Abstract
Genomic sequence and gene expression association studies in animals and humans have identified genes that may be integral in the pathogenesis of various diseases. CD14 (cluster of differentiation 14)—a cell surface protein involved in innate immune system activation—is one such gene associated with cardiovascular and hypertensive disease. We previously showed that this gene is upregulated in renal macrophages of Dahl salt-sensitive animals fed a high-salt diet; here we test the hypothesis that CD14 contributes to the elevated pressure and renal injury observed in salt-sensitive hypertension. Using CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat–associated 9), we created a targeted mutation in the CD14 gene on the Dahl SS (SS/JrHSDMcwi) background and validated the absence of CD14 peptides via mass spectrometry. Radiotelemetry was used to monitor blood pressure in wild-type and CD14−/− animals challenged with high salt and identified infiltrating renal immune cells via flow cytometry. Germline knockout of CD14 exacerbated salt-sensitive hypertension and renal injury in female animals but not males. CD14−/− females demonstrated increased infiltrating macrophages but no difference in infiltrating lymphocytes. Transplant of CD14+/+ or CD14−/− bone marrow was used to isolate the effects of CD14 knockout to hematopoietic cells and confirmed that the differential phenotype observed was due to knockout of CD14 in hematopoietic cells. Ovariectomy was used to remove the influence of female sex hormones, which completely abrogated the effect of CD14 knockout. These studies provide a novel treatment target and evidence of a new dichotomy in immune activation between sexes within the context of hypertensive disease where CD14 regulates immune cell activation and renal injury.
Keywords: bone marrow, hypertension, immunity, kidney, sex characteristics
Association studies, such as GWASs, have identified various genes of interest that may be involved in cardiovascular disease.1 Working from this list of genes, we have previously shown that the identified genes SH2B3 (LNK) and CD247 play mechanistic roles in immunity and the development of salt-sensitive hypertension.2–4 Herein, we investigated an alternatively identified gene CD14 (cluster of differentiation 14) using the Dahl SS (SS/JrHSDMcwi) rat animal model. Previous studies have implicated a clinical significance for CD14 in the context of hypertension and renal injury. It was first demonstrated that CD14 was associated with cardiovascular disease in 1999,5 and this observation has been repeatedly confirmed by others in genome-wide association studies.6,7 Additionally, a specific C(−260)T CD14 promoter variant associates with acute coronary syndrome, where patients produce greater amounts of the inflammatory cytokine IL (interleukin)-6,8 which we have previously shown to be important in salt-sensitive hypertension.9 These observations imply that individuals with variants in the CD14 gene have an increased risk for developing cardiovascular disease; however, this does not provide information on whether variant CD14 actively contributes to the disease or is unable to provide an appropriate compensatory response.
Canonically, CD14 is a glycosylphosphatidylinositol-anchored cell surface protein10 that functions in the TLR (Toll-like receptor) 4 complex to initiate proinflammatory signaling events in response to gram-negative bacteria through recognition of lipopolysaccharide.11 The few studies using a CD14 knockout mouse have shown altered response to lipopolysaccharide,12 attenuation in obesity-related cardiovascular dysfunction,13 and an attenuation of necrosis in hepatic ischemia reperfusion injury.14 CD14 has also been shown to be upregulated during renal ischemia reperfusion injury15 and in tubular epithelial cells of the kidney after unilateral ureteral obstruction.16 In human populations, soluble CD14 protein in the circulation is inversely correlated with estimated glomerular filtration rate17 and can also be used as a predictor of hypertension in patients with HIV.18 Mutations in the CD14 gene are associated with increased arterial stiffness19 and prevalence of hypertension in patients with IgA nephropathy.20 In addition, independent of hypertensive state, CD14 polymorphisms can be used to predict progression of IgA nephropathy.21
A recent report from the Systolic Blood Pressure Intervention Trial trial demonstrated that intensive blood pressure–lowering treatment was accompanied by a distinctly greater decrease in albumin-to-creatinine ratio and urinary inflammatory markers compared with standard treatment, illustrating the direct relationship between hypertensive phenotype, renal inflammation, and renal damage.22 Of hypertensives, ≈50% are considered salt sensitive—a term used to describe those whose blood pressure responds to changes in dietary salt.23 These individuals are at a greater risk for developing cardiovascular disease24 and demonstrate reduced survival.25 The Dahl SS rat is an animal model that demonstrates salt-sensitive hypertension accompanied by distinct renal damage and renal immune cell infiltration.26 We have shown that immunosuppression reduces this infiltration, concurrent with an attenuation in the hypertensive response to dietary salt.27 A transcriptomic analysis of renal outer medullary tissue in the Dahl SS rat revealed that a number of genes related to innate immunity pathways are upregulated when these animals are on a high-salt diet and develop renal damage.28 We further confirmed that innate immunity genes in TLR signaling pathways are upregulated specifically in infiltrating macrophages in the kidneys29 of this model. Of particular note, CD14, Ly96, and TLR4, all encoding proteins that function in the TLR4 protein complex, were upregulated in these renal infiltrating macrophages in response to a high-salt diet.
We hypothesize that CD14—a gene that associates with hypertension in human populations and upregulated in renal macrophages of the Dahl SS rat on a high-salt diet—contributes to the pathogenesis of salt-sensitive hypertension. To investigate this, we have created a genetic knockout of CD14 on the Dahl SS rat background, which we have validated by mass spectrometry. We have performed studies on these SS-CD14−/− rats using a high-salt diet model of salt-sensitive hypertension and renal injury.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Animals
Some experiments were performed on age-matched male and female, inbred Dahl SS rats (SS/JrHSDMcwi). Other experiments were performed on littermate male and female, wild type, or homozygous knockout for CD14 animals born from heterozygous breeders (CD14em2Mcwi; Rat Genome Database ID:12790610). This strain of SS rats was created by the MCW Gene Editing Rat Resource Center using CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat–associated 9) mutagenesis as we have described previously.30,31 Briefly, these animals were produced by injecting a pX330 plasmid32 encoding SpCas9 and sgRNA targeting the CD14 coding sequence GGAGTACCTTCCTAAAGCGTGTGG (protospacer adjacent motif underlined) into SS rat embryos. Founder animals were genotyped by Cel-1 assay and confirmed by Sanger sequencing to identify a founder animal harboring a net 7-bp deletion (deleted TAAAGCGT, inserted C; Figure 2A) within the CRISPR target site. This founder was backcrossed to the parental SS strain, and a breeding colony was established. Offspring will be referred to as CD14+/+ or CD14−/−. The colony of breeders was maintained on a 0.4% NaCl diet (AIN-76A; Dyets). For high-salt challenge studies, animals received indwelling carotid catheter radiotelemeter implantation at 7 weeks of age as described below. After recovery and baseline pressure recordings, rats were switched to a 4.0% NaCl diet (AIN-76A) for 21 days. At the end of the study period, animals were deeply anesthetized under isoflurane gas inhalation and the kidneys flushed and excised. For bone marrow transplant experiments, recipients received total body irradiation (11 Gy) as described previously.2,33 Donor animals were euthanized by barbiturate overdose (beauthanasia-D; Midwest Veterinary Supply), both femurs extracted, and bone marrow collected in Dulbecco’s phosphate buffered saline. Bone marrow solution (0.3 mL/recipient) was transplanted into the recipients via conscious tail vein injection. Animals were allowed to recover for 2 weeks before being implanted with telemeters as in the high-salt challenge protocol. To assess the role of female sex hormones, ovariectomy was performed at 6 weeks of age and the female rats allowed to recover before receiving radiotelemeter implantation and high-salt challenge. All experimental animal procedures were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
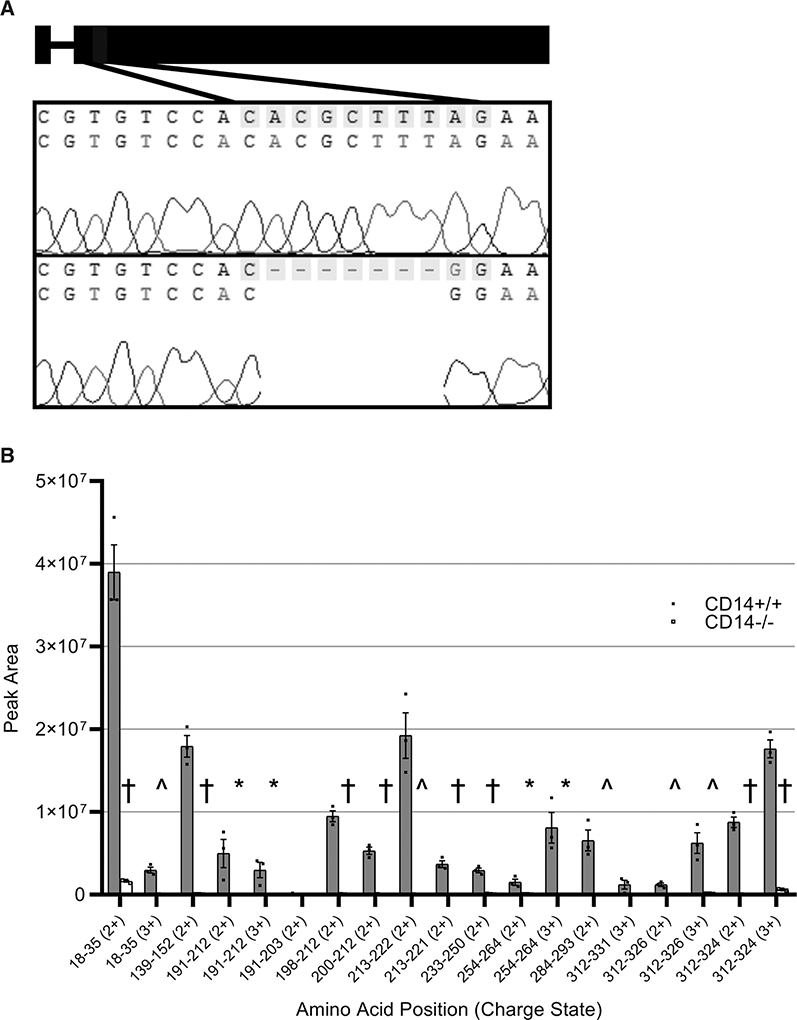
Figure 2. Sequencing and protein validation of CD14 knockout.
Schematic for the CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat–associated 9) target site and the resultant 8 base pair deletion and 1 base pair insertion, which causes transcription of a predicted premature stop codon in the CD14 (cluster of differentiation 14) gene on the Dahl SS (SS/JrHSDMcwi) background (A). Parallel reaction monitoring was used to measure the presence of peptide sequences from the CD14 protein in isolated peritoneal macrophages (B). n=3/3. Charge state (2+, 3+) is indicated for each peptide monitored. †P<0.001, ^P<0.01, *P<0.05.
Surgical Procedures
Blood Pressure Monitoring
At 7 weeks of age, rats underwent a carotid telemeter implantation surgery as described previously.4,34 Briefly, the rats were deeply anesthetized under isoflurane gas inhalation. Using aseptic technique, the carotid artery was exposed, and a telemetry catheter was inserted into the artery. Telemetry units (HDS10; Data Sciences International, St. Paul, MN) were placed under the skin at the nape of the neck. Analgesics (0.3 mg/kg buprenorphine SR) and antibiotics (25 mg/kg cefazolin) were administered postsurgically.
Ovariectomy
For some experiments, female CD14+/+ and CD14−/− rats received an ovariectomy in the sixth week of age. To do this, animals were deeply anesthetized under isoflurane gas inhalation, bilateral flank incisions made, the ovaries located, and a ligature tied tightly around the superior-most end of each horn of the uterus. The ovaries were excised, uterus horns returned to the peritoneum, muscle layer sutured, and the skin closed with absorbable suture. Analgesics (0.3 mg/kg buprenorphine SR) and antibiotics (25 mg/kg cefazolin) were administered postsurgically.
Urinalysis
For experiments in SS parental animals, overnight urine collections were performed while on the low-salt diet or at the end of the high-salt period, whereas in experiments on CD14 wild-type and knockout animals, collections were performed on days-1, 7, 14, and 21 of the experimental protocol. Urine creatinine values were measured by an autoanalyzer (ACE; Alfa Wasserman, Fairfield, NJ) with an assay based on the Jaffé reaction. Urine albumin was quantified with a fluorescent assay that utilized Albumin Blue 580 dye (Sigma-Aldrich, St. Louis, MO) and a fluorescent plate reader (FL-600; BioTek, Winooski, VT). Urinary protein was quantified utilizing Weichselbaum biuret reagent and an autoanalyzer (Alfa Wasserman). Urine CD14 (Lifespan Bioscience), KIM-1 (kidney injury molecule-1; R&D Systems), and nephrin (Ethos Biosciences) were measured by ELISA according to the manufacturers’ instructions.
Immune Cell Isolation From the Kidney
Immune cells in the kidney were isolated as described previously.34,35 Briefly, the left kidney was minced and incubated in RPMI 1640 media containing L-glutamine, HEPES, collagenase type IV, and DNase. The solution was then passed through a series of 100, 70, and 40-μm filters. Mononuclear cells were separated by Percoll density gradient centrifugation (400g×30 minutes at room temperature) and washed with a wash buffer (2% HI-FBS, 5 mmol/L EDTA DPBS). Cells from the kidney were then pelleted and resuspended in the wash buffer solution, and the concentration of cells was determined by counting on a hemocytometer.
Flow Cytometry
General characterization of immune cell types was performed as described previously.34,35 Mononuclear cells were incubated with Fc receptor blocking CD32 for 5 minutes followed by an incubation for 30 minutes in a solution containing antibodies for the following extracellular markers: anti-CD3 (eBioscience) for T cells, anti-CD4 (BioLegend) for helper T cells, anti-CD8 (BioLegend) for cytotoxic T cells, anti-CD45R (BD Bioscience) for B cells, and anti-CD11b/c (eBioscience) for monocytes and macrophages. All cells were then analyzed by flow cytometry (LSRII Becton Dickinson) with FACSDIVA software (Becton Dickinson) and FlowJo Software (TreeStar). The gating strategy can be found in Figure S2 in the Data Supplement.
Mass Spectrometry Validation of CD14 Knockout on the Dahl SS Genetic Background
Peritoneal macrophages were isolated from CD14+/+ and CD14−/− animals and flash frozen. Cells were lysed and proteins digested with trypsin. The resulting peptide mixture was analyzed by data-dependent acquisition to inform identifiable peptides corresponding to CD14. Subsequently, peptide sequences identified as matching wild-type or mutant CD14 sequences were then assayed by parallel reaction monitoring for targeted peptide detection and relative abundance determination. A detailed description of these methods is provided in the Data Supplement.36–38
Statistical Analysis
One-way ANOVA with a Holm-Sidak post hoc, 2-way ANOVA, 2-way Repeated Measures-ANOVA, or Student t test was used where appropriate. Data are expressed as means±SE. A value of P<0.05 was considered statistically significant. SigmaPlot 12.5 software (Systat Software, Inc) or Prism 8 software (GraphPad) was used for all statistical analyses.
RESULTS
Urinary and Renal CD14 Protein Is Increased in Response to a High-Salt Diet in Parental Dahl SS Rats
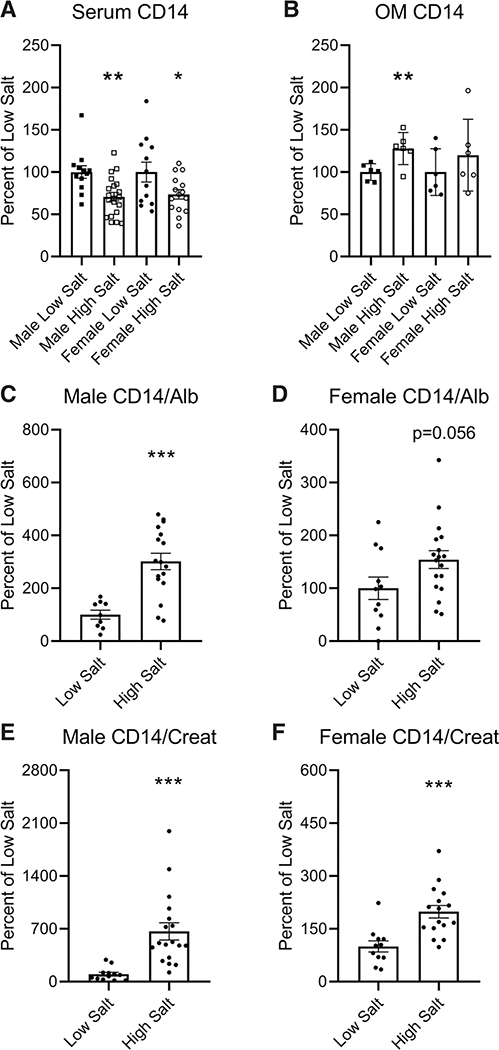
Parental Dahl SS males and females on a high-salt diet demonstrated lower serum CD14 compared with those maintained on a low-salt diet (Figure 1A; P<0.01 in males, P<0.05 in females). Males also exhibited elevated renal outer medullary CD14 when on the high-salt diet (Figure 1B; P<0.01). Urinary CD14 was increased in male rats fed high salt relative to urinary albumin (Figure 1C; P<0.001) and creatinine (Figure 1E; P<0.001)—a trend consistent in females (Figure 1D and 1F).
Figure 1. Levels of serum and urinary CD14 in male and female Dahl SS rats on either a low or high-salt diet.
Serum (A), kidney outer medulla (OM) homogenate (B), and urine (C–F) were collected from male and female, parental SS (SS/JrHSDMcwi) rats maintained on either a low-salt or high-salt diet for 3 wk and CD14 (cluster of differentiation 14) protein measured by ELISA. Alb indicates Albumin; and Creat, Creatinine. n=6 to 20. *P<0.05, **P<0.01, ***P<0.001 vs low salt.
CRISPR/Cas9 Knockout of CD14 on the Dahl SS Genetic Background Validated by Mass Spectrometry
CRISPR/Cas9 was used to target CD14 early in the second exon, which caused an indel resulting from an 8-base pair deletion and 1-base pair insertion (Figure 2A). This frameshift deletion at bases 248 to 254 results in a predicted premature stop codon. Targeted analysis of peptide sequences by parallel reaction monitoring demonstrated that genetic knockout of CD14 eliminated detectable CD14 protein as seen by loss of almost all CD14 tryptic peptides (Figure 2B).
Knockout of CD14 in Female but Not Male Animals of the Dahl SS Background Confers Exacerbated Salt-Sensitive Renal Damage and Hypertension
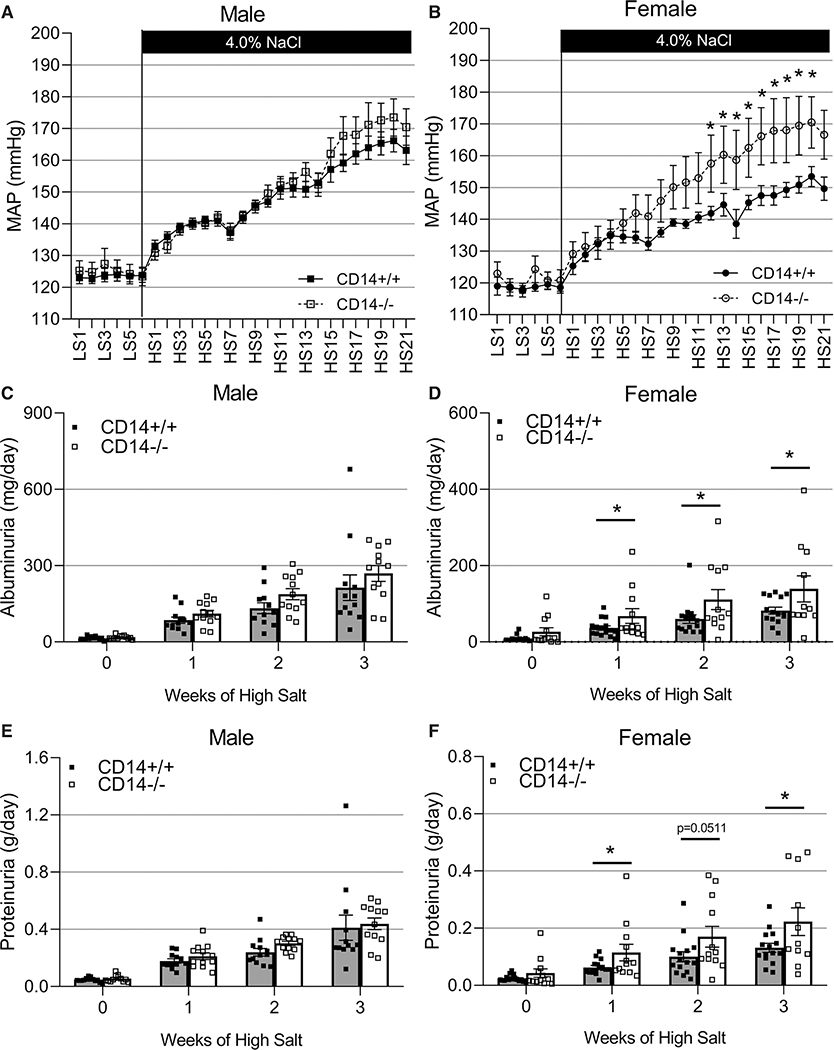
CD14 knockout did not significantly affect the salt-sensitive increase in blood pressure (Figure 3A; P>0.05) nor the amount of renal damage as assessed by proteinuria and albuminuria (Figure 3C and 3E; P>0.05) in male animals. In contrast, CD14−/− females demonstrated an augmentation of the salt-induced blood pressure response (Figure 3B; P<0.05) and an exacerbation in renal damage assessed by albuminuria and proteinuria (Figure 3D and 3F; P<0.05) compared with CD14+/+ littermates. At the end of the 21-day high-salt challenge, urinary nephrin excretion was increased in CD14−/− females (P<0.01) but not CD14−/− male animals (Figure S1A and S1B) indicating damage to the glomerulus. Excretion of KIM-1 was not statistically different between the CD14+/+ and CD14−/− rats of either sex (Figure S1C and S1D).
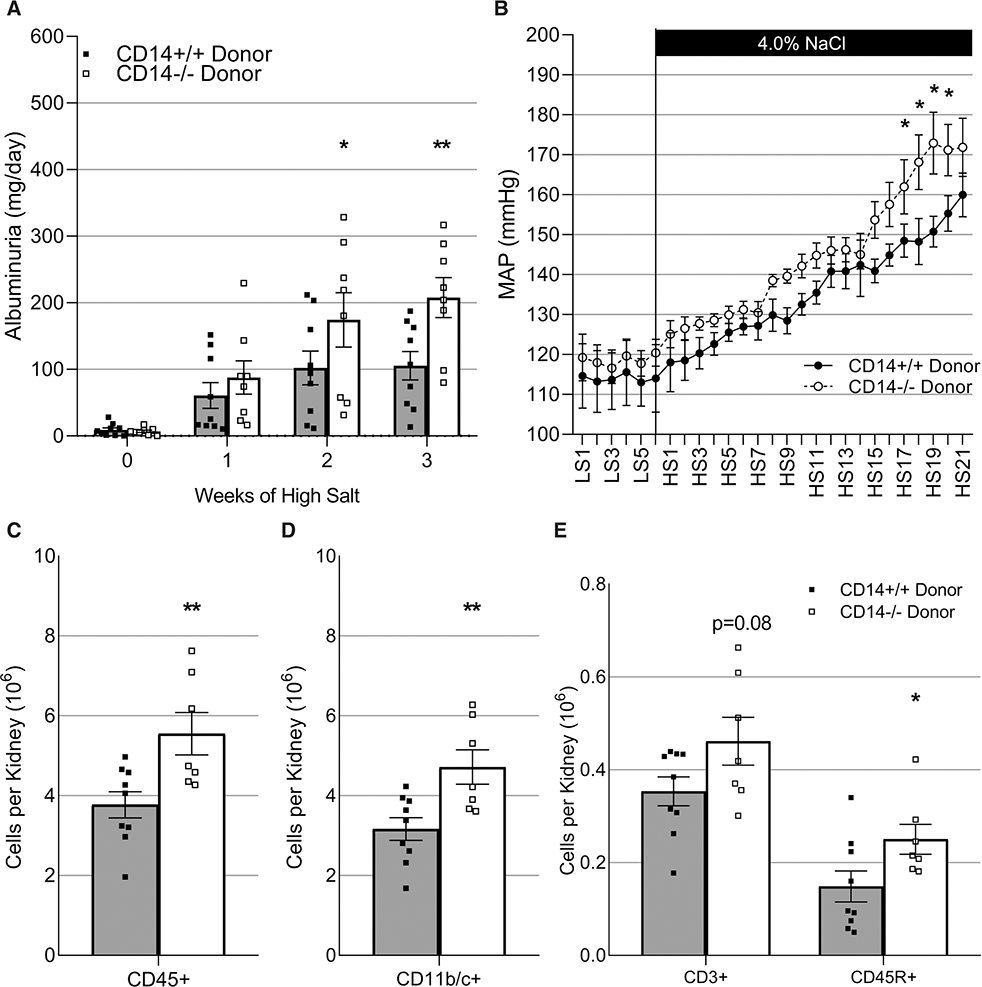
Figure 3. Effect of CD14 knockout on salt-sensitive hypertension in male and female Dahl SS rats.
Radiotelemetry was used to monitor 6 d of low-salt baseline and 21 d of high-salt diet mean arterial blood pressure (MAP) recordings in male (n=11/12, A) and female (n=7/11, B) CD14+/+ and CD14−/− Dahl SS (SS/JrHSDMcwi) rats. Weekly overnight urine collections were used to measure albuminuria (C and D) and proteinuria (E and F; male, n=12/12; female, n=12/15; 2-way Repeated Measures-ANOVA; *P<0.05). CD14 indicates cluster of differentiation 14; HS, high salt; and LS, low salt.
Increased Macrophages in Female CD14−/− Dahl SS Rats
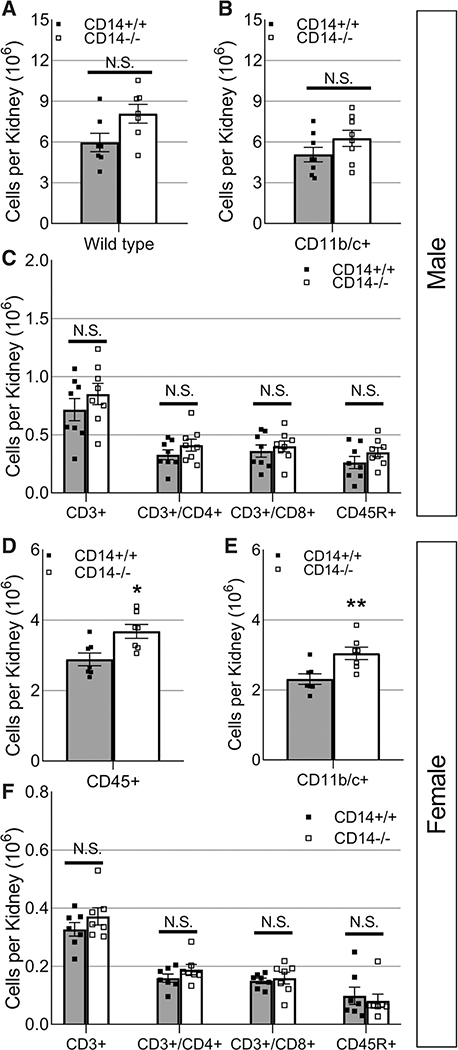
After the high-salt challenge, the number of infiltrating immune cells in the kidney was evaluated by flow cytometry. CD14+/+ and CD14−/− male animals demonstrated a similar number of infiltrating renal immune cells (Figure 4A through 4C; P>0.05). In contrast, knockout of CD14 in females resulted in an elevation in infiltrating macrophages (Figure 4E; P<0.01) independent of changes to the number of infiltrating lymphocytes (Figure 4F; P>0.05).
Figure 4. Effect of CD14 knockout on the number of infiltrating renal immune cells in male and female Dahl SS rats in response to a high-salt diet.
Infiltrating immune cells in the kidneys of male (n=8/8, A–C) and female (n=7/7, D–F) CD14+/+ and CD14−/− Dahl SS (SS/JrHSDMcwi) rats including CD45+ leukocytes (A and D), CD11b/c+ macrophages (C and E), CD3+ T cells, CD3+/CD4+ helper T cells, CD3+/CD8+ cytotoxic T cells, and CD45R+ B cells (C and F). CD14 indicates cluster of differentiation 14; and NS, not significant. *P<0.05, **P<0.01 vs CD14+/+.
CD14 Knockout in Hematopoietic Cells of Female Dahl SS Rats Exacerbates Salt-Sensitive Renal Damage and Hypertension
As CD14 is a gene expressed across various tissues,39 our next goal was to determine whether loss of CD14 specifically in circulating immune cells was responsible for the augmented salt-sensitive response observed in the CD14−/− female rats. CD14+/+ female recipients received total body irradiation to eliminate their host immune system followed by bone marrow transfer from either CD14+/+ or CD14−/− donor females. The resultant chimeras were CD14+/+ in all solid tissue but either CD14+/+ or CD14−/− in hematopoietic cells. Consistent with what was observed in the germline-whole body knockout, those deficient in CD14 only in hematopoietic cells demonstrated exacerbated renal damage (Figure 5A; P<0.05), augmented blood pressure response (Figure 5B; P<0.05), and renal macrophage infiltration (Figure 5D; P<0.01). An additional elevation in infiltrating B cells was also observed (Figure 5E; P<0.05). Representative flow cytometry plots of these renal infiltrating immune cell populations can be found in Figure S2.
Figure 5. Bone marrow from either CD14+/+ or CD14−/− Dahl SS (SS/JrHSDMcwi) donors was transferred into total body irradiated CD14+/+ recipients who were then subjected to a high-salt challenge.
Albuminuria (A). Mean arterial pressure (MAP; B). Infiltrating CD45+ leukocytes (C), CD11b/c+ macrophages (D), CD3+ T cells, and CD45R+ B cells (E). n=6 to 9. CD14 indicates cluster of differentiation 14; HS, high salt; and LS, low salt. *P<0.05, **P<0.01 vs CD14+/+ donor.
Exacerbation of Salt-Sensitive Phenotypes in CD14−/− Female Dahl SS Rats Is Female Sex Hormone Dependent
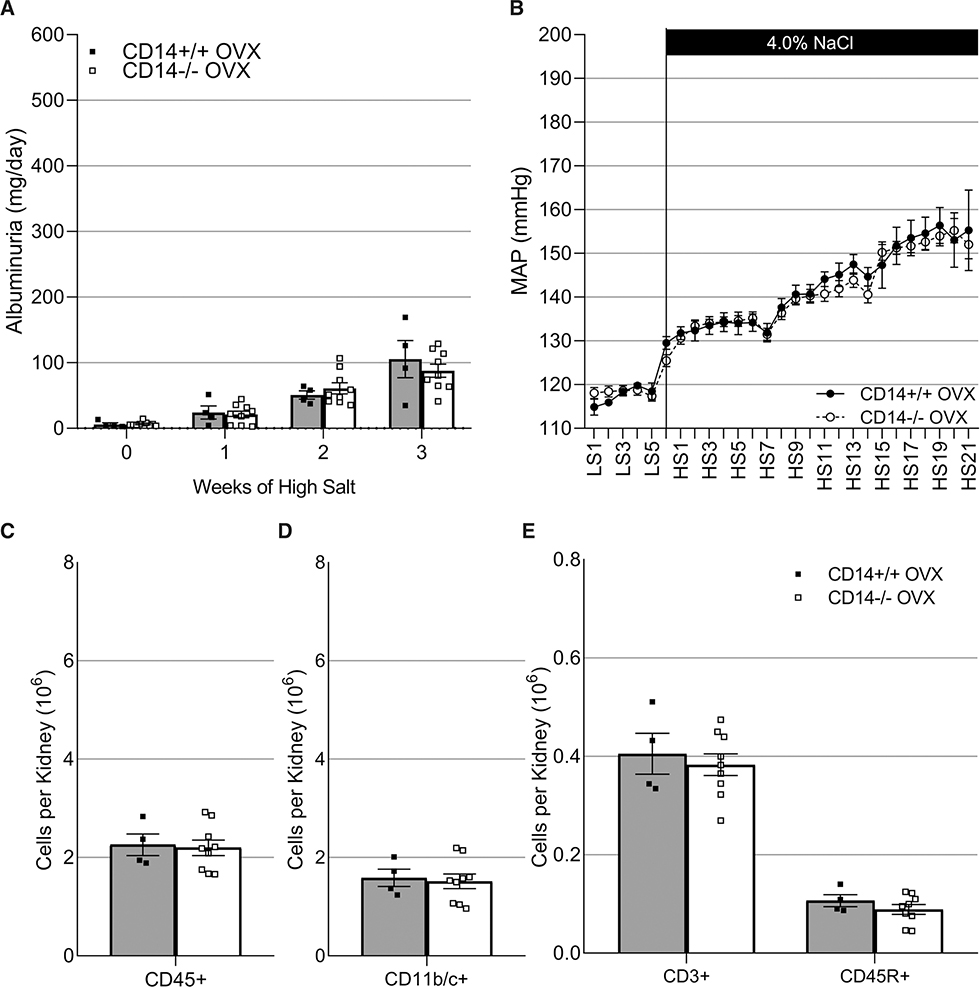
Due to the sexual dimorphic effect of knocking out CD14, we assessed whether female sex hormones contribute to this dichotomy. Ovariectomy was performed on CD14+/+ and CD14−/− females who were then subjected to a high-salt challenge. Unlike what was observed in Figures 3 through 5, CD14+/+ and CD14−/− OVX females no longer demonstrated differential renal damage (Figure 6A; P>0.05), elevation in blood pressure (Figure 6B; P>0.05), or immune cell infiltration (Figure 6C through 6E; P>0.05).
Figure 6. Ovariectomy (OVX) was performed on CD14+/+ and CD14−/− Dahl SS (SS/JrHSDMcwi) rats who were then subjected to a high-salt challenge.
Albuminuria (A). Mean arterial pressure (MAP; B). Infiltrating CD45+ leukocytes (C), CD11b/c+ macrophages (D), CD3+ T cells, and CD45R+ B cells (E). n=4/8. No significant differences were identified. CD14 indicates cluster of differentiation 14.
DISCUSSION
Due to an established relationship between CD14 and hypertension in human populations, we aimed to understand its role in a model of salt-sensitive hypertension, the Dahl SS rat. An initial experiment demonstrated that Dahl SS rats on a high-salt diet have depressed levels of soluble CD14 in the serum compared with those on a low-salt diet. This was accompanied by a reciprocal effect on the urinary levels of CD14, where a high-salt diet in SS rats raised urinary CD14. The increase in renal CD14 is consistent with our previous reports28,29 and is similar to the reported increased urinary soluble CD14 protein observed in patients with rheumatoid arthritis, another condition of sterile inflammation.40,41 In these studies, a highly sensitive mass spectrometry technique was used to confirm a robust and complete knockout of the CD14 protein by CRISPR/Cas9. This approach provided antibody-independent evidence that truncated protein products were not being produced—a frequent concern that arises in genetic knockout studies.
This work demonstrated that genetic deletion of CD14 on the Dahl SS background exacerbates salt-sensitive phenotypes including renal damage, hypertension, and macrophage infiltration, establishing a regulatory role for CD14-dependent signaling. It is notable that this exacerbation was only observed in female animals, which would implicate that the regulatory role of CD14 may be estrogen dependent. This conclusion is consistent with the fact that ovariectomy eliminated the differential response to the salt challenge observed in CD14−/− females. These studies have also shown that CD14-dependent signaling specifically in hematopoietic cells plays this regulatory role. It is interesting to note that mean arterial pressure in female CD14−/− rats reached similar levels to that of male rats even though the absolute magnitude of urinary renal damage markers and renal immune cell infiltration was approximately half of what was observed in males. As female rats are more often smaller than males, we were not surprised to observe a smaller magnitude in albumin excretion even when blood pressures reached similar levels, consistent with previous reports of Dahl SS rats.42 Likewise, female kidneys are considerably smaller than male kidneys, which is reflected in a smaller absolute number of infiltrating immune cells in females. Nonetheless, the effect of CD14 knockout in females is clear and eliminates, to some degree, the protection normally observed.
Though not assessed in these studies, CD14−/− mice show enhanced neutrophil recruitment to sites of infection, which provides a beneficial clearance of bacteria.43 It is possible that, in our model, CD14−/− female rats may show increased renal neutrophil recruitment, which may cause sterile inflammation to persist. Additional literature has shown that CD14 plays a regulatory role in host immunity. CD14−/− macrophages show enhanced TNFα (tumor necrosis factor alpha) production in response to Borrelia burgdorferi and importantly, a failure to upregulate negative regulators such as SOCS genes through the p38/MAPK (mitogen-activated protein kinases) pathway.44 Interestingly, SOCS3 is one of those negative regulators that is upregulated in the renal outer medulla28 and renal T cells45 when Dahl SS animals are on a high-salt diet. Work by these authors has also shown that CD14-null animals show persistent inflammation in Lyme disease46 and postulate that CD14-independent signaling is more “destructive” than CD14-dependent signaling.
One of the more striking observations in these studies was that CD14−/− females showed an exacerbated salt-sensitive response, whereas knockout in male animals had no effect, indicating that female sex hormones may be involved. This was then confirmed by performing ovariectomy before the high-salt challenge, which eliminated the differential response to salt between CD14+/+ and CD14−/− females. A possible explanation for these results is that female sex hormones augment inflammatory mechanisms and CD14-dependent signaling regulates this enhancement, where these 2 factors play opposing roles in regulating inflammatory activation. This contradicts the well-established protective role of estrogens observed in the Dahl SS rat.47
Estradiol (E2) has been shown to upregulate surface expression of the CD14 protein.48 Treating primary microglia (a type of macrophage) with E2 enhances the proinflammatory response to lipopolysaccharide in ovariectomized female rats.49 E2 also promotes M1 proinflammatory activation upregulating iNOS (inducible NO synthase) and IL-1β and prevents M2 activation by downregulating IL-10 and arginase. This increase in inflammation seen with E2 treatment may be due to increased cadherin-11–dependent signaling,50 another gene upregulated in the renal outer medulla of Dahl rats on a high-salt diet.28 Two important studies by Calippe et al51,52 demonstrated that the inflammatory response to E2 was via estrogen receptor alpha, which increased NFκB (nuclear factor kappa B) p65 transcriptional activity, downregulated the inhibitory PI3K/AKT pathway, and increased the production of IL-1β, IL-6, and TNFα. Notably, these effects were observed with chronic E2 treatment but not with acute treatment. Though these results are from animal models, peritoneal macrophages isolated from human patients showed the exact same effects of enhanced IL-6 and TNFα production with E2 treatment. This was even more pronounced if macrophages came from patients with endometriosis—a state where there is chronic inflammation.53
Though female sex is associated with protection from cardiovascular disease, females are more likely to be diagnosed with an autoimmune disease. Various investigators have documented that polymorphisms in the human CD14 gene are associated with increased incidence of autoimmune diseases such as autoimmune thyroid disease,54 Parkinson disease,55 celiac disease,56–58 and systemic lupus erythematosus (SLE).59 Interestingly, multiple reports have shown there is no such relationship between CD14 polymorphisms and rheumatoid arthritis.59–63 Women with SLE have reduced monocyte surface expression of CD14 compared with healthy controls, and expression is also reduced when comparing females with SLE to males with SLE.64 This is consistent with the results of the present studies where loss of CD14 enhanced the disease process. As reviewed by Moulton,65 estrogen signaling, particularly through ERα, appears to be proinflammatory and contributes to the inflammatory activity of SLE, again consistent with the present results. Though there is a pronounced increased incidence of hypertension in women with SLE,66 it is unclear how or whether CD14 contributes to this relationship. The results of the present studies may be informative for understanding the relationship between biological sex and prevalence of autoimmune diseases. The mechanistic connection between CD14 and female sex hormones is not yet clear, and we do not yet know whether the effects of these signaling pathways on inflammatory balance are through shared or parallel pathways. Importantly, these studies reinforce the relationship between increased arterial blood pressure, renal immune cell infiltration, and renal injury. Consistent with previous work,67 increased renal perfusion pressure drives immune cells to infiltrate into the kidney interstitium, which we speculate is in response to increasing pressure-induced tissue damage. These infiltrating immune cells then contribute to the accumulating tissue damage and renal dysfunction, further potentiating the hypertensive phenotype.
PERSPECTIVES
CD14—a gene associated with cardiovascular disease in human populations—is also upregulated in Dahl SS rats when on a high-salt diet. We created a genetic knockout of CD14 on the Dahl SS background to demonstrate a novel regulatory role for CD14-dependant signaling in hematopoietic cells. In addition, we show that this is female specific, and the exacerbation of disease seen in CD14−/− animals is eliminated by ovariectomy. This sexual dimorphism in innate immune system activation was previously unappreciated and provides a new understanding of the difference in pathogenesis between males and females, as well as a targetable pathway.
Supplementary Material
Novelty and Significance.
What Is New?
Salt-sensitive Dahl SS rats exhibit increased renal CD14 (cluster of differentiation 14) when on a high-salt diet.
Global knockout of CD14 results in a female-specific exacerbation of salt-sensitive phenotypes including salt-induced elevation in blood pressure, renal damage, and renal macrophage infiltration.
Ovariectomy abrogates the effect of CD14 knockout implicating female sex hormones in potentiating innate immune system activation in this model.
What Is Relevant?
Mutations in CD14 have been associated with hypertension in genome-wide association and gene expression studies in human populations. These results point to a sexually dimorphic mechanism by which the immune system affects salt-sensitive phenotypes.
Summary
In this study, we demonstrate that CD14—a protein involved innate immune system activation—interacts with female sex hormone signaling to modulate the inflammatory activity contributing to salt-sensitive hypertension and renal damage in Dahl SS rats.
Acknowledgments
D.J. Fehrenbach, D.L. Mattson, R.L. Gundry, M.R. Dwinell, M. Cherian-Shaw, and A.M. Geurts conceived the experiments. D.J. Fehrenbach, J.M. Abais-Battad, J.H. Dasinger, J. Zemaj, T. Keppel, and H. Lund performed the experiments. D.J. Fehrenbach analyzed the data and prepared the figures. D.J. Fehrenbach drafted the manuscript. All authors contributed to the editing process and approved the final draft of the manuscript.
Sources of Funding
This work was supported by R24HL114474, HL-116264, HL-137748, 18PRE3400038, 1F31HL144084-01, 18POST33990140, and the Georgia Research Alliance.
Nonstandard Abbreviations and Acronyms
- CD14
cluster of differentiation 14
- CRISPR/Cas9
clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat-associated 9
- E2
estradiol
- IL
interleukin
- iNOS
inducible NO synthase
- KIM-1
kidney injury molecule-1
- NFκB
nuclear factor kappa B
- SLE
systemic lupus erythematosus
- SS
SS/JrHSDMcwi
- TLR
Toll-like receptor
- TNFα
tumor necrosis factor alpha
Footnotes
Disclosures
None.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.120.14928.
Contributor Information
Daniel J. Fehrenbach, Department of Physiology, Medical College of Wisconsin, Wauwatosa, WI; Medical College of Wisconsin, Wauwatosa, WI. Department of Physiology, Augusta University and the Medical College of Georgia, Augusta, GA.
Justine M. Abais-Battad, Medical College of Wisconsin, Wauwatosa, WI. Department of Physiology, Augusta University and the Medical College of Georgia, Augusta, GA
John Henry Dasinger, Medical College of Wisconsin, Wauwatosa, WI. Department of Physiology, Augusta University and the Medical College of Georgia, Augusta, GA.
Hayley Lund, Department of Physiology, Medical College of Wisconsin, Wauwatosa, WI.
Theodore Keppel, Center for Biomedical Mass Spectrometry Research, Medical College of Wisconsin, Wauwatosa, WI.
Jeylan Zemaj, Department of Physiology, Medical College of Wisconsin, Wauwatosa, WI.
Mary Cherian-Shaw, Medical College of Wisconsin, Wauwatosa, WI. Department of Physiology, Augusta University and the Medical College of Georgia, Augusta, GA.
Rebekah L. Gundry, Center for Biomedical Mass Spectrometry Research, Medical College of Wisconsin, Wauwatosa, WI; CardiOmics Program, Center for Heart and Vascular Research, University of Nebraska Medical Center, Omaha, NE; Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE; Division of Cardiovascular Medicine, University of Nebraska Medical Center, Omaha, NE.
Aron M. Geurts, Department of Physiology, Medical College of Wisconsin, Wauwatosa, WI; Genomic Sciences and Precision Medicine Center, Medical College of Wisconsin, Wauwatosa, WI.
Melinda R. Dwinell, Department of Physiology, Medical College of Wisconsin, Wauwatosa, WI Genomic Sciences and Precision Medicine Center, Medical College of Wisconsin, Wauwatosa, WI.
David L. Mattson, Medical College of Wisconsin, Wauwatosa, WI. Department of Physiology, Augusta University and the Medical College of Georgia, Augusta, GA
REFERENCES
- 1.Kessler T, Vilne B, Schunkert H. The impact of genome-wide association studies on the pathophysiology and therapy of cardiovascular disease. EMBO Mol Med. 2016;8:688–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of sh2b3 (LNK), a genome-wide association study candidate for hypertension, attenuates dahl salt-sensitive hypertension via inflammatory modulation. Hypertension. 2015;65:1111–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudemiller NP, Mattson DL. Candidate genes for hypertension: insights from the Dahl S rat. Am J Physiol Renal Physiol. 2015;309:F993–F995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL; PhysGen Knockout Program. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. doi: 10.1161/HYPERTENSIONAHA.113.02191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubacek JA, Rothe G, Pit’ha J, Skodova Z, Stanek V, Poledne R, Schmitz G. C(−260)-->T polymorphism in the promoter of the CD14 monocyte receptor gene as a risk factor for myocardial infarction. Circulation. 1999;99:3218–3220. [DOI] [PubMed] [Google Scholar]
- 6.Xu JJ, Liu KQ, Ying ZM, Zhu XW, Xu XJ, Zhao PP, Bai WY, Qiu MC, Zhang XW, Zheng HF. Effect of CD14 polymorphisms on the risk of cardiovascular disease: evidence from a meta-analysis. Lipids Health Dis. 2019;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiner AP, Lange EM, Jenny NS, Chaves PH, Ellis J, Li J, Walston J, Lange LA, Cushman M, Tracy RP. Soluble CD14: genomewide association analysis and relationship to cardiovascular risk and mortality in older adults. Arterioscler Thromb Vasc Biol. 2013;33:158–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzello V, Liuzzo G, Trabetti E, Di Giannuario G, Brugaletta S, Santamaria M, Piro M, Boccanelli A, Pignatti PF, Biasucci LM, et al. Role of the CD14 C(−260)T promoter polymorphism in determining the first clinical manifestation of coronary artery disease. J Cardiovasc Med (Hagerstown). 2010;11:20–25. [DOI] [PubMed] [Google Scholar]
- 9.Hashmat S, Rudemiller N, Lund H, Abais-Battad JM, Van Why S, Mattson DL. Interleukin-6 inhibition attenuates hypertension and associated renal damage in Dahl salt-sensitive rats. Am J Physiol Renal Physiol. 2016;311:F555–F561. doi: 10.1152/ajprenal.00594.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 11.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311 [DOI] [PubMed] [Google Scholar]
- 12.Fenton MJ, Golenbock DT. LPS-binding proteins and receptors. J Leukoc Biol. 1998;64:25–32. doi: 10.1002/jlb.64.1.25 [DOI] [PubMed] [Google Scholar]
- 13.Roncon-Albuquerque R Jr, Moreira-Rodrigues M, Faria B, Ferreira AP, Cerqueira C, Lourenço AP, Pestana M, von Hafe P, Leite-Moreira AF. Attenuation of the cardiovascular and metabolic complications of obesity in CD14 knockout mice. Life Sci. 2008;83:502–510. doi: 10.1016/j.lfs.2008.07.021 [DOI] [PubMed] [Google Scholar]
- 14.Cai C, Shi X, Korff S, Zhang J, Loughran PA, Ruan X, Zhang Y, Liu L, Billiar TR. CD14 contributes to warm hepatic ischemia-reperfusion injury in mice. Shock. 2013;40:115–121. doi: 10.1097/SHK.0b013e318299d1a7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correa-Costa M, Azevedo H, Amano MT, Gonçalves GM, Hyane MI, Cenedeze MA, Renesto PG, Pacheco-Silva A, Moreira-Filho CA, Câmara NO. Transcriptome analysis of renal ischemia/reperfusion injury and its modulation by ischemic pre-conditioning or hemin treatment. PLoS One. 2012;7:e49569. doi: 10.1371/journal.pone.0049569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrissey J, Guo G, McCracken R, Tolley T, Klahr S. Induction of CD14 in tubular epithelial cells during kidney disease. J Am Soc Nephrol. 2000;11:1681–1690. [DOI] [PubMed] [Google Scholar]
- 17.Poesen R, Ramezani A, Claes K, Augustijns P, Kuypers D, Barrows IR, Muralidharan J, Evenepoel P, Meijers B, Raj DS. Associations of soluble CD14 and endotoxin with mortality, cardiovascular disease, and progression of kidney disease among patients with CKD. Clin J Am Soc Nephrol. 2015;10:1525–1533. doi: 10.2215/CJN.03100315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manner IW, Baekken M, Kvale D, Oektedalen O, Pedersen M, Nielsen SD, Nowak P, Os I, Trøseid M. Markers of microbial translocation predict hypertension in HIV-infected individuals. HIV Med. 2013;14:354–361. doi: 10.1111/hiv.12015 [DOI] [PubMed] [Google Scholar]
- 19.Kheradmand M, Niimura H, Kuwabara K, Nakahata N, Nakamura A, Ogawa S, Mantjoro EM, Shimatani K, Nerome Y, Owaki T, et al. Association of inflammatory gene polymorphisms and conventional risk factors with arterial stiffness by age. J Epidemiol. 2013;23:457–465. doi: 10.2188/jea.je20130054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Sun Y, Fu Y, Yu X, Li M. The effects of both single-locus and multi-locus interaction on the clinical manifestations of IgA nephropathy in Southern Han Chinese. Nephrol Dial Transplant. 2014;29:550–555. doi: 10.1093/ndt/gft468 [DOI] [PubMed] [Google Scholar]
- 21.Yoon HJ, Shin JH, Yang SH, Chae DW, Kim H, Lee DS, Kim HL, Kim S, Lee JS, Kim YS. Association of the CD14 gene −159C polymorphism with progression of IgA nephropathy. J Med Genet. 2003;40:104–108. doi: 10.1136/jmg.40.2.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang WR, Craven TE, Malhotra R, Cheung AK, Chonchol M, Drawz P, Sarnak MJ, Parikh CR, Shlipak MG, Ix JH; SPRINT Research Group. Kidney damage biomarkers and incident chronic kidney disease during blood pressure reduction: a Case-Control Study. Ann Intern Med. 2018;169:610–618. doi: 10.7326/M18-1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(6 pt 2):II127–II134. doi: 10.1161/01.hyp.8.6_pt_2.ii127 [DOI] [PubMed] [Google Scholar]
- 24.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1 [DOI] [PubMed] [Google Scholar]
- 25.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37(2 pt 2):429–432. doi: 10.1161/01.hyp.37.2.429 [DOI] [PubMed] [Google Scholar]
- 26.De Miguel C, Das S, Lund H, Mattson DL. T lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1136–R1142. doi: 10.1152/ajpregu.00298.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29 [DOI] [PubMed] [Google Scholar]
- 28.Geurts AM, Mattson DL, Liu P, Cabacungan E, Skelton MM, Kurth TM, Yang C, Endres BT, Klotz J, Liang M, et al. Maternal diet during gestation and lactation modifies the severity of salt-induced hypertension and renal injury in Dahl salt-sensitive rats. Hypertension. 2015;65:447–455. doi: 10.1161/HYPERTENSIONAHA.114.04179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fehrenbach DJ, Abais-Battad JM, Dasinger JH, Lund H, Mattson DL. Salt-sensitive increase in macrophages in the kidneys of Dahl SS rats. Am J Physiol Renal Physiol. 2019;317:F361–F374. doi: 10.1152/ajprenal.00096.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spires D, Ilatovskaya DV, Levchenko V, North PE, Geurts AM, Palygin O, Staruschenko A. Protective role of Trpc6 knockout in the progression of diabetic kidney disease. Am J Physiol Renal Physiol. 2018;315:F1091–F1097. doi: 10.1152/ajprenal.00155.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siebers EM, Choi MJ, Tinklenberg JA, Beatka MJ, Ayres S, Meng H, Helbling DC, Takizawa A, Bennett B, Garces AM, et al. Sdha+/− rats display minimal muscle pathology without significant behavioral or biochemical abnormalities. J Neuropathol Exp Neurol. 2018;77:665–672. doi: 10.1093/jnen/nly042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, et al. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abais-Battad JM, Lund H, Dasinger JH, Fehrenbach DJ, Cowley AW Jr, Mattson DL. NOX2-derived reactive oxygen species in immune cells exacerbates salt-sensitive hypertension. Free Radic Biol Med. 2020;146:333–339. doi: 10.1016/j.freeradbiomed.2019.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Alsheikh AJ, Mattson DL. Parental dietary protein source and the role of CMKLR1 in determining the severity of Dahl salt-sensitive hypertension. Hypertension. 2019;73:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abais-Battad JM, Lund H, Fehrenbach DJ, Dasinger JH, Mattson DL. Rag1-null Dahl SS rats reveal that adaptive immune mechanisms exacerbate high protein-induced hypertension and renal injury. Am J Physiol Regul Integr Comp Physiol. 2018;315:R28–R35. doi: 10.1152/ajpregu.00201.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waas M, Bhattacharya S, Chuppa S, Wu X, Jensen DR, Omasits U, Wollscheid B, Volkman BF, Noon KR, Gundry RL. Combine and conquer: surfactants, solvents, and chaotropes for robust mass spectrometry based analyses of membrane proteins. Anal Chem. 2014;86:1551–1559. doi: 10.1021/ac403185a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waas M, Pereckas M, Jones Lipinski RA, Ashwood C, Gundry RL. SP2: rapid and automatable contaminant removal from peptide samples for proteomic analyses. J Proteome Res. 2019;18:1644–1656. doi: 10.1021/acs.jproteome.8b00916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Fuscoe JC, Zhao C, Guo C, Jia M, Qing T, Bannon DI, Lancashire L, Bao W, Du T, et al. A rat RNA-Seq transcriptomic BodyMap across 11 organs and 4 developmental stages. Nat Commun. 2014;5:3230. doi: 10.1038/ncomms4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park YJ, Yoo SA, Hwang D, Cho CS, Kim WU. Identification of novel urinary biomarkers for assessing disease activity and prognosis of rheumatoid arthritis. Exp Mol Med. 2016;48:e211. doi: 10.1038/emm.2015.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang MJ, Park YJ, You S, Yoo SA, Choi S, Kim DH, Cho CS, Yi EC, Hwang D, Kim WU. Urinary proteome profile predictive of disease activity in rheumatoid arthritis. J Proteome Res. 2014;13:5206–5217. doi: 10.1021/pr500467d [DOI] [PubMed] [Google Scholar]
- 42.Moreno C, Kaldunski ML, Wang T, Roman RJ, Greene AS, Lazar J, Jacob HJ, Cowley AW Jr. Multiple blood pressure loci on rat chromosome 13 attenuate development of hypertension in the Dahl S hypertensive rat. Physiol Genomics. 2007;31:228–235. doi: 10.1152/physiolgenomics.00280.2006 [DOI] [PubMed] [Google Scholar]
- 43.Metkar S, Kim KS, Silver J, Goyert SM. Differential expression of CD14-dependent and independent pathways for chemokine induction regulates neutrophil trafficking in infection. J Leukoc Biol. 2012;92:389–396. doi: 10.1189/jlb.0112011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sahay B, Patsey RL, Eggers CH, Salazar JC, Radolf JD, Sellati TJ. CD14 signaling restrains chronic inflammation through induction of p38-MAPK/SOCS-dependent tolerance. PLoS Pathog. 2009;5:e1000687. doi: 10.1371/journal.ppat.1000687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abais-Battad JM, Alsheikh AJ, Pan X, Fehrenbach DJ, Dasinger JH, Lund H, Roberts ML, Kriegel AJ, Cowley AW Jr, Kidambi S, et al. Dietary effects on Dahl salt-sensitive hypertension, renal damage, and the T lymphocyte transcriptome. Hypertension. 2019;74:854–863. doi: 10.1161/HYPERTENSIONAHA.119.12927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benhnia MR, Wroblewski D, Akhtar MN, Patel RA, Lavezzi W, Gangloff SC, Goyert SM, Caimano MJ, Radolf JD, Sellati TJ. Signaling through CD14 attenuates the inflammatory response to Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 2005;174:1539–1548. doi: 10.4049/jimmunol.174.3.1539 [DOI] [PubMed] [Google Scholar]
- 47.Hinojosa-Laborde C, Craig T, Zheng W, Ji H, Haywood JR, Sandberg K. Ovariectomy augments hypertension in aging female Dahl salt-sensitive rats. Hypertension. 2004;44:405–409. doi: 10.1161/01.HYP.0000142893.08655.96 [DOI] [PubMed] [Google Scholar]
- 48.Rettew JA, Huet YM, Marriott I. Estrogens augment cell surface TLR4 expression on murine macrophages and regulate sepsis susceptibility in vivo. Endocrinology. 2009;150:3877–3884. doi: 10.1210/en.2009-0098 [DOI] [PubMed] [Google Scholar]
- 49.Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, Berkelhammer D, Day HE, Maier SF, Watkins LR. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37:1688–1699. doi: 10.1016/j.psyneuen.2012.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kou XX, Li CS, He DQ, Wang XD, Hao T, Meng Z, Zhou YH, Gan YH. Estradiol promotes M1-like macrophage activation through cadherin-11 to aggravate temporomandibular joint inflammation in rats. J Immunol. 2015;194:2810–2818. doi: 10.4049/jimmunol.1303188 [DOI] [PubMed] [Google Scholar]
- 51.Calippe B, Douin-Echinard V, Laffargue M, Laurell H, Rana-Poussine V, Pipy B, Guéry JC, Bayard F, Arnal JF, Gourdy P. Chronic estradiol administration in vivo promotes the proinflammatory response of macrophages to TLR4 activation: involvement of the phosphatidylinositol 3-kinase pathway. J Immunol. 2008;180:7980–7988. doi: 10.4049/jimmunol.180.12.7980 [DOI] [PubMed] [Google Scholar]
- 52.Calippe B, Douin-Echinard V, Delpy L, Laffargue M, Lélu K, Krust A, Pipy B, Bayard F, Arnal JF, Guéry JC, et al. 17Beta-estradiol promotes TLR4-triggered proinflammatory mediator production through direct estrogen receptor alpha signaling in macrophages in vivo. J Immunol. 2010;185:1169–1176. doi: 10.4049/jimmunol.0902383 [DOI] [PubMed] [Google Scholar]
- 53.Khan KN, Kitajima M, Inoue T, Fujishita A, Nakashima M, Masuzaki H. 17β-estradiol and lipopolysaccharide additively promote pelvic inflammation and growth of endometriosis. Reprod Sci. 2015;22:585–594. doi: 10.1177/1933719114556487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jia X, Wang B, Yao Q, Li Q, Zhang J. Variations in CD14 gene are associated with autoimmune thyroid diseases in the Chinese population. Front Endocrinol (Lausanne). 2018;9:811. doi: 10.3389/fendo.2018.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lin JJ, Chen CH, Yueh KC, Chang CY, Lin SZ. A CD14 monocyte receptor polymorphism and genetic susceptibility to Parkinson’s disease for females. Parkinsonism Relat Disord. 2006;12:9–13. doi: 10.1016/j.parkreldis.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 56.Catamo E, Segat L, Lenarduzzi S, Petix V, Morgutti M, Crovella S. CD14 polymorphisms correlate with an augmented risk for celiac disease in Italian patients. Genes Immun. 2012;13:489–495. doi: 10.1038/gene.2012.23 [DOI] [PubMed] [Google Scholar]
- 57.Boniotto M, Braida L, Ventura A, Percopo S, Amoroso A, Crovella S. Promoter polymorphisms of the CD14 gene in Italian patients with coeliac disease. J Med Genet. 2003;40:e108. doi: 10.1136/jmg.40.9.e108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dezsofi A, Szebeni B, Hermann CS, Kapitány A, Veres G, Sipka S, Körner A, Madácsy L, Korponay-Szabó I, Rajczy K, et al. Frequencies of genetic polymorphisms of TLR4 and CD14 and of HLA-DQ genotypes in children with celiac disease, type 1 diabetes mellitus, or both. J Pediatr Gastroenterol Nutr. 2008;47:283–287. doi: 10.1097/MPG.0b013e31816de885 [DOI] [PubMed] [Google Scholar]
- 59.Dhaouadi T, Sfar I, Haouami Y, Abdelmoula L, Turki S, Hassine LB, Zouari R, Khedher A, Khalfallah N, Abdallah TB, et al. Polymorphisms of toll-like receptor-4 and CD14 in systemic lupus erythematosus and rheumatoid arthritis. Biomark Res. 2013;1:20. doi: 10.1186/2050-7771-1-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takeuchi F, Nakaue N, Kobayashi N, Kuwata S, Murayama T, Kawasugi K, Mori M, Matsuta K. Genetic contribution of the CD14 −159C/T dimorphism in the promoter region in Japanese RA. Clin Exp Rheumatol. 2008;26:337–339. [PubMed] [Google Scholar]
- 61.de la Fontaine L, Schwarz MJ, Riedel M, Dehning S, Douhet A, Spellmann I, Kleindienst N, Zill P, Plischke H, Gruber R, et al. Investigating disease susceptibility and the negative correlation of schizophrenia and rheumatoid arthritis focusing on MIF and CD14 gene polymorphisms. Psychiatry Res. 2006;144:39–47. [DOI] [PubMed] [Google Scholar]
- 62.de la Fontaine L, Schwarz M, Plischke H, Kleindienst N, Gruber R. Lack of association of the CD14/C-159T polymorphism with susceptibility and serological activity parameters of rheumatoid arthritis. Scand J Rheumatol. 2006;35:20–22. [DOI] [PubMed] [Google Scholar]
- 63.Mikuls TR, LeVan TD, Sayles H, Yu F, Caplan L, Cannon GW, Kerr GS, Reimold AM, Johnson DS, Thiele GM. Soluble CD14 and CD14 polymorphisms in rheumatoid arthritis. J Rheumatol. 2011;38:2509–2516. doi: 10.3899/jrheum.110378 [DOI] [PubMed] [Google Scholar]
- 64.Jiang W, Zhang L, Lang R, Li Z, Gilkeson G. Sex differences in monocyte activation in systemic lupus erythematosus (SLE). PLoS One. 2014;9:e114589. doi: 10.1371/journal.pone.0114589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moulton VR. Sex hormones in acquired immunity and autoimmune disease. Front Immunol. 2018;9:2279. doi: 10.3389/fimmu.2018.02279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shaharir SS, Mustafar R, Mohd R, Mohd Said MS, Gafor HA. Persistent hypertension in lupus nephritis and the associated risk factors. Clin Rheumatol. 2015;34:93–97. doi: 10.1007/s10067-014-2802-0 [DOI] [PubMed] [Google Scholar]
- 67.Evans LC, Petrova G, Kurth T, Yang C, Bukowy JD, Mattson DL, Cowley AW Jr. Increased perfusion pressure drives renal T-cell infiltration in the Dahl salt-sensitive rat. Hypertension. 2017;70:543–551. doi: 10.1161/HYPERTENSIONAHA.117.09208 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.