Take Home Message
In this report, we describe the technique and results of en bloc kidney transplantation. We found that results are excellent for renal function and patient survival. We conclude that en bloc kidney transplantation should be considered to increase the number of grafts.
Keywords: Renal transplantation, En bloc kidney transplantation, Pediatric donor
Abstract
Background
Renal transplantation is facing a shortage of grafts. En bloc kidney transplantation (EBKT) from pediatric donors could increase the number of available grafts.
Objective
To describe the surgical technique as well as the long-term functional and morphological results of EBKT.
Design, setting, and participants
We performed a retrospective study of all the EBKT procedures performed in Lyon between 2002 and 2020. Electronic medical records were checked with an analysis of demographics, and peri- and postoperative results.
Outcome measurements and statistical analysis
A descriptive analysis of donor and recipient characteristics, perioperative data, complications, and renal function was performed.
Results and limitations
Between 2002 and 2020, 21 EBKT procedures were performed. Donors had a mean weight of 8.6 kg and a mean age of 12 mo, with a mean cold ischemia time of 11 h and 30 min. Receivers had a mean age of 30 yr and a body mass index of 20. The mean follow-up time was 62 mo, with patient survival of 100% and graft survival of 95%. There were 13 reinterventions comprising one early unilateral transplantectomy for thrombosis. Renal function was excellent, and the morphological findings described an important growth in size in the first 2 yr before attaining the adult size. This study’s limitations include its retrospective nature and a small number of participants.
Conclusions
The present study reports excellent results with EBKT and supports the pursuit and spread of this technique.
Patient summary
In this report, we describe the technique and results of en bloc kidney transplantation. We found that results are excellent for renal function and patient survival. We conclude that en bloc kidney transplantation should be considered to increase the number of grafts.
1. Introduction
Critical organ shortage has led kidney transplantation teams to consider kidney grafts from extremes of age to expand the donor pool [1], [2]. The concept of transplanting two kidneys en bloc in a single recipient was first described experimentally in 1908 by Carrel [3] from Lyon, France. It was then performed successfully in humans by Martin et al [4] in 1969 and shortly followed by Meakins and colleagues [5] in 1972. However, there has been initial reluctance to use pediatric donors when considering the increased risk of vascular [6] and urinary complications [7], [8], hyperfiltration injury [9], and decreased function [10]. Nonetheless, en bloc kidney transplantation (EBKT) has more recently been reported to have better outcomes than single kidney transplantation from very small pediatric donors [11], [12]. Moreover, authors have demonstrated better results with en bloc kidneys than with standard kidney [13] and similar results to living donors [14], [15]. However, pediatric kidneys from small-weight donors remain an underused resource [11], [16], [17]. Long-term results of EBKT are scarce [13], [18]. We relate our single-center 18-yr experience of EBKT from small pediatric donors with perioperative and long-term outcomes and description of the technique.
2. Patients and methods
We performed a retrospective study over the past 18 yr of EBKT procedures in our center. Data were collected from the medical records regarding donor and recipient characteristics, peri- and postoperative results, and long-term outcomes. A data analysis was approved by our local institutional review board, and the study’s protocol was approved by the French Association of Urology’s ethics committee. A written letter of information was sent to all participants explaining the purpose and nature of the study.
2.1. Recipient selection
There were no specific criteria for recipients’ age and body mass index; however, our tendency was to prefer young adults with a low body mass index. We selected patients who had had no prior transplantation and no major comorbidities. Patients with uncontrolled hypertension, impaired bladder capacity, HIV infection, hepatitis C, prior pelvic irradiation, or vascular calcifications were excluded. A low immunological profile was preferred but not mandatory. Nonetheless, same blood type as that of the donor was required. Patients were informed of the possibility of receiving a pediatric en bloc graft, and they gave an oral consent.
2.2. Donor selection and organ procurement
Donors were exclusively pediatric brain-dead donors weighing ≤20 kg who had been discarded for pediatric transplantation or for whom no pediatric recipient had been found. There were no age criteria. Donors with acute kidney injury (AKI) were excluded. The procurement was performed either in our center by our surgical team or in a distant geographical site by a different team and then sent to our center. Ideal procurement was performed en bloc with both kidneys, abdominal aorta and vena cava, the ureters, and the full bladder (Fig. 1A). The vessels needed sufficient suprarenal length to permit the closure and as long as possible distally. The graft was flushed with IGL-1 (Institut-Gustave-Lopez 1) preservation solution and placed in a hypothermic static preservation container at 4 °C.
Fig. 1.
(A) En bloc procurement. (B) Suprarenal aorta and vena cava sealing. (C) Bladder patch technique. (D) Liech-Gregoire anastomoses.
2.3. Backbench procedure and surgical technique
The surgery was performed in almost all cases by the same highly experienced surgeon. Aortic lumbar vessels were ligated carefully. Suprarenal aorta and vena cava were sealed by a continuous suture of polypropylene 5.0 (Fig. 1B). Whenever the suprarenal aorta was too short, an iliac artery graft from the donor was used to close the aorta. No hilar dissection was performed, and some perinephric fat was left. The bladder was opened and both ureters were kept attached to a trigonal bladder patch. The recipient iliac fossa was approached in the standard fashion, preferably on the right side. The kidneys were anastomosed end to side with the donor distal aorta and vena cava to the external iliac (or primary) artery and external iliac (or primary) vein, respectively, with two hemicontinuous sutures of polydiaxone 5.0. The kidneys were fixed temporarily during the anastomoses either by their perinephric fat, which was attached to the pelvic wall, or with a sterile pad used as a hammock to prevent any vascular twist. Intravenous heparine was administered during the vascular anastomoses. The urinary anastomosis was performed by an anastomosis of the trigonal patch to the recipient’s bladder dome (Fig. 1C), by two separate ureterovesical anastomoses following the Liech-Gregoire’s technique (Fig. 1D), or by a pyeloureterostomy to the recipient’s native ureter for one of the kidneys and a ureterovesical anastomosis following Liech-Gregoire for the second kidney. Sutures were performed with polydiaxone 4.0. Ureteral stents were placed exclusively when performing ureterovesical anastomoses and pyeloureterostomy, but not in the case of the bladder patch technique. The kidneys were then fixed to the psoas or the peritoneum to prevent vascular torsion, kinking, or twisting.
2.4. Immunosuppression and perioperative treatments
Either basiliximab or antithymocyte globulin were used for induction therapy followed by triple therapy with mycophenolate mofetil, steroids, and either cyclosporine or tacrolimus. Residual cyclosporine target was 150–300 μg/l in the initial phase and 100–200 μg/l after 6 mo. Long-term antiplatelet therapy was systematically prescribed as soon as the first postoperative day. Additional anticoagulant therapy was prescribed in case of very small donor vessels. Antihypertensive treatment was prescribed in case of hypertension with a target of blood pressure inferior to 140/90 mmHg.
2.5. Definitions
Delayed graft function was defined as the need for at least one dialysis in the week following kidney transplantation. Primary nonfunction was defined as failure to decrease plasmatic creatinine in the context of preemptive transplantation, failure to stop dialysis, or early transplantectomy. The need to undertake permanent dialysis, double transplant nephrectomy, or the patient’s death with a functional graft was considered as an allograft loss. Acute rejection was suspected clinically when facing an unexplained elevation of creatinine and was confirmed by a percutaneous biopsy. Warm ischemia time was defined as the duration of the vascular sutures during which the transplant was removed from the ice and not yet perfused.
2.6. Histological assessment
We performed no systematic biopsies. Ultrasound (US)-guided percutaneous biopsies were performed during follow-up in case of an unexplained increase of creatinine to detect rejection. The Banff classification was followed to determine rejection. In case of rejection, steroids were prescribed.
2.7. US measurements
An US study was performed by a radiologist the day following transplantation, at 15 d postoperatively, and then at 1, 6, and 12 mo postoperatively. Afterwards, the US study was performed yearly. An US measurement was also performed “on demand” in case of an increase of serum creatinine or a urinary tract infection (UTI). For each study, craniocaudal size was measured for both kidneys. A mean resistive index was given for each kidney based on automated measurements in the parenchyma (the systolic peak of velocity minus the end of diastole velocity divided by the systolic peak of velocity). Velocities in the donor aorta and renal arteries were also measured.
2.8. Follow-up
Patients were recalled after 1 mo and every 3 mo thereafter for 2 yr, and yearly afterward. A physical examination was performed with a verification of body weight, blood pressure, and a search for skin and general neoplasia. Biological assessment consisted of creatinine and estimated clearance, screening of infectious diseases, and search of donor-specific antibody (DSA). A US evaluation was performed at each consultation.
2.9. Statistical method
Continuous variables are expressed as means and standard deviations, and categorical variables are expressed as numbers and percentages. A two-sided p value of <0.05 was considered significant. An analysis of data was performed using RStudio version 1.2.5033 (RStudio, Inc., PBC, Boston, MA) and Microsoft Excel version 16.38 (Microsoft Corporation, One Microsoft WayRedmond, WA 98052-7329 USA).
3. Results
3.1. Donor and recipient characteristics
From 2002 to 2020, we performed 21 EBKT procedures from small pediatric donors in our institution. Donor and recipient characteristics are shown in Table 1, Table 2, respectively. Data could not be retrieved for the first three donors. Organ procurement was performed mostly in a geographically distant site. It was performed by the local surgical team, but in two cases, our surgical team had to be transported by air because no local team had enough experience. All donors were brain dead; however, up to half of the donors had presented a cardiac arrest, which recovered rapidly. No donor presented AKI; nonetheless, oligoanuria and proteinuria were often present in the intensive care unit.
Table 1.
Donor characteristics
| Female, n (%) | 9 (50) |
| Age (mo), mean ± SD | 12 ± 7 |
| ICU length (d), mean ± SD | 2 ± 1 |
| Weight (kg), mean ± SD | 8.6 ± 2.1 |
| Height (cm), mean ± SD | 75 ± 9 |
| Distant donor site, n (%) | 14 (78) |
| Cause of death, n (%) | |
| Anoxia | 9 (50) |
| Head trauma | 6 (33) |
| Meningitis | 1 (6) |
| Cerebrovascular disease | 2 (11) |
| Vasopressive drug | 14 (78) |
| Recovered cardiac arrest | 10 (56) |
| Initial creatinine (μmol/l), mean ± SD | 34 ± 13 |
| Final creatinine (μmol/l), mean ± SD | 28 ± 11 |
| Proteinuria (g/l), mean ± SD | 0.71 ± 1.72 |
| Initial diuresis (ml/h), mean ± SD | 105 ± 113 |
| Final diuresis (ml/h), mean ± SD | 43 ± 34 |
ICU = intensive care unit; SD = standard deviation.
Results are shown for 18 of the 21 donors. Data could not be retrieved for the remaining three donors.
Table 2.
Recipient characteristics
| Female, n (%) | 11 (52) |
| BMI (kg/m2), mean ± SD | 20 ± 1.9 |
| Age (yr), mean ± SD | 30 ± 10 |
| First transplant, n (%) | 21 (100) |
| ASA score, mean ± SD | 2.3 ± 0.5 |
| CCI | 2.2 ± 0.6 |
| Primary renal disease, n (%) | |
| APKD | 3 (14) |
| Berger’s disease | 2 (10) |
| Unknown | 7 (33) |
| Urological malformation | 2 (10) |
| Other | 7 (33) |
| Hypertension, n (%) | 14 (67) |
| Number of antihypertensive treatment, mean ± SD | 1 ± 1.1 |
| Diabetes, n (%) | 0 (0) |
| Blood type, n (%) | |
| A | 9 (42) |
| B | 2 (10) |
| O | 8 (38) |
| Missing data | 2 (10) |
| Preemptive transplantation, n (%) | 3 (14) |
| Hemodialysis, n (%) | 15 (71) |
| Peritoneal dialysis, n (%) | 3(14) |
| Duration of dialysis (yr), mean ± SD | 2 ± 1.9 |
| Residual diuresis (ml), mean ± SD | 961 ± 787 |
APKD = autosomal polycystic kidney disease; ASA = American Society of Anesthesiologists; BMI = body mass index; CCI = Charlson Comorbidity Index; SD = standard deviation.
3.2. Peri- and postoperative outcomes
Perioperative outcomes are described in Table 3. The bladder was not retrieved systematically by the surgical teams from distant geographic sites. The technique for the urinary anastomosis depended greatly on the quality of the bladder patch and distal ureters, and on the surgeon’s appreciation. The first three recipients were treated with cyclosporine, whereas all the following recipients were treated with tacrolimus.
Table 3.
Perioperative outcomes
| CIT (h), mean ± SD | 11.4 ± 4.3 |
| WIT (min), mean ± SD | 33 ± 10 |
| Intraoperative blood loss (ml), mean ± SD | 320 ± 170 |
| Hospitalization time (d), mean ± SD | 16 ± 9 |
| Urinary anastomosis, n (%) | |
| Bladder patch | 15 (71) |
| Two ureterovesical anastomoses | 5 (24) |
| Ureterovesical + pyeloureterostomy | 1 (5) |
| Induction, n (%) | |
| Basiliximab | 12 (57) |
| ATG | 8 (38) |
| Data missing | 1 (5) |
| Immunosuppression, n (%) | |
| Cyclosporine | 3 (14) |
| Tacrolimus | 18 (86) |
ATG = antithymocyte globulin; CIT = cold ischemia time; SD = standard deviation; WIT = warm ischemia time.
The postoperative and late complications are summarized in Table 4. There were one transplantectomy of both kidneys at postoperative day 1 due to a venous thrombosis and an early transplantectomy of a single kidney due to an arterial thrombosis; the remaining kidney was functional. Postoperative complications requiring a reintervention occurred mostly within the first 3 mo and were dominated by urological features. Out of the five ureteral reimplantations, three where necessary after a necrosis of the bladder patch, while the other two were consequences of stenoses of the ureterovesical anastomoses. Among the other reinterventions, two were performed for a kinking or twist of the vascular pedicle, one for intravesical blood clot, one for extravesical hematoma, and one for a surgical drain caught in the deep sutures. As for the nonsurgical complications, one-third of the patients were treated for a UTI and nearly half of the patients required a peri- or postoperative blood transfusion. One patient presented acute pulmonary edema and underwent dialysis the week following transplantation. In the long term, two acute rejections occurred, which responded favorably to an increase in steroids. However, one of these two patients developed a fistula after biopsy and had to be treated by selective radiological embolization. Out of the five patients who presented an arterial stenosis, only one necessitated angioplasty and stenting with a favorable outcome. To date, no patient developed any neoplasia. Three patients had antiviral treatment following cytomegalovirus activation. One single patient produced DSA without functional repercussions. Finally, one recipient presented kidney stones in one of the kidney grafts 18 yr after the transplantation and will be undergoing surgical treatment.
Table 4.
Postoperative complications
| DGF, n (%) | 1 (5) |
| PNF, n (%) | 1 (5) |
| 90-d Clavien-Dindo grade, n (%) | |
| <3 | 10 (48) |
| ≥3 | 10 (48) |
| 3A | 1 (5) |
| 3B | 6 (29) |
| 4A | 3 (14) |
| Vascular thrombosis, n (%) | 2 (10) |
| 90-d urological complication, n (%) | 7 (33) |
| Vesical clotting | 2 (10) |
| Bladder patch necrosis | 3 (14) |
| Ureteral stenosis | 1 (5) |
| Surgical drain caught in deep sutures | 1 (5) |
| UTI, n (%) | 9 (43) |
| Blood transfusion, n (%) | 7 (33) |
| Rejection, n (%) | 2 (10) |
| Arterial stenosis, n (%) | 5 (24) |
| 90-d readmissions, n (%) | 8 (38) |
| 90-d reoperations, n (%) | |
| Unilateral transplantectomy | 1 (5) |
| Bilateral transplantectomy | 1 (5) |
| Ureteral reimplantation | 5 (24) |
| Other | 5 (24) |
| Graft loss, n (%) | |
| Thrombosis | 1 (5) |
| Death with a functioning graft | 0 (0) |
| Late rejection | 0 (0) |
| Follow-up (mo), mean ± SD | 62 ± 55 |
DGF = delayed graft function; PNF = primary nonfunction; UTI = urinary tract infection.
3.3. Functional outcomes
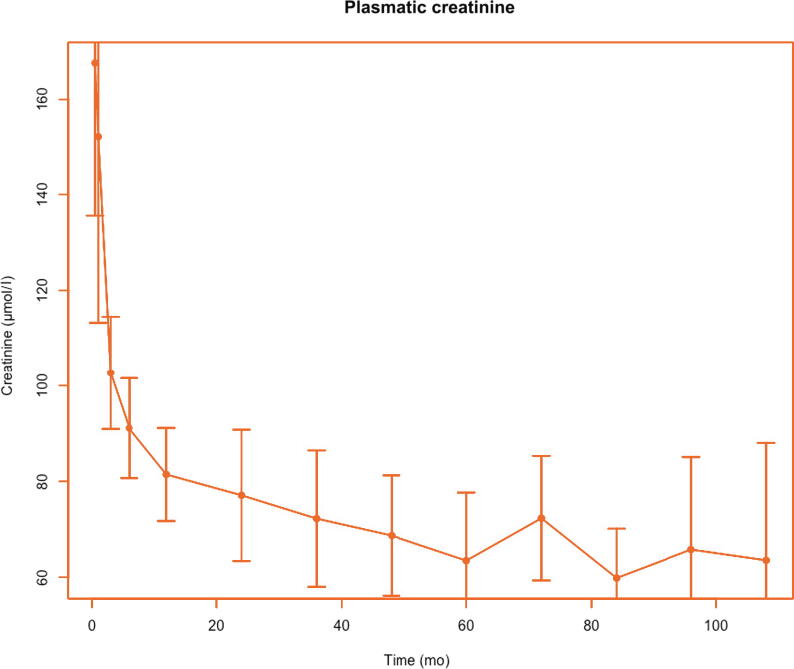
Patient survival was excellent (100%) with a mean follow-up of 65 mo (range 7–220 mo). Overall, there was one single graft loss (7%), which was a result of the early transplantectomy for venous thrombosis. To date, all the remaining transplants have satisfactory function. Evolution of plasmatic creatinine and creatinine clearance are shown in Fig. 2, Fig. 3, respectively. Creatinine clearance increased during the first 3 yr before reaching stabilization. At 10 yr, the mean creatinine clearance was 112 ml/min (95% confidence interval 107–117). As for graft size, the mean graft size increased in the first 2 yr after transplantation until reaching an adult size (Fig. 4). A few patients presented temporary proteinuria in the early post-transplant period, but it disappeared rapidly and spontaneously in all cases (data not shown).
Fig. 2.
Mean plasmatic creatinine over time with 95% confidence interval.
Fig. 3.
Mean creatinine clearance over time with 95% confidence interval.
Fig. 4.
Mean graft size over time with 95% confidence interval.
4. Discussion
The studies reporting results on EBKT are scarce. The historical first negative results [9], [10], [19] have contributed to the rejection of the technique in the past. Nonetheless, all recent studies have reported favorable outcomes with EBKT. Furthermore, Kizilbash et al [20] recently highlighted the fact that lower survival was seen for EBKT in the old transplant era (before 1997) but not afterward.
The present study is the first to present results concerning EBKT in France as no other center has, to our knowledge, performed this technique in our country. Our strength is the length of our follow-up, with the program having started nearly 20 yr ago. Moreover, graft size was evaluated continuously over time, providing results for a very long period. There have been very few studies evaluating graft size after pediatric kidney transplantation in adults and concerned mostly single kidneys with a mean follow-up rarely over 24 mo [1], [21], [22], [23]. Nonetheless, our results are comparable with previous studies and show a rapid growth in the 1st year, before reaching the final size at 24 mo and remaining stable thereafter. Foss et al [21] found a 2.6-fold increase in volume at 12 mo and Nghiem et al [23] a three-fold at 6 mo. Our very late results in graft size concern a small number of patients, and the small decrease is accompanied by an increase in the width of the 95% confidence interval, which does not allow drawing any conclusions.
Our results show excellent patient (100%) and graft (93%) survival after EBKT. However, we experienced 33% of urological complications, with many patients needing a reintervention. Nevertheless, Fananapazir et al [8] demonstrated that however common in EBKT, urological complications did not have an impact on graft survival. They reported a 9.8% rate of strictly urological complications (after excluding vascular complications and early graft loss). López-González et al [18] reported a vascular complication rate of 23.8% and 16.7% of graft loss.
This study is subject to the limit of being retrospective and of small size. However, the number of EBKT procedures performed in our institution is relatively respectable when comparing with similar studies. Maluf and colleagues [11], in their national American retrospective study, stated that centers were regarded as large-volume centers of EBKT after having performed five or more EBKT procedures.
A potential downside of our study was the lack of standardization of the surgical technique. However, this greatly depends on the quality of the organs that were harvested and cannot be controlled. On the contrary, it should appear as a strength to have been able to adapt our technique to the organs procured. The complication rate did not decrease over time despite growing EBKT experience in our center and remained stable (data not shown).
Recently, Troppmann et al [24], [25], [26] reported favorable results with EBKT after donors’ circulatory arrest and even AKI. Moreover, the same team presented the possibility of using hypothermic pulsatile perfusion and pediatric en bloc transplants [27]. These results are in favor of “extending” criteria for pediatric en bloc grafts and thereby extending the donor pool.
The choice between EBKT and single kidney transplantation from pediatric donors remains an issue. Several studies have shown superiority of EBKT over single kidneys from very small pediatric donors [11], [15], [16], [28], but it appears that single kidney transplantation could be a valid option in certain cases [29]. In our experience, one en bloc kidney graft presented arterial thrombosis requiring a transplantectomy of one single kidney; the outcomes were favorable in terms of graft function for the remaining kidney.
5. Conclusions
We consider EBKT to be a valid option to expand the donor pool. Long-term function and survival are excellent. There was a high rate of postoperative complications. However, the complications were managed successfully with a low impact on survival and should not restrain transplant teams from using this technique.
Author contributions: Emilien Seizilles de Mazancourt had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Seizilles de Mazancourt, Matillon, Badet, Terrier.
Acquisition of data: Seizilles de Mazancourt, Matillon, Badet, Codas Duarte.
Analysis and interpretation of data: Seizilles de Mazancourt, Matillon, Badet.
Drafting of the manuscript: Seizilles de Mazancourt, Matillon.
Critical revision of the manuscript for important intellectual content: Crouzet, Morelon, Badet.
Statistical analysis: Seizilles de Mazancourt.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: Matillon, Badet.
Other: None.
Financial disclosures: Emilien Seizilles de Mazancourt certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: None.
Funding/Support and role of the sponsor: None.
Associate Editor: Silvia Proietti
References
- 1.Sanchez-Fructuoso A.I., Prats D., Perez-Contin M.J., et al. Increasing the donor pool using en bloc pediatric kidneys for transplant. Transplantation. 2003;76:1180–1184. doi: 10.1097/01.TP.0000090395.98045.09. [DOI] [PubMed] [Google Scholar]
- 2.Rogers J., Farney A.C., Orlando G., et al. Dual kidney transplantation from donors at the extremes of age. J Am Coll Surgeons. 2019;228:690–705. doi: 10.1016/j.jamcollsurg.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Carrel A. Transplantation in mass of the kidneys. J Exp Med. 1908;10:98–140. doi: 10.1084/jem.10.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin L.W., Gonzalez L.L., West C.D., Swartz R.A., Sutorius D.J. Homotransplantation of both kidneys from an anencephalic monster to a 17 pound boy with Eagle-Barrett syndrome. Surgery. 1969;66:603–607. [PubMed] [Google Scholar]
- 5.Meakins J.L., Smith E.J., Alexander J.W. En bloc transplantation of both kidneys from pediatric donors into adult patients. Surgery. 1972;71:72–75. [PubMed] [Google Scholar]
- 6.Memel D.S., Dodd G.D., Shah A.N., et al. Imaging of en bloc renal transplants: normal and abnormal postoperative findings. AJR Am J Roentgenol. 1993;160:75–81. doi: 10.2214/ajr.160.1.8416653. [DOI] [PubMed] [Google Scholar]
- 7.Routh J.C., Yu R.N., Kozinn S.I., Nguyen H.T., Borer J.G. Urological complications and vesicoureteral reflux following pediatric kidney transplantation. J Urol. 2013;189:1071–1076. doi: 10.1016/j.juro.2012.09.091. [DOI] [PubMed] [Google Scholar]
- 8.Fananapazir G., Tse G., Di Geronimo R., et al. Urologic complications after transplantation of 225 en bloc kidneys from small pediatric donors ≤20 kg: incidence, management, and impact on graft survival. Am J Transplant. 2020;20:2126–2132. doi: 10.1111/ajt.15792. [DOI] [PubMed] [Google Scholar]
- 9.Hayes J.M., Steinmuller D.R., Streem S.B., Novick A.C. The development of proteinuria and focal-segmental glomerulosclerosis in recipients of pediatric donor kidneys. Transplantation. 1991;52:813–817. doi: 10.1097/00007890-199111000-00011. [DOI] [PubMed] [Google Scholar]
- 10.Ruder H., Schaefer F., Gretz N., Möhring S., Schärer K. Donor kidneys of infants and very young children are unacceptable for transplantation. Lancet. 1989;334:168. doi: 10.1016/s0140-6736(89)90236-5. [DOI] [PubMed] [Google Scholar]
- 11.Maluf D.G., Carrico R.J., Rosendale J.D., Perez R.V., Feng S. Optimizing recovery, utilization and transplantation outcomes for kidneys from small, ≤20 kg, pediatric donors. Am J Transplant. 2013;13:2703–2712. doi: 10.1111/ajt.12410. [DOI] [PubMed] [Google Scholar]
- 12.Bresnahan B.A., McBride M.A., Cherikh W.S., Hariharan S. Risk factors for renal allograft survival from pediatric cadaver donors: an analysis of united network for organ sharing data. Transplantation. 2001;72:256–261. doi: 10.1097/00007890-200107270-00016. [DOI] [PubMed] [Google Scholar]
- 13.Thomusch O., Tittelbach-Helmrich D., Meyer S., Drognitz O., Pisarski P. Twenty-year graft survival and graft function analysis by a matched pair study between pediatric en bloc kidney and deceased adult donors grafts. Transplantation. 2009;88:920–925. doi: 10.1097/TP.0b013e3181b74e84. [DOI] [PubMed] [Google Scholar]
- 14.Sharma A., Fisher R.A., Cotterell A.H., King A.L., Maluf D.G., Posner M.P. En bloc kidney transplantation from pediatric donors: comparable outcomes with living donor kidney transplantation. Transplantation. 2011;92:564–569. doi: 10.1097/TP.0b013e3182279107. [DOI] [PubMed] [Google Scholar]
- 15.Sureshkumar K.K., Habbach A., Tang A., Chopra B. Long-term outcomes of pediatric en bloc compared to living donor kidney transplantation: a single-center experience with 25 years follow-up. Transplantation. 2018;102:e245–e248. doi: 10.1097/TP.0000000000002104. [DOI] [PubMed] [Google Scholar]
- 16.Pelletier S.J., Guidinger M.K., Merion R.M., et al. Recovery and utilization of deceased donor kidneys from small pediatric donors. Am J Transplant. 2006;6:1646–1652. doi: 10.1111/j.1600-6143.2006.01353.x. [DOI] [PubMed] [Google Scholar]
- 17.Kayler L.K., Magliocca J., Fujita S., et al. Recovery factors affecting utilization of small pediatric donor kidneys. Am J Transplant. 2009;9:210–216. doi: 10.1111/j.1600-6143.2008.02447.x. [DOI] [PubMed] [Google Scholar]
- 18.López-González J.A., Beamud-Cortés M., Bermell-Marco L., et al. A 20-year experience in cadaveric pediatric en bloc kidney transplantation in adult recipients. Actas Urol Esp. 2022;46:85–91. doi: 10.1016/j.acuroe.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Gourlay W., Stothers L., McLoughlin M.G., Manson A.D., Keown P. Transplantation of pediatric cadaver kidneys into adult recipients. J Urol. 1995;153:322–325. doi: 10.1097/00005392-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Kizilbash S.J., Evans M.D., Chinnakotla S., Chavers B.M. Survival benefit of en bloc transplantation of small pediatric kidneys in children. Transplantation. 2020;104:2435–2443. doi: 10.1097/TP.0000000000003158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foss A., Line P.-D., Brabrand K., Midtvedt K., Hartmann A., et al. A prospective study on size and function of paediatric kidneys (<10 years) transplanted to adults. Nephrol Dialysis Transplant. 2007;22:1738–1742. doi: 10.1093/ndt/gfm080. [DOI] [PubMed] [Google Scholar]
- 22.Zhu L., Fu C., Chen S., et al. Successful single-kidney transplantation in adult recipients using pediatric donors aged 8 to 36 months: comparable outcomes with those using pediatric donors aged >3 years. Transplantation. 2019;103:2388–2396. doi: 10.1097/TP.0000000000002618. [DOI] [PubMed] [Google Scholar]
- 23.Nghiem D.D., Hsia S., Schlosser J.D. Growth and function of en bloc infant kidney transplants: a preliminary study. J Urol. 1995;153:326–329. doi: 10.1097/00005392-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Troppmann C., Santhanakrishnan C., Fananapazir G., Troppmann K., Perez R. Pediatric en bloc kidney transplantation from very small (≤10 kg) donation after circulatory death (versus brain death) donors: single-center matched-pair analysis of 130 transplants. Am J Transplant. 2018;18:2811–2817. doi: 10.1111/ajt.14914. [DOI] [PubMed] [Google Scholar]
- 25.Troppmann C., Santhanakrishnan C., Troppmann K., Fananapazir G., Perez R. Does acute kidney injury contraindicate transplantation of kidneys from very small pediatric donors? Single-center analysis of 68 en bloc kidney transplants from donors ≤15 kg. Transplantation. 2018;102:S175. [Google Scholar]
- 26.Troppmann C., Santhanakrishnan C., Fananapazir G., Sageshima J., Troppmann K.M., Perez R.V. Short- and long-term outcomes of kidney transplants from very small (≤15 kg) pediatric donors with acute kidney injury. Transplantation. 2021;105:430–435. doi: 10.1097/TP.0000000000003230. [DOI] [PubMed] [Google Scholar]
- 27.Troppmann C., Daily M.F., McVicar J.P., Perez R.V. Hypothermic pulsatile perfusion of small pediatric en bloc kidneys: technical aspects and outcomes. Transplantation. 2009;88:289–290. doi: 10.1097/TP.0b013e3181acc8b3. [DOI] [PubMed] [Google Scholar]
- 28.Bhayana S., Kuo Y.-F., Madan P., et al. Pediatric en bloc kidney transplantation to adult recipients: more than suboptimal? Transplantation. 2010;90:248–254. doi: 10.1097/TP.0b013e3181e641f8. [DOI] [PubMed] [Google Scholar]
- 29.Balachandran V.P., Aull M.J., Goris M., Figueiro J., Leeser D.B., Kapur S. Successful transplantation of single kidneys from pediatric donors weighing less than or equal to 10 kg into standard weight adult recipients. Transplantation. 2010;90:518–522. doi: 10.1097/TP.0b013e3181e98d35. [DOI] [PubMed] [Google Scholar]