Abstract
Intervertebral disc degeneration (IDD) is a high incidence disease of musculoskeletal system that often leads to stenosis, instability, pain and even deformity of the spinal segments. IDD is an important cause of discogenic lower back pain and often leads to large economic burden to families and society. Currently, the treatment of IDD is aimed at alleviating symptoms rather than blocking or reversing pathological progression of the damaged intervertebral disc. Resveratrol (RSV) is a polyphenol phytoalexin first extracted from the Veratrum grandiflflorum O. Loes and can be found in various plants and red wine. Owing to the in‐depth study of pharmacological mechanisms, the therapeutic potential of RSV in various diseases such as osteoarthritis, neurodegenerative diseases, cardiovascular diseases and diabetes have attracted the attention of many researchers. RSV has anti‐apoptotic, anti‐senescent, anti‐inflammatory, anti‐oxidative, and anabolic activities, which can prevent further degeneration of intervertebral disc cells and enhance their regeneration. With high safety and various biological functions, RSV might be a promising candidate for the treatment of IDD. This review summarizes the biological functions of RSV in the treatment of IDD and to facilitate further research.
Keywords: Apoptosis, Autophagy, Extracellular matrix degradation, Intervertebral disc degeneration, Resveratrol, Senescence
The intervertebral disc acts as the load‐bearing component of the spine and is composed of three closely connected parts: inner nucleus pulposus (NP), peripheral annulus fibrosus (AF) and outer cartilage endplate (CEP). IDD is a complex disease including multiple pathological processes. The pathogenesis of IDD mainly involves degradation of ECM, NP cells senescence, apoptosis, autophagy, inflammatory responses and oxidative stress. NP cells senescence, apoptosis, inflammation and oxidative stress result in the promotion of the ECM degradation. What is more, activation of autophagy can also influence the activity of NP cells, thereby regulating ECM homeostasis. According to the literatures, the RSV showed protective effects on IDD through a variety of mechanisms.
Introduction
Intervertebral disc degeneration (IDD) is a high incidence disease of musculoskeletal system that often leads to stenosis, instability, pain and even deformity of the spinal segments. 1 , 2 , 3 Many IDD patients lose their ability to work, thereby imposing a large economic burden to their families and society. 4 , 5 , 6 , 7 Current treatments mainly focus on alleviating the symptoms rather than limiting or reversing pathological changes of disc degeneration, which means the treatment outcomes and prognosis are far from ideal. 8 , 9 The pathogenesis of IDD is related to nucleus pulposus (NP) cells apoptosis, NP cells senescence, inflammation, oxidative stress, and extracellular matrix (ECM) degradation. Therefore, suppressing the inflammatory response and oxidative stress, inhibiting NP cells senescence and apoptosis and promoting the biosynthesis of the ECM are pivotal to IDD treatment. 10 , 11
Resveratrol (RSV, C14H12O3) is a polyphenol phytoalexin with a relative molecular weight of 228. It was first extracted from Veratrum grandiflorum O. Loes by Takaoka in 1939 and can be found in various plants and red wine. 12 It has both cis‐ and trans‐isomers when found in nature, of which the trans‐isomer provides the main biological benefits due to the lower steric hindrance of its side chains. 13 , 14 Modern pharmacological studies show that RSV has protective effects against various diseases, such as osteoarthritis, neurodegenerative diseases, cardiovascular diseases, and diabetes. 15 , 16 , 17 , 18 The therapeutic effects of RSV on IDD are related to its antioxidant and anti‐inflammatory activities. 19 , 20 , 21 Moreover, RSV also affects NP cells apoptosis, autophagy, and ECM biosynthesis through multiple signal transduction pathways. 19 , 20 , 22 , 23 At present, researches of RSV on the treatment of IDD are limited to preclinical studies. This review summarizes the biological functions of RSV in IDD treatment. The limitations of current studies and new insights for further studies are also provided at the end of article.
Pharmacokinetics and Toxicity
In 40 healthy volunteers orally administered with single doses of 0.5, 1, 2.5, or 5 g RSV, the mean maximum plasma concentration (Cmax) at the highest dose was 539 ng/mL, which was achieved within 1.5 h post dose (Tmax). 24 The elimination half‐life of RSV was 8.52 h, while the total exposure (AUC0–∞) was 1319 ng h/mL. 24 The distribution research in animal models indicates that the highest tissue concentration of RSV was found in the brain, liver, intestine, and fat. 25 After absorption through passive diffusion or carrier‐mediated processes, RSV is mainly metabolized in enterocytes and hepatocytes. 26 , 27 It can be rapidly metabolized into RSV‐glucuronides and RSV‐sulfates via uridine‐5′‐diphosphate‐glucuronosyltransferase and sulfotransferase, respectively. 28 , 29 In addition, intestinal bacteria can produce a dehydroxylated form of RSV, which can also pass through enterocytes and be metabolized into RSV‐glucuronides and RSV‐sulfates. 30 RSV is mainly excreted by the urinary system. 31 , 32 Five metabolites of RSV have been detected in human urine: RSV monosulfate; two isomeric forms of RSV monoglucuronide; monosulfate dihydroresveratrol; and monoglucuronide dihydroresveratrol. 14 Rapid absorption, poor bioavailability, and low aqueous solubility are some of the crucial limitations of the clinical application of RSV. Therefore, novel methodological approaches, such as nanoparticles and nanobubbles, have been used to improve the poor aqueous solubility and the low bioavailability of RSV. 33 , 34 , 35 , 36
The toxicity of RSV mainly depends on its dosage. At single dose of <1 g RSV showed no obvious side effects. 37 , 38 Upon continuous administration of RSV at 2.5 g/day for a month, side effects such as nausea, vomiting, diarrhea, and liver dysfunction may appear. 39 Pollack et al. 40 reported frequent diarrhea upon treatment with 2 g RSV twice daily. When the dose was reduced to 1 g twice daily there were no further gastrointestinal side effects. Overall, RSV is generally considered to be safe at dose of ≤1 g/day.
Molecular Actions of RSV in the Prevention of IDD
The intervertebral disc acts as the load‐bearing component of the spine and is composed of three closely connected parts: inner NP, peripheral annulus fibrosus (AF) and outer cartilage endplate (CEP). 41 , 42 , 43 IDD is a complex disease including multiple pathological processes. The pathogenesis of IDD mainly involves degradation of ECM, NP cells senescence, apoptosis, autophagy, inflammatory responses and oxidative stress. 44 NP cells senescence, apoptosis, inflammation and oxidative stress result in the promotion of the ECM degradation. 10 , 45 , 46 What is more, activation of autophagy can also influence the activity of NP cells, thereby regulating ECM homeostasis. 47 According to the literatures, the RSV showed protective effects on IDD through a variety of mechanisms (Table 1, Fig. 1).
TABLE 1.
Summary of the in vitro researches about pharmacological activities of RSV in IDD
| Type of model | Dose | Findings | Molecular mechanism | Reference |
|---|---|---|---|---|
| Human NP cells | 10, 20, 50 μM | HSP90↑, N‐cadherin↑, aggrecan↑, collagen II↑, collagen X↓, IL‐6↓, p‐JAK1↓, p‐STAT3↓ | Promoted ECM synthesis and inhibited NP cells apoptosis by blocking IL‐6/JAK/STAT3 pathway | 54 |
| SD rats NP cells | 100 μM RSV |
caspase‐3↓, Bcl‐2↑, Bax↓, cleaved PARP↓, p‐Akt/Akt↑ |
Inhibited IL‐1β‐induced NP cells apoptosis through activation of PI3K/Akt pathway | 21 |
| SD rats NP cells | 100 μM RSV | ROS↓, SA‐β‐Gal activity↓, telomerase activity↑, p16↓, p53↓, MMP‐3↓, MMP‐13↓, ADAMTS4↓, collagen II↑, aggrecan↑ | Inhibited NP cells senescence in inflammatory environment | 55 |
| Pig NP tissues | 50 and 100 μM | GAG↑, HYP↑, aggrecan↑, collagen II↑, p‐Akt/Akt↑, SOX‐9↑ | Promoted ECM biosynthesis through activating the PI3K/Akt signaling pathway under mechanical compression | 69 |
| Human NP Cells | 50 μM RSV | SIRT1↑, aggrecan↑, collagen II ↑, MMP‐1↑ | Promoted ECM biosynthesis by activating the SIRT1 expression | 73 |
| Human NP Cells | 25 μM |
SIRT1↑, collagen II ↑, MMP‐13↓, ADAMTS‐5↓, p21↓, p16↓ |
Promoted ECM biosynthesis, suppressed cellular senescence, increased cell proliferation, and restrained the apoptosis by activating the SIRT1 expression | 72 |
| Human NP Cells | 100 μM | aggrecan↑, collagen II↑, SIRT1↑, β‐catenin↓ | Promoted ECM biosynthesis by activating the SIRT1 expression via Wnt/β‐catenin signaling pathway | 22 |
| SD rats NP cells | 1 μM E2, 10 μM, 100 μM and 200 μM RSV | collagen II↑, aggrecan↑, MMP‐3↓, MMP‐13↓, p‐Akt/Akt↑, caspase‐3↓ | Combined with E2 inhibited NP cells apoptosis and ECM degradation by activating PI3k/Akt pathway | 74 |
| Bovine NP cells | 10, 50, 200 μM RSV | MMP‐13↓, ADAMTS4↓, PG↑, CREB↓ | Slowed the progression of IDD by inhibiting catabolism and promoting PG synthesis in NP cells | 75 |
| SD rats NP cells | 100 μM RSV | caspase‐3↓, caspase‐9↓, mitochondrial membrane potential↑, ROS↓ | Inhibited SNP induced NP cells apoptosis by scavenging ROS | 20 |
| Pig NP tissues | 50 μM, 100 μM RSV |
Caspase‐9 activity↓, Caspase‐3 activity↓, Bcl‐2↑, Bax↓, caspase‐3↓, p‐ERK1/2↓ |
Inhibited mechanical compression induced NP cells apoptosis through the inhibition of ERK1/2 pathway | 79 |
| SD rats NP cells | 100 μM |
Bcl‐2↑, Bax↓, caspase‐3↓, p16↓, p53↓, p‐Akt/Akt↑ |
Inhibited high glucose induced NP cells apoptosis and senescence by activating the PI3K/Akt pathway | 82 |
| Human NP cells |
100 μM RSV 1 mM E2 |
caspase‐3↓, p‐mTOR/mTOR↑, P‐GSK‐3b/GSK‐3b↑, NF‐κB↓ | Combined with E2 prevented IL‐1β induced NP cells apoptosis via the PI3K/AKT/mTOR and PI3K/AKT/ GSK‐3β Pathway | 84 |
| Human NP cells | 50 μM | mitochondrial membrane potential↑, ROS↓, LC3II/LC3 I↑, p62↓ | Attenuated oxidative stress induced mitochondrial dysfunction by activation of autophagy | 87 |
| SD rats NP cells | 50 μM | aggrecan↑, collagen II↑, LC3II/LC3 I↑, beclin‐1↑, GAG↑, p‐Akt/Akt↑ | Promoted ECM biosynthesis through activating autophagy via the PI3K/Akt pathway under oxidative damage | 23 |
| Human NP cells | 24 μM RSV | MMP‐3↓, SIRT1↑, p‐AMPK↑, beclin‐1↑, LC3II/LC3 I↑ | Inhibited TNF‐a–induced MMP‐3 expression by activating autophagy via SIRT1/AMPK pathway | 88 |
| Human NP cells | 8 μM RSV | LC3II/LC3 I↑, Nampt↑, NAD + ↑, SIRT1↑ | Promoted autophagy of NP cells by activating Nampt/NAD+/SIRT1 signaling pathway | 89 |
| SD rats NP cells | 30 μM, 60 μM RSV | SA‐β‐Gal activity↓, ROS↓, telomerase activity↑, p16↓, p53↓ | Inhibited NP cells senescence induced by mechanical overload through inhibition of ROS/NF‐κB pathway | 19 |
| SD rats CEP cells | 10 μM, 20 μM, 30 μM, 40 μM RSV | TNF‐α↓, IL‐10↑, HMGB1↓, p‐ERK↓, Bax ↓, Bcl‐2↑ | Inhibited CEP cells apoptosis by suppressing HMGB1/ERK signaling pathway | 95 |
Fig. 1.
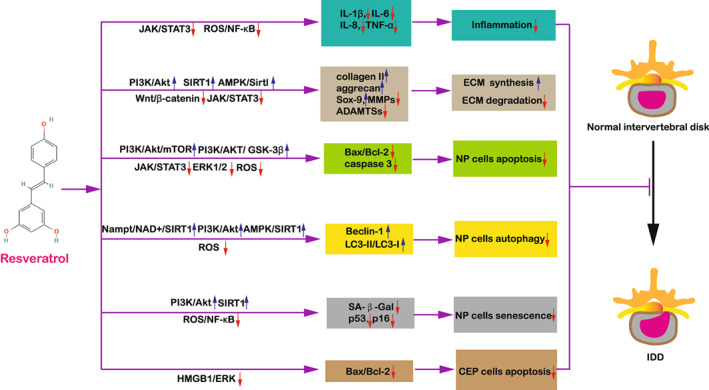
Effects of RSV on IDD. RSV treatment leads to up‐regulation of ECM biosynthesis, down‐regulation of ECM degradation, inhibition of NP cells apoptosis, activation of NP cells autophagy, and inhibition of NP cells senescence, thus attenuating IDD progression
Anti‐Inflammation Effects
Inflammatory response directly participates in the progression of IDD. It can also lead to secondary low back pain and radicular symptoms. 48 The expression of pro‐inflammatory cytokines, such as TNF‐α, IL‐1β and IL‐6 are found to be increased in the intervertebral disc tissues of IDD patients. 49 , 50 , 51 They have been shown to involve in various disease processes of IDD, including ECM degradation, cell apoptosis and senescence. 52 , 53 Wu et al. 54 revealed that RSV could increase the expression of heat shock protein 90 (HSP90) and N‐Cadherin in NP cells and promoted ECM biosynthesis by blocking IL‐6‐JAK/ STAT3 positive feedback loop. Jiang et al. 21 found that RSV exhibited its anti‐inflammatory effects on IL‐1β‐mediated NP cells via activating PI3K/Akt signaling pathway, thereby attenuating inflammation induced apoptosis of NP cells. Li et al. 55 discovered that RSV could inhibit NP cells senescence in IL‐1β and TNF‐α induced inflammation environment. Wuertz et al. 56 reported that the administration of RSV significantly inhibited the expression of IL‐6 and IL‐8 in the human NP. In another study, RSV could reduce TNF‐a and IL‐1 levels in radiculopathic pain model rats, leading to improved pain behavior in animal models. 57 Taken together, RSV can inhibit the production of pro‐inflammatory cytokines such as IL‐1β, IL‐6, IL‐8 and TNF‐α, and thus ameliorate IDD progression mediated by inflammation.
Induction of the ECM Metabolic Homoeostasis of NP Cells
The NP is a hydrated gel‐like tissue, which is the main functional structure of intervertebral disc and can be adjusted according to mechanical stress stimuli. 58 , 59 , 60 Studies have reported that the preservation of normal intervertebral disc mechanical function is dependent on the ECM homeostasis, and reduced biosynthesis and increased degradation of the ECM during IDD leads to mechanical dysfunction of the disc and aggravates disc degeneration. 61 , 62 , 63 NP cells (the main cell type in NP) are responsible for the biosynthesis and maintenance of the ECM. Matrix metalloproteinases (MMPs) and a disintegrin and metalloproteinases with thrombo‐spondin motif proteins (ADAMTSs) are the main force of ECM remolding during IDD. 64 , 65 At the cellular level, mechanical load, oxidative stress, high glucose and inflammatory response can induce ECM catabolism. 65 , 66 , 67 , 68 Han et al. 69 investigated the effects of RSV on ECM under mechanical compression. The results showed high‐magnitude mechanical compression seriously decreased ECM content, while treatment with RSV could increase the proteoglycan content, biochemical content (glycosaminoglycan (GAG) and hydroxyproline (HYP)) and anabolic factors (Sox‐9, aggrecan and collagen II) of ECM. Further research indicated that RSV reversed mechanical load induced ECM degradation by activating the PI3K/Akt pathway in a dose‐dependent manner. 69 Previous studies showed that activating Sirtuin 1(SIRT1), a NAD (+)‐dependent deacetylase, could also promote ECM biosynthesis. 70 , 71 , 72 Wu et al. 73 compared the expression of SIRT1 and ECM biosynthesis‐related factors in the NP tissues removed from patients with IDD and lumbar fracture (control group). The results of immunohistochemistry and quantitative fluorescence PCR showed that the expressions of SIRT1, collagen II and aggrecan in IDD patients were lower, while the expression of MMP‐1 was higher than those in control patients. However, after RSV treatment, the above results were reversed. Therefore, they suggested that RSV promotes ECM expression by altering MMP‐1 and SIRT1 expression, thereby reversing IDD progression. 73 Similarly, Guo et al. 72 extracted NP cells from elderly patients with lumbar disc herniation and treated the degenerative NP cells with RSV. The results showed that RSV could down‐regulate catabolic facros including MMP‐3 and MMP‐13 as well as up‐regulate the anabolic factors including collagen II and aggrecan by activating SIRT1.Another study showed that RSV activated SIRT1 through inhibiting Wnt/β‐catenin signaling pathway, and thus ameliorated degradation of the ECM and promoted ECM biosynthesis. 22 Inflammatory response is another important factor leading to ECM degradation. In a study by Yang et al., 74 combined use of 17β‐estradiol (E2) and RSV could reverse the down‐regulation of collagen II and aggrecan induced by IL‐1β in rat NP cells via PI3K/Akt/caspase‐3 pathway. Wu et al. 54 found that RSV promoted the expression of collagen II and aggrecan by suppressing JAK/STAT3 phosphorylation and decreasing IL‐6 production. In addition, Li et al. 75 found RSV can effectively suppress the IL‐1β induced up‐regulation of catabolic factors including MMP‐1, MMP‐3, MMP‐13, and ADAMTS‐4 and enhance anabolism of proteoglycan. These studies demonstrated that RSV could improve ECM homeostasis under inflammatory environment. 54 , 74 , 75
Inhibition of NP Cells Apoptosis
The occurrence of IDD is accompanied by high rates of apoptosis, leading to decreasing cell numbers in NP tissue, and therefore disturb the homeostasis of ECM. 76 , 77 Hence, inhibiting the apoptosis of NP cells can be an effective approach for IDD treatment. 78 Oxidative stress accelerates IDD by influencing NP cells apoptosis, autophagy, senescence and DNA methylation. 11 Li et al. 20 induced an oxidative stress injury model of NP cells using sodium nitroprusside (SNP). The results showed that RSV could reverse the promotion of NP cells apoptosis and overproduction of reactive oxygen species (ROS) and nitric oxide (NO). Actin‐Tracker Green and Tubulin‐Tracker red staining showed RSV could protect NP cells from disruption of cytoskeletal and morphological structure induced by SNP. Notably, its effect on ROS scavenging is comparable to another common ROS scavenger n‐acetyl cysteine (NAC). Zhang et al. 79 identified a significant increase in apoptosis ratio of NP cells under mechanical compression in a dose‐dependent manner, and further signaling pathway research indicated RSV treatment appeared to be effective in alleviating NP cells apoptosis via ERK1/2 pathway. Recent research has confirmed that diabetes is a potential causative factor of IDD. 80 , 81 Wang et al. 82 treated high glucose induced human NP cells with RSV and RSV + PI3K inhibitor LY294002, respectively. The results showed that the apoptosis ratio of NP cells treated with RSV was lower than those treated with RSV + LY294002, while the Akt phosphorylation (p‐Akt) level showed the opposite trend. Therefore, they concluded that RSV inhibited high glucose‐induced NP cells apoptosis by activating the PI3K/Akt signaling pathway. Research has also demonstrated that inflammation response is directly related with NP cells apoptosis. 82 Inhibiting inflammation may be another important way to suppress NP cells apoptosis. 10 , 83 Jiang et al. 21 induced NP cells apoptosis using IL‐1β and found that the expression of caspase‐3 decreased in NP cells after RSV treatment but increased after LY294002 treatment, so that RSV attenuated inflammation mediated NP cells apoptosis by activating the PI3K/Akt signaling pathway. In addition, Wu et al. 54 found that RSV inhibited NP cells apoptosis by blocking IL‐6/JAK/STAT3 signaling pathway. Yang et al. 74 found that single application of E2 or RSV both inhibited IL‐1β induced NP cells apoptosis. However, combined use of E2 and RSV reduced the cytotoxic effect of IL‐1β on NP cells more efficiently. Furthermore, Bai et al. 84 discovered that RSV combined with E2 enhanced PI3K and Akt expression, and subsequently promoted the activation of p‐mTOR and p‐GSK‐3b, which contributed to decreased caspase‐3 levels. They concluded that combined use of RSV and E2 attenuated IL‐1β‐induced cell apoptosis and recovered cell viability via activating PI3K/AKT/mTOR and PI3K/AKT/GSK‐3β signaling pathways.
Promotion of NP Cells Autophagy
Autophagy is a self‐protective cellular mechanism that removes damaged or senescent organelles, and considered to be an important cellular metabolic process. 85 , 86 The exact role of autophagy in IDD remains controversial. At present, most studies reported that the activation of autophagy could ameliorate IDD progression, while relatively few studies showed that excessive activation of autophagy could accelerate IDD progression. 47 The impacts of RSV on autophagy were investigated in different studies. Zhang et al. 87 discovered that H2O2 enhanced intracellular ROS expression and induced mitochondrial dysfunction in human NP cells, which was characterized by down‐regulation of ATP and mitochondrial membrane potential levels. However, RSV treatment promoted autophagic flux as well as exerting protective effects on mitochondrial dysfunction and cell apoptosis induced by H2O2. Therefore, they concluded that RSV attenuated oxidative stress induced mitochondrial dysfunction by activation of autophagy. 87 In another study, Gao et al. 23 found RSV promoted ECM biosynthesis through stimulating autophagy via the PI3K/Akt pathway under oxidative damage. Wang et al. 88 discovered that RSV activated autophagy through up‐regulating SIRT1 expression via stimulating upstream regulator AMPK in TNF‐a treated human NP cells and thereby inhibited the expression of MMP‐3. They concluded that RSV attenuated the catabolic effect through down‐regulating TNF‐a induced MMP‐3 expression via stimulating autophagy mediated by the AMPK/SIRT1 signaling pathway. In addition, Shi et al. 89 found that RSV can promote autophagy of NP cells by activating Nampt/NAD+/SIRT1signaling pathway and thus accelerating the progression of IDD.
Inhibition of NP Cells Senescence
NP cells senescence is a common feature during IDD progression and is often demonstrated to be positively correlated with IDD grade. 90 , 91 Age‐related β‐galactosidase (SA‐β‐Gal) is considered as a reliable marker of cellular senescence. 92 , 93 Recent research has shown that RSV attuned cellular senescence by down‐regulation of SA‐β‐Gal activity, inhibits G0/1 cell cycle arrest, and production of senescent markers (p53 and p16) and up‐regulation of telomerase activity. 19 , 55 , 82 For further mechanism research, Jiang et al. 19 discovered that RSV alleviated NP cell senescence by inhibiting ROS generation and activity of the NF‐κB under mechanical compression in a dose‐dependent manner. Wang et al. 82 found that RSV activated the PI3K/Akt pathway by reducing ROS production, and thereby inhibiting high glucose‐induced cellular senescence. Li et al. 55 demonstrated that RSV reversed the increase of NP cell senescence and promoted ECM biosynthesis in TNF‐α and IL‐1β induced inflammatory environment. Additionally, Guo et al. 72 found that the RSV suppressed cellular senescence and attenuated the apoptosis of NP cells through activating SIRT1.
Inhibition of CEP Cells Apoptosis
The CEP plays an important role in maintaining the normal shape of the vertebral body, biomechanical stabilization and solute transportation. The calcification or cell apoptosis of CEP hinders nutrient supplement, oxygen transmission and excretion of metabolic wastes from NP, and in depth study of the mechanism of CEP degeneration can provide a novel idea for the prevention and treatment of IDD. 94 Presently, there is only one study investigating the effects of RSV on CEP. Hu et al. 95 reported that RSV could inhibit TNF‐ α expression, increase IL‐10 expression, reduce Bax/Bcl‐2 and therefore inhibit CEP cells apoptosis. Gene chip analysis indicated that the regulatory mechanism of RSV on CEP cells apoptosis was mediated by inhibiting HMGB1/ERK signaling pathway. 95
Effects of RSV on IDD in Animal Studies
Researchers investigated the effects of RSV on IDD in vivo using rodent and rabbit models (Table 2). Kwon96 induced IDD by annular puncture of lumbar discs in New Zealand white rabbits. MRI showed that compared to the vehicle (DMSO) group, the RSV treatment group had an increased T2 weighted image signal, and the modified Thompson MRI grade was lower than that of the vehicle group. Examination of IDD related gene expression showed aggrecan expression in the RSV group was higher than that of the vehicle group, while the expression level of MMP‐13 was lower than that of the vehicle group. Hematoxylin–eosin (HE) staining showed RSV could ameliorate the cellular characteristics of IDD caused by annular puncture, such as fibroblast‐like cells and severe fibrosis of extracellular components. In another study by Zhang et al., 87 Safranine O/fast green staining showed that the OD value of central NP tissue in the RSV injection group was higher than that of the operation group, which indicated the beneficial effects of RSV on ECM biosynthesis. Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining showed that the proportion of NP cells apoptosis in the operation group was higher than that of the sham operation group, while injection of RSV reduced the ratio of apoptotic cells. Transmission electron microscope (TEM) results showed that the mitochondria in the NP cells of the operation group were swollen, indicating that mitochondria were damaged, while the ratio of damaged mitochondrial damage was ameliorated by RSV injection. These results showed that RSV injection reduces the apoptosis of NP cells by reducing mitochondrial damage and therefore effectively prevented the progression of IDD. Xia et al. 97 induced an C57BL/6J mice IDD model using intervertebral disc puncture. MRI examination showed that RSV reduced the modified Thompson MRI grade scores. The immunohistochemical staining also showed that RSV could promote the expression of collagen II and PCNA as well as decrease the number of p16 positive cells. Furthermore, knockout of the SIRT1 gene could reverse the above results. In conclusion, they suggested that SIRT1 plays a protective role in IDD progression, and down‐regulated expression of SIRT1 could lead to NP cells senescence, thereby accelerating IDD progression. RSV also showed preventative role against IDD by promoting cells proliferation and inhibiting the senescence of NP cells through activating SIRT1 and its downstream molecule, p16.
TABLE 2.
Summary of the in vivo researches about pharmacological activities of RSV in IDD
| Type of model | Route | Dose | Duration | Findings | Reference |
|---|---|---|---|---|---|
| New Zealand rabbits IDD model by percutaneous annular puncture | Injection | 15 μL of 100 μM RSV | once every 2 weeks for 4,8 and 16 weeks | modified Thompson MRI grade scores↓, MMP‐13↓, aggrecan↑, histological grades↓ | 96 |
| New Zealand rabbit IDD model by annulus fibrosus puncture method | Injection | 50 μL of 50 μM RSV | 8 weeks | MRI index↑, ECM synthesis ↑, proportion of NP cells apoptosis ↓, proportion of damaged mitochondria↓ | 87 |
| C57BL/6J mice IDD model by the annulus needle puncture | Oral | 100 mg RSV/kg body weight per day | At 1 and 4 weeks after puncture, lasted for 7 days each time | SIRT1↑, modified Thompson MRI grade scores↓, histological grades↓, collagen II↑, p16(+) cells↓, PCNA (+) cells↑ | 97 |
| SD rats lumbar radiculopathy model induced by autologous NP | Injection | 0.1 mL of 50 μM RSV | 21 days | pain threshold↑, pro‐inflammatory cytokine levels↓ | 56 |
| SD rats lumbar radiculopathy model induced by autologous NP | Injection | 0.1 mL of 50 μM RSV | 21 days | pain threshold↑, cell edema↓, focal hyperaemia↓, pro‐inflammatory cytokine levels↓ | 57 |
| New Zealand rabbits IDD model by annular puncture | Injection | 25 μL of 100 mg/ml RSV/PLGA NBs | 8 weeks | disc area↑, MRI index↑, aggrecan↑, SIRT1 (+) cells↑ | 36 |
Radiculopathic pain is the main symptom of IDD. The von Frey filament test is commonly used to evaluate paw withdrawal threshold, and lower withdrawal thresholds are considered as sign of mechanical hypersensitivity, which is correlated to pain behavior in animal models. 98 , 99 Researchers applied autologous NP on the rat dorsal root ganglion (DRG) to conduct IDD pain model. The results showed that compared to the vehicle (saline) group, RSV promoted withdrawal thresholds for up to 14 days after injection, which indicated that RSV could attenuate NP‐mediated pain. 56 , 57 Inflammation inhibition are known as potential targets for ameliorating pain in IDD. 100 Wuerts et al. 56 found that RSV suppressed the expression of IL‐6, IL‐8, MMP1, MMP3, and MMP13 and thereby induced anti‐inflammatory and anti‐catabolic effects. They believed that decreased pro‐inflammatory cytokine levels may be the underlying mechanism of pain reduction. 56 Lin et al. 57 used HE staining to show that RSV could improve cell structure, with decreased edema and focal hyperaemia, which indicated the inflammatory response was suppressed. Additionally, immunohistochemical staining showed that RSV could reduce TNF‐a and IL‐1 levels caused by neuron surgery. 57
The application potential of RSV is limited by its poor water solubility, poor stability, fast metabolism, and difficulty in reaching an effective blood concentration in intervertebral discs. 101 To solve this problem, the target release of RSV by ultrasound (US)‐mediated poly (lactic‐co‐glycolic acid) nanobubbles (NBs) have been investigated by Shen et al. 36 The RSV‐embedded NBs were synthesized using a double‐emulsion method. The active NP cell‐targeting biomarker CDH2 antibody (AbCDH2) was further conjugated to the NBs using a carbodiimide method. 36 The results showed that the RSV/AbCDH2 NBs had a high loading capacity and drug release efficiency. Furthermore, US can also enhance the release of RSV from RSV/AbCDH2 NBs and increase local blood drug concentration. In a rabbit IDD model, local injection of US mediated RSV/AbCDH2 NBs effectively improved characteristics of IDD progression including MRI index, aggrecan expression, and SIRT1 expression compared with using RSV alone. 36
At present, rodent trauma models are the main animal models used to investigate the effects of RSV on IDD, which do not reflect the biomechanical characteristics of the natural degeneration of the human body. 102 The recent mouse standing model may provide a better direction for related researches. 103 , 104 For instance, Lao et al. 103 found that the movement pattern of mice was similar to that of humans when standing and jumping with their lower limbs. The custom‐made hot plate cage was used to provide the axial biomechanical load of the spine. The fissures in AF increased, the height of intervertebral disc and CEP decreased, and the metabolic homeostasis of ECM was disturbed with the accumulation of biomechanical loading and time, forming an IDD model. What's more, notochordal cells are retained in rodent NP tissues, which disappear in human adults. At present, no suitable molecules have been found to distinguish notochord cells in NP tissues. 102 , 105 Therefore, the existence of notochord cells may affect the research results. In order to eliminate the gap between in vivo models and humans, a more clinically relevant animal model should be developed to better mimic the complexity of the human intervertebral disc and IDD progression.
Conclusion and Perspective
In conclusion, RSV shows substantial protective roles in the progression IDD. Mechanism researches reveal that RSV could effectively inhibit the apoptosis and senescence as well as promote autophagy of NP cells. It also exerts anabolic and anti‐catabolic effects on ECM, which are crucial for the regeneration of damaged intervertebral disc. Multiple signaling pathways, such as PI3K/Akt, NF‐κB, AMPK/SIRT1, and ERK1/2, are the common target signaling pathways of RSV in IDD treatment. What is more, inflammatory and oxidative stress could also be suppressed by RSV treatment. However, the published studies are limited to preclinical studies, and evidence of RSV‐containing drugs for the therapy of IDD has yet to be investigated in clinical trials to confirm the preliminary results obtained from previous researches. And the current studies are mainly focused on NP, and the effects of RSV on AP and CEP also need systematic investigation. In addition, more studies should be performed to verify the synergistic effects of RSV combined with traditional drugs in the treatment of IDD in order to enhance efficacy and decrease the drug resistance and side effects. Moreover, the poor aqueous solubility and rapid metabolism of RSV might restrict its clinical application. As previously reported, RSV released by US‐mediated RSV/AbCDH2 NBs is a promising strategy to enhance its bioavailability and pharmacological efficacy in IDD treatment. With this consideration, mechanisms to promote the absorption of RSV are also worthy of investigation.
Author Contributions
The concept of the manuscript was devised by Yan‐zheng Gao. Ming‐yang Liu and Kai‐guang Zhang performed the overall literature searches. Ming‐yang Liu and Liang Zhang were in charge of writing. Tables and Figure were designed by Ming‐yang Liu. Hai‐jun Li, Wei‐dong Zang and Yan‐zheng Gao discussed the content of the article and gave suggestions.
Acknowledgment
This work was supported by the Thousand Talents Plan of Central Plains (ZYQR201912122).
Contributor Information
Ming‐yang Liu, Email: liumy410@126.com.
Yan‐zheng Gao, Email: gaoyz410@126.com.
References
- 1. Roberts S. Disc morphology in health and disease. Biochem Soc Trans. 2002;30(Pt 6):864–9. [DOI] [PubMed] [Google Scholar]
- 2. Navone SE, Marfia G, Giannoni A, et al. Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol Histopathol. 2017;32(6):523–42. [DOI] [PubMed] [Google Scholar]
- 3. Wu PH, Kim HS, Jang IT. Intervertebral disc diseases PART 2: a review of the current diagnostic and treatment strategies for intervertebral disc disease. Int J Mol Sci. 2020;21(6):1‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu MY, Wang HB, Liu SW, Zhang GP, Liu JG, Yang C. Dimensional changes of lumbar intervertebral foramen in direct anterior approach‐specific hyperextension supine position. Orthop Surg. 2020;12(4):1173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker A, Held H, Redaelli M, et al. Implementation of a guideline for low back pain management in primary care: a cost‐effectiveness analysis. Spine. 2012;37(8):701–10. [DOI] [PubMed] [Google Scholar]
- 6. Jöud A, Petersson IF, Englund M. Low back pain: epidemiology of consultations. Arthritis Care Res. 2012;64(7):1084–8. [DOI] [PubMed] [Google Scholar]
- 7. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008;8(1):8–20. [DOI] [PubMed] [Google Scholar]
- 8. Mirza SK, Deyo RA. Systematic review of randomized trials comparing lumbar fusion surgery to nonoperative care for treatment of chronic back pain. Spine. 2007;32(7):816–23. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez‐Moure J, Moore CA, Kim K, et al. Novel therapeutic strategies for degenerative disc disease: review of cell biology and intervertebral disc cell therapy. SAGE Open Med. 2018;6:2050312118761674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamali A, Ziadlou R, Lang G, et al. Small molecule‐based treatment approaches for intervertebral disc degeneration: current options and future directions. Theranostics. 2021;11(1):27–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao G, Yang S, Cao J, et al. The role of oxidative stress in intervertebral disc degeneration. Oxid Med Cell Longev. 2022;2022:2166817–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pezzuto JM. Resveratrol: twenty years of growth, development and controversy. Biomol Ther. 2019;27(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wenzel E, Somoza V. Metabolism and bioavailability of trans‐resveratrol. Mol Nutr Food Res. 2005;49(5):472–81. [DOI] [PubMed] [Google Scholar]
- 14. Gambini J, Inglés M, Olaso G, et al. Properties of resveratrol: in vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid Med Cell Longev. 2015;2015:837042–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Deng Z, Li Y, Liu H, et al. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci Rep. 2019;39(5):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moussa C, Hebron M, Huang X, et al. Resveratrol regulates neuro‐inflammation and induces adaptive immunity in Alzheimer's disease. J Neuroinflammation. 2017;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang DD, Shi G, Jiang Y, Yao C, Zhu C. A review on the potential of resveratrol in prevention and therapy of diabetes and diabetic complications. Biomed Pharmacother. 2020;125:109767. [DOI] [PubMed] [Google Scholar]
- 18. Dyck GJB, Raj P, Zieroth S, Dyck JRB, Ezekowitz JA. The effects of resveratrol in patients with cardiovascular disease and heart failure: a narrative review. Int J Mol Sci. 2019;20(4):1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang Y, Dong G, Song Y. Nucleus pulposus cell senescence is alleviated by resveratrol through regulating the ROS/NF‐kappaB pathway under high‐magnitude compression. Biosci Rep. 2018;38(4):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20. Li K, Li Y, Mi J, Mao L, Han X, Zhao J. Resveratrol protects against sodium nitroprusside induced nucleus pulposus cell apoptosis by scavenging ROS. Int J Mol Med. 2018;41(5):2485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jiang Y, Xie Z, Yu J, Fu L. Resveratrol inhibits IL‐1beta‐mediated nucleus pulposus cell apoptosis through regulating the PI3K/Akt pathway. Biosci Rep. 2019;39(3):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Liu H, Shen J, Zhou H, Xu S, Hu Z. Resveratrol regulate the extracellular matrix expression via Wnt/beta‐catenin pathway in nucleus pulposus cells. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2018;32(4):476–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gao J, Zhang Q, Song L. Resveratrol enhances matrix biosynthesis of nucleus pulposus cells through activating autophagy via the PI3K/Akt pathway under oxidative damage. Biosci Rep. 2018;38(4):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24. Boocock DJ, Faust GE, Patel KR, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. 2007;16(6):1246–52. [DOI] [PubMed] [Google Scholar]
- 25. Andres‐Lacueva C, Macarulla MT, et al. Distribution of resveratrol metabolites in liver, adipose tissue, and skeletal muscle in rats fed different doses of this polyphenol. J Agric Food Chem. 2012;60(19):4833–40. [DOI] [PubMed] [Google Scholar]
- 26. Nunes S, Danesi F, Del Rio D, Silva P. Resveratrol and inflammatory bowel disease: the evidence so far. Nutr Res Rev. 2018;31(1):85–97. [DOI] [PubMed] [Google Scholar]
- 27. Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. [DOI] [PubMed] [Google Scholar]
- 28. Patel KR, Andreadi C, Britton RG, et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci Transl Med. 2013;5(205):205ra133. [DOI] [PubMed] [Google Scholar]
- 29. De Santi C, Pietrabissa A, Spisni R, Mosca F, Pacifici GM. Sulphation of resveratrol, a natural product present in grapes and wine, in the human liver and duodenum. Xenobiotica. 2000;30(6):609–17. [DOI] [PubMed] [Google Scholar]
- 30. Chaplin A, Carpéné C, Mercader J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients. 2018;10(11):1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jannin B, Menzel M, Berlot JP, Delmas D, Lançon A, Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: plasmatic protein binding and cell uptake. Biochem Pharmacol. 2004;68(6):1113–8. [DOI] [PubMed] [Google Scholar]
- 32. Qiu Z, Yu J, Dai Y, et al. A simple LC‐MS/MS method facilitated by salting‐out assisted liquid‐liquid extraction to simultaneously determine trans‐resveratrol and its glucuronide and sulfate conjugates in rat plasma and its application to pharmacokinetic assay. Biomed Chromatogr. 2017;31(11):e4001. [DOI] [PubMed] [Google Scholar]
- 33. Wang W, Zhou M, Xu Y, et al. Resveratrol‐loaded TPGS‐resveratrol‐solid lipid nanoparticles for multidrug‐resistant therapy of breast cancer: in vivo and in vitro study. Front Bioeng Biotechnol. 2021;9:762489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahman M, Almalki WH, Afzal O, et al. Cationic solid lipid nanoparticles of resveratrol for hepatocellular carcinoma treatment: systematic optimization, in vitro characterization and preclinical investigation. Int J Nanomed. 2020;15:9283–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gumireddy A, Christman R, Kumari D, Tiwari A, North EJ, Chauhan H. Preparation, characterization, and in vitro evaluation of curcumin‐ and resveratrol‐loaded solid lipid nanoparticles. AAPS PharmSciTech. 2019;20(4):145. [DOI] [PubMed] [Google Scholar]
- 36. Shen J, Zhuo N, Xu S, et al. Resveratrol delivery by ultrasound‐mediated nanobubbles targeting nucleus pulposus cells. Nanomedicine. 2018;13(12):1433–46. [DOI] [PubMed] [Google Scholar]
- 37. Abbott JA, Medina‐Bolivar F, Martin EM, et al. Purification of resveratrol, arachidin‐1, and arachidin‐3 from hairy root cultures of peanut (Arachis hypogaea) and determination of their antioxidant activity and cytotoxicity. Biotechnol Prog. 2010;26(5):1344–51. [DOI] [PubMed] [Google Scholar]
- 38. Abboud MR, Ghanem K, Muwakkit S. Acute lymphoblastic leukemia in low and middle‐income countries: disease characteristics and treatment results. Curr Opin Oncol. 2014;26(6):650–5. [DOI] [PubMed] [Google Scholar]
- 39. Brown VA, Patel KR, Viskaduraki M, et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin‐like growth factor axis. Cancer Res. 2010;70(22):9003–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pollack RM, Barzilai N, Anghel V, et al. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J Gerontol, Ser A. 2017;72(12):1703–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochem Soc Trans. 2007;35(Pt 4):652–5. [DOI] [PubMed] [Google Scholar]
- 42. Molladavoodi S, McMorran J, Gregory D. Mechanobiology of annulus fibrosus and nucleus pulposus cells in intervertebral discs. Cell Tissue Res. 2020;379(3):429–44. [DOI] [PubMed] [Google Scholar]
- 43. Thonar E, An H, Masuda K. Compartmentalization of the matrix formed by nucleus pulposus and annulus fibrosus cells in alginate gel. Biochem Soc Trans. 2002;30(Pt 6):874–8. [DOI] [PubMed] [Google Scholar]
- 44. Zhang HJ, Liao HY, Bai DY, Wang ZQ, Xie XW. MAPK /ERK signaling pathway: a potential target for the treatment of intervertebral disc degeneration. Biomed Pharmacother. 2021;143:112170. [DOI] [PubMed] [Google Scholar]
- 45. Banala RR, Vemuri SK, Dar GH, et al. Efficiency of dual siRNA‐mediated gene therapy for intervertebral disc degeneration (IVDD). Spine J. 2019;19(5):896–904. [DOI] [PubMed] [Google Scholar]
- 46. Zhang GZ, Deng YJ, Xie QQ, et al. Sirtuins and intervertebral disc degeneration: roles in inflammation, oxidative stress, and mitochondrial function. Clin Chim Acta. 2020;508:33–42. [DOI] [PubMed] [Google Scholar]
- 47. Gong CY, Zhang HH. Autophagy as a potential therapeutic target in intervertebral disc degeneration. Life Sci. 2021;273:119266. [DOI] [PubMed] [Google Scholar]
- 48. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014;10(1):44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liang H, Yang X, Liu C, Sun Z, Wang X. Effect of NF‐kB signaling pathway on the expression of MIF, TNF‐α, IL‐6 in the regulation of intervertebral disc degeneration. J Musculoskelet Neuronal Interact. 2018;18(4):551–6. [PMC free article] [PubMed] [Google Scholar]
- 50. Tian Y, Ji Y, Mei X, et al. Lower plasma melatonin in the intervertebral disk degeneration patients was associated with increased proinflammatory cytokines. Clin Interv Aging. 2021;16:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee JM, Song JY, Baek M, et al. Interleukin‐1β induces angiogenesis and innervation in human intervertebral disc degeneration. J Orthop Res. 2011;29(2):265–9. [DOI] [PubMed] [Google Scholar]
- 52. Wang Y, Che M, Xin J, Zheng Z, Li J, Zhang S. The role of IL‐1β and TNF‐α in intervertebral disc degeneration. Biomed Pharmacother. 2020;131:110660. [DOI] [PubMed] [Google Scholar]
- 53. Yang W, Yu XH, Wang C, et al. Interleukin‐1β in intervertebral disk degeneration. Clin Chim Acta. 2015;450:262–72. [DOI] [PubMed] [Google Scholar]
- 54. Wu C, Ge J, Yang M, et al. Resveratrol protects human nucleus pulposus cells from degeneration by blocking IL‐6/JAK/STAT3 pathway. Eur J Med Res. 2021;26(1):81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Li X, Lin F, Wu Y, et al. Resveratrol attenuates inflammation environment‐induced nucleus pulposus cell senescence in vitro. Biosci Rep. 2019;39(5):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56. Wuertz K, Quero L, Sekiguchi M, et al. The red wine polyphenol resveratrol shows promising potential for the treatment of nucleus pulposus‐mediated pain in vitro and in vivo. Spine. 2011;36(21):E1373–84. [DOI] [PubMed] [Google Scholar]
- 57. Lin B, Yu H, He Y, et al. Protective effects of resveratrol on autologous nucleus pulposus model of radiculopathy. Exp Ther Med. 2016;12(6):3917–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kepler CK, Ponnappan RK, Tannoury CA, Risbud MV, Anderson DG. The molecular basis of intervertebral disc degeneration. Spine J. 2013;13(3):318–30. [DOI] [PubMed] [Google Scholar]
- 59. Dou Y, Sun X, Ma X, Zhao X, Yang Q. Intervertebral disk degeneration: the microenvironment and tissue engineering strategies. Front Bioeng Biotechnol. 2021;9:592118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Roughley PJ, Melching LI, Heathfield TF, Pearce RH, Mort JS. The structure and degradation of aggrecan in human intervertebral disc. Eur Spine J. 2006;15(Suppl 3):S326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang GZ, Liu MQ, Chen HW, et al. NF‐κB signalling pathways in nucleus pulposus cell function and intervertebral disc degeneration. Cell Prolif. 2021;54(7):e13057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Luo Z, Ma Y, Di T, et al. DNMT3B decreases extracellular matrix degradation and alleviates intervertebral disc degeneration through TRPA1 methylation to inhibit the COX2/YAP axis. Aging. 2021;13(16):20258–76. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63. Chen Z, Zhang W, Deng M, Li Y, Zhou Y. CircGLCE alleviates intervertebral disc degeneration by regulating apoptosis and matrix degradation through the targeting of miR‐587/STAP1. Aging. 2020;12(21):21971–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE award competition in basic science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11(4):308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13(3):331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liu Y, Lin J, Wu X, et al. Aspirin‐mediated attenuation of intervertebral disc degeneration by ameliorating reactive oxygen species in vivo and in vitro. Oxid Med Cell Longev. 2019;2019:7189854–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Vergroesen PP, Kingma I, Emanuel KS, et al. Mechanics and biology in intervertebral disc degeneration: a vicious circle. Osteoarthr Cartil. 2015;23(7):1057–70. [DOI] [PubMed] [Google Scholar]
- 68. Bai X, Wang D, Zhou M, et al. Noninvasive cumulative axial load may induce intervertebral disc degeneration‐a potential rabbit model. Exp Ther Med. 2017;13(4):1438–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Han X, Leng X, Zhao M, et al. Resveratrol increases nucleus pulposus matrix synthesis through activating the PI3K/Akt signaling pathway under mechanical compression in a disc organ culture. Biosci Rep. 2017;37(6):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70. Wang D, He X, Wang D, et al. Quercetin suppresses apoptosis and attenuates intervertebral disc degeneration via the SIRT1‐autophagy pathway. Front Cell Dev Biol. 2020;8:613006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cai WT, Guan P, Lin MX, Fu B, Wu B. Sirt1 suppresses MCP‐1 production during the intervertebral disc degeneration by inactivating AP‐1 subunits c‐Fos/c‐Jun. Eur Rev Med Pharmacol Sci. 2020;24(11):5895–904. [DOI] [PubMed] [Google Scholar]
- 72. Guo J, Shao M, Lu F, Jiang J, Xia X. Role of Sirt1 plays in nucleus pulposus cells and intervertebral disc degeneration. Spine. 2017;42(13):E757–e66. [DOI] [PubMed] [Google Scholar]
- 73. Wu JW, Wang JJ, Chen JB, et al. Resveratrol could reverse the expression of SIRT1 and MMP‐1 in vitro. Genet Mol Res. 2015;14(4):12386–93. [DOI] [PubMed] [Google Scholar]
- 74. Yang SD, Ma L, Yang DL, Ding WY. Combined effect of 17beta‐estradiol and resveratrol against apoptosis induced by interleukin‐1beta in rat nucleus pulposus cells via PI3K/Akt/caspase‐3 pathway. PeerJ. 2016;4:e1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Li X, Phillips FM, An HS, et al. The action of resveratrol, a phytoestrogen found in grapes, on the intervertebral disc. Spine. 2008;33(24):2586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ding F, Shao ZW, Yang SH, Wu Q, Gao F, Xiong LM. Role of mitochondrial pathway in compression‐induced apoptosis of nucleus pulposus cells. Apoptosis. 2012;17(6):579–90. [DOI] [PubMed] [Google Scholar]
- 77. Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis. 2006;11(12):2079–88. [DOI] [PubMed] [Google Scholar]
- 78. Xie L, Huang W, Fang Z, et al. CircERCC2 ameliorated intervertebral disc degeneration by regulating mitophagy and apoptosis through miR‐182‐5p/SIRT1 axis. Cell Death Dis. 2019;10(10):751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhang Z, Wen F, He C, Yu J. Resveratrol attenuates mechanical compression‐induced nucleus pulposus cell apoptosis through regulating the ERK1/2 signaling pathway in a disc organ culture. Biosci Rep. 2018;38(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 80. Feng Y, Wang H, Chen Z, Chen B. High glucose mediates the ChREBP/p300 transcriptional complex to activate proapoptotic genes puma and BAX and contributes to intervertebral disc degeneration. Bone. 2021;153:116164. [DOI] [PubMed] [Google Scholar]
- 81. Kong CG, Park JB, Kim MS, Park EY. High glucose accelerates autophagy in adult rat intervertebral disc cells. Asian Spine J. 2014;8(5):543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang W, Li P, Xu J, et al. Resveratrol attenuates high glucose‐induced nucleus pulposus cell apoptosis and senescence through activating the ROS‐mediated PI3K/Akt pathway. Biosci Rep. 2018;38(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83. Cazzanelli P, Wuertz‐Kozak K. MicroRNAs in intervertebral disc degeneration, apoptosis, inflammation, and mechanobiology. Int J Mol Sci. 2020;21(10):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Bai X, Guo X, Zhang F, Zheng L, Ding W, Yang S. Resveratrol combined with 17beta‐estradiol prevents IL‐1beta induced apoptosis in human nucleus Pulposus via the PI3K/AKT/Mtor and PI3K/AKT/GSK‐3beta pathway. J Invest Surg. 2021;34(8):904–11. [DOI] [PubMed] [Google Scholar]
- 85. Revuelta M, Matheu A. Autophagy in stem cell aging. Aging Cell. 2017;16(5):912–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Liu MY, Liu F, Li YJ, et al. Ginsenoside Rg5 inhibits human osteosarcoma cell proliferation and induces cell apoptosis through PI3K/Akt/mTORC1‐related LC3 autophagy pathway. Oxid Med Cell Longev. 2021;2021:5040326–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhang B, Xu L, Zhuo N, Shen J. Resveratrol protects against mitochondrial dysfunction through autophagy activation in human nucleus pulposus cells. Biochem Biophys Res Commun. 2017;493(1):373–81. [DOI] [PubMed] [Google Scholar]
- 88. Wang XH, Zhu L, Hong X, et al. Resveratrol attenuated TNF‐alpha‐induced MMP‐3 expression in human nucleus pulposus cells by activating autophagy via AMPK/SIRT1 signaling pathway. Exp Biol Med. 2016;241(8):848–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shi Q, Xu L, Zeng X. Sirtuin 1 participates in intervertebral disc degeneration via the nicotinamide phosphoribosyl transferase/nicotinamide adenine dinucleotide/sirtuin 1 pathway responsible for regulating autophagy of nucleus pulposus cells. Exp Ther Med. 2022;23(4):267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016;15(13):1674–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Wang F, Cai F, Shi R, Wang XH, Wu XT. Aging and age related stresses: a senescence mechanism of intervertebral disc degeneration. Osteoarthr Cartil. 2016;24(3):398–408. [DOI] [PubMed] [Google Scholar]
- 92. Mohamad Kamal NS, Safuan S, Shamsuddin S, Foroozandeh P. Aging of the cells: insight into cellular senescence and detection methods. Eur J Cell Biol. 2020;99(6):151108. [DOI] [PubMed] [Google Scholar]
- 93. Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence‐associated beta‐galactosidase assay. Methods Mol Biol. 2007;371:21–31. [DOI] [PubMed] [Google Scholar]
- 94. Cheng Z, Xiang Q, Wang J, Zhang Y. The potential role of melatonin in retarding intervertebral disc ageing and degeneration: a systematic review. Ageing Res Rev. 2021;70:101394. [DOI] [PubMed] [Google Scholar]
- 95. Hu H, Li L, Liu Y, Wang S, Xie S, Sun J. Effect of resveratrol on high mobility group box‐1 protein signaling pathway in cartilage endplate degeneration caused by inflammation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2022;36(4):461–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kwon YJ. Resveratrol has anabolic effects on disc degeneration in a rabbit model. J Korean Med Sci. 2013;28(6):939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Xia X, Guo J, Lu F, Jiang J. SIRT1 plays a protective role in intervertebral disc degeneration in a puncture‐induced rodent model. Spine. 2015;40(9):E515–24. [DOI] [PubMed] [Google Scholar]
- 98. Li W, Zhang Y, Xing C, Zhang M. Tanshinone IIA represses inflammatory response and reduces radiculopathic pain by inhibiting IRAK‐1 and NF‐κB/p38/JNK signaling. Int Immunopharmacol. 2015;28(1):382–9. [DOI] [PubMed] [Google Scholar]
- 99. Krupkova O, Sekiguchi M, Klasen J, et al. Epigallocatechin 3‐gallate suppresses interleukin‐1β‐induced inflammatory responses in intervertebral disc cells in vitro and reduces radiculopathic pain in rats. Eur Cell Mater. 2014;28:372–86. [DOI] [PubMed] [Google Scholar]
- 100. Molinos M, Almeida CR, Caldeira J, Cunha C, Gonçalves RM, Barbosa MA. Inflammation in intervertebral disc degeneration and regeneration. J R Soc Interface. 2015;12(108):20150429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chimento A, De Amicis F, Sirianni R, et al. Progress to improve Oral bioavailability and beneficial effects of resveratrol. Int J Mol Sci. 2019;20(6):1‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Daly C, Ghosh P, Jenkin G, Oehme D, Goldschlager T. A review of animal models of intervertebral disc degeneration: pathophysiology, regeneration, and translation to the clinic. Biomed Res Int. 2016;2016:5952165–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lao YJ, Xu TT, Jin HT, et al. Accumulated spinal axial biomechanical loading induces degeneration in intervertebral disc of mice lumbar spine. Orthop Surg. 2018;10(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ao X, Wang L, Shao Y, et al. Development and characterization of a novel bipedal standing mouse model of intervertebral disc and facet joint degeneration. Clin Orthop Relat Res. 2019;477:1492–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Alini M, Eisenstein SM, Ito K, et al. Are animal models useful for studying human disc disorders/degeneration? Eur Spine J. 2008;17(1):2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


