Abstract
Rationale & Objective
Patients with advanced kidney disease are at risk for cognitive impairment, which may persist after kidney transplantation. We sought to understand changes in neurocognitive function domains utilizing comprehensive cognitive assessments.
Study Design
Prospective cohort study.
Setting & Population
Single-center study of patients undergoing kidney transplantation.
Exposure
Kidney transplantation.
Outcomes
Changes in neurocognitive function as measured by the Repeatable Battery for Assessment of Neuropsychological Status (RBANS) and the Trail Making Test Parts A and B (TRAIL A and B) before transplantation (baseline) and compared to 3 months and 12 months posttransplant.
Analytical Approach
Wilcoxon signed-rank and linear mixed effect models were utilized to assess changes in neurocognitive scores at 3 months and 12 months compared to baseline.
Results
Thirty-two patients were included with a mean age of 45 years, 47% female, 85% White, and 62% with at least some college education. Hypertension and diabetes were etiologies of kidney disease in 31% and 25% of patients, respectively. Baseline RBANS and TRAIL A and B scores averaged 84.7 ± 14, 40.4 ± 9.9, and 41 ± 11.5, respectively. Although there were posttransplant improvements in immediate and delayed memory at 3 months, these were not sustained at 12 months. There were no significant differences from baseline at 3 months and 12 months in RBANS index scores for language, visuospatial/constructional abilities, and attention. Compared to baseline, TRAIL A scores were not significantly different at 3 months but were significantly improved at 12 months, whereas TRAIL B scores improved significantly at both 3 months and 12 months.
Limitations
Single-center design and small sample size.
Conclusions
Utilizing comprehensive cognitive assessments, we found improvements in attention and executive function in the first posttransplant year as measured by TRAIL A and B. However, there was no significant change in global cognition as measured by RBANS. These findings identify cognitive domains for potential intervention in the posttransplant population.
Index Words: Kidney transplant, neurocognitive function, RBANS, TRAIL A, TRAIL B
Plain-Language Summary.
Patients with advanced kidney disease are at risk for cognitive impairment, which may persist after kidney transplantation. This study measured neurocognitive function in 32 patients before transplant and at 3 and 12 months posttransplant utilizing the Repeatable Battery for Assessment of Neuropsychological Status and the Trail Making Test Parts A and B. The study found that there were improvements in attention and executive function in the first posttransplant year as measured by the Trail Making Test Parts A and B. However, there was no significant change in global cognition as measured by the Repeatable Battery for Assessment of Neuropsychological Status test. These findings identify cognitive domains for potential intervention in the posttransplant population.
Editorial, • • •
Persons with kidney failure and earlier stages of chronic kidney disease are known to be at increased risk for cognitive impairment when compared to the general population.1, 2, 3, 4, 5, 6 The COGNITIVE impairment in adults with end-stage kidney disease treated with HemoDialysis (COGNITIVE-HD) study showed that up to 71% of patients treated by maintenance hemodialysis have impairment in at least 1 cognitive domain, including motor function, executive function, language, learning and memory, orientation, and complex attention.2 A meta-analysis in 2019 demonstrated that mild cognitive impairment could be overlooked, and some of our traditional methods for screening may not detect mild expressions of impairment in the pre-dialysis and dialysis population, underestimating the significant burden cognitive impairment has on this patient population.3 Multiple mechanisms have been proposed to account for the increased risk of cognitive impairment because of kidney disease, including increased uremic toxins, hyperparathyroidism, and Klotho deficiency.7 Unfortunately, the initiation of dialysis in the chronic kidney disease population does not necessarily reverse these deficits despite correcting metabolic abnormalities and may contribute to further cognitive decline.7,8 In fact, the prevalence of cognitive impairment in kidney transplant candidates ranges from 6%-10%.9, 10, 11
Kidney transplantation can correct the metabolic derangements and eliminate the fluid and osmotic shifts that patients can experience on dialysis; however, cognitive impairments still persist posttransplant.10 This has important implications for patient and graft survival as cognitive impairment at time of transplantation has been associated with an increased risk of graft loss and mortality in kidney transplant recipients.9,12 In the current literature, there are mixed findings on the impact of transplantation on global cognitive function and individual cognitive domains.7,9,12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23 Multiple studies assessing global cognitive function only utilized screening tests such as the Modified Mini-Mental State (3MS) test or Mini-Mental State Examination rather than more comprehensive cognitive assessments.9,12,17,18,21,23 A limited meta-analysis evaluated the impact of kidney transplant on different cognitive domains and determined that transplant recipients had improved cognitive performance in abilities including processing speed, verbal and visual memory, and spatial reasoning compared to dialysis patients or those with chronic kidney disease; however, they continued to perform below matched controls and did not improve in domains of attention, executive function, and verbal fluency.15
Therefore, although it is established that kidney transplant candidates are at risk for pre-existing cognitive impairment, further research is warranted to better understand the impact of kidney transplantation on a wide array of cognitive domains, as previous investigations have largely focused on global cognition using brief screening tools. In this prospective study, we aimed to assess the trajectory of and point-specific change in global cognition, attention, and executive function through comprehensive cognitive assessments that evaluate diverse and clinically relevant cognitive domains in kidney transplant recipients before transplantation and during the first posttransplant year.
Methods
Study Setting and Participants
This was a prospective, single-center study that enrolled patients to undergo neurocognitive performance testing before transplantation and at 3 months and 12 months posttransplant. The study occurred from July 2017 to June 2019. Inclusion criteria were: (1) age greater than or equal to 18 years, (2) eligible for kidney transplantation as determined by the surgical and medical teams, 3) admitted to the hospital for an impending deceased donor kidney transplant or seen in clinic for a pre-operative evaluation before a scheduled living donor kidney transplant, and 4) able to provide informed consent. Patients who were to receive a dual-organ transplant or who were non-English–speaking were excluded. Patients were screened for eligibility through the electronic medical record. The final cohort (N=32) included participants who had baseline data and at least 1 posttransplant observation for the Repeatable Battery for Assessment of Neuropsychological Status (RBANS) and/or the Trail Making Test Part A (TRAIL A) and/or Part B (TRAIL B). The study was approved by the Vanderbilt University Institutional Review Board (IRB#170144). The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Data Collection
Patient and transplant characteristics were obtained from the electronic medical record. Neurocognitive performance scores were tabulated and computed after completion of testing. Study data were collected and managed using Research Electronic Data Capture hosted at Vanderbilt University.24,25 Research Electronic Data Capture is a secure, web-based software platform designed to support data capture for research studies.
Neurocognitive Performance Testing
We assessed global cognition and attention and executive function before transplantation (baseline) and at 3 and 12 months posttransplant utilizing the RBANS and the TRAIL A and B) (Table 1). The RBANS is a broad-based neuropsychological battery measuring global cognition and tests 5 individual domains: attention, language, visuospatial/constructional abilities, and immediate and delayed memory. It consists of 12 subtests that yield 5 index scores, one for each domain tested, and a total scale score. General population age-adjusted mean and standard deviation (SD) reference values are 100 ± 15 (possible range 40-160), with lower scores indicating worse performance. Scores on the RBANS of < 78 are considered to reflect significant cognitive impairment.26 The TRAIL A and TRAIL B tests measure attention and executive function, specifically cognitive flexibility and set shifting, respectively. They provide information on visual search, scanning, speed of processing, mental flexibility, and executive functions. T-scores on TRAIL A and TRAIL B tests are adjusted for age, sex, and education and have a mean of 50 and an SD of 10 (lower scores reflect worse performance). T-scores of ≤ 35 are considered to reflect significant cognitive impairment.27 Tests comprising our battery are psychometrically robust and suitable for use with a broad array of medical populations. All cognitive tests were administered by trained research personnel and took approximately 45-60 minutes to complete.
Table 1.
Neurocognitive Tests
| Neurocognitive Test | Description |
|---|---|
| TRAIL Making Test A and B | A measurement of attention and executive function. TRAIL A is a test of cognitive flexibility and TRAIL B tests set shifting. Combined, they provide information on visual search, scanning, speed of processing, mental flexibility, and executive functions. |
| Repeatable Battery for Assessment of Neuropsychological Status (RBANS) | A measurement of global cognition and tests 5 individual domains: attention, language, visuospatial/constructional abilities, and immediate and delayed memory. |
Outcome
The primary outcome of interest was change in neurocognitive function as measured by RBANS total and the 5 RBANS index scores and TRAIL A and TRAIL B scores at 3 and 12 months posttransplant in comparison to pretransplant baseline scores.
Statistical Analysis
Summary statistics were computed. Categorical variables were described using frequencies and percentages while continuous variables were expressed as medians (with interquartile ranges) or means (with SDs). Neurocognitive performance scores were referenced to population-based adjusted means. Consistent with the standard convention, cognitive impairment was defined as having a score 1.5 SDs or more below the population mean for each measure.26 Among patients having data at all 3 time points, differences in median scores at 3 and 12 months posttransplant were assessed for all cognitive function measures as within-subjects planned comparisons versus baseline scores using Wilcoxon signed-rank tests. To comprehensively evaluate outcomes, and to incorporate all available data among patients having baseline and at least 1 posttransplant observation for a given measure, linear mixed effects models were used to assess the effect of posttransplant time point (3 and 12 months; reference pretransplant baseline) for all neurocognitive measures. Data at each posttransplant time point (referenced to pretransplant data) and an intercept were modeled as fixed effects, with a random effect specified at the patient level. To address concerns regarding Type I error, given that analyses were conducted for 8 outcome measures (RBANS total score, 5 RBANS index scores, TRAIL A and TRAIL B), data were evaluated as 2 planned comparisons (3 and 12 months posttransplant versus baseline), and a P value of < 0.01 was considered statistically significant. All analyses were performed using IBM SPSS Statistics for Windows, version 27 (IBM Corp).
Results
Study Cohort
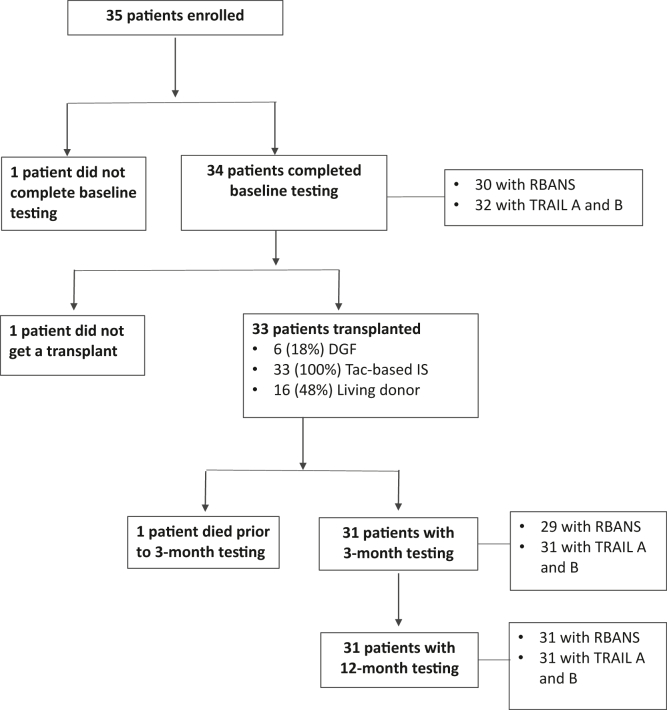
During the study period, 35 patients in total were enrolled. Three were excluded, as indicated (Fig 1). Of the remaining 32 patients, 30 completed baseline RBANS assessment and all 32 completed baseline TRAIL A and TRAIL B tests. Baseline testing was completed within 24 hours for recipients of deceased donor kidneys or at the time of standard pre-operative evaluation for living kidney recipients. Repeat testing was conducted at a mean of 3.5 ± 0.7 months posttransplant for the 3-month assessment and at a mean of 12.5 ± 1.3 months for the 12-month assessment. Twenty-nine patients completed RBANS testing at 3 months posttransplant and 31 patients completed RBANS testing at 12 months. Thirty-one patients completed follow-up testing for TRAIL A and TRAIL B at 3 and 12 months. Table 2 outlines the characteristics of the 32 patients included in the study. The cohort had an average age of 45 years, 47% were female, and 87% were White. Sixty-two percent of patients had, at minimum, some college education, and 29% completed their education as high school graduates. Twenty-five percent of patients had preemptive transplants. Hypertension and diabetes represented the predominant etiologies of kidney failure at 31% and 25%, respectively.
Figure 1.
Patient enrollment and testing. DGF, delayed graft dunction; IS, immunosuppression; RBANS, Repeatable Battery for Assessment of Neuropsychological Status; Tac, tacrolimus; TRAIL A and B, Trail Making Test Parts A and B.
Table 2.
Baseline Patient Characteristics
| Characteristics | All Patientsa N = 32 |
RBANSbScore <78c n = 10 |
RBANSb Score ≥78c n = 20 |
|---|---|---|---|
| Age, y | 44.9 ± 12.1 | 41.1 ± 14.2 | 47.3 ± 11.2 |
| Sex | |||
| Female | 15 (47%) | 3 (30%) | 11 (55%) |
| Male | 17 (53%) | 7 (70%) | 9 (45%) |
| Race | |||
| Black | 4 (13%) | 3 (30%) | 1 (5%) |
| White | 28 (87%) | 7 (70%) | 19 (95%) |
| Marital status | |||
| Single | 9 (28%) | 2 (20%) | 6 (30%) |
| Married | 21 (66%) | 8 (80%) | 13 (65%) |
| Divorced | 2 (6%) | 0 (0%) | 1 (5%) |
| Insurance | |||
| Medicare only | 9 (28%) | 1 (10%) | 6 (30%) |
| Medicare and Medicaid | 3 (9%) | 1 (10%) | 3 (15%) |
| Medicare and private | 14 (44%) | 7 (70%) | 6 (30%) |
| Private only | 6 (19%) | 1 (10%) | 5 (25%) |
| Education | |||
| Some high school | 1 (3%) | 1 (10%) | 0 (0%) |
| High school graduate | 8 (25%) | 3 (30%) | 5 (25%) |
| Some college | 12 (37%) | 3 (30%) | 8 (40%) |
| College graduate | 6 (19%) | 3 (30%) | 3 (15%) |
| Postgraduate degree | 5 (16%) | 0 (0%) | 4 (20%) |
| Dialysis modality | |||
| Hemodialysis | 18 (56%) | 7 (70%) | 10 (50%) |
| Peritoneal dialysis | 6 (19%) | 1 (10%) | 4 (20%) |
| Not on dialysis | 8 (25%) | 2 (20%) | 6 (30%) |
| Dialysis duration, yd | 2.5 ± 2.0 | 2.0 ± 1.3 | 2.7 ± 2.4 |
| Prior kidney transplant | 5 (16%) | 2 (20%) | 2 (10%) |
| Cause of kidney disease | |||
| Diabetes | 7 (22%) | (30%) | 4 (20%) |
| Hypertension | 11 (34%) | 4 (40%) | 6 (30%) |
| Glomerular disease | 7 (22%) | 1 (10%) | 5 (25%) |
| Polycystic kidney disease | 5 (16%) | 1 (10%) | 4 (20%) |
| Other | 2 (6%) | 1 (10%) | 1 (5%) |
Note: Table entries are mean ± standard deviation or frequency (%).
Patients with baseline RBANS or TRAIL A or B testing and at least one posttransplant observation (N=32)
RBANS, Repeatable Battery for Assessment of Neuropsychological Status
Patients with baseline RBANS and at least one posttransplant observation (N=30)
Patients on hemodialysis or peritoneal dialysis with available data (n=20)
Cognitive Function Pretransplant
Using all available data, RBANS scores at baseline averaged 84.7 ± 14. TRAIL A and TRAIL B scores averaged 40.4 ± 9.9 and 41 ± 11.5 at baseline, respectively. Seventeen persons (53%) had impaired pretransplant cognitive function based on one or more metrics including the RBANS total score, TRAIL A, and TRAIL B. Among those with any baseline cognitive impairment, 8 of 17 (47%) were impaired on 1 measure, 3 of 17 (18%) were impaired on 2 measures, and 6 of 17 (35%) had impaired pretransplant cognitive function on all 3 measures. Among all patients, 33% (10 of 30) had cognitive impairment before transplantation based on the RBANS total score, 41% (13 of 32) had impairment based on TRAIL A and 28% (9 of 32) were cognitively impaired based on TRAIL B scores.
Global Cognitive Function Posttransplant (RBANS)
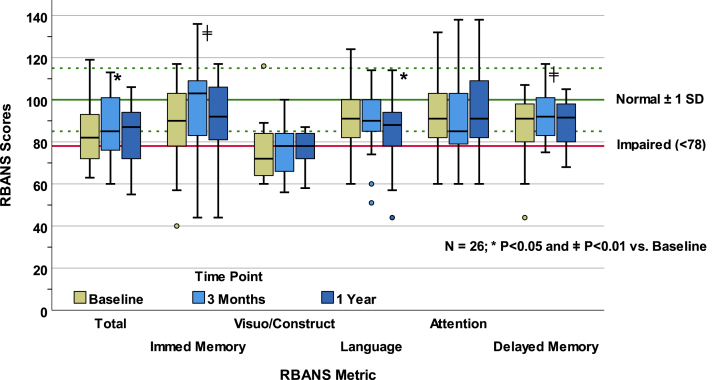
Using all available data, RBANS scores at 3 months and 12 months posttransplant averaged 87.5 ± 14.3 and 85.06 ± 14.2, respectively. Twenty-six patients completed RBANS testing at all 3 time points. Paired comparisons of median RBANS total scores demonstrated no significant improvement from baseline at 3 months or at 12 months posttransplant (all P > 0.01; Fig 2). Paired comparisons among RBANS index scores showed statistically significant improvement from baseline in immediate memory (P = 0.005) and delayed memory (P = 0.008) at 3 months, but these changes were not sustained at month 12 (both P > 0.75 versus baseline). There were no significant differences from baseline in the index scores for language, visuospatial/constructional abilities, and attention at 3 months or at 12 months (all P > 0.02).
Figure 2.
RBANS scores and paired tests. This figure shows longitudinal summary data for persons having RBANS scores at all 3 measurement points. The general population norm ± 1 SD, which pertains to all metrics, is indicated in green by the solid and dashed reference lines, respectively. The solid red line indicates the threshold below which scores are considered impaired. RBANS, Repeatable Battery for Assessment of Neuropsychological Status; SD, standard deviation.
Findings from the linear mixed effects models aligned with the paired, nonparametric analyses and are outlined in Table 3, with time-specific contrasts referenced to pretransplant baseline scores. There were no significant differences from baseline in RBANS total scores at 3 or 12 months posttransplant (both P > 0.06). On average, immediate memory index scores improved by 8.8 points at 3 months (P = 0.003), but this was not sustained at month 12 (P = 0.89). No other RBANS index scores demonstrated statistically robust effects in comparison to baseline (all P ≥ 0.013).
Table 3.
Mixed Effect Models
| 3 Mos (ref. Baseline) |
12 Mos (ref. Baseline) |
Intercept |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | P-value | Estimate | 95% CI | P-value | Estimate | 95% CI | P-value | |
| RBANS (n = 86 datapoints, 30 persons) | |||||||||
| Total | 2.7 | -0.2, 5.5 | 0.065 | -0.7 | -3.4, 2.1 | 0.632 | 84.8 | 79.7, 89.9 | <0.001 |
| Immediate memory | 8.8 | 3.1, 14.4 | 0.003 | -0.4 | -5.9, 5.1 | 0.893 | 90.8 | 83.8, 97.9 | <0.001 |
| Visuospatial/constructional | 1.4 | -2.9, 5.7 | 0.522 | 1.7 | -2.6, 5.9 | 0.432 | 75.0 | 71.0, 78.9 | <0.001 |
| Language | -1.1 | -5.5, 3.3 | 0.618 | -5.5 | -9.8, -1.2 | 0.013 | 91.0 | 86.0, 96.0 | <0.001 |
| Attention | 0.0 | -4.1, 4.2 | 0.985 | 2.9 | -1.2, 6.9 | 0.159 | 93.8 | 86.7, 100.9 | <0.001 |
| Delayed memory | 4.1 | 0.8, 7.5 | 0.017 | -0.7 | -4.0, 2.5 | 0.656 | 88.9 | 84.8, 93.0 | <0.001 |
| TRAIL (n = 94 datapoints, 32 persons) | |||||||||
| TRAIL A | 4.4 | 1.0, 7.9 | 0.012 | 5.6 | 2.1, 9.0 | 0.002 | 40.4 | 36.5, 44.3 | <0.001 |
| TRAIL B | 7.6 | 4.0, 11.2 | <0.001 | 5.7 | 2.1, 9.2 | 0.002 | 41.0 | 36.9, 45.1 | <0.001 |
Abbreviations: CI, confidence interval; RBANS, Repeatable Battery for Assessment of Neuropsychological Status; TRAIL A, Trail Making Test A; TRAIL B, Trail Making Test B.
Executive Function Posttransplant (TRAIL A & B)
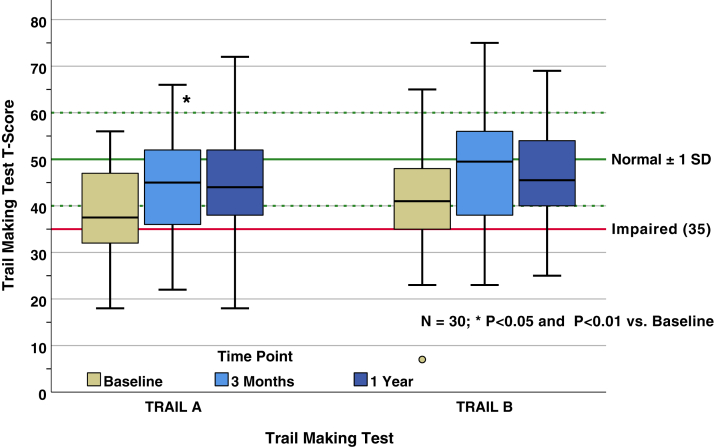
Using all available data, TRAIL A scores averaged 44.7 ± 11 at 3 months and 45.6 ± 11.9 at 12 months. Average TRAIL B scores were 48.4 ± 12.2 at 3 months and 46.6 ± 11.3 at 12 months. Thirty persons completed TRAIL A and B tests at all 3 time points (Fig 3). Paired comparisons of median scores for the TRAIL A test demonstrated no significant difference from baseline at 3 months posttransplant (P = 0.02); however, there was a significant improvement at 12 months posttransplant compared to baseline (P = 0.002). Paired comparisons demonstrated that TRAIL B scores improved from baseline at 3 months posttransplant (P = 0.001) and remained improved at 12 months posttransplant (P < 0.001).
Figure 3.
TRAIL A and TRAIL B scores and paired tests. This figure shows longitudinal summary data for persons having TRAIL A and TRAIL B scores at all 3 measurement points. The general population norm ± 1 SD, which pertains to both metrics, is indicated in green by the solid and dashed reference lines, respectively. The solid red line indicates the threshold below which scores are considered impaired. SD, standard deviation; TRAIL A and B, Trail Making Test Parts A and B.
Linear mixed effects model analyses for TRAIL A and TRAIL B tests aligned with the paired, nonparametric analyses and are outlined in Table 3. At 3 months, scores on the TRAIL A test were not significantly different from baseline (P = 0.01), but TRAIL B scores had improved by approximately 7.6 points (P < 0.001). There was a statistically significant improvement from baseline, of approximately 6 points, in TRAIL A and TRAIL B scores at 12 months posttransplant (both P = 0.002).
Discussion
In this study we found an improvement in attention and executive function 12 months posttransplant based on TRAIL A and TRAIL B tests, respectively. Improvements on these measures were not only statistically significant but clinically significant as well, reflecting changes of over half a SD, likely significant enough to have actual impacts on a patient’s daily life. There was not significant improvement in global cognition, as reflected by the RBANS. However, it is worth noting that in our cohort, the average total RBANS score at baseline was comparable to patients with mild cognitive impairment, further reinforcing the cognitive deficits seen in this population.26
The current literature is inconclusive with regard to the impact of transplantation on cognitive function, with studies reporting highly variable findings. In contrast to our findings, prior studies do not support improvement in executive function, assessed by TRAIL A and/or TRAIL B, at 6-12 months posttransplant in comparison to patients with kidney failure treated by hemodialysis.12,16,19,20 Other studies do suggest improvement in executive function based on various measurements in addition to TRAIL A and TRAIL B such as P300 latencies and components of the Complex Reactionmeter Drenovac series.14,17,18,21,22 Consistent with our results, most studies have not shown clear improvement in global cognition 3-19 months posttransplant. 12,17,18,20,21 The largest study, involving 665 kidney transplant patients, did not demonstrate a clinically significant improvement in global cognition based on the 3MS assessment.23 This finding could be because of the mean scores of the transplant recipients in these studies not meeting criteria for cognitive impairment at baseline, as they tested global cognitive function with either Mini-Mental State Examination or 3MS tests, which are limited in their ability to detect mild decrements. These screening tests may fail to detect improvement in cognitive function rather than more comprehensive assessments. Our study has the benefit of utilizing a more comprehensive assessment of global cognitive function and did identify mild cognitive impairment.
Overall, there is a wide variability in the types of cognitive assessments used and there are conflicting results in which cognitive domains improve posttransplant. A meta-analysis of the available studies found that transplant recipients had improvement in general cognitive status, information and motor speed, spatial reasoning, verbal memory, and visual memory in comparison to their pretransplant scores.15 When comparing transplant recipients to patients with kidney failure treated by hemodialysis, transplant patients scored better in the same 5 domains and had no difference in attention, executive function, language, and verbal fluency.15 This meta-analysis was limited because of a small number of included studies, small sample sizes within each study, and difficulty comparing the different cognitive tests used across studies.
Our findings support that there is an improvement in executive function, in particular, set shifting, by 12 months posttransplant. As a practical matter, such improvement is important and clinically relevant in light of the fact that executive dysfunction alters patients’ ability to live independently, manage medication, handle personal finances, and perform job-related tasks.28, 29, 30, 31 This improvement could be because of a specific mechanism—namely, patients no longer being exposed to intradialytic hypotension. Prior studies have indicated a high prevalence of cognitive impairment in the population of patients with kidney failure treated by hemodialysis,1, 2, 3, 4, 5, 6 particularly in areas of orientation and attention, memory, and executive function, when compared to the general population. Dialysis initiation has been associated with worsening cognitive function, particularly a loss in executive function, with a continued decline over time.8,32 The observed decrease in executive function and attention deficits, as well as frontal lobe atrophy, have been associated with intradialytic hypotension.7 It is possible that the baseline executive function scores in our study were affected by the variability of timing of dialysis before cognitive testing. One study showed significant improvement in executive function in dialysis-dependent patients after 1 dialysis session.33
The observed improvement in executive function is supported by studies on functional changes after kidney transplant.18,34,35 Gupta et al18 demonstrated improvement in white matter integrity in the tracts involved with memory and executive function 3 months posttransplant, corresponding with improvement in cognitive measures. Chen et al35 showed improvement in psychomotor speed, attention and visual memory at 6 months posttransplant with improved functional connectivity in several resting-state subnetworks, including the central executive network. The improved connectivity seen in the central executive function network was no longer different from functional activity when compared to controls at 6 months. There has also been evidence of changes in cerebral blood flow, neurochemical concentrations, and white matter integrity in kidney transplant recipients that suggests some reversibility to brain abnormalities seen in kidney failure patients treated by hemodialysis.18,35, 36, 37
Previous findings of improvement in the initial transplant period and then sustained at 1 year are consistent with aspects of our findings. The slight increase in 3MS scores seen in Chu et al23 occurred at 3 months, and then cognitive function remained stable in nonfrail patients over the 4-year follow-up period. This finding of initial improvement at 3 months with sustained improvement at 12 months was also shown by Gupta et al.18 This persistent cognitive impairment in kidney transplant recipients could be due to neurotoxicity secondary to immunosuppressive medications.7
There are several strengths to our study. Compared to other studies of similar populations, we moved beyond the use of simple screening tools and incorporated a battery, the RBANS, coupled with brief but sensitive measures of attention and executive function (TRAIL A and TRAIL B). In doing so, we created a comprehensive set of cognitive outcomes allowing us to identify even subtle deficits across a wide range of abilities. Second, despite being a small sample size, we did have a good distribution of patients on hemodialysis, peritoneal dialysis, or patients who had a preemptive transplant. We also did not exclude patients with diabetes or hypertension, allowing our findings to be more generalizable to transplant patients.
Our study does have limitations. It is a small sample size and was conducted at a single center. The majority of our study participants completed some college education, and this could limit the generalizability to the general population. We did not compare our findings to a cohort of patients with kidney failure who remained on dialysis and rather used the reported adjusted population means of the assessments for comparison. Although the RBANS assesses 5 domains over the course of approximately 45 minutes (when used with medical populations) and is significantly more comprehensive than simple screening tests such as the Montreal Cognitive Assessment or the 3MS, it is not as definitive or broad in scope as a clinical neuropsychological battery. Although there are obvious advantages to conducting a lengthier assessment, we concluded that this was likely not feasible because of the nature of the postoperative evaluation for recipients of deceased donor kidney transplants; therefore, longer evaluation may not be feasible in this setting. The performance on the neurocognitive tests could have been affected because of the stress of being called in for possible transplantation as well as not being well rested at the time of evaluation as many patients travel long distances.
In conclusion, utilizing relatively comprehensive cognitive assessments in a small number of patients, we found improvements in attention and executive function in the first posttransplant year as measured by TRAIL A and B. However, there was no significant improvement in global cognition as measured by the RBANS. Although there may be a natural trend toward improvement in domains like attention and executive functioning after transplant, our study serves as a reminder of the importance of fostering enhanced ability in these domains. Although our investigation was not interventional, future research may include the integration of approaches like cognitive rehabilitation or computerized brain training, both of which may be effective in improving neuropsychological ability and functioning.38 Cognitive impairment is also a barrier for patients to be listed for a kidney transplant.39 Transplant teams should be mindful that some domains of cognitive function improve after transplantation, and as such, cognitive impairment should not necessarily be used as an exclusion criteria for transplantation. Domain-based assessments as performed in this study will be useful in identifying patients who will benefit from a kidney transplant despite cognitive impairment pretransplant.
Additional research is warranted to further assess the impact of transplant and the durability of any cognitive improvement seen in the initial posttransplant period. It would be beneficial to further expand on our findings by including other validated assessments of the different subdomains of executive function, as this could have important implications for transplant recipients’ posttransplant care. The majority of studies have had up to 1 year as the outcome time point, and more longitudinal studies would help to strengthen our current understanding on the long-term effect transplantation can have on cognitive function.
Article Information
Authors’ Full Names and Academic Degrees
Laura A. Binari, MD, Amy L. Kiehl, MA, LPC-MHSP, James C. Jackson, PsyD, Irene D. Feurer, PhD, Scott A. Rega, MS, MMHC, Tareq M. Altuhaifi, MBBS, Rita P. Yankyera, FNP-BC, Malia Reed, RN, Mohammed Sika, PhD, Julie Van, BA, Erin M. Collar, MPH, Rachel C. Forbes, MD, MBA6,∗, and Beatrice P. Concepcion, MD, MS1,∗
Authors’ Contributions
Research area and study design: LAB, ALK, JCJ, RCF, BPC; Data acquisition: LAB, ALK, TMA, RPY, MR, MS, JV, EMC, RCF, BPC; Data analysis and interpretation: ALK, JCJ, IDF, SAR, JV, EMC, BPC; Statistical analysis: IDF, SAR, BPC; Supervision and mentorship: JCJ, IDF, RCF, BPC. RCF and BPC contributed equally to this work. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Grant funding for this study was provided by Dialysis Clinic, Inc. The funders of this study did not have a role in study design; collection, analysis, and interpretation of data; writing the report; and the decision to submit the report for publication.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
This project was also supported by award No. UL1TR000445 from NCATS/NIH. Its contents are solely the responsibility of the authors and do not necessarily represent official views of the National Center for Advancing Translational Sciences or the National Institutes of Health.
Peer Review
Received April 9, 2022. Evaluated by 1 external peer reviewer, with direct editorial input from the Statistical Editor, an Associate Editor, and the Editor-in-Chief. Accepted in revised form August 28, 2022.
Footnotes
Complete author and article information provided before references.
References
- 1.Murray A.M., Tupper D.E., Knopman D.S., et al. Cognitive impairment in hemodialysis patients is common. Neurology. 2006;67(2):216–223. doi: 10.1212/01.wnl.0000225182.15532.40. [DOI] [PubMed] [Google Scholar]
- 2.van Zwieten A., Wong G., Ruospo M., et al. Prevalence and patterns of cognitive impairment in adult hemodialysis patients: the COGNITIVE-HD study. Nephrol Dial Transplant. 2018;33(7):1197–1206. doi: 10.1093/ndt/gfx314. [DOI] [PubMed] [Google Scholar]
- 3.Vanderlinden J.A., Ross-White A., Holden R., Shamseddin M.K., Day A., Boyd J.G. Quantifying cognitive dysfunction across the spectrum of end-stage kidney disease: A systematic review and meta-analysis. Nephrology (Carlton) 2019;24(1):5–16. doi: 10.1111/nep.13448. [DOI] [PubMed] [Google Scholar]
- 4.Shea Y.F., Lee M.C., Mok M.M., Chan F.H., Chan T.M. Prevalence of cognitive impairment among peritoneal dialysis patients: a systematic review and meta-analysis. Clin Exp Nephrol. 2019;23(10):1221–1234. doi: 10.1007/s10157-019-01762-1. [DOI] [PubMed] [Google Scholar]
- 5.Kalirao P., Pederson S., Foley R.N., et al. Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis. 2011;57(4):612–620. doi: 10.1053/j.ajkd.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger I., Wu S., Masson P., et al. Cognition in chronic kidney disease: a systematic review and meta-analysis. BMC Med. 2016;14(1):206. doi: 10.1186/s12916-016-0745-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Sandwijk M.S., Ten Berge I.J., Majoie C.B., et al. Cognitive changes in chronic kidney disease and after transplantation. Transplantation. 2016;100(4):734–742. doi: 10.1097/TP.0000000000000968. [DOI] [PubMed] [Google Scholar]
- 8.Kurella Tamura M., Vittinghoff E., Hsu C.Y., et al. Loss of executive function after dialysis initiation in adults with chronic kidney disease. Kidney Int. 2017;91(4):948–953. doi: 10.1016/j.kint.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas A.G., Ruck J.M., Shaffer A.A., et al. Kidney transplant outcomes in recipients with cognitive impairment: a national registry and prospective cohort study. Transplantation. 2019;103(7):1504–1513. doi: 10.1097/TP.0000000000002431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta A., Mahnken J.D., Johnson D.K., et al. Prevalence and correlates of cognitive impairment in kidney transplant recipients. BMC Nephrol. 2017;18(1):158. doi: 10.1186/s12882-017-0570-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu N.M., Shi Z., Haugen C.E., et al. Cognitive function, access to kidney transplantation, and waitlist mortality among kidney transplant candidates with or without diabetes. Am J Kidney Dis. 2020;76(1):72–81. doi: 10.1053/j.ajkd.2019.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma A., Yabes J., Al Mawed S., et al. Impact of cognitive function change on mortality in renal transplant and end-stage renal disease patients. Am J Nephrol. 2016;44(6):462–472. doi: 10.1159/000451059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaya Y., Ozturkeri O.A., Benli U.S., Colak T. Evaluation of the cognitive functions in patients with chronic renal failure before and after renal transplantation. Acta Neurol Belg. 2013;113(2):147–155. doi: 10.1007/s13760-012-0139-5. [DOI] [PubMed] [Google Scholar]
- 14.Radić J., Ljutić D., Radić M., Kovačić V., Dodig-Ćurković K., Šain M. Kidney transplantation improves cognitive and psychomotor functions in adult hemodialysis patients. Am J Nephrol. 2011;34(5):399–406. doi: 10.1159/000330849. [DOI] [PubMed] [Google Scholar]
- 15.Joshee P., Wood A.G., Wood E.R., Grunfeld E.A. Meta-analysis of cognitive functioning in patients following kidney transplantation. Nephrol Dial Transplant. 2018;33(7):1268–1277. doi: 10.1093/ndt/gfx240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gelb S., Shapiro R.J., Hill A., Thornton W.L. Cognitive outcome following kidney transplantation. Nephrol Dial Transplant. 2008;23(3):1032–1038. doi: 10.1093/ndt/gfm659. [DOI] [PubMed] [Google Scholar]
- 17.Kramer L., Madl C., Stockenhuber F., et al. Beneficial effect of renal transplantation on cognitive brain function. Kidney Int. 1996;49(3):833–838. doi: 10.1038/ki.1996.115. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A., Lepping R.J., Yu A.S., et al. Cognitive function and white matter changes associated with renal transplantation. Am J Nephrol. 2016;43(1):50–57. doi: 10.1159/000444334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griva K., Thompson D., Jayasena D., Davenport A., Harrison M., Newman S.P. Cognitive functioning pre- to post-kidney transplantation—a prospective study. Nephrol Dial Transplant. 2006;21(11):3275–3282. doi: 10.1093/ndt/gfl385. [DOI] [PubMed] [Google Scholar]
- 20.Dixon B.S., VanBuren J.M., Rodrigue J.R., et al. Cognitive changes associated with switching to frequent nocturnal hemodialysis or renal transplantation. BMC Nephrol. 2016;17:12. doi: 10.1186/s12882-016-0223-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harciarek M., Biedunkiewicz B., Lichodziejewska-Niemierko M., Dębska-Ślizień A., Rutkowski B. Continuous cognitive improvement 1 year following successful kidney transplant. Kidney Int. 2011;79(12):1353–1360. doi: 10.1038/ki.2011.40. [DOI] [PubMed] [Google Scholar]
- 22.Posselt J., Harbeck B., Rahvar A.H., Kropp P., Haas C.S. Improved cognitive function after kidney transplantation compared to hemodialysis. Ther Apher Dial. 2021;25(6):931–938. doi: 10.1111/1744-9987.13625. [DOI] [PubMed] [Google Scholar]
- 23.Chu N.M., Gross A.L., Shaffer A.A., et al. Frailty and changes in cognitive function after kidney transplantation. J Am Soc Nephrol. 2019;30(2):336–345. doi: 10.1681/ASN.2018070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandharipande P.P., Girard T.D., Jackson J.C., et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heaton R.K., Miller S.M., Taylor M.J., Grant I. Psychological Assessment Resources; 2004. Revised Comprehensive Norms for an Expanded Halstead-Reitan Battery. [Google Scholar]
- 28.Boyle P.A., Cohen R.A., Paul R., Moser D., Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. Int J Geriatr Psychiatry. 2002;17(2):164–169. doi: 10.1002/gps.539. [DOI] [PubMed] [Google Scholar]
- 29.Cahn-Weiner D.A., Boyle P.A., Malloy P.F. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9(3):187–191. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 30.Boyle P.A., Paul R.H., Moser D.J., Cohen R.A. Executive impairments predict functional declines in vascular dementia. Clin Neuropsychol. 2004;18(1):75–82. doi: 10.1080/13854040490507172. [DOI] [PubMed] [Google Scholar]
- 31.Boyle P.A., Malloy P.F., Salloway S., Cahn-Weiner D.A., Cohen R., Cummings J.L. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–221. [PubMed] [Google Scholar]
- 32.Drew D.A., Weiner D.E., Tighiouart H., et al. Cognitive decline and its risk factors in prevalent hemodialysis patients. Am J Kidney Dis. 2017;69(6):780–787. doi: 10.1053/j.ajkd.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schneider S.M., Malecki A.K., Müller K., et al. Effect of a single dialysis session on cognitive function in CKD5D patients: a prospective clinical study. Nephrol Dial Transplant. 2015;30(9):1551–1559. doi: 10.1093/ndt/gfv213. [DOI] [PubMed] [Google Scholar]
- 34.Findlay M.D., Dawson J., Dickie D.A., et al. Investigating the relationship between cerebral blood flow and cognitive function in hemodialysis patients. J Am Soc Nephrol. 2019;30(1):147–158. doi: 10.1681/ASN.2018050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen H.J., Wen J., Qi R., et al. Re-establishing brain networks in patients with ESRD after successful kidney transplantation. Clin J Am Soc Nephrol. 2018;13(1):109–117. doi: 10.2215/CJN.00420117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lepping R.J., Montgomery R.N., Sharma P., et al. Normalization of cerebral blood flow, neurochemicals, and white matter integrity after kidney transplantation. J Am Soc Nephrol. 2021;32(1):177–187. doi: 10.1681/ASN.2020050584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Sandwijk M.S., Ten Berge I.J.M., Caan M.W.A., et al. Cognitive improvement after kidney transplantation is associated with structural and functional changes on MRI. Transplant Direct. 2020;6(3) doi: 10.1097/TXD.0000000000000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anguera J.A., Boccanfuso J., Rintoul J.L., et al. Video game training enhances cognitive control in older adults. Nature. 2013;501(7465):97–101. doi: 10.1038/nature12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gupta A., Montgomery R.N., Bedros V., et al. Subclinical cognitive impairment and listing for kidney transplantation. Clin J Am Soc Nephrol. 2019;14(4):567–575. doi: 10.2215/CJN.11010918. [DOI] [PMC free article] [PubMed] [Google Scholar]