Abstract
Background
In 2012, the World Health Organization (WHO) released the Global Plan for Insecticide Resistance Management in malaria vectors to stress the need to address insecticide resistance. In a prospective multi-centric study commissioned by the Indian Council of Medical Research (ICMR), we assessed the insecticide susceptibility status of the primary malaria vectors in India from 2017 through 2019.
Methods
The insecticide susceptibility status of the prevalent primary malaria vectors – An. culicifacies, An. fluviatilis, An. stephensi, An. minimus and An. baimaii and secondary malaria vectors - An. aconitus, An. annularis and An. philippinensis/nivepes from 328 villages in 79 districts of 15 states of India were assessed following the WHO method mainly to insecticides used in vector control, organochlorine (DDT), organophosphate (malathion), and other pyrethroids (alpha-cypermethrin, cyfluthrin, lambda-cyhalothrin and permethrin). The study sites were selected as suggested by the National Vector Borne Disease Control Programme.
Results
The primary malaria vector An. culicifacies showed resistance to DDT (50/50 districts including two districts of Northeastern India), malathion (27/44 districts), and deltamethrin (17/44 districts). This species was resistant to DDT alone in 19 districts, double resistant to DDT-malathion in 16 districts, double resistant to DDT-deltamethrin in 6 districts, and triple resistant to DDT-malathion-deltamethrin in 9 districts. An. minimus and An. baimaii were susceptible in Northeastern India while An. fluviatilis and the secondary malaria vector An. annularis was resistant to DDT in Jharkhand.
Conclusion
In this study we report that among the primary vectors An. culicifacies is predominantly resistant to multiple insecticides. Our data suggest that periodic monitoring of insecticide susceptibility is vital. The national malaria program can take proactive steps for insecticide resistance management to continue its push toward malaria elimination in India.
Keywords: Anopheles, India, Insecticide-resistance, Malaria vectors
Anopheles; India; Insecticide-resistance; Malaria vectors.
1. Introduction
The World Malaria Report 2021 estimated ∼241 million malaria cases worldwide in 2020, with India accounting for ∼83% of the total malaria cases -i.e ∼4.8 million within the WHO South-East Asia region, and a maximum region-wide decrease in the number of cases was registered, with 23 million in 2000 to about 5 million in 2020 [1]. Insecticides have been the mainstay of vector control in India and elsewhere among various vector control strategies [2, 3]. Currently, 83 vector control products have been prequalified by World Health Organization vector control product prequalification system [4]. In India, currently, the following insecticides are deployed against malaria vectors – (1) for control of adult malaria vector - dichloro-diphenyl-trichloroethane (DDT), malathion, and five pyrethroid insecticides (alpha-cypermethrin, bifenthrin, cyfluthrin, deltamethrin, lambda-cyhalothrin); (2) for larval control – temephos, two bacterial pesticides (Bacillus thuringiensis var. israelensis, B. sphaericus), two insect growth regulator (IGR) compounds (diflubenzuron, pyriproxyfen), and (3) for space spray, malathion (technical) and formulations of deltamethrin, cyphenothrin (synthetic pyrethroid) or natural pyrethrum extract [5]. For control of adult malaria vectors deltamethrin and alpha-cypermethrin impregnated long-lasting insecticidal nets (LLINs) are used [2]. A comprehensive analysis of insecticide resistance data from India between 1991 to 2016 indicated an increase in resistance and the potential to derail the progress made in reducing malaria transmission [6]. The present study was conducted from 2017 to 2019 in the select districts suggested by the National Vector Borne Disease Control Programme (NVBDCP) that had lacked insecticide resistance data in recent years. The data have been generated for some districts after a gap of a decade or more.
WHO encourages countries to develop local data-based insecticide resistance management plans. Data on insecticide resistance, including the frequency and intensity of malaria vectors and the resistance mechanism, is scarce from malaria-endemic regions. According to the World Malaria Report 2021, of the 88 countries that have provided data on insecticide resistance from 2010 to 2020, ∼80% of countries reported resistance to pyrethroids, ∼64% to organochlorines, ∼34% to carbamates, and ∼28% countries to organophosphates [1]. Further, reports on Global Insecticide Resistance (2010–16) have indicated an increased intensity and time to kill 50% of the exposed mosquitoes [7].
India has six primary malaria vectors – An. culicifacies, An. fluviatilis, An. stephensi, An. baimaii, An. minimus and An. sundaicus; and four secondary malaria vectors – An. annularis, An. philippinensis/nivepes, An. jeyporiensis and An. varuna. [8] Amongst these, An. culicifacies (rural and peri-urban areas) contributes ∼67%, An. fluviatilis (forested areas) ∼15% and An. stephensi (urban areas) ∼12% of the malaria cases in India [6]. Annually, ∼93% of the malaria cases were contributed by these three primary malaria vector species and, ∼7% of cases are from An. minimus (foothills in the east), An. baimaii (forested areas in northeastern states) and An. sundaicus (coastal areas of the Andaman and Nicobar Islands).
Malaria is endemic in 36 states/UTs in India. The Indian Council of Medical Research (ICMR) commissioned multi-centric field studies from 2017 to 2019 to determine the insecticide resistance status of primary and secondary malaria vectors across India. The study was conducted in 15 states as suggested by the NVBDCP. The states were Odisha, Jharkhand, Madhya Pradesh, Karnataka, Maharashtra, Gujarat, Haryana, Uttar Pradesh, and 7 Northeastern Indian states – Arunachal Pradesh, Assam, Manipur, Meghalaya, Mizoram, Nagaland, and Tripura. The districts suggested in these states reportedly lacked recent insecticide susceptibility data for major malaria vectors. Our data sheds light on the insecticide susceptibility in vectors during the years 2017–2019 across India and has implications for the ongoing efforts at malaria elimination in India.
2. Materials and methods
2.1. Study area
The multi-centric field-based insecticide susceptibility study covered 328 villages in 79 districts under 15 Indian states to ensure inclusion of major topographies (forest, forested hills, plains, and coastal areas) and different malaria eco-epidemiological settings (Table 1). Susceptibility to different classes of insecticides was determined for prevalent primary malaria vectors – An. culicifacies, An. minimus, An. baimaii in the Northeastern states in India and An. culicifacies, An. fluviatilis and An. stephensi in other Indian states and additionally to secondary malaria vectors, An. aconitus, An. annularis and An. philippinensis/nivepes (Figure 1). Mosquitoes were mainly exposed to insecticides in use in vector control in Indian vector control programme, DDT, malathion and deltamethrin/alpha-cypermethrin/lambda-cyhalothrin/cyfluthrin and in some locations to other WHO-approved insecticides for use in vector control namely, fenitrothion and permethrin. The data on vector control interventions, i.e., DDT/SP-IRS and LLINs employed in the 13 Indian states, except that Odisha (coastal) and Uttar Pradesh which received focal spray with DDT-IRS, were compiled (Table 1).
Table 1.
Table showing states, districts surveyed, geolocations, interventions, number of villages surveyed, topography, primary and secondary vectors.
| S. No. | State (location in India) | District | Location in the state | Geolocation | Vector intervention/s (adult) last 5 years | No. of Villages surveyed | Villages (Topography) | Primary and Secondary vector Species |
|---|---|---|---|---|---|---|---|---|
| 1 | Odisha (East India) | Kalahandi | South | 20.083°N 83.2°E | DDT-IRS & LLIN | 4 | Plain, Foothill, Forest | Primary -An. culicifacies, An. fluviatilis |
| Angul | Central | 20°50′17″N 85°05′44″E | 4 | |||||
| Cuttack | East | 20°31′25″N 85°47′17″E | Focal Spray | 4 | Coastal | |||
| Khurda | East | 20.18°N 85.62°E | 4 | |||||
| Puri | East | 19°48′38″N 85°49′53″E | 4 | |||||
| Bhadrak | East | 21.06°N 86.50°E | 4 | |||||
| Jagatsinghpur | East | 20.266°N 86.166°E | 4 | |||||
| Balasore | East | 21.5°N 86.9°E | 4 | |||||
| Kendrapara | East | 20.50°N 86.42°E | 4 | |||||
| Jajpur | East | 20.85°N 86.33°E | 4 | |||||
| Mayurbhanj | East | 21.933°N 86.733°E | 4 | |||||
| 2 | Jharkhand (East India) | Simdega | South | 22.62°N 84.52°E | DDT-IRS & LLIN | 6 | Plain, Foothill, Hills, Hilltop | Primary- An. culicifacies, An. fluviatilis, Secondary-An. annularis (local importance), An. minimus |
| Gumla | South | 23°N 84.50°E | 8 | Plain, Forest, Hilltop | ||||
| Khunti | South | 23.0140203°N 85.2724457°E | 5 | Plain, Forest, Foothill, Hills | ||||
| West Singhbum | South | 21.97°N 86.90°E | 7 | Forest, Hills | ||||
| Giridih | North | 24°10′48″N 86°19′12″E | 8 | Plain, Foothill, Hills | ||||
| Latehar | West | 23°43′48″N 84°28′12″E | 7 | Foothill, Forest | ||||
| Palamu | North | 24°1′48″N 84°4′12″E | 8 | Plain, Forest | ||||
| Koderma | North | 24.47°N 85.6°E | 9 | |||||
| Chatra | North | 24°12 0″N 84°52′12″E | 6 | Plain, Foothill | ||||
| Dhanbad | East | 23°47′24″N 86°25′48″E | 6 | |||||
| Godda | East | 24.83°N 87.22°E | SP-IRS & LLIN | 4 | Plain, Forest, Foothill | |||
| Sahibganj | East | 25.25°N 87.65°E | 5 | Plain, Foothill, Hills | ||||
| 3 | Madhya Pradesh (Central India) | Umaria | East | 23°31′36.65″N 80°50′17.18″E | DDT-IRS | 6 | Plain, Foothill, Hilltop | Primary -An. culicifacies |
| Bhind | North | 26°33′51.12″ N 78°47′17.88″E | 7 | Plain, Foothill | ||||
| Anuppur | East | 23.1°N 81.68°E | DDT-IRS & LLIN | 6 | Plain, Foothill, Hilltop | |||
| Khargone | South | 21°49′23″N 75°36′37″E | SP-IRS | 6 | ||||
| Shivpuri | North | 25.43°N 77.65°E | 7 | |||||
| Hoshangabad | South | 22°40′0″N 77°30′0″E | SP-IRS & LLIN | 6 | ||||
| Panna | East | 24°43′12″N 80°10′48″ E | 6 | |||||
| Singrouli | East | 24°12′0″N 82°40′12″E | 6 | |||||
| Alirajpur | West | 22°18′18″N 74°21′36″E | 6 | |||||
| Dhar | West | 22°36′0″N 75°18′0″E | 6 | |||||
| Sidhi | East | 24°25′0.12″N 81°52′59.88″E | 8 | Plain, Forest, Foothill | ||||
| Tikamgarh | North | 24°44′24″N 78°49′48″E | (NI) | 3 | Plain, Forest | |||
| Datia | North | 25°40′12″N 78°27′36″E | 6 | Plain, Forest | ||||
| 4 | Karnataka (South India) | Kalaburgi | North | 17.33°N 76.83°E | Malathion & SP - IRS & LLIN | 3 | stone quarry, irrigation area, riverine | Primary -An. culicifacies, An. fluviatilis |
| 5 | Maharashtra (West India) | Gadchiroli | East | 20°11′10″N 80°00′19″E | SP-IRS & LLIN | 12 | Forest, Foothill, Plain | Primary -An. culicifacies |
| 6 | Gujarat (West India) | Kheda | Central | 22°45′0″N 72°41′0″E | SP-IRS & LLIN | 4 | Riverine and canal irrigated plain | Primary -An. culicifacies |
| Panchmahal | Central | 22°45′N 73°36′E | 4 | |||||
| 7 | Uttar Pradesh (North India) | Gautam Budh Nagar | North | 28.34°N 77.19°E | DDT-IRS | 7 | Plain | Primary -An. culicifacies |
| Saharanpur | North | 29.97°N 77.55°E | DDT-IRS-Focal | 9 | ||||
| Badaun | Central | 28.2°N 79.7°E | 9 | |||||
| Hathras | West | 27.26°N 78.3°E | 10 | |||||
| Jhansi | South | 25.25°N 78.34°E | 13 | |||||
| Banda | South | 25.30°N 80.30°E | 14 | |||||
| Kanpur Dehat | Central | 26.24°N 79.59°E | 16 | |||||
| Prayag Raj | South | 25.27°N 81.50°E | 13 | Rocky | ||||
| Mirzapur | South | 27.40°N 29.33°E | 15 | Forest, Hills | ||||
| Sonebhadra | South | 24.42°N 83.4°E | 12 | |||||
| 8 | Haryana (North India) | Nuh | South | 28°6′0″N 77°0′0″E | DDT-IRS | 3 | Plain and agriculture land | Primary -An. culicifacies, An. stephensi |
| Palwal | South | 28.143°N 77.329°E | 3 | |||||
| 9 | Arunachal Pradesh (North East India) | Changlang/3 | South | 27°7′48″N 95°44′24″E | DDT-IRS & LLIN | 3 | Hilltop, Plains | Primary - An. annularis, Secondary -An. minimus, An. philippinensis/nivipes, An. baimaii |
| Tirap | South | 26°59′26.52″N 95°30′10.08″E | 3 | Hilltop, Foothill | ||||
| Namsai | South | 27.6689°N 95.8714°E | 5 | Hill, Hilltop, Forested Hill | ||||
| 10 | Assam (North East India) | Kokrazhar | West | 26° 24′ 0″ N, 90° 16′ 12″ E | DDT-IRS & LLIN | 1 | Plain Forest | Primary - An. culicifacies, An. annularis, Secondary - An. minimus,An. philippinensis/nivipes, An. baimaii,An. aconitus |
| Baksa | North | 26° 34′ 51″ N, 91° 25′ 13″ E | 1 | |||||
| Bongaigaon | West | 26.4667°N 90.5667°E | 1 | |||||
| Kamrup (M) | West | 26°11′0″N 91°44′0″E | 2 | Foothills | ||||
| Goalpara | West | 26° 26′ 0″ N, 90° 22′ 0″ E | 1 | |||||
| Udalguri | North | 26° 44′ 42.72″ N, 92° 5′ 46.32″ E | 5 | Plain forest, foothills | ||||
| Kamrup (R) | West | 26°20′N 91°15′E | 1 | Plain | ||||
| Nalbari | West | 26° 27′ 0″ N, 91° 26′ 24″ E | 1 | |||||
| Tinsukia | East | 27.500°N 95.367°E | 2 | |||||
| Dibrugarh | East | 27.48°N 95°E | 4 | Forested foothill, Forested Plains, Foothill | ||||
| Jorhat | East | 26.75°N 94.22°E | 4 | Forested foothill, Foothill, Plain, Forested Plain | ||||
| Golaghat | East | 26° 0′ 0″ N, 93° 0′ 0″ E | 6 | Forested Plain, Foothill, Plain | ||||
| KarbiAnglong | Central | 26° 11′ 0″ N, 93° 34′ 0″ E | 3 | Forested foothill, Forested Plain, Hilltop | ||||
| Sivasagar | East | 26.98°N 94.63°E | 6 | Plain, Foothill | ||||
| 11 | Manipur (North East India) | Temenglong | West | 24°59′26.39″N 93°30′3.26″E | DDT-IRS & LLIN | 6 | Hilltop, Foothill, Plain | Secondary -An. annularis |
| 12 | Meghalaya (North East India) | Ri Bhoi | North | 25° 54′ 0″ N, 91° 53′ 0″ E | DDT-IRS & LLIN | 1 | Foothills | Primary - An. baimaii, Secondary -An. annularis,An. minimus,An. philippinensis/nivipes |
| South West Garo Hills | South | 1 | ||||||
| EastGaro Hills | West | 25° 29′ 43.66″ N, 90° 37′ 0.55″ E | 1 | |||||
| South Garo Hills | South | 25° 12′ 0″ N, 90° 38′ 0″ E | 7 | Foothill, Hilltop | ||||
| West Garo Hills | West | 25° 31′ 0.12″ N, 90° 13′ 0.12″ E | 6 | |||||
| 13 | Mizoram | Kolasib | North | 24°13′48″N 92°40′48″E | DDT-IRS & LLIN | 8 | Foothill, Forested Plain, Hilltop, Forested foothill, Plain, Forested Hilltop | Primary -An. minimus, Secondary - An. annularis, An. philippinensis/nivipes, An. aconitus |
| Mamit | West | 23.9294° N, 92.4906° E | 4 | Hilltop, Plain | ||||
| 14 | Nagaland (North East India) | Mokokchung | North | 26° 19′ 12″ N, 94° 30′ 0″ E | DDT-IRS & LLIN | 15 | Hilltop, Foothill, Forested Plain, Plain, Forested Hilltop | Primary -An. baimaii, Secondary - An. annularis, An. philippinensis/nivipes, An. aconitus |
| Dimapur | West | 25.92°N 93.73°E | 6 | Foothill, Plain, Hilltop | ||||
| Peren | South | 25°31′N 93°44′E | 4 | Plain, Foothill | ||||
| 15 | Tripura (North East India) | Dhalai | East | 23° 56′ 0″ N, 91° 51′ 0″ E | DDT-IRS & LLIN | 5 | Forested hill, Foothill | Primary - An. baimaii Secondary -An. annularis, |
| South Tripura | South | 23° 32′ 0″ N, 91° 29′ 0″ E | 5 | Plain |
Figure 1.
The distribution of the primary and secondary Anopheles vectors in several districts (shown in brackets) from the fifteen states for insecticide resistance studies.
2.2. Mosquito collection
Mosquitoes were collected following standard WHO methods [9]. In the districts selected for surveillance, villages with good mosquito productivity were identified. A team of ∼3 people collected mosquitoes from human dwellings and cattle sheds. Anophelines resting indoors in human habitats and cattle holding sheds were collected by hand catch using an aspirator and torch in the early morning hours (0600 h–0800 h), spending 15 min per structure. The collected mosquitoes were transferred to a cloth cage and brought to the laboratory (wrapped in a wet towel). The mosquitoes were identified based on specific morphological characteristics, and female vector species were separated and used for susceptibility tests.
2.3. Insecticide susceptibility tests
Mixed-age field-collected preferably full-fed stage mosquitoes were used in the study. Primarily, mosquitoes were exposed to insecticides in use in vector control in public health; namely DDT, malathion, and deltamethrin, and exposure to other insecticides was made to assess susceptibility to insecticides in the same or different classes of insecticides approved for vector control for more qualitative data. These tests were conducted using the standard WHO methods and kits to WHO specified discriminatory dosages of insecticide-impregnated papers. These kits and insecticide-impregnated and control papers were procured from Universiti Sains Malaysia (USM), Penang, Malaysia. Four replicates of 20–25 female mosquitoes were exposed for 1 h (except fenitrothion for 2 h) to the WHO prescribed discriminating dosages of insecticides (DDT 4%, malathion 5%, fenitrothion 1%, deltamethrin 0.05%, alpha-cypermethrin 0.05%, permethrin 0.75%, cyfluthrin 0.15%, lambda-cyhalothrin 0.05%). Two control replicates of 20–25 female mosquitoes were run simultaneously alongside the test replicates (Supplementary Table 1). However, fewer mosquitoes were exposed in case of species having low densities. The susceptibility tests were conducted in a base laboratory in the field at the prescribed temperature (27 ± 2 °C) and humidity conditions (75 ± 10%). After 24 h, the dead and alive mosquitoes were scored, and percent mortality in test and control replicates was determined.
The states, districts and villages included in the study and their respective geolocations and vector control intervention strategies are listed in Table 1. The number of adult mosquito vector species collected to determine the percentage mortality and susceptibility to different insecticides for each state is briefly mentioned below and provided in Supplementary Table 1A–D.
In the state of Odisha, An. culicifacies (n = 1380) and An. fluviatilis (n = 800) were exposed to DDT, malathion, deltamethrin and cyfluthrin in 9 districts (three ecotypes namely plain, foothill and forest). In the state of Jharkhand, the insecticide susceptibility status to different classes of insecticides was determined for three primary malaria vectors – An. culicifacies (n = 10,194; 12 districts), An. fluviatilis (n = 3155; 9 districts), An. minimus (n = 120; 1 district) and secondary malaria vector An. annularis (n = 2500; 6 districts). Collections were made from five major ecotypes (plain, forest, foothill, hilly, and hilltop). The insecticide susceptibility data in Madhya Pradesh were determined only for major malaria vector An. culicifacies (n = 5740) from 13 districts with four major ecotypes (plain, forest, foothill and hilltop). An. culicifacies (n = 203) was studied for insecticide resistance in the district Kalaburgi of Karnataka. Insecticide susceptibility studies for An. culicifacies (n = 1490) were performed in the district Gadchiroli of Maharashtra with three major ecotypes (plain, foothill, and forest topographies). Studies were conducted for An. culicifacies (n = 1280) in two districts, Kheda and Panchmahal of Gujarat state for malathion, deltamethrin, permethrin, and alpha-cypermethrin. An. culicifacies (n = 805) and An. stephensi (n = 795) were tested for insecticide susceptibility status in 2 districts (Nuh and Palwal) with plain and agricultural ecotypes from Haryana. Susceptibility status of An. culicifacies (n = 5030) was determined in 10 districts of Uttar Pradesh state with three different ecotypes (plain, rocky, hilly forested). Prayagraj with a rocky terrain; Mirzapur and Sonebhadra both with hilly-forested terrain, and the rest of seven districts had plain terrain. Studies were conducted in 29 districts of 7 Northeastern states against two primary malaria vectors An. minimus and An. baimaii and in Assam at two locations against An. culicifacies. The major topography in different states was mostly forested and in some plain ecotype. Insecticide susceptibility tests were conducted against DDT, fenitrothion, malathion, deltamethrin, lambda-cyhalothrin, and permethrin. Besides these, malaria vectors of secondary importance - An. annularis, An. philippinensis/nivipes and An. aconitus were tested in some states, mostly Northeastern states, depending upon their prevalence.
2.4. Data analysis
The pooled data of the test replicates for a given insecticide were compiled and analyzed.
Percent mortality in the insecticide susceptibility tests was calculated using the formula:
Tests with control mortality exceeding 20% were discarded. If the mortality in control was found 5%–20%, then the mortality in the test was corrected using Abbott's formula [10],
The susceptibility status of the mosquito species against the tested insecticide is categorized as: (1) susceptible (98%–100% mortality); (2) possible resistance (90%–97% mortality), and (3) confirmed resistance (<90% mortality) [9].
2.5. Statistical analysis
Mosquito mortality data were subjected to Chi-square/Fisher exact tests using the SPSS Software package (Ver 20) to assess possible correlation.
2.6. Patient and public involvement
Patients were not involved in the current study.
3. Results
A total of 43,862 female malaria vector mosquitoes (primary and secondary) belonging to An. culicifacies, An. fluviatilis, An. stephensi, An. baimaii, An. minimus, An. aconitus, An. annularis, An. philippinensis/nivepes were exposed to insecticides of different classes. State-wise details and results are given below.
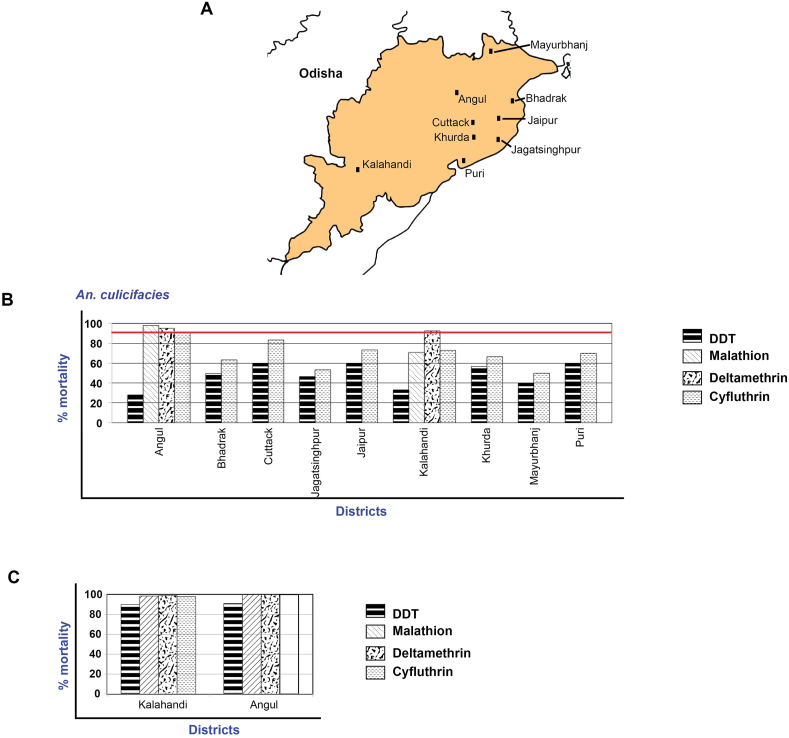
3.1. State of Odisha
An. culicifacies was resistant to DDT (% mortality – 30%–60%) and cyfluthrin (% mortality – 50%–90%) in all the nine districts (Figure 2A). An. culicifacies was possibly resistant to deltamethrin in both Angul and Kalahandi. On the other hand, it was susceptible to malathion in Angul and resistant to malathion in Kalahandi (mortality – 70%) (Figure 2B). An. fluviatilis was susceptible to malathion, cyfluthrin, and deltamethrin in the two tribal districts (mortality – 98–100%) but possible resistant to DDT (mortality – 90–91%) (Figure 2C).
Figure 2.
Map of Odisha showing the location of study districts (A) and susceptibility status of An. culicifacies (B) and An. fluviatilis (C). % mortality of 98-100 % suggests the vector species are susceptible to the insecticide (shown in red line).
3.2. State of Jharkhand
An. culicifacies was found resistant to DDT in the surveyed 12 districts (Figure 3A) (mortality – 4.5%–47%), to malathion in 6 (mortality – 69%–89.5%); and to deltamethrin in 10 districts (mortality – 58.5%–87.5%) (Supplementary Table 1A, Figure 3B). Further, An. culicifacies was resistant to permethrin in 5 districts (mortality – 65.5%–89%), lambda-cyhalothrin in 4 districts, and cyfluthrin in 3 districts (mortality 40%–86.5%) (Supplementary Table 1A, Figure 3B). Possible resistance was recorded in one district each for malathion, deltamethrin and lambda-cyhalothrin, in two districts for cyfluthrin, and five districts for permethrin (Supplementary Table 1A). The observed insecticide susceptibility data from 12 districts for six insecticides were analyzed for correlation with five ecotypes. A significant association was found in all the five ecotypes, and the data for each district with correlation status is listed in Supplementary Table 2. More importantly, category-wise analysis of insecticide resistance data of An. culicifacies (confirmed resistance, possible resistance, and susceptible) to find a correlation with topographies was found significant for malathion only (p = .037, p < .05).
Figure 3.
Map of Jharkhand showing the location of study districts (A) and susceptibility status of An. culicifacies to different insecticides in different districts (B).
An. fluviatilis was found resistant to DDT in all the nine districts (mortality range - 18.5%–80%) and susceptible to malathion, deltamethrin, and permethrin in 6 districts (Supplementary Table 1B). The study districts represented a combination of 2 major topographies of the 5, namely: plain, forest, foothill, hilly, and hilltop. Studies were undertaken in Simdega and Gumla that represented plain and hilltop, Khunti, Palamu (plain and forested), Sahibganj, Giridih (plain and foothills), West Singhbhum (forested and hilly) and Godda and Latehar (forested and foothills; Table 1). An fluviatilis susceptibility data for all the insecticides was statistically not significant for correlation between mortality rate and ecotypes, unlike the case for An. culicifacies. The insecticide susceptibility studies were conducted in 6 districts for the secondary malaria vector An. annularis. An. annularis was found susceptible to DDT, malathion, deltamethrin, and permethrin in the surveyed districts. At the same time, An. annularis showed possible resistance in 3 districts of Jharkhand towards malathion and confirmed resistance in 6 districts of Jharkhand towards DDT (Supplementary Table 1D). The other important vector An. minimus was possible resistant to DDT and susceptible to malathion, deltamethrin, and permethrin (Supplementary Table 1C).
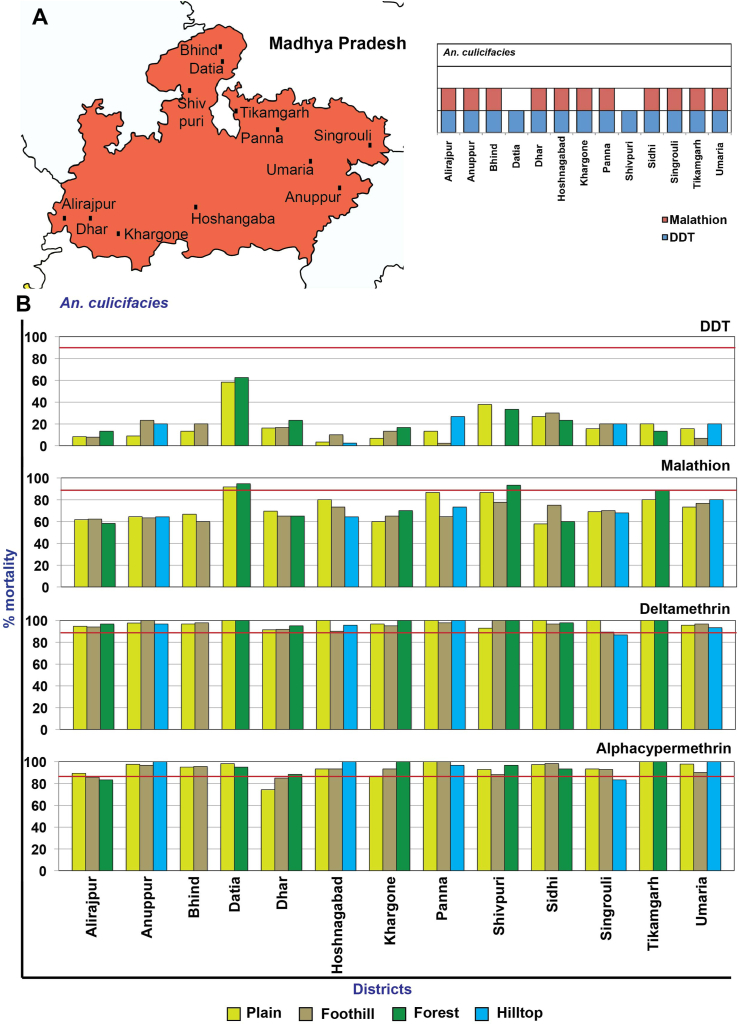
3.3. State of Madhya Pradesh
The pooled data on susceptibility to the three insecticides (DDT, malathion, and alpha cypermethrin) from different villages in the four topographies did not show an association (p < 0.05). An. culicifacies was observed as resistant to DDT (in 13 districts) (Figure 4A) (mortality – 7.6%–60%), to malathion (in 12 districts) (mortality – 58.1%–87%) (except Datia, where the species showed possible insecticide resistance), while to alpha-cypermethrin (in 2 districts) (Dhar and Alirajpur) (mortality – 82.9% and 85.7%, respectively). The species was susceptible to alpha-cypermethrin in 3 districts while it showed possible resistance in 8 districts. An. culicifacies was possible resistant to deltamethrin in 8 districts but was susceptible in 5 districts (Figure 4B).
Figure 4.
Map of Madhya Pradesh showing the location of study districts and the An. culicifacies resistant insecticides represented by a box for each district (A), % mortality of the An. culicifacies to different insecticides in different districts (B).
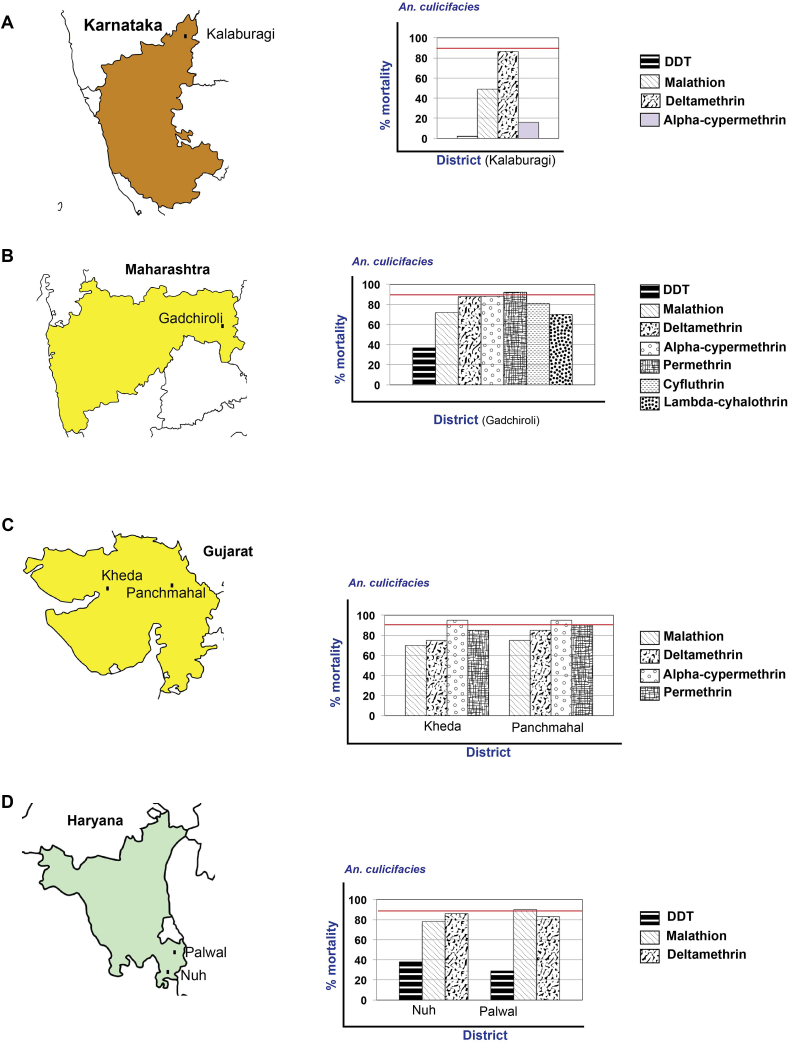
3.4. State of Karnataka
An. culicifacies was found resistant to all the four insecticides studied - DDT (mortality – 2%), malathion (mortality – 49%), deltamethrin (mortality – 86.5%) and alpha-cypermethrin (mortality – 16%) (Figure 5A). The data were generated from single terrain of stone quarry area.
Figure 5.
Map of Karnataka (A), Maharashtra (B), Gujarat (C), and Haryana (D), showing the location of study districts and susceptibility status of An. culicifacies to different insecticides in different districts in the states. % mortality of 90% suggests the vector species are susceptible to the insecticide (shown in red line).
3.5. State of Maharashtra
Data showed that An. culicifacies was resistant to the six insecticides used in India, i.e., DDT (mortailty – 37%), malathion (mortailty – 71.9%), deltamethrin (mortailty – 87.8%), alpha-cypermethrin (mortailty – 88.2%), cyfluthrin (mortailty – 81%), and lambda-cyhalothrin (mortailty – 70.1%), with possible resistance to permethrin (mortality – 92.2%) (Figure 5B).
3.6. State of Gujarat
An. culicifacies was resistant to malathion in Kheda and Panchmahal (mortality – 70% and 75%) and deltamethrin (mortality – 75% and 85%). In Kheda, An. culicifacies was resistant to permethrin, while in Panchmahal, it showed possible resistance (mortality – 85% and 90%). It showed possible resistance to alpha-cypermethrin in both the districts (mortality – 95%) (Figure 5C). Both the districts represented the common topography of the riverine and canal irrigated plain. The susceptibility status to the four insecticides (malathion, deltamethrin permethrin, and alpha-cypermethrin) was not significant at p < 0.05.
3.7. State of Haryana
In both the districts (Nuh and Palwal) An. culicifacies was resistant to DDT (mortality – 38% and 29%), malathion (mortality – 78% and 90%) and deltamethrin (mortality – 86% and 83%) (Figure 5D). Similarly, An.stephensi was resistant to DDT (mortality – 31% and 48%, respectively), malathion (mortality – 69 and 81%) and to deltamethrin (mortality – 85% and 72%, respectively) (Figure 5D). Both the districts have common topography, and the mortalities in An. culicifacies with DDT and deltamethrin were not significant, while they were significant for malathion at p < 0.05. For An. stephensi, the mortalities were significant for all three insecticides at p < .05 (see Supplementary Table 2).
3.8. State of Uttar Pradesh
An culicifacies in Uttar Pradesh was found resistant to DDT in all the ten districts (% mortality – 0 to 6.9% (Figure 6A and B). In 8 districts, this species was in the possible resistance category to malathion (mortality – 92.2%–96.7%), but was resistant to malathion in Gautam Budh Nagar district and susceptible in the district Badaun with 98.9% mortality. For deltamethrin, the species showed possible resistance in 10 districts with a mortality range of 91.5%–97.5%, while An culicifacies was resistant in the Banda district, registering 86.7% mortality. An. culicifacies was susceptible to cyfluthrin in 5 districts and showed possible resistance (mortality – 92.4%–96.9%) in 5 districts (Figure 6B). The mortality correlation of DDT and cyfluthrin susceptibility among plain, rocky and hilly-forested topographies was significant at p < 0.05. For malathion and deltamethrin, the differences between the mortalities among the three topographies was not significant (see Supplementary Table 2).
Figure 6.
Map of Uttar Pradesh showing the location of study districts and the An. culicifacies resistant insecticides represented by a box for each district (A), % mortality of the An. culicifacies to different insecticides in different districts (B).
3.9. The Northeastern states
The study revealed that for DDT, An. culicifacies (n = 442) showed resistance in 2 study districts of Assam (Supplementary Table 1A), while An. minimus (n = 244) remained susceptible in 11 districts in 4 states (Supplementary Table 1C). The other vector An. baimaii (n = 365) was susceptible to DDT in 13 districts from 6 states (Supplementary Table 1C). The primary malaria vector species were completely susceptible except An. culicifacies in districts Udalgiri and Kamrup(R) of Assam with plain forest, foothill and plain ecotypes. An. culicifacies did not show any significant difference in mortality for DDT and malathion across the topographies at p < 0.05. The secondary malaria vector An. annularis (n = 2,310) was tested for insecticide susceptibility in 19 districts in 7 states. An. annularis was susceptible to DDT and deltamethrin, and it was susceptible to malathion in one district of Meghalaya and 2 districts of Tripura (Supplementary Table 1D). An. philippinensis/nivepes (n = 6,929) showed susceptibility to DDT, malathion and deltamethrin in 23 districts of the 5 states (Arunachal Pradesh, Assam, Meghalaya, Mizoram, and Nagaland) (Figure 7). An. philippinensis/nivepes was susceptible to fenitrothion, lambda-cyhalothrin, and permethrin in 13 districts of 4 states (Arunachal Pradesh, Assam, Mizoram, and Nagaland, Supplementary Table 1D). An. aconitus (n = 80) collected from 1 district each of Assam, Mizoram and Nagaland states was susceptible to DDT (Supplementary Table 1D).
Figure 7.
Maps of the Northeastern states showing the location of study districts in Arunachal Pradesh (A), Assam (B), Manipur (C), Meghalaya (D), Mizoram (E), Nagaland (F), and Tripura (G).
To summarize, in the present study from 15 states of India, An. culicifacies was found to be resistant to DDT alone in 19 districts of 5 states. It was double resistant to DDT-malathion in 16 districts of 5 states, to malathion-deltamethrin in 2 districts of 1 state, and DDT-deltamethrin in 6 districts of 2 states. An. culicifacies was triple resistant to DDT-malathion-deltamethrin in 9 districts in 4 states. Four out of the fifteen states included in this study, i.e., Haryana, Jharkhand, Karnataka, and Maharashtra reported triple resistance. In contrast, An. minimus and An. baimaii that are vectors of prominence in Northeastern states [11] were susceptible to all the insecticides including DDT. An. culicifacies in Northeastern region was resistant to DDT in Assam.
4. Discussion
Genetic, biological, and operational factors influence the evolution of insecticide resistance in disease vectors [12, 13, 15]. The operational factors include coverage of interventions, the optimum dosage of application, and resultant selection pressure [14, 15]. Operational factors associated with interventions such as indoor residual spray with improper dosage and non-uniformity may lead to uneven coverage resulting in differential selection pressure. This might alter susceptibility levels in the same species in varied topographies, as observed in this study. Here we report the insecticide susceptibility status of the critical Indian malaria vectors in 328 villages within 79 districts from 15 states with varied ecotypes. In this study, An. culicifacies was found to be resistant to at least one insecticide in 52 districts (Supplementary Table 3). It showed variation in susceptibility categories (susceptible, possible, and confirmed resistance), indicating the dynamic status of insecticide resistance in the population. Further, significant differences were found between the ecotype and insecticide susceptibility to some insecticides in Jharkhand and Uttar Pradesh. Madhya Pradesh and other states did not show significant differences across the species in susceptibility. Topographies possibly influence the preponderance of a given species and behavioural differences. Previous studies on An. culicifacies sibling species (provisionally designated as A, B, C, D, and E) have suggested variations in sympatric, seasonal prevalence, host feeding preference, vector potential, and insecticide susceptibility [16, 17, 18, 19, 20]. Differential prevalence of sibling species in a given topography could be one of the reasons for the observed variations in susceptibility to insecticides.
In India, changes in the vector control policy have been brought over the years based on insecticide resistance data. DDT was introduced in IRS in the early 1950s, HCH in 1958, malathion in 1969, and synthetic pyrethroids in the mid 1990s [21]. Currently for adult vector control, insecticide DDT (organo chlorine), malathion (organophosphate) and deltamethrin, alpha-cypermethrin, and lambda-cyhalothrin (pyrethroids) are mostly used for indoor residual spray (IRS), and deltamethrin and alpha-cypermethrin for impregnated LLINs. For larval control temephos, bacterial pesticides (Bacillus thuringiensis var. israelensis, B. sphaericus), and IGR compounds (diflubenzuron, pyriproxyfen); and for space spray malathion (technical) and formulations of deltamethrin (pyrethroid)/natural pyrethrum extracts are in use. Vector control in India per se is control of An. culicifacies because of its wide distribution. It transmits nearly two-thirds of new malaria cases each year, and has also developed multiple resistance to different classes of insecticides [8]. The current vector control strategies rely on pyrethroids both for IRS and LLINs. Thus, there is an impending threat of the development of widespread pyrethroid resistance [8]. It was observed earlier in India that the critical reason for malaria resurgence in the mid-1970s was insecticide resistance in An. culicifacies [22]. This prompts for implementing vector control strategies with effective tools to evade or defer the onset of resistance [23]. In India, multiple insecticide resistance in vector species is observed, which may persist for a variable duration of time [12, 13, 14, 21]. Generally, an insecticide-unselected population will have insects that are fully susceptible with resistance gene in rarity, possible due to “fitness cost” [24].
The effective strategy suggested for managing insecticide resistance is rotation, mosaics, mixtures, and combinations of insecticides [25]. Though not in use in India at present, improved LLINs with synergist piperonyl butoxide and insecticide deltamethrin mixture on the roof, and deltamethrin in different concentrations on panels, and a mosaic of the same was reportedly effective for resistance management compared to deltamethrin alone net [26]. Another possibility for resistance management is using insecticide mixtures from different classes with different modes of action to promote the efficacy of either of the components [27]. Such products for IRS and LLINs, such as deltamethrin + clothianidin mixtures for IRS [28, 29, 30, 31, 32] and deltamethrin/permethrin + synergists for LLINs [33, 34, 35, 36] are getting available in India, though they are not registered as yet. The above interventions are of special importance in the run-up for malaria elimination as the vectors have developed multiple resistance to insecticides, including pyrethroids. The combination of two vector control tools is another management strategy used with the presumption that the mosquito should have the propensity for simultaneous exposure to both the vector control tools resulting in choice for the efficacy such as pyrethroid LLINs, and non-pyrethroid IRS.
In the present study An. culicifacies showed single resistance to DDT in 19 districts, double resistance to DDT-malathion in 16 districts, malathion-deltamethrin in 2 districts, to DDT-deltamethrin in 6 districts and triple resistance to DDT-malathion-deltamethrin in 9 districts. This presents an alarming scenario for India for malaria control. For example, the triple resistance to DDT, malathion, and deltamethrin in Haryana, Jharkhand, Karnataka, and Maharashtra needs urgent attention for resistance management. In contrast, An. minimus and An. baimaii (from Northeastern states) were susceptible to all the insecticides. However, a limitation of the present study in the Northeastern states was that we could not test An. minimus and An. baimai mosquitoes in sufficient numbers due to low density in the field in some sites, and therefore we could expose only a few mosquitoes. Other researchers have encountered this situation as well [6].
The Northeastern states had regular IRS with DDT since the initiation of the control programme in the 1950s and for about 2 decades in a combination with pyrethroid LLINs. The combination was very effective for the control of An. minimus. An. baimaii is an exophilic and exophagic mosquito by behaviour [37] and thus was devoid of continued insecticide selection pressure and possibly remained susceptible to all insecticides. Recently, An. baimaii has shown endophilic behavior but its epidemiological significance is yet to be ascertained [38]. Thus, there is a need for a renewed focus on malaria control in the Northeastern states. As India hurtles towards malaria elimination by 2030, it is vital that the gains so far are retained for the foreseeable future in the context of vector control. In areas with widespread multiple insecticide-resistant vectors, the mitigation option is resistance management by using the insecticides with alternative modes of action, different modes of deployment and to introduce new vector control tools when available. The strategy management should limit the geographical spread of resistance to new areas by using effective vector control tools focusing on novel insecticides and should target to mitigate the impact of resistance. The enzyme families being investigated for malaria parasites can be explored as potent drug targets for malaria vectors [39, 40, 41, 42]. It is important that data generated on insecticide resistance in vectors and data on other entomological surveillance indicators are regularly shared with the national malaria programme preferably via a digital platform [43]. The work presented here will help formulate policies that are urgently required to prevent the widespread dissemination of insecticide-resistant vectors.
Declarations
Author contribution statement
Kamaraju Raghavendra: Conceived and designed the experiments.
Manju Rahi: Designed the study, analyzed and interpreted the data and wrote the paper.
Vaishali Verma, Poonam Sharma Velamuri: Analyzed and interpreted the data; Wrote the paper.
Divya Kamaraju: Analyzed and interpreted the data.
Jyoti Chhibber-Goel: Analyzed and interpreted the data; Wrote the paper.
Kalpana Baruah: Provided national programme data, analysis tools or data.
Amit Sharma: Analyzed and interpreted the data, designed the flow of figures and wrote the paper.
Funding statement
Dr Amit Sharma was supported by Department of Science and Technology [JCB41].
Jyoti Chhibber-Goel was supported by Department of Biotechnology [BT/PR30603/BIC/101/1104/2018].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors sincerely thank the investigators - Dr. A.K. Mishra, Dr. M.K. Das, Dr. S.A. Khan, Dr R. Baharia, Dr. R.K. Hazra, Dr. Gyan Chand, Dr. U. Sreehari, Dr. S.P. Singh, Dr. Kuldeep Singh, Dr. A.K. Gupta and the supporting staff for generating the data. Authors thank Ms. Pooja Dey for assistance in data compilation and type setting of manuscript. Authors acknowledge with thanks for the support of the Director General, Indian Council of Medical Research, for facilitating and funding task force studies on insecticide resistance.
Contributor Information
Manju Rahi, Email: drmanjurahi@gmail.com.
Amit Sharma, Email: amit.icgeb@gmail.com.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Malaria Report. World Health Organization; Geneva: 2021. [Google Scholar]
- 2.National Framework for Malaria Elimination in India (2016–2030) National Vector Borne Disease Control Programme; Delhi, India: Delhi: 2016. http://nvbdcp.gov.in/Doc/National [Google Scholar]
- 3.Corbel V., N’Guessan R. In: Anopheles Mosquitoes - New Insights into Malaria Vectors. Manguin S., editor. InTech; 2013. Distribution, mechanisms, impact and management of insecticide resistance in malaria vectors: a pragmatic review. [Google Scholar]
- 4.List of WHO Prequalified Vector Control Products, updated on 26/08/2020. https://www.who.int/pq-vector-control/prequalified-lists/en/.
- 5.Insecticides – Formulations and Dosages (IRS and Larvicides). https://nvbdcp.gov.in/WriteReadData/l892s/40056316231597136390.pdf, (www.nvbdcp.gov.in-1840276/2020/NVBDCP).
- 6.Raghavendra K., Velamuri P.S., Verma V., et al. Temporo-spatial distribution of insecticide-resistance in Indian malaria vectors in the last quarter-century: need for regular resistance monitoring and management. J. Vector Borne Dis. 2017;54:111–130. [PubMed] [Google Scholar]
- 7.Global report on insecticide resistance in malaria vectors: 2010–2016. 2017.https://apps.who.int/iris/bitstream/handle/10665/272533/9789241514057-eng.pdf.
- 8.Raghavendra K., Barik T.K., Reddy B.P.N., et al. Malaria vector control: from past to future. Parasitol. Res. 2011;108:757–779. doi: 10.1007/s00436-010-2232-0. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . 2016. Test Procedures for Insecticide Resistance Monitoring in Malaria Vector Mosquitoes. [Google Scholar]
- 10.Abbott W.S. A method of computing the effectiveness of an insecticide. 1925. J. Am. Mosq. Control Assoc. 1987;3:302–303. [PubMed] [Google Scholar]
- 11.Akhtar N., Nagpal B.N., Kapoor N., et al. Role of An. culicifacies as a vector of malaria in changing ecological scenario of Northeastern states of India. J. Vector Borne Dis. 2016;53:264–271. [PubMed] [Google Scholar]
- 12.Georghiou G.P., Taylor C.E. Genetic and biological influences in the evolution of insecticide resistance. J. Econ. Entomol. 1977;70:319–323. doi: 10.1093/jee/70.3.319. [DOI] [PubMed] [Google Scholar]
- 13.Georghiou G.P., Taylor C.E. Operational influences in the evolution of insecticide resistance. J. Econ. Entomol. 1977;70:653–658. doi: 10.1093/jee/70.5.653. [DOI] [PubMed] [Google Scholar]
- 14.Denholm I., Rowland M.W. Tactics for managing pesticide resistance in arthropods: theory and practice. Annu. Rev. Entomol. 1992;37:91–112. doi: 10.1146/annurev.en.37.010192.000515. [DOI] [PubMed] [Google Scholar]
- 15.Verma V., Agrawal O.P., Velamuri P.S., et al. A laboratory simulation study on suppression of resistance genes by differential exposures to an insecticide in Anopheles stephensi Liston population. J. Vector Borne Dis. 2018;55:184–188. doi: 10.4103/0972-9062.249126. [DOI] [PubMed] [Google Scholar]
- 16.Subbarao S.K. The Anopheles culicifacies complex and control of malaria. Parasitol. Today. 1988;4:72–75. doi: 10.1016/0169-4758(88)90199-8. [DOI] [PubMed] [Google Scholar]
- 17.Joshi H., Vasantha K., Subbarao S.K., et al. Host feeding patterns of Anopheles culicifacies species A and B. J. Am. Mosq. Control Assoc. 1988;4:248–251. [PubMed] [Google Scholar]
- 18.Subbarao S.K., Adak T., Vasantha K., et al. Susceptibility of Anopheles culicifacies species A and B to Plasmodium vivax and Plasmodium falciparum as determined by immunoradiometric assay. Trans. R. Soc. Trop. Med. Hyg. 1988;82:394–397. doi: 10.1016/0035-9203(88)90132-0. [DOI] [PubMed] [Google Scholar]
- 19.Raghavendra K., Vasantha K., Subbarao S.K., et al. Resistance in Anopheles culicifacies sibling species B and C to malathion in Andhra Pradesh and Gujarat States, India. J. Am. Mosq. Control Assoc. 1991;7:255–259. [PubMed] [Google Scholar]
- 20.Raghavendra K., Subbarao S.K., Vasantha K., et al. Differential selection of malathion resistance in Anopheles culicifacies A and B (Diptera: Culicidae) in Haryana State, India. J. Med. Entomol. 1992;29:183–187. doi: 10.1093/jmedent/29.2.183. [DOI] [PubMed] [Google Scholar]
- 21.Raghavendra K., Verma V., Srivastava H.C., et al. Persistence of DDT, malathion & deltamethrin resistance in Anopheles culicifacies after their sequential withdrawal from indoor residual spraying in Surat district, India. Indian J. Med. Res. 2010;132:260–264. [PubMed] [Google Scholar]
- 22.Sharma G. Review of Malaria and its Control in India in Proceedings of Indo-UK Workshop on Malaria ed. V.P. Sharma.
- 23.WHO Global Malaria Programme: Global Plan for Insecticide Resistance Management in Malaria Vectors. 2012. http://whqlibdoc.who.int/publications/2012/9789241564472_eng.pdf
- 24.Berticat C., Boquien G., Raymond M., et al. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet. Res. 2002;79:41–47. doi: 10.1017/s001667230100547x. [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization . 2004. Global Strategic Framework for Integrated Vector Management. [Google Scholar]
- 26.Killeen G.F., Okumu F.O., N’Guessan R., et al. The importance of considering community-level effects when selecting insecticidal malaria vector products. Parasites Vectors. 2011;4:160. doi: 10.1186/1756-3305-4-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curtis C. Theoretical models of the use of insecticide mixtures for management of resistance. Bull. Entomol. Res. 1985;75:259–265. [Google Scholar]
- 28.Oxborough R.M., Seyoum A., Yihdego Y., et al. Susceptibility testing of Anopheles malaria vectors with the neonicotinoid insecticide clothianidin; results from 16 African countries, in preparation for indoor residual spraying with new insecticide formulations. Malar. J. 2019;18:264. doi: 10.1186/s12936-019-2888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agossa F.R., Padonou G.G., Ajyh Fassinou, et al. Small-scale field evaluation of the efficacy and residual effect of Fludora® Fusion (mixture of clothianidin and deltamethrin) against susceptible and resistant Anopheles gambiae populations from Benin, West Africa. Malar. J. 2018;17:484. doi: 10.1186/s12936-018-2633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuseini G., Phiri W.P., von Fricken M.E., et al. Evaluation of the residual effectiveness of FludoraTM fusion WP-SB, a combination of clothianidin and deltamethrin, for the control of pyrethroid-resistant malaria vectors on Bioko Island, Equatorial Guinea. Acta Trop. 2019;196:42–47. doi: 10.1016/j.actatropica.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Fongnikin A., Houeto N., Agbevo A., et al. Efficacy of Fludora® Fusion (a mixture of deltamethrin and clothianidin) for indoor residual spraying against pyrethroid-resistant malaria vectors: laboratory and experimental hut evaluation. Parasites Vectors. 2020;13:466. doi: 10.1186/s13071-020-04341-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamaraju R., Pant C.S., Uragayala S., et al. Small-scale field evaluation of the entomological efficacy and the residual activity of Fludora ® Fusion WP-SB indoor residual spraying against Anopheles culicifacies s.l. in Gujarat, India. Trop. Med. Int. Health. 2021;26:469–477. doi: 10.1111/tmi.13549. [DOI] [PubMed] [Google Scholar]
- 33.Oumbouke W.A., Rowland M., Koffi A.A., et al. Evaluation of an alpha-cypermethrin + PBO mixture long-lasting insecticidal net VEERALIN® LN against pyrethroid resistant Anopheles gambiae s.s.: an experimental hut trial in M’bé, central Côte d’Ivoire. Parasites Vectors. 2019;12:544. doi: 10.1186/s13071-019-3796-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gleave K., Lissenden N., Richardson M., et al. Cochrane Database of Systematic Reviews Published Online First. 2018. Piperonyl butoxide (PBO) combined with pyrethroids in insecticide-treated nets to prevent malaria in Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Staedke S.G., Gonahasa S., Dorsey G., et al. Effect of long-lasting insecticidal nets with and without piperonyl butoxide on malaria indicators in Uganda (LLINEUP): a pragmatic, cluster-randomised trial embedded in a national LLIN distribution campaign. Lancet. 2020;395:1292–1303. doi: 10.1016/S0140-6736(20)30214-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Camara S., Ahoua Alou L.P., Koffi A.A., et al. Efficacy of Interceptor ® G2, a new long-lasting insecticidal net against wild pyrethroid-resistant Anopheles gambiae s.s. from Côte d’Ivoire: a semi-field trial. Parasite. 2018;25:42. doi: 10.1051/parasite/2018042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prakash A., Bhattacharyya D.R., Mohapatra P.K., Mahanta J. Proceedings of the Second Symposium on Vectors & Vector Borne Diseases. 1997. Studies on bionomics of Anopheles dirus peyton and Harrison, 1997 (Diptera: Culicidae) in Assam, India; pp. 236–246. [Google Scholar]
- 38.Subbarao S.K., Nanda N., Rahi M., et al. Biology and bionomics of malaria vectors in India: existing information and what more needs to be known for strategizing elimination of malaria. Malar. J. 2019;18:396. doi: 10.1186/s12936-019-3011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pazhayam N.M., Chhibber-Goel J., Sharma A. New leads for drug repurposing against malaria. Drug Discov. Today. 2019;24:263–271. doi: 10.1016/j.drudis.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Manickam Y., Chaturvedi R., Babbar P., et al. Drug targeting of one or more aminoacyl-tRNA synthetase in the malaria parasite Plasmodium falciparum. Drug Discov. Today. 2018;23:1233–1240. doi: 10.1016/j.drudis.2018.01.050. [DOI] [PubMed] [Google Scholar]
- 41.Jain V., Sharma A. Repurposing of potent drug candidates for multiparasite targeting. Trends Parasitol. 2017;33:158–161. doi: 10.1016/j.pt.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Kato N., Comer E., Sakata-Kato T., et al. Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature. 2016;538:344–349. doi: 10.1038/nature19804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahi M., Sharma A. For malaria elimination India needs a platform for data integration. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.