Abstract
We previously reported that a liposome-mannan vaccine (L-mann) of Candida albicans induces production of mouse antibodies that protect against disseminated candidiasis and vaginal infection. Immunoglobulin M (IgM) monoclonal antibody (MAb) B6.1, specific for a C. albicans cell surface β-1,2-mannotriose, protects mice against both infections. Another IgM MAb, termed B6, which is specific for a different cell surface mannan epitope, does not protect against disseminated candidiasis. The B6.1 epitope is displayed homogeneously over the entire cell surface, compared to a patchy distribution of the B6 epitope. To determine if protection is restricted to an IgM class of antibody, we tested an IgG antibody. MAb C3.1 was obtained from L-mann-immunized mice. By results of sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis, capture enzyme-linked immunosorbent assay, and immunodiffusion tests, MAb C3.1 is an IgG3 isotype. By epitope inhibition assays, we determined that MAb C3.1 is specific for same mannotriose as MAb B6.1. As expected by the results of the inhibition assays, immunofluorescence microscopy showed that the C3.1 epitope is distributed on the yeast cell surface in a pattern identical to that of the B6.1 epitope. Kidney CFU and mean survival times of infected mice pretreated with MAb C3.1 indicated that the antibody enhanced resistance of mice against disseminated candidiasis. Mice in pseudoestrus that were given MAb C3.1 prior to vaginal infection developed fewer vaginal Candida CFU than control animals that received buffered saline instead of the antibody. The finding that an IgG3 antibody is protective is consistent with our hypothesis that epitope specificity and complement activation are related to the ability of an antibody to protect against candidiasis.
In experimental animals, host defense against cryptococcosis and candidiasis involves virtually all aspects of the immune system, including antibodies (7). The role of antibodies in defense against these diseases in humans is not known, but suggestive evidence has been obtained that certain antibodies may be protective (6, 7, 19). Because of the possible utility in prevention and treatment of human fungal diseases, continued efforts are needed for gaining a clear definition of protective antibodies. In studies on experimental cryptococcosis, both antibody specificity and isotype are apparently important considerations. Monoclonal antibodies (MAbs) specific for epitopes within the glucuronoxylomannan capsular structure are protective. However, whereas immunoglobulin M (IgM) and IgG1 MAbs specific for the glucuronoxylomannan protect against experimental disease, an IgG3 MAb does not protect even though all three antibodies have identical levels of epitope recognition (25, 26).
We have been investigating the role of Candida-specific antibodies in host defense against various forms of candidiasis. Using an intravenous (i.v.) immunization route for administering liposomes containing Candida albicans surface mannan complexes, mice responded by making a protective antibody response against both disseminated candidiasis (11, 12) and Candida vaginal infection (14). Recently, we developed a more efficient vaccine formula made of mannan-protein conjugates. The conjugate vaccine requires fewer boosters and is administered by an intraperitoneal (i.p.) route (15). Both vaccines induced protective antibody responses that could be passively transferred to naive animals. During this work, we isolated an IgM MAb, termed MAb B6.1, which enhances resistance of normal, SCID, and neutropenic mice against disseminated disease (11, 12) and protected the animals against Candida vaginal infection (14). MAb B6.1 is specific for a β-1,2-mannotriose (13), which is an acid-labile component of the phosphomannan complex (PMC) of the C. albicans cell walls (23, 24). This oligomannosyl epitope appears to be expressed uniformly over the entire cell surface (13). In contrast to MAb B6.1, a nonprotective IgM MAb, termed MAb B6, has a patchy distribution over the cells (13). The B6 epitope is apparently associated with the acid-stable part of the PMC (13). Whereas these data show that antibody specificity is a key consideration, they do not address the relevance of Ig isotype to protection. In view of the critical role that antibody isotype plays in protection against cryptococcosis, as cited above, and in other diseases, including those due to group B streptococci (16), we investigated whether an IgG MAb can protect against experimental candidiasis. The results of these studies should help in the elucidation of mechanisms by which protective antibodies assist the host in defense against candidiasis.
In this study, we compared the protective efficacies of the IgM MAb B6.1 and an IgG3 MAb, termed C3.1, against experimental candidiasis. We show that the two MAbs are specific for the same mannotriose and both are protective against candidiasis in mice.
MATERIALS AND METHODS
Organism and culture conditions.
C. albicans CA-1 was started from frozen glycerol stocks, grown at 37°C, washed and suspended in cold Dulbecco's phosphate-buffered saline (DPBS; Sigma Chemical Co., St. Louis, Mo.), and used to infect mice as previously described (11, 14, 18).
Mice.
BALB/c female mice (NCI Animal Production Program, Frederick Cancer Research Center, Frederick, Md.) 6 to 8 weeks old were used throughout the study. In all experiments, mice were maintained in accordance with institutional regulations in an Association for Assessment and Accreditation of Laboratory Animal Care-certified animal facility at Montana State University.
Anti-C. albicans MAbs C3.1 and B6.1.
MAb C3.1 was one of several MAbs, including the protective MAb B6.1, which were isolated through hybridoma techniques from mice vaccinated with a mannan-liposome preparation as described elsewhere (11). MAb C3.1 was produced from a hybridoma clone grown in the absence of antibiotics either in RPMI 1640 (Sigma) plus 10% fetal bovine serum or in serum-free medium (HB101 Liquid kit; Irvine Scientific, Santa Ana, Calif.) without antibiotics. The MAb was concentrated by precipitation in 50% ammonium sulfate. MAb B6.1, which served as a positive control antibody, was produced by LigoCyte Pharmaceuticals, Inc. (Bozeman, Mont.) as before, and both MAbs were quantified as described previously (12, 14).
ELISA titer of MAbs.
Wells of a polystyrene microtiter plate (Costar 96-well microtiter plate; Costar Corning, Corning, N.Y.) were coated with mannan extract by adding to each well 100 μl of mannan (10 μg of mannan/ml) solubilized in 0.06 M carbonate buffer (pH 9.6), with incubation for 3 h at 37°C and at 4°C overnight. The wells were washed with deionized water, and each received 250 μl of 0.01 M Tris-buffered saline (0.01 M TBS) (0.01 M Trizma-HCl [Sigma] plus 0.15 N NaCl [pH 7.4]) with 3% bovine serum albumin (BSA) (fraction V; Sigma) for 1 h at 21 to 23°C. The buffer was replaced by 50 μl of either MAb appropriately diluted in 0.01 M TBS-BSA for 1 h at 37°C, washed three times with 0.01 M TBS containing 0.05% Tween 20 (0.01 M TBST), and washed three times with 0.01 M TBS. The 0.01 M TBS was replaced by 50 μl of peroxidase-conjugated anti-mouse polyvalent Igs (1.6 mg/ml; diluted 1:1,000 in 0.01 M TBS-BSA; Sigma) for 1 h at 21 to 23°C, washed with 0.01 M TBS, and replaced by 100 μl of substrate. The substrate was a solution made of 25 ml of 0.1 M citrate buffer (pH 5.0), 0.5 ml of a water solution of o-phenylenediamine (50 mg/ml; Sigma), and 10 μl of 30% H2O2. The color was allowed to develop for 30 min, at which time 100 μl of 10% H2SO4 was added as a stop to each well, and the optical density (OD) for each was determined at 490 nm in a microtiter plate reader (model 450; Bio-Rad, Richmond, Calif.). Negative control wells that were coated with or without the mannan extract, respectively, received only diluent (0.01 M TBS-BSA) instead of the test MAbs, and the remaining steps of the procedure were exactly as described above. Background absorbance from the negative control wells was subtracted from the test well to obtain final OD readings. The enzyme-linked immunosorbent assay (ELISA) titer was taken as the reciprocal of the last antibody dilution that gave a positive OD reading.
Standardization of antibody concentrations.
Antibodies were titered by agglutination against either whole C. albicans yeast cells or mannan-coated latex beads (11, 14) and by ELISA (14) as described above. By these various measures, the antibody concentrations and titers were determined, and working solutions of each were prepared as follows. MAb C3.1 prepared at 2.14 mg/ml gave the same agglutinin and ELISA titers as MAb B6.1 prepared at 0.21 mg/ml (40 and 2,000, respectively). For the protection assays in mice, each animal received 0.5 ml of these antibody preparations.
SDS-PAGE.
MAb C3.1 was evaluated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12.5% polyacrylamide) analysis under reducing (β-mercaptoethanol) conditions to determine sizes of heavy and light chains of the antibody. The sizes were compared to those of known IgG MAb 54.1, specific for human neutrophil cytochrome b (a gift from Jim Burritt and Al Jesaitis, Montana State University), and IgM MAb B6.1, specific for C. albicans cell wall mannan (LigoCyte). Locations of the protein bands were determined by silver staining and compared to known molecular markers (Sigma) of 205-, 116-, 97-, 66-, 45-, and 29-kDa molecules corresponding to myosin, β-galactose, phosphorylase b, albumin (bovine plasma), albumin (egg), and carbonic anhydrase, respectively.
Isotyping of MAb C3.1.
The isotype of MAb C3.1 was determined by a capture ELISA as follows. One half of the wells in a polystyrene microtiter plate (Costar 3396 EIA/RIA plate) received 100 μl of affinity-purified heavy-chain-specific goat, rabbit, or rat anti-mouse Ig. All anti-mouse Igs used in these assays were prepared at 10 μg/ml in 0.06 M carbonate buffer (pH 9.6). Anti-mouse IgA was purchased from Zymed (San Francisco, Calif.), IgE was obtained from Southern Biotechnology Associates, Inc. (Birmingham, Ala.), and anti-mouse IgG and IgM were obtained from Sigma. For negative controls, each of the remaining wells received 100 μl of the carbonate buffer solution. The plate was incubated at 21 to 23°C for 3 h. The wells were washed with Trizma (Sigma)-buffered saline solution (0.05 M Trizma-HCl [Sigma] plus 0.15 mM NaCl [pH 7.5]) three times, blocked with 1% BSA and 5% nonfat dry milk in TBST (blocking buffer), and incubated at 5 to 8°C overnight. The wells were washed once with TBST and then three times with TBS. One hundred microliters of MAb C3.1 (diluted 1:200 in blocking buffer) was added to the appropriate wells, which were then incubated at 21 to 23°C for 2 h. Negative control wells received only 100 μl of blocking buffer. After the wells were washed with TBST and TBS as described above, 100 μl of heavy-chain-specific biotinylated goat anti-mouse Ig (diluted 1:4,000 in blocking buffer) was put into the appropriate wells. Biotinylated anti-mouse IgE was obtained from Southern Biotechnology Associates, biotinylated anti-mouse IgA and IgM were from Sigma, and the remaining biotin-conjugated anti-mouse IgG, IgG1, IgG2a, IgG2b, and IgG3 were from Zymed. The wells were washed with TBST and TBS as described above, 100 μl of streptavidin-alkaline phosphatase (diluted 1:1,000 in TBST; Zymed) was added to each, and the plate was incubated at 21 to 23°C for 1 h and washed as described above. For these experiments, the substrate was prepared by dissolving 50 mg of p-nitrophenol phosphate (Sigma) in 1 ml of distilled water and diluting the solution 1:100 in substrate buffer (8.2% diethanolamine [Fisher Scientific, Fair Lawn, N.J.], 60 mM HCl, 1 mM MgCl2). One hundred microliters of substrate was added to each well, the plate was incubated in the dark at 21 to 23°C for 15 min for color development, and the results were read at 405 nm in a microtiter plate reader (model 450; Bio-Rad). As positive controls, MAb B6.1 (IgM) and a mouse IgG3 (Zymed) were used under conditions identical to those described above. In some tests, an IgG3 and an IgG1 specific for a Cryptococcus neoformans capsular glucuronoxylomannan epitope (a generous gift from A. Casadevall) (8) were used as positive controls.
In addition to the ELISA, double immunodiffusion in agarose was used to confirm the isotype of MAb C3.1. In brief, appropriate wells were made in a plate containing 1% agarose (Amresco, Solon, Ohio)-buffered saline (15 mM Trizma, 150 mM NaCl, 5 mM KCl, 1 mM EDTA, 5 mM NaN3 [pH 7.4]); 5 μl of MAb C3.1 (4.3 mg/ml) was put into a center well, and the indicated anti-mouse Igs were put into appropriate peripheral wells. The plate was incubated at 21 to 23°C under humid conditions for 48 h, and precipitin bands were stained with Coomassie blue. As a positive control, in one configuration, mouse IgG (1.0 mg/ml; Sigma) was placed in the center well. Goat anti-mouse antibodies (IgG [whole molecule], IgG1, IgG2a, IgG2b, IgG3, and IgM) placed in the peripheral wells were obtained from Sigma and were appropriately diluted in the buffered saline solution.
Mannan extraction, acid hydrolysis, and identification of MAb C3.1 epitope.
The same batch of affinity column-purified PMC, acid hydrolysate of the PMC, and P-2 column-fractionated components of the hydrolysate were used in these experiments as described previously for work on the identification of the B6.1 epitope (13). The individual fractions from the P-2 column, each of which represented a predominance of one size of a β-1,2-linked oligomannoside, were tested for the ability to react with MAb C3.1 by the mannotriose inhibition assay described for identification of the epitope recognized by MAb B6.1 (13). That is, solutions containing various amounts of each fraction per milliliter were prepared in DPBS containing 1% BSA and 0.02% sodium azide. Each fraction (10 μl) at the indicated concentrations was mixed with 10 μl of MAb C3.1 (4.3 mg/ml; 1:20 diluted in DPBS). Following mixing in solution of the various fractions with antibody, 10 μl of mannan-coated latex beads (30 × 107 beads per ml) was added to the solutions, mixed, and observed for agglutination of the beads. As a positive control for agglutination, antibody was mixed with the mannan-coated latex beads in the absence of the column fractions. As controls for specificity, the irrelevant sugars raffinose (a triose; Sigma) and stachyose (a tetraose; Sigma) were tested for the ability to inhibit agglutination of the latex-mannan beads. Concentrations of the irrelevant sugars used were the same as those indicated with the fractions above.
Fluorescence microscopy.
Distribution of the C3.1 epitope on yeast cells was determined by indirect immunofluorescence. Two hundred microliters of MAb C3.1 (at 107.5 μg/ml of DPBS) was added to a pellet of C. albicans hydrophilic yeast cells (5 × 106) that were prewashed with cold DPBS three times. The yeast cells were suspended in the antibody solution and incubated on ice for 30 min. During the incubation, the suspension was mixed by inverting the tube every 3 min. The yeast cells were washed with cold DPBS three times, suspended in 200 μl of goat anti-mouse IgG (γ-chain specific; Sigma) (stock solution, 0.8 mg/ml; working solution, 26.7 μg/ml of DPBS) and incubated on ice as described above. After washing, the yeast cells were suspended in 500 μl of fluorescein-labeled anti-goat IgG (heavy and light chains; 1:200 diluted in DPBS; Jackson ImmunoResearch Lab, West Grove, Pa.) and incubated on ice for 20 min. The yeast cells were washed and suspended in 200 μl of cold DPBS. The cells were observed by fluorescence and phase-contrast microscopy (model Labophot, series no. 234142; Nikon, Tokyo, Japan). The distribution of the C3.1 epitope on the yeast cell surface was compared to that obtained with yeast cells fluorescently stained for detection of the B6.1 epitope as described previously (13).
Protection against Candida infections by passive transfer.
MAbs C3.1 and B6.1 were used without pretreatment, pretreated at 56°C for 30 min before use, or absorbed with C. albicans yeast cells (11, 14, 15). Untreated and treated MAbs C3.1 and B6.1 were compared for the ability to protect mice against experimental disseminated candidiasis and Candida vaginal infection as previously described (11, 12, 14, 15) with one modification. Test animals were given antibody only before infection with C. albicans, rather than 4 h before and 20 h after infection. Specifically, for testing against disseminated candidiasis, the MAbs (0.5 ml) were given to mice by an i.p. route 4 h before the animals were infected by an i.v. route with yeast cells (5 × 105 per mouse). Forty-eight hours after antibody treatment, groups of animals were sacrificed and their kidneys were assessed for CFU as before (11, 12, 15). Other groups of mice were monitored for determination of mean survival times.
For testing against vaginal infection, each mouse was pretreated with 0.5 mg of estradiol (Sigma) subcutaneously. Seventy-two hours later, these mice received MAb C3.1 by an i.p. (0.5 ml/mouse) or intravaginal (i.vg.; 10 μl/mouse) route. Four hours later, the animals were infected i.vg. with yeast cells (5 × 105 per mouse in a 10-μl volume); 48 h after infection, vaginal CFU were enumerated as previously described (14). The protective effect of MAb B6.1 given i.p. was previously tested in the mouse vaginal infection model (14), but in the present study this antibody was tested only by the i.vg. route at a dose of 10 μl/mouse.
Statistical analyses.
In all cases, the minimum number of animals per group was five, and each experiment was repeated at least three times. Statistical significance of differences of survival times was measured by use of the Kaplan-Meier test (Systat 7.0; SPSS Inc., Chicago, Ill.). Survival experiments were terminated at day 27. For calculation of mean survival time, survivors were assigned a survival time of 27 days. For other analyses, Student's t test was used. P values were considered statistically significant if they were less than 0.05.
RESULTS
MAb C3.1 is an IgG3.
SDS-PAGE run under reducing conditions revealed molecular sizes of heavy and light chains of MAb C3.1 of approximately 50 and 27 kDa, respectively (gels not shown), the former of which is consistent with the expected size of an IgG heavy chain (2). Under identical electrophoretic conditions, MAb B6.1 produced heavy and light chains of 76 and 25 kDa, the former of which is consistent with the expected size of an IgM heavy chain.
Results of the capture ELISA and double immunodiffusion in agar showed that the isotype of MAb C3.1 is an IgG3. By ELISA, MAb C3.1 gave a color development of about 0.77 OD unit at 405 nm when interacted with anti-IgG3, whereas the OD was <0.02 following an interaction of MAb C3.1 with anti-IgM, anti-IgG1, anti-IgG2a, anti-IgG2b, anti-IgA, and anti-IgE. The control antibody, MAb B6.1 (IgM), reacted specifically with anti-IgM (OD405 of approximately 1.0). Results obtained for MAb C3.1 were the same as those for the IgG3 MAb specific for a glucuronoxylomannan capsular epitope from C. neoformans. The double immunodiffusion (gels not shown) was confirmatory in that visible precipitin bands occurred between the well containing MAb C3.1 and peripheral wells containing whole anti-IgG and anti-IgG3 but not with the peripheral well containing anti-IgM. The pooled mouse IgG control produced obvious precipitin bands with whole anti-IgG, anti-IgG1, and anti-IgG2b but not with anti-IgM.
Identification of the MAb C3.1 epitope.
For isolation of the epitope recognized by MAb C3.1 (i.e., the C3.1 epitope), the same PMC preparation was used in these studies as in those for identification of the B6.1 epitope (13). That is, the PMC was hydrolyzed in 10 mM HCl to release the acid-labile oligomannosyl residues from the remaining acid-stable part. The resulting oligomannosyl residues were separated on a P-2 column. The column resolved four acid-stable fractions and seven oligomannosyl acid-labile peaks (fractions I to VII). Acid-labile fractions III and IV inhibited agglutination of mannan-coated latex beads (Table 1), whereas none of the other acid-labile moieties or the acid-stable part of the PMC inhibited agglutination. Likewise, raffinose (triose) and stachyose (tetraose) did not affect the agglutination (Table 1). These results are identical to those obtained on studies of the epitope recognized by MAb B6.1, indicating that MAb C3.1 is also specific for β-1,2-mannotriose.
TABLE 1.
P-2 column fractions (III and IV) contain a β-1,2-mannotriose and inhibit MAb C3.1-dependent agglutination of mannan-coated latex beadsa
| Expt | Fraction | Agglutinationb at fraction concn (μg/ml) of:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2,000 | 1,800 | 1,600 | 1,400 | 1,200 | 1,000 | 500 | 250 | 200 | 20 | 2 | ||
| 1A | I, II, and V–VII | NI | NI | NI | NI | |||||||
| III | I | NI | NI | NI | ||||||||
| IV | I | NI | NI | NI | ||||||||
| Acid-stable PMCc | NI | NI | NI | NI | ||||||||
| Raffinose (triose control) | NI | NI | NI | NI | ||||||||
| Stachyose (tetraose control) | NI | NI | NI | NI | ||||||||
| 1B | III | I | I | I | I | NI | NI | NI | NI | |||
| IV | I | I | I | I | NI | NI | NI | NI | ||||
| Mannan (whole) | I | I | I | NI | NI | NI | NI | NI | ||||
| 2 | I, II, and V–VII | NI | NI | NI | NI | NI | NI | NI | ||||
| III | I | I | I | NI | NI | NI | NI | |||||
| IV | I | I | I | NI | NI | NI | NI | |||||
| Acid-stable PMC | NI | NI | NI | NI | NI | NI | NI | |||||
Oligomannosyl residues that were released from the PMC as a result of acid hydrolysis (acid-labile components) were separated by size on a P-2 (acrylamide gel) column. Each fraction designation denotes the number of mannose units as determined from mass spectral analysis and control elution profiles (13). For two or more mannose units per fraction, the mannose units are β-1,2 linked as determined by two-dimensional nuclear magnetic resonance analysis (13). Each fraction (10 μl) at the indicated concentrations was mixed with 10 μl of MAb C3.1, and then a 10-μl volume of mannan-coated latex beads was added, mixed, and observed for agglutination of the beads. In experiments 1A and 1B, the mannan-latex agglutination titer of MAb C3.1 was 40; for experiment 2, the titer was 5.
NI, not inhibited; I, inhibited.
The part of the PMC that is devoid of the β-1,2-linked oligomannosyl chains that are bonded to the acid-stable part of the complex via phosphodiester bonds.
Cell surface distribution of the C3.1.
Immunofluorescence microscopy was used to approximate the cell surface distribution pattern of the C3.1 epitope. Stationary-phase yeast cells displayed a uniform distribution of epitope over the entire cell surface of C. albicans (Fig. 1). This distribution was essentially identical to the distribution of the B6.1 epitope (13). Comparing yeast cells observed by bright-field microscopy with those that fluoresced showed that some yeast cells and all of the blastoconidia were nonreactive with MAb C3.1. These results are also identical to those with MAb B6.1.
FIG. 1.
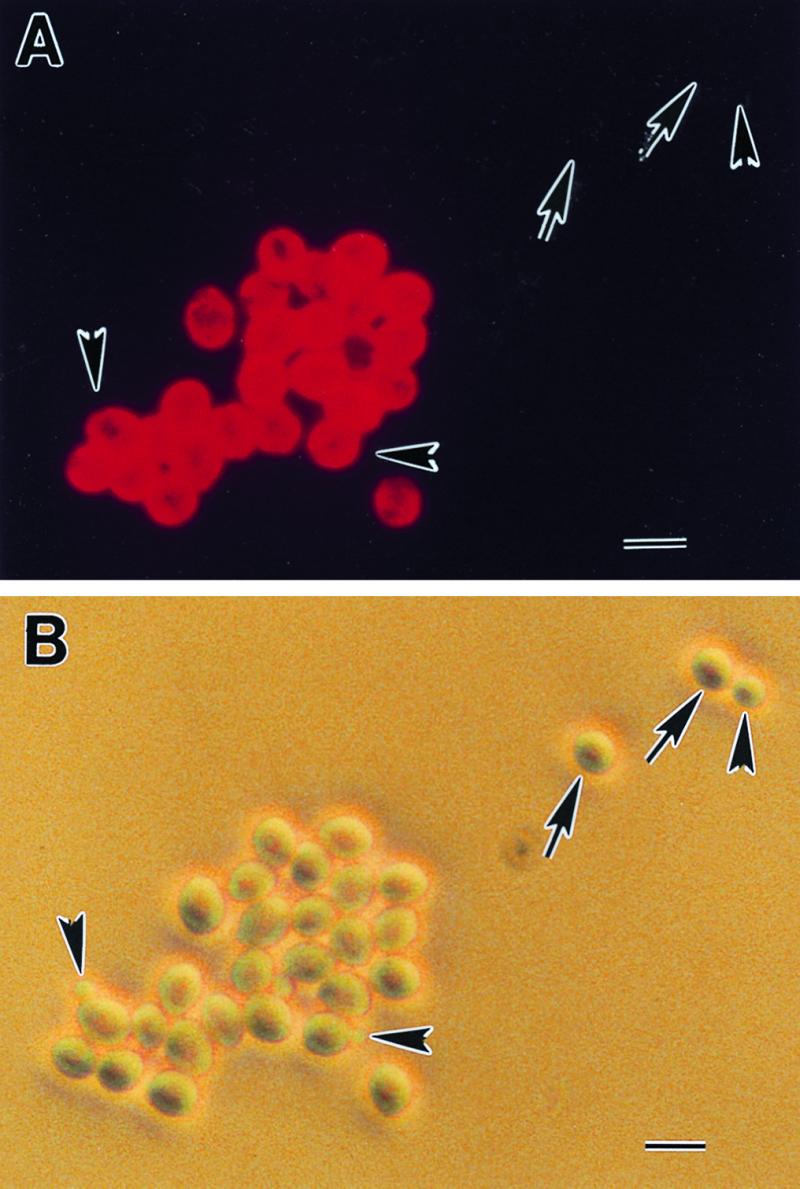
The C3.1 epitope is uniformly distributed on the cell surface of yeast forms of C. albicans. Hydrophilic stationary-phase yeast cells were reacted with MAb C3.1, washed, and counterreacted with fluorescence-labeled anti-mouse IgG. The C3.1 epitope is located over the entire cell surface (A). The same field was photographed under phase-contrast microscopy (B). The C3.1 epitope distribution displays the same homogeneous pattern over the cell surface as the epitope recognized by the IgM protective antibody, MAb IgM B6.1. These results provide further evidence that the IgG3 antibody is specific for the same oligomannoside as the protective IgM. Note that some yeast cells (arrows) and all of the blastoconidia (arrowheads) were nonreactive with MAb C3.1. The importance of nonreactive cells in pathogenesis and protection experiments is unknown. Bar, 10 μM.
MAb C3.1 transfers protection against disseminated candidiasis.
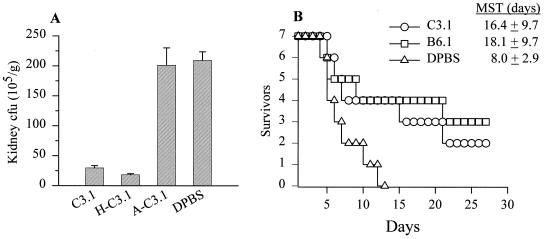
BALB/c mice were given unheated MAb C3.1 (C3.1), MAb C3.1 heated at 56°C for 30 min (H-C3.1), C. albicans-absorbed MAb C3.1 (A-C3.1), or DPBS (buffer diluent) by an i.p. route and were then infected with viable yeast cells i.v. (Fig. 2A). In other experiments, mice were given C3.1, MAb B6.1, or DPBS to compare the prophylactic potentials of the two MAbs (Fig. 2B). Susceptibility to disseminated disease was assessed by determining kidney CFU (Fig. 2A) and by survival curves (Fig. 2B). The mean CFU values (105) per gram of kidney ± standard error for mice given C3.1, H-C3.1, or A-C3.1, and DPBS were 30 ± 6, 25 ± 6, 200 ± 50, and 210 ± 25. That is, mice that received either C3.1 or H-C3.1 had 86 and 88% fewer CFU, respectively, than control mice that received DPBS (P < 0.001). Mice that were given the A-C3.1 developed almost the same number of CFU as the control animals. Analyses of mean survival times (Fig. 2B) showed that mice given C3.1 had extended survival times similar to those for animals given MAb B6.1. These survival times were significantly longer than those for animals given DPBS (P < 0.05).
FIG. 2.
MAb C3.1 transfers protection against disseminated candidiasis. (A) Mice given unheated MAb C3.1 (C3.1), H-C3.1, A-C3.1, or DPBS (buffer diluent) i.p.; (B) mice given MAb C3.1, MAb B6.1, or DPBS as a control. In both panels, the animals were challenged i.v. with 5 × 105 viable yeast cells, and susceptibility to disseminated disease was assessed by determining kidney CFU (A) and by survival curves (B). (A) Mice that received the unheated or heated MAb C3.1 had 86 and 88% fewer CFU, respectively, than mice that received DPBS (P < 0.001). Mice that were given the absorbed serum developed almost the same number of CFU as mice that received DPBS (control). Bars show standard errors. (B) Mice given MAb C3.1 had survival times similar to those of mice given MAb B6.1. Their mean survival times were significantly longer than for animals given DPBS (P < 0.05).
MAb C3.1 has a prophylactic effect against Candida vaginal infection.
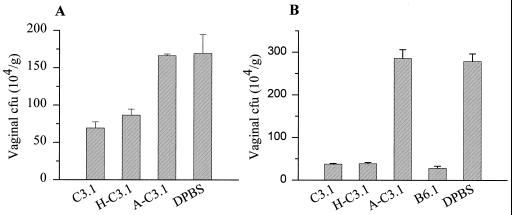
Pseudoestrus mice were given MAb C3.1 i.p. or i.vg. before an i.vg. infection with 5 × 105 yeast cells (Fig. 3). Vaginal CFU counts were compared with CFU from animals that were given unheated C3.1, H-C3.1, A-C3.1, or DPBS (diluent). Mice that received MAb C3.1 or H-C3.1 i.p. developed 60 and 49% fewer CFU, respectively, than DPBS control mice. Mice that received A-C3.1 developed CFU to levels similar to those for control mice that received DPBS i.vg. prior to infection (Fig. 3A). Mice that received either C3.1, H-C3.1, or MAb B6.1 developed approximately 86% fewer CFU than DPBS control mice (Fig. 3B). The differences between antibody-treated mice and DPBS controls were significant (P < 0.05), whereas mice that received A-C3.1 developed approximately the same number of vaginal CFU as the DPBS-treated mice.
FIG. 3.
MAb C3.1 has a prophylactic effect against Candida vaginal infection. Pseudoestrus mice were given MAb C3.1 i.p. (A) or i.vg. (B) before an i.vg. challenge with 5 × 105 yeast cells. Vaginal CFU counts were compared with those from animals given unheated C3.1, H-C3.1, A-C3.1, or DPBS (diluent). Mice that received the unheated or heated C3.1 developed 60 and 49% fewer CFU, respectively, than control mice that received DPBS. Mice that received A-C3.1 or DPBS developed similar numbers of CFU (A). Mice that received the unheated or heated MAb C3.1 developed approximately 86% fewer CFU than DPBS control mice, whereas CFU numbers in mice given A-C3.1 were similar to those in the DPBS control mice (B). In both panels, significant differences were found between mice that received either the unheated MAb C3.1 or heated MAb C3.1 and DPBS controls (P < 0.05). Bars show standard errors.
DISCUSSION
The hybridoma cell line that produces MAb C3.1 was obtained from the splenocyte-myeloma fusion that yielded the IgM MAb B6.1-producing cell line (11). At that time, the C3.1 hybridoma was cloned twice by limiting dilution, the isotype class was determined as an IgG (unpublished data), and the clone was stored in liquid nitrogen. Interest in the C3.1 clone peaked with current speculation on mechanisms by which MAb B6.1 protects experimental animals against both hematogenously disseminated candidiasis and vaginal infection. In work on the IgM antimannan MAbs B6.1 and B6, we clearly established that antibody specificity is a critical criterion for consideration of a protective antibody against candidiasis (11, 13, 14).
Based on suggestive evidence that MAb B6.1 was found to enhance neutrophil candidacidal activity only in the presence of complement (4) and other unpublished observations, we have hypothesized that rapid complement activation is critical to the protective activity by the antibody. A prediction based on this hypothesis is that an IgG3 antibody, which is also a potent activator of complement (21), should be protective. The purpose of this report was to establish the rationale for using MAb C3.1 to extend our knowledge of mechanisms by which antibodies protect against candidiasis. These studies necessitated establishing the identity of the isotype subclass of MAb C3.1, comparing its specificity with that of MAb B6.1, and testing MAb C3.1 for protective activities.
By different measures, the subtype of MAb C3.1 was unequivocally an IgG3. SDS-PAGE analysis gave a heavy-chain molecular size consistent with that reported for an IgG molecule (2). The IgG3 subclass was established by similar results obtained through a capture ELISA and by an Ouchterlony double-diffusion test. Both analyses showed that the C3.1 hybridoma clone produced immune globulin reactive with antibody specific for the γ3 heavy chain but not with antibodies that recognize other IgG subtypes or other Ig isotypes. These results were not surprising. The splenocytes used in the fusion that yielded the C3.1 clone were obtained from animals immunized against a cell wall extract rich in PMC (11). Although the subclass isotype of MAb C3.1 was not previously determined, an IgG3 would be expected because this may be the predominant IgG subclass produced by mice in response to polysaccharide antigens (5, 10, 20).
A strategy similar to that used for identification of the B6.1 epitope was used for identification of C3.1 specificity. The basis of this analysis is that the PMC is composed of α- and β-mannan (24), the latter of which is phosphodiester linked to the former. The phosphodiester bond is readily hydrolyzed by 10 mM HCl, which results in release of the β-oligomannosyl chains from the α-mannan. The β-oligomannosyl chains are of varying lengths based on the number of mannose units of each, but β-1,2-glycosidic bonds connect all of the mannose units regardless of length. Because these chains are readily released from the PMC by acid hydrolysis, these β-1,2-linked oligomannosyl residues are referred to as acid-labile parts of the complex; the α-mannan is called the acid-stable part (17, 22). Following acid hydrolysis, each part of the PMC and the various β-1,2-linked oligomannosyl residues may be separated by size exclusion column chromatography. In previous work, mass spectroscopy was used to confirm the molecular mass and purity of each of the column fractions (13). The various parts of the complex may be tested for reactivity with antibodies by mixing each of the column fractions with antibody and determining which prevents the antibody from agglutinating latex beads coated with nonhydrolyzed phosphomannan. By this approach, the B6.1 epitope was defined as an acid-labile component composed specifically of a β-1,2-mannotriose (13). By an identical analysis, we determined that this is the same oligomannoside that is recognized by MAb C3.1. As suggestive confirmatory evidence, we tested the C3.1 epitope distribution on the surface of C. albicans. Our expectation was that the distribution should be uniform as with the B6.1 epitope, rather than punctate and patchy as with the B6 epitope (13). By the same immunofluorescence analysis that was used for distribution studies of the B6.1 and B6 epitopes, we found that the C3.1 epitope distribution is identical to that of the B6.1 epitope display on the yeast cell surface. By these various findings, we concluded that MAb C3.1 is an IgG3 and the antibody is specific for the same oligomannoside as MAb B6.1. MAb C3.1 was then tested for its ability to protect mice against candidiasis to determine whether an IgM isotype (i.e., MAb B6.1) is required for this activity.
The preventive or prophylactic activity of MAb C3.1 against disseminated candidiasis was compared to that of MAb B6.1. The concentrations of each antibody were standardized on the basis of agglutination titer and previous dose response information from other studies (12, 14). A significant departure from these studies compared to our previous work is that a single dose of either antibody was given i.p. 4 h before infection with live C. albicans yeast cells, rather than one dose 4 h before infection and another 24 h after the first dose (11, 14). Based on mean survival times, both antibodies showed similar protective activities. As with MAb B6.1, MAb C3.1 protective activity was resistant to pretreatment at 56°C, but the activity was removed by preabsorption with yeast cells. Both of these characteristics are consistent with antibody properties and tend to rule out nonspecific protective factors that might be present in the antibody preparations.
In addition to enhancing protection against hematogenously disseminated candidiasis, MAb B6.1 was previously shown to enhance resistance against vaginal infection in mice (14). For effective results, the antibody could be given either i.p., as above in the disseminated candidiasis studies, or i.vg. 4 h before infection. By use of the same concentration of MAb C3.1 as was used in the disseminated disease studies involving this antibody, significant protective effects were observed when MAb C3.1 was given either i.p. or i.vg. prior to a vaginal infection with viable yeast cells.
Our results show that experimental animals can be protected against candidiasis by antibodies with specificity for a β-1,2-mannotriose, but the protective antibody may be either an IgM or an IgG3. Since interleukin-12 administration to mice causes enhanced IgG3 responses to antigens presented in the presence of alum (9), it will be interesting to examine the efficacy of our mannan-protein vaccine (15) administered as an alum preparation to mice given multiple doses of the cytokine.
Our finding that an IgG3 antibody is protective is in contrast to the reported findings that an IgG1 but not an IgG3 MAb protects against cryptococcosis (25, 26). In addition, protective and nonprotective IgG3 MAbs have been described in studies on experimental rickettsial (1) and Pseudomonas aeruginosa infections (3). Obviously, the key consideration is the underlying mechanism of protection. In our work, an IgG3 protective response is consistent with our findings and hypothesis that complement activation may be involved in the mechanism of resistance against disseminated disease. Regardless of the mechanism, the immediate implication of these findings is that it should be possible to develop an immunization protocol that leads to memory B-cell responses and long-term immunity against various forms of candidiasis.
ACKNOWLEDGMENTS
The technical assistance of Soo Kyung Han and Sheila E. Beery is gratefully acknowledged.
This work was supported by grants RO1 AI24912 and PO1 AI37194 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Anacker R L, List R H, Mann R E, Hayes S F, Thomas L A. Characterization of monoclonal antibodies protecting mice against Rickettsia rickettsii. J Infect Dis. 1985;151:1052–1060. doi: 10.1093/infdis/151.6.1052. [DOI] [PubMed] [Google Scholar]
- 2.Andrew S M, Titus J A. Purification and fragmentation of antibodies. In: Coligan J E, Kruisbeek A M, Margulies D H, Shevach E M, Strober W, editors. Current protocols in immunology. I. New York, N.Y: John Wiley & Sons, Inc.; 1994. p. 2.7.4. [Google Scholar]
- 3.Barclay G R, Yap P L, McClelland D B L, Jones R J, Roe E A, McCann M C, Micklem L R, James K. Characterisation of mouse monoclonal antibodies produced by immunisation with a single serotype component of a polyvalent Pseudomonas aeruginosa vaccine. J Med Microbiol. 1986;21:87–90. doi: 10.1099/00222615-21-1-87. [DOI] [PubMed] [Google Scholar]
- 4.Caesar-TonThat T C, Cutler J E. A monoclonal antibody to Candida albicans enhances mouse neutrophil candidacidal activity. Infect Immun. 1997;65:5354–5357. doi: 10.1128/iai.65.12.5354-5357.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caldwell H D, Hitchcock P J. Monoclonal antibody against a genus-specific antigen of Chlamydia species: location of the epitope on chlamydial lipopolysaccharide. Infect Immun. 1984;44:306–314. doi: 10.1128/iai.44.2.306-314.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casadevall A. Antibody immunity and invasive fungal infections. Infect Immun. 1995;63:4211–4218. doi: 10.1128/iai.63.11.4211-4218.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casadevall A, Cassone A, Bistoni F, Cutler J E, Magliani W, Murphy J W, Polonelli L, Romani L. Antibody and/or cell-mediated immunity, protective mechanisms in fungal disease: an ongoing dilemma or an unnecessary dispute? Med Mycol. 1998;36:95–105. [PubMed] [Google Scholar]
- 8.Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 9.Germann T, Bongartz M, Dlugonska H, Hess H, Schmidt E, Kolbe L, Kolsch E, Podlaski F J, Gately M K, Rude E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 10.Greenspan N S, Cooper J N. Intermolecular cooperativity: a clue to why mice have IgG3? Immunol Today. 1992;13:164–168. doi: 10.1016/0167-5699(92)90120-V. [DOI] [PubMed] [Google Scholar]
- 11.Han Y, Cutler J E. Antibody response that protects against disseminated candidiasis. Infect Immun. 1995;63:2714–2719. doi: 10.1128/iai.63.7.2714-2719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han Y, Cutler J E. Assessment of a mouse model of neutropenia and the effect of an anti-candidiasis monoclonal antibody in these animals. J Infect Dis. 1997;175:1169–1175. doi: 10.1086/516455. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Kanbe T, Cherniak R, Cutler J E. Biochemical characterization of Candida albicans epitopes that can elicit protective and nonprotective antibodies. Infect Immun. 1997;65:4100–4107. doi: 10.1128/iai.65.10.4100-4107.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han Y, Morrison R P, Cutler J E. A vaccine and monoclonal antibodies that enhance mouse resistance to Candida albicans vaginal infection. Infect Immun. 1998;66:5771–5776. doi: 10.1128/iai.66.12.5771-5776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han Y, Ulrich M A, Cutler J E. Candida albicans mannan extract-protein conjugates induce a protective immune response against experimental candidiasis. J Infect Dis. 1998;179:1477–1484. doi: 10.1086/314779. [DOI] [PubMed] [Google Scholar]
- 16.Hill H R, Kelsey D K, Gonzales L A, Raff H V. Monoclonal antibodies in the therapy of experimental neonatal group B streptococcal disease. Clin Immunol Immunopathol. 1992;62:87–91. doi: 10.1016/0090-1229(92)90046-q. [DOI] [PubMed] [Google Scholar]
- 17.Kanbe T, Cutler J E. Evidence for adhesin activity in the acid-stable moiety of the phosphomannoprotein complex of Candida albicans. Infect Immun. 1994;62:1662–1668. doi: 10.1128/iai.62.5.1662-1668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanbe T, Han Y, Redgrave B, Riesselman M H, Cutler J E. Evidence that mannans of Candida albicans are responsible for adherence of yeast forms to spleen and lymph node tissue. Infect Immun. 1993;61:2578–2584. doi: 10.1128/iai.61.6.2578-2584.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matthews R, Burnie J. Antibodies against Candida: potential therapeutics? Trends Microbiol. 1996;4:354–358. doi: 10.1016/0966-842x(96)10056-1. [DOI] [PubMed] [Google Scholar]
- 20.Perlmutter R M, Hansburg D, Briles D E, Nicolotti R A, Davie J M. Subclass restriction of murine anti-carbohydrate antibodies. J Immunol. 1978;121:566–572. [PubMed] [Google Scholar]
- 21.Schreiber J R, Cooper L J N, Diehn S, Dahlhauser P A, Tosi M F, Glass D D, Patawaran M, Greenspan N S. Variable region-identical monoclonal antibodies of different IgG subclass directed to Pseudomonas aeruginosa lipopolysaccharide O-specific side chain function differently. J Infect Dis. 1993;167:221–226. doi: 10.1093/infdis/167.1.221. [DOI] [PubMed] [Google Scholar]
- 22.Shibata N, Arai M, Haga E, Kikuchi T, Najima M, Satoh T, Kobayashi H, Suzuki S. Structural identification of an epitope of antigenic factor 5 in mannans of Candida albicans NIH B-792 (serotype B) and J-1012 (serotype A) as beta-1,2-linked oligomannosyl residues. Infect Immun. 1992;60:4100–4110. doi: 10.1128/iai.60.10.4100-4110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shibata N, Hisamichi K, Kikuchi T, Kobayashi H, Okawa Y, Suzuki S. Sequential nuclear magnetic resonance assignment of β-1,2-linked mannooligosaccharides isolated from the phosphomannan of the pathogenic yeast Candida albicans NIH B-792 strain. Biochemistry. 1992;31:5680–5686. doi: 10.1021/bi00139a036. [DOI] [PubMed] [Google Scholar]
- 24.Shibata N, Ikuta K, Imai T, Satoh Y, Satoh R, Suzuki A, Kojima C, Kobayashi H, Hisamichi K, Suzuki S. Existence of branched side chains in the cell wall mannan of pathogenic yeast, Candida albicans. Structure-antigenicity relationship between the cell wall mannans of Candida albicans and Candida parapsilosis. J Biol Chem. 1995;270:1113–1122. doi: 10.1074/jbc.270.3.1113. [DOI] [PubMed] [Google Scholar]
- 25.Yuan R, Casadevall A, Spira G, Scharff M D. Isotype switching from IgG3 to IgG1 converts a nonprotective murine antibody to Cryptococcus neoformans into a protective antibody. J Immunol. 1995;154:1810–1816. [PubMed] [Google Scholar]
- 26.Yuan R R, Spira G, Oh J, Paizi M, Casadevall A, Scharff M D. Isotope switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infect Immun. 1998;66:1057–1062. doi: 10.1128/iai.66.3.1057-1062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]