Abstract
A number of pathogens of the upper respiratory tract express an unusual prokaryotic structure, phosphorylcholine (ChoP), on their cell surface. We tested the hypothesis that ChoP, also found on host membrane lipids in the form of phosphatidylcholine, acts so as to decrease killing by antimicrobial peptides that target differences between bacterial and host membranes. In Haemophilus influenzae, ChoP is a phase-variable structure on the oligosaccharide portion of the lipopolysaccharide (LPS). There was a bactericidal effect of the peptide LL-37/hCAP18 on a nontypeable H. influenzae strain, with an increasing selection for the ChoP+ phase as the concentration of the peptide was raised from 0 to 10 μg/ml. Moreover, constitutive ChoP-expressing mutants of unrelated strains showed up to 1,000-fold-greater survival compared to mutants without ChoP. The effect of ChoP on resistance to killing by LL-37/hCAP18 was dependent on the salt concentration and was observed only when bacteria were grown in the presence of environmental choline, a requirement for the expression of ChoP on the LPS. Further studies established that there is transcription of the LL-37/hCAP18 gene on the epithelial surface of the human nasopharynx in situ and inducible transcription in epithelial cells derived from the upper airway. The presence of highly variable amounts of LL-37/hCAP18 in normal nasal secretions (<1.2 to >80 μg/ml) was demonstrated with an antibody against this peptide. It was concluded that ChoP alters the bacterial cell surface so as mimic host membrane lipids and decrease killing by LL-37/hCAP18, an antimicrobial peptide that may be expressed on the mucosal surface of the nasopharynx in bactericidal concentrations.
Innate immunity is a key component of host defense in the respiratory tract. It is now recognized that one aspect of innate immunity in this host environment is the broad-spectrum bactericidal activity of antibiotic peptides (3, 4, 9, 12, 29). Several antimicrobial peptides, including human β-defensins 1 and 2 and a structurally distinct peptide of the cathelicidin family, hCAP18, have been isolated from the normally sterile lower respiratory tract, where there is synthesis by the epithelial cells lining the larger airways (3, 4, 12, 29). The human β-defensins have also been detected in the airway surface fluid from the nasopharynx (7). The effect of antimicrobial peptides on the normal microbial flora colonizing the upper respiratory tract, however, has not been examined. Since this mucosal surface is heavily colonized, these peptides either are inactive in this environment or are not present in sufficient quantities in the uninflamed state. An alternative possibility is that the bacteria that inhabit the mucosal surface of the nasopharynx are resistant to the peptides expressed at this site.
These small cationic peptides are thought to preferentially target the negatively charged surface membrane of bacteria (5). Host cell membranes are less sensitive to these peptides at least in part because of their more positive surface charge resulting from constituents like the quaternary amine on choline, one of the major constituents of eukaryotic membrane lipids in the form of phosphatidylcholine (11). The focus of this study is the linear, inducible peptide LL-37, which is the biologically active C-terminal 37 amino acids of hCAP18 (1, 10). The biological activity of this peptide correlates with its pH- and anion-dependent α-helical content (16). Interaction with lipopolysaccharide (LPS) confers a conformational change from a random coil to an amphipathic α helix which is thought to allow the initial step in cytotoxicity, insertion and disruption of the cell envelope (16, 33).
This as well as other laboratories have shown that members of the oral streptococci, pneumococci, hemophili, mycoplasmas, and neisseriae express surface structures containing choline, previously thought to be an unusual structural feature of prokaryotes (8, 19, 22, 39; L. Serino and M. Virji, presented at the 11th International Pathogenic Neisseria Conference, 1998). The association of choline in the form of phosphorylcholine (ChoP) with species that reside primarily within the respiratory system and also represent the major pathogens originating from this site suggested that this structure may contribute to survival and pathogenicity in this particular host environment.
Haemophilus influenzae was used in our studies because of the availability of genetically defined mutants with and without ChoP (20). In H. influenzae, choline is obtained from the growth medium, phosphorylated, and attached as ChoP to a terminal hexose residue on the LPS (39). The rough-type oligosaccharide of H. influenzae shows inter- and intrastrain variation in structure (33). One source of this variability is ChoP, which may be linked to different hexoses in different strains (25, 26). In addition to heterogeneity in the position of ChoP, there is on-off phase variation in expression of ChoP on the LPS due to a translational switch based on slip-stranded mispairing of multiple tandem repeats of 5′-CAAT-3′ within the open reading frame of licA, a putative choline kinase gene (36, 39). Only phase variants displaying ChoP are able to persist for extended periods in animals models of nasopharyngeal carriage (38). In addition, direct analysis of clinical specimens from the human respiratory tract that contained H. influenzae by PCR followed by sequencing across the repeat region in licA showed that >90% had a number of copies of the repeat that indicated the phase-on, ChoP+ phenotype. The predominance of the ChoP+ phenotype in the human respiratory tract was an unexpected finding, since ChoP renders the organism sensitive to killing by at least two mechanisms. There is activation of the classical pathway of complement mediated by both the abundant naturally acquired antibodies recognizing ChoP and C-reactive protein (CRP), which binds specifically to ChoP (6, 24, 38). The selection for the ChoP+ phenotype in vivo despite the targeting of this structure by innate and adaptive immune mechanisms again emphasized that ChoP must confer advantages for bacterial survival.
In this report, we consider the hypothesis that bacterial mimicry of host membrane lipids by the cell surface expression of ChoP serves to decrease killing by the antimicrobial peptide LL-37/hCAP18. In addition, we show that LL-37/hCAP18 is expressed by the epithelium of the nasopharynx and may be present at bactericidal concentrations in nasal secretions.
MATERIALS AND METHODS
Bactericidal assays with LL-37/hCAP18.
H. influenzae was grown in brain heart infusion medium supplemented with 2% Fildes enrichment (sBHI; Difco Laboratories, Detroit, Mich.) to an optical density at 620 nm (OD620) of 0.3. The cells were then kept at 4°C and washed in an equal volume of nonnutrient medium E (0.8 mM MgSO4, 9.6 mM citric acid, 57.4 mM K2HPO4, 16.7 mM NaH4HPO4) (16). LL-37/hCAP18 was chemically synthesized, analyzed as previously described, and diluted from a stock concentration of 1.0 mg/ml in water into a buffer of 0.01% acetic acid and 0.02% bovine serum albumin (4). Bactericidal assay mixtures consisted of 90 μl of bacteria diluted 1,000-fold in medium E and 10 μl of 0.01% acetic acid and 0.02% bovine serum albumin with various amounts of diluted peptide; 0.1% gelatin was added to the medium E used in bactericidal assays to maintain bacterial viability. H. influenzae was treated for 60 min at 37°C without shaking and then maintained at 4°C while serial dilutions in duplicate were plated on sBHI solidified with 1% agar for viable counts. All assays included viable counts in the absence of peptide at times 0 and 60 min to ensure that bacterial viability was maintained in these assay conditions. For each strain, mutant, or variant tested, incubation in medium E containing gelatin over the 60-min period at 37°C had no significant effect on viability. Since longer incubation periods had little effect on killing by the peptide and viability counts decreased with incubation periods exceeding 3 h, all assays were limited to 60 min. When specified, H. influenzae was grown at 37°C for 16 h in a chemically defined medium which lacks choline with or without added choline chloride (5 μg/ml) (21). In this case, bacteria were first grown in sBHI and then washed in an equal volume of phosphate-buffered saline (PBS), and a 1-in-100 inoculum was added to the chemically defined medium. In some bactericidal assays, NaCl at the concentration specified was added to the medium E with gelatin buffer.
Expression of phase-variable LPS structures by H. influenzae.
The proportion of clinical isolate H233 expressing or not expressing ChoP was determined by immunoblotting of colonies from viable counts (39). Colonies lifted onto nitrocellulose membranes were incubated with monoclonal antibody (MAb) HAS (Statens Seruminstitut, Copenhagen, Denmark), a murine immunoglobulin M (IgM) myeloma that recognizes ChoP, followed by an antiserum to mouse IgM conjugated to alkaline phosphatase as previously described (37). The construction of constitutive mutants with an in-frame deletion of the 5′-CAAT-3′ repeats (ChoP-on) or insertion mutation in licD (ChoP-off), required for ChoP expression, was previously described (20). Constitutive mutants included H446 (ChoP-off) and H491 (ChoP-on) in unencapsulated type d strain Rd as well as H445 (ChoP-off) and H512 (ChoP-on) in encapsulated type b strain Eagan. Western blot analysis of H491 grown to an OD620 of 0.2 in a chemically defined medium with and without choline was carried out with bacteria washed in an equal volume of PBS, pelleted, and treated for 5 min at 100°C in a 1/10 volume of gel loading buffer; 2-μl aliquots of the whole cell lysates were then separated on sodium dodecyl sulfate (SDS)–18% polyacrylamide gels prior to transfer to Immobilon (Millipore Co., Bedford, Mass.) for immunoblotting with MAb HAS as described above. The expression of Galα1-4Gal was determined by colony immunoblotting using MAb 4C4 followed by an antiserum to mouse IgG conjugated to alkaline phosphatase as previously described (15, 37).
Binding of LL-37/hCAP18 to H. influenzae.
Constitutive mutants H445, H446, H491, and H512 were grown to an OD620 of 0.3, washed in medium E, and resuspended at a concentration of 108 CFU/ml in medium E. The cells were incubated with twofold dilutions of LL-37/hCAP18 from 0.03 to 2.0 μg/ml (final concentration) for 15 min at 37°C. Unbound peptide was removed by two washes in an equal volume of medium E. The cells were then pelleted, resuspended in 1/10 volume of gel loading buffer, and treated for 5 min at 100°C. The relative binding of LL-37/hCAP18 in whole cell lysates was determined as described below for Western blot analysis of nasal secretions. Loading of equivalent numbers of bacteria in each lane was assessed using Ponseau S staining of membranes prior to immunoblotting.
Analysis of LL-37/hCAP18 transcription.
In situ hybridization was carried out on paraformaldehyde-fixed human nasal polyp sections (7 μm) obtained from the pathology department at The Children's Hospital of Philadelphia. After dewaxing through a series of xylene and graded ethanol washes, the sections were acid treated, incubated with a solution of 20 μg of proteinase K per ml (7 min at 25°C), washed in 0.4% glycine in PBS (twice for 2 min each time), and then washed in 0.9% NaCl (2 min). A postfixation step included incubation in cold 4% paraformaldehyde in PBS (15 min at 25°C) followed by PBS (twice for 2 min each time) and 0.9% NaCl (2 min). Finally, the slides were treated in acetic anhydride in 0.2 M triethanolamine-HCl (pH 8.0) (twice for 5 min each time) and rinsed with distilled H2O. Prehybridization was performed in 50% formamide–25% dextran sulfate–0.3 M NaCl–10 mM NaH2PO4–5 mM EDTA–0.2% Ficoll 400–0.2% polyvinylpyrrolidone–1 M dithiothreitol–5 mg of polyadenylic acid–250 μM α-S-thio-ATP–50 μg of yeast tRNA per ml–10 mM Tris-HCl (pH 7.6) in a humidified chamber (60 min at 50°C). Sections were hybridized in the prehybridization solution containing 0.15 ng of the [35S]UTP-labeled sense or antisense riboprobe per μl (16 h at 50°C). Probes were synthesized using in vitro transcription by the T3 or T7 RNA polymerase (Boehringer Mannheim, Mannheim, Germany) of full-length LL-37/hCAP18 cDNA cloned in pBluescript II KS (4). After hybridization, the slides were washed in 50% formamide with 2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–20 mM β-mercaptoethanol (65°C twice for 30 min each time) and then with 4× SSC with 20 mM Tris-HCl (pH 7.6)–1 mM EDTA (two times for 10 min each at 37°C), followed by digestion with RNase A (10 μg/ml) (10 min at 37°C) in 4× SSC–20 mM Tris-HCl (pH 7.6)–1 mM EDTA, followed by 4× SSC–20 mM Tris-HCl (pH 7.6)–1 mM EDTA–20 mM β-mercaptoethanol (10 min at 37°C), followed by 50% formamide–2× SSC–20 mM β-mercaptoethanol (45 min at 65°C, twice) and 2× SSC (10 min at 37°C). The dried slides were then dipped in NTB-2 photoemulsion (Kodak Co., Rochester, N.Y.), dried, and stored at 4°C in the dark. After developing, the slides were counterstained with a solution of 2 μg of Hoechst 33258 per ml, mounted, and analyzed by dark-field microscopy and UV fluorescence.
RT-PCR.
Detroit 562 pharyngeal carcinoma cells (CCL 138; American Type Tissue Collection, Manassas, Va.) maintained in minimal essential medium with 5% fetal calf serum were used as the source of total RNA. After reaching confluence, monolayers were infected with 106 CFU of strain H491 per ml in PBS or with PBS alone for 4 h prior to harvesting the cells with trypsin. Total RNA was isolated using an RNeasy minikit as instructed by the manufacturer (Qiagen Inc., Torrance, Calif.). Reverse transcription-PCR (RT-PCR) was performed using a pd(N)6 primer purchased from Amersham Pharmacia Biotech Inc. (Piscataway, N.J.) and Moloney murine leukemia virus reverse transcriptase as instructed by the manufacturer (Promega Co., Madison, Wis.). This cDNA was used as a template in PCRs with primers 5′-CCATGAAGACCCAAAGGAATGG-3′ (forward) and 5′-AATCCTCTGGTGACTGCTGTGTCG-3′ (reverse) designed based on the LL-37/hCAP18 cDNA sequence (GenBank accession no. Z38026). Controls used primers 5′-AAGGTCGGAGTCAACGGATTTGG-3′ (forward) and 5′-GAGATGATGACCCTTTTGGCTCCC-3′ (reverse) based on the sequence of glyceraldehyde 3-phosphate dehydrogenase cDNA to demonstrate the adequacy of the cDNA template. The PCR products were analyzed in 1.0% agarose gels.
Western blot analysis of nasal secretions.
Nasal secretions were collected without chemical stimulation from normal volunteers. LL-37/hCAP18 was extracted in acetonitrile (final concentration, 60%) and trifluoroacetic acid (final concentration, 1.0%) for 16 h at 25°C. After insoluble debris was removed by centrifugation at 1,500 × g for 10 min, the solution was lyophilized. The extracted material was resuspended by sonication in water at the original volume; 1.0 M Tris-HCl (pH 7.5) was added until the solution was no longer acidic. Gel loading buffer was added to 10-μl aliquots of material extracted from nasal secretions or purified LL-37/hCAP18 controls, treated at 100°C for 5 min, and loaded onto SDS–18% polyacrylamide gels. After transfer to Immobilon membranes (Millipore Co., Bedford, Mass.), LL-37/hCAP18 was detected using a previously described rabbit antiserum raised to this peptide (4). Bound antibody was visualized by incubation of the membrane with a monoclonal antibody to rabbit IgG conjugated to alkaline phosphatase (Sigma Chemical Co., St. Louis, Mo.) as previously described (2). The concentration of the peptide in the nasal secretions was calculated by comparison to a standard curve consisting of twofold dilutions of known amounts of chemically synthesized LL-37/hCAP18 (0.0125 to 1.6 μg/lane) run in parallel Western blots and measured by digitalization with an AlphaImager gel documentation system. Total protein content of the extracted material was determined by a micro-bicinchoninic acid assay as instructed by the manufacturer (Pierce Chemical Co., Rockford, Ill.).
RESULTS
Bacterial ChoP confers resistance to killing by LL-37/hCAP18.
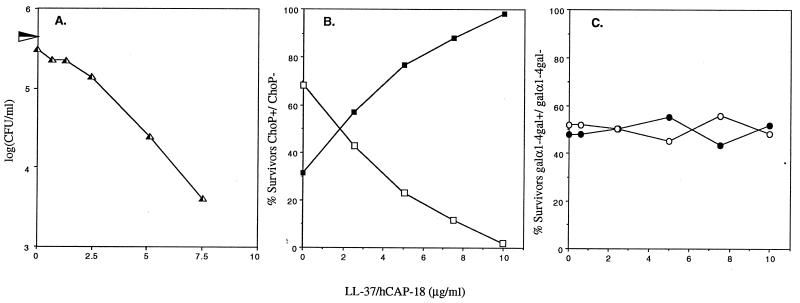
Because LL-37/hCAP18 is linear, unlike other peptide antibiotics known to be in the human respiratory tract (e.g., β-defensins), we were able to obtain sufficient quantities of synthetically produced peptide for these studies. This peptide was used in bactericidal assays in a low-ionic-strength buffer (medium E) previously shown to optimize the α-helical content and antibacterial activity of LL-37/hCAP18 (16). Initial studies examined the effects of LL-37/hCAP18 on a clinical isolate of nontypeable H. influenzae, H233. There were no survivors when bacteria at a density of 105 CFU/ml were treated for 60 min with >10 μg of peptide per ml (Fig. 1A). The phenotype of bacteria surviving treatment with 0 to 10 μg of LL-37/hCAP18 per ml was determined in colony immunoblots using MAbs HAS and 4C4, recognizing phase-variable oligosaccharide structures ChoP and Galα1-4Gal, respectively (15, 37). As the concentration of peptide was increased, the proportion of ChoP+ phase variants among survivors rose from 32% without peptide to 98% in 10 μg of LL-37/hCAP18 per ml (Fig. 1B). In contrast, there was no selective effect for organisms expressing or not expressing Galα1-4Gal in this isolate due to LL-37/hCAP18 (Fig. 1C). This demonstrated that under identical assay conditions, LPS variants expressing ChoP were more resistant to the peptide than the ChoP− phenotype for this wild-type strain.
FIG. 1.
Selection for LPS phase variants in the presence of LL-37/hCAP18. Nontypeable clinical isolate H233 shows a dose-dependent killing caused by LL-37/hCAP18. Numbers of bacteria surviving treatment for 60 min in the concentration of LL-37/hCAP18 indicated on the x axis are shown in panel A. The horizontal arrowhead along the y axis indicates the total number of bacteria present at the beginning of the incubation period (0 min). H233 is a mixed population of phase variants with (solid symbols) and without (open symbols) ChoP or Galα1-4Gal on their LPS (37). Survivors showed a dose-related increase in the proportion of ChoP+ compared to ChoP− phase variants as determined by colony immunoblotting (B). There was, however, no selection for phase variants with or without the Galα1-4Gal structure (C). Results are the mean of a representative experiment in duplicate.
The hypothesis that ChoP confers resistance to LL-37/hCAP18 was then tested by comparison of mutants of unencapsulated strain Rd with constitutive phenotypes. H446, which is ChoP-off due to an insertion mutation in licD, was compared to H491, which is ChoP-on due to in-frame deletion of the 5′-CAAT-3′ repeat region (20). The ChoP-on mutant showed a >1,000-fold increased survival in amounts of LL-37/hCAP18 below the concentration giving complete killing under the conditions tested (Fig. 2A). This difference in survival was a result of the activity of the peptide, since there was no difference in survival between ChoP-on and ChoP-off phenotypes incubated in the nonnutrient medium E with gelatin in the absence of LL-37/hCAP18. Similar assays were carried out with an unrelated strain, Eagan, an encapsulated type b isolate, containing analogous mutations to generate ChoP-off and ChoP-on phenotypes (Fig. 2B) (20). Although both phenotypes of this strain were sensitive to the peptide, there was a significantly greater survival among the ChoP-on bacteria with >1.2 μg of LL-37/hCAP18 per ml. Therefore, for the three unrelated strains tested, H233, Rd, and Eagan, the expression of ChoP on the LPS conferred significantly increased resistance to the bactericidal effects of LL-37/hCAP18.
FIG. 2.
Effect of ChoP on susceptibility to the bactericidal activity of LL-37/hCAP18. ChoP-on (solid symbols) and ChoP-off (open symbols) constitutive mutants were compared in unrelated strains with different LPS structures, unencapsulated type d strain Rd (A) and type b strain Eagan (B). Cells were treated for 60 min at 37°C with LL-37/hCAP18 at the concentrations indicated on the x axis, and the number of survivors was determined in viable counts. The horizontal arrowheads along the y axis indicate the number of bacteria of each type present at the beginning of the incubation period (0 min). Numbers represent the mean of at least four determinations + standard deviation.
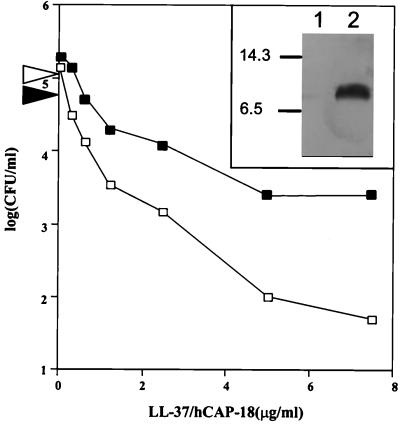
Finally, we confirmed that it is the incorporation of choline and its conversion to ChoP on the LPS that accounts for the relative increase in resistance of H. influenzae. Constitutive ChoP-on mutant H491 was grown in a chemically defined medium lacking choline that is suitable for strain Rd (21). Only when exogenous choline is provided in this medium is ChoP expressed on the LPS of H491 (Fig. 3, insert). This strain was generally more sensitive to the peptide when grown in chemically defined medium than when grown in nutrient medium. H491 grown in the chemically defined medium in the presence of choline was more resistant to killing by LL-37/hCAP18 than was H491 grown under identical conditions in the absence of choline (Fig. 3). This result confirmed that incorporation of choline decreases susceptibility of H. influenzae to this peptide.
FIG. 3.
Effect of environmental choline on ChoP expression on the LPS and susceptibility to the bactericidal activity of LL-37/hCAP18. Constitutive ChoP-on mutant H491 was grown in a chemically defined medium for H. influenzae with (solid symbols) or without (open symbols) supplemental choline (5 μg/ml), and the sensitivity to killing by LL-37/hCAP18 (for 60 min at 37°C) was determined. The horizontal arrows along the y axis indicate the number of bacteria grown with or without choline present at the beginning of the incubation period (0 min). Western blot analysis (insert) of whole cell lysates using a MAb against ChoP shows the presence of ChoP on the LPS when grown with choline in the medium (lane 2) but not in the same medium without added choline (lane 1). Size markers are in kilodaltons.
Factors affecting the bactericidal activity of LL-37/hCAP18.
A further consideration was the position of ChoP on the LPS since strains Rd and Eagan have ChoP positioned on different hexoses on different chain extensions on the oligosaccharide (20). The observation that mutants with ChoP were more resistant to LL-37/hCAP18 for both strains Rd and Eagan demonstrated that the position of ChoP and other differences in oligosaccharide structure were not the determining factors in sensitivity to LL-37/hCAP18.
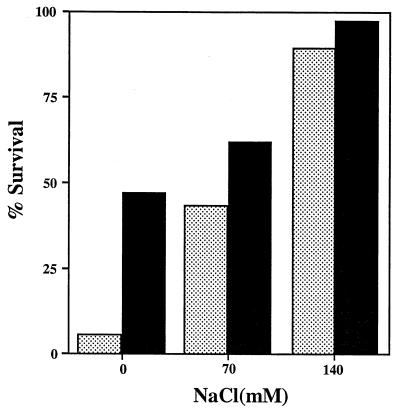
The salt concentration has been implicated as a factor in the antimicrobial activity of LL-37/hCAP18 (4, 32). The addition of NaCl (0 to 140 mM) to low-ionic-strength medium E (16.7 mM Na+) used in bactericidal assays resulted in a dose-related increase in the amount of peptide required to give an equivalent antimicrobial effect. With the addition of 140 mM NaCl, the difference in susceptibility between ChoP-on and ChoP-off mutants of strain Eagan was insignificant (Fig. 4). It was concluded that the selective effect of ChoP on susceptibility to LL-37/hCAP18 was more pronounced in lower concentrations of salt.
FIG. 4.
Effect of salt concentration on the bactericidal activity of LL-37/hCAP18. ChoP-on (H512; solid bars) and ChoP-off (H445; stippled bars) constitutive mutants of strain Eagan were treated for 60 min at 37°C with LL-37/hCAP18 (final concentration, 5 μg/ml), and the number of survivors was determined in viable counts. NaCl was added to medium E (16.7 mM Na+) in bactericidal assays at the final concentrations indicated at the bottom. Results are expressed in relation to the same conditions without peptide and are the mean of two determinations in duplicate.
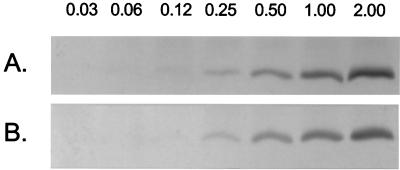
The possibility that the decreased killing of ChoP+ bacteria was due to diminished binding of the peptide was considered. ChoP-on (H491 and H512) and ChoP-off (H446 and H445) mutants were incubated in equal twofold dilutions of LL-37 (0.03 to 2.0 μg/ml) in medium E at a cell density (108/ml) at which there was no significant bactericidal activity. After 15 min at 37°C, the unbound peptide was removed and the lysed cells were examined by Western blotting using an antiserum to LL-37/hCAP18. There was a dose-related binding of the peptide to bacterial cells, but the difference between ChoP-on and ChoP-off mutants in the amount of cell-associated peptide was less than twofold (Fig. 5). The lack of a significant difference in amount of peptide bound suggested that the effect of ChoP on susceptibility to LL-37/hCAP18 was the result of qualitative differences in interaction of the peptide with the bacterial cell.
FIG. 5.
Binding of LL-37/hCAP18 to H. influenzae Rd with and without LPS-ChoP. Equivalent numbers of constitutive ChoP-off strain H446 (A) or ChoP-on strain H491 (B) were incubated with the concentration (in μg/ml) of LL-37/hCAP18 indicated above each lane. After removal of unbound peptide, the binding of LL-37/hCAP18 to the bacteria was determined in whole cell lysates. Following separation on SDS–18% polyacrylamide gels, the amount of bound peptide was determined by Western blotting using an antiserum raised against LL-37/hCAP18.
LL-37/hCAP18 is expressed in the human nasopharynx.
The above experiments established that peptides such as LL-37/hCAP18 could account for the selection of ChoP+ bacteria seen in vivo. The possibility that this peptide is expressed on the mucosal surface of the nasopharynx, the environmental niche for H. influenzae, was examined using in situ hybridization on tissue sections of human nasal polyps. Sense and antisense RNA probes generated from LL-37/hCAP18 cDNA were labeled with [35S]UTP and hybridized to tissue sections. Hybridization occurred predominantly along the epithelial surface of the nasal polyp and was specific to the antisense probe (Fig. 6A). Additional evidence for transcription of the LL-37/hCAP18 gene by the epithelium of the upper respiratory tract came from RT-PCR experiments using RNA isolated from Detroit 562 pharyngeal carcinoma cells in culture, which demonstrated a single band of the predicted size (Fig. 7). The LL-37/hCAP18 transcript, however, was detected only after these cells were infected with H. influenzae. This finding suggested that the presence of bacteria may induce expression of this peptide by epithelial cells lining the respiratory tract.
FIG. 6.
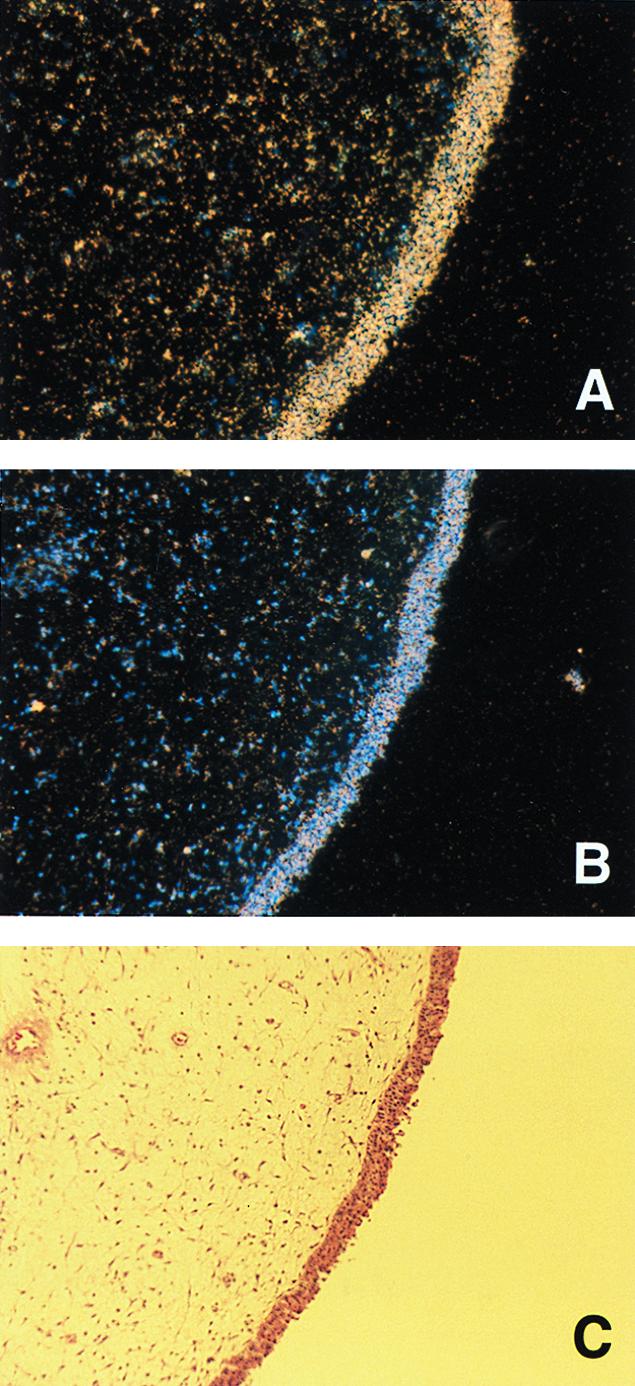
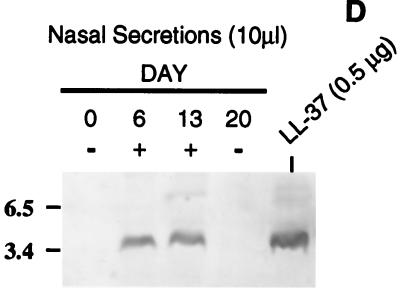
In situ hybridization of LL-37/hCAP18 in a human nasal polyp. Tissue sections of the same polyp were hybridized with a [35S]UTP-labeled RNA antisense (A) or sense (B) probe. The nasal epithelial surface is visualized by the blue fluorescence of cell nuclei with the Hoechst 33258 counterstain (B) and by the dense band of staining with hematoxylin and eosin (C). Magnification, ×10. (D) Western blot showing LL-37/hCAP18 in nonpurulent nasal secretions collected from the same individual over a 3-week period. Ten microliters of solubilized nasal secretions collected on the day indicated was separated on an SDS–18% polyacrylamide gel, transferred to a membrane, and immunoblotted with an antiserum raised to LL-37/hCAP18. The control consists of 0.5 μg of chemically synthesized peptide. + and − denote whether the untreated secretions were both grossly torpid and contained >5 mg of total protein per ml. Size markers are in kilodaltons.
FIG. 7.
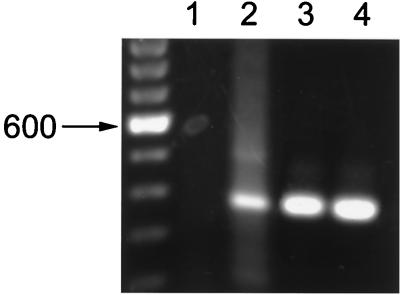
Detection of LL-37/hCAP18 mRNA in H. influenzae-infected Detroit 562 pharyngeal carcinoma cells in culture. RNA was isolated from uninfected cells (lanes 1 and 3) or cells infected for 4 h with 106 CFU of strain H491 per ml (lanes 2 and 4) and subjected to RT-PCR using primers specific to the gene for LL-37/hCAP18 (lanes 1 and 2). RT-PCR using primers to the glyceraldehyde 3-phosphate dehydrogenase gene served as controls for the quality of the template (lanes 3 and 4). The PCR products were visualized using ethidium bromide after separation on a agarose gel. Molecular weight markers consisted of a 100-bp ladder.
To estimate the concentration of LL-37/hCAP18 on the mucosal surface of the nasopharynx, unstimulated, nonpurulent nasal secretions were collected from eight healthy volunteers. Western blot analysis of solubilized secretions with a polyclonal antiserum to LL-37/hCAP18 was used to detect the peptide and estimate its concentration by comparison to controls with known amounts of synthetic LL-37/hCAP18. The calculated concentration of LL-37/hCAP18 in individual samples varied considerably (<1.2 to >80 μg per ml of nasal fluid). This variability was examined in more detail by testing additional specimens obtained from one donor with initially undetectable amounts of the peptide (Fig. 6D). Specimens collected 6 and 13 days after the first sample both contained a calculated peptide concentration of 21 μg/ml, while a final specimen obtained on day 20 again had undetectable amounts of the peptide. Only the two specimens with detectable peptide were grossly torpid and had total protein concentrations of >5 mg/ml. It was concluded that there is individual-to-individual as well as temporal variability in amounts of LL-37/hCAP18 in the nasal airway fluid. However, based on in vitro results with H. influenzae, this antimicrobial peptide may be present on the mucosal surface of the nasopharynx at concentrations sufficient to have a selective effect on the flora colonizing this site.
DISCUSSION
All antibiotics target physiologic or structural differences between host and microbial cells. This is also the case for natural antibiotics, which include an array of different cationic peptides expressed in a variety of tissues and host surfaces (5). Many of these peptides have been identified in normally sterile sites such as the lower airway, where they may provide an important protective function. For antimicrobial peptides that are also expressed on heavily colonized mucosal surfaces, there must be a mechanism that allows for coexistence with the normal flora. It seems plausible that one strategy for evading their antibacterial activity would involve modification of the bacterial surface, since antimicrobial peptides act by nonspecific partitioning to the membrane and disturbing its barrier function (30). One example of a strategy to increase resistance to cationic antimicrobial peptides used by Salmonella enterica serovar Typhimurium appears to involve changing the lipid A portion of the LPS by the addition of aminoarabinose and 2-hydroxymyristate (13).
The impetus for this study was the observation that a diverse group of bacteria that colonize and infect the human respiratory tract express a host-like structure, ChoP, on either the LPS in gram-negative bacteria, the teichoic acids in gram-positive bacteria, or the cytoplasmic membrane of mollicutes (8, 19, 27, 38; Serino and Virji, presented at the 11th International Pathogenic Neisseria Conference, 1998). Our approach was to test the contribution of ChoP to resistance for an organism commonly colonizing the mucosal surface against a human antimicrobial peptide present in the same host environment. In this regard, we showed that the peptide LL-37/hCAP18, previously reported to be expressed in bone marrow, testis, lung, and the squamous epithelia of the mouth, tongue, esophagus, cervix, and vagina, is also expressed in the nasal epithelium and is present in secretions collected from the human nasopharynx (1, 4, 23).
The hypothesis that ChoP on the oligosaccharide region of the LPS of H. influenzae increases resistance to the antimicrobial peptide LL-37/hCAP18 was examined. This was the single peptide associated with the respiratory tract for which sufficient quantities for these assays could be generated. In addition, H. influenzae was the only respiratory tract pathogen for which isogenic mutants with and without ChoP were available to test this hypothesis. These studies used bactericidal activity rather than growth inhibition as a more rigorous demonstration of concentration-dependent antimicrobial effect (28). Three lines of investigation supported the effect of ChoP in augmenting the relative resistance to killing by LL-37/hCAP18. In a wild-type strain there was a dose-dependent selection for ChoP+ phase variants from the mixed population consisting of ChoP+ and ChoP− phenotypes. In two unrelated strains with a defined, nonvarying phenotype, mutants with ChoP required a greater dose of peptide for the equivalent bactericidal effect and in higher concentrations of LL-37/hCAP18 showed as much as a 1,000-fold increased survival. Finally, increased resistance to the peptide required the presence of environmental choline, which is necessary for the expression of ChoP on the LPS (39). This effect was specific to ChoP, as other variations in the oligosaccharide portion of the LPS did not significantly affect susceptibility to the peptide. Although a bactericidal effect of LL-37/hCAP18 occurred with and without ChoP, the decreased susceptibility associated with the expression of ChoP could lead to a selection for this phenotype in environments with sufficient quantities of this or other, similar antimicrobial peptides. Results of this study, therefore, could explain the finding that ChoP+ phase variants predominate in H. influenzae within the human respiratory tract (38).
Innate immunity based on antibiotic peptides may be a determining factor in the characteristics of bacteria colonizing the mucosal surface. In the case of ChoP, the selection of ChoP+ phase variants in the presence of LL-37/hCAP18 may be balanced by the targeting of this same structure by a separate arm of the innate immune system involving CRP and complement (37, 38). Peptides such as LL-37/hCAP18 may act primarily on the mucosal surface, whereas the contribution of CRP together with complement may be significant only in the presence of serum.
Our findings may be relevant to other species that colonize the upper respiratory tract of humans and have ChoP on their cell surface. We have shown that Streptococcus pneumoniae variants expressing increased amounts of ChoP-containing teichoic acid are more efficient at colonization in an animal model of carriage (18, 35). This variant was also more resistant to killing by LL-37/hCAP18 than were variants of the same strain with lower amounts of ChoP (unpublished data). Because ChoP affects many aspects of the pneumococcal cell surface, including amounts of capsular polysaccharide and an array of proteins noncovalently anchored to choline, it was not possible to conclude that this observation is a direct effect of ChoP (34). In contrast, in H. influenzae, ChoP expression does not appear to affect other surface structures and the observed effect of LL-37/hCAP18 was independent of encapsulation. The relevance of these observations to other classes of antimicrobial peptides, such as the β-defensins also present in airway fluid from the human nasopharynx, has not been examined because of limited availability of these substances (7). Another factor in the activity of antibiotic peptides and the contribution of ChoP to resistance is salt concentration. In nasal airway fluid, the concentration of Na+ has been estimated at 40 to 135 mM (A. Cole, personal communication), which is similar to the concentration in airway surface fluid reported in the lower human respiratory tract (17). Results from this study suggest that these concentrations would be permissive for bactericidal levels of LL-37/hCAP18 activity.
This study did not specifically address the mechanism responsible for the effect of ChoP on killing by LL-37/hCAP18. There is, however, evidence that this peptide interacts with LPS, albeit from another species, and that the structure of the LPS affects sensitivity to antimicrobial peptides in general (13, 14, 32). When ChoP is expressed in H. influenzae, it is an abundant cell surface constituent, with >90% of LPS molecules having this surface-exposed residue regardless of its specific linkage on the oligosaccharide region (20). Although ChoP is zwitterionic, the positively charged quaternary amine on choline would be predicted to orient toward the exterior of the cell. Since mutants or phase variants lacking ChoP have no charged residue replacing this structure, it is plausible that the decreased killing by cationic antimicrobial peptides is primarily due to the effect of ChoP on the surface charge. This hypothesis is consistent with data showing that the magnitude of the effect of ChoP on resistance is diminished as the salt concentration is increased. ChoP did not appear to affect the quantity of peptide that associates with the bacterium. It may, however, interfere with the ability of the amphipathic peptide to insert properly into the LPS on the outer membrane. In this respect, bacterial ChoP behaves similarly to phosphatidylcholine on eukaryotic membranes, which are inherently more resistant to antimicrobial peptides. Modeling of host membranes with vesicles showed that phosphatidylcholine was more resistant to lysis with cationic peptides compared to less positively charged membranes containing phosphatidylserine (30).
This study was not designed to define the precise concentration of LL-37/hCAP18 on the mucosal surface. Factors controlling the expression of this peptide are not completely understood. Based on a limited collection of specimens analyzed in this study, LL-37/hCAP18 may be present in unstimulated nasopharyngeal surface fluid, although the concentration appears to vary widely from specimen to specimen. Amounts of this peptide in at least some samples were sufficient to show substantial bactericidal activity against H. influenzae under the assay conditions examined in this study. Our results also show transcription of the gene for LL-37/hCAP18 by the normal human nasal epithelium in situ as well as epithelial cells derived from the upper respiratory tract in culture. This suggests the possibility that peptide detected in nasal secretion is from local production by epithelial cells lining the upper airway. Since LL-37/hCAP18 is also expressed by other cells such as leukocytes that may be present in this environment, it is unclear what proportion of the peptide comes from synthesis by nasal epithelial cells (31). An additional factor in the expression of this peptide by epithelial cells may be its induction by environmental or inflammatory signals. In this study, the presence of bacteria was shown to signal the transcription of the LL-37/hCAP18 gene in Detroit 562 pharyngeal carcinoma cells.
In summary, we show that bacterial mimicry of host membranes based on expression of ChoP contributes to resistance of H. influenzae to at least one human antimicrobial peptide, LL-37/hCAP18, found in the same host environment in concentrations that may be bactericidal.
ACKNOWLEDGMENTS
We thank Eduardo Richelli for providing sections of nasal polyps and Rebecca Oakey for guidance with in situ hybridization experiments. MAb 4C4 was generously provided by Eric Hansen.
This work was supported by grants from the Public Health Service (AI38436 and AI44231 to J.N.W.; HL49040 and DK47757 to J.M.W.) and the Cystic Fibrosis Foundation (J.M.W.).
REFERENCES
- 1.Agerberth B, Gunne H, Odeberg J, Kogner P, Bowman H, Gudmundsson G. FALL-39, a putative human peptide antibiotic, is cysteine-free and expressed in bone marrow and testis. Proc Natl Acad Sci USA. 1995;92:195–199. doi: 10.1073/pnas.92.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1999. [Google Scholar]
- 3.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. Human β-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bals R, Wang X, Zasloff M, Wilson J M. The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA. 1998;95:9541–9546. doi: 10.1073/pnas.95.16.9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boman H. Antibacterial peptides: key components needed in immunity. Cell. 1991;65:205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- 6.Briles D E, Scott G, Gray B, Crain M J, Blaese M, Nahm M, Scott V, Haber P. Naturally occurring antibodies to phosphocholine as a potential index of antibody responsiveness to polysaccharides. J Infect Dis. 1987;155:1307–1314. doi: 10.1093/infdis/155.6.1307. [DOI] [PubMed] [Google Scholar]
- 7.Cole A, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deutsch J, Salman M, Rottem S. An unusual polar lipid from the cell membrane of Mycoplasma fermentans. Eur J Biochem. 1995;227:897–902. doi: 10.1111/j.1432-1033.1995.tb20216.x. [DOI] [PubMed] [Google Scholar]
- 9.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frohm M, Agerberth B, Ahangari G, Stahle-Backdahl M, Liden S, Wigzell H, Gudmundsson G. The expression of the gene coding for the antibacterial peptide LL-37 is induced in human keratinocytes during inflammatory disorders. J Biol Chem. 1997;272:15258–15263. doi: 10.1074/jbc.272.24.15258. [DOI] [PubMed] [Google Scholar]
- 11.Gazit E, Boman A, Boman H, Shai Y. Interaction of the mammalian antibacterial peptide cecropin P1 with phospholipid vesicles. Biochemistry. 1995;34:11479–11488. doi: 10.1021/bi00036a021. [DOI] [PubMed] [Google Scholar]
- 12.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human β-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 13.Guo L, Lim K, Gunn J S, Bainbridge B, Darveau R, Hackett M, Miller S I. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science. 1996;276:250–253. doi: 10.1126/science.276.5310.250. [DOI] [PubMed] [Google Scholar]
- 14.Guo L, Lim K, Poduje C, Daniel M, Gunn J, Hackett M, Miller S. Lipid A acylation and bacterial resistance against vertebrate antimicrobial peptides. Cell. 1998;95:189–198. doi: 10.1016/s0092-8674(00)81750-x. [DOI] [PubMed] [Google Scholar]
- 15.High N J, Deadman M E, Moxon E R. The role of a repetitive DNA motif (5′-CAAT-3′) in the variable expression of the Haemophilus influenzae lipopolysaccharide epitope αGal(1-4)βGal. Mol Microbiol. 1993;9:1275–1282. doi: 10.1111/j.1365-2958.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- 16.Johansson J, Gudmundsson G H, Rottenberg M E, Berndt K D, Agerberth B. Conformation-dependent antibacterial activity of the naturally occurring human peptide LL-37. J Biol Chem. 1998;273:3718–3724. doi: 10.1074/jbc.273.6.3718. [DOI] [PubMed] [Google Scholar]
- 17.Joris L, Dab I, Quinton P. Elemental composition of human airway surface fluid in healthy and diseased airways. Am Rev Respir Dis. 1993;148:1633–1637. doi: 10.1164/ajrccm/148.6_Pt_1.1633. [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Weiser J. Association of intrastrain phase variation in quantity of capsular polysaccharide and teichoic acid with the virulence of Streptococcus pneumoniae. J Infect Dis. 1998;177:368–377. doi: 10.1086/514205. [DOI] [PubMed] [Google Scholar]
- 19.Kolberg J, Holby E A, Jantzen E. Detection of the phosphorylcholine epitope in streptococci, Haemophilus and pathogenic neisseriae by immunoblotting. Microb Pathog. 1997;22:321–329. doi: 10.1006/mpat.1996.0114. [DOI] [PubMed] [Google Scholar]
- 20.Lysenko, E., J. Richards, A. Cox, M. Kapoor, and J. Weiser. The position of phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae affects binding and sensitivity to C-reactive protein mediated killing. Mol. Microbiol., in press. [DOI] [PubMed]
- 21.Michalka J, Goodgal S. Genetic and physical map of the chromosome of Hemophilus influenzae. J Mol Biol. 1969;45:407–421. doi: 10.1016/0022-2836(69)90115-6. [DOI] [PubMed] [Google Scholar]
- 22.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 23.Nilsson M, Sandstedt B, Sorensen O, Weber G, Borregaard N, Stahle-Backdahl M. The human cationic antimicrobial protein (hCAP18), a peptide antibiotic, is widely expressed in the human squamous epithelia and colocalizes with interleukin-6. Infect Immun. 1999;67:2561–2566. doi: 10.1128/iai.67.5.2561-2566.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nordenstam G, Andersson B, Briles D, Brooks J W J, Oden A, Svanborg A, Eden C S. High anti-phosphorylcholine antibody levels and mortality associated with pneumonia. Scand J Infect Dis. 1990;22:187–195. doi: 10.3109/00365549009037901. [DOI] [PubMed] [Google Scholar]
- 25.Risberg A, Masoud H, Martin A, Richards J, Moxon E, Schweda E. Structural analysis of the lipopolysaccharide oligosaccharide epitopes expressed by a capsule-deficient strain of Haemophilus influenzae Rd. Eur J Biochem. 1999;261:171–180. doi: 10.1046/j.1432-1327.1999.00248.x. [DOI] [PubMed] [Google Scholar]
- 26.Risberg A, Schweda E K H, Jansson P-E. Structural studies of the cell-envelope oligosaccharide from lipopolysaccharide of Haemophilus influenzae strain RM 118-28. Eur J Biochem. 1997;243:701–707. doi: 10.1111/j.1432-1033.1997.00701.x. [DOI] [PubMed] [Google Scholar]
- 27.Schenkein H, Gunsolley J, Best A, Harrison M, Hahn C, Wu J, Tew J. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect Immun. 1999;67:4814–4818. doi: 10.1128/iai.67.9.4814-4818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvestro L, Gupta K, Weiser J N, Axelson P H. The concentration-dependent membrane activity of cecropin A. Biochemistry. 1997;36:11452–11460. doi: 10.1021/bi9630826. [DOI] [PubMed] [Google Scholar]
- 29.Singh P, Jia H, Wiles K, Hesselberth J, Liu L, Conway B, Greenberg E, Valore E, Welsh M, Ganz T, Tack B, McCray P., Jr Production of beta-defensins by human airway epithelia. Proc Natl Acad Sci USA. 1998;95:14961–14966. doi: 10.1073/pnas.95.25.14961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitaram N, Nagaraj R. Interaction of the 47-residue antibacterial peptide seminalplasmin and its 13-residue fragment which has antibacterial and hemolytic activities with model membranes. Biochemistry. 1993;32:3124–3130. doi: 10.1021/bi00063a026. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen O, Arnljots K, Cowland J, Bainton D, Borregaard N. The human antibacterial cathelicidin, hCAP-18, is synthesized in myelocytes and metamyelocytes and localized to specific granules in neutrophils. Blood. 1997;90:2796–2803. [PubMed] [Google Scholar]
- 32.Turner J, Cho Y, Dinh N, Waring A, Lehrer R. Activities of LL-37, a cathelin-associated antimicrobial peptide of human neutrophils. Antimicrob Agents Chemother. 1998;42:2206–2214. doi: 10.1128/aac.42.9.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weiser J N. The oligosaccharide of Haemophilus influenzae. Microb Pathog. 1993;13:335–342. doi: 10.1016/0882-4010(92)90077-2. [DOI] [PubMed] [Google Scholar]
- 34.Weiser J N. Phase variation of Streptococcus pneumoniae. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 1999. pp. 225–231. [Google Scholar]
- 35.Weiser J N, Austrian R, Sreenivasan P K, Masure H R. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect Immun. 1994;62:2582–2589. doi: 10.1128/iai.62.6.2582-2589.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiser J N, Love J M, Moxon E R. The molecular mechanism of phase variation of H. influenzae lipopolysaccharide. Cell. 1989;59:657–665. doi: 10.1016/0092-8674(89)90011-1. [DOI] [PubMed] [Google Scholar]
- 37.Weiser J N, Pan N. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol. 1998;30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]
- 38.Weiser J N, Pan N, McGowan K L, Musher D, Martin A, Richards J C. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J Exp Med. 1998;187:631–640. doi: 10.1084/jem.187.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiser J N, Shchepetov M, Chong S T H. Decoration of lipopolysaccaride with phosphorylcholine: a phase-variable characteristic of Haemophilus influenzae. Infect Immun. 1997;65:943–950. doi: 10.1128/iai.65.3.943-950.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]