Abstract
Context
Considering the accumulation of recent studies investigating the health effects of walnut consumption, both including and beyond cardiovascular health effects, a systematic review of this literature to investigate the strength of the evidence is warranted.
Objective
To investigate associations between walnut consumption and outcomes with public health relevance (specifically all-cause mortality, type 2 diabetes, CVD, metabolic syndrome, obesity, cancer, neurological and mental health, musculoskeletal, gastrointestinal, and maternal disorders) and the effect on associated disease risk markers, reported in studies published from 2017 to present.
Data Sources
MEDLINE, FSTA, CENTRAL, and Scopus were searched from 1 January 2017 to 5 May 2021.
Data Extraction
Human studies (cohort studies and RCTs) ≥3 weeks in duration comparing consumption of walnuts (whole, pieces, or 100% butter) to a control and measuring associations with relevant public health outcomes and disease risk markers were assessed. Key study characteristics were extracted independently by 2 investigators using a standardized table. The quality of the studies was assessed using the Cochrane Risk-of-Bias tool 2.0 and the Newcastle–Ottawa Scale.
Data Analysis
Only 1 RCT was considered to be at low risk of bias for any of its outcomes. The cohort studies were considered to be of moderate or high quality. The results were synthesized using vote counting, based on the direction of effect. Thirty-three articles, 23 describing RCTs (walnut dose ∼10–99 g/day, 1,948 subjects) and 10 describing cohort studies (∼675,928 subjects), were included. Vote counting could be performed for the blood lipids, cardiovascular function, inflammation- and hemostatic-related factors, markers of glucose metabolism, and body weight and composition outcome groupings. The results are presented in effect direction plots. With respect to blood lipids, results from 8/8 RCTs favoured walnuts, in accordance with associations with a reduced risk of CVD suggested by cohort studies; results from 6/6 RCTs favoured control with respect to body weight and composition, although most of these effects were small. This was contrary to cohort study results suggesting small benefits of walnut consumption on body weight. There was no overall consistent direction of effect for cardiovascular function, markers of glucose metabolism, or inflammation- and hemostatic-related factors.
Conclusions
Evidence published since 2017 is consistent with previous research suggesting that walnut consumption improves lipid profiles and is associated with reduced CVD risk. Evidence is accumulating in other areas, such as cognitive health, although more research is needed to draw firm conclusions.
Systematic Review Registration
PROSPERO registration no. CRD4202122.
Keywords: cardiovascular disease, public health, systematic review, walnuts
INTRODUCTION
Analysis from the Global Burden of Disease study suggests that poor diet was responsible for 188 million disability-adjusted life-years (DALYs) and 7.94 million deaths among adults aged 25 years and older in 2019.1 Diets low in nuts and seeds (defined as average daily consumption of less than 10–19 g) were highlighted by the researchers as 1 of 15 dietary risk factors contributing to mortality and DALYs, with an estimated 6% of ischemic heart disease and 2% of diabetes deaths being attributable to low intake.2 Data from a number of prospective cohort studies suggest an association between higher nut consumption and lower all-cause and cardiovascular disease (CVD) mortality3 and cancer risk.4,5
Nuts are among foods that are encouraged as part of national and international food-based dietary guidelines6–11 and are a common feature of healthy dietary patterns such as the Mediterranean and Dietary Approaches to Stop Hypertension (DASH) diets.12 Nuts have been described as a nutrient-dense food7 and provide a range of nutrients, including fiber, often lacking in typical Western diets,13,14 and essential micronutrients (eg, copper, manganese) as well as plant bioactives, including phenolic compounds.15–18 These nutritional properties, as well as their physical structure, which requires considerable mastication and reduces the bioaccessibility of some of the energy, mean that nuts are thought to be advantageous for health, despite being relatively calorie dense.19 An association has been observed between better overall dietary quality and regular nut consumption, which may in part be due to displacement of less healthy foods.20 Nuts and nut butters are typically listed as examples of healthier snacks by health organizations.21–24
Nuts additionally provide plant protein, and the need to shift to more plant-based dietary patterns for the sake of planetary health is becoming increasingly recognized.25–28 The EAT-Lancet Commission review recommends increasing the consumption of a variety of plant-based foods, including consuming 25 g of tree nuts (definitions of which vary, but typically includes almonds, walnuts, pistachios, cashews, hazelnuts, pecans, macadamias, and Brazil nuts29) per day. In addition, the optimal level of intake of nuts estimated from the Global Burden of Disease study, based on the level of intake associated with the lowest risk of mortality in prospective cohort studies, was 21 g/day.30 However, current consumption is considerably lower than this, at an estimated 3 g/day globally.30 In the United Kingdom, on average, consumption of nuts and seeds combined is estimated to be 6 g/day including nut butters31 and 4.6 g/day excluding nut butters,32 and in the US, average daily nut consumption has been estimated to be 8–13 g based on large cohort studies.33
The fatty acid composition of nuts varies widely.16 In comparison with other nuts, walnuts are higher in polyunsaturated fatty acids (PUFAs), particularly the essential fatty acids, alpha-linolenic acid (ALA; 18:3, n-3) and linoleic acid (18:2, n-6).15,16 Replacing saturated fats with unsaturated fats in the diet has been shown to reduce low-density-lipoprotein cholesterol (LDL-C),34 and replacing saturated fat with polyunsaturated fat appears to be a useful strategy in reducing cardiovascular events.35 In the PREDIMED study (examined as an observational cohort), subjects who consumed >3 servings of walnuts/week at baseline had a lower risk of cardiovascular mortality (hazard ratio [HR] 0.53; 0.29–0.98) during a median follow-up of 4.8 years (adjusted for intervention group).36 Several randomized controlled trials have also demonstrated improvements in cardiovascular risk markers, including lipid profiles following walnut consumption.37,38 Such research has led to an approved US Food and Drug Administration health claim for walnuts reducing risk of coronary heart disease (CHD) (supporting the inclusion of 1.5 ounces [43 g] walnuts daily).39 Vascular function is also observed to improve after walnut consumption,40,41 with a claim authorized for use in the European Union and the Great Britain based on improvements in endothelium-dependent vasodilation (the beneficial effect is obtained with a daily intake of 30 g of walnuts42–44). The literature often points to the fatty acid composition of walnuts in relation to reported health benefits, with their relative content of other bioactive compounds, including phytosterols, also highlighted.
Reviews examining the health effects of walnut consumption published in the last 30 years have largely focused on CVD risk markers and end points.45–52 Additionally, more limited research relates to other major global health concerns, including obesity53–55 and age-related cognitive decline,56–58 which is likely to become increasingly important for aging populations.59 There is a lack of recent systematic reviews that amalgamate many different areas of walnut and health research, ie, including both more established and emerging risk markers and outcomes, and some reviews have focused on particular walnut components such as polyphenols or micronutrients rather than consumption of whole nuts.51,52,60
A 2018 systematic review comprehensively examined the effect of walnut consumption on cardiovascular disease risk markers, including blood lipids, body weight and blood pressure, and included studies published up to January of that year.37 However, the evidence base is rapidly expanding, including research on emerging areas such as the gut microbiota.61,62 With the publication of analyses from several large prospective cohort studies and results from large randomized controlled trials in the last few years,33,63–66 a systematic review of the literature to investigate the strength of the evidence relating to walnut consumption and health effects more broadly is therefore timely.
The objectives of this review are to address the following questions:
What is the association between walnut consumption and public health outcomes of relevance in higher/middle income countries (based on causes and risk factors for death and DALYs according to the Global Burden of Disease study,2 that may be modifiable by diet), specifically all-cause mortality, type 2 diabetes, CVD, metabolic syndrome (MetS), obesity, cancer, neurological and mental health disorders, musculoskeletal disorders, gastrointestinal disorders, and maternal disorders?
What is the effect of walnut consumption on risk markers of these conditions in humans, compared with a control?
In order to consider these questions, we carried out a systematic review of cohort studies and randomized controlled trials (RCTs) investigating walnut consumption, compared with no or lower walnut consumption, including those with subjects from within the general population and those with existing health conditions, published from 2017 onwards. Vote counting based on the direction of effect was used for data synthesis in order to combine data from diverse but related outcome measures (eg, body weight and waist circumference), as well as results reported using different metrics, in order to produce an overview and as a way of visualizing the data and promoting transparent links between the data and the narrative.67
METHODS
This review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement,68 taking into account the general principles for synthesizing and presenting findings using methods other than meta-analysis set out in the Cochrane Handbook69 and the Synthesis Without Meta-analysis (SWiM) in Systematic Reviews reporting guideline.70 The protocol was prospectively registered in an international registry of systematic reviews (PROSPERO registration number CRD42021225340). An expert steering group was convened to comment on the review methodology and findings.
Study eligibility
The inclusion and exclusion criteria for study characteristics (in the PICOS format) can be seen in Table 1. Details are provided for: Population of interest (P); Intervention (I); Comparisons (C); Outcome (O); Study type (S). Articles published from 2017 until the end of the search period in English in peer-reviewed journals were eligible for inclusion. The review protocol was amended (as detailed on the PROSPERO record) during full-text screening to add “excluding drugs” to the “Comparator(s)/control” field, as members of the review team agreed that drugs were inappropriate comparators within the context of this review.
Table 1.
PICOS criteria for inclusion and exclusion of studies
| Parameter | Inclusion and exclusion criteria |
|---|---|
| Participants | Studies carried out in humans were included, specifically adults and children both from within the general population and those with existing health conditions (eg, type 2 diabetes, CVD). |
| Interventions |
|
| Comparators | No walnut consumption (any comparator, excluding drugs) or lower walnut consumption |
| Outcomes |
|
| Study design | Cohort studies and randomized controlled trials with the amount and frequency of walnuts clearly defined were included. Cross-sectional, animal, in vitro, and ex vivo studies were excluded. |
Abbreviations: BMI, body mass index; CVD, cardiovascular disease; FMD, flow-mediated dilation; HDL-C, high-density lipoprotein cholesterol; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; LDL, low-density lipoprotein; LDL-C, low-density lipoprotein cholesterol; RCTs, randomized controlled trials.
Search strategy and data sources
MEDLINE (PubMed, coverage 1946–present), Scopus (coverage 1788–present), Food Science and Technology Abstracts (EBSCO and Web of Science, coverage [both] 1968–present), and the Cochrane Central Register of Controlled Trials (CENTRAL, no inception date) were searched to identify articles describing human studies published in English, in peer-reviewed journals from 2017 onwards. A broad search strategy was employed, using variations of the search terms “walnut” and “Juglans”. Further details can be found in Table S1 in the Supporting Information online. The initial searches were completed on January 13, 2021. Reference lists from relevant review articles identified in the searches and articles included in the review were screened to identify any additional eligible studies not captured by the database searches. To help ensure completeness, the steering group was consulted, and abstract lists supplied by the project funders were hand-searched. Update searches were performed on May 5, 2021.
Study selection
Records generated from the searches were imported into the evidence synthesis software Covidence (Melbourne, Australia), with duplicates automatically detected and removed. Titles and abstracts were screened for acceptance against the inclusion/exclusion criteria by 2 investigators independently. For each of the relevant abstracts that appeared to meet the inclusion criteria, or where there was uncertainty, full publications were retrieved for evaluation by 2 investigators independently. Uncertainty and discrepancies regarding study eligibility were discussed with a third researcher from within the review team and resolved through consensus, with the steering group being consulted if consensus could not be reached.
Data extraction
Two investigators independently performed data extraction, entering information into an electronic form within Covidence (Melbourne, Australia). All results that were compatible with outcomes of interest as listed and grouped in the review PICOS (established a priori), using any measure, were eligible for inclusion. The following information was extracted from articles describing RCTs: citation; location of the study; subject number; subject characteristics (including age, sex, health status, body mass index [BMI] / body weight); intervention (form of walnuts); dose; duration; control; study design (parallel group/crossover, length of run-in and wash-out periods); outcome measures; results; and adverse events. The following information was extracted from articles describing cohort studies: citation; location(s) of the cohort; subject number; subject characteristics (including age, sex, health status, BMI/body weight); method of assessing level of exposure and frequency of data collection; length of follow-up; analysis strategy (eg, comparison of tertiles, adjustment for confounding factors); outcome measures; and results.
Where available, data derived from intention-to-treat analysis and fully adjusted results were extracted preferentially. Where multiple articles reported results for the same outcome measure from the same RCT, cited protocol articles were retrieved and consulted in order to ascertain methodological details where necessary, and results were extracted from the article that reported data for the largest number of subjects, or the later time point, in order to avoid double counting. Where multiple articles analyzing data from the same cohort(s) reported the same outcome, results were extracted from the article with the greatest number of cases of the outcome of interest. For RCTs that involved multiple arms, the 2 arms were selected that would most clearly allow the effect of walnut consumption to be isolated and compared with a suitable control. Consensus checking was performed by 1 investigator, and discrepancies were resolved through consensus.
Quality assessment
Two researchers independently undertook quality assessment for included RCTs and cohort studies. The Cochrane Collaboration Risk-of-Bias tool 2.0 (RoB 2.0) was used for RCTs,71 with the effect of assignment to the interventions considered and assessments conducted per outcome (and individual result, if more than 1 was reported per outcome). RoB 2.0 addresses 5 specific domains: (1) bias arising from the randomization process; (2) bias due to deviations from intended interventions; (3) bias due to missing outcome data; (4) bias in measurement of the outcome; and (5) bias in selection of the reported results. Studies were judged to be at “low” or “high” risk of bias or to raise “some concerns”. The Newcastle–Ottawa scale (NOS) was used to assess study quality for cohort studies.72 Studies are judged on 3 broad perspectives: the selection of the study groups; the comparability of the groups; and the ascertainment of the outcome of interest. Scores of 0–3, 4–6, and 7–9 were regarded as low, moderate, and high quality, respectively. Where there was disagreement in any quality assessment, a third investigator from within the review team was consulted and decision reached by consensus. Further details on how risk-of-bias assessments were conducted can be found in Appendix S1 in the Supporting Information online.
Data synthesis
Data from RCTs and cohort studies were considered separately. Following the example set in the SACN Carbohydrates and Health Report,73 for each outcome group, data were considered suitable for synthesis if results from RCTs that were relevant to an outcome group were reported by at least 3 studies. The method of vote counting based on the direction of effect was used in this review as an alternative synthesis method, outlined within the Cochrane Handbook for Systematic Reviews of Interventions.67,69,70 Related outcomes were first grouped into outcome domains.67 Outcome groupings used for synthesizing the results (eg, “blood lipids”, established based on the groupings listed in the protocol after data extraction was complete) are listed in Table S2 in the Supporting Information online.
Using the results included within each domain, individual effects (ie, from a single outcome measure) were categorized as positive (ie, favoring walnut consumption), negative (ie, favoring the control), or having no effect (ie, no difference between walnut consumption and control, based on reported data). Neither statistical significance, nor magnitude of effect are considered, as set out within the Cochrane Handbook for Systematic Reviews of Interventions.69 Further details of the methodology used can be found in Appendix S2 in the Supporting Information online. The overall effect direction for a particular study, the number of outcomes contributing to the overall effect direction, the study size, the study design (ie, parallel group or crossover trial), and the risk of bias are displayed using direction of effect plots, using a published template67 to summarize the direction of health impact. Results from studies that did not provide data from which effect direction could be ascertained could not be included in the direction of effect plot and have been described narratively.
The overall proportion of studies showing a positive effect direction was calculated, and the sign test used to calculate the probability of observing the given number of positive and negative results if the null hypothesis (an equal number of positive and negative effects, ie, no effect of walnut intervention) were true.69 Further details can be found in Appendix S2 in the Supporting Information online. Harvest plots were used to further display the results (categorized as positive, negative, or no effect) for individual outcome measures that were reported by 3 or more studies if the result of the sign test was significant for an outcome group.
Vote counting based on effect direction was not considered suitable for use when the clinical significance of the direction of effect was uncertain. In relation to results pertaining to gut microbiota (eg, alpha-diversity, beta-diversity, bacterial abundance), the precise classifications of changes at the taxonomic level in relation to health are still emerging and not conclusive.74,75 Similarly, it was felt to be inappropriate to include lipoprotein subclasses, subfractions, and cholesterol efflux within the vote counting for the blood lipids outcome group, since meaningful categorization of effect directions (ie, as “positive” or “negative”) in relation to health have also not been robustly determined. Results for these outcome groups were therefore discussed narratively.
Planned stratifications for different outcomes were: study duration, health status of participants, age group (ie, adults [defined as individuals aged 18 years and above] or children [defined as individuals aged below 18 years]), walnut dose, and sex. As such, the results for the cardiovascular function group were stratified according to walnut dose (<30 g per day and ≥30 g) to reflect the conditions of use of the health claim authorized for use in the European Union and Great Britain,42–44 as well as baseline blood pressure. The results for blood lipids were stratified by study duration (<8 weeks and ≥8 weeks) in line with guidance from the European Food Safety Authority (EFSA),76 as well as baseline LDL-C and walnut dose. The results for body weight and composition were stratified by study duration (<12 weeks and ≥12 weeks) in line with guidance from EFSA,77 as well as dose and baseline BMI. The results for glucose control were stratified by study duration (<12 weeks and ≥12 weeks) in line with guidance from EFSA,77 as well as dose and baseline BMI. Where stratifications could be performed, these are presented within effect direction plots. Since the RoB 2.0 tool calls for risk of bias to be assessed per outcome rather than per study, in the small number of cases where the risk of bias was judged to be different for outcomes within the same outcome group, the highest risk of bias judgement across all outcomes within the group for that study is displayed within effect direction plots.
RESULTS
Study selection
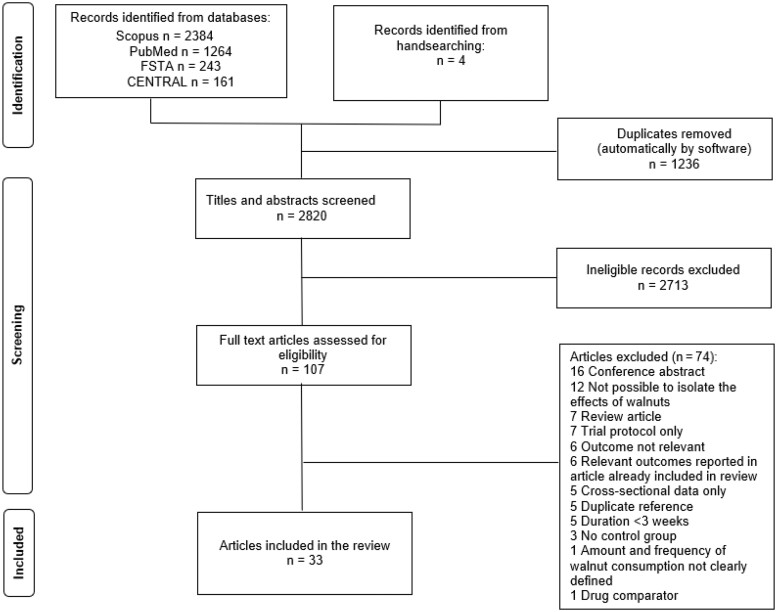
The searches and hand-searching yielded 4056 records in total. After the removal of duplicates, 2820 abstracts were screened and 107 full-text articles were assessed for eligibility (see PRISMA flow chart, Figure 1). Seventy-four full-text articles were excluded as they did not meet the inclusion criteria. A summary of the exclusion reasons is listed in the PRISMA flow chart and a detailed list of all excluded full-text articles and associated reasons can be seen in Table S3 in the Supporting Information online. Thirty-three articles met the inclusion criteria and were included in this systematic review.
Figure 1.
PRISMA flow chart.
Characteristics of included studies
This review includes 33 articles describing RCTs and cohort studies that met the inclusion criteria; 23 articles describing 13 RCTs enrolling 1948 subjects63–65,78–97; and 10 articles describing 8 cohorts, including data from 675 928 subjects (note, some articles only report subject numbers for analysis related to total nut consumption, undertaken on a larger group of subjects in some cases).33,66,98–105 Articles were published between 2017 and 2021.
Characteristics of the RCTs can be seen in Table S4 in the Supporting Information online. Eight of the RCTs were parallel group studies,63,64,78,79,82–89,92–94 and 5 employed a crossover design.65,80,81,90,91,95–97 RCTs were conducted in Australia, Cyprus, Germany, Iran, South Korea, Spain, Tunisia, Turkey, and the United States. The number of enrolled subjects ranged from 15 to 708, and study duration ranged from 3 weeks to 2 years. Two RCTs exclusively enrolled older adults.78,92 No RCTs involved children. One RCT was in chronic kidney disease patients.95 Mean baseline BMI ranged from normal (18.5–24.9 kg/m2) to obesity class I (30.0–34.9 kg/m2),106 and 4 RCTs exclusively recruited participants with overweight and/or obesity (BMI 25.0–40.0 kg/m2).64,65,87,94 The Walnuts and Healthy Ageing (WAHA) study was the largest RCT (n = 708 subjects enrolled across 2 centers) and had the longest duration (2 years).63,78,83,85,86,88
The RCTs investigated the effect of consuming whole walnuts or pieces, in doses ranging from equivalent of ∼10–99 g/day. None of the RCTs provided walnut butter as the intervention. The control diets varied. Nine RCTs compared walnut consumption with a walnut- (or nut)-free diet.63,64,78,79,81–83,85,86,88–90,92,94 In 1 RCT the comparator was oily fish,87 in 2 RCTs the comparator was white bread,91,95 and in another the comparator was a diet that replaced the amount of ALA contributed by walnuts with oleic acid.65 Included RCTs providing additional interventions alongside walnut consumption also contained a group that received the additional interventions without walnuts, which served as the comparator group for the purposes of this review. In 1 RCT exercise classes were provided (with or without walnuts),92 1 RCT prescribed an energy-restricted diet alongside advice to consume walnuts (or not),87 and 2 RCTs supplied dietary counseling, advice to increase physical activity, and psychological coaching with the aim of inducing weight loss, with or without walnuts.64,94 In total >190 unique outcomes that were relevant to the review were reported across the RCTs (summarized in Table S2 in the Supporting Information online). Results reported in the RCTs are tabulated in Tables S5–S14 in the Supporting Information online. The planned stratifications of adults versus children and male versus female could not be completed, as none of the studies included any subjects below the age of 18, and 10 out of the 13 RCTs included both male and female subjects. Any reported differences in findings by sex are outlined in Appendix S3 in the Supporting Information online.
The characteristics of the cohort studies can be seen in Table S15 in the Supporting Information online. Cohort studies were conducted in the United States (Nurses’ Health Study [NHS], Nurses’ Health Study II [NHS II], Health Professionals’ Follow-up study [HPFS],33,66,99,103,104 Coronary Artery Risk Development in Young Adults [CARDIA] study,102 Health and Retirement and Health Care Nutrition studies101) Iran (Golestan Cohort Study, Tehran Lipid and Glucose Study100,105), and 1 measured walnut consumption in Argentina, Bangladesh, Brazil, China, Iran, Poland, Saudi Arabia, Turkey, and the United Arab Emirates (the Prospective Urban and Rural Epidemiology [PURE] study98) All assessed walnut intake using food frequency questionnaires, which were self-administered in 6 cohorts, and interviewer-administered in 2 cohorts (CARDIA study102 and Tehran Lipid and Glucose Study105). One cohort study exclusively enrolled older adults.101 Relevant reported outcome measures in the cohort studies included CVD, CHD), stroke, “healthy aging”, cognitive status, physical function impairment, body weight / BMI classification, esophageal squamous cell carcinoma, hepatocellular carcinoma, blood pressure, heart function, MetS, and all-cause mortality. Length of follow-up ranged from ∼3 years to 28 years. Results for the cohort studies can be seen in Table S16 in the Supporting Information online.
Quality assessments
A summary of risk-of-bias assessments for RCTs by outcome group can be viewed in Figure S1 in the Supporting Information online. Only 1 of the RCTs was at low risk of bias for any of its outcomes.65,96,97 A lack of information regarding allocation sequence concealment was common. The scores indicating the quality of the cohort studies, according to the NOS, can be seen in Table S15 in the Supporting Information online. Differences in the amount and type of information reported relating to adequacy of follow-up for cohort studies made assessment of this question challenging. All cohort studies were judged to be of either moderate or high quality.
Effect of walnut consumption on study outcomes
Results have been ordered based on interrelated aspects of health and the amount of available evidence (in descending order). Hard disease end points and intermediate or proxy end points for the disease are noted as relevant.
Cardiometabolic health
CVD (hard end points): Pooled analysis from 3 large prospective cohort studies (NHS, NHS II, and HPFS, n = 210 566), with up to 14 years of follow-up, was used to report the association of total and specific types of nut consumption with total CVD and CHD.66 Consuming walnuts at least once per week (0 vs ≥ 1 serving/wk; 1 serving = 28 g) was associated with a lower risk of total CVD (myocardial infarction, stroke or fatal CVD [fatal stroke, fatal myocardial infarction, and cardiovascular death], multivariate HR 0.81, 95% confidence interval [CI] 0.72–0.92, P for trend <0.001), and CHD (fatal and nonfatal myocardial infarction, multivariate HR 0.79, 95% CI 0.66–0.94, P for trend = 0.01) compared with those who never or almost never consumed walnuts. Walnut intake of 1 or more times per week was associated with a 17% lower risk of stroke (95% CI 0.71–0.96, P = 0.10). Hazard ratios for CVD, CHD, and stroke per 28 g increase were 0.71 (95% CI 0.52–0.97), 0.63 (95% CI 0.41–0.94), and 0.83 (95% CI 0.53–1.30), respectively. The data for total CVD was additionally adjusted for consumption of other nuts, which attenuated the results (relative risk 0.89, 95% CI 0.77–1.03, comparing at least once per week vs never or almost never, P for trend = 0.28).66
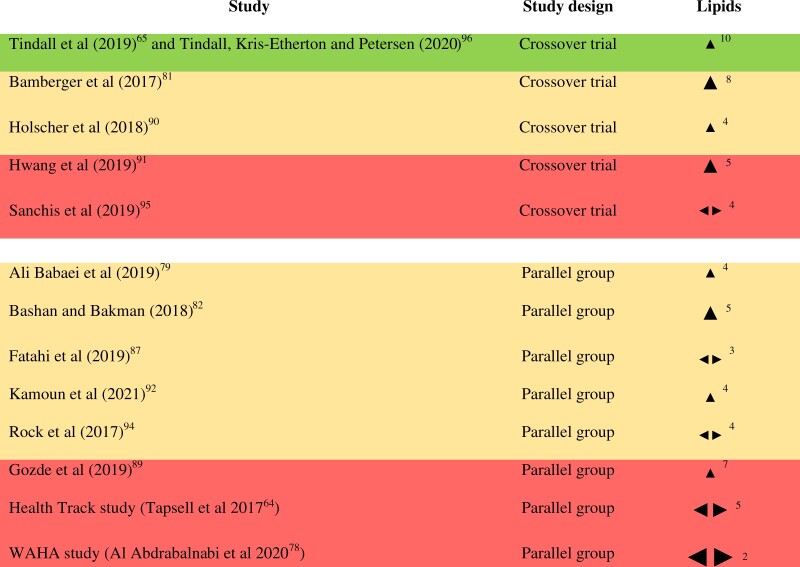
Blood lipids: All of the 13 RCTs (enrolling 1948 subjects in total) included in this review measured blood lipids. Baseline LDL-C among the subjects ranged from “optimal” (<100 mg/dL) to “high” (160–189 mg/dL), with most falling into the near/above optimal category (100–129 mg/dL).107 The number of RCTs reporting each outcome in this group is as follows: high-density lipoprotein cholesterol (HDL-C) (13), triglycerides (13), LDL-C (12), total cholesterol (TC, 11), very low-density lipoprotein cholesterol (VLDL-C) (4), the TC:HDL-C ratio (3), lipoprotein(a) (2), nonHDL-C (2), apolipoprotein B (apoB) (2), intermediate-density lipoprotein cholesterol (IDL-C) (1), and the LDL-C:HDL-C ratio (1). Five of the RCTs reported mixed effects on various lipid outcomes (ie, inconsistent effects on various outcomes, with no consistent direction across the effects [using the figure of ≥70% as a majority], see Appendix S2 in the Supporting Information online). Results from all of the remaining 8 RCTs favored walnuts (100%, result of sign test calculation P = 0.0078). Of these, 2 studies were judged to be at high risk of bias, 5 were judged to have “some concerns,” and 1 was judged to be at low risk of bias for this outcome group (see Figure 2). Results stratified by baseline LDL-C, study duration, and walnut dose can be seen in Figure S2 in the Supporting Information online. Based on visual inspection of the effect direction plots, overall effect direction appears to be more consistent (ie, almost all studies showing a favorable direction, compared with a mixture of favorable and conflicting effect directions) across studies in subjects with high/borderline–high LDL-C at baseline, compared with those with optimal or near/above optimal LDL-C, although only 3 studies fall into the high/borderline–high categories. Overall, effect direction appears to be more consistent across studies of less than 8 weeks duration, compared with those of longer duration, and in studies using doses of at least 40 g/day.To provide greater insight into the results of the synthesis, individual effect directions for blood lipid parameters reported by ≥3 RCTs (TC, HDL-C, LDL-C, triglycerides, VLDL-C, and TC:HDL-C ratio) can be seen in Figure S3 in the Supporting Information online. All RCTs reported a favorable direction of effect on TC (ie, had a more favorable effect [of any size] in comparison with control). Similarly, all RCTs reporting VLDL-C found a favorable effect direction, but results were more inconsistent for HDL-C, triglycerides, and TC:HDL-C ratio. With respect to LDL-C, 10 out of 12 RCTs showed a positive effect direction. It is worth noting that only 1 RCT reported a negative effect direction for LDL-C (and also reported a negative effect direction for triglycerides), and in this study oily fish was the comparator (300 g of oily fish/week vs 18 walnuts/week).87 A 12-month study that found no effect of walnut consumption on median LDL-C at the end of a multidisciplinary weight loss intervention, compared with the multidisciplinary intervention alone, showed a positive effect direction after 3 months, and there was a lower overall mean for TC in this group (P = 0.037), with the authors noting that this was in the context of a significantly different dietary polyunsaturated:saturated fatty acid ratio.64 The size of effects (between-group differences) ranged from −17.0 to −2.0 mg/dL for TC, −6.0 to +4.5 mg/dL for HDL-C, −15.9 to +1.9 mg/dL for LDL-C, −31.3 to +7.0 mg/dL for triglycerides, −6.0 to −0.7 mg/dL for VLDL-C, and −0.34 to +0.1 for the TC:HDL-C ratio.In addition, 1 study measured lipoprotein subclasses, cholesterol efflux and proprotein convertase subtilisinkexin type 9 (PCSK9).96 LDLreal (LDL-C minus Lipoprotein(a) [Lp(a)] and IDL) was significantly lower after the walnut diet versus control, but there were no significant differences in HDL-C, IDL-C, or VLDL-C subclasses, remnant lipoproteins, ATP-binding cassette transporter 1–mediated or global cholesterol efflux and PCSK9.Overall, results from the data synthesis and sign test calculation suggesting favorable effect directions for blood lipids after a walnut intervention versus control are supported by the harvest plots of effect on individual blood lipid parameters.
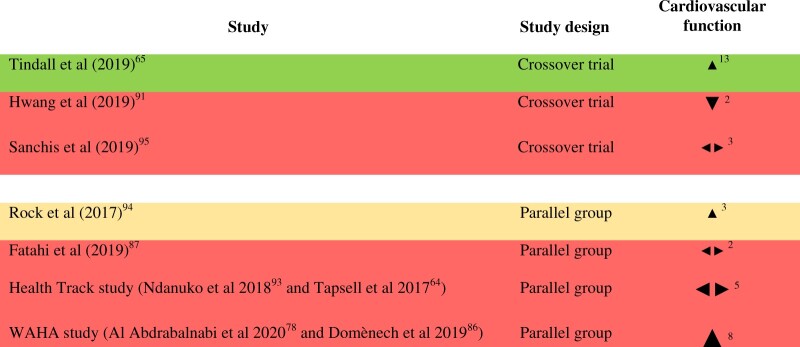
Measures of cardiovascular function: Seven RCTs enrolling 1464 subjects in total measured markers of cardiovascular function.64,65,78,86,87,91,93–95 Of these, 6 studies measured office blood pressure,64,78,87,91,93–95 and the WAHA study additionally measured ambulatory blood pressure.86 The Health Track study measured urinary sodium and potassium and reported urinary sodium-to-potassium ratio.64,93 Two studies measured resting heart rate,65,95 and 1 study measured heart rate after the step test.94 Tindall and colleagues used the SphygmoCor system that allows assessment of the central arterial pressure and pulse wave velocity, as well as an assessment of arterial stiffness.65 The effect direction plot for cardiovascular function can be seen in Figure 3. Three studies reported mixed effects. Results from three of the remaining 4 studies favored walnuts (75%, result of sign test calculation P = 0.625), of which 1 was judged to be at high risk of bias, 1 was judged to have “some concerns” and 1 was judged to be at low risk of bias for this outcome group. Cardiovascular function results stratified by dose and baseline blood pressure can be seen in Figure S4 in the Supporting Information online. There did not appear to be a relationship between effect direction and walnut dose or baseline blood pressure, based on visual inspection of the effect direction plots.One cohort study (n = 3341; walnut consumers n = 340, nonconsumers n = 3001; mean age 45 years) compared heart structure and function measured by echocardiography, to assess risk for heart failure, in walnut consumers versus nonconsumers.102 Diastolic blood pressure was significantly lower among walnut consumers (P = 0.013), but there were no significant differences in systolic blood pressure or pulse pressure. Walnut consumers had a significantly lower heart rate and significantly better values for some diastolic function parameters (A wave, E/A ratio, septal e’ and lateral e’), but there were no significant differences in E wave, deceleration time, left ventricular mass index, or systolic function parameters. In summary, the majority of the evidence for measures of cardiovascular function identified derived from blood pressure data from RCTs, with mixed results.
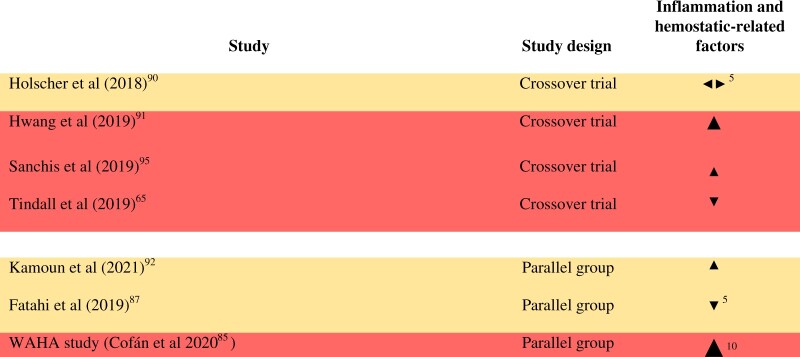
Inflammation- and hemostatic-related factors: Eight RCTs enrolling 1237 subjects in total measured inflammation and hemostatic-related factors.65,81,85,87,90–92,95 The number of RCTs reporting each outcome in this group is as follows: high sensitivity C-reactive protein (hsCRP) (6), interleukin-6 (IL-6) (3), soluble vascular cell adhesion molecule-1 (sVCAM-1) (3), C-reactive protein (CRP) (2),serum amyloid A (2), tumor necrosis factor alpha (TNF-α) (2), soluble intercellular adhesion molecule-1 (sICAM-1) (2), D-dimer (1), fibrinogen (1), endothelin-1 (1), granulocyte–monocyte colony-stimulating factor (1), IL-1β (1), sE-selectin (1), and interferon gamma (IFN-γ) (1). Bamberger and colleagues reported that walnut consumption did not affect hsCRP, soluble vascular cell adhesion molecule-1 (sVCAM-1), or endothelin-1, but the manuscript does not provide data for inclusion in the vote counting.81 The effect direction plot for inflammation- and hemostatic-related factors can be seen in Figure 4. Of the 7 studies that could be included in the vote counting, 1 reported mixed findings. Results from four of the remaining 6 studies favored walnuts (67%, result of sign test calculation P = 0.688), and of these 3 were judged to be at high risk of bias and 1 was judged to have “some concerns” for this outcome group. Results for inflammation-related factors stratified by dose can be seen in Figure S5 in the Supporting Information online. There appeared to be no relationship between dose and effect direction, based on visual inspection of the effect direction plot.
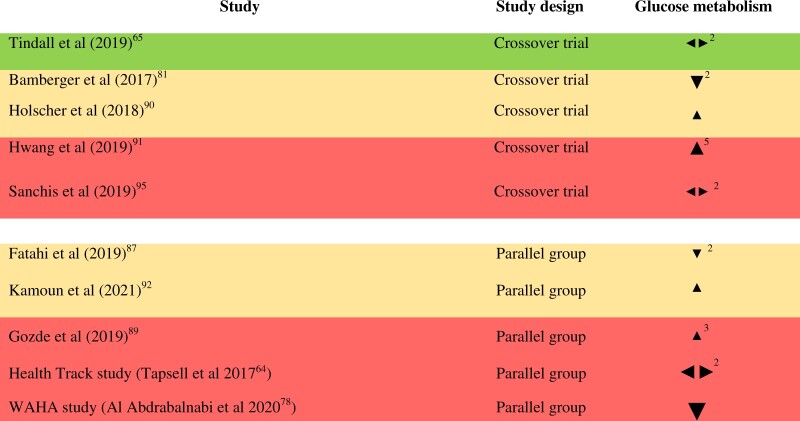
Glucose metabolism: Ten RCTs enrolling 1653 subjects in total measured markers of glucose metabolism.64,65,78,81,87,89–92,95 The number of RCTs reporting each outcome in this group is as follows: fasting glucose (10), HbA1c (4), fasting insulin (3), leptin (2), Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) (1), and adiponectin (1). The effect direction plot for markers of glucose metabolism can be seen in Figure 5. Three of the 10 studies reported mixed effects. Results from four of the remaining 7 studies favored walnuts (57%, result of sign test calculation P = 1.0). Of these, 2 were judged to be at high risk of bias and 2 were judged as raising “some concerns” for this outcome group. Results for glucose metabolism stratified by baseline BMI, study duration, and walnut dose can be seen in Figure S6 in the Supporting Information online. Based on visual inspection of the effect direction plots, there do not appear to be clear patterns linking effect direction with study duration, baseline BMI, or walnut dose. No cohort studies were identified as part of this review that examined the association between walnut consumption and the development of type 2 diabetes.
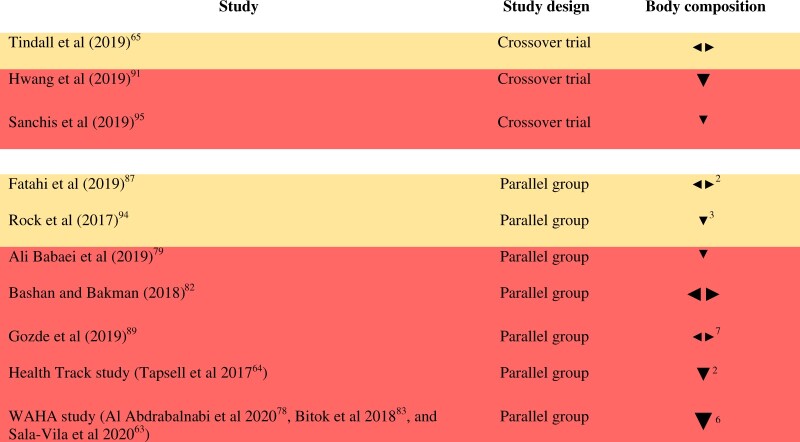
Body weight and composition: Eleven RCTs enrolling 1902 subjects in total measured body weight and/or composition.63–65,78,79,81–83,87,89,91,94,95 Nine RCTs measured body weight, 6 measured waist circumference, 6 measured BMI, 3 measured body fat, 2 articles reported waist-to-hip ratio, 1 article reported hip circumference, 1 measured fat-free mass, and 1 measured lean body mass.Bamberger and colleagues reported that body weight and BMI remained stable during both walnut and control diets, but the article did not provide data for inclusion in the vote counting.81 Similarly, Bashan and Bakman reported no significant changes in BMI or waist circumference, and the article did not provide data for inclusion in the vote counting, though figures for body weight were reported.82 The effect direction plot for body weight and composition can be seen in Figure 6. Of the 10 RCTs that could be included in the vote counting, 2 reported mixed effects (1 reported a negative effect direction for waist circumference and a positive effect direction for body weight; the other reported a positive effect direction for body fat, fat-free mass, and waist circumference, a negative effect direction for weight and BMI, and no effect on hip circumference and waist-to-hip ratio) and 2 reported no effect (on body weight in both cases). Results from all of the remaining 6 RCTs favored control (100%, result of sign test calculation P = 0.03125). Of these, 5 were judged to be at high risk of bias, and 1 was judged to raise “some concerns” for this outcome group. There does not appear to be a clear pattern linking walnut dose with effect direction, based on visual inspection of the effect direction plot (Figure S7 in the Supporting Information online). Study duration ranged from 4 weeks to 2 years. Overall effect direction appears to be more consistent across studies >12 weeks in duration, with one quarter of the shorter studies showing mixed effects and one quarter showing no effect, compared with only one sixth of the longer studies showing mixed effects and one sixth showing no effect (Figure S7 in the Supporting Information online). There is also some suggestion of less consistent effect direction in studies involving subjects with obesity (with 2 out of 5 studies showing a negative effect direction, 2 showing no effect, and 1 showing conflicting findings), and a negative effect direction being more consistently found in studies of normal weight subjects (Figure S7 in the Supporting Information online).To provide greater insight into the results of the synthesis, effect direction for parameters reported by ≥3 RCTs (ie, body weight, waist circumference, and BMI) are displayed in harvest plots (Figure S8 in the Supporting Information online). Across all of the RCTs included in the vote counting, all 4 studies that measured BMI, 5 out of 8 studies measuring body weight, and 4 out of 5 studies measuring waist circumference reported an unfavorable effect direction. However, effect sizes tended to be small, with between-group differences ranging from −0.4 to +1.9 kg for body weight, −0.12 to +1.66 cm for waist circumference, and +0.01 to +0.2 kg/m2 for BMI.The association between intake of walnuts (and other nuts) and longer-term weight changes were evaluated in cohort analysis by Liu and colleagues, who investigated the relationship between changes in nut consumption over 4-year intervals and concurrent weight change over 20–24 years of follow-up, using data from the NHS, the NHS II, and the HPFS (n = 144 885).33 Across all 3 cohorts, the average weight gain was 0.32 kg per year. Increased consumption of 0.5 servings (14 g/day) of walnuts was associated with less weight gain per 4 year interval (−0.37 kg, 95% CI −0.45 to −0.29, multivariate adjusted, P < 0.001), a lower risk of moderate weight gain (≥2 kg, multivariate-adjusted relative risk (RR) = 0.90, 95% CI 0.88–0.92, P < 0.01, and ≥5 kg, RR not reported, P < 0.01), and a lower risk of becoming obese (BMI ≥ 30 kg/m2, multivariate-adjusted RR = 0.85, 95% CI 0.80–0.89, P = 0.0002). As average 4-year changes in walnut consumption were 0.0 (0.2) servings/day in the HPFS, 0.0 (0.1) servings/day in the NHS, and 0.0 (0.2) servings/day in the NHS II (mean [standard deviation (SD)] standardized to the age distribution of the study population), increased consumption of 0.5 servings/day likely only relates to a small proportion of the subjects.Overall, data from 3 large cohorts reported favorable associations between walnut intake and body weight, but this was not reflected in the RCTs, where synthesis of results using vote counting based on direction of effect suggested a more favorable effect direction for control (or no difference between the 2 groups).
AppetiteI: n a 6-month weight loss intervention (n = 100) prescribing either a standard reduced energy diet or a walnut-enriched reduced energy diet, differences in eating behaviors between groups were studied using the 3-factor Eating Inventory, a 51-item questionnaire across 3 scales (dietary restraint, disinhibition, and hunger). External locus of hunger (ie, hunger that is triggered by external cues) was significantly higher in the walnut group vs the control at 6 months (P = 0.04), but there were no significant differences in other eating behaviors.84 Participants were also asked to self-report satiety on a visual analogue scale rating hunger, fullness, and anticipated prospective consumption at 3 earlier time points. There were no significant differences in self-reported hunger ratings before lunch and before dinner at weeks 1, 6, and 12 between the 2 groups, but fullness rating was significantly lower in the walnut group compared with the control at week 12 (P = 0.04).94
Metabolic syndrome: Two RCTs enrolling 827 subjects in total78,91 and 1 cohort study (the Tehran Lipid and Glucose Study, n = 1915105) examined walnut consumption and MetS status. There were no significant differences in the odds of MetS reversion or incidence among participants of the WAHA study after 2 years of intervention (with BMI used as a surrogate marker for waist circumference).78 Hwang and colleagues reported that all subjects completing their study were classified as having MetS at baseline, but 64% had an improvement in 1 or more MetS components, and 51% had reverted to normal status after 16 weeks of walnut consumption.91 Analyses of data from the Tehran Lipid and Glucose study suggested a significant inverse association between walnut consumption and MetS (HR 0.78, 95% CI 0.63–0.96, comparing ≥16.6 g/week vs ≤4.5 g/week [energy-adjusted], fully adjusted model, P for trend = 0.01; 0.93 (0.89–0.98) per 10 g increment).105
Figure 2.
Effect direction plot for blood lipids, presented by study design. Effect direction: Upward arrow  = positive health impact, downward arrow
= positive health impact, downward arrow  = negative health impact, sideways arrow
= negative health impact, sideways arrow  = no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow
= no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow  indicates >300; medium arrow
indicates >300; medium arrow  indicates 50–300; small arrow
indicates 50–300; small arrow  indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
Figure 3.
Effect direction plot for cardiovascular function, presented by study design. Effect direction: upward arrow  = positive health impact, downward arrow ▼ = negative health impact, sideways arrow
= positive health impact, downward arrow ▼ = negative health impact, sideways arrow  = no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow
= no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow  indicates >300; medium arrow
indicates >300; medium arrow  indicates 50–300; small arrow
indicates 50–300; small arrow  indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
Figure 4.
Effect direction plot for inflammation and hemostatic-related factors, presented by study design. Effect direction: upward arrow  = positive health impact, downward arrow
= positive health impact, downward arrow  = negative health impact, sideways arrow
= negative health impact, sideways arrow  = no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow
= no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow  indicates >300; medium arrow
indicates >300; medium arrow  indicates 50–300; small arrow
indicates 50–300; small arrow  indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
Figure 5.
Effect direction plot for glucose metabolism, presented by study design. Effect direction: upward arrow  = positive health impact, downward arrow
= positive health impact, downward arrow  = negative health impact, sideways arrow
= negative health impact, sideways arrow  = no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow
= no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow  indicates >300; medium arrow
indicates >300; medium arrow  indicates 50–300; small arrow
indicates 50–300; small arrow  indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
Figure 6.
Effect direction plot for body weight and composition, presented by study design. Effect direction: upward arrow  = positive health impact, downward arrow ▼ = negative health impact, sideways arrow
= positive health impact, downward arrow ▼ = negative health impact, sideways arrow  = no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow
= no change/mixed effects/conflicting findings. Final sample size (individuals) in intervention group: large arrow  indicates >300; medium arrow
indicates >300; medium arrow  indicates 50–300; small arrow
indicates 50–300; small arrow  indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
indicates <50. Study quality, denoted by row color: green = low risk of bias; amber = some concerns; red = high risk of bias. The numbers in superscript in column 1 denote the reference numbers, and the numbers in superscript in column 3 denote the number of outcomes that contributed to the effect direction (if >1). A color version of this figure appears in the online version of this article.
Markers of aging.
Studies that were identified investigating the effect of walnut intake on biomarkers of aging were limited to 1 RCT enrolling 708 subjects (the WAHA study)63,88 and 3 cohort studies.99,101,104
In the RCT, the effect of walnut consumption (30–60 g/day) on cognitive function among older adults was investigated using a cognitive test battery.63 There were no significant differences between the walnut-consuming and the control groups in adjusted composite scores of global cognition, memory, language, perception, or frontal function (California and Barcelona centers), indicating that walnut consumption did not delay cognitive decline. However, post hoc analyses showed that among subjects at the Barcelona site, there were significant differences in global cognition (P = 0.040) and perception (P = 0.011) over the 2-year intervention period, although no significant differences were observed between groups at the California site.
In addition, in a subset of subjects at the Barcelona site, structural and functional MRI was undertaken to examine brain structure, resting state connectivity, blood flow, and the expression of functional brain networks during cognitive demands. No significant differences were observed between the walnut-consuming and the control groups on structural outcomes, indicating similar rates of brain atrophy. There were also no differences in change in white matter hyperintensity ratings, brain perfusion, or scores on a working memory task. However, there was a significant group × time interaction for reaction time scoring, which increased in the control group but remained unchanged in the walnut group, suggesting attenuation of the age-related decline in working memory efficiency networks. There was also a significant group × time interaction for blood oxygenation level–dependent signal values at the region of interest. The control group exhibited increases in brain activity over time in brain regions outside the original task-related areas, but this was not observed in the walnut-consuming group, suggesting greater brain efficiency in the walnut-consuming group. Telomere length, a suggested biomarker of aging108 was also measured in a subgroup of participants at the Barcelona center.88 There was no significant time × intervention interaction for (adjusted) leukocyte telomere length (P = 0.079), though the increase in “short telomeres” (<3 kb) was significantly lower in the walnut-consuming group versus control (P = 0.048). The authors suggested this may indicate a potential effect of walnut consumption in preventing telomere attrition, but this exploratory finding should be confirmed in trials with adequate statistical power.
Freitas-Simoes and colleagues reported a significant positive association between walnut consumption and odds of healthy aging (defined as surviving beyond 65 years of age with no history of 11 chronic diseases, no self-reported memory impairment, no physical disabilities, and intact mental health) among participants of the NHS (odds ratio [OR] = 1.20, 95% CI 1.00–1.44, comparing ≥2 servings/week vs no walnut consumption, P for trend = 0.0001, fully adjusted model).104 Higher consumption of walnuts was significantly associated with lower odds of physical function impairment (measured using the physical function domain of the SF-36 questionnaire, which assesses physical limitations in performing daily activities) among participants of the HPFS (OR = 0.80, 95% CI 0.68–0.94, comparing ≥2 servings/week vs never or <1 serving/month, P for trend = 0.01, fully adjusted model).99 Finally, secondary analysis of the Health and Retirement Study and Health Care and Nutrition Study reported an association between walnut consumption and cognitive function.101 Global cognitive scores were significantly higher (P < 0.001) among consumers reporting any walnut consumption (low and moderate) versus nonconsumers at 3 time points (2012, 2014, and 2016), but there was no significant association between walnut consumption and change in cognitive scores over time.
This review only identified 1 RCT assessing the impact of walnut consumption on markers of healthy aging. This study did not find significant effects of walnuts on global cognition after 2 years of walnut consumption, although improvements in some subdomains were reported after subgroup analysis. Observational studies found significant, positive associations between walnut consumption and cognitive function, physical function, and “healthy aging,” but nonuniformity of tests for aging-related outcomes mean that definitive conclusions regarding the effect of walnut consumption on aging cannot be reached.
Gut microbiota.
Three RCTs enrolling 268 subjects in total reported outcomes relevant to the gut microbiota.80,90,97 All 3 studies reported no significant differences in measures of α-diversity. Two studies reported that walnut consumption significantly affected β-diversity compared with controls,80,90 whereas 1 study reported no distinct shaping or clustering between groups.97 Two studies reported no significant shifts in the relative abundance of predominant phyla,80,97 and 1 study reported that walnut consumption significantly increased the relative abundance of Firmicutes and decreased the relative abundance of Actinobacteria.90 The latter study found no significant differences in the relative abundance of Bacteroidetes, Proteobacteria, or Verrucomicrobia, nor in arachea, or fungal abundances.90
All studies however, reported significant changes in a number of bacterial genera; Holscher and colleagues reported significant increases in the abundance of Faecalibacterium, Roseburia, Clostridium, and Dialister and significant decreases in Ruminococcus, Oscillospira, Dorea, and Bifidobacterium.90 Tindall and colleagues also reported enrichment in Roseburia versus control, in addition to Defluviitaleaceae UCG_011 and Defluviitaleaceae identified down to the family level. Bamberger and colleagues reported significant increases in the abundance of 2 unknown species of the genus Ruminococcus spp. (Clostridium Cluster IV) and in Bifidobacterium spp., and a significant decrease in the relative abundance of an Anaerostipes and a Blautia species. Holscher and colleagues measured bile acids within fecal samples and reported that there was no significant difference in primary bile acids, but the microbially derived secondary bile acids, deoxycholic and lithocholic acids, were significantly reduced after walnut consumption versus control.
Cancer.
No RCTs measuring cancer markers were identified, while 2 cohort studies reported no association between walnut intake and 2 specific cancer types. Hashemian and colleagues found no significant association between walnut consumption and risk of esophageal squamous-cell carcinoma among participants of the Golestan Cohort Study (n = 48 284; HR 0.71, 95% CI 0.45–1.14, comparing 0.86 g/1000 kcal/day vs nonconsumers, P for trend = 0.16, fully adjusted model).100 Sui and colleagues reported no significant association between walnut consumption and risk of hepatocellular carcinoma among participants of the NHS and HPFS (n = 140 275) (HR 0.71, 95% CI 0.45–1.12, comparing mean 0.62 servings/week vs nonconsumers, P for trend = 0.23, fully adjusted model).103
Mental health.
The Health Track study (n = 377), which applied psychological assessments using questionnaires,64 reported no significant group × time interactions for the Acceptance and Action Questionnaire for Weight-Related Difficulties, Quality of life (SF-12, mental summary), or Depression Anxiety Stress Scale (short-form 21 questions).
Mortality.
One cohort study identified within the present review examined the association between walnut consumption and mortality. De Souza and colleagues reported a significant inverse association between walnut consumption and mortality among participants of the PURE study (HR 0.79, 95% CI 0.67–0.92, comparing ≥30 g/week to <30 g per month, P for trend = 0.0017, fully adjusted model).98
Bone and muscle health, maternal health, and oxidative stress.
No RCTs or cohort studies were identified that measured markers of bone and muscle health, musculoskeletal disorders, markers of maternal health, maternal disorders, or markers of oxidative stress.
Adverse events.
The majority of the included RCTs did not report any adverse events that were likely to be linked to walnut consumption. Two studies noted gastrointestinal symptoms among small numbers of subjects (Appendix S4 in the Supporting Information online).
DISCUSSION
This review summarizes recent evidence investigating the link between walnut consumption and health or risk markers for health outcomes, including data from 33 articles describing results from 8 cohorts and 13 RCTs published from 2017 onwards. There was sufficient, suitable evidence for results to be synthesized for body weight and composition, blood lipids, cardiovascular function, glucose metabolism, and inflammation and hemostatic factors, employing the vote counting based on the direction of effect method. Smaller numbers of studies were identified reporting results related to total mortality, CVD (hard end points), MetS, cancer, aging, appetite, gut microbiota, and mental health, but no formal synthesis could be performed. No recent RCTs or cohort studies were identified investigating the effects of walnuts alone on oxidative stress, bone and muscle health, maternal health, or type 2 diabetes.
Cardiometabolic health
CVD (hard end points)
As with research published in this area prior to 2017, the majority of the studies included in this review assessed outcomes related to cardiometabolic health. In an analysis performed by Guasch-Ferré and colleagues, total nut consumption was inversely associated with total CVD and CHD,66 which was in line with results from a meta-analysis that included data from 18 prospective cohort studies.109 In the PREDIMED study, subjects who were randomized to a Mediterranean diet supplemented with mixed nuts (including 15 g of walnuts, as well as 7.5 g of hazelnuts and 7.5 g of almonds per day) had a 36% lower risk (95% CI 0.47–0.88) of myocardial infarction, stroke, and death from cardiovascular events after a median follow-up of 4.8 years, compared with a control diet (advice to reduce dietary fat).110 For comparison, subjects who consumed a Mediterranean diet supplemented with 4 tablespoons of extra-virgin olive oil per day had a 34% lower risk (95% CI 0.49–0.89) of the composite outcome. Interestingly, a metabolite profile (including lipids, purines, acylcarnitines, and amino acids) associated with walnut consumption was associated with a lower risk of incident CVD (and type 2 diabetes) (calculated per 1-SD increase in correlated multimetabolite score) among PREDIMED subjects.111
When considering walnut consumption specifically, a pooled analysis from 3 large US cohorts included within the present review found that consuming walnuts at least once per week was associated with a lower risk of CVD, CHD, and stroke.66 Further analysis of data from the same cohorts investigated changes in walnut consumption over 4-year periods and cardiovascular end points in the subsequent 4 years.112 This found an increased intake of walnuts by 0.5 servings/day to be associated with lower risk of CVD and stroke. A recent analysis of data from 2 of the cohorts included in the 2 aforementioned analyses (NHS and HPFS) published after our final searches were performed reported a 14% (HR 0.86, 95% CI 0.79–0.94) reduced risk of death from CVD per 0.5 serving/day increase in walnut consumption.113 These findings from large US cohorts and the PREDIMED study, based on a large number of subjects, are promising, suggesting reductions in CVD risk with walnut intake. Analysis of data from additional large cohorts reflecting diverse populations from different parts of the world exploring the association between walnut consumption specifically and CVD end points would be useful.
Blood lipids.
The RCTs identified in our review showed a beneficial effect of walnut consumption on blood lipids (results from 8 out of 8 RCTs that could be included in the sign test calculation favored walnuts, P = 0.0078), with effect direction being most consistent for TC, LDL-C, and VLDL-C. A recent network meta-analysis that only included studies of ≥3 weeks duration (a duration that satisfies the minimum follow-up requirement of the US Food and Drug Administration for lipid-lowering health claims114) reported that walnut consumption significantly reduced triglycerides (mean difference (MD) 7.97 [−9.74 to −6.2] mg/dL, 7% greater reduction), LDL-C (MD −3.48 [−4.64 to −2.71] mg/dL, 3% greater reduction), and TC (MD −5.03[−6.19 to −4.25] mg/dL, 3% greater reduction) compared with control diets,115 but there were no significant effects on HDL-C (MD 0.00 [−0.77, 0.77] mg/dL) (results converted from mmol/L, see Table S5 in the Supporting Information online for conversion factors). A 2018 meta-analysis that included 26 RCTs reported a significantly greater reduction in TC (weighted mean difference (WMD) −6.99 [−9.39 to −4.58] mg/dL, 3.25% greater decrease) and LDL-C (WMD −5.51 [−7.72 to −3.29] mg/dL, 3.73% greater decrease) after walnut consumption versus control diets.37 Significant reductions in the LDL-C:HDL-C ratio (WMD −0.14 mg/dL, P = 0.01), triglycerides (WMD −4.69 [−8.93, −0.45] mg/dL 5.52% greater decrease), and apoB (−3.74 mg/dL, P = 0.008) were also identified. There were no significant differences for changes in HDL-C, TC:HDL-C ratio, VLDL-C, nonHDL-C, or apoA. The authors observed a linear dose–response relationship between walnut intake and TC. There were no differences in effects between studies in subjects with hypercholesterolemia and those involving normocholesterolemic subjects. Our analysis, however, suggested a more consistently positive effect direction in subjects with high/borderline–high LDL-C compared with optimal and near/above optimal LDL-C (based on National Cholesterol Education Program Expert Panel classifications) at baseline, although only 3 studies fall into the high/borderline–high categories.
Our review also suggested a more consistent beneficial effect on blood lipids (across different outcomes) in RCTs of less than 8 weeks’ duration, compared with longer studies. Guidance from EFSA states that with respect to blood lipids, evidence of the sustainability of the effect with continuous consumption of a food/constituent over longer periods of time (eg, 8 weeks) should be provided due to the time needed for blood lipids to stabilize after a nutritional intervention.76 However, improvements were seen in all but 2 RCTs measuring LDL-C, regardless of duration. A meta-analysis of RCTs by Guasch-Ferré and colleagues found smaller effects of walnut consumption on LDL-C (but not TC, triglycerides, or HDL-C) in studies of ≥8 weeks in duration vs shorter studies.37 This could be due to increased adherence over shorter periods or habituation. Interestingly, results from the 2-year WAHA study (both sites), published after our searches were completed, showed a significant reduction in TC and LDL-C after walnut consumption compared with the control group, with no differences in triglycerides and HDL-C.116
Replacing foods high in saturates with unsaturated fats in the diet has been shown to reduce LDL-C.34 The lipid-lowering effects of walnuts may be linked to their ALA content, which has been demonstrated to enrich LDL-particles, facilitating receptor-mediated LDL clearance due to increased affinity of LDL particles to the LDL receptor.117 However, Muñoz and colleagures found that this effect only explained 30% of the LDL decrease in their study,117 pointing to additional mechanisms. A more recent study reported that 6 weeks of walnut consumption did not increase cholesterol efflux, nor change circulating PCSK9, a protein involved in the degradation of LDL receptors.96
Roles for fiber and bioactive compounds such as tocopherols, phenolics (which are concentrated in the pellicle [seed coat/skin]118–120) and phytosterols within walnuts, the latter of which can hinder intestinal cholesterol absorption, have also been suggested.37,50,96 Indeed, observed reductions in LDL-C are reportedly greater than would be predicted based on their fatty acid profile alone65,121; so, due to their high nutrient-density, walnuts may also impact on lipids by improving overall dietary quality, eg, by helping to increase fiber intake, replacing less healthy foods. Significant effects on blood lipids have been reported regardless of whether walnuts were consumed as a snack or during a meal.81 A recent umbrella review concluded that there was moderate evidence that walnut consumption (approximate weighted mean dose 46 g/day or 16% of energy) results in a reduction in LDL-C of <0.20 mmol/L (7.73 mg/dL), which was in line with results for wholegrains (approximate weighted mean dose 90 g/day).122
Measures of cardiovascular function.
In the present review, 3 out of 4 studies that contributed toward the vote counting with respect to markers of cardiovascular function favored walnuts; however, there was no overall consistent direction of effect (sign test calculation result P = 0.625). Predominantly, the effects that contributed toward this outcome group were measures of blood pressure. Recent meta-analyses of RCTs have not found that walnut-enriched diets lead to significant differences in systolic or diastolic blood pressure compared with control diets.38,47,123,124 However, a health claim linking a daily intake of 30 g of walnuts with improvements in endothelium-dependent vasodilation was authorized for use in the European Union in 2012 (and applies in Great Britain post-Brexit).42 Indeed, recent meta-analyses have found significant improvements in endothelial function (predominantly measured using flow mediated dilation).40,41,48,125 Our review identified only 1 RCT with measurement of vascular function (walnut dose 57–99 g/day), which reported no significant diet effects on arterial stiffness (carotid-femoral pulse wave velocity), augmentation index, or pulse transit time (flow-mediated dilation, the focus of the authorized European Union health claim, was not measured).65 One cohort study we identified reported significantly more favorable values for some heart function parameters among walnut consumers compared with nonconsumers, which the authors deemed to be potentially important for early detection of changes in left ventricular diastolic function, though results for both groups were within normal ranges.102
Inflammation- and hemostatic-related factors.
When considering markers of inflammation and hemostatic-related factors, results from 4 out of 6 studies that could be included in the vote counting in the present review favored walnuts; however, there was no overall consistent direction of effect (result of the sign test calculation P = 0.688), and there appeared to be no relationship between dose and effect direction. These results are in line with meta-analyses that reported no significant effects of walnut consumption on CRP37,38,40 or TNF-α, IL-6, inter-cellular adhesion molecule-1 (ICAM-1), or vascular cell adhesium molecule-1 (VCAM-1).40
Glucose metabolism.
In relation to markers of glucose metabolism, results from 4 out of 7 studies that could be included in the sign test calculation in the present review favored walnuts; however, there was no overall consistent direction of effect (P = 1.0). Similarly, 2 recent meta-analyses reported that consumption of walnuts did not result in significant changes in fasting blood glucose, fasting insulin, HbA1c, and HOMA-IR.126,127 A further meta-analysis also found no effects on fasting blood glucose, fasting insulin, HbA1c, and additionally assessed effects on leptin and adiponectin, reporting a significant increase in both outcomes, with significant heterogeneity among studies in both cases.128 Only 1 study included within the present review measured adiponectin91 (reporting a positive effect direction), and 2 measured leptin,87,91 with mixed findings (1 reporting a positive, 1 reporting a negative effect direction). Studies less than 12 weeks in duration may not be expected to detect a change in markers of glucose control, eg, due to the rate of HbA1c turnover,129 In the present review there is no clear pattern linking effect direction with study duration, baseline BMI, or dose, based on visual inspection of the effect direction plots. In another systematic review, meta-regression also found no significant relationship between markers of glucose control and walnut dose or study duration as continuous variables.127
No cohort studies that investigated associations between walnut consumption and type 2 diabetes published since 2017 were identified as part of the present review. A meta-analysis of prospective cohort studies found no association between consumption of total nuts, tree nuts, or peanuts and type 2 diabetes incidence, but a significant inverse association with peanut butter was reported (RR 0.87; 0.77–0.98; 2 studies [female subjects only]).130 The only study identified as part of that review examining walnut consumption was a 2013 analysis of data from the NHS that reported that subjects consuming ≥2 servings/week had a 15% lower risk of developing type 2 diabetes than those who never or almost never consumed walnuts (fully adjusted model including BMI) over 10 years of follow-up.131
Body weight and composition.
A pooled analysis using data from 3 cohorts of US healthcare professionals included in our review suggested that increasing walnut consumption was associated with less weight gain and lower risk of moderate weight gain and becoming obese.33 The same relationships have been noted in other cohort studies for total nut consumption, including the European Prospective Investigation into Cancer and Nutrition (EPIC) study.132 The Seguimiento Universidad de Navarra (SUN) cohort study reported that more frequent nut consumers had a significantly lower risk of weight gain, but there was no association with incident overweight/obesity.133 In substitution analysis by Liu and colleagues, it was estimated that eating 0.5 servings of walnuts instead of 0.5 servings of refined grains, red meat, processed meat, desserts, French fries, and chips (crisps) would be associated with less weight gain.33
Conversely, our review found evidence from RCTs that walnut consumption had a less favorable effect direction on body weight and composition in comparison with control diets (results from 6 out of 6 studies that could be included in the sign test calculation favored control, P = 0.03125, see Figure 6 and Figure S8 in the Supporting Information online). It is important to note that many of the effects were very small in size (see Table S9 in the Supporting Information online). None of the reported (between-group) effects were statistically significant (where statistics were reported) (Table S9 in the Supporting Information online). Furthermore, taking a closer look at the included studies, in 5 of the 8 studies reporting body weight data, both groups lost weight compared with baseline. For example, in a weight loss study employing subjects with a BMI of 32 (29–35) kg/m2 at baseline, the intervention plus walnut group lost 3.5 kg, and the intervention group lost 5.4 kg after 12 months.64 While greater weight loss is likely to be advantageous in such a group, the weight loss in both groups is potentially clinically relevant, because it has been estimated that if all individuals who are overweight or living with obesity in the United Kingdom lost 2.5 kg, this could save the National Health Service £105 million over the next 5 years134; while 5% weight loss is typically considered to be clinically meaningful for individuals with obesity,135 there has been a shift in the focus of messaging towards smaller amounts of weight loss also being worthwhile.136 In 2 of the studies included in this review, the amount of weight loss was identical between the 2 groups, in 2 studies the amount of weight loss was greater in the control group, and in 1 study the amount of weight loss was greater in the walnut-consuming group. In 2 studies, the walnut-consuming group gained weight and the control group lost weight; the walnut-consuming group gained 0.26 kg in a 4-week study in which energy from walnuts was in addition to the usual diet,79 and the walnut-consuming group gained 0.05 kg in the 2-year WAHA study employing older adults.63 In a 6-week study, both groups gained weight (the walnut-consuming group gained 0.34 kg).89 Among the 5 studies that reported data for waist circumference, in 3 studies waist circumference reduced in both groups (albeit to a greater extent in the control group). In the WAHA study, waist circumference increased in the walnut group (by 0.3 cm)83 and did not change in the control group, and in 1 study waist circumference increased in the control group and remained unchanged in the walnut group. The WAHA study found small changes in BMI of 0.04 kg/m2 (0.01–0.07) in the walnut-consuming group and 0.03 kg/m2 (0.0–0.06) in the control group, respectively.63,78 Recent meta-analyses have reported no effect on mean body weight, BMI, waist circumference, fat mass, or percentage body fat when comparing walnut-containing diets with control diets37,55,137 (see Table 2.37,55,138) In a network meta-analysis, subgroup analyses including only RCTs designed to assess whether nut consumption affected weight loss found that walnuts were associated with a reduced percentage body fat138 (see Table 2). In a meta-analysis by Fang and colleagues, benefit was seen on body weight, BMI, and waist circumference, but not fat mass, after walnut consumption among studies employing doses of up to 35 g/day, but doses ≤35 g/day were only employed in a minority of studies included both in this and our review.55 Furthermore, body weight was significantly reduced in studies up to 50 weeks in duration, whereas there were no significant relationships between study duration and BMI, waist circumference, or fat mass.55
Table 2.
Results from meta-analyses of RCTs investigating the effect of walnut consumption, and body weight and composition
| Body weight | BMI | Waist circumference | Fat mass | Percentage body fat | |
|---|---|---|---|---|---|
| Guasch-Ferré et al (2018)37 | WMD: −0.12 (−2.12, 1.88) kg | WMD: −0.11 (−1.15, 0.92) kg/m2 | |||
| Fang et al (2020)55 | WMD: 0.083 (−0.032, 0.198) kg | WMD: −0.40 (−0.244, 0.164) kg/m2 | WMD: −0.193 (−1.03, 0.64) cm | WMD: 0.28 (−0.49, 1.06)% | |
| Fernandez-Rodriguez et al (2021)137 | SMD: 0.03 (−0.05, 0.11) | SMD: 0.04 (−0.06, 0.14) | SMD: <−0.01 (−0.12, 0.11) | SMD: −0.16 (−0.40, 0.09) |
Abbreviations: BMI, body mass index; RCTs, randomized controlled trials; SMD, standardized mean difference; WMD, weighted mean difference.
Walnuts are an energy-dense food, with a 43 g serving (the daily amount indicated by the approved US health claim for walnuts reducing risk of CHD39) providing 296 kcal (1238 kJ).16 In our review, there did not appear to be a relationship between whether or not the walnut-enriched diet and the control diets were isocaloric and effect direction for body weight and composition. However, actual dietary intakes (and level of physical activity) of study participants can differ from those prescribed according to the study design, and it could be that in shorter-term controlled studies increases in walnut consumption increase energy intake temporarily before subjects adjust their intake of other foods to compensate. For example, Bamberger and colleagues found subjects did not maintain an isocaloric diet as recommended, but increased energy intake during walnut consumption, yet body weight and waist circumference did not change (data not reported).81 Walnuts have been reported to have a 21% lower actual metabolizable energy value compared with those found in food composition tables,138 thought to be due to structural features limiting the accessibility of the lipids,19,139 which may distort the interpretation of the estimated energy intake from walnuts in relation to weight loss (though large interindividual variability has been reported in metabolizable energy values from studies of other nut types140,141). The WAHA study provided no advice on energy restriction to subjects consuming walnuts, apart from suggesting that missed doses should be made up for the following day. While energy intake was 228 kcal/day higher in the walnut-consuming group, it was 53 kcal lower than expected, since 19% of the energy provided by other foods in the diet was displaced,142 an effect that has previously been noted in relation to walnut consumption.143
As well as their lower metabolizable energy and the potential displacement of foods high in saturated fat and sugar from the diet, other mechanisms proposed to counteract any negative impact of walnut consumption on body weight include their fiber and protein contents, which may promote satiety,144 and their unsaturated fat content, which may increase thermogenesis.145 One study included in the present review measured self-reported satiety and eating attitudes and behaviors in response to consumption of walnuts in the context of a reduced energy diet.84,94 While there were significant changes from baseline in almost all of the parameters (as would be expected during a weight loss study), most results were similar between the walnut-consuming and control groups. External locus of hunger was significantly higher and fullness was significantly lower in the walnut-consuming group, but the amount of weight loss was similar (8.7 kg in the walnut-consuming group and 8.8 kg in the control group). Acute studies have reported that satiety responses and energy intake at a subsequent meal are similar after walnut-containing meals versus a variety of comparators.145–148 However, interestingly, an RCT in subjects with obesity reported a reduction in the quantity of food participants felt they could eat, reduced hunger, and increased activation of the right insula (an area of the brain providing representations of taste concentration, pleasantness, and satiety) in response to highly desirable food cues after consuming walnut smoothies for 5 days,149 indicating that there may be more to discover in this area.
Metabolic syndrome.
Findings with respect to MetS included in the present review were mixed. The only identified cohort study investigating MetS, carried out in Iran, suggested a significant inverse association between walnut consumption and MetS.105 This is in line with results from the SUN Spanish cohort study that reported a significantly lower risk of developing MetS among those consuming ≥2 servings of nuts/week compared with those consuming nuts never or almost never over 6 years of follow-up.150 While consuming 45 g of walnuts/day for 16 weeks in a crossover RCT among subjects with MetS in South Korea resulted in a reversion rate of 51% and a reversion rate of 29%–53% for individual MetS components compared with baseline,91 the 2-year WAHA intervention study found no significant differences in MetS reversion or incidence.78 This was in line with a 12-week RCT that reported no difference in incidence or reversion of MetS when comparing outcomes for a walnut consumption group with those for a control group.151 The PREDIMED study also reported no difference in incidence of MetS between the Mediterranean diet plus nuts intervention group vs controls after 4.8 years, but MetS reversion was significantly more likely to occur in the intervention group (based on original analysis).152 The authors of the WAHA study proposed that their null findings may be due to the older age of the subjects, since CVD risk factors are more likely to worsen in later years, as well as the fact that they did not ask subjects in the walnut-consumption group to reduce their energy intake in order to compensate.78
Markers of aging
Use of the telephone-based cognitive function interviews among participants of the NHS suggested that higher long-term total nut intake was associated with better average cognitive status for all cognitive outcomes. The difference was approximately equivalent to 2 years of cognitive aging, but long-term intake of nuts was not associated with rates of cognitive decline over time, and there was no significant overall trend of increasingly better cognitive performance specifically with increasing walnut intake.153 However, results suggesting lower odds of physical function impairment99 and higher odds of ‘healthy aging’104 are interesting.
The WAHA study was the only recent RCT that examined the effect of walnut consumption specifically on various markers of aging, collecting detailed information related to cognitive decline after a 2-year intervention in older adults. While there were no significant differences in cognitive scores versus control in the group as a whole (Barcelona and California centers), post hoc analysis identified significant improvements in global cognition and perception (but no differences in memory, language, or frontal function) at the Barcelona site, which was calculated to be equivalent to 1.24 years of cognitive aging.63 Although there was an absence of structural change, functional magnetic resonance imaging (fMRI) examinations in a subset of the subjects at the Barcelona center reported improvement in some functional parameters (reaction time scoring and brain activity in response to a task), but not all. The subjects at the Barcelona center were less well educated and had a lower baseline ALA status, which may explain these results. Prior to 2017, an 8-week intervention study (60 g walnuts/day) in young adults found a significant increase in inferential verbal reasoning, but no significant differences in nonverbal reasoning or memory,154 and data from observational studies have suggested a positive association between walnut consumption and cognitive function among older adults.155,156 There is also some observational evidence that benefits extend to younger age groups too.156
Overall, the cognitive improvements and a decrease in short telomeres after 2 years of walnut consumption found in the WAHA study,88 combined with results from prospective cohort studies,99,104 point to some promising findings in this area. However, more consistency in the outcome measures used would make it easier to draw firm conclusions, particularly in relation to cognitive decline.56,157
Mental health
One study identified within the present review assessed mental health, although this was in the context of a weight loss intervention, which may make it difficult to detect any effects of walnut consumption (scores improved over time in all groups).64 There are a limited number of additional human studies in this area that did not meet the inclusion criteria for the present review, including a cross-sectional analysis of National Health and Nutrition Examination Survey (NHANES) data, which reported that walnut consumers showed lower depression scores compared with non-nut consumers.158,159
Gut microbiota
The impact of walnut consumption, and indeed other of types of nuts, on the gut microbiota is emerging, but to date there have only been a small number of human studies published in this area.61,62 Increased microbial diversity is thought to be associated with more favorable health outcomes, with lower diversity often seen in disease states.160 Of the 3 studies we identified, all reported that there were no significant effects of walnut consumption on alpha-diversity,80,90,97 with 2 reporting significant effects on beta-diversity. A recent meta-analysis found no significant impact of nut consumption on any alpha-diversity metric overall.61 However, sensitivity analysis revealed a significant effect on the Shannon index (an indicator of alpha-diversity) that was exclusive to almonds, though the impact of almond consumption on beta-diversity was inconclusive.61 One short-term study reported that alpha-diversity (indicated by the Chao1 index) significantly decreased after 3 days of walnut consumption (33 g/day).161
Abundance of Roseburia and Clostridium seem to be influenced by nut type. Two of the 3 studies identified as part of the present review reported significant increases in the abundance of Roseburia spp.90,97 These microbes ferment fiber, producing butyrate,162 which is thought to benefit host health163 and can metabolize fatty acids, including linoleic acid.164 Interestingly, results of a recent meta-analysis investigating nut consumption and the gut microbiota found that the overall significant increase in Roseburia was being driven by walnut studies.61 Reduction of secondary bile acids, which are associated with several diseases,165,166 by Roseburia may be beneficial.90 While 1 study reported a significant increase in the abundance of Bifidobacterium spp.,80 a genus thought to be beneficial for health,167,168 another reported a significant decrease.90 The area of gut microbiome research is rapidly expanding, and there is still much to be discovered. If, and how, any shifts in relative abundance of bacterial phyla or species that may occur after dietary changes would impact health remains to be determined.
Cancer
No new RCTs investigating the effect of walnut consumption on cancer markers were identified as part of the present review. A limited number of RCTs published pre-2017 focused on prostate cancer. A small study reported that phenolic metabolites can reach and enter the human prostate gland after consumption of 35 g/day of walnuts for 3 days.169 However, neither 8 weeks’ consumption of 75 g of walnuts/day nor consumption of 35 g of walnuts/day for 6 months significantly reduced prostate-specific antigen concentrations compared with control diets.170,171 A small study (n = 10) in women with breast cancer found that consumption of ∼56 g of walnuts/day for the ∼2 week period between a diagnostic biopsy and subsequent surgery modified gene expression in tumors in ways that might be expected to slow proliferation, reduce inflammation, reduce metastasis, and increase cancer cell death.172 The authors stated that many of the genes that were modified are promotional for all types of cancers, suggesting a potential benefit beyond breast cancer specifically.
The 2 analyses of data from cohort studies in Iran and the United States identified as part of the present review did not find significant associations between walnut consumption and esophageal squamous-cell carcinoma or hepatocellular carcinoma, respectively.100,103 Similarly, a recent analysis of data from the NHS and HPFS reported that walnut consumption was not associated with cancer mortality in multivariate-adjusted analyses.114 However, Hashemian and colleagues did find that the highest tertile of total nut consumption (tree nuts, peanuts, walnuts, and seeds) was associated with lower risk of developing esophageal squamous-cell carcinoma compared with nonconsumers (HR 0.60, 0.39–0.93, P = 0.02),100 and Sui and colleagues observed a significant inverse association between tree nut intake and hepatocellular carcinoma risk (HR 0.64, 95% CI 0.43–0.95).103 A 2021 meta-analysis of observational studies reported that both total (tree nut and peanut) and tree nut consumption were associated with decreased risk of cancer.173 This included 14% (38 studies) and 13% (7 studies) lower risk when comparing the highest versus the lowest level of consumption for total and tree nuts, respectively. For cancer mortality, 13% and 8% lower risk was reported when comparing the highest versus the lowest level of consumption for total and tree nuts, respectively.174 Each 5 g/day increase in total nut consumption was associated with a 3% lower risk of cancer overall (22 studies). There were significant inverse associations between total nut intake and risk of pancreatic, lung, and colon cancer, but there were no significant associations between total nut intake and risk of esophageal, liver, colorectal, rectal, prostate, gastric, ovarian, endometrial, or breast cancers or glioma, nor between tree nut intake and risk of specific types of cancer.174
Mortality
The only cohort study identified as part of the present review examining the relationship between walnut consumption and total mortality was carried out across 9 different middle- and high-income countries (the PURE study) and reported a significant inverse association.98 This result is in line with the PREDIMED study (examined as an observational cohort), which found that participants who consumed >3 servings of walnuts per week at baseline had significant reductions in total mortality risk of 45% compared with those who “rarely” or “never” consumed nuts (fully adjusted model, including adjustment for intervention group).36 In addition, a recent analysis of data from the NHS and HPFS reported a HR for total mortality of 0.86 (95% CI 0.79–0.93, P for trend <0.0001) and a greater life expectancy at age 60 (+1.30 years in women and +1.26 years in men) for those consuming ≥5 servings of walnuts/week versus nonconsumers.114 Findings from the PURE and PREDIMED studies also suggest that a higher intake of total nuts was associated with a lower risk of mortality, which is in line with several meta-analyses.5,174–177
Bone and muscle health, maternal health, and oxidative stress
We did not identify any studies in the areas of bone and muscle health, oxidative stress, or maternal health that met the inclusion criteria for the present review, and existing research investigating the effect of consuming walnuts on these outcomes in humans is scant.178–184
Summary
The results of this review point to a considerable increase in recent evidence in relation to walnut consumption and cardiometabolic health. Taken together with research published prior to 2017, it would appear that the association with reduced CVD risk seen in large cohort studies may be mainly attributable to their lipid-lowering effects, in addition to improvements in endothelial function. Evidence pointing to effects on blood pressure, inflammation, hemostatic markers, and glucose metabolism as yet remains conflicting. Evidence from human studies showing that walnut consumption may benefit cognitive health, which is needed to corroborate findings from animal studies,185,186 is now beginning to accumulate. Animal data points towards the polyphenols and essential fatty acids within walnuts conferring benefits, including neuroprotection (and possibly acting synergistically), but research in humans is inconsistent.51,187 It has been suggested that crossover in pathophysiology between CVD and neurodegenerative disorders (oxidative stress, inflammation, and vascular damage) makes dual benefit plausible.187 Further studies of the effects of walnut consumption on cancer development and the gut microbiota are also warranted.
Limitations of this review
Despite having the advantage of producing an overview, the vote counting based on the direction of effect method that was used to synthesize the results within this review has a number of limitations that must be acknowledged. First, the method answers the question “Is there any evidence of an effect?” rather than “What is the average intervention effect?”, and so it provides no information on the magnitude or statistical significance of effects, and does not take into account differences in the relative sizes of the studies. This means that in some instances an effect direction relates to results showing that the walnut intervention and control had a very similar effect on a particular outcome (ie, the same direction, but with a very small difference in magnitude). Second, the power of the sign test that is employed is limited if the number of included studies is small. The sign test is further limited by the requirement to exclude studies with an overall mixed effect direction, ie, only studies reporting ≥70% of effects in the same direction can be included in the calculation, reducing the number of studies included. Therefore, the effect direction plot has the strength of being able to represent all studies included in a review that report data for a particular outcome group, but the sign test does not.67 Furthermore, while advantageous for making use of a large quantity of data from a diverse range of similar outcomes, grouping outcome measures may obscure important individual outcome results from studies that show mixed effects overall. In addition, differences in the number of outcomes reported within a particular group and therefore contributing to the overall effect direction for each study are also worth considering in the context of meeting the ≥70% threshold for assigning a consistent direction of effect. We have therefore set our findings in the context of other reviews.
A further limitation of this review is that some of the identified studies, despite meeting our inclusion criteria, were designed using dietary interventions to answer a very specific research question, such as which component of walnuts might be responsible for specific health effects (ie, fatty acids, fiber, phenolic compounds)65,96,97 or to examine differences in bioactivity between marine and plant n-3 fatty acids.87 Therefore, between-group comparisons may have produced different findings from studies simply comparing walnuts with simply “no walnuts.” In addition, we included results in the synthesis that were not the primary outcome for the particular study and, in a small number of cases, were also not listed by authors as a secondary outcome, but were reported within the results section of an article.
It is important to note that many of the outcomes from the included RCTs were judged to be at high risk of bias. When considering the evidence that was identified from cohort studies, it was often the case that the main focus of the analysis was total nut consumption, and so fewer results pertaining to walnut consumption were available to inform the present review. This is a consideration for future analyses of data from cohort studies. While datasets from cohort studies are typically adjusted for many dietary and lifestyle factors, residual confounding cannot be completely ruled out. Furthermore, bias due to reverse causation cannot be eliminated. In addition, measurement error in self-reported dietary intake is possible, and social desirability bias may be introduced when considering walnut intake specifically. All of the included cohort studies assessed nut intake using validated food frequency questionnaires (FFQs), with 3 of the included articles citing a correlation coefficient of 0.75 for the specific FFQ employed (for the NHS, NHS II, and HPFS) relative to diet diaries33,66,103 and another reporting a correlation coefficient of 0.54 and 0.39 in males and females, respectively, relative to 24-hour recalls (for the Tehran Lipid and Glucose study)105 for nut consumption. The reported correlation coefficients are described as “reasonable” and “good,” respectively by the authors, due to the fact that differing classification systems are used for their interpretation.187–190 While FFQs have been subject to some debate with regards to their methodological limitations in producing good quality dietary data, they may have an advantage over 3–4 day diet diaries for capturing foods such as nuts, which may be infrequently consumed. However, FFQs may be problematic for use in particular groups (eg, older adults with cognitive decline), who may have impaired ability to complete a questionnaire.191 As a result of drawbacks with self-reporting food consumption, biomarkers of intake may be preferable. While ALA is present in significantly higher amounts in walnuts compared with other nuts and is used as a biomarker to assess compliance in RCTs, rapeseed, and flaxseed oils also contain high amounts of ALA. The use of multimetabolite biomarker models is being explored as an objective method for assessing walnut intake.112,192 Data regarding the form in which the nuts are consumed (eg, salted, roasted, etc) is not always available from FFQs, and results from a particular cohort may not be generalizable to other groups. Finally, it is important to emphasize the fact that the results presented here only relate to evidence from articles published from January 1, 2017 to May 5, 2021.
Concluding remarks
Nuts provide important nutrients, including fiber, unsaturated fats, and micronutrients, as well as plant bioactives such as polyphenols (see Table 315,16 and Table 416–18). Walnuts (in 100 g) are a source of iron, zinc, potassium, niacin, pantothenic acid, and fiber, and they are high in vitamin E, thiamin, vitamin B6, folate, biotin, magnesium, phosphorus, copper, and manganese.16 Currently in the United Kingdom, fiber intake is low (19.7 g on average among adults aged 19–64 years13), with only 9% of adults meeting the recommendation to consume 30 g of fiber per day. While nuts and seeds are sources of fiber, because of their relatively low consumption they only contribute on average around 2% of fiber in the United Kingdom diet.13 There is evidence that both healthcare professionals and consumers193–195 may have negative perceptions of nuts (eg, due to their energy density), or have low awareness of the potential health benefits or nutritional attributes of nuts as part of a healthy balanced diet196–199 (eg, cardioprotective effects and the fact that nuts are a good source of fiber). Consumer studies have also identified cost and dentition issues as barriers to nut consumption.199 Furthermore, strong public health messages relating to obesity and calories may have prevented consideration of the overall nutritional quality of foods, so they have been viewed simply in terms of their calorific value,200 which may also have had a negative impact upon nut consumption. Evidence from observational studies and intervention trials demonstrates that eating nuts is associated with, and results in, better diet quality,143,201–207 eg, lower intakes of sodium and saturates, and higher intakes of dietary fiber and a number of micronutrients. In the RCTs included within this review, compliance in consuming walnuts (measured in most studies by self-report/return of empty walnut packets, and confirmed in some studies by the assessment of the red blood cell or plasma content of ALA,63,91,94) was reported to be good, and walnuts were generally well tolerated (apart from gastrointestinal upset in a small number of cases). Consistently favorable effect directions across blood lipid measures were evident at doses of 40 g/day and above, with a minimum duration of 3 weeks (Figure S2 in the Supporting Information online). However, it is worth considering whether the higher doses used in some studies (eg, 57–99 g/day) could reasonably be expected to fit within the average person’s daily diet. Pooled analysis from 3 large prospective cohort studies found that consuming walnuts at least once per week (1 serving = 28 g) was associated with a lower risk of total CVD and CHD (though no other analyses of cohort study data were identified as part of this review for comparison). Previous meta-analyses of RCTs as well as recent cohort studies included in this review, report that consuming a walnut-enriched diet has either no or favourable effects on bodyweight, and whilst some of the synthesised RCT data in this review suggested an unfavourable direction of effect on weight compared to control, these effects were small. Combined with the evidence for improvements in blood lipids after walnut consumption, as a healthier plant-based snack or component of main meals, walnuts can be incorporated as part of a more plant-based healthy, balanced diet. There are a number of key areas for future research on walnut consumption and health outcomes. With the keen interest in healthy aging, further research should investigate associations between walnut intake and cognitive health, using measurements that would allow for comparison between studies. Future studies are also needed to have a clearer understanding of the impact of walnut consumption on gut microbiota, incorporating clinical and functional outcomes. Studies exploring health outcomes linked to walnut consumption in children (including the form of nuts and the amount) are warranted. As well as information on total nuts, it would be useful if future analyses of data from large cohorts also focused on individual nut types in order to better tease out any unique effects. Finally, it would be prudent to better understand the facilitators for and barriers to nut consumption in different populations to inform potential strategies for encouraging the consumption of nuts in a healthy balanced diet.
Table 3.
Macronutrient composition of different nut types
| Nut type (per 100 g) | Energy (kcal) | Protein (g) | Carbohydrate (g) | Fiber (g) | Total fat (g) | SFAs (g) | MUFAs (g) | Total PUFAs (g) | LA (g) | ALA (g) |
|---|---|---|---|---|---|---|---|---|---|---|
| Almond | 554 | 21.2 | 5.3 | 12.5 | 49.9 | 3.8 | 31.5 | 12.3 | 12.3 | Tr |
| Brazil | 683 | 14.3 | 3.1 | 5.7a | 68.2 | 17.4 | 22.4 | 25.4 | 25.4 | 0 |
| Hazelnut | 650 | 14.1 | 6.0 | 6.9 | 63.5 | 4.6 | 49.2 | 6.6 | 6.5 | 0.1 |
| Mixed nuts | 581 | 23.8 | 11.6 | 8.2a | 49.1 | 7.7 | 27.2 | 11.8 | NR | NR |
| Peanut | 564 | 25.8 | 12.5 | 8.2a | 46.0 | 8.7 | 22.0 | 13.1 | 12.8 | 0.4 |
| Walnut | 688 | 14.7 | 3.3 | 4.7a | 68.5 | 7.5 | 10.7 | 46.8 | 39.3 | 7.5 |
Sources: McCance and Widdowson’s composition of foods integrated dataset (Public Health England 202116) and Fatty Acids: Seventh Supplement to the Fifth Edition of McCance and Widdowson’s The Composition of Foods (Ministry of Agriculture Fisheries and Food London [United Kingdom] 1998)15. aEstimated from NSP fiber values. Abbreviations: ALA, alpha linolenic acid; LA, linoleic acid; MUFAs, monounsaturated fatty acids; NR, not reported; NSP, nonstarch polysaccharide; PUFAs, polyunsaturated fatty acids; SFAs, saturated fatty acids.
Table 4.
Micronutrient composition and total polyphenol content of different nut types
| Nut type (per 100 g) | Iron (mg) | Zinc (mg) | Potassium (mg) | Magnesium (mg) | Phosphorus (mg) | Calcium (mg) | Copper (mg) | Selenium (µg) | Thiamin (mg) | Riboflavin (mg) | Niacin equivalent (mg) | Vitamin B6 (mg) | Vitamin E (mg) | Folate (µg) | Biotin (µg) | Total polyphenols (mg)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Almond | 3.7 | 3.1 | 733 | 270 | 481 | 269 | 1.0 | 4 | 0.21 | 1.14 | 7.0 | 0.14 | 25.6 | 44 | 64.0 | 287 |
| Brazil | 2.5 | 4.2 | 660 | 410 | 590 | 170 | 1.8 | 254 | 0.67 | 0.03 | 3.3 | 0.31 | 7.2 | 21 | 11.0 | 244 |
| Hazelnut | 3.2 | 2.1 | 730 | 160 | 300 | 140 | 1.2 | 2 | 0.43 | 0.16 | 5.1 | 0.59 | 25.0 | 72 | 76.0 | 671 |
| Mixed nuts | 2.9 | 3.5 | 696 | 222 | 452 | 94 | 1.1 | 5 | 0.89 | 0.22 | 15.0 | 0.51 | 12.8 | 93 | 66.1 | NR |
| Peanut | 2.5 | 3.5 | 670 | 210 | 430 | 60 | 1.0 | 3 | 1.14 | 0.10 | 19.3 | 0.59 | 10.1 | 110 | 72.0 | 406 |
| Walnut | 2.9 | 2.7 | 450 | 160 | 380 | 94 | 1.3 | 3 | 0.40 | 0.14 | 4.0 | 0.67 | 3.9 | 66 | 19.0 | 1574 |
Supplementary Material
Acknowledgments
We would like to thank the members of the steering group, Dr Sarah Berry (King’s College London), Dr Wendy Hall (King’s College London) and Dr Duane Mellor (Aston University), for their expert advice. We would like to thank Covidence (Melbourne, Australia) for providing free trial access to their software for use in the conduct of this review. We would like to thank IFIS Publishing for providing access to the FSTA—Food Science and Technology Abstracts database and for developing a human study filter for our use so that searches could be performed for the purpose of this review.
Author contributions. A.S. and S.A.S. conceptualized the systematic review. A.S., S.A.S., and S.L. acquired the funding. S.L., A.S., S.A.S., and A.d.l.H. developed the methodology. S.L. conducted the literature search. S.L. and A.d.l.H. screened the articles. S.L., A.d.l.H. and S.S. extracted the data and performed the quality assessment. A.S. and S.A.S. were consulted in order to resolve conflicting views over study eligibility and quality, and reviewed data extraction tables. S.L. and A.d.l.H. performed the data synthesis. S.L. drafted the manuscript. S.S. and S.L. prepared tables and figures. A.S., S.A.S., A.d.l.H. and S.S. provided critical revision of the manuscript.
Funding. This review was funded by the California Walnut Commission. The funding body did not have input into the final design or conduct of the review or the drafting of the manuscript.
Declaration of interest. The authors are all employed by the British Nutrition Foundation. Funding to support the British Nutrition Foundation’s charitable aims and objectives comes from a range of sources, including membership, donations and project grants from food producers and manufacturers, retailers, and food service companies; contracts with government departments; conferences, publications and training; overseas projects; funding from grant providing bodies, trusts and other charities. Further information about the British Nutrition Foundation’s activities and funding can be found at www.nutrition.org.uk/our-work/. At the time of writing, the California Walnut Commission is not a current corporate member of the British Nutrition Foundation.
Contributor Information
Stacey Lockyer, are employed by the British Nutrition Foundation, London, UK.
Anne E de la Hunty, are employed by the British Nutrition Foundation, London, UK.
Simon Steenson, are employed by the British Nutrition Foundation, London, UK.
Ayela Spiro, are employed by the British Nutrition Foundation, London, UK.
Sara A Stanner, are employed by the British Nutrition Foundation, London, UK.
Supporting Information
The following Supporting Information is available through the online version of this article at the publisher’s website.
Table S1 Electronic search strategies
Appendix S1 Further details of methodology used for quality assessments
Table S2 Outcome groupings for results from RCTs
Appendix S2 Methodology for vote counting based on the direction of effect and the sign test calculation
Table S3 Reasons for excluding reviewed full-text articles, collated using Covidence
Table S4 Characteristics of the RCTs
Table S5 Results from RCTs reporting blood lipids and related factors
Table S6 Results from RCTs reporting markers of cardiovascular function
Table S7 Results from RCTs reporting inflammation and hemostatic-related factors
Table S8 Results from RCTs reporting markers of glucose metabolism
Table S9 Results from RCTs reporting markers of body weight and composition
Table S10 Results from RCTs reporting markers of appetite
Table S11 Results from RCTs reporting metabolic syndrome
Table S12 Results from RCTs reporting markers of aging
Table S13 Results from RCTs reporting measures related to the gut microbiota
Table S14 Results from RCTs reporting markers of mental health
Appendix S3 Results from RCTs reported by sex
Table S15 Characteristics of the cohort studies
Table S16 Results from cohort studies
Figure S1 Risk of bias summary for results from RCTs, presented as a proportion of all outcomes included within the review contributing to each outcome group
Figure S2 Effect direction plot for blood lipids displaying stratifications
Figure S3 Harvest plots showing effect directions for total cholesterol, HDL-C, LDL-C, triglycerides, VLDL-C, and total cholesterol: HDL-C ratio
Figure S4 Effect direction plots for cardiovascular function displaying stratifications
Figure S5 Effect direction plot for inflammation and hemostatic-related factors stratified by walnut dose
Figure S6 Effect direction plots for markers of glucose metabolism displaying stratifications
Figure S7 Effect direction plots for body weight and composition displaying stratifications
Figure S8 Harvest plots showing effect directions for body weight, BMI, and waist circumference
Appendix S4 Adverse events
AVAILABILITY OF DATA, CODE AND OTHER MATERIALS
Data extracted from the included studies is available in Tables S4–S16 and Appendix S3 in the Supporting Information online.
REFERENCES
- 1. GBD 2019 Risk Factors Collaborators. Global Burden of Disease 2019 risk factor summaries—dietary risks—Level 2 risk. Lancet 2020;396:S268. [Google Scholar]
- 2. Institute for Health Metrics and Evaluation (IHME). GBD Compare. Seattle, WA: IHME, University of Washington; 2020. http://vizhub.healthdata.org/gbd-compare. Published 2020. Accessed March 18, 2022. . [Google Scholar]
- 3. Coates AM, Hill AM, Tan SY.. Nuts and cardiovascular disease prevention. Curr Atheroscler Rep. 2018;20:48. [DOI] [PubMed] [Google Scholar]
- 4. Wu L, Wang Z, Zhu J, et al. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73:409–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aune D, Keum N, Giovannucci E, et al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose–response meta-analysis of prospective studies. BMC Med. 2016;14:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Santé Publique France. Recommendations concerning diet, physical activity and sedentary behaviour for adults. https://www.santepubliquefrance.fr/content/download/186842/2320236. Published 2019. Accessed March 18, 2022.
- 7. U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. Published 2020. Accessed March 18, 2022.
- 8. Government of Canada. Canada’s Food Guide. https://food-guide.canada.ca/en/. Published 2019. Accessed March 18, 2022.
- 9. National Food Administration (Sweden). The Swedish Dietary Guidelines. https://www.livsmedelsverket.se/globalassets/publikationsdatabas/andra-sprak/kostraden/kostrad-eng.pdf?AspxAutoDetectCookieSupport=1. Published 2015. Accessed March 18, 2022.
- 10. Herforth A, Arimond M, Álvarez-Sánchez C, et al. A global review of food-based dietary guidelines. Adv Nutr. 2019;10:590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization. Healthy diet. https://www.who.int/news-room/fact-sheets/detail/healthy-diet. Published 2020. Accessed March 18, 2022.
- 12. Cena H, Calder PC.. Defining a healthy diet: evidence for the role of contemporary dietary patterns in health and disease. Nutrients. 2020;12:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Public Health England. NDNS: results from years 9 to 11 (2016 to 2017 and 2018 to 2019). https://www.gov.uk/government/statistics/ndns-results-from-years-9-to-11-2016-to-2017-and-2018-to-2019. Published 2020. Accessed March 18, 2022.
- 14. U.S. Department of Agriculture. What we eat in America, NHANES 2017–2018. https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/wweianhanes-overview/. Published 2021. Accessed March 18, 2022.
- 15. Ministry of Agriculture Fisheries and Food London (United Kingdom). Fatty Acids: Seventh Supplement to the Fifth Edition of McCance and Widdowson’s The Composition of Foods. Vol 7. Cambridge, United Kingdom: Royal Society of Chemistry; 1998.
- 16. Public Health England. McCance and Widdowson’s composition of foods integrated dataset 2021. https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid. Published 2021. Accessed March 18, 2022.
- 17. Rothwell JA, Perez-Jimenez J, Neveu V, et al. Phenol-explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database (Oxford). 2013;2013:bat070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Phenol-Explorer. Database on polyphenol content in foods. http://phenol-explorer.eu/. Published 2015. Accessed March 18, 2022.
- 19. McArthur BM, Mattes RD.. Energy extraction from nuts: walnuts, almonds and pistachios. Br J Nutr. 2020;123:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Coe S. Nuts in the diet and bodyweight: what’s the relationship? Nutr Bull. 2020;45:25–34. [Google Scholar]
- 21. Better Health. Healthier snacks for kids. https://www.nhs.uk/change4life/food-facts/healthier-snacks-for-kids/100-calorie-snacks. Published 2021. Accessed March 18, 2022.
- 22. World Cancer Research Fund. Snack smart while at home. https://www.wcrf-uk.org/uk/blog/articles/2020/05/snack-smart-while-home. Published 2020. Accessed March 18, 2022.
- 23. American Heart Association. Healthy Snacking. https://www.heart.org/en/healthy-living/healthy-eating/add-color/healthy-snacking. Published 2016. Accessed March 18, 2022.
- 24. Diabetes UK, Healthy swaps: snacks. https://www.diabetes.org.uk/guide-to-diabetes/enjoy-food/eating-with-diabetes/healthy-swaps/healthy-swaps-snacks. Published 2021. Accessed March 18, 2022.
- 25. Willett W, Rockström J, Loken B, et al. Food in the anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393:447–492. [DOI] [PubMed] [Google Scholar]
- 26. Committee on Climate Change. Land use: policies for a Net Zero UK. https://www.theccc.org.uk/publication/land-use-policies-for-a-net-zero-uk/. Published 2020. Accessed March 18, 2022.
- 27. FAO and WHO. Sustainable healthy diets—guiding principles. Rome; 2019.
- 28. Steenson S, Buttriss JL.. Healthier and more sustainable diets: what changes are needed in high-income countries? Nutr Bull. 2021;46:279–309. [Google Scholar]
- 29. Codex Committee on Food Labelling. Annex 2—allergen labelling. https://ec.europa.eu/food/system/files/2018-07/codex_ccfl_cl-2018-24_ann-02.pdf. Published 2018. Accessed March 18, 2022.
- 30. Afshin A, Sur PJ, Fay KA, et al. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet. 2019;393:1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Public Health England and the Food Standards Agency. National Diet and Nutrition Survey Years 1 to 9 of the Rolling Programme (2008/2009–2016/2017): time trend and income analyses. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/772434/NDNS_UK_Y1-9_report.pdf. Published 2019. Accessed March 18, 2022.
- 32. Department for Environment, Food and Rural Affairs. Family food datasets—UK household purchases. https://www.gov.uk/government/statistical-data-sets/family-food-datasets. Published 2020. Accessed March 18, 2022.
- 33. Liu X, Li Y, Guasch-Ferré M, et al. Changes in nut consumption influence long-term weight change in US men and women. BMJ Nutr Prev Health. 2019;2:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Scientific Advisory Committee on Nutrition. Saturated fats and health. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/814995/SACN_report_on_saturated_fat_and_health.pdf. Published 2019. Accessed March 18, 2022.
- 35. Hooper L, Martin N, Jimoh OF, et al. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst Rev. 2020;8:CD011737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guasch-Ferré M, Bulló M, Martínez-González MÁ, et al. Frequency of nut consumption and mortality risk in the PREDIMED nutrition intervention trial. BMC Med. 2013;11:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guasch-Ferré M, Li J, Hu FB, et al. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: an updated meta-analysis and systematic review of controlled trials. Am J Clin Nutr. 2018;108:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Del Gobbo LC, Falk MC, Feldman R, et al. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose–response of 61 controlled intervention trials. Am J Clin Nutr. 2015;102:1347–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Food and Drug Administration. Qualified Health Claims: Letter of Enforcement Discretion – Walnuts and Coronary Heart Disease (Docket No 02P-0292). http://wayback.archive-it.org/7993/20171114183725/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072910.htm. Published 2004. Accessed March 18, 2022.
- 40. Neale EP, Tapsell LC, Guan V, et al. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7:e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao Y, Huang W, Peng C, et al. Effect of nut consumption on vascular endothelial function: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37:831–839. [DOI] [PubMed] [Google Scholar]
- 42. EFSA Panel on Dietetic Products Nutrition and Allergies. Scientific opinion on the substantiation of health claims related to walnuts and maintenance of normal blood LDL-cholesterol concentrations (ID 1156, 1158) and improvement of endothelium-dependent vasodilation (ID 1155, 1157) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9:2074. [Google Scholar]
- 43. Department of Health and Social Care. Great Britain nutrition and health claims (NHC) register. https://www.gov.uk/government/publications/great-britain-nutrition-and-health-claims-nhc-register. Published 2021. Accessed March 18, 2022.
- 44. European Commission. EU register of nutrition and health claims made on foods (v.3.6). https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/. Published 2021. Accessed March 18, 2022.
- 45. Banel DK, Hu FB.. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bolling BW, Chen CY, McKay DL, et al. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24:244–275. [DOI] [PubMed] [Google Scholar]
- 47. Guasch-Ferre M, Li J, Hu FB, et al. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: an updated meta-analysis and systematic review of controlled trials. Am J Clin Nutr. 2018;108:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mohammadi-Sartang M, Bellissimo N, Totosy de Zepetnek JO, et al. Effects of walnuts consumption on vascular endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. 2018;28:52–58. [DOI] [PubMed] [Google Scholar]
- 49. Feldman EB. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J Nutr. 2002;132:1062S–1101S. [DOI] [PubMed] [Google Scholar]
- 50. Kris-Etherton PM. Walnuts decrease risk of cardiovascular disease: a summary of efficacy and biologic mechanisms. J Nutr. 2014;144:547S–554S. [DOI] [PubMed] [Google Scholar]
- 51. Ros E, Izquierdo-Pulido M, Sala-Vila A.. Beneficial effects of walnut consumption on human health: role of micronutrients. Curr Opin Clin Nutr Metab Care. 2018;21:498–504. [DOI] [PubMed] [Google Scholar]
- 52. Sanchez-Gonzalez C, Ciudad CJ, Noe V, et al. Health benefits of walnut polyphenols: an exploration beyond their lipid profile. Crit Rev Food Sci Nutr. 2017;57:3373–3383. [DOI] [PubMed] [Google Scholar]
- 53. Jackson CL, Hu FB.. Long-term associations of nut consumption with body weight and obesity. Am J Clin Nutr. 2014;100(suppl 1):408S–411S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pahlavani N, Rostami D, Ebrahimi F, et al. Nuts effects in chronic disease and relationship between walnuts and satiety: review on the available evidence. Obes Med. 2020;17:100173. [Google Scholar]
- 55. Fang Z, Dang M, Zhang W, et al. Effects of walnut intake on anthropometric characteristics: a systematic review and dose–response meta-analysis of randomized controlled trials. Complement Ther Med. 2020;50:102395. [DOI] [PubMed] [Google Scholar]
- 56. Cahoon D, Shertukde SP, Avendano EE, et al. Walnut intake, cognitive outcomes and risk factors: a systematic review and meta-analysis. Ann Med. 2021;53:971–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Poulose SM, Miller MG, Shukitt-Hale B.. Role of walnuts in maintaining brain health with age. J Nutr. 2014;144:561S–566S. [DOI] [PubMed] [Google Scholar]
- 58. Gorji N, Moeini R, Memariani Z.. Almond, hazelnut and walnut, three nuts for neuroprotection in Alzheimer’s disease: a neuropharmacological review of their bioactive constituents. Pharmacol Res. 2018;129:115–127. [DOI] [PubMed] [Google Scholar]
- 59. World Health Organization. World Report on Ageing and Health. https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf;jsessionid=09567FC5D6557D9B24BEA242A9BED076?sequence=1. Published 2015. Accessed March 18, 2022.
- 60. Ni Z-J, Zhang Y-G, Chen S-X, et al. Exploration of walnut components and their association with health effects. Crit Rev Food Sci Nutr. 2021;1–17. Advance Access published February 11, 2021, doi: 10.1080/10408398.2021.1881439 Pub [DOI] [PubMed] [Google Scholar]
- 61. Creedon AC, Hung ES, Berry SE, et al. Nuts and their effect on gut microbiota, gut function and symptoms in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2020;12:2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fitzgerald E, Lambert K, Stanford J, et al. The effect of nut consumption (tree nuts and peanuts) on the gut microbiota of humans: a systematic review. Br J Nutr. 2021;125:508–520. [DOI] [PubMed] [Google Scholar]
- 63. Sala-Vila A, Valls-Pedret C, Rajaram S, et al. Effect of a 2-year diet intervention with walnuts on cognitive decline. The Walnuts And Healthy Aging (WAHA) study: a randomized controlled trial. Am J Clin Nutr. 2020;111:590–600. [DOI] [PubMed] [Google Scholar]
- 64. Tapsell LC, Lonergan M, Batterham MJ, et al. Effect of interdisciplinary care on weight loss: a randomised controlled trial. BMJ Open. 2017;7:e014533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tindall AM, Petersen KS, Skulas-Ray AC, et al. Replacing saturated fat with walnuts or vegetable oils improves central blood pressure and serum lipids in adults at risk for cardiovascular disease: a randomized controlled-feeding trial. J Am Heart Assoc. 2019;8:e011512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guasch-Ferre M, Liu X, Malik VS, et al. Nut consumption and risk of cardiovascular disease. J Am Coll Cardiol. 2017;70:2519–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Boon MH, Thomson H.. The effect direction plot revisited: application of the 2019 Cochrane Handbook guidance on alternative synthesis methods. Res Synth Methods. 2021;12:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372: n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. McKenzie JE, Brennan SE, Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). https://training.cochrane.org/handbook/current/chapter-12. Published 2021. Accessed March 18, 2022.
- 70. Campbell M, McKenzie JE, Sowden A, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ. 2020;368:L6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sterne JA, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:L4898. [DOI] [PubMed] [Google Scholar]
- 72. Wells BS GA, O’Connell D, Peterson J, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Published 2021. Accessed April 22, 2021.
- 73. Scientific Advisory Committee on Nutrition. Carbohydrates and health. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/445503/SACN_Carbohydrates_and_Health.pdf Published 2015. Accessed March 18, 2022.
- 74. Fan Y, Pedersen O.. Gut microbiota in human metabolic health and disease. Nat Rev Microbiol. 2021;19:55–71. [DOI] [PubMed] [Google Scholar]
- 75. Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. EFSA Panel on Dietetic Products Nutrition and Allergies. Guidance for the scientific requirements for health claims related to antioxidants, oxidative damage and cardiovascular health: (Revision 1). EFSA J. 2018;16:e05136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. EFSA Panel on Dietetic Products Nutrition and Allergies. Guidance on the scientific requirements for health claims related to appetite ratings, weight management, and blood glucose concentrations. EFSA J. 2012;10:2604. [Google Scholar]
- 78. Al Abdrabalnabi A, Rajaram S, 1 E, et al. Effects of supplementing the usual diet with a daily dose of walnuts for two years on metabolic syndrome and its components in an elderly cohort. Nutrients. 2020;12:451–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ali Babaei A, Tavakoli Ghouchani H, Khoshghamat N, et al. Effects of walnut consumption on lipid profile of female undergraduate students. J Nutr Fast Health. 2019;7:92–96. [Google Scholar]
- 80. Bamberger C, Rossmeier A, Lechner K, et al. A walnut-enriched diet affects gut microbiome in healthy Caucasian subjects: a randomized, controlled trial. Nutrients. 2018;10:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Bamberger C, Rossmeier A, Lechner K, et al. A walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutrients. 2017;9:1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bashan İ, Bakman M.. The effect of daily walnut consumption on dyslipidemia. J Food Qual. 2018;2018:1–6. [Google Scholar]
- 83. Bitok E, Rajaram S, Jaceldo-Siegl K, et al. Effects of long-term walnut supplementation on body weight in free-living elderly: results of a randomized controlled trial. Nutrients. 2018;10:1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rock CL, Flatt SW, Nichols JF, et al. Changes in disinhibition, restraint and hunger and associated characteristics during a weight loss intervention. J Obes Weight Loss Ther. 2017;7:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cofán M, Rajaram S, Sala-Vila A, et al. Effects of 2-year walnut-supplemented diet on inflammatory biomarkers. J Am Coll Cardiol. 2020;76:2282–2284. [DOI] [PubMed] [Google Scholar]
- 86. Domènech M, Serra-Mir M, Roth I, et al. Effect of a walnut diet on office and 24-hour ambulatory blood pressure in elderly individuals: findings from the WAHA randomized trial. Hypertension. 2019;73:1049–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Fatahi S, Haghighatdoost F, Larijani B, et al. Effect of weight reduction diets containing fish, walnut or fish plus walnut on cardiovascular risk factors in overweight and obese women. Archiv Iran Med. 2019;22:574–583. [PubMed] [Google Scholar]
- 88. Freitas-Simoes T-M, Cofán M, Blasco MA, et al. Walnut consumption for two years and leukocyte telomere attrition in Mediterranean elders: results of a randomized controlled trial. Nutrients. 2018;10:1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Gozde O, Mercanligil SM, Kabaran S, et al. Effects of walnut-enriched diet on blood lipids and glucose profiles in hyperlipidemic subjects: a randomized-controlled trial. Prog Nutr. 2019;21:825–835. [Google Scholar]
- 90. Holscher HD, Guetterman HM, Swanson KS, et al. Walnut consumption alters the gastrointestinal microbiota, microbially derived secondary bile acids, and health markers in healthy adults: a randomized controlled trial. J Nutr. 2018;148:861–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hwang H-J, Liu Y, Kim H-S, et al. Daily walnut intake improves metabolic syndrome status and increases circulating adiponectin levels: randomized controlled crossover trial. Nutr Res Pract. 2019;13:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kamoun A, Hammouda O, Turki M, et al. Moderate walnut consumption improved lipid profile, steroid hormones and inflammation in trained elderly men: a pilot study with a randomized controlled trial. Biol Sport. 2021;38:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Ndanuko RN, Tapsell LC, Charlton KE, et al. Effect of individualised dietary advice for weight loss supplemented with walnuts on blood pressure: the HealthTrack study. Eur J Clin Nutr. 2018;72:894–903. [DOI] [PubMed] [Google Scholar]
- 94. Rock CL, Flatt SW, Barkai H-S, et al. Walnut consumption in a weight reduction intervention: effects on body weight, biological measures, blood pressure and satiety. Nutr J. 2017;16:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sanchis P, Molina M, Berga F, et al. A pilot randomized crossover trial assessing the safety and short-term effects of walnut consumption by patients with chronic kidney disease. Nutrients. 2019;12:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tindall AM, Kris-Etherton PM, Petersen KS.. Replacing saturated fats with unsaturated fats from walnuts or vegetable oils lowers atherogenic lipoprotein classes without increasing lipoprotein (a). J Nutr. 2020;150:818–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tindall AM, McLimans CJ, Petersen KS, et al. Walnuts and vegetable oils containing oleic acid differentially affect the gut microbiota and associations with cardiovascular risk factors: follow-up of a randomized, controlled, feeding trial in adults at risk for cardiovascular disease. J Nutr. 2020;150:806–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. de Souza RJ, Dehghan M, Mente A, PURE study investigators, et al. Association of nut intake with risk factors, cardiovascular disease, and mortality in 16 countries from 5 continents: analysis from the Prospective Urban and Rural Epidemiology (PURE) study. Am J Clin Nutr. 2020;112:208–219. [DOI] [PubMed] [Google Scholar]
- 99. Hagan KA, Grodstein F.. The alternative healthy eating index and physical function impairment in men. J Nutr Health Aging. 2019;23:459–465. [DOI] [PubMed] [Google Scholar]
- 100. Hashemian M, Murphy G, Etemadi A, et al. Nut consumption and the risk of oesophageal squamous cell carcinoma in the Golestan Cohort Study. Br J Cancer. 2018;119:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bishop NJ, Zuniga KE.. Investigating walnut consumption and cognitive trajectories in a representative sample of older US adults. Public Health Nutr. 2021;24:1741–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Steffen LM, Yi SY, Duprez D, et al. Walnut consumption and cardiac phenotypes: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Nutr Metab Cardiovasc Dis. 2021;31:95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sui J, Yang W, Ma Y, et al. A prospective study of nut consumption and risk of primary hepatocellular carcinoma in the US women and men. Cancer Prev Res (Phila). 2019;12:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Freitas-Simoes T-M, Wagner M, Samieri C, et al. Consumption of nuts at midlife and healthy aging in women. J Aging Res. 2020;2020:5651737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hosseinpour-Niazi S, Bakhshi B, Mirmiran P, et al. Socioeconomic and lifestyle factors modifies the association between nut consumption and metabolic syndrome incidence. Clin Nutr. 2021;40:4055–4064. [DOI] [PubMed] [Google Scholar]
- 106. World Health Organization. Body mass index – BMI. https://www.who.int/europe/news-room/fact-sheets/item/a-healthy-lifestyle. Published 2021. Accessed March 18, 2022.
- 107. National Cholesterol Education Program Expert Panel. Third Report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults https://www.ahajournals.org/doi/pdf/10.1161/circ.106.25.3143. Published 2002. Accessed March 18, 2022.
- 108. López-Otín C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153:1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Luo C, Zhang Y, Ding Y, et al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100:256–269. [DOI] [PubMed] [Google Scholar]
- 110. Estruch R, Ros E, Salas-Salvadó J, et al. ; PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378:E34. [DOI] [PubMed] [Google Scholar]
- 111. Guasch-Ferré M, Hernández-Alonso P, Drouin-Chartier J-P, et al. Walnut consumption, plasma metabolomics, and risk of type 2 diabetes and cardiovascular disease. J Nutr. 2021;151:303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Liu X, Guasch‐Ferré M, Drouin‐Chartier JP, et al. Changes in nut consumption and subsequent cardiovascular disease risk among US men and women: 3 large prospective cohort studies. J Am Heart Assoc. 2020;9:e013877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu X, Guasch-Ferré M, Tobias DK, et al. Association of walnut consumption with total and cause-specific mortality and life expectancy in U.S. adults. Nutrients. 2021;13:2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Food and Drug Administration. Guidance for industry: evidence-based review system for the scientific evaluation of health claims. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-evidence-based-review-system-scientific-evaluation-health-claims. Published 2009. Accessed March 20, 2022.
- 115. Liu K, Hui S, Wang B, et al. Comparative effects of different types of tree nut consumption on blood lipids: a network meta-analysis of clinical trials. Am J Clin Nutr. 2020;111:219–227. [DOI] [PubMed] [Google Scholar]
- 116. Rajaram S, Cofán M, Sala-Vila A, et al. Effects of walnut consumption for 2 years on lipoprotein subclasses among healthy elders: findings from the WAHA Randomized Controlled Trial. Circulation. 2021;144:1083–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Muñoz S, Merlos M, Zambón D, et al. Walnut-enriched diet increases the association of LDL from hypercholesterolemic men with human HepG2 cells. J Lipid Res. 2001;42:2069–2076. [PubMed] [Google Scholar]
- 118. Arcan I, Yemenicioğlu A.. Antioxidant activity and phenolic content of fresh and dry nuts with or without the seed coat. J Food Compos Anal. 2009;22:184–188. [Google Scholar]
- 119. Jahanban-Esfahlan A, Ostadrahimi A, Tabibiazar M, et al. A comparative review on the extraction, antioxidant content and antioxidant potential of different parts of walnut (Juglans regia L.) fruit and tree. Molecules. 2019;24:2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhang Y-G, Kan H, Chen S-X, et al. Comparison of phenolic compounds extracted from Diaphragma juglandis fructus, walnut pellicle, and flowers of Juglans regia using methanol, ultrasonic wave, and enzyme assisted-extraction. Food Chem. 2020;321:126672. [DOI] [PubMed] [Google Scholar]
- 121. Sabate J, Fraser GE, Burke K, et al. Effects of walnuts on serum lipid levels and blood pressure in normal men. N Engl J Med. 1993;328:603–607. [DOI] [PubMed] [Google Scholar]
- 122. Schoeneck M, Iggman D.. The effects of foods on LDL cholesterol levels: a systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr Metab Cardiovasc Dis. 2021;31:1325–1338. [DOI] [PubMed] [Google Scholar]
- 123. Li J, Jiang B, Santos HO, et al. Effects of walnut intake on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2020;34:2921–2931. [DOI] [PubMed] [Google Scholar]
- 124. Mohammadifard N, Salehi-Abargouei A, Salas-Salvado J, et al. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am J Clin Nutr. 2015;101:966–982. [DOI] [PubMed] [Google Scholar]
- 125. Huang Y, Zheng S, Wang T, et al. Effect of oral nut supplementation on endothelium-dependent vasodilation—a meta-analysis. Vasa. 2018;47:203–208. [DOI] [PubMed] [Google Scholar]
- 126. Neale EP, Guan V, Tapsell LC, et al. Effect of walnut consumption on markers of blood glucose control: a systematic review and meta-analysis. Br J Nutr. 2020;124:641–653. [DOI] [PubMed] [Google Scholar]
- 127. Tindall AM, Johnston EA, Kris-Etherton PM, et al. The effect of nuts on markers of glycemic control: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109:297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang L, Guo Z, Qi S, et al. Walnut intake may increase circulating adiponectin and leptin levels but does not improve glycemic biomarkers: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. 2020;52:102505. [DOI] [PubMed] [Google Scholar]
- 129. Venos E, de Koning L.. Chapter 6—Endocrine markers of diabetes and cardiovascular disease risk. In: Sadrzadeh H, Kline G, eds. Endocrine Biomarkers. Amsterdam, the Netherlands; Oxford, United Kingdom; Cambridge: Elsevier; 2017:251–299. [Google Scholar]
- 130. Becerra-Tomás N, Paz-Graniel I, Hernández-Alonso P, et al. Nut consumption and type 2 diabetes risk: a systematic review and meta-analysis of observational studies. Am J Clin Nutr. 2021;113:960–971. [DOI] [PubMed] [Google Scholar]
- 131. Pan A, Sun Q, Manson JE, et al. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr. 2013;143:512–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Freisling H, Noh H, Slimani N, et al. Nut intake and 5-year changes in body weight and obesity risk in adults: results from the EPIC-PANACEA study. Eur J Nutr. 2018;57:2399–2408. [DOI] [PubMed] [Google Scholar]
- 133. Bes-Rastrollo M, Sabaté J, Gómez-Gracia E, et al. Nut consumption and weight gain in a Mediterranean cohort: the SUN study. Obesity (Silver Spring). 2007;15:107–107. [DOI] [PubMed] [Google Scholar]
- 134. Department of Health and Social Care. Policy paper. Tackling obesity: Empowering adults and children to live healthier lives. https://www.gov.uk/government/publications/tackling-obesity-government-strategy/tackling-obesity-empowering-adults-and-children-to-live-healthier-lives#fn:18. Published 2020. Accessed March 18, 2022.
- 135. Jensen MD, Ryan DH, Apovian CM, Obesity Society, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. National Health Service. Obesity. https://www.nhs.uk/conditions/obesity/. Published 2022. Accessed March 18, 2022.
- 137. Fernández-Rodríguez R, Mesas AE, Garrido-Miguel M, et al. The relationship of tree nuts and peanuts with adiposity parameters: a systematic review and network meta-analysis. Nutrients. 2021;13:2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Baer D, Gebauer S, Novotny J.. Atwater factors overestimate the calorie content of walnuts (371.1). FASEB J. 2014;28:2251. [Google Scholar]
- 139. Baer DJ, Gebauer SK, Novotny JA.. Walnuts consumed by healthy adults provide less available energy than predicted by the Atwater factors. J Nutr. 2016;146:9–13. [DOI] [PubMed] [Google Scholar]
- 140. Baer DJ, Gebauer SK, Novotny JA.. Measured energy value of pistachios in the human diet. Br J Nutr. 2012;107:120–125. [DOI] [PubMed] [Google Scholar]
- 141. Novotny JA, Gebauer SK, Baer DJ.. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am J Clin Nutr. 2012;96:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. 1 E, Jaceldo-Siegl K, Rajaram S, et al. Favourable nutrient intake and displacement with long-term walnut supplementation among elderly: results of a randomised trial. Br J Nutr. 2017;118:201–209. [DOI] [PubMed] [Google Scholar]
- 143. Kranz S, Hill A, Fleming J, et al. Nutrient displacement associated with walnut supplementation in men. J Hum Nutr Diet. 2014;27:247–254. [DOI] [PubMed] [Google Scholar]
- 144. Brennan AM, Sweeney LL, Liu X, Mantzoros CS.. Walnut consumption increases satiation but has no effect on insulin resistance or the metabolic profile over a 4-day period. Obesity (Silver Spring). 2010;18:1176–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Casas-Agustench P, López-Uriarte P, Bulló M, et al. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28:39–45. [DOI] [PubMed] [Google Scholar]
- 146. Burton-Freeman B. Sex and cognitive dietary restraint influence cholecystokinin release and satiety in response to preloads varying in fatty acid composition and content. J Nutr. 2005;135:1407–1414. [DOI] [PubMed] [Google Scholar]
- 147. Probst A, Humpeler S, Heinzl H, et al. Short-term effect of macronutrient composition and glycemic index of a yoghurt breakfast on satiety and mood in healthy young men. Forsch Komplementmed. 2012;19:247–251. [DOI] [PubMed] [Google Scholar]
- 148. Rock CL, Flatt SW, Barkai H-S, et al. A walnut-containing meal had similar effects on early satiety, CCK, and PYY, but attenuated the postprandial GLP-1 and insulin response compared to a nut-free control meal. Appetite. 2017;117:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Farr OM, Tuccinardi D, Upadhyay J, et al. Walnut consumption increases activation of the insula to highly desirable food cues: a randomized, double-blind, placebo-controlled, cross-over fMRI study. Diabetes Obes Metab. 2018;20:173–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Fernández-Montero A, Bes-Rastrollo M, Beunza JJ, et al. Nut consumption and incidence of metabolic syndrome after 6-year follow-up: the SUN (Seguimiento Universidad de Navarra, University of Navarra Follow-up) cohort. Public Health Nutr. 2013;16:2064–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wu H, Pan A, Yu Z, et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr. 2010;140:1937–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Babio N, Toledo E, Estruch R, et al. Mediterranean diets and metabolic syndrome status in the PREDIMED randomized trial. CMAJ. 2014;186:E649–E657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. O’Brien J, Okereke O, Devore E, et al. Long-term intake of nuts in relation to cognitive function in older women. J Nutr Health Aging. 2014;18:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Pribis P, Bailey RN, Russell AA, et al. Effects of walnut consumption on cognitive performance in young adults. Br J Nutr. 2012;107:1393–1401. [DOI] [PubMed] [Google Scholar]
- 155. Arab L, Ang A.. A cross sectional study of the association between walnut consumption and cognitive function among adult US populations represented in NHANES. J Nutr Health Aging. 2015;19:284–290. [DOI] [PubMed] [Google Scholar]
- 156. Valls-Pedret C, Lamuela-Raventós RM, Medina-Remón A, et al. Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk. J Alzheimers Dis. 2012;29:773–782. [DOI] [PubMed] [Google Scholar]
- 157. Theodore LE, Kellow NJ, McNeil EA, et al. Nut consumption for cognitive performance: a systematic review. Adv Nutr. 2021;12:777–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Arab L, Guo R, Elashoff D.. Lower depression scores among walnut consumers in NHANES. Nutrients. 2019;11:275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Pribis P. Effects of walnut consumption on mood in young adults—a randomized controlled trial. Nutrients. 2016;8:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Heiman ML, Greenway FL.. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol Metab. 2016;5:317–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. García-Mantrana I, Calatayud M, Romo-Vaquero M, et al. Urolithin metabotypes can determine the modulation of gut microbiota in healthy individuals by tracking walnuts consumption over three days. Nutrients. 2019;11:2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Louis P, Flint HJ.. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett. 2009;294:1–8. [DOI] [PubMed] [Google Scholar]
- 163. Canani RB, Di Costanzo M, Leone L, et al. Potential beneficial effects of butyrate in intestinal and extraintestinal diseases. World J Gastroenterol. 2011;17:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Devillard E, McIntosh FM, Duncan SH, et al. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J Bacteriol. 2007;189:2566–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Staley C, Weingarden AR, Khoruts A, et al. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl Microbiol Biotechnol. 2017;101:47–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Louis P, Hold GL, Flint HJ.. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12:661–672. [DOI] [PubMed] [Google Scholar]
- 167. Tojo R, Suárez A, Clemente MG, et al. Intestinal microbiota in health and disease: role of bifidobacteria in gut homeostasis. World J Gastroenterol. 2014;20:15163–15176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. O’Callaghan A, Van Sinderen D.. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. 2016;7:925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. González-Sarrías A, Giménez-Bastida JA, García-Conesa MT, et al. Occurrence of urolithins, gut microbiota ellagic acid metabolites and proliferation markers expression response in the human prostate gland upon consumption of walnuts and pomegranate juice. Mol Nutr Food Res. 2010;54:311–322. [DOI] [PubMed] [Google Scholar]
- 170. Simon JA, Tanzman JS, Sabaté J.. Lack of effect of walnuts on serum levels of prostate specific antigen: a brief report. J Am Coll Nutr. 2007;26:317–320. [DOI] [PubMed] [Google Scholar]
- 171. Spaccarotella KJ, Kris-Etherton PM, Stone WL, et al. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr J. 2008;7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Hardman WE, Primerano DA, Legenza MT, et al. Dietary walnut altered gene expressions related to tumor growth, survival, and metastasis in breast cancer patients: a pilot clinical trial. Nutr Res. 2019;66:82–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Naghshi S, Sadeghian M, Nasiri M, et al. Association of total nut, tree nut, peanut, and peanut butter consumption with cancer incidence and mortality: a comprehensive systematic review and dose–response meta-analysis of observational studies. Adv Nutr. 2021;12:793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mayhew AJ, de Souza RJ, Meyre D, et al. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr. 2016;115:212–225. [DOI] [PubMed] [Google Scholar]
- 175. Grosso G, Yang J, Marventano S, et al. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr. 2015;101:783–793. [DOI] [PubMed] [Google Scholar]
- 176. Schwingshackl L, Schwedhelm C, Hoffmann G, et al. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105:1462–1473. [DOI] [PubMed] [Google Scholar]
- 177. Kwok CS, Gulati M, Michos ED, et al. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prev Cardiol. 2019;26:1415–1429. [DOI] [PubMed] [Google Scholar]
- 178. Griel AE, Kris-Etherton PM, Hilpert KF, et al. An increase in dietary n-3 fatty acids decreases a marker of bone resorption in humans. Nutr J. 2007;6:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Al Wattar BH, Dodds J, Placzek A, for the ESTEEM study group, et al. Mediterranean-style diet in pregnant women with metabolic risk factors (ESTEEM): a pragmatic multicentre randomised trial. PLOS Med. 2019;16:e1002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rashid AI, Mohammed IK, Kadhem RA, et al. The metabolic effect of walnut in polycystic ovarian syndrome. Int J Drug Deliv Technol. 2019;9:593–596. [Google Scholar]
- 181. Berryman CE, Grieger JA, West SG, et al. Acute consumption of walnuts and walnut components differentially affect postprandial lipemia, endothelial function, oxidative stress, and cholesterol efflux in humans with mild hypercholesterolemia. J Nutr. 2013;143:788–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Haddad EH, Gaban-Chong N, Oda K, et al. Effect of a walnut meal on postprandial oxidative stress and antioxidants in healthy individuals. Nutr J. 2014;13:4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. McKay DL, Chen CO, Yeum K-J, et al. Chronic and acute effects of walnuts on antioxidant capacity and nutritional status in humans: a randomized, cross-over pilot study. Nutr J. 2010;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Zambón D, Sabaté J, Munoz S, et al. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: a randomized crossover trial. Ann Intern Med. 2000;132:538–546. [DOI] [PubMed] [Google Scholar]
- 185. Chauhan A, Chauhan V.. Beneficial effects of walnuts on cognition and brain health. Nutrients. 2020;12:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Rajaram S, Jones J, Lee GJ.. Plant-based dietary patterns, plant foods, and age-related cognitive decline. Adv Nutr. 2019;10:S422–S436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Siddiqui S, Zainal H, Harun SN, et al. Dietary assessment of pre-diabetic patients by using food frequency questionnaire. A systematic review of study quality, study outcome, study questionnaire and their relative validity and reliability. Clin Nutr ESPEN. 2019;29:213–223. [DOI] [PubMed] [Google Scholar]
- 188. Sierra-Ruelas É, Bernal-Orozco MF, Macedo-Ojeda G, et al. Validation of semiquantitative FFQ administered to adults: a systematic review. Public Health Nutr. 2021;24:3399–3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Saravia L, Miguel-Berges ML, Iglesia I, et al. Relative validity of FFQ to assess food items, energy, macronutrient and micronutrient intake in children and adolescents: a systematic review with meta-analysis. Br J Nutr. 2021;125:792–818. [DOI] [PubMed] [Google Scholar]
- 190. Yue Y, Petimar J, Willett WC, et al. Dietary flavonoids and flavonoid-rich foods: validity and reproducibility of FFQ-derived intake estimates. Public Health Nutr. 2020;23:3295–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Cade J, Thompson R, Burley V, et al. Development, validation and utilisation of food-frequency questionnaires—a review. Public Health Nutr. 2002;5:567–587. [DOI] [PubMed] [Google Scholar]
- 192. Garcia-Aloy M, Hulshof PJM, Estruel-Amades S, et al. Biomarkers of food intake for nuts and vegetable oils: an extensive literature search. Genes Nutr. 2019;14:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Pawlak R, London H, Colby S, et al. Perception of nut intake among individuals with or at risk for heart disease and/or diabetes. J Behav Health. 2012;1:185–188. [Google Scholar]
- 194. Brown RC, Gray AR, Yong LC, et al. A comparison of perceptions of nuts between the general public, dietitians, general practitioners, and nurses. PeerJ. 2018;6:e5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Brown RC, Yong LC, Gray AR, et al. Perceptions and knowledge of nuts amongst health professionals in New Zealand. Nutrients. 2017;9:220.28257045 [Google Scholar]
- 196. Yong LC, Gray AR, Chisholm A, et al. Barriers to and facilitators and perceptions of nut consumption among the general population in New Zealand. Public Health Nutr. 2017;20:3166–3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lockyer S, Spiro A, Stanner S.. Dietary fibre and the prevention of chronic disease – should health professionals be doing more to raise awareness? Nutr Bull. 2016;41:214–231. [Google Scholar]
- 198. The Institute of Grocery Distribution. Consumer Research on the awareness and understanding of Fibre within the UK. https://www.igd.com/Portals/0/Downloads/Charitable%20Impact/IGD-Consumer-research-into-fibre.pdf. Published 2018. Accessed March 18, 2022.
- 199. Neale EP, Tran G, Brown RC.. Barriers and facilitators to nut consumption: a narrative review. IJERPH. 2020;17:9127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Lockyer S, Cade J, Darmon N, et al. Proceedings of a roundtable event ‘Is communicating the concept of nutrient density important?’ Nutr Bull. 2020;45:74–97. [Google Scholar]
- 201. Brown RC, Tey SL, Gray AR, et al. Nut consumption is associated with better nutrient intakes: results from the 2008/09 New Zealand Adult Nutrition Survey. Br J Nutr. 2016;115:105–112. [DOI] [PubMed] [Google Scholar]
- 202. O’Neil CE, Keast DR, Fulgoni VL, et al. Tree nut consumption improves nutrient intake and diet quality in US adults: an analysis of National Health and Nutrition Examination Survey (NHANES) 1999–2004. Asia Pac J Clin Nutr. 2010;19:142–150. [PubMed] [Google Scholar]
- 203. O’Neil CE, Keast DR, Nicklas TA, et al. Out-of-hand nut consumption is associated with improved nutrient intake and health risk markers in US children and adults: National Health and Nutrition Examination Survey 1999–2004. Nutr Res. 2012;32:185–194. [DOI] [PubMed] [Google Scholar]
- 204. Sterling SR, Bertrand B, Judd S, et al. Longitudinal analysis of nut-inclusive diets and body mass index among overweight and obese African American women living in rural Alabama and Mississippi, 2011–2013. Prev Chronic Dis. 2017;14:E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Tey SL, Brown R, Gray A, et al. Nuts improve diet quality compared to other energy-dense snacks while maintaining body weight. J Nutr Metab. 2011;2011:357350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Wibisono C, Probst Y, Neale E, et al. Changes in diet quality during a 12 month weight loss randomised controlled trial. BMC Nutr. 2017;3:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Dikariyanto V, Berry SE, Pot GK, et al. Tree nut snack consumption is associated with better diet quality and CVD risk in the UK adult population: National Diet and Nutrition Survey (NDNS) 2008–2014. Public Health Nutr. 2020;23:3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.