Abstract
Dyslipidemia is a common feature of type 2 diabetes mellitus and is characterised by elevated triglyceride, decreased HDL cholesterol, and increased small dense LDL cholesterol levels. The underlying causes appears to be associated with insulin resistance, increased free fatty acid reflux, and low-grade inflammation, resulting in increased hepatic lipogenesis, and altered lipoprotein metabolism. Improved glycaemic control has been shown to have a positive effect on lipoprotein levels in diabetics. This can be achieved through medications/therapeutics and life style changes. Several classes of pharmacologic agents are currently in use to treat dyslipidemia. However, they may have dangerous long-term side effects, including an increased risk of liver dysfunction, weight gain, and cardiovascular diseases. Therefore, stronger alternatives with fewer side effects are required to reduce the diabetes associated complications. Many secondary plant metabolites have been shown to improve glucose homeostasis and lower lipid levels. Aloe vera and its constituents have long been used in a traditional medicine system for a diverse range of biological activities, including hypoglycaemic, antioxidant, anticarcinogenic, anti-inflammatory, and wound healing effects through various mechanisms and they have been covered well in literature. However, studies on the potential role of Aloe vera in the treatment of diabetic dyslipidemia are scanty. Therefore, in this systematic review, we focussed on the potential effect of Aloe vera and its active components in alleviating diabetic dyslipidemia, as well as their mechanism of action in pre-clinical and clinical studies.
Keywords: Aloe vera, Diabetic dyslipidemia, Blood glucose levels, Triglycerides, VLDL
Abbreviations: A.vera, Aloe vera; ASCVD, Atherosclerotic cardiovascular disease; apoB, Apolipoprotein B; BAS, Bile acid sequestrants; CKD, Chronic kidney disease; CF, Carbohydrate fraction; CETP, Cholesterol ester transport protein; FBS, Fasting blood glucose; FFA, Free fatty acid; HOMA-β, Homeostatic model assessment β-cell function; HbA1C, Glycated haemoglobin; HDL, High-density lipoprotein; HOMA-IR, Homeostatic model assessment of insulin resistance; IR, Insulin resistance; LDL-C, Low-density lipoprotein-Cholesterol; MNT, Medical nutrition therapy; PPF, Polypeptide fraction; PCSK9, Proprotein Convertase Subtilisin/Kexin Type 9 inhibitors; TG, Triglycerides; T2DM, Type 2 Diabetes mellitus; VLDL, Very low-density lipoprotein
Graphical abstract
1. Introduction
There has been an increased prevalence of type 2 diabetes mellitus and metabolic syndrome globally due to the result of easy access to energy-rich food combined with sedentary lifestyles in genetically susceptible individuals [1]. Diabetes currently affects 573 million people, and this figure is expected to rise to 643 million by 2030, posing a significant global threat [2]. One of the hallmarks of hyperglycemia is abnormal lipid and lipoprotein levels, which impairs hepatic lipogenesis and results in diabetic dyslipidemia. It is very common in type 2 diabetes (T2DM), affecting approximately 70% of patients [3]. To treat dyslipidemia, a variety of pharmacologic agents are used. According to a meta-analysis of clinical trials, statins could be used as both primary and secondary interventions in the diabetics [4]. However, the wide range of treatment interventions makes prioritization of drug therapy difficult. Also, these medications may have dangerous long-term effects including, an increased risk of liver dysfunction, weight gain, and cardiovascular disease [5,6]. Therefore, stronger alternatives with fewer side effects are a better approach to reducing diabetes complications. Many secondary plant metabolites have been found to have lipid-lowering properties as well as a better glycaemic control [7,8]. Among the several herbal preparations, A. vera and its components play a significant role in traditional medicine systems and plant-derived food. It has a wide range of biological activities, including hypoglycaemic, antioxidant, antibacterial, anti-inflammatory, and hypolipidemic properties [9,10]. Numerous studies have demonstrated that A. vera has the potential to reduce blood glucose levels by protecting pancreatic cells and/or improving insulin sensitivity. Furthermore, the lipid-lowering impacts of A. vera may be contributed to lipid catabolism or/and anabolism regulation [[11], [12], [13]]. There are review articles on the effect of A. vera on wound healing, its laxative properties, and its effects in dentistry. However, scanty reviews are available for the treatment of diabetic dyslipidemia with A. vera. Therefore, we focused on the potential effect of A. vera and its active components, which play an important role in diabetic dyslipidemia, as well as their mechanism of action in pre-clinical and clinical studies.
1.1. Aloe vera phytocomponents and their potential role in metabolic management
A.vera is a scrumptious plant that belongs to the Liliaceae family, which has over 420 species. Aloe barbadensis Miller, the most common species, is known colloquially as A. vera [14]. Several potentially active bio-constituents including flavonoids, saccharides, polyphenols, anthraquinones, chromone, phytosterols, proteins, and trace minerals have been identified (Table 1) [15]. However, much remains unknown about their mechanism of action. The rich chemical composition of the plant is influenced by a variety of factors, including location, ripening stage, climatic conditions, harvest time, and harvesting method [16].
Table 1.
| Major classes | Components | Mechanism of action |
|---|---|---|
| Anthraquinones | Aloe-emodin, aloetic acid, emodin, aloin A, aloin B, 6′-O-acetyl-aloin A, 6′-O-acetyl-aloin B, 10-hydroxyaloins A, 10-hydroxyaloins B, aloinoside A, aloinoside B, 7-hydroxyaloin A, 7-hydroxyaloin B, 7-hydroxy-8-O-methylaloin A, 7-hydroxy-8-O-methylaloin B, 6′-malonylnataloin A, 6′-malonylnataloin B, homonataloside B, elgonica dimer A, elgonica dimer B, aloindimer A, aloindimer B, aloindimer C, aloindimer D, aloe-emodin-11-O-rhamnoside, chrysophanol, emodin, physcione, aloe-emodin, nataloeemodin, aloesaponarin I, aloesaponarin II, madagascine, 3-Geranyloxyemodin, rhein | Anti-oxidant and anti-inflammatory activity |
| Saccharides | Pure mannan, acetylated mannan, acetylated glucomannan, glucogalactomannan, galactan, galactogalacturan, arabinogalactan, galactoglucoarabinomannan, pectic substance, xylan, cellulose, Veracylglucan B and veracylglucan C, glucose, galactose, mannose, and arabinose | Regulates glucose metabolism by activating glycogenesis and inhibiting gluconeogenesis. Modulates immune function |
| Flavonoids | Apigenin, luteolin, isovitexin, isoorientin, saponarin, lutonarin, kaempferol, quercetin, myricetin, quercitrin, rutin, catechin, epicatechin | Protects beta cells from oxidative damage, increasing islet cell proliferation, stimulating glucose-stimulated insulin secretion, and inhibiting the α-glucosidase enzyme |
| Phytosterols | Cycloartanol, 24-methylene-cycloartanol, lophenol, 24-methyl-lophenol, 24-ethyl-lophenol, lupeol, β-sitosterol, campesterol | Reduces serum-free fatty acid, triglyceride levels, PPARɣ levels and improved glucose homeostasis and lipid metabolism |
| Chromones | Aloesin, aloeresin E, isoaloeresin D, aloeresin D, rabaichromone, aloeresin K, aloesinol | Anti-oxidant activity |
| Polyphenols | Cinnamic acid, p-coumaric, caffeic acid, ferulic acid, sinapic acid, 3-(4-hydroxyphenyl) propanoic acid, methyl 3-(4-hydroxyphenyl) propionate, 7-demethylsiderin, feralolide, dihydrocoumarin, Aloenin A, aloenin B, p-coumaroyl aloenin, aloveroside A, feroxidin 1-(2,4-dihydroxy-6-methylphenyl) ethenone, p-anisaldehyde, salicylaldehyde, p-cresol, pyrocatechol, gentisic acid, gallic acid, vanillic acid, syringic acid, ascorbic acid | Improves insulin sensitivity, anti-oxidant property |
| Proteins | Lectins and lectin like substances, polypetide fractions | Improved glucose and lipid metabolism, Inhibits DPP-IV, reduces intestinal permeability, anti-inflammatory |
| Trace minerals | Calcium, copper, zinc, magnesium, potassium, manganese, iron, choline, chromium, phosphorous, sodium, vanadium | Insulinotropic effect |
The main component of A. vera gel is carbohydrates, particularly mannose-containing polysaccharides and their content varies depending on the age of the plant [17]. Acetylated mannan, also known as acemannan, is largely responsible for its mucilaginous properties and has been reported to have anti-diabetic properties as well as the ability to modulate immune function [18]. Recent research has shown that A. vera carbohydrate-rich fraction (AVCF) regulates glucose metabolism in diabetic rats by activating glycogenesis and inhibiting gluconeogenesis, as well as modulating the immune functions [19,20].
Polyphenols are another important phytochemical found in A. vera, known to exert their effect through their antioxidant properties. Over the last decade, their health effects have been extensively researched, and they are thought to be the most active constituents in alleviating metabolic syndrome in vitro and in vivo [21]. Administration of a polyphenol-rich extract from A. vera containing 181.7 mg/g aloin and 3.6 mg/g Aloe-emodin for a period of 28 days improves insulin sensitivity in experimentally induced diabetic mice [11], implying that, in addition to antioxidative properties, their biological activity is the result of various other complex mechanisms.
Flavonoids have been linked to a variety of health benefits. This is due to their antioxidative and anti-inflammatory properties, in addition to their ability to regulate key cellular enzyme functions [22]. Direct scavenging of free radicals is one way flavonoids protect against free radical damage [23]. With the confirmation of an increasing number of plant flavonoids with anti-diabetic potential, the mechanisms of action of these bioactive constituents are being meticulously elucidated. They play a protect beta cells from oxidative damage by increasing islet cell proliferation, stimulating glucose-stimulated insulin secretion, and inhibiting the α-glucosidase enzyme [24].
Sterols found in the leaf and the gel of the Aloe plant appear to influence islet functions. Five phytosterols were isolated and identified from A. vera gel. At a dose of 1 μg/day, they were able to reduce FBG and HbA1c levels in db/db mice when compared to the control group, implying that sterols from A. vera could be used to treat type 2 diabetes [25]. Furthermore, phytosterols (lophenol and cycloartanol) isolated from A. vera reduced serum-free fatty acid, triglyceride levels, PPARɣ levels and improved hyperglycemia and hyperlipidaemia, as well as visceral fat accumulation in Zucker diabetic fatty rats [26]. Phytosterols may have hypolipidemic effects because they are not prevalently absorbed from the intestine and can bind to cholesterol and prevent its absorption. A. vera gel containing sterols was known to be possibly involved in brown adipose tissue (BAT) activation in high-fat diet treated mice, and the treated group had increased expression of Ucp1, Adrb3, and Cidea in comparison to the untreated group, implying that anti-obesity potential of sterols from A. vera in diet-induced model is partially contributed by BAT adipogenesis [27].
Anthraquinones are anthracene derivatives of the quinone group that are typically found in the epidermis of the plant. They are well-known for their laxative properties. They are inadequately absorbed in the small intestine and are broken down in the large intestine into active metabolites such as aloe-emodin and aloe-emodin-9-anthrone. [17]. The primary anthraquinones found in A. vera are aloe-emodin and aloin. The concentration of these components determines the antioxidant property of A. vera [28]. At higher concentrations, aloe-emodin acts as an antioxidant, and at lower concentrations, it acts as a prooxidant [29] and its anti-inflammatory effect was comparable to that of kaempferol and quercetin [30].
In comparison to other constituents, little is known about protein or glycoprotein; however, lectins are a type of glycoprotein found in A. vera that differs in the connection between oligosaccharide groups and polypeptide chains [17]. Recent research indicated that the peptide/polypeptide-rich fraction (PPF) inhibited the DPP-IV enzyme in a dose-dependent manner. Furthermore, the fraction was able to restore FBG levels and important enzymes involved in restoring glucose homeostasis and lipid metabolism, implying that PPF from A. vera could be used as alternative for diabetes mellitus treatment due to its ability to decrease intestinal permeability [31], improve lipid profile and reduce pro-inflammatory cytokines [9].
Aloesin, aloeresin A, isoaloerersin D and aloesinol are the most significant chromones isolated and identified from A. vera and approximately 29 chromone derivatives have been identified, but the absolute configuration has not yet been determined [15]. Usually, they are known to have antioxidant activity, however, the concentrations of chromones determine whether they act as prooxidants or antioxidants [32].
Aloe minerals are reported to have insulinotropic effects, playing a direct or indirect role in insulin secretion in a synergistic way. Earlier studies have reported that the presence of these trace minerals plays a significant role in the anti-diabetic potential of A. vera. Zinc is an important mineral that acts as a cofactor and improves insulin effectiveness. The presence of zinc in A. vera can modulate insulin activity targets [33]. Furthermore, the mineral content of A. vera has been shown to have hypoglycaemic properties in streptozotocin-induced diabetic rats, where potassium functions as an insulin secretagogue, vanadium elicits glucose levels, copper is associated in insulin binding, and chromium is important in carbohydrate metabolism [34]. To date, a great number of research works and reviews have addressed the bio-efficacy of A. vera gel extract and its components on blood sugar levels and lipid profile in vitro, in vivo and in clinical trials; however, the diversity in its composition and bioavailability varies in its beneficial effect on different signalling pathways.
2. Methods used for literature collection
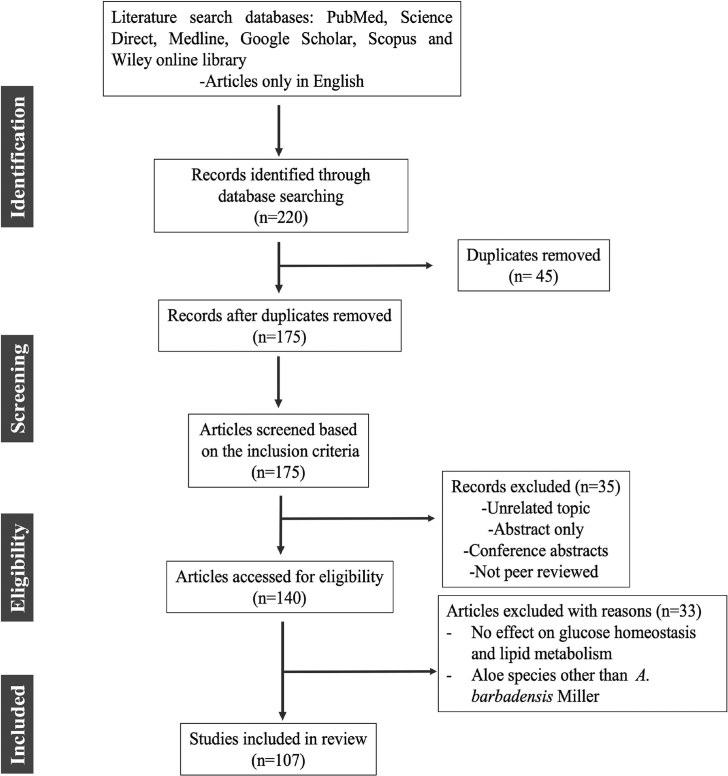
A literature search for this review was conducted using a variety of reputable and authentic databases, including Google Scholar, Medline, PubMed, Science Direct, Scopus and Wiley online library. The major keywords used in various combinations included: diabetic dyslipidemia, therapeutic strategies, Aloe vera, anti-diabetic activity, hypoglycaemic effects, hypolipidemic activity and phytocomponents. The full-length articles from peer-reviewed journals related to the subject of interest, published between (1983–2021) and written in English were included in the review process. Given the large volume of scientific research on the anti-diabetic potential of A. vera, the search has been refined using the following inclusion and exclusion criteria: in vivo approaches that describe the effect of A. vera on glucose-lowering effects via restoration of lipoprotein metabolism and glucose homeostasis. Case-control studies and randomized or controlled clinical trials with therapeutic evidence of A. vera in the management of hyperglycemia and hyperlipidaemia were included in this review. There were no restrictions on the sample size, study design, or method of exposure. Duplicated studies, conference abstracts, letters, and guidance articles, which were only available as abstracts but not full texts, as well as those did not fall within the purview of the search were excluded. Aloe species other than A. barbadensis Miller were also excluded from this review. Out of the 220 articles, 107 articles were included in this review by following inclusion and exclusion criteria as described in Fig. 1.
Fig. 1.
Methodology for search.
2.1. Diabetic dyslipidemia
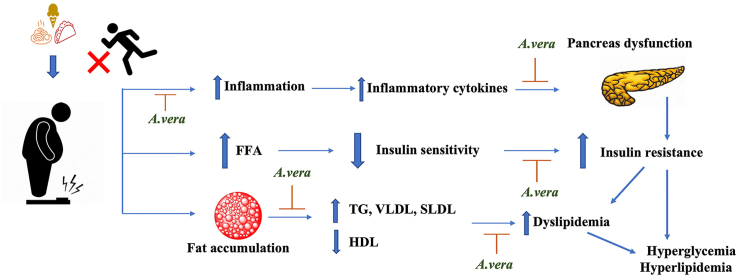
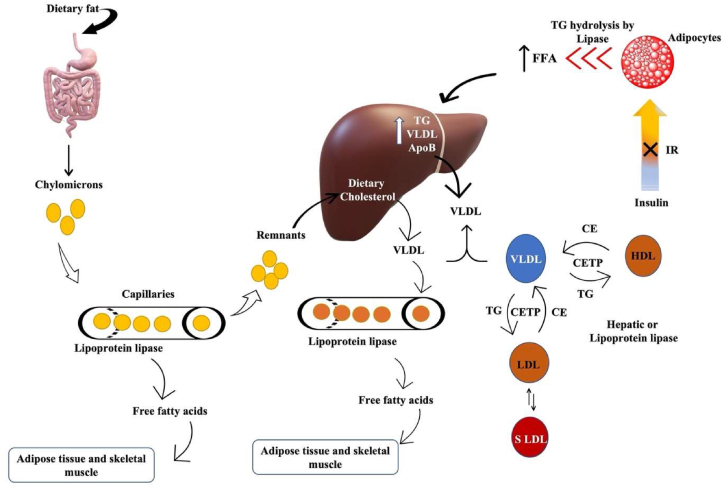
Dyslipidemia is a common feature of type 2 diabetes mellitus and is characterised by elevated triglyceride levels, decreased HDL cholesterol, and increased small dense LDL cholesterol levels. The reference values for diagnosis of dyslipidemia in adults over the age of 20 [37] are mentioned in Table 2. Dyslipidemia appears to be caused by an insulin-resistant state itself rather than by high insulin levels or obesity [38]. Furthermore, these modifications are linked to increased FFA reflux as a result of the insulin resistance [39]. Insulin deficiency or resistance stimulates intracellular hormone-sensitive lipase, increasing FA release from triglycerides stored in more metabolically active centrally distributed adipose tissue [40]. Excess FFA accumulate in the liver, which has at least three options to deal with them: 1) β-oxidation, 2) storage or 3) export via VLDL (TG-rich) particles Fig. 2. The intestine produces chylomicrons after food consumption, which are high in TG; however, chylomicrons should dissipate within a few hours and should not be detected in a fasting blood sample. These TG-rich lipoproteins (VLDL particles and chylomicrons) work together to deliver fatty acids to tissues such as muscle and adipose tissue Fig. 2 [41]. Insulin resistance, as previously stated, increases hepatic triglyceride production, which is linked to increased apoB secretion. Furthermore, the normal inhibitory effect of insulin on hepatic apoB production and triglyceride secretion in VLDL is lost, resulting in a larger and more triglyceride-rich VLDL secreted [42]. Lipoprotein lipase regulates the rate at which triglycerides are removed from circulation. This lipoprotein lipase, unlike intracellular hormone-sensitive lipase, may be downregulated when there is either insulin deficiency or insulin resistance [43], contributing to post-prandial lipemia [44]. As a result, hypertriglyceridemia has been identified as the impaired blood lipid component most commonly associated with obesity and insulin resistance, which is caused by the liver producing excess VLDL particles, slow clearance from circulation, or both. Furthermore, chylomicrons contribute to hypertriglyceridemia if they are not effectively cleared from the blood after a meal [45].
Table 2.
Reference values for diagnosis of dyslipidemia in adults over the age of 20 [37].
| Lipids | Values | Level |
|---|---|---|
| Triglycerides | <150 | Ideal |
| 150–200 | Borderline | |
| 201–499 | High | |
| ≥500 | Very High | |
| LDL-Cholesterol | <100 | Ideal |
| 100–129 | Desirable | |
| 130–159 | Borderline | |
| ≥190 | Very High | |
| HDL-Cholesterol | <40 | Low |
| >60 | High | |
| <150 | Ideal |
Fig. 2.
Diabetic dyslipidemia: Insulin resistance increases TG hydrolysis from adipocytes and increases hepatic triglyceride production and secretion of VLDL & apolipoprotein B. Also promotes lipid exchange via cholesterol ester transport protein resulting in triglyceride-rich HDL particles which are depleted in cholesterol. Similar lipid exchange results in an increase in small dense LDL-cholesterol particles. Chylomicrons are formed by the intestine after consuming food from dietary fats and are cleared within a few hours. Remnants from chylomicrons are transported back to the liver for VLDL production.
Another feature of dyslipidemia is low HDL-C and an increase in the production of small, LDL-C levels Fig. 2. Although the liver produces HDL particles, a significant portion of them are formed from remnant TG-rich lipoproteins particles as they are metabolized. This metabolism is impaired in diabetes, reducing HDL-C production from this source [46]. Furthermore, CETP transports cholesterol esters away from the HDL particles in exchange for TG from VLDL particles and this will likely result in triglyceride-rich, cholesterol-depleted HDL particles. This lowers HDL-C in the blood. Similarly, lipid exchange by CETP transports cholesterol away from LDL particles resulting in the increased accumulation of small dense LDL-C particles. These changes, when combined, steer the body in an unfavourable direction [47]. Additionally, in diabetic dyslipidemia, inflammatory markers increase insulin resistance and therefore increase the dyslipidemia [48]. Improved blood glucose levels generally have a positive effect on lipoprotein levels in diabetes, with lower cholesterol and triglyceride levels due to decreased circulating VLDL and increased catabolism of LDL due to reduced glycation and up-regulation of LDL receptors [49]. Thus, altered plasma lipid levels in diabetics are one of the key factors that can be addressed for intervention.
2.2. Therapeutic strategies
There are two types of treatment for diabetic dyslipidemia: non-pharmacological and pharmacological. Non-pharmacological treatment options include weight loss, MNT, and physical activity. While pharmacological therapies include drugs such as statins, niacin, BAS, cholesterol absorption inhibitors, PCSK9 inhibitors, omega-3 fatty acids, fibrates, etc. Table 3[39,50]. Due to the long-term side effects associated with these pharmacological agents, there is a need for an alternative therapy that is both safe and cost-effective for the treatment of diabetic dyslipidemia.
Table 3.
Pharmacologic agents for treating dyslipidaemias
| Drug class | Mechanism of action | Clinical effectiveness | Adverse impacts | Reference |
|---|---|---|---|---|
| Statins | HMG coenzyme A reductase Inhibition | Very effective | Myalgia, myositis, rhabdomyolysis, elevation in liver enzymes | [51] |
| Ezetimibe | Reduced intestinal cholesterol absorption by binding to C1-like 1 protein | Moderately effective, Safe in addition to statin therapy | Nasopharyngitis, diarrhea, upper respiratory tract infection | [52] |
| Inhibitors of PCSK9 | Inhibition of PCSK9 | Extremely effective in combination with statin therapy | Injection site reaction includes itching, swelling, erythema, and pain | [53] |
| BAS | Prevent reabsorption of bile acids by binding them in the small intestine | Moderately effective, safe in addition to statin therapy, not recommended if triglycerides are >400 mg/dL | Constipation, bloating, abdominal pain, drug malabsorption | [54] |
| Nicotinic acid | Lowers LDL 5–25%, TG 20–50%, and small dense LDL. Increases HDL 15–35% | Clinical efficacy and safety uncertain | Hot flashes, hyperglycemia, hyperuricemia, hepatotoxicity | [55] |
| Fibrates | Activates PPAR-α and lowers triglycerides by 30–50%. Increases HDL | Moderately effective but should be used with prudence in patients with CKD | Dyspepsia, gallstones, hepatotoxicity, myopathy | [56] |
| Omega-3 fatty acid | Lowers TG | Moderately effective, could be used in conjugation with statins in patients with CVD and increased TG | Gastrointestinal disturbances | [57] |
2.3. Plausible role of Aloe vera in adipose tissue, liver, and muscle
Obesity and impaired insulin sensitivity in targeted tissues, primarily the liver, muscle and adipose tissue, remain major risk factors for T2D progression. Insulin resistance impairs glucose disposal, causing an increase in endogenous insulin production to compensate. This leads to weight gain, which perpetuates insulin resistance.
This vicious cycle continues until pancreatic beta-cell activity can no longer meet the insulin demand adequately created by insulin resistance, resulting in impaired glucose homeostasis [58]. The metabolic repercussions of insulin resistance include dyslipidemia, visceral adiposity, elevated oxidative stress and inflammatory markers. Impaired carbohydrate utilisation accelerates lipolysis, resulting in excess triglyceride synthesis in the liver and lipid accumulation [59]. Numerous articles discuss the relationship between obesity, dyslipidemia, and diabetes progression. It is beyond the scope of this review to go into detail about very diverse mechanisms and pathways through which progression occurs. Readers are encouraged to follow some of the publications [60,61]. Here, the plausible role of A. vera in adipose tissue, liver and muscle is discussed further in terms of improving insulin sensitivity and dyslipidemia.
2.4. Adipose tissue
Obesity is regarded as a major contributor to metabolic disorders and the syndrome of energy equilibrium. It is well known that obesity is associated with deranged lipid metabolism and altered insulin-mediated glucose uptake [62]. Previous research has shown that adipose tissue becomes dysfunctional and is associated with hyperplasia and hypertrophy, particularly in the visceral depot [63]. This leads to additional cellular and molecular changes, resulting in inflammation and an altered pattern of adipokine secretion [64]. It has been demonstrated that A. vera reduces visceral fat accumulation, and phytosterols derived from A. vera gel extract can mitigate hyperlipidaemia and total abdominal fat tissue weight [26]. Further, aloe sterols were found to be involved in brown adipose tissue adipogenesis, and the treated group had higher levels of uncoupling protein-1 (Ucp1) expression than the untreated group [27]. The AMP-activated protein kinase plays an important role in glucose and fatty oxidation. Aloe QDM complex [Aloe formulation containing Chrome (Cr)] suppressed receptor expression levels on adipose tissue macrophages in HFD obese mice, demonstrating its efficacy in fat reduction. Also, the findings point to a reduction in obesity-induced inflammatory cytokines and the translocation of NF–B p65 from the cytosol in the white adipose tissue (WAT) [65]. Furthermore, A. vera improved insulin resistance and increased HOMA-β values in non-insulin-dependent genetically obese rats. In addition, the treated group had more lean body mass and a lower fat percentage than the other group's [8]. Perez et al. reported that A. vera gel containing a high concentration of polyphenols when administered for 4 weeks was able to reduce body weight and improve insulin resistance when compared to the untreated group and that A. vera could be effective for the control of insulin resistance [11]. Furthermore, an ethanolic extract of A. vera demonstrated an insulin mimicking effect as evidenced by time-dependent increases in the expression of pIRS1 and pAkt. There was also increased expression of GLUT-4, indicating its potential as a therapeutic [66].
2.5. Liver
In an insulin-resistant state, both gluconeogenesis and lipogenesis increase in hepatocytes [67], and administration of A. vera for 15 days in alloxan-induced diabetic rats improved hepatic glycogen content. This could be due to inhibition of glycogenolysis and stimulation of insulin release, which aids in the mobilisation of blood glucose towards liver glycogen reserve or storage. The histopathological analysis also revealed that A. vera treatment reduces hepatocyte degeneration and cellular infiltration. There was also a significant decrease in lipogenesis, which could be attributed to improved insulin secretion and action [66]. Hyperglycemia can result in increased oxidative stress and changes the redox potential of glutathione due to increased utilization of antioxidants [68]. A. vera treatment improved GSH and SOD levels in the treated group and that lipid peroxidation was significantly decreased [66]. Another study reported that administration of A. vera for 21 days in STZ-induced diabetic rats resulted in a significant reduction in hepatic transaminases and liver triglycerides, free fatty acids and phospholipids. The altered liver fatty acid composition analysed by gas chromatography in the diabetic group was brought to normal following treatment with A. vera [69]. Further, A. vera and its polypeptide-rich fraction (PPF) reduced triglycerides, cholesterol, and hepatic transaminases, possibly due to a reduction in hepatic lipogenesis via the AMP-K pathway. PPF fraction elevated hexokinase activity and liver glycogen levels, with a significant decrease in glucose-6-phosphatase activity (a key enzyme in the gluconeogenesis pathway) which further reduces the production of glucose in the liver. Increased insulin levels after PPF treatment may be responsible for increased enzyme activities and glycogen content because insulin stimulates glucose uptake in the liver via the IRS/PI3K/Akt pathway. PPF treatment improved the architecture of the liver hepatocytes and reduced the damage caused by STZ, according to histopathological observations [9]. A. vera carbohydrate-rich fraction (AVCF) reduced FBG, glucagon, and glucose-6 phosphatase levels while increasing insulin, hexokinase, glycogen synthase, and glycogen content, according to another study conducted by the same group. These findings are consistent with the increased hepatic glycogen content observed in the PAS-stained histological section of the liver of diabetic rats given AVCF [20].
2.6. Muscle
In the muscle, defective glucose metabolism, reduction in glucose uptake by GLUT-4 and impaired insulin signalling for peripheral glucose uptake, including insulin receptors and downstream mediators, play a major role in the pathogenesis of the insulin resistance [70]. Further, hyperglycemia can upregulate markers of chronic inflammation and increased oxidative stress may cause interference with glucose uptake and reduce insulin sensitivity [71]. Therefore, the improved glucose uptake and the reduction of pro-inflammatory cytokine secretion by A. vera could manifest in a positive effect on IR [11]. A. vera treatment for 4 weeks in STZ-induced mice indicated a hypoglycaemic effect and an increase in GLUT-4 mRNA synthesis in mouse embryonic cell lines [72]. Another study reported that A. vera treatment increased GLUT-4 expression as well as pAkt and pIRS1 expression, indicating insulin signalling activation after treatment, implying that A. vera can be an effective anti-diabetic drug by improving insulin action and thus increasing glucose uptake [66]. High levels of TNF-α and IL-6 can also negatively regulate insulin levels leading to increased glucose levels. Numerous studies have reported that A. vera and its phytocomponents have decreased pro-inflammatory cytokines, restored insulin levels and reduced blood glucose levels and these effects might be mediated through improved insulin sensitivity [9,19,73].
2.7. Aloe vera in diabetic dyslipidemia
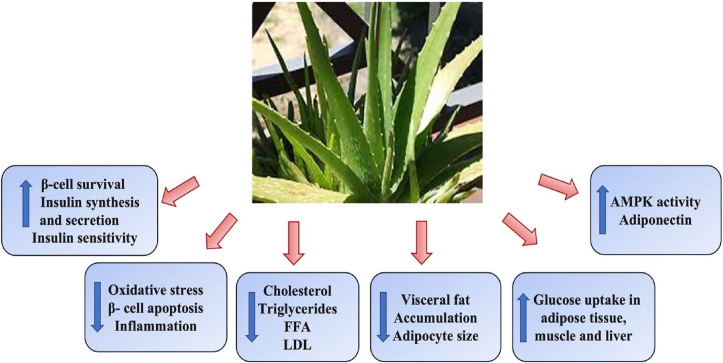
A.vera and its active components have been shown to have numerous benefits, including hypoglycaemic and hypolipidemic properties in animal models and human studies Fig. 3 [74]. A plethora of reports have revealed various properties of A. vera, including the ability to reduce hepatic tissue damage caused by diabetic complications [75] and oxidative damage [76]. It is conjectured that A. vera can restore normal fatty acid distribution in the blood by controlling lipid metabolism in the liver. Furthermore, A. vera extract can be used to construct non-saturated fatty acids, which remove free radicals from the bloodstream and regulate fat metabolism in the body [69].
Fig. 3.
Beneficial effects of A. vera on hyperglycemia and hyperlipidaemia: A. vera imparts its beneficial effects on hyperglycemia and hyperlipidaemia by increasing insulin synthesis and secretion, decreased oxidative stress, beta-cell apoptosis, inflammation, lowering cholesterol and lipoprotein levels, decreasing adipocyte size and visceral fat accumulation, increase glucose uptake and increased AMPK activity and adiponectin levels.
2.8. Pre-clinical studies
A.vera has been studied extensively in various animal models for its hypoglycaemic and hypolipidemic effects [74] (Table 4). The hypoglycaemic action of A. vera is attributed to an increase in pancreatic cell function in terms of insulin synthesis and its secretion [77], and the hypolipidemic effect is associated with phytosterols present in A. vera inhibiting cholesterol absorption in the intestine [78]. In diabetic rats, administration of A. vera ethanolic extract (300 mg/kg body weight) restored blood glucose levels while increasing insulin levels. Lipid levels, liver cholesterol, and kidney triglyceride levels were also reduced [69]. Similar findings were reported by another study where A. vera extract had beneficial effects comparable to glibenclamide in diabetic models [79]. A 10-KDa fraction powder from aloe leaf skin (Kidachi) showed protective effects against streptozotocin-induced pancreatic β-cell damage, which could be attributed to the presence of anthrachinonic derivatives like Aloenin and barbaloin. These beneficial effects of anthrone derivatives and phenolic plant components are exerted via metabolization in the intestine and then enter the circulation [80]. Furthermore, Noor et al. reported that oral ingestion of A. vera extract for 21 days restored FPG levels to normalcy with an increase in insulin levels in STZ-induced Wistar rats and restored pancreatic beta-cell function [81,82].
Table 4.
Hypoglycaemic and hypolipidemic effects of A. vera in pre-clinical studies.
| Study type | Duration | Beneficial effects | Target organ | Reference |
|---|---|---|---|---|
| Animal study (STZ induced rats) | 21 days | Improved membrane bound phosphatases and lysosomal hydrolases | Liver Kidney |
[79] |
| Animal study (STZ induced rats) | 21 days | Reduced FBG, hepatic transaminases Improved Fatty acid composition in liver and kidney and lipid profile | Liver Kidney Pancreas |
[69] |
| Animal study (Alloxan induced mice) | 4 days | Insulin synthesis and secretion | Pancreas | [77] |
| Animal study (STZ induced mice) | 73 days | Protective effects on pancreatic beta cells | Pancreas | [80] |
| Animal study (STZ induced rats) | 21 days | Restored FBG, Insulin levels, Qualitative and quantitative restoration of islet cells | Pancreas | [81,82] |
| Animal study (Zucker diabetic fatty rats) | 44 days | Reduce hyperglycemia and visceral fat accumulation. Reduced Triglycerides, FFA, improved insulin sensitivity | Pancreas Adipocytes |
[26] |
| Animal study NIDDM mice | 56 days | Reduced FBG, triglycerides in plasma and liver, improved insulin sensitivity Decrease adipocyte size | Pancreas Liver Adipocytes |
[83] |
| Animal study (STZ induced rats) | 28 days | Reduced FBG, serum cholesterol, And LDL-C levels Antioxidant activity | Pancreas | [84] |
| Animal study (db/db and HFD-mice) | 70 days | Reduce FBG, triglycerides, restored insulin levels, improved insulin sensitivity, increased adiponectin levels | Pancreas Adipocytes |
[85] |
| Animal study (STZ induced rats) | 21 days | Reduced FPG, triglycerides, cholesterol levels, Increased adiponectin, restored apolipoprotein levels | Pancreas | [31]. |
| Animal study (High-fat fed diet obese mice) | 54 days | Reduced body weight, FPG, leptin levels, improved glucose tolerance, increase adiponectin levels, AMPK activity, improved insulin sensitivity, reduced PPARγ/LXRα | Pancreas Liver Adipose tissue Muscles |
[65,92] [93] |
| Animal study (Diet induced obese rats) | 56 days | Lowering triglycerides, cholesterol, FFA, decrease visceral fat accumulation, inhibit pancreatic lipase and improved oxidative stress | Adipose tissue | [13] |
| Animal study (STZ induced genetically obese rats) | 28 days | Reduced FBG, triglycerides, VLDL, TG:HDL, restored insulin levels, DPP-IV activity, reduced HOMA-IR, restored HOMA-B values. Increased LBM | Pancreas Adipose tissue |
[8] |
The phytosterols identified from A. vera were reported to reduce hyperglycemia and visceral fat accumulation in ZDF rats (ZDF mutation in the leptin receptor, which spontaneously develops severe obesity, hyperglycemia, hyperlipidaemia, and IR), and could lower serum free fatty acid and triglyceride levels except for total cholesterol. Even though FFA is engaged in the development of IR, reducing serum FFA levels benefits insulin sensitivity and secretion as well. Furthermore, the lack of effect on TC could be attributed to the A. vera phytosterol administration dosage. Treatment with A. vera, on the other hand, may increase energy expenditure, and phytosterols derived from A. vera may be useful for hyperglycemia and hyperlipidaemia improvement [26]. According to one study, a maximum dose of 50 mg/kg bw of A. vera did not have a significant effect on cholesterol levels in diabetic rats, but it did improve blood glucose levels. This implies that the A. vera dose required to reduce serum cholesterol levels is greater than the dose required to reduce blood glucose levels [25]. Intriguingly, 8 weeks of administration of processed A. vera gel prevented the progression of diet-induced non-insulin-dependent diabetes mellitus in C57BL/6 J mice and it can improve blood glucose levels by decreasing IR. It also reduced triglyceride levels in the liver and plasma, and histopathological analysis of the per-epididymal fat pad revealed a decrease in adipocyte average size, implying that it could be useful in the treatment of diet-induced obesity [83].
As previously stated, A. vera contains a variety of phytoconstituents that are known to work synergistically and/or alone to impart therapeutic effects. Oral administration of polyphenolic and flavonoid-enriched A. vera skin extract for 4 weeks significantly reduced serum glucose levels, with a 25% reduction in total cholesterol levels and a 69% reduction in LDL-C levels after treatment [84]. UP780, a chromone-enriched aloe composition formulated with A. vera gel polysaccharides at 200 mg/kg bw, showed a reduction in fasting triglyceride and glucose levels after 10 weeks of treatment and improved insulin sensitivity [85]. Peptides or polypeptides and carbohydrates are a few of the components which exert their antidiabetic activity via various mechanisms. It was found that treatment of STZ-induced Wistar rats with PPF and CF reduced the levels of FPG by 74.8% and 64.9% respectively, as well as cholesterol levels by 40.4%, and 33.3%, and the triglycerides levels by 58.5% and 52.3%, respectively [31].
The role of adipose tissue as an endocrine organ in the progression of dyslipidemia has become increasingly popular [86]. Adiponectin, a major adipokine, is exclusively found in adipose tissue and has insulin-sensitizing, antiapoptotic, and anti-inflammatory properties [87]. It actively regulates glucose levels by increasing uptake through its receptors found in adipose tissue, skeletal muscle, and the liver [88]. The expression of adiponectin and its plasma concentrations are all inversely related to IR, fatty acid oxidation, lipid metabolism, and obesity [89]. Decreased expression of adiponectin and its receptors in the visceral and subcutaneous tissue is linked with diabetic dyslipidemia [90]. Interestingly, adiponectin levels were increased by 82.8%, and 81% post-treatment with CF and PPF. Furthermore, CF and PPF have restored apolipoproteins, which play an important role in glucose homeostasis and lipid metabolism, to normal/near normal levels, lowering the risk of diabetes-related cardiomyopathy [31]. Adiponectin levels are significantly related to insulin levels, inferring that increased insulin levels may have caused a spike in adiponectin levels, resulting in lower cholesterol and triglyceride levels [91]. Previously, dietary Aloe QDM complex [Aloe formulation containing Chrome (Cr)] administered for 54 days to high-fat fed diet C57BL/6 obese mice reduced body weight, FBG, plasma insulin, and leptin levels, as well as markedly reduced impairment of glucose tolerance. Additionally, AMPK activity in muscles increased plasma adiponectin levels and insulin sensitivity. Simultaneously, PPARγ/LXR and scavenger receptor mRNA and protein levels in white adipose tissue decreased. Thus, the Aloe QDM complex reduces obesity-induced glucose tolerance by suppressing PPARγ/LXR but increasing AMPK activity, both of which are important for peripheral tissues influencing the IR [65,92]. Furthermore, there was a reduction in obesity-induced inflammatory cytokine (IL-1β, −6, −12, TNF-α) and chemokine (CX3CL1, CCL5) levels, as well as macrophage infiltration and hepatic triglycerides levels. PPARγ/LXRα and 11β-HSD1 mRNA and protein levels were reduced in both liver and white adipose tissue by Aloe QDM [93].
Pancreatic lipase is a key lipid-digesting enzyme that aids in the absorption of dietary triglycerides by hydrolyzing triacylglycerols to monoacylglycerols and fatty acids [94]. As a result, inhibiting pancreatic lipase is an intriguing step toward developing potent agents that can reduce dietary fat absorption. In diet-induced obese rats, A. vera has been shown to improve lipid profile by lowering triglycerides, total cholesterol, and free fatty acids while decreasing adipose tissue accumulation, inhibiting pancreatic lipase, and improving oxidative stress [13]. These findings are consistent with our previous study in which A. vera showed beneficial effects on diabetes and dyslipidemia in WNIN/GR-Ob rats vis-à-vis β-cell dysfunction. The A. vera treated group significantly decreased TG, VLDL, and the TG: HDL ratio, along with fasting blood glucose levels and DPP-IV activity, with a concurrent elevation of serum insulin levels. A. vera was also efficient in decreasing HOMA-IR and increasing HOMA- βvalues. In comparison to the other groups, the treated group had more LBM and decreased fat per cent [8]. Unpublished findings from our lab showed that A. vera can inhibit pancreatic lipase and that Aloenin-a, a glycoside found in A. vera, can competitively inhibit this enzyme. These findings are supported both by molecular docking studies and biochemical experiments, which suggested that the anti-hyperlipidemic effects of A. vera on pancreatic lipase can be attributed in part to the presence of Aloenin-a. There have been numerous reports on the effects of A. vera on hypoglycaemic and hypolipidemic properties; however, animal experiments are not a substitute for clinical trials in determining efficacy.
2.9. Clinical studies
Over a 5 years trial, the impact of A. vera on 5000 diabetics showed a significant decrease in TC, TG, LDL-C, fasting, and postprandial blood glucose levels. The level of HDL-C increased dramatically within 60 days of treatment. Interestingly, no untoward side effects were reported during the study [95]. A 12-week controlled clinical trial on 60 patients with hyperlipidaemia who had previously not responded to dietary interventions found that 20 ml of A. vera reduced serum cholesterol by 15.4%, TG by 31.9%, and LDL by 18.2%. There was no significant difference in the group treated with 10 ml of A. vera. Because this trial was only available as a conceptual, there was no mention of intergroup comparisons, random sampling, or blinding [96]. Another study divided 72 diabetic women who were not on medication into two groups. For 42 days, they were given one tablespoon of A. vera gel or a placebo. Fasting blood glucose levels in the experimental group reduced from 250 mg to 141 mg %, while controls showed no significant changes. Other variables such as TC, serum triglycerides, weight, and appetite were also assessed. Except for triglyceride levels, which decreased significantly in the actively treated group from 220 mg to 123 mg%; these variables remained constant across both groups. There was no randomization in this study, and neither the patient nor the investigator was blinded [97].
A.vera was found to be effective in another clinical trial involving 36 patients with type 2 diabetes, where it could lower TG levels but did not affect TC levels after 6 weeks of regular use of one tablespoon of A. vera along with glibenclamide [98]. As previously stated, high blood glucose levels result in elevated blood lipid levels in diabetics. Perhaps, improved glycaemic control in patients receiving glibenclamide and A. vera may have reduced triglyceride levels, though the effect remained modest in magnitude. Another study found that consuming a high molecular weight fraction of A. vera containing less than 10 ppm of barbaloin and polysaccharide (MW: 1000 kDa) with glycoprotein, verectin (MW: 29 kDa) for 12 weeks (2 tablespoons three times a day) could lower serum triglyceride levels without affecting cholesterol levels, with no evidence of renal or hepatic toxicity [99].
Another study found contradictory results, with 300 mg of A. vera extract given to diabetics resistant to oral hypoglycaemic agents and using insulin for two months showing a significant decrease in FBG and HbA1c levels without any significant effects on the lipid profile. The lack of response in this study could be attributed to chronic hyperglycemia in patients, which can cause oxidative stress, or to the low dose of A. vera [100]. Over 8 weeks, a pilot study of two aloe products (UP780 and AC952) in patients with prediabetes/metabolic syndrome reported that the AC952 resulted in significant reductions in TC and LDL-C levels, as well as concomitant reductions in blood glucose levels, HbA1C levels, and improved insulin sensitivity. However, it was not found to be effective in lowering triglyceride levels and increasing HDL-C levels in the serum [101]. These disparities in the therapeutical effects of different A. vera preparations could be attributed to several factors, including inconsistencies among studies in terms of standardization of the A. vera manufacturing process, dosing frequency, length of treatment, laboratory test units, and race of the patients chosen. Such variation complicates the interpretation of clinical findings [102]. Interestingly, another study reported that non-insulin-dependent diabetics supplemented with 100 mg and 200 mg of A. vera gel powder for 3 months combined with healthy lifestyles experienced a significant reduction in FBG levels of 11.4% and 15.4%, respectively, and postprandial glucose level of 18.5% and 27.8%. TC was reduced by 8.6% and 10.1%, TG by 9.6% and 12.2%, LDL-C by 12.8% and 14.6%, VLDL by 9.6% and 12.2%, and an increase in HDL-C by 7.3% and 9.4% was observed. Total cholesterol to HDL-C ratio reduced from 5.6 to 4.8 and 6.1 to 5.0, respectively, and LDL-C to HDL-C ratio decreased from 3.7 to 3.0 and 4.1 to 3.1. According to these findings, a 200 mg dose was more effective in improving blood glucose and lipoprotein metabolism in the diabetics [12]. In a randomized controlled trial of pre-diabetics, A. vera 300 mg (AL300), 500 mg (AL500), and placebo (PL) capsules were taken twice daily. FBS, HbA1C, and lipid profiles were measured at baseline, 4 weeks, and 8 weeks. It was observed that using A. vera in pre-diabetics can significantly regulate blood glucose levels within four weeks and revert lipid profile levels within eight weeks [103]. This implies that the A. vera dose needed to normalize lipid levels is higher than the dose required to regulate blood glucose levels. These results are consistent with the findings of Choudhary et al. and that the duration of treatment may also play a role in regulating blood lipid levels [12]. The phytosterols present in A. vera, which are similar in structure to cholesterol and aid in lowering serum cholesterol concentrations by reducing the absorption of cholesterol from the gut by competing for the limited space for cholesterol in mixed micelles, could be attributed to the per cent reduction in TC. Increased hormone-sensitive lipase activity as a result of IR increases free fatty acid release from fat tissue. As a result of the FFA accumulation in plasma, the hepatic production of phospholipids and cholesterol increases. This can raise the level of triglycerides in the blood, which raises the level of lipoproteins in the blood. It has been postulated that A. vera can lower blood lipid levels by regulating fat metabolism in the liver [104]. Furthermore, two studies investigated the effects of polyherbal formulations containing A. vera in addition to conventional drugs on diabetic patients with uncontrolled dyslipidemia. Serum TG, total cholesterol, LDL, and HbA1c levels improved significantly in the intervention group, and the tested compound, as an add-on, was efficient in decreasing serum lipids in diabetics with uncontrolled dyslipidemia [105,106]. In both studies, no untoward effects on the liver and kidney were observed. In obese individuals with prediabetes or early untreated diabetes, administration of Aloe QDM complex for 8 weeks reduced fasting blood glucose, body weight, body fat mass, and IR [107]. Another randomized double-blind placebo-controlled clinical trial suggested that 300 mg of Aloe gel capsule for 2 months in 30 hyperlipidemic type 2 diabetics significantly reduced FBG, HbA1c, TC and LDL levels without any significant effects on liver/kidney function tests suggesting it to be safe for antihyperglycemic and anti-hypercholesteraemic agent [108] Table 5 briefly summarizes the clinical trials.
Table 5.
Hypoglycaemic and hypolipidemic effects of A. vera in clinical studies.
| Study type | Duration | Beneficial effects | Study population | Reference |
|---|---|---|---|---|
| Randomized controlled clinical trial | 5 years | Decrease in TC, TG,LDL-C, FBG and postprandial blood glucose | 5000 diabetics | [95] |
| Controlled clinical trial | 84 days | Reduced TC by 15.4%, TG by 31.9% and, LDL by 18.2% | 60 hyperlipidemic patients | [96] |
| Controlled clinical trial | 42 days | Reduced FBG, triglycerides | 72 diabetics | [97] |
| Controlled clinical trial | 42 days | Reduced FBG, triglyceride levels | 36 diabetics | [98] |
| Uncontrolled clinical trial | 84 days | Decreased blood glucose and triglyceride levels. Improved HbA1c levels | 15 diabetics | [99] |
| Double blind-placebo controlled clinical trial | 61 days | Decrease in FBG and HbA1c | 35 diabetics | [100] |
| Double blind-placebo controlled clinical trial | 56 days | Decrease in FBG, HbA1c, TC, LDL-C | 45 pre-diabetics | [101] |
| Placebo controlled clinical trial | 90 days | Reduced FBG, TC, TG, VLDL, LDL-C, HbA1c, TC:HDL and increase in HDL-C | 90 diabetics | [12] |
| Open label phase 1 trial | 40 days | Decreased FBG, HbA1c, TC, LDL and triglycerides | 30 diabetics | [105] |
| Randomized controlled trial | 84 days | Decreased HbA1c, TC, LDL and triglycerides | 50 diabetics | [106] |
| Randomized controlled trial | 56 days | Reduced FBG, insulin resistance, body weight and body fat mass | 136 pre-diabetics | [107] |
| Randomized-double blind placebo controlled clinical trial | 61 days | Reduced FBG, HbA1c, TC and LDL levels | 30 diabetics | [108] |
3. Conclusion
From this review, shreds of evidence strongly suggest that the oral administration of A. vera may be effective in improving blood glucose homeostasis and lipid metabolism. However, many studies are associated with limitations. The most significant limitation is that not all of the preparations of A. vera included reviewed here were assumed to be equivalent in composition and bioactivity, which could result in variations in glucose-lowering and hypolipidemic effects. Given the widespread use of A. vera, the lack of controlled clinical trials is perhaps the most surprising finding. Other limitations include a lack of random sampling, a lack of blinding, and the sample size in various studies. Because of the variation in the dosage used in clinical trials, determining the minimum effective dose of A. vera that can produce beneficial effects in clinical studies is difficult. Except for one 5-year study, the selected trials used A. vera for 6–12 weeks. Given the short duration of these studies, the long-term safety of A. vera consumption appears to be uncertain. Despite some limitations, the current findings are promising and can be useful for future research. Thus, prospective trial investigators must include surveillance time - frame in clinical trials to oversee any medium-to long-term adverse events associated with A. vera use.
Furthermore, future research should focus on the effect of A. vera on other lipid metabolism targets by modulating adipogenesis and adipolysis-related transcriptional factors such as peroxisome proliferator-activated receptors (PPARs) and UCP-1 (mitochondrial uncoupling protein). Its beneficial effects on Ghrelin, Leptin, Neuropeptide Y, and adiponectin, all of which are important targets for obesity, can also be investigated further. Integrating modern diagnostic procedures with complementary and/or alternative medicine would go a long way toward achieving any society's health and well-being.
Source of funding
None.
Authors contribution
Neha Deora: Lead author carried out conceptualization, review of literature, data collection, curation, validation, manuscript writing, reviewing, editing text and art work.
Krishnan Venkataraman: Conceptualization, review of literature, manuscript, editing text, art work and correspondence of the manuscript.
Both the authors have read the manuscript and accepted the content for publication.
Declaration of Competing Interest
None.
Acknowledgement
The study author (N.D) would like to thank VIT, Vellore for their help and support with the research.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Unger R.H. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 2.https://diabetesatlas.org/ IDF diabetes atlas | tenth edition.
- 3.Vergès B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–899. doi: 10.1007/s00125-015-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney P.M., Blackwell L., Collins R., Keech A., Simes J., Peto R., et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–125. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 5.Dyslipidemia | National Health Portal of India https://www.nhp.gov.in/dyslipidemia_mtl (accessed June 10, 2022)
- 6.Tziomalos K., Athyros V.G., Karagiannis A., Mikhailidis D.P. Dyslipidemia induced by drugs used for the prevention and treatment of vascular diseases. Open Cardiovasc Med J. 2011;5:85–89. doi: 10.2174/1874192401105010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayaraman R., Subramani S., Sheik Abdullah S.H., Udaiyar M. Antihyperglycemic effect of hesperetin, a citrus flavonoid, extenuates hyperglycemia and exploring the potential role in antioxidant and antihyperlipidemic in streptozotocin-induced diabetic rats. Biomed Pharmacother. 2018;97:98–106. doi: 10.1016/j.biopha.2017.10.102. [DOI] [PubMed] [Google Scholar]
- 8.Deora N., Sunitha M.M., Satyavani M., Harishankar N., Vijayalakshmi M.A., Venkataraman K., et al. Alleviation of diabetes mellitus through the restoration of β-cell function and lipid metabolism by Aloe vera (L.) Burm. f. extract in obesogenic WNIN/GR-Ob rats. J Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113921. [DOI] [PubMed] [Google Scholar]
- 9.Babu S.N., Govindarajan S., Vijayalakshmi M.A., Noor A. Role of zonulin and GLP-1/DPP-IV in alleviation of diabetes mellitus by peptide/polypeptide fraction of Aloe vera in streptozotocin- induced diabetic wistar rats. J Ethnopharmacol. 2021;272 doi: 10.1016/j.jep.2021.113949. [DOI] [PubMed] [Google Scholar]
- 10.Nejatzadeh-Barandozi F. Antibacterial activities and antioxidant capacity of Aloe vera. Org Med Chem Lett. 2013;3:5. doi: 10.1186/2191-2858-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez Y.Y., Jiménez-Ferrer E., Zamilpa A., Hernández-Valencia M., Alarcón-Aguilar F.J., Tortoriello J., et al. Effect of a polyphenol-rich extract from Aloe vera gel on experimentally induced insulin resistance in mice. Am J Chin Med. 2007;35:1037–1046. doi: 10.1142/S0192415X07005491. [DOI] [PubMed] [Google Scholar]
- 12.Choudhary M., Kochhar A., Sangha J. Hypoglycemic and hypolipidemic effect of Aloe vera L. in non-insulin dependent diabetics. J Food Sci Technol. 2014;51:90–96. doi: 10.1007/s13197-011-0459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahoui W., Merzouk H., El Haci I.A., Bettioui R., Azzi R., Benali M. Beneficial effects of Aloe vera gel on lipid profile, lipase activities and oxidant/antioxidant status in obese rats. J Funct Foods. 2018;48:525–532. doi: 10.1016/j.jff.2018.07.050. [DOI] [Google Scholar]
- 14.Surjushe A., Vasani R., Saple D.G. Aloe vera: a short review. Indian J Dermatol. 2008;53:163–166. doi: 10.4103/0019-5154.44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahramanoğlu İ., Chen C., Chen J., Wan C. Chemical constituents, antimicrobial activity, and food preservative characteristics of aloe vera gel. Agronomy. 2019;9:831. doi: 10.3390/agronomy9120831. [DOI] [Google Scholar]
- 16.Hęś M., Dziedzic K., Górecka D., Jędrusek-Golińska A., Gujska E. Aloe vera (L.) webb.: natural sources of antioxidants - a review. Plant Foods Hum Nutr. 2019;74:255–265. doi: 10.1007/s11130-019-00747-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster M., Hunter D., Samman S. In: Herbal medicine: biomolecular and clinical aspects. 2nd ed. Benzie I.F.F., Wachtel-Galor S., editors. CRC Press/Taylor & Francis; Boca Raton (FL): 2011. Evaluation of the nutritional and metabolic effects of aloe vera. [Google Scholar]
- 18.Hamman J.H. Composition and applications of Aloe vera leaf gel. Molecules. 2008;13:1599–1616. doi: 10.3390/molecules13081599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannakoudakis D.A., Hosseini-Bandegharaei A., Tsafrakidou P., Triantafyllidis K.S., Kornaros M., Anastopoulos I. Aloe vera waste biomass-based adsorbents for the removal of aquatic pollutants: a review. J Environ Manag. 2018;227:354–364. doi: 10.1016/j.jenvman.2018.08.064. [DOI] [PubMed] [Google Scholar]
- 20.Ulbricht C., Armstrong J., Basch E., Basch S., Bent S., Dacey C., et al. An evidence-based systematic review of Aloe vera by the natural standard research collaboration. J Herb Pharmacother. 2007;7:279–323. doi: 10.1080/15228940802153339. [DOI] [PubMed] [Google Scholar]
- 21.Noor A. Carbohydrate fraction of Aloe vera ameliorates inflammation through suppression of pro-inflammatory mediators and oxidative stress in vitro and rats with Freund's adjuvant induced paw edema. IJEB Vol59(3) [March 2021] 2021.
- 22.Govindarajan S., Babu S.N., Vijayalakshmi M.A., Manohar P., Noor A. Aloe vera carbohydrates regulate glucose metabolism through improved glycogen synthesis and downregulation of hepatic gluconeogenesis in diabetic rats. J Ethnopharmacol. 2021;281 doi: 10.1016/j.jep.2021.114556. [DOI] [PubMed] [Google Scholar]
- 23.Chen L., Cao H., Xiao J. Elsevier; 2018. Polyphenols. Polyphenols: properties, recovery, and applications; pp. 45–67. [DOI] [Google Scholar]
- 24.Panche A.N., Diwan A.D., Chandra S.R. Flavonoids: an overview. J Nutr Sci. 2016;5:e47. doi: 10.1017/jns.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korkina L.G., Afanas’ev I.B. Antioxidant and chelating properties of flavonoids. Adv Pharmacol. 1997;38:151–163. doi: 10.1016/s1054-3589(08)60983-7. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Mangelinckx S., Adams A., Wang Z.-T., Li W.-L., De Kimpe N. Natural flavonoids as potential herbal medication for the treatment of diabetes mellitus and its complications. Nat Prod Commun. 2015;10:187–200. [PubMed] [Google Scholar]
- 27.Tanaka M., Misawa E., Ito Y., Habara N., Nomaguchi K., Yamada M., et al. Identification of five phytosterols from Aloe vera gel as anti-diabetic compounds. Biol Pharm Bull. 2006;29:1418–1422. doi: 10.1248/bpb.29.1418. [DOI] [PubMed] [Google Scholar]
- 28.Misawa E., Tanaka M., Nomaguchi K., Yamada M., Toida T., Takase M., et al. Administration of phytosterols isolated from Aloe vera gel reduce visceral fat mass and improve hyperglycemia in Zucker diabetic fatty (ZDF) rats. Obes Res Clin Pract. 2008;2:I–II. doi: 10.1016/j.orcp.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 29.Tada A., Misawa E., Tanaka M., Saito M., Nabeshima K., Yamauchi K., et al. Investigating anti-obesity effects by oral administration of aloe vera gel extract (AVGE): possible involvement in activation of Brown adipose tissue (BAT) J Nutr Sci Vitaminol. 2020;66:176–184. doi: 10.3177/jnsv.66.176. [DOI] [PubMed] [Google Scholar]
- 30.Spoorthy N.B., Ayesha N. Bioactive constituents of the genus Aloe and their potential therapeutic and pharmacological applications: a review. J Appl Pharmaceut Sci. 2020 doi: 10.7324/JAPS.2020.101118. [DOI] [Google Scholar]
- 31.Cock I.E. The genus aloe: phytochemistry and therapeutic uses including treatments for gastrointestinal conditions and chronic inflammation. Prog Drug Res. 2015;70:179–235. doi: 10.1007/978-3-0348-0927-6_6. [DOI] [PubMed] [Google Scholar]
- 32.Park M.-Y., Kwon H.-J., Sung M.-K. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci Biotechnol Biochem. 2009;73:828–832. doi: 10.1271/bbb.80714. [DOI] [PubMed] [Google Scholar]
- 33.Babu S.N., Govindarajan S., Noor A. Aloe vera and its two bioactive constituents in alleviation of diabetes -proteomic & mechanistic insights. J Ethnopharmacol. 2021;280 doi: 10.1016/j.jep.2021.114445. [DOI] [PubMed] [Google Scholar]
- 34.Gomes A., Neuwirth O., Freitas M., Couto D., Ribeiro D., Figueiredo A.G.P.R., et al. Synthesis and antioxidant properties of new chromone derivatives. Bioorg Med Chem. 2009;17:7218–7226. doi: 10.1016/j.bmc.2009.08.056. [DOI] [PubMed] [Google Scholar]
- 35.Rajendran A., Narayanan V., Gnanavel I. Study on the analysis of trace elements in Aloe vera and its biological importance. J Appl Sci Res. 2007;3:1476–1478. [Google Scholar]
- 36.Rajasekaran S., Sivagnanam K., Subramanian S. Mineral contents of aloe vera leaf gel and their role on streptozotocin-induced diabetic rats. Biol Trace Elem Res. 2005;108:185–195. doi: 10.1385/BTER:108:1-3:185. [DOI] [PubMed] [Google Scholar]
- 37.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., et al. ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howard B.V. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;84:28J–32J. doi: 10.1016/s0002-9149(99)00355-0. [DOI] [PubMed] [Google Scholar]
- 39.Mooradian A.D. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metabol. 2009;5:150–159. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 40.Nikkilä E.A., Kekki M. Plasma triglyceride transport kinetics in diabetes mellitus. Metab Clin Exp. 1973;22:1–22. doi: 10.1016/0026-0495(73)90024-3. [DOI] [PubMed] [Google Scholar]
- 41.Alves-Bezerra M., Cohen D.E. Triglyceride metabolism in the liver. Compr Physiol. 2017;8:1–8. doi: 10.1002/cphy.c170012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McEneny J., O'Kane M.J., Moles K.W., McMaster C., McMaster D., Mercer C., et al. Very low density lipoprotein subfractions in Type II diabetes mellitus: alterations in composition and susceptibility to oxidation. Diabetologia. 2000;43:485–493. doi: 10.1007/s001250051333. [DOI] [PubMed] [Google Scholar]
- 43.Taskinen M.R. Diabetic dyslipidaemia: from basic research to clinical practice. Diabetologia. 2003;46:733–749. doi: 10.1007/s00125-003-1111-y. [DOI] [PubMed] [Google Scholar]
- 44.Chen Y.D., Swami S., Skowronski R., Coulston A., Reaven G.M. Differences in postprandial lipemia between patients with normal glucose tolerance and noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab. 1993;76:172–177. doi: 10.1210/jcem.76.1.8421086. [DOI] [PubMed] [Google Scholar]
- 45.https://www.diabetesincontrol.com/dyslipidemia-in-insulin-resistance-hypertriglyceridemia-and-low-hdl-cholesterol/ Dyslipidemia in insulin resistance: hypertriglyceridemia and low HDL cholesterol.
- 46.Taskinen M.R. Lipoprotein lipase in diabetes. Diabetes Metab Rev. 1987;3:551–570. doi: 10.1002/dmr.5610030208. [DOI] [PubMed] [Google Scholar]
- 47.Stadler J.T., Marsche G. Obesity-related changes in high-density lipoprotein metabolism and function. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lasselin J., Magne E., Beau C., Ledaguenel P., Dexpert S., Aubert A., et al. Adipose inflammation in obesity: relationship with circulating levels of inflammatory markers and association with surgery-induced weight loss. J Clin Endocrinol Metab. 2014;99:E53–E61. doi: 10.1210/jc.2013-2673. [DOI] [PubMed] [Google Scholar]
- 49.Pietri A.O., Dunn F.L., Grundy S.M., Raskin P. The effect of continuous subcutaneous insulin infusion on very-low-density lipoprotein triglyceride metabolism in type I diabetes mellitus. Diabetes. 1983;32:75–81. doi: 10.2337/diab.32.1.75. [DOI] [PubMed] [Google Scholar]
- 50.Jialal I., Singh G. Management of diabetic dyslipidemia: an update. World J Diabetes. 2019;10:280–290. doi: 10.4239/wjd.v10.i5.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson P.D., Panza G., Zaleski A., Taylor B. Statin-associated side effects. J Am Coll Cardiol. 2016;67:2395–2410. doi: 10.1016/j.jacc.2016.02.071. [DOI] [PubMed] [Google Scholar]
- 52.Katsiki N., Theocharidou E., Karagiannis A., Athyros V.G., Mikhailidis D.P. Ezetimibe therapy for dyslipidemia: an update. Curr Pharmaceut Des. 2013;19:3107–3114. doi: 10.2174/13816128113199990314. [DOI] [PubMed] [Google Scholar]
- 53.Robinson J.G., Farnier M., Krempf M., Bergeron J., Luc G., Averna M., et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 54.Jialal I., Amess W., Kaur M. Management of hypertriglyceridemia in the diabetic patient. Curr Diabetes Rep. 2010;10:316–320. doi: 10.1007/s11892-010-0124-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landray M.J., Haynes R., Hopewell J.C., Parish S., Aung T., Jomson T., et al. HPS2-THRIVE Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 56.Superko H.R., Berneis K.K., Williams P.T., Rizzo M., Wood P.D. Gemfibrozil reduces small low-density lipoprotein more in normolipemic subjects classified as low-density lipoprotein pattern B compared with pattern A. Am J Cardiol. 2005;96:1266–1272. doi: 10.1016/j.amjcard.2005.06.069. [DOI] [PubMed] [Google Scholar]
- 57.Bhatt D.L., Steg P.G., Miller M., Brinton E.A., Jacobson T.A., Ketchum S.B., et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 58.Freeman A.M., Pennings N. StatPearls Publishing; StatPearls, Treasure Island (FL): 2022. Insulin resistance. [PubMed] [Google Scholar]
- 59.Vekic J., Zeljkovic A., Stefanovic A., Jelic-Ivanovic Z., Spasojevic-Kalimanovska V. Obesity and dyslipidemia. Metab Clin Exp. 2019;92:71–81. doi: 10.1016/j.metabol.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Sangeetha K.N., Sujatha S., Muthusamy V.S., Anand S., Shilpa K., Kumari P.J., et al. Current trends in small molecule discovery targeting key cellular signaling events towards the combined management of diabetes and obesity. Bioinformation. 2017;13:394–399. doi: 10.6026/97320630013394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J Diabetes. 2015;6:456–480. doi: 10.4239/wjd.v6.i3.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brahma Naidu P., Uddandrao V.V.S., Ravindar Naik R., Suresh P., Meriga B., Begum M.S., et al. Ameliorative potential of gingerol: promising modulation of inflammatory factors and lipid marker enzymes expressions in HFD induced obesity in rats. Mol Cell Endocrinol. 2016;419:139–147. doi: 10.1016/j.mce.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 63.Giridharan N.V. Animal models of obesity & their usefulness in molecular approach to obesity. Indian J Med Res. 1998;108:225–242. [PubMed] [Google Scholar]
- 64.Singh H., Ganneru S., Malakapalli V., Chalasani M., Nappanveettil G., Bhonde R.R., et al. Islet adaptation to obesity and insulin resistance in WNIN/GR-Ob rats. Islets. 2014;6 doi: 10.1080/19382014.2014.998099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shin E., Shin S., Kong H., Lee S., Do S.-G., Jo T.H., et al. Dietary aloe reduces adipogenesis via the activation of AMPK and suppresses obesity-related inflammation in obese mice. Immune Netw. 2011;11:107–113. doi: 10.4110/in.2011.11.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Singh V., Singh S.P., Singh M., Gupta A.K., Kumar A. Combined potentiating action of phytochemical(s) from Cinnamomum tamala and Aloe vera for their anti-diabetic and insulinomimetic effect using in vivo rat and in vitro NIH/3T3 cell culture system. Appl Biochem Biotechnol. 2015;175:2542–2563. doi: 10.1007/s12010-014-1448-3. [DOI] [PubMed] [Google Scholar]
- 67.Bazotte R.B., Silva L.G., Schiavon F.P. Insulin resistance in the liver: deficiency or excess of insulin? Cell Cycle. 2014;13:2494–2500. doi: 10.4161/15384101.2014.947750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Irshad M., Chaudhuri P.S. Oxidant-antioxidant system: role and significance in human body. Indian J Exp Biol. 2002;40:1233–1239. [PubMed] [Google Scholar]
- 69.Rajasekaran S., Ravi K., Sivagnanam K., Subramanian S. Beneficial effects of aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 70.Cai D. NFkappaB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle. 2009;8:2542–2548. doi: 10.4161/cc.8.16.9386. [DOI] [PubMed] [Google Scholar]
- 71.Luc K., Schramm-Luc A., Guzik T.J., Mikolajczyk T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70 doi: 10.26402/jpp.2019.6.01. [DOI] [PubMed] [Google Scholar]
- 72.Kumar R., Sharma B., Tomar N.R., Roy P., Gupta A.K., Kumar A. In vivo evaluation of hypoglycemic activity of Aloe spp. and identification of its mode of action on GLUT-4 gene expression in vitro. Appl Biochem Biotechnol. 2011;164:1246–1256. doi: 10.1007/s12010-011-9210-6. [DOI] [PubMed] [Google Scholar]
- 73.Babu S., Noor A. Aloe barbadensis Miller peptide/polypeptide fraction alleviates inflammation through inhibition of proinflammatory cytokines and mediators in vitro and in rats with Freund's adjuvant-induced hind paw edema. Asian Pac J Trop Biomed. 2019;9:524. doi: 10.4103/2221-1691.271726. [DOI] [Google Scholar]
- 74.Pothuraju R., Sharma R.K., Onteru S.K., Singh S., Hussain S.A. Hypoglycemic and hypolipidemic effects of aloe vera extract preparations: a review. Phytother Res. 2016;30:200–207. doi: 10.1002/ptr.5532. [DOI] [PubMed] [Google Scholar]
- 75.Can A., Akev N., Ozsoy N., Bolkent S., Arda B.P., Yanardag R., et al. Effect of Aloe vera leaf gel and pulp extracts on the liver in type-II diabetic rat models. Biol Pharm Bull. 2004;27:694–698. doi: 10.1248/bpb.27.694. [DOI] [PubMed] [Google Scholar]
- 76.Parihar M.S., Chaudhary M., Shetty R., Hemnani T. Susceptibility of hippocampus and cerebral cortex to oxidative damage in streptozotocin treated mice: prevention by extracts of Withania somnifera and Aloe vera. J Clin Neurosci. 2004;11:397–402. doi: 10.1016/j.jocn.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 77.Ajabnoor M.A. Effect of aloes on blood glucose levels in normal and alloxan diabetic mice. J Ethnopharmacol. 1990;28:215–220. doi: 10.1016/0378-8741(90)90031-n. [DOI] [PubMed] [Google Scholar]
- 78.Kamal-Eldin A., Moazzami A. Plant sterols and stanols as cholesterol-lowering ingredients in functional foods. Recent Pat Food, Nutr Agric. 2009;1:1–14. doi: 10.2174/2212798410901010001. [DOI] [PubMed] [Google Scholar]
- 79.Rajasekaran S., Sriram N., Arulselvan P., Subramanian S. Effect of aloe vera leaf gel extract on membrane bound phosphatases and lysosomal hydrolases in rats with streptozotocin diabetes. Pharmazie. 2007;62:221–225. [PubMed] [Google Scholar]
- 80.Beppu H., Shimpo K., Chihara T., Kaneko T., Tamai I., Yamaji S., et al. Antidiabetic effects of dietary administration of Aloe arborescens Miller components on multiple low-dose streptozotocin-induced diabetes in mice: investigation on hypoglycemic action and systemic absorption dynamics of aloe components. J Ethnopharmacol. 2006;103:468–477. doi: 10.1016/j.jep.2005.10.034. [DOI] [PubMed] [Google Scholar]
- 81.Noor A., Gunasekaran S., Manickam A.S., Vijayalakshmi M. Antidiabetic activity of Aloe vera and histology of organs in streptozotocin-induced diabetic rats. Curr Sci. 2008;94:1070–1076. [Google Scholar]
- 82.Noor A., Gunasekaran S., Vijayalakshmi M.A. Improvement of insulin secretion and pancreatic β-cell function in streptozotocin-induced diabetic rats treated with aloe vera extract. Pharmacogn Res. 2017;9:S99–S104. doi: 10.4103/pr.pr_75_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim K., Kim H., Kwon J., Lee S., Kong H., Im S.-A., et al. Hypoglycemic and hypolipidemic effects of processed Aloe vera gel in a mouse model of non-insulin-dependent diabetes mellitus. Phytomedicine. 2009;16:856–863. doi: 10.1016/j.phymed.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 84.Moniruzzaman M., Rokeya B., Ahmed S., Bhowmik A., Khalil M.I., Gan S.H. In vitro antioxidant effects of Aloe barbadensis Miller extracts and the potential role of these extracts as antidiabetic and antilipidemic agents on streptozotocin-induced type 2 diabetic model rats. Molecules. 2012;17:12851–12867. doi: 10.3390/molecules171112851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yimam M., Zhao J., Corneliusen B., Pantier M., Brownell L.A., Jia Q. UP780, a chromone-enriched aloe composition improves insulin sensitivity. Metab Syndr Relat Disord. 2013;11:267–275. doi: 10.1089/met.2012.0135. [DOI] [PubMed] [Google Scholar]
- 86.Ronti T., Lupattelli G., Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol. 2006;64:355–365. doi: 10.1111/j.1365-2265.2006.02474.x. [DOI] [PubMed] [Google Scholar]
- 87.Robinson K., Prins J., Venkatesh B. Clinical review: adiponectin biology and its role in inflammation and critical illness. Crit Care. 2011;15:221. doi: 10.1186/cc10021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chang L.-C., Huang K.-C., Wu Y.-W., Kao H.-L., Chen C.-L., Lai L.-P., et al. The clinical implications of blood adiponectin in cardiometabolic disorders. J Formos Med Assoc. 2009;108:353–366. doi: 10.1016/S0929-6646(09)60079-6. [DOI] [PubMed] [Google Scholar]
- 89.Ghoshal K., Bhattacharyya M. Adiponectin: probe of the molecular paradigm associating diabetes and obesity. World J Diabetes. 2015;6:151–166. doi: 10.4239/wjd.v6.i1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghoshal K., Chatterjee T., Chowdhury S., Sengupta S., Bhattacharyya M. Adiponectin genetic variant and expression coupled with lipid peroxidation reveal new signatures in diabetic dyslipidemia. Biochem Genet. 2021;59:781–798. doi: 10.1007/s10528-021-10030-5. [DOI] [PubMed] [Google Scholar]
- 91.Ahlstrom P., Rai E., Chakma S., Cho H.H., Rengasamy P., Sweeney G. Adiponectin improves insulin sensitivity via activation of autophagic flux. J Mol Endocrinol. 2017;59:339–350. doi: 10.1530/JME-17-0096. [DOI] [PubMed] [Google Scholar]
- 92.Shin S., Kim S., Oh H.-E., Kong H., Shin E., Do S.-G., et al. Dietary aloe QDM complex reduces obesity-induced insulin resistance and adipogenesis in obese mice fed a high-fat diet. Immune Netw. 2012;12:96–103. doi: 10.4110/in.2012.12.3.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shin E., Shim K.-S., Kong H., Lee S., Shin S., Kwon J., et al. Dietary aloe improves insulin sensitivity via the suppression of obesity-induced inflammation in obese mice. Immune Netw. 2011;11:59–67. doi: 10.4110/in.2011.11.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lowe M.E. The triglyceride lipases of the pancreas. J Lipid Res. 2002;43:2007–2016. doi: 10.1194/jlr.r200012-jlr200. [DOI] [PubMed] [Google Scholar]
- 95.Agarwal O.P. Prevention of atheromatous heart disease. Angiology. 1985;36:485–492. doi: 10.1177/000331978503600801. [DOI] [PubMed] [Google Scholar]
- 96.Nasiff Hadad A., Fajardo Ferra R., Velez Fernández ME. de P. Efecto del aloe sobre la hiperlipedemia en pacientes refractarios a la dieta. Rev Cubana Med Gen Integr. 1993;9:43–51. [Google Scholar]
- 97.Yongchaiyudha S., Rungpitarangsi V., Bunyapraphatsara N., Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice. I. Clinical trial in new cases of diabetes mellitus. Phytomedicine. 1996;3:241–243. doi: 10.1016/S0944-7113(96)80060-2. [DOI] [PubMed] [Google Scholar]
- 98.Bunyapraphatsara N., Yongchaiyudha S., Rungpitarangsi V., Chokechaijaroenporn O. Antidiabetic activity of Aloe vera L. juice II. Clinical trial in diabetes mellitus patients in combination with glibenclamide. Phytomedicine. 1996;3:245–248. doi: 10.1016/S0944-7113(96)80061-4. [DOI] [PubMed] [Google Scholar]
- 99.Yagi A., Hegazy S., Kabbash A., Wahab E.A.-E. Possible hypoglycemic effect of Aloe vera L. high molecular weight fractions on type 2 diabetic patients. Saudi Pharmaceut J. 2009;17:209–215. doi: 10.1016/j.jsps.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fallah H., Kianbakht S., Hajiaghaee R., Afkhami A., Bonakdaran A., Hashem D. 2012. Aloe vera leaf gel in treatment of advanced type 2 diabetes mellitus needing insulin therapy: a randomized double-blind placebo-controlled clinical trial; pp. 19–27. [Google Scholar]
- 101.Devaraj S., Yimam M., Brownell L.A., Jialal I., Singh S., Jia Q. Effects of Aloe vera supplementation in subjects with prediabetes/metabolic syndrome. Metab Syndr Relat Disord. 2013;11:35–40. doi: 10.1089/met.2012.0066. [DOI] [PubMed] [Google Scholar]
- 102.Zhang Y., Liu W., Liu D., Zhao T., Tian H. Efficacy of aloe vera supplementation on prediabetes and early non-treated diabetic patients: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8 doi: 10.3390/nu8070388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alinejad-Mofrad S., Foadoddini M., Saadatjoo S.A., Shayesteh M. Improvement of glucose and lipid profile status with Aloe vera in pre-diabetic subjects: a randomized controlled-trial. J Diabetes Metab Disord. 2015;14:22. doi: 10.1186/s40200-015-0137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lim B.O., Seong N.S., Choue R.W., Kim J.D., Lee H.Y., Kim S.Y., et al. Efficacy of dietary aloe vera supplementation on hepatic cholesterol and oxidative status in aged rats. J Nutr Sci Vitaminol. 2003;49:292–296. doi: 10.3177/jnsv.49.292. [DOI] [PubMed] [Google Scholar]
- 105.Zarvandi M., Rakhshandeh H., Abazari M., Shafiee-Nick R., Ghorbani A. Safety and efficacy of a polyherbal formulation for the management of dyslipidemia and hyperglycemia in patients with advanced-stage of type-2 diabetes. Biomed Pharmacother. 2017;89:69–75. doi: 10.1016/j.biopha.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 106.Ghorbani A., Zarvandi M., Rakhshandeh H. A randomized controlled trial of a herbal compound for improving metabolic parameters in diabetic patients with uncontrolled dyslipidemia. Endocr, Metab Immune Disord: Drug Targets. 2019;19:1075–1082. doi: 10.2174/1871530319666190206213420. [DOI] [PubMed] [Google Scholar]
- 107.Choi H.-C., Kim S.-J., Son K.-Y., Oh B.-J., Cho B.-L. Metabolic effects of aloe vera gel complex in obese prediabetes and early non-treated diabetic patients: randomized controlled trial. Nutrition. 2013;29:1110–1114. doi: 10.1016/j.nut.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 108.Huseini H.F., Kianbakht S., Hajiaghaee R., Dabaghian F.H. Anti-hyperglycemic and anti-hypercholesterolemic effects of Aloe vera leaf gel in hyperlipidemic type 2 diabetic patients: a randomized double-blind placebo-controlled clinical trial. Planta Med. 2012;78:311–316. doi: 10.1055/s-0031-1280474. [DOI] [PubMed] [Google Scholar]