tissue was cut into 2–3 mm3 cubes and microscopic observation of cultured tenocytes was performed on days 7–10. Primary tenocytes (Te, black arrow) migrated from the tendon tissue (Ti) and proliferated extensively in the culture dish. Tenocyte morphology was spindle-like, resembling fibroblasts. (B) Fixed frame and in vitro rESWT treatment of cultured tenocytes.
2.3. Immunocytochemistry
Immunocytochemical staining was performed in vitro to characterize human primary tenocytes following a standard protocol [11], using antibodies against COL1 (1:200), COL3 (1:200), vimentin (1:200), SCX (1:400), CD34 (1:200), and OCT4 (1:200). When using mouse primary antibodies, normal goat serum (Solarbio; A8020) was used for blocking and a goat anti-mouse secondary antibody conjugated with fluorescein isothiocyanate (FITC) (Bioss; bs-0296G-FITC) was applied. For rabbit primary antibodies, normal goat serum (Solarbio) and a goat anti-rabbit secondary antibody (Bioss; bs-0295G-AF594) were used. Cell nuclei were counterstained with DAPI-containing mounting media (Solarbio; S2110) and analyzed under a fluorescence microscope (Olympus BX53; Olympus Corporation, Tokyo, Japan). The montages were created using Adobe Photoshop (version CC2017; Adobe, San Jose, CA, USA).
2.4. Stimulation with TNF-α
Recombinant TNF-α was dissolved in 1 × phosphate buffer saline (PBS) and diluted to concentrations of 10, 50, and 100 μg/mL. For exposure, TNF-α solutions were prepared at 1:1000 in 1% fetal bovine serum (FBS) in DMEM/F12 (1 μL TNF-α + 1 mL 1% FBS in DMEM/F12) to final concentrations of 10, 50, and 100 ng/mL. To measure cell proliferation assays, stimulation durations (time points) of 24, 72, and 120 h were selected. For quantitative proteomics and western blotting, the stimulation duration (time point) was 72 h. The same volume of 1 × PBS (1 μL 1 × PBS +1 mL 1% FBS in DMEM/F12) was used for the control and unstimulated tenocytes.
2.5. In vitro rESWT for cultured tenocytes
To testify the effects of rESWT on cell viability, the P2–P5 primary cultured tenocytes were divided into nine groups (energy flux density (EFD) × impulses: 0.09 mJ/mm2 × 400 impulses, 0.09 mJ/mm2 × 800 impulses, 0.09 mJ/mm2 × 1200 impulses, 0.29 mJ/mm2 × 400 impulses, 0.29 mJ/mm2 × 800 impulses, 0.29 mJ/mm2 × 1200 impulses, 0.49 mJ/mm2 × 400 impulses, 0.49 mJ/mm2 × 800 impulses, 0.49 mJ/mm2 × 1200 impulses) and untreated tenocytes were used as controls.
To perform proteomics and western blotting, P2–P5 primary cultured tenocytes were divided into four groups: TNF-α, rESWT + TNF-α, rESWT, and untreated groups as controls. Tenocytes were exposed to rESWT under the protocol based on previous selection.
Shockwaves were generated using a ballistic shock wave therapy device with a standard 15 mm applicator (Gymna ShockMaster 300, GymnaUniphy NV, Bilzen, Belgium). A 5-mL polypropylene tube (Taizhou, China) containing tenocyte suspension with 1 × 105–1 × 106 cells/mL was placed on a shockwave applicator with a common ultrasonic coupling agent. The 5-mL polypropylene tube was sterilized using pressure steam prior to use.
The device applicator with the same diameter as the tube cover (15 mm) was selected. Based on a previous experiment, the headpiece of the ESWT device was firmly clamped by three rings on a fixed frame [12]. During the process, the tube was always vertically inverted on the applicator. The operator fixed the tube on the applicator by hand and kept the tube in close contact with the applicator (Figure 1B). The pressure between the tube and applicator was maintained at 2800–3200 N as measured by a pressure sensor (Figure S1).
2.6. Cell viability and proliferation assay
Immediately after rESWT intervention (<1 min), rESWT-treated and untreated tenocytes were quantified using a hemocytometer. Cell viability was measured using a trypan blue exclusion assay [13]. The number of unstained tenocytes was quantified under an inverted Axio Imager A2 microscope (Zeiss, Oberkochen, Germany).
After rESWT intervention, the aforementioned four groups were seeded into 96-well culture plates (1.5 × 104 cells/cm2) and were grown in 1% FBS in DMEM/F12. Cell proliferation was assessed at 24, 72, and 120 h. Cell proliferation was measured using a CCK-8 assay following the manufacturer's instructions. The absorbance of the formazan produced was measured at 450 nm using a microplate reader (Multiskan, Thermo Scientific, MA, USA).
2.7. Cytokine detection in culture medium
After rESWT intervention, the aforementioned four groups were seeded into 96-well culture plates (1.5 × 104 cells/cm2) and were grown in 1% FBS in DMEM/F12. Cytokines in the medium were assessed at 24, 72, and 120 h. IL-1β levels in culture medium supernatant from the 96-well plate were measured using multiplex bead-based flow fluorescent immunoassay. The protocol provided by the manufacturer was followed. Finally, the flow tubes were read using a flow cytometer (NAVIOS; Beckman-Coulter, CA, USA).
2.8. Proteomics strategy and bioinformatics analysis
For quantitative proteomics, after rESWT intervention, the aforementioned four groups were seeded into 150 mm culture dishes (2.0 × 104 cells/cm2) and collected at 72 h. The corresponding tenocytes from three patients were used for quantitative proteomics in PTM Biolabs (Hangzhou, China). The experiment included TMT labeling, HPLC fractionation, affinity enrichment, and mass spectrometry based quantitative proteomics. Bioinformatics analysis was then performed to annotate the quantifiable targets, such as Gene Ontology (GO) enrichment.
2.9. Western blotting
Tenocytes were harvested for western blotting 72 h after rESWT intervention. Tenocytes from four 100 mm culture dishes (80%–90% confluence, cell density 2.0 × 104 cells/cm2) were detached and resuspended, and divided into four groups: TNF-α, rESWT + TNF-α, rESWT, and untreated groups. The medium with/without TNF-α (10 ng/mL) and rESWT-treated were inoculated into four 100 mm petri dishes. After 72 h of incubation, the medium was discarded, washed twice with ice-cold PBS, and 100 μL of cell lysis buffer (RIPA lysis buffer: protein phosphatase inhibitor mixture: PMSF = 100:2:1) was added to each Petri dish (100 mm). The lysis procedure was as follows: Lyse on ice for 5 min and the lysed cells were collected in a 1.5 mL Eppendorf tube with a cell scraper. Then, continued to lyse on ice for 15 min, centrifuged at 14,000 rpm at 4 °C for 15 min. The supernatant was aspirated, loading buffer was added, and heated at 100 °C for 5 min.
Proteins (30 μg per tenocyte sample) were added to each lane and separated on 4–20% HEPES-Tris precast gels. Electrophoresis was performed, followed by transfer to nitrocellulose membranes and immunodetection. Nonspecific antibody binding was blocked with 5% nonfat milk overnight at 4 °C. Immunoblotting was performed with antibodies against ITGA2 (1:1000), SELS (1:1000), and NLRC4 (1:1000), followed by secondary antibody peroxidase-conjugated AffiniPure goat anti-rabbit IgG (H + L) (1:10000). Blots were performed using a 1:1 solution of enhanced chemiluminescence kit (Biosharp). The bands were visualized via an electrochemiluminescence detection system (Thermo Fisher Scientific, MA, USA) and analyzed using Image Lab 5.0 software (Bio-Rad, CA, USA).
2.10. Statistical analysis
SPSS (version 19.0; IBM Corp., NY, USA) was used for data analysis. All values are expressed as means ± standard deviation (SD). Statistical analysis was performed using either one-way analysis of variance (ANOVA) (equal variance) or Welch's (unequal variance). The least significant difference (LSD) or Dunnett test (2-sided) was used for multiple comparisons. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Primary tenocytes express tenocyte markers
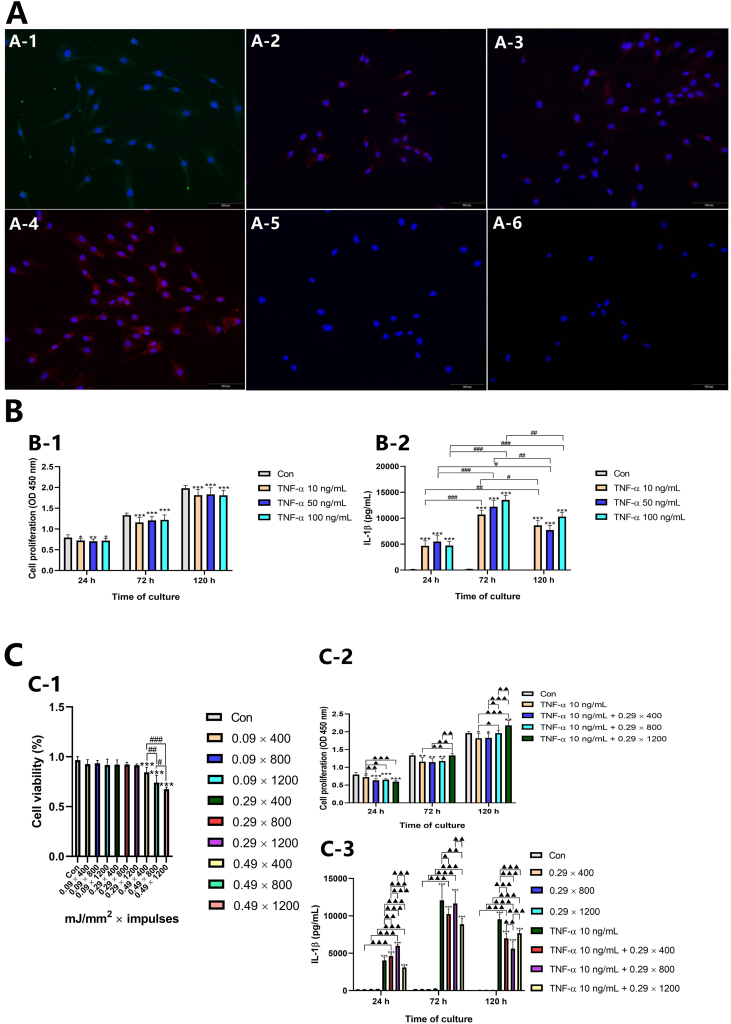
The immunocytochemistry results revealed COL1, COL3, vimentin, and SCX immunoreactivity (Figure 2A-1‒A-4), but limited immunopositive responses were observed for the stemness marker OCT4 and endothelial marker CD34 (Figure 2A-5, A-6).
Figure 2.
Immunofluorescence staining and rESWT effects on cell proliferation and pro-inflammatory cytokines. (A) Primary tenocytes immunostained for COL1 (A-1), COL3 (A-2), vimentin (A-3), SCX (A-4), OCT4 (A-5), and CD34 (A-6). (B) Changes in OD values (B-1) and IL-1β levels (B-2) at different time points after intervention with different concentrations of TNF-α. Data are expressed as means ± SD and were analyzed using one-way ANOVA (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus the control. #p < 0.05, ##p < 0.01, ###p < 0.001 indicate intragroup comparisons at different time points. (C) Effects of rESWT on tenocyte viability and reversal of TNF-α-induced decline in tenocyte proliferation and increase in IL-1β levels. (C-1) Effects of rESWT waves on tenocyte viability. (C-2) rESWT reversal of TNF-α-induced decline in tenocyte proliferation. (C-3) rESWT reversal of TNF-α-induced increase in IL-1β. Data are expressed as means ± SD and were analyzed using one-way ANOVA (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 versus the control. ▲ p < 0.05, ▲▲ p < 0.01, ▲▲▲ p < 0.001 indicate intergroup comparisons (except the control). #p < 0.05, ##p < 0.01, ###p < 0.001 indicate intragroup comparisons at different time points.
3.2. Effects of rESWT on cell viability
The data revealed that 0.09 mJ/mm2 (400, 800, and 1200 impulses) and 0.29 mJ/mm2 (400, 800, and 1200 impulses) resulted in normal cell viability; however, 0.49 mJ/mm2 (400, 800, and 1200 impulses) significantly suppressed cell viability in a dose-dependent manner (Figure 2C-1). In combination with clinical practice, 0.29 mJ/mm2 (400, 800, and 1200 impulses) was initially selected for subsequent experiments.
3.3. TNF-α decreases cell proliferation and increases IL-1β
Results revealed a significant decrease in optical density (OD) values at 450 nm at different concentrations of TNF-α (10, 50, and 100 ng/mL) compared to control values after 24, 72, and 120 h (Figure 2B-1). However, this decrease did not exhibit a time dependence, and tenocytes incubated with different concentrations of TNF-α demonstrated a normal rate of proliferation. Therefore, 10 ng/mL of TNF-α was selected for subsequent experiments.
With regard to IL-1β levels, results revealed a significant increase in culture medium treated with 10, 50, and 100 ng/mL of TNF-α compared with controls after 24, 72, and 120 h of exposure (Figure 2B-2). The level of IL-1β released from tenocytes peaked 72 h after intervention with TNF-α.
3.4. Effects of rESWT on cell proliferation and IL-1β levels in the culture medium in a TNF-α-induced model of acute inflammation
The rESWT regimen with an EFD of 0.29 mJ/mm2 (400, 800, and 1200 impulses) was selected to explore the effect of rESWT on the cell proliferation and IL-1β levels in the culture medium in an acute inflammation model of tenocytes induced by TNF-α.
As shown in Figure 2C-2, a significant decrease in cell proliferation (presented as OD values), was observed in cells treated with 10 ng/mL TNF-α when compared with the control after 24, 72, and 120 h of rESWT intervention. Cell proliferation was significantly suppressed by an EFD of 0.29 mJ/mm2 (400, 800, and 1200 impulses) compared to that in cells treated with 10 ng/mL of TNF-α alone after 24 h of shockwave exposure, however this effect was not dose-dependent, while an EFD of 0.29 mJ/mm2 (1200 impulses) significantly reversed the decline in cell proliferation after 72 and 120 h of shockwave exposure. Moreover, 0.29 mJ/mm2 with 400, 800, and 1200 impulses exerted positive stimulatory effects in a dose-dependent manner after 120 h of shockwave exposure.
IL-1β levels were significantly increased in the culture medium of cells treated with 10 ng/mL TNF-α compared with the control after 24, 72, and 120 h of exposure (Figure 2C-3). No significant changes were observed in IL-1β levels of cells treated with rESWT (0.29 mJ/mm2 with 400, 800, and 1200 impulses) compared with the control after 24, 72, and 120 h of exposure (Figure 2C-3). An EFD of 0.29 mJ/mm2 with 400 and 800 impulses significantly increased IL-1β levels compared to that in cells treated with 10 ng/mL TNF-α alone after 24 h of shockwave exposure, whereas application of 0.29 mJ/mm2 with 1200 impulses significantly reversed the TNF-α-induced increase in IL-1β level. Moreover, treatment with 0.29 mJ/mm2 with 400 and 1200 impulses significantly reversed the TNF-α-induced increase in IL-1β levels compared to that in cells treated with 10 ng/mL TNF-α alone after 72 h of shockwave exposure, whereas application of 0.29 mJ/mm2 with 800 impulses did not significantly affect IL-1β levels. After 120 h of shockwave exposure, application of 0.29 mJ/mm2 with 400, 800, and 1200 shockwaves significantly reversed the TNF-α-induced increase in IL-1β compared with that in cells treated with 10 ng/mL TNF-α alone, but this effect was not in a dose-dependent manner (Figure 2C-3). Levels of IL-1β released from tenocytes peaked at 72 h after treatment with TNF-α. Therefore, combining the results mentioned above, 0.29 mJ/mm2 with 1200 shockwaves was initially selected for quantitative proteomics.
3.5. Identification of DEPs
To screen for significant DEPs, strict criteria were applied as follows: 1) proteins must be observed in all three biological replicates; 2) at least one unique peptide must be contained in the protein; 3) a fold change >1.20 or <0.83 was used as the meaningful cutoff representing significant differences [14, 15]. In this study, the fold-change cutoff value indicates the ratio of the protein expression level of rESWT + TNF-α group to TNF-α group; 4) statistical analysis was conducted between the two groups with relative quantification p-values <0.05.
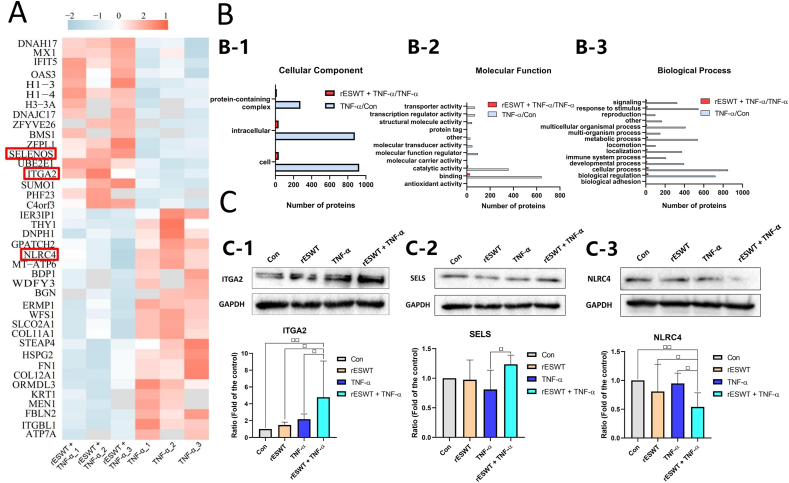
Based on the above criteria, 1028 proteins displayed significant differences between the TNF-α and control groups, of which 650 proteins were upregulated and 378 proteins were downregulated (Table S1). Compared with those in the TNF-α group, 40 proteins were significantly altered in the rESWT + TNF-α group, of which 17 proteins were upregulated and 23 proteins were downregulated (Table S2). Among the 40 dysregulated proteins, 3 related to cell inflammation were significantly enriched (Table S3, Figure 3A).
Figure 3.
Proteomics outcomes and identification. (A) Heatmap of DEPs identified in the rESWT + TNF-α group versus the TNF-α group. The pink and gray clusters represent upregulated and downregulated proteins, respectively. The target proteins are marked in red. (B) Histogram plots of GO analysis of DEPs identified in the rESWT + TNF-α versus TNF-α groups (red) and TNF-α versus control groups (gray). (B-1) Cellular component; (B-2) Molecular function; (B-3) Biological process. (C) Western blotting for three selected DEPs including (C-1) ITGA2, (C-2) SELS, and (C-3) NLRC4 among the control, rESWT, TNF-α, and rESWT + TNF-α groups. Data are expressed as means ± SD and were analyzed using one-way ANOVA (n = 3). □ p < 0.05, □□ p < 0.01 indicate intergroup comparisons. The uncropped images of (C) were referred to in Figure S2.
3.6. Bioinformatics analysis of DEPs
Gene ontology categories were assigned to further elucidate the functions of DEPs between the TNF-α and control groups and between the rESWT + TNF-α and TNF-α groups. In the comparison of TNF-α and control groups, the cellular component (CC) of GO analysis revealed that the highest proportion of DEPs was located in the cell, followed by the intracellular component (Figure 3B-1). In terms of molecular function (MF), GO enrichment revealed that most DEPs in the TNF-α and control groups were associated with two principal functions: binding and catalytic activity (Figure 3B-2). Regarding biological process (BP), GO analysis revealed that the DEPs between the TNF-α and control groups were predominantly involved in cellular processes, biological regulation, responses to stimuli, metabolic processes, and multicellular organismal processes (Figure 3B-3). Gene ontology categories of DEPs in the rESWT + TNF-α and TNF-α groups were similar to those in the TNF-α and control groups in terms of CC, MF, and BP (Figure 3B).
3.7. Validation of selected DEPs using western blot
Western blotting was performed on three DEPs: ITGA2, SELS, and NLRC4. The levels of ITGA2 and SELS in tenocytes were significantly higher but NLRC4 levels were significantly lower in the rESWT + TNF-α group than in the TNF-α group (Figure 3C).
4. Discussion
Tendinopathy is a disabling musculoskeletal disorder related to the inflammatory response, which may serve as an early initiation factor. Tumor necrosis factor-α and IL-1β are classic pro-inflammatory cytokines and play pivotal roles in extracellular matrix remodeling and reduction of type I collagen production in tendinopathy [5]. In this study, TNF-α treatment significantly decreased proliferation and increased IL-1β levels in the culture medium, indicating a successful model of acute inflammation in primary human tenocytes in tendinopathy.
In this study, low-to moderate-energy rESWT resulted in fewer immediate cytodestructive effects and greater stimulation of cell proliferation, which are in agreement with previous findings [16]. We observed that rESWT reversed the TNF-α-induced decline in primary human tenocyte proliferation and increase in IL-1β levels. Notably, no change in IL-1β levels was observed in normal tenocytes after rESWT exposure, which is in disagreement with findings from a previous study [13]. These differences may be due to the different protocols used. By referring to the relevant guideline [17], the above-mentioned three rESWT energy regimens represent the three levels of low energy, moderate energy and high energy used in clinical practice. In fact, the high-energy regimen of rESWT may result in adverse reactions such as exacerbation of pain, ecchymosis, and swelling at the affected site. Therefore, the high energy regimen of rESWT is rarely used in clinical practice for tendinopathy. In clinical practice, rESWT with a moderate energy regimen is the most commonly-used regimen for the treatment of tendinopathy. This is an important reason why we finally selected the moderate energy regimen of rESWT for the proteomics test.
Integrins serve as mediators of ESWT effects and ITGA2 is a member of the integrin family; ESWT increases the expression of α2, α6, and β1 integrin subunits [18]. However, no biological effects of rESWT regulating integrins have been reported. Moreover, few studies have further explored the downstream molecular pathways of fESWT in regulating integrins [19]. Integrins bind to focal adhesion kinase (FAK) and recruit kinases to activate pathways that ultimately result in mitogen-activated protein kinase (MAPK) pathway phosphorylation [20]. The MAPK signal transduction pathway is involved in the inflammatory mechanisms of tendinopathy [21]. In the present study, ITGA2 expression was significantly upregulated in the tenocytes of the rESWT + TNF-α group versus the TNF-α group, suggesting that ITGA2 may be a potential anti-inflammatory protein target of rESWT in a TNF-α-induced model of acute inflammation in primary human tenocytes. Due to the lack of catalytic activity in integrins, FAK/protein-tyrosine kinase (PTK) activation may play a major role in integrin-mediated signal transduction [22]. Phosphorylation is the main pathway for FAK and MAPK activation, which could underlie the failure of quantitative proteomics to detect changes in the expression of phosphorylated FAK and MAPK.
A key transmembrane selenoprotein, SELS, is closely associated with inflammation and can relieve inflammatory damage to tissues or cells induced by IL-1β or TNF-α [23]. Previous experimental results demonstrated that SELS forms a regulatory loop between pro-inflammatory cytokines, nuclear factor-kappa B (NF-κB), and regulation of inflammatory responses [24]. SELS expression was significantly upregulated in the tenocytes of the ESWT + TNF-α group versus the TNF-α group.
A major type of inflammasome, NLRC4, which comprises a family of cytosolic multiprotein complexes that modulate the activation of cysteine-aspartate-specific protease 1 (caspase-1) and promotes the maturation and secretion of IL-1β and IL-18, leads to an inflammatory response [25]. The inflammasome plays a key role in infectious diseases as well as auto-inflammatory diseases. However, there is a paucity of evidence of the existence and activation of inflammasome-mediated inflammation in tendinopathy. A limited number of studies have indicated that the degradation fragments of the tendon matrix may act as damage-associated molecular patterns to trigger the inflammasome pathway [26]. NLRC4 expression can be upregulated by TNF-α stimulation [27]. In this study, NLRC4 expression in the tenocytes of the TNF-α group was upregulated 1.326-fold compared to that in the control group, although this did not reach statistical significance (p = 0.053) (Table S1 in red font). Compared to that of the TNF-α group, NLRC4 expression in the tenocytes of the rESWT + TNF-α group was significantly downregulated.
In summary, we speculate that ITGA2, SELS, and NLRC4 are potential anti-inflammatory protein targets of rESWT in a TNF-α-induced model of acute inflammation in primary human tenocytes.
Radial extracorporeal shock wave therapy is generally employed for chronic conditions. However, in vivo and in vitro studies have demonstrated that rESWT may be beneficial in the treatment of acute muscle injuries [28, 29]. Therefore, we hypothesize that rESWT can prevent tendinopathy from progressing to the chronic stage by regulating the inflammatory response in the acute or subacute stages, which may open new opportunities for clinical treatment.
There are some limitations in this study. First, it is more convincing if tendinopathic tenocytes from tendinopathy samples were used in this study. However, the number of patients with acute tendon injuries requiring surgery in clinic is seldom, therefore it's quite difficult to obtain tendon tissue samples in acute tendon injuries. Second, whether rESWT exerts biological effects via different molecular pathways remains unclear, and this should be considered as important research for future study as it is of great significance to understanding the role of rESWT at the molecular level.
The present study demonstrated that rESWT reversed the TNF-α-induced decline in primary human tenocyte proliferation and increase in IL-1β levels. Quantitative proteomics identified ITGA2, SELS, and NLRC4 as crucial proteins serving as molecular targets for the anti-inflammatory effects of rESWT (Figure 4). However, the effectiveness of rESWT for the treatment of acute tendon injury and its underlying molecular mechanisms warrant further investigation in in vivo studies.
Figure 4.
Potential molecular mechanisms underscoring the anti-inflammatory effects of rESWT in tenocytes. Solid lines indicate potential targets have been validated in previous studies, while dashed lines represent the speculated targets. “X?” refers to a substance that mediates rESWT's inhibitory effect on NLRC4.
Declarations
Author contribution statement
Ruidong Ge: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Qianzheng Zhu: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Die Liu: Performed the experiments; Analyzed and interpreted the data.
Qi Zhang and Shan Jiang: Conceived and designed the experiments.
Xueying Yu, Jun Shu and Fuqiang Gao: Performed the experiments.
Jingwei Guo and Beiyao Gao: Contributed reagents, materials, analysis tools or data.
Shengxuan Chen: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Dr. Ruidong Ge was supported by Fundamental Research Funds for the Central Universities [2020064], the National High Level Hospital Clinical Research Funding [2022-NHLHCRF-PY-10]. Dr. Shan Jiang was supported by Natural Science Foundation of Beijing Municipality [7212102], Elite Medical Professionals Project of China-Japan Friendship Hospital [ZRJY2021-BJ04].
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We are grateful to Dr. Shenglou Ni (Beijing University of Chinese Medicine) for his suggestions regarding this paper.
Contributor Information
Qi Zhang, Email: zhangqi@zryhyy.com.cn.
Shan Jiang, Email: landjiang@126.com.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Riley G. Tendinopathy – from basic science to treatment. Nat. Clin. Pract. Rheumatol. 2008;4:82e89. doi: 10.1038/ncprheum0700. [DOI] [PubMed] [Google Scholar]
- 2.McGonagle D., Marzo-Ortega H., Benjamin M., Emery P. Report on the second international enthesitis workshop. Arthritis Rheum. 2003;48:896e905. doi: 10.1002/art.10841. [DOI] [PubMed] [Google Scholar]
- 3.Legerlotz K., Jones E.R., Screen H.R., Riley G.P. Increased expression of IL-6 family members in tendon pathology. Rheumatology. 2012;51:1161e1165. doi: 10.1093/rheumatology/kes002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Sadi O., Schulze-Tanzil G., Kohl B., Lohan A., Lemke M., Ertel W., John T. Tenocytes, pro-inflammatory cytokines and leukocytes: a relationship? Muscles. Ligaments. Tendons. J. 2012;1:68e76. [PMC free article] [PubMed] [Google Scholar]
- 5.Millar N.L., Murrell G.A., McInnes I.B. Inflammatory mechanisms in tendinopathy - towards translation. Nat. Rev. Rheumatol. 2017;13:110e122. doi: 10.1038/nrrheum.2016.213. [DOI] [PubMed] [Google Scholar]
- 6.Alkhawashki H.M. Shock wave therapy of fracture nonunion. Injury. 2015;46 doi: 10.1016/j.injury.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 7.Mei J., Pang L., Jiang Z. The effect of extracorporeal shock wave on osteonecrosis of femoral head: a systematic review and meta-analysis. Physiother. Sport. 2021:1e9. doi: 10.1080/00913847.2021.1936685. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz C., Császár N.B., Milz S., Schieker M., Maffulli N., Rompe J.D., Furia J.P. Efficacy and safety of extracorporeal shock wave therapy for orthopedic conditions: a systematic review on studies listed in the PEDro database. Br. Med. Bull. 2015;116:115e138. doi: 10.1093/bmb/ldv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martini L., Giavaresi G., Fini M., Borsari V., Torricelli P., Giardino R. Early effects of extracorporeal shock wave treatment on osteoblast-like cells: a comparative study between electromagnetic and electrohydraulic devices. J. Trauma. 2006;61:1198e1206. doi: 10.1097/01.ta.0000203575.96896.34. [DOI] [PubMed] [Google Scholar]
- 10.Schulze-Tanzil G., Mobasheri A., Clegg P.D., Sendzik J., John T., Shakibaei M. Cultivation of human tenocytes in high-density culture. Histochem. Cell Biol. 2004;122:219e228. doi: 10.1007/s00418-004-0694-9. [DOI] [PubMed] [Google Scholar]
- 11.Lu H.X., Hao Z.M., Jiao Q., Xie W.L., Zhang J.F., Lu Y.F., Cai M., Wang Y.Y., Yang Z.Q., Parker T., Liu Y. Neurotrophin-3 gene transduction of mouse neural stem cells promotes proliferation and neuronal differentiation in organotypic hippocampal slice cultures. Med. Sci. Mon. Int. Med. J. Exp. Clin. Res. 2011;17:305e311. doi: 10.12659/MSM.882039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Y., Chen X., Guo A., Liu S., Hu G. Quantitative assessments of mechanical responses upon radial extracorporeal shock wave therapy. Adv. Sci. 2018;5 doi: 10.1002/advs.201700797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han S.H., Lee J.W., Guyton G.P., Parks B.G., Courneya J.P., Schon L.C. Effect of extracorporeal shock wave therapy on cultured tenocytes. Foot Ankle Int. 2009;30:93e98. doi: 10.3113/FAI-2009-0093. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X., Yang Q., Zhao L., et al. Crosstalk between proteins expression and lysine acetylation in response to patulin stress in Rhodotorula mucilaginosa. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-14078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xiao H., Zhang Y., Kim Y., et al. Differential proteomic analysis of human saliva using tandem mass tags quantification for gastric cancer detection. Sci. Rep. 2016;6 doi: 10.1038/srep22165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frairia R., Berta L. Biological effects of extracorporeal shock waves on fibroblasts. A review, Muscles. Ligaments. Tendons. J. 2012;1:138e147. [PMC free article] [PubMed] [Google Scholar]
- 17.Shockwave Medical Association of Chinese Research Hospital Association Chinese guidelines for extracorporeal shock waves therapy for musculoskeletal disorders (2019 version) Chin. J. Frontiers. Med. Sci. Electr. Version. 2019;11:1e10. [Google Scholar]
- 18.Rinella L., Marano F., Paletto L., Fraccalvieri M., Annaratone L., Castellano I., Fortunati N., Bargoni A., Berta L., Frairia R., Catalano M.G. Extracorporeal shock waves trigger tenogenic differentiation of human adipose-derived stem cells, Connect. Tissue. Res. 2018;59:561e573. doi: 10.1080/03008207.2018.1424147. [DOI] [PubMed] [Google Scholar]
- 19.Xu J.K., Chen H.J., Li X.D., Huang Z.L., Xu H., Yang H.L., Hu J. Optimal intensity shock wave promotes the adhesion and migration of rat osteoblasts via integrin 1-mediated expression of phosphorylated focal adhesion kinase. J. Bio. Chem. 2012;287 doi: 10.1074/jbc.M112.349811. 26200e26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iqbal J., Zaidi M. Molecular regulation of mechanotransduction. Biochem. Biophys. Res. Commun. 2005;328:751e755. doi: 10.1016/j.bbrc.2004.12.087. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz A.J., Sarver D.C., Sugg K.B., Dzierzawski J.T., Gumucio J.P., Mendias C.L. p38MAPK signaling in postnatal tendon growth and remodeling. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaepfer D.D., Hunter T. Focal adhesion kinase overexpression enhances Ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Bio. Chem. 1997;272 doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 23.Labunskyy V.M., Hatfield D.L., Gladyshev V.N. Selenoproteins: molecular pathways and physiological roles. Physiol. Rev. 2014;94:739e777. doi: 10.1152/physrev.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addinsall A.B., Wright C.R., Andrikopoulos S., van der Poel C., Stupka N. Emerging roles of endoplasmic reticulum-resident selenoproteins in the regulation of cellular stress responses and the implications for metabolic disease. Biochem. J. 2018;475:1037e1057. doi: 10.1042/BCJ20170920. [DOI] [PubMed] [Google Scholar]
- 25.Wen J., Xuan B., Liu Y., Wang L., He L., Meng X., Zhou T., Wang Y. Updating the NLRC4 inflammasome: from bacterial infections to autoimmunity and cancer. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.702527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thankam F.G., Roesch Z.K., Dilisio M.F., Radwan M.M., Kovilam A., Gross R.M., Agrawal D.K. Association of inflammatory responses and ECM disorganization with HMGB1 upregulation and NLRP3 inflammasome activation in the injured rotator cuff tendon. Sci. Rep. 2018;8:8918. doi: 10.1038/s41598-018-27250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez O., Pipaon C., Fernandez-Luna J.L. Ipaf is upregulated by tumor necrosis factor-alpha in human leukemia cells, FEBS. Letture. 2004;568:79e82. doi: 10.1016/j.febslet.2004.04.095. [DOI] [PubMed] [Google Scholar]
- 28.Morgan J.P.M., Hamm M., Schmitz C., Brem M.H. Return to play after treating acute muscle injuries in elite football players with radial extracorporeal shock wave therapy. J. Orthop. Surg. Res. 2021;16:708. doi: 10.1186/s13018-021-02853-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zissler A., Steinbacher P., Zimmermann R., Pittner S., Stoiber W., Bathke A.C., Sänger A.M. Extracorporeal shock wave therapy accelerates regeneration after acute skeletal muscle injury. Am. J. Sports Med. 2017;45:676e684. doi: 10.1177/0363546516668622. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.