Abstract
Lipopolysaccharides (LPS) are proinflammatory bacterial products implicated in the pathogenesis of gram-negative sepsis and septic shock. Polymyxin B (PMB), a cyclic, cationic peptide antibiotic, inhibits biological activities of LPS through high-affinity binding to the lipid A moiety. Small synthetic peptides have been designed to mimic the primary and secondary structures of PMB to determine structural requirements for binding and detoxification of lipid A and to assess possible therapeutic potential. The purpose of this study was to compare and contrast the endotoxin-neutralizing activities of two synthetic antiendotoxin peptides (SAEP-2 and SAEP-4), PMB, and an LPS core-specific monoclonal antibody (MAb), WN1 222-5, based on their abilities to inhibit CD14-mediated target cell uptake of fluorescein isothiocyanate (FITC)-conjugated LPS, detected by flow cytometry and confocal microscopy, and LPS-induced production of the proinflammatory cytokines, interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), as measured by bioassays. PMB and SAEP-4 produced dose-dependent inhibition of FITC-LPS uptake by CD14-transfected Chinese hamster ovary fibroblasts (CHO-CD14 cells) and by human peripheral blood mononuclear cells. The anti-LPS MAb, WN1 222-5, also blocked LPS uptake by these cells and synergized with PMB and SAEP-4. LPS-induced IL-6 release was inhibited by PMB, SAEP-4, and MAb WN1 222-5, and these inhibitory activities were additive or synergistic. LPS-induced TNF-α release by PBMC was also inhibited by PMB and SAEP-4 alone and in combination with anti-LPS MAb. SAEP-2, in contrast, produced comparatively minor decrements in cellular uptake of LPS and LPS-induced cytokine responses, and did so only in the absence of serum, while a nonsense peptide exerted no discernible inhibitory effect on LPS uptake or LPS-induced cytokine expression in the presence or absence of serum. Thus, PMB and SAEP-4, like the LPS-reactive MAb, WN1 222-5, block proinflammatory activities of LPS in part by preventing LPS recognition by membrane-bound CD14-expressing target cells. Differences in peptide structure, however, like those exemplified by SAEP-2 and SAEP-4, may differentially affect the endotoxin-neutralizing potency of these peptides despite similar binding activity against lipid A, reflecting possible differences in peptide solubility or peptide regulation of intracellular signal transduction.
Lipopolysaccharides (LPS), or endotoxins, are major structural and functional components of the outer membrane of gram-negative bacteria (24). These complex macromolecules exhibit a variety of toxic and proinflammatory activities that are associated with the lipid A moiety and are causally related to the pathogenesis of gram-negative sepsis and septic shock (17, 18).
Many of the local and systemic pathophysiologic phenomena produced by LPS in the exposed host result from the ability of LPS to activate host inflammatory cells (7), including monocytes, macrophages, and polymorphonuclear leukocytes. Recent attention has focused on putative LPS receptors found on the surfaces of these cells, the relation of these receptors to LPS-induced signal transduction, and the role of each in the development of proinflammatory responses.
Membrane-bound CD14 (mCD14), a glycosyl phosphatidylinositol-anchored protein expressed on myeloid cells, is the best characterized LPS receptor identified to date (9, 33, 37). mCD14 appears to be part of a multicomponent LPS receptor functionally linked to the initiation of intracellular signaling events related to LPS-induced cell activation (29). The signaling unit of the LPS receptor is comprised of members of the Toll-like receptor family of transmembrane proteins characterized by their amphiphilic properties and leucine-rich repeats (31, 36). Serum-associated LPS-binding protein (LBP), which forms complexes with LPS through high-affinity attachment to the lipid A moiety, catalyzes LPS recognition by mCD14, resulting in the generation of LPS-induced proinflammatory signals (12, 14).
Recent experiments have attempted to define the roles of mCD14 and LBP in LPS-related septic events as well as the possible protective or therapeutic activities of proteins, including antibodies, that neutralize LPS by interrupting its proinflammatory interactions with mCD14 and LBP.
We previously showed that LPS-specific monoclonal antibodies (MAbs) are capable of neutralizing cytokine- and transcription factor-inducing activities of LPS by inhibiting the binding of LPS to mCD14 expressed on human peripheral blood monocytes (PBMC) and on CD14-transfected Chinese hamster ovary fibroblasts (CHO-CD14 cells) (20, 21).
Polymyxin B (PMB), a cationic, cyclic peptide antibiotic, inhibits biological activities of LPS through high-affinity binding to the lipid A moiety (1, 15). Small synthetic peptides comprised of l-amino acids have been designed to mimic the primary and secondary structures of PMB in part to determine the structural requirements for binding and detoxification of lipid A (2, 27). Like PMB, these peptides, termed synthetic antiendotoxin peptides (SAEPs), form complexes with lipid A. Moreover, high-affinity binding by SAEPs to lipid A from different LPSs, like binding by PMB, can result in LPS detoxification.
Various peptide-related factors are responsible for optimal binding of peptide structures to lipid A and resulting lipid A (or LPS) detoxification. These factors include amphipathic and cationic characteristics of the primary amino acid sequence of the peptide, the size of the peptide structure, and the peptide conformation (27).
Mapping of the lipid A binding site of PMB based upon the structures of synthetic peptides that mimic primary and secondary structures of this antibiotic has revealed sequences of 8 to 13 amino acids that are similar to sequences found in naturally occurring LPS-binding proteins (22, 23, 27). The latter include LBP of humans and rabbits, CD14 of humans and mice (37), bactericidal/permeability-increasing protein of humans (4, 13), and the Limulus anti-LPS factor of horseshoe crabs (25, 37).
The purpose of this study was to evaluate the LPS-neutralizing activity of SAEPs by documenting their ability to block mCD14-mediated LPS uptake and LPS-induced proinflammatory responses by LPS-sensitive target cells in vitro. A further purpose was to compare and contrast the modulating effects of SAEPs and anti-LPS MAbs, used singly and in combination, on the cellular uptake of LPS and LPS-induced cell activation. The target cell uptake of fluorescein isothiocyanate (FITC)-conjugated LPS was analyzed by flow cytometry and laser scanning confocal microscopy. The term “uptake,” as used here, denotes both membrane-bound and internalized LPS (11, 21). SAEP-mediated modification of LPS uptake was correlated with alterations in LPS-induced interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) production, as measured by bioassays.
The long-term goals of this study are to better understand cellular mechanisms of endotoxin neutralization by peptides that exhibit high-affinity binding to the lipid A moiety of LPS and to evaluate the therapeutic potential of SAEPs in gram-negative sepsis associated with endotoxemia.
MATERIALS AND METHODS
LPS and FITC-conjugated LPS.
Purified Escherichia coli O111:B4 LPS was purchased from List Biological Laboratories (Campbell, Calif.). FITC-conjugated LPS was prepared as previously described (20, 28). FITC-LPS conjugates typically contained 2 to 10 mg of FITC/mg of LPS. FITC-conjugated and unconjugated LPS exhibited similar enzyme-linked immunosorbent assay (ELISA) reactivities with anti-LPS MAbs, Limulus amebocyte lysate-gelating activity, in vitro TNF-α-inducing activity for PBMC, and ability to cross-compete for anti-CD14-inhibitable binding sites on elutriated PBMC (20).
PMB and SAEPs.
PMB, SAEP-2, and SAEP-4 were prepared and characterized as previously described (27). All three peptides bind to lipid A with similar, high affinity. SAEP-2 has a 10-amino-acid, cyclic structure, and SAEP-4 is characterized by 9 amino acids arranged in a linear sequence (Fig. 1). A 6-amino-acid “nonsense” peptide, which exhibits no lipid A reactivity or neutralizing activity, was employed as a negative control (Fig. 1). PMB and SAEP were diluted to a concentration of 1 mg/ml in LPS-free normal saline and stored at −35°C prior to use. In preliminary experiments, PMB and SAEP exerted no effect on membrane-bound CD14 expression in either PBMC or CHO-CD14 cells. Nor did the peptides induce IL-6 release by these cells.
FIG. 1.
Amino acid sequences of peptides employed in this study. DAB, α,γ-diaminobutyric acid; X, 6-methyl/heptanoyloctanoyl; Thr, threonine; Phe, phenylalanine; Leu, leucine; Lys, lysine; Cys, cysteine.
Anti-LPS MAb.
The murine immunoglobulin G2a MAb, WN1 222-5, cross-reacts with LPS produced by all E. coli and Salmonella species serotypes, including both rough and smooth phenotypes, and exhibits endotoxin-neutralizing properties (3). It appears to be specific for a conserved epitope on the core oligosaccharides of LPS with which it reacts.
Sera.
Sera from 10 normal adult donors were screened by ELISA for antibody reactivity with E. coli O111:B4 LPS. The serum sample with the lowest binding activity was used in subsequent experiments requiring LPS and fresh normal human serum (NHS). Sera were frozen at −35°C prior to use.
CHO-CD14 and CHO-NEO cells.
CHO-K1 cells were cotransfected, as described earlier (5), with the cDNA for human CD14 using the mammalian expression vector pcDNAI (pCD14) and the plasmid kKoNeo, which encodes the gene for neomycin phosphotransferase and confers resistance to G418. This cotransfected cell line expresses mCD14 and is referred to as CHO-CD14. A second, control cell line, CHO-NEO, was transfected with the pKoNeo plasmid alone and does not express mCD14. CHO-CD14 and CHO-NEO cells were incubated in Ham's F-12 medium (BioWhittaker, Walkersville, Md.) containing 10% fetal calf serum (HyClone, Logan, Utah) and 100 mg of ciprofloxacin per ml.
PBMC.
PBMC were obtained by leukapheresis of fresh human blood, and monocytes were purified by counterflow centrifugal elutriation, as described elsewhere (20). Elutriated PBMC were enriched to >90%, as determined by cell morphology, nonspecific esterase staining, and detection of appropriate cell markers by flow cytometry. The purification procedure did not result in PBMC activation, as documented by the fact that <2% of cells incubated overnight at 37°C were IL-2 receptor-positive, a sensitive measure of monocyte activation (37).
Uptake of FITC-conjugated LPS by CHO-CD14 cells, CHO-NEO cells, and PBMC.
Our operational definition of LPS uptake, i.e., cell-associated fluorescence in excess of background, did not distinguish fluorescence signal originating inside the cell (internalized LPS) from that externally attached to the cell (membrane-bound LPS) (21). CHO-CD14 and CHO-NEO cells were washed twice with Hanks balanced salt solution (HBSS) and adjusted to 107 cells/ml in cold HBSS immediately prior to use. Elutriated PBMC were washed twice with HBSS, resuspended in HBSS containing 1% fetal calf serum, and incubated for 2 h at 37°C in an atmosphere containing 5% CO2. Nonadherent cells were removed by vigorous washing with warm HBSS, and adherent PBMC were recovered by using a rubber policeman and adjusted to 107 cells/ml in RPMI 1640. FITC-conjugated LPS (1 μg/ml) were preincubated for 15 min at 37°C with PMB or SAEP at concentrations of 100, 10, and 1 μg/ml in the presence or absence of NHS at concentrations of 10, 1.0, and 0.1% (vol/vol). These mixtures were then added to 106 cells and further incubated for 30 min at 37°C. To evaluate the combined effects of SAEP and MAb WN1 222-5 on the cell uptake of LPS, mixtures of FITC-conjugated LPS and SAEP were incubated for 15 min at 37°C with equal volumes of serially diluted MAb WN1 222-5 prior to the addition of cells and further incubation for 30 min at 37°C. Postincubation, the cells were washed twice with cold HBSS, fixed in 1% paraformaldehyde solution, and examined by flow cytometry or laser scanning confocal microscopy (see below).
Flow cytometry.
The fluorescence emission of 5,000 cells per gated sample of PBMC, CHO-CD14 cells, or CHO-NEO cells was quantified, as previously described (21), by using a flow cytometer and Consort 30 software (FACScan; Immunocytochemistry Systems Division, Becton Dickinson, Oxnard, Calif.).
Laser scanning confocal microscopy.
Three hundred thousand CHO-CD14 cells were exposed to 1 μg of FITC-conjugated E. coli O111:B4 LPS per ml for 30 min at 37°C in the presence of 10% NHS following preincubation of the LPS at 37°C for 15 min with 100 μg of SAEP, nonsense peptide, PMB, or medium per ml. After incubation, further LPS uptake was prevented by the addition of 2 ml of HBSS at 4°C. The cells were then washed twice with phosphate-buffered saline and resuspended in 1% paraformaldehyde solution. One-micron optical slices of CHO-CD14 cells at their greatest diameter were generated with a Zeiss Axiovert 35, Laser-Sharp MRC-600 confocal microscope (Bio-Rad Laboratories, Glattbrugg, Switzerland). Further computer image processing was performed with Confocal Assistant 3.10 (Bio-Rad) and Adobe Photoshop (Adobe Systems, Salinas, Calif.).
IL-6 assay.
CHO-CD14 or CHO-NEO cells were suspended in Ham's F-12 medium (BioWhittaker) containing 10% fetal calf serum and dispensed into 24-well culture plates (Costar, Cambridge, Mass.) at a cell density of 5 × 105 cells per 500 μl of medium. The cells were then incubated overnight at 37°C in an atmosphere containing 5% CO2 and washed twice with serum-free Ham's F-12 medium prior to use. Similarly, 106 freshly obtained PBMC, suspended in serum-free Dulbecco's modified essential medium (BioWhittaker), were dispensed into 24-well plates prior to use. Unlabeled E. coli O111:B4 LPS was preincubated at a concentration of 1 μg/ml for 15 min at 37°C with PMB, SAEPs, or nonsense peptide at final concentrations of 100, 10, 1.0, and 0.1 μg/ml in the presence of NHS at final concentrations of 10, 1, and 0.1% (vol/vol). Medium controls were included with each assay. The incubation mixtures containing LPS and inhibitors were then added to CHO-CD14 cells or PBMCs and further incubated at 37°C for 2 h. Cell-free supernatants were collected and stored at −35°C prior to assay. IL-6 determinations were performed on supernatants from LPS-exposed CHO-CD14 cells and PBMC employing the B9 cell bioassay as previously described (19).
TNF-α bioassay.
TNF-α bioactivity was measured as cytolysis on actinomycin D-sensitized L-929 fibroblasts according to the procedure of Ruff and Gifford (26). One unit of TNF-α bioactivity was expressed as the reciprocal of the titer that produced 50% cytolysis, based upon twofold dilutions of tissue culture supernatants. This endpoint corresponded to 1 to 10 pg of recombinant human TNF-α standard (Endogen, Boston, Mass.) per ml. The data were expressed as a percentage of TNF-α present in a control sample obtained from cells stimulated with 1 μg of LPS per ml in the absence of peptides and antibodies. The specificity of the assay was verified by using a neutralizing MAb against recombinant human TNF-α (Endogen).
Statistics.
Mean IL-6 or TNF-α concentrations of triplicate samples were compared by an unpaired Student's t test (Microsoft Excel 5.0; Microsoft, Redmond, Wash.). Representative histograms generated by flow cytometric analysis of 5,000 cell samples were compared statistically by the Kolmogorov-Smirnov test employed in conjunction with FACScan Consort 30 software to confirm apparent differences in median fluorescence intensity (MFI). The statistical significance of peptide, serum, and antibody effects on LPS uptake and LPS-induced cytokine release was determined by two-way analysis of variance (ANOVA). In addition, the effects of SAEP-2, SAEP-4, PMB, and nonsense peptide on LPS uptake and LPS-induced cytokine secretion in the absence of serum were evaluated by one-way ANOVA.
RESULTS
Inhibitory effects of PMB, SAEPS, and anti-LPS MAb on target cell uptake of FITC-conjugated LPS.
PBMC were employed here because they are naturally occurring LPS target cells and express mCD14, a functionally active LPS receptor. CHO-CD14 cells were evaluated in parallel with PBMC because transfection of human CD14 into CHO fibroblasts transfers monocyte/macrophage-like responsiveness to otherwise LPS-unresponsive cells. CD14-transfected CHO cells that express this single leukocyte-specific gene product on their surface provide a nonleukocyte cell line that produces no other known LPS binding or signaling proteins. Moreover, the vector-transfected CHO-NEO cell line, which does not express mCD14, is an ideal control for CD14-transfected CHO cells.
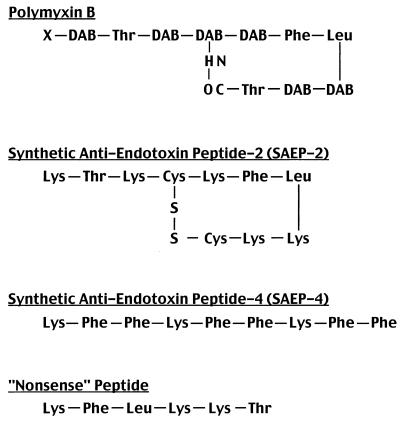
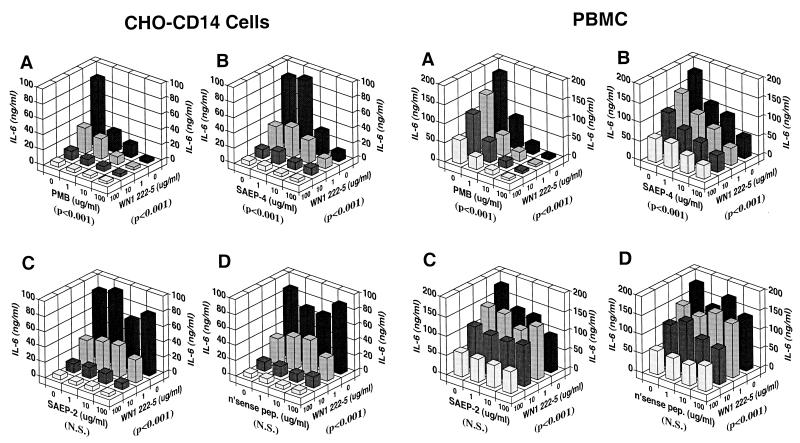
CHO-CD14 cells (left side of Fig. 2) and PBMC (right side of Fig. 2) exhibited similar patterns of uptake of FITC-conjugated E. coli O111:B4 LPS, characterized by marked serum enhancement consistent with the previously documented mediation of most of this uptake by serum-associated LBP and cell-associated mCD14. Although the magnitude of LPS uptake was greater among CHO-CD14 cells than in PBMC, uptake was inhibited in a dose-dependent manner in the case of both cell types by PMB and by SAEP-4 (Fig. 2, A and B). SAEP-2, in contrast (Fig. 2C) inhibited only low-level LPS uptake noted in the absence of serum (Table 1), while nonsense peptide (Fig. 2D) produced no significant inhibition of LPS uptake in the presence or absence of serum (Fig. 2, Table 1).
FIG. 2.
Inhibitory effects of various peptides on the uptake of FITC-conjugated E. coli O111:B4 LPS by CHO-CD14 cells (left side of figure) and by human PBMC (right side of figure) in the presence of different concentrations of NHS. FITC-conjugated LPS (1 μg/ml) was preincubated with peptides, in the presence or absence of NHS, at 37°C for 15 min, and the mixture was then added to cells and incubated at 37°C for an additional 30 min. Cell-associated fluorescence was measured by flow cytometry, and the data are expressed as the MFI. Panels A to D document the influence of PMB, SAEP-4, SAEP-2, and “nonsense peptide,” respectively, on the cell uptake of LPS. The P value shown in parentheses below each horizontal axis indicates the statistical significance, determined by two-way ANOVA, of differences in MFI produced by the material specified for that axis. N.S., not significant (P > 0.05).
TABLE 1.
Inhibitory effects of peptides on cell uptake of LPS and LPS-induced cytokine secretion in the absence of serum
| LPS-related activity | Significance of peptide-mediated inhibition (P)a
|
|||
|---|---|---|---|---|
| Polymyxin B | SAEP-4 | SAEP-2 | Nonsense peptide | |
| Uptake by CHO-CD14 cells | <0.0001 | <0.0001 | 0.0387 | 0.2506 |
| Uptake by PBMC | <0.0001 | <0.0001 | 0.0294 | 0.3583 |
| Uptake by CHO-NEO cells | <0.0001 | <0.0001 | 0.0894 | 0.4376 |
| IL-6 secretion by CHO-CD14 cells | 0.0147 | 0.0013 | 0.0098 | 0.8283 |
| IL-6 secretion by PBMC | <0.0001 | <0.0001 | 0.0473 | 0.2202 |
| IL-6 secretion by CHO-NEO cells | 0.0064 | 0.0139 | 0.0684 | 0.6037 |
| TNF-α secretion by PBMC | <0.0001 | <0.0001 | 0.3872 | 0.4461 |
P value based upon one-way ANOVA.
In contrast with CHO-CD14 cells, vector-transfected CHO-NEO cells took up substantially less LPS, and this uptake was inhibited rather than enhanced by serum (data not shown). Despite the distinctive features of low-level LPS uptake by CHO-NEO cells, this uptake was inhibited by PMB and SAEP-4 (data not shown; also see Table 1) but not by SAEP-2 or nonsense peptide (data not shown; also see Table 1).
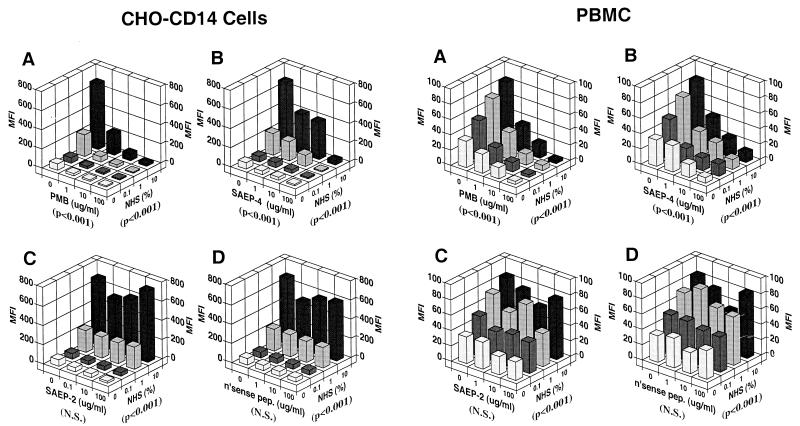
In a separate set of experiments (Fig. 3), various concentrations of synthetic peptides and LPS core-specific MAb were incubated, singly or in combination, with 1 μg of FITC-conjugated LPS per ml in 10% NHS, prior to addition of the LPS-containing mixtures to CHO-CD14 cells or PBMC and further incubation at 37°C for 30 min. Under these serum conditions, PMB and SAEP-4 (Fig. 3A and B), but not SAEP-2 or nonsense peptide (Fig. 3C and D), inhibited the uptake of FITC-LPS by CHO-CD14 cells and PBMC in a dose-dependent fashion. Also documented was dose-related inhibition of LPS uptake by the endotoxin core-specific MAb, WN1 222-5 (Fig. 3A to D), and the additive or synergistic inhibitory effects of MAb and PMB or SAEP-4 (Fig. 3A and B), but not SAEP-2 or nonsense peptide (Fig. 3C and D).
FIG. 3.
Inhibitory effects of various peptides, alone or in combination with LPS core-specific MAb (WN1 222-5) on the uptake of FITC-conjugated E. coli O111:B4 LPS by CHO-CD14 cells (left side of figure) and by PBMC (right side of figure). See Fig. 2 legend for experimental procedures, except that NHS was held constant in this experiment at 10% (vol/vol) and WN1 222-5 was introduced at various concentrations as shown. Panels A to D document the influence of PMB, SAEP-4, SAEP-2, and “nonsense peptide,” respectively, on the cell uptake of LPS. The P values shown in parentheses below each horizontal axis indicate the statistical significance, as determined by two-way ANOVA, of differences in MFI produced by the material specified for that axis. N.S., not significant (P > 0.05).
Inhibitory effects of PMB, SAEPS, and anti-LPS MAb on LPS-induced cytokine release by LPS target cells.
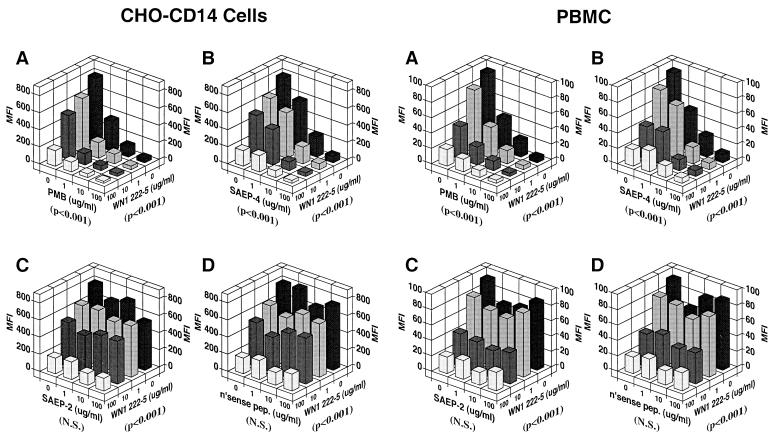
CHO-CD14 cells and PBMC both released IL-6 in response to stimulation with LPS (Fig. 4). In both cases, the pattern of IL-6 release corresponded to that observed for LPS uptake. Like LPS uptake, LPS-induced IL-6 release was enhanced by serum and inhibited by PMB and SAEP-4 (Fig. 4A and B). Moreover, SAEP-2 inhibited low-level LPS-induced IL-6 release only in the absence of serum (Fig. 4C and Table 1) compared with nonsense peptide, which produced no significant change in the production of LPS-induced IL-6 (Fig. 4D and Table 1). LPS-induced IL-6 release by CHO-NEO cells followed the pattern of low-level LPS uptake exhibited by these cells, namely, serum-independence or slight serum-inhibition and susceptibility to inhibition by PMB and SAEP-4 (data not shown) but not by SAEP-2 or nonsense peptide (data not shown; also see Table 1). Like LPS uptake, LPS-induced IL-6 release by CHO-CD14 cells and PBMC was susceptible to inhibition by the LPS core-specific MAb, WN1 222-5, and the inhibitory activity of this MAb appeared to be additive or synergistic with that of PMB and SAEP-4 (Fig. 5A and B), but not with SAEP-2 or nonsense peptide (Fig. 5C and D).
FIG. 4.
Inhibitory effects of various peptides on LPS-induced IL-6 production by CHO-CD14 cells (left side of figure) and by human PBMC (right side of figure) in the presence of different concentrations of NHS. E. coli O111:B4 LPS at 1 μg/ml was preincubated with peptides in the presence or absence of NHS at 37°C for 15 min, and the mixture was then added to cells and incubated at 37°C for an additional 2 h in an atmosphere containing 5% CO2. The supernatants were collected and IL-6 concentration measured by using the B9 cell proliferation assay. The data shown are representative of those obtained in three similar experiments. Panels A to D document the influence of PMB, SAEP-4, SAEP-2, and “nonsense peptide,” respectively, on LPS-induced IL-6 release. The P values shown in parentheses below each horizontal axis indicate the statistical significance, as determined by two-way ANOVA, of differences in IL-6 concentration produced by the material specified for that axis. N.S., not significant (P > 0.05).
FIG. 5.
Inhibitory effect of various peptides, alone or in combination with LPS core-specific MAb (WN1 222-5), on LPS-induced IL-6 production by CHO-CD14 cells (left side of figure) and by human PBMC (right side of figure). See Fig. 4 legend for experimental procedures, except that in this experiment the concentration of NHS was held constant at 10% (vol/vol), and the concentration of WN1 222-5 was varied as indicated. The data shown are representative of those obtained in three similar experiments. Panels A to D document the influence of PMB, SAEP-4, SAEP-2, and “nonsense peptide,” respectively, on LPS-induced IL-6 release. The P values shown in parentheses below each horizontal axis indicate the statistical significance, as determined by two-way ANOVA, of differences in IL-6 concentration produced by the material specified for that axis. N.S., not significant (P > 0.05).
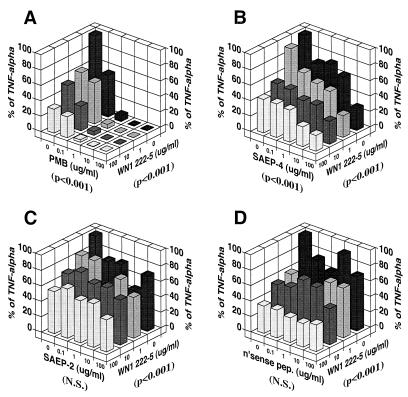
LPS-induced TNF-α release by PBMC (Fig. 6) was also susceptible to inhibition by PMB and SAEP-4 alone and in combination with anti-LPS MAb, suggesting a pattern and mechanism of inhibition similar to that produced in the case of IL-6.
FIG. 6.
Inhibitory effects of various peptides, alone or in combination with LPS core-specific MAb (WN1 222-5), on LPS-induced TNF-α production by human PBMC. See Fig. 4 legend for experimental procedures, except for the following: (i) in this experiment the concentration of NHS was held constant at 10% (vol/vol), and the concentration of WN1 222-5 was varied as indicated, and (ii) supernatant TNF-α concentrations were measured by using the L929 cell cytotoxicity assay. The P values shown in parentheses below each horizontal axis indicate the statistical significance, as determined by two-way ANOVA, of differences in TNF-α concentration produced by the material specified for that axis. N.S., not significant (P > 0.05).
Laser scanning confocal microscopic images of CHO-CD14 cells exposed to FITC-LPS in the presence or absence of PMB, SAEPS, and anti-LPS MAb.
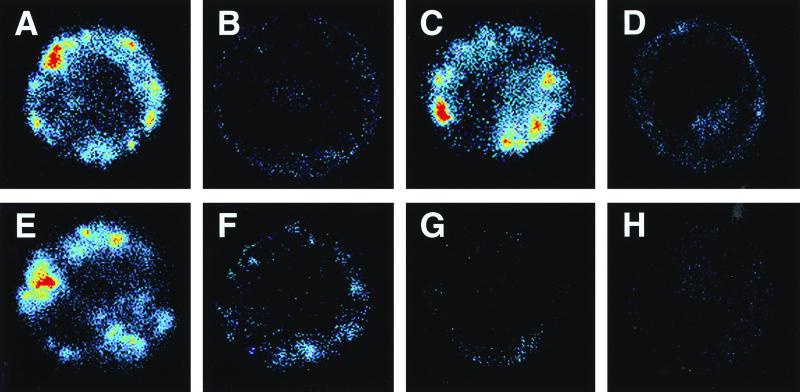
Confocal microscopy of LPS-exposed CHO-CD14 cells was carried out to confirm flow cytometry data and to establish patterns of LPS uptake and the impact on that uptake of synthetic peptides and anti-LPS MAb. After 30 min of incubation at 37°C in the presence of 1 μg of FITC-conjugated E. coli O111:B4 LPS per ml and 10% NHS, CHO-CD14 cells exhibited marked accumulations of fluorescently labeled LPS in association with both the cell surface and cell interior (Fig. 7A). Preincubation of LPS with 100 μg of PMB (panel B), SAEP-4 (panel D), or MAb WN1 222-5 (panel F), but not SAEP-2 or nonsense peptide (panels C and E), per ml substantially reduced the amount of labeled LPS associated with external and internal cell surfaces. Preincubation of FITC-LPS with a combination of MAb WN1 222-5 and PMB (Fig. 7G) or SAEP-4 (panel H) appeared to produce greater inhibition of LPS uptake than did any individual agent.
FIG. 7.
Laser scanning confocal microscopic images of representative CHO-CD14 cells exposed to 1 μg of FITC-conjugated E. coli O111:B4 LPS per ml in the presence or absence of various peptides and LPS core-reactive MAb WN1 222-5. LPS was preincubated at 37°C for 15 min, with or without peptide and/or anti-LPS MAb, in the presence of 10% (vol/vol) NHS. The mixture was then added to CHO-CD14 cells, followed by further incubation at 37°C for 30 min. Panels A to H show reaction mixtures containing, in addition to FITC-LPS, 10% NHS, and cells, the following reactants: A, none; B, PMB; C, SAEP-2; D, SAEP-4; E, “nonsense peptide”; F, anti-LPS MAb; G, PMB plus anti-LPS MAb; and H, SAEP-4 plus anti-LPS MAb. Single cells averaging 15 μm in diameter appear in each panel. A representative cell is shown for each specified set of conditions, selected from among at least 50 randomly observed cells. The experiment was repeated twice with similar results. Fluorescence intensity is color coded on a linear scale as follows: red > yellow > green > blue > black (background).
DISCUSSION
We previously documented that certain LPS core- and O-side-chain-specific antibodies, including the WN1 222-5 core-specific MAb evaluated here, were capable of blocking CD14-mediated cell recognition and uptake of LPS in vitro (20, 21). This resulted in the modulation of LPS-induced TNF-α production by PBMC and the inhibition of nuclear factor-κB translocation in CHO-CD14 fibroblasts (20, 21). In the present study, analogous antiendotoxin activities were expressed by the PMB-like synthetic peptide, SAEP-4, to a lesser extent by SAEP-2, and by PMB itself. These peptide molecules, especially SAEP-4 and PMB, downregulated serum-dependent LPS uptake by target cells expressing mCD14, and this correlated with the inhibition of LPS-induced release of IL-6 (CHO-CD14 cells and PBMC) and TNF-α (PBMC). Both SAEP-4 and PMB appeared to act additively or synergistically with anti-LPS MAb to produce enhanced endotoxin neutralization. SAEP-2, in contrast, produced relatively minor decrements in LPS uptake and LPS-induced cytokine release, in the absence of serum.
The majority of LPS uptake and LPS-induced cytokine responses blocked by SAEP-4 and PMB appeared to be CD14-mediated and serum (LBP)-dependent (9, 14, 30). Low-level, serum- and CD14-independent LPS responses by vector-transfected CHO-NEO cells were partially inhibited by SAEP-4 and PMB. SAEP-2, like SAEP-4 and PMB, exhibited comparatively minor yet significant anti-LPS activities in the absence of serum (Table 1), suggesting that peptide-associated endotoxin neutralization may occur in conjunction with LPS recognition factors other than CD14.
In this study, serum augmented LPS uptake and LPS-induced IL-6 generation by CHO-CD14 cells and PBMC, while appearing to enhance the inhibitory effects of PMB and SAEP-4 on these LPS-related activities. This was in striking contrast with certain previous studies (2, 38) which reported that serum actually attenuated the LPS binding and neutralizing capacity of PMB and SAEPs. These apparent discrepancies may have been based simply on differences in the order in which reactants were combined in various studies. In our experiments, for example, LPS and peptides were combined prior to the addition of serum and cells, while in the earlier studies serum was added to LPS before the addition of matrix-bound peptides (2). In the latter case, it has been suggested that LBP contained in serum competed with synthetic peptides for binding sites on LPS and in so acting attenuated the LPS-inhibitory function of the peptides (38).
Our in vitro data comparing SAEP-2 and SAEP-4 suggest that peptides with analogous physical properties (22, 23, 27), including similarly high binding affinities for LPS (lipid A), may exhibit quite different inhibitory activities against LPS uptake by CD14-expressing target cells and the resulting LPS-induced release of proinflammatory cytokines. The greater anti-LPS activity of SAEP-4 compared with SAEP-2 revealed in these in vitro assays may be accounted for in part by the lower solubility of SAEP-4 (32), a characteristic which likely facilitates the precipitation and removal of SAEP-LPS complexes once formed.
The antiendotoxin activities of SAEPs may also appear discordant in in vitro settings compared with in vivo settings. In the present study, for example, SAEP-2 failed to inhibit cellular uptake of LPS and LPS-induced cytokine release in vitro, in the presence of serum, while in previous in vivo studies (2, 27), SAEP-2 inhibited LPS-induced TNF-α release, and in an unpublished series of in vivo experiments (N. Koles and M. Pollack, unpublished data), SAEP-2 significantly reduced LPS-related mortality in galactosamine-sensitized mice. In contrast, while SAEP-4 consistently inhibited cell uptake of LPS and LPS-induced cytokine expression in vitro, this peptide demonstrated weak or nonexistent protection against LPS-induced mortality in mice (Koles and Pollack, unpublished).
The striking and somewhat unexpected functional differences between SAEP-2 and SAEP-4 with respect to in vitro and in vivo anti-LPS activity prompt speculation concerning alternative mechanisms of peptide-mediated protection unrelated to the inhibition of LPS uptake by cells via mCD14. One possible starting point for such speculation is the previous observation that PMB inhibits protein kinase C (PKC), a key intracellular signal-transducing molecule implicated in cell activation induced by LPS (6, 8) and by phorbol myristate acetate (10, 16). Preliminary results from our laboratory (A. Iwagaki, unpublished data) indicate that SAEP-2, SAEP-4, and PMB all inhibit PKC, and this PKC-inhibitory activity correlates with peptide-mediated inhibition of PMA- or LPS-induced TNF-α production by PBMC and by THP-1 macrophages. Particularly striking in these experiments is the fact that SAEP-2 proved to be a more potent inhibitor of PKC compared with SAEP-4, suggesting a possible basis for the apparent differences between the anti-endotoxin activities of these two peptides.
In summary, certain SAEPs exhibiting high-affinity binding to the phylogenetically conserved lipid A moiety of LPS, like the prototype molecule, PMB, and the LPS core-reactive MAb, WN1 222-5, are capable of blocking LPS uptake by cells expressing membrane-bound CD14 and thereby preventing the LPS-induced release of proinflammatory cytokines. SAEP-mediated endotoxin neutralization is enhanced, in general, by low concentrations of human serum, and is additive or synergistic with antibody-mediated neutralization. Apparent differences among SAEPs with regard to the inhibition of LPS uptake and LPS-induced cytokine release in vitro may relate in part to physical properties other than simple peptide affinity for lipid A, or to differential peptide influences on proinflammatory cell responses not based solely on the recognition and uptake of LPS per se. The endotoxin-neutralizing, antiinflammatory, and nontoxic properties of selected SAEPs make them attractive candidates for adjuvant therapy in gram-negative sepsis.
ACKNOWLEDGMENTS
We thank Douglas T. Golenbock for supplying the CHO-CD14 and CHO-NEO cell lines, Franco Di Padova for donating the WN1 222-5 MAb, and Larry M. Wahl for giving us human peripheral blood monocytes.
This work was supported by grant RO83IZ from the Uniformed Services University of the Health Sciences.
REFERENCES
- 1.Coyne C P, Fenwick B W. Inhibition of lipopolysaccharide-induced macrophage tumor necrosis factor-α synthesis by polymyxin B sulfate. Am J Vet Res. 1993;54:305–314. [PubMed] [Google Scholar]
- 2.Demitri M T, Velucchi M, Bracci L, Rustici A, Porro M, Villa P, Ghezzi P. Inhibition of LPS-induced systemic and local TNF production by a synthetic anti-endotoxin peptide (SAEP-2) J Endotoxin Res. 1996;3:445–454. [Google Scholar]
- 3.Di Padova F E, Brade H, Barclay G R, Poxton I R, Liehl E, Schuetze E, Kocher H P, Ramsay G, Schreier M H, McClelland D B, Rietschel E T. A broadly cross-protective monoclonal antibody binding to Escherichia coli and Salmonella lipopolysaccharide. Infect Immun. 1993;61:3863–3872. doi: 10.1128/iai.61.9.3863-3872.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans T J, Carpenter A, Moyes D, Martin R, Cohen J. Protective effects of a recombinant amino-terminal fragment of human bactericidal/permeability-increasing protein in an animal model of gram-negative sepsis. J Infect Dis. 1995;171:153–160. doi: 10.1093/infdis/171.1.153. [DOI] [PubMed] [Google Scholar]
- 5.Golenbock D T, Liu Y, Millham F H, Freeman M W, Zoeller R A. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 6.Hegemann L, van Rooijen L A A, Traber J, Schmidt B H. Polymyxin B is a selective and potent antagonist of calmodulin. Eur J Pharmacol. 1991;207:17–22. doi: 10.1016/s0922-4106(05)80032-x. [DOI] [PubMed] [Google Scholar]
- 7.Heumann D, Gallay P, Barras C, Zaech P, Ulevitch R J, Tobias P S, Glauser M-P, Baumgartner J D. Control of lipopolysaccharide (LPS) binding and LPS-induced tumor necrosis factor secretion in human peripheral blood monocytes. J Immunol. 1992;148:3505–3512. [PubMed] [Google Scholar]
- 8.Jun C-D, Choi B-M, Kim H-M, Chung H-T. Involvement of protein kinase C during taxol-induced activation of murine peritoneal macrophages. J Immunol. 1995;154:6541–6547. [PubMed] [Google Scholar]
- 9.Kirkland T N, Finley F, Leturcq D, Moriarty A, Lee J-D, Ulevitch R J, Tobias P S. Analysis of lipopolysaccharide binding by CD14. J Biol Chem. 1993;268:24818–24823. [PubMed] [Google Scholar]
- 10.Kiss Z, Deli E, Girard P, Pettit R, Kuo J. Comparative effects of polymyxin B, phorbol ester and bryostatin on protein phospholylation, protein kinase C translation, phospholipid metabolism and differentiation of HL60 cells. Biochem Biophys Res Commun. 1987;146:208–215. doi: 10.1016/0006-291x(87)90712-1. [DOI] [PubMed] [Google Scholar]
- 11.Kitchens R L, Ulevitch R J, Munford R S. Lipopolysaccharide (LPS) partial structures inhibit responses to LPS in a human macrophage cell line without inhibiting LPS uptake by a CD14-mediated pathway. J Exp Med. 1992;176:485–494. doi: 10.1084/jem.176.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landmann R, Ludwig C, Obrist R, Obrecht J P. Effect of cytokines and lipopolysaccharide on CD14 antigen expression in human monocytes and macrophages. J Cell Biochem. 1991;47:317–329. doi: 10.1002/jcb.240470406. [DOI] [PubMed] [Google Scholar]
- 13.Marra M N, Au-Young J, Lin L, Lane J C, Snable J L, Thornton M B, McKelligon B M, Seilhamer J J, Scott R W. Identification of bactericidal/permeability increasing protein (BPI) and lipopolysaccharide binding protein (LBP) domains which are important for LPS neutralizing or transducing activity and circulating half-life. J Endotoxin Res. 1994;1(Suppl. 1):18. [Google Scholar]
- 14.Martin T R, Mathison J C, Tobias P S, Leturcq D J, Moriarty A M, Maunder R J, Ulevitch R J. Lipopolysaccharide binding protein enhances the responsiveness of alveolar macrophages to bacterial lipopolysaccharide. Implications for cytokine production in normal and injured lungs. J Clin Investig. 1992;90:2209–2219. doi: 10.1172/JCI116106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morrison D C, Jacobs D M. Binding of polymyxin B to the lipid A portion of bacterial lipopolysaccharides. Immunochemistry. 1976;13:813–818. doi: 10.1016/0019-2791(76)90181-6. [DOI] [PubMed] [Google Scholar]
- 16.Naccache P, Molski M, Sha'afi R. Polymyxin B inhibits phorbol 12-myristate 13-acetate, but not chemotactic factor, induced effects in rabbit neutrophils. FEBS Lett. 1985;192:227–230. doi: 10.1016/0014-5793(85)80157-5. [DOI] [PubMed] [Google Scholar]
- 17.Natanson C, Parrillo J E. Septic shock. Anesthesiol Clin N Am. 1988;6:73–85. [Google Scholar]
- 18.Parrillo J E. Septic shock in humans: clinical evaluation, pathogenesis, and therapeutic approach. In: Shoemaker W C, Anres S, Grenvik A, et al., editors. Textbook of critical care. W. B. Philadelphia, Pa: Saunders Co.; 1989. pp. 1006–1024. [Google Scholar]
- 19.Pedersen M R, Jensen S, Christensen J D, Hansen E W. Lipopolysaccharide in concentrations above 40 ng/ml stimulates proliferation of the IL-6-dependent B9 cell line. J Immunol Methods. 1995;180:159–163. doi: 10.1016/0022-1759(94)00311-j. [DOI] [PubMed] [Google Scholar]
- 20.Pollack M, Espinoza A M, Guelde G, Koles N L, Wahl L M, Ohl C A. Lipopolysaccharide (LPS)-specific monoclonal antibodies regulate LPS uptake and LPS-induced tumor necrosis factor-α responses by human monocytes. J Infect Dis. 1995;172:794–804. doi: 10.1093/infdis/172.3.794. [DOI] [PubMed] [Google Scholar]
- 21.Pollack M, Ohl C A, Golenbock D T, DiPadova F, Wahl L M, Koles N L, Guelde G, Monks B G. Dual effects of lipopolysaccharide (LPS) antibodies on cellular uptake of LPS and LPS-induced proinflammatory functions. J Immunol. 1997;151:3519–3530. [PubMed] [Google Scholar]
- 22.Porro M. Structural basis of endotoxin recognition by natural polypeptides. Trends Microbiol. 1994;2:65–67. doi: 10.1016/0966-842x(94)90530-4. [DOI] [PubMed] [Google Scholar]
- 23.Porro M. Cyclic or linear conformations of sequences binding lipid A: does it really matter? Trends Microbiol. 1994;2:338–339. doi: 10.1016/0966-842x(94)90454-5. [DOI] [PubMed] [Google Scholar]
- 24.Rietschel E T, Kirikae T, Schade F U, Ulmer A J, Holst O, Brade H, Schmidt G, Mamat U, Grimmecke H-D, Kusumoto S, Zähringer U. The chemical structure of bacterial endotoxin in relation to bioactivity. Immunobiology. 1993;187:169–190. doi: 10.1016/S0171-2985(11)80338-4. [DOI] [PubMed] [Google Scholar]
- 25.Roth R I, Tobias P S. Lipopolysaccharide-binding proteins of Limulus amebocyte lysate. Infect Immun. 1993;61:1033–1039. doi: 10.1128/iai.61.3.1033-1039.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruff M, Gifford G. Purification and physicochemical characterization of rabbit tumor necrosis factor. J Immunol. 1980;125:1671–1677. [PubMed] [Google Scholar]
- 27.Rustici A, Velucchi M, Faggioni R, Sironi M, Ghezzi P, Quataert S, Green B, Porro M. Molecular mapping and detoxification of the lipid A binding site by synthetic peptides. Science. 1993;259:361–365. doi: 10.1126/science.8420003. [DOI] [PubMed] [Google Scholar]
- 28.Skelly R R, Munkenbeck P, Morrison D C. Stimulation of T-independent antibody responses by hapten-lipopolysaccharides without repeating polymeric structure. Infect Immun. 1979;23:287–293. doi: 10.1128/iai.23.2.287-293.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobias P S, Soldau K, Gegner J A, Mintz D, Ulevitch R J. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 30.Tobias P S, Ulevitch R J. Lipopolysaccharide binding protein and CD14 in LPS dependent macrophage activation. Immunobiology. 1993;187:227–232. doi: 10.1016/S0171-2985(11)80341-4. [DOI] [PubMed] [Google Scholar]
- 31.Ulevitch R J. Endotoxin opens the Tollgates to innate immunity. Nat Med. 1999;5:144–145. doi: 10.1038/5504. [DOI] [PubMed] [Google Scholar]
- 32.Vaara M, Porro M. Group of peptides that act synergistically with hydrophobic antibodies against gram-negative enteric bacteria. Antimicrob Agents Chemother. 1996;40:1801–1805. doi: 10.1128/aac.40.8.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viriyakosol S, Kirkland T N. A region of human CD14 required for lipopolysaccharide binding. J Biol Chem. 1995;270:361–368. doi: 10.1074/jbc.270.1.361. [DOI] [PubMed] [Google Scholar]
- 34.Wahl S M, McCartney-Francis N, Hunt D A, Smith P D, Wahl L M, Katona I M. Monocyte interleukin 2 receptor gene expression and interleukin 2 augmentation of microbicidal activity. J Immunol. 1987;139:1342–1347. [PubMed] [Google Scholar]
- 35.Warren H S, Glennon M L, Wainwright N, Amato S F, Black K M, Kirsch S J, Riveau G R, Whyte R I, Zapol W M, Novitsky T J. Binding and neutralization of endotoxin by Limulus antilipopolysaccharide factor. Infect Immun. 1992;60:2506–2513. doi: 10.1128/iai.60.6.2506-2513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright S D. Toll, a new piece in the puzzle of innate immunity. J Exp Med. 1999;189:605–609. doi: 10.1084/jem.189.4.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 38.Zhang G H, Mann D M, Tsai C M. Neutralization of endotoxin in vitro and in vivo by a human lactoferrin-derived peptide. Infect Immun. 1999;67:1353–1358. doi: 10.1128/iai.67.3.1353-1358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]