Abstract
In this study, we investigated the baseline characteristics and “trajectories” of clinical response in men and women after cardiac resynchronization therapy (CRT) implantation. Although women enjoy improved echocardiographic response after CRT compared with men, the kinetics of this response and its relation to functional performance and outcomes are less clear. We identified 592 patients who underwent CRT implantation at our center between 2004 and 2017 and were serially followed in a multidisciplinary clinic. Longitudinal linear mixed effects regression for cardiac response was specified, including interaction terms between time after CRT and sex , and Cox regression models were used to assess differences in all-cause mortality by gender after CRT. Women in our cohort were younger than men, had less frequent ischemic etiology of heart failure (24% vs 60% in men), a shorter QRS (151 vs 161 ms) and more frequent left bundle branch block (77% vs 52%) at baseline. Women had a greater improvement in left ventricular ejection fraction that was evident starting at approximately 1-month after CRT. We did not observe effect modification by gender in New York Heart Association class or 6-minute walk distance after CRT. Although women had improved mortality after CRT, after adjustment for potential confounders, gender was not associated with mortality after CRT. In conclusion, women were more likely to have CRT implantation for left bundle branch block and exhibited improved echocardiographic but not functional response within the first year after CRT. Clinical outcomes after CRT were not associated with gender in adjusted analysis.
Introduction
Nearly 50% of patients with symptomatic advanced heart failure (HF) with reduced left ventricular (LV) ejection fraction (LVEF) exhibit ventricular dyssynchrony, with improved quality of life, functional capacity, and HF prognosis after cardiac resynchronization therapy (CRT).1-4 Previous studies suggest that women may have a better clinical response to CRT, accompanied by greater echocardiographic evidence of reverse ventricular remodeling than men,5 although the exact mechanisms are not fully understood. Nonetheless, women remain markedly under-represented in patients referred for CRT6-8 and in in most clinical trials of CRT.9-11 Given the importance of functional cardiac recovery (by ejection fraction or volumes) in the pathophysiology of improvement after CRT, we evaluated changes in LVEF, 6-minute walk distance, and all-cause mortality in a multidisciplinary clinic from our center and sought to determine underlying clinical heterogeneity by gender.
Methods
We identified patients who received implantation of a CRT device and were followed for device and medication optimization in the Resynchronization and Advanced Cardiac Therapeutics (ReACT) program (a multidisciplinary clinic across advanced HF and electrophysiology) at our center between 2004 and 2017. Patients are referred to the clinic both before and after CRT implantation. The construction of our study subsample is shown in Supplementary Figure 1. We identified 608 patients with any logged data for at least 3 timepoints (baseline previous implant, first month after implant, sixth month after implant). From these 608 patients that were initially eligible to be included in our study, 12 patients underwent heart transplantation and 4 had unsuccessful/complicated CRT implantation and were excluded because they did not complete the scheduled follow-ups in this clinic. From the remaining 592 patients, 98 patients had incomplete clinical information (e.g., clinical characteristics, echocardiography before implantation, medication list, electrocardiograph before implantation), and 41 patients had an LVEF >35% at time of CRT. All remaining 453 patients met the current accepted class I or IIa indications for CRT implantation for clinical indications (class II to IV HF with LvEF ≤35% and QRS duration ≥120 ms with left bundle branch block [LBBB] or QRS ≥150 ms with non-LBBB),12,13 and 411 of these patients had echocardiographic measurements at all 3 time points. A subset of these patients (n = 378) also had functional data (6-minute walk test [6MWT]) at all 3 time points (Supplementary Figure 1).
Patients in our program are typically seen by a multidisciplinary team of physicians including a cardiac electrophysiologist, a HF physician, and device technicians for serial visits at 1, 3, and 6 months after device implantation. The clinic visit involves detailed assessment of 6-minute walk distance, transthoracic echocardiography in patients (with CRT optimization by published methods14,15 in nonresponders), and clinical assessment.16-18
We extracted demographic, clinical, echocardiographic, and electrophysiologic data from the electronic medical record where available (data abstracted by MZ, VVS, DV, MPL, MZ, EL over 2018 to 2020). Diabetes, hypertension, chronic obstructive pulmonary disease, and etiology of HF were determined by clinical diagnosis or medical history in the electronic medical record. Transthoracic echocardiography was performed before CRT implantation (within 1 month before CRT, median 12 days before implant). As part of our clinic protocol, echocardiography was also obtained at 2 other time points after CRT: approximately 1 month (median time from implant 39 days, interquartile range [IQR] 30-55) and 6 months after implantation (median time from implant 200 days, IQR 183- 239). A 6MWT was performed on a level hallway surface, blinded to the results of the echocardiogram of the corresponding visit.19,20 New York Heart Association (NYHA) class was assessed at every visit. Medications and device programming changes were performed by the HF or electrophysiology clinicians at the time of the ReACT clinic visit. Death was ascertained by review of the Service Set Identifier database and were cross-validated using the electronic medical record and on-device data registries for each of the device companies (Medtronic, Boston Scientific, and Biotronik). Patients not found on any of these databases were contacted to verify status. Time-to-death was defined as the time from implantation of the CRT device until death or date of censoring (final collection of follow-ups was on January 1, 2017).
Baseline clinical and demographic variables were compared using Wilcoxon or t test (continuous) or the Fisher’s exact test (categoric). We constructed serial linear mixed effects regressions for LVEF as a function of age, gender, diabetes, hypertension, atrial fibrillation (AF), LBBB, QRS, etiology of HF (ischemic vs nonischemic), and chronic obstructive pulmonary disease. Sex and etiology of HF were introduced as multiplicative linear interaction terms with time point after CRT (modeled as baseline, 1 month, and 6 month). A per-patient random effect was included. For the regression models, continuous variables were neither standardized nor mean-centered as they were originally normally distributed. We constructed similar models for 6-minute walk distance (not available before CRT) and NYHA class. Finally, we performed Cox models for all-cause mortality, for which age and LVEF where mean-centered and standardized. R version 4.0.0 (2020/4/24) and STATA (Stata/IC 16.1,TX: StataCorp LP) were used for analysis, and a 2-sided p <0.05 was used as a measure of statistical significance.
Results
The baseline characteristics of our analytic sample are shown in Table 1. In general, our population was predominantly male, with class II to III HF and LV systolic dysfunction (average LVEF 25.45%). The patients had an even distribution of ischemic and nonischemic etiology of HF. Medications included in guideline-directed medical therapy for LV systolic dysfunction were prescribed to most of the patients. Many co-morbidities were less prevalent in women: in particular, women were slightly younger than men (65 vs 69, p <0.001), had lower QRS values at baseline (151 vs 161 ms, p <0.001), less AF (27% vs 45%, p <0.001), and ischemic etiology of HF (24% vs 60%, p <0.001). Interestingly, women referred for CRT were more likely to have lBbB (70.1% vs 54.3%, p <0.001). The preimplant degree of cardiac dysfunction and functional limitation was similar between women and men, as was medical therapy (except for a greater prevalence of aldosterone antagonists use in women). Our data suggested that men were more likely to have CRT implantations for a non-LBBB indication than women (48.6% vs 34.4%).
Table 1.
Patient demographics, clinical, echocardiographic and functional characteristics.
| Total study population | Total | Men Number (%) or mean (SD) |
Women | Missing (n) (Total/M/W) |
P |
|---|---|---|---|---|---|
| N (total/M/W) | 592 | 417 | 175 | ||
| Age (years) | 67.75 (12.56) | 60.080 (12.16) | 64.60 (12.97) | 0/0/0 | 0.001 |
| Clinical characteristics | |||||
|
Hypertension (588/413/175) |
393(67%) | 288 (69.7%) | 105 (60%) | 4/4/0 | 0.02 |
|
Smoking (586/412/174) |
398 (50.9%) | 228 (55.3%) | 70 (40.2%) | 6/5/1 | 0.01 |
|
Diabetes (587/412/175) |
194 (33%) | 133 (32.3%) | 60 (34.3%) | 5/5/0 | 0.70 |
|
Atrial fibrillation (569/396/173) |
224 (39.4%) | 178 (44.9%) | 46 (26.6%) | 23/21/2 | <0.001 |
|
Ischemic etiology (582/408/174) |
289(49.7%) | 247 (60.5%) | 42 (24.1%) | 10/9/1 | <0.001 |
| Medications | |||||
|
Loop diuretic (570/399/171) |
422 (74%) | 303 (75.9%) | 119 (69.6%) | 22/18/4 | 0.139 |
|
ACE-I (575/402/173) |
322 (56%) | 224 (55.7%) | 98 (56.6%) | 17/15/2 | 0.910 |
|
ARBs (568/396/172) |
123 (21.7%) | 81 (20.5%) | 42 (24.4%) | 24/21/3 | 0.346 |
|
Beta-blocker (574/401/173) |
591 (85.5%) | 339 (84.5%) | 152 (87.9%) | 18/16/2 | 0.363 |
|
Aldosterone antagonist (574/401/173) |
183 (31.9%) | 111 (27.7%) | 72 (41.6%) | 18/16/2 | 0.001 |
| Echocardiographic and electrocardiographic findings | |||||
|
Baseline LVEF % (mean, SD) (494/352/142) |
25.45 (8.74) | 25.49 (8.53) | 25.32 (9.28) | 98/65/3 | 0.845 |
|
LVEF at 1st month % (mean, SD) (457/330/127) |
30.64 (10.9) | 30.031 (10.75) | 32.28 (11.16) | 135/87/48 | 0.045 |
|
LVEF at 6th month % (mean, SD) (445/323/122) |
32.26 (11.15) | 31.57 (10.92) | 34.07 (11.60) | 147/94/53 | 0.035 |
|
QRS (msec) (mean, SD) (549/376/173) |
158 (27) | 161 (28.28) | 151 (22.24) | 43/41/2 | 0.001 |
|
QRS at baseline >150ms (549/376/168) |
343 (62.5) | 248 (66) | 95 (54.9) | 43/41/2 | 0.02 |
|
LBBB (538/370/168) |
305 (56.7%) | 193 (52.2%) | 112 (66.7%) | 54/47/7 | 0.002 |
|
6-Min walk at 1st month
ft (mean, SD) (378/276/102) |
1032 (386) | 1063 (378) | 947 (397) | 214/141/73 | 0.01 |
|
6-Min walk at 6th month
ft (mean, SD) (386/286/100) |
1104 (415) | 1121(411) | 1056 (422) | 206/131/75 | 0.177 |
|
NYHA score
(mean, SD) (575/408/167) |
2,76 (0.58) | 2.79 (0.59) | 2.64(0.54) | 17/9/8 | 0.271 |
The data available for the number of total subjects, as well as the number of men (M) or women (W) in the data set are provided in the first column under each variable name. Correspondingly, the number of subjects with missing data are noted in the penultimate column.
Abbreviations: M: men; W: women; ACE-I: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; LVEF: left ventricular ejection fraction; LBBB: left bundle branch block; NYHA: New York Heart Association.
Patients in the ReACT clinic historically have had the option of echocardiogram-based optimization of device programming at the discretion of the attending electrophysiology physician (including the option not to change any programming), although this practice has evolved over time on the basis of newer clinical findings.21 Nonetheless, 68% of the women and 67.8% of men had testing and optimization (if needed) under echocardiographic guidance at their first visit after implantation (p = 0.974, by chi-square). At their 6-month visit, 65.6% of women and 62.2% of men had echocardiographic-guided optimization (p = 0.485). Our data did not reveal any significant differences in the treatment of men and women in the ReACT clinic after CRT implantation.
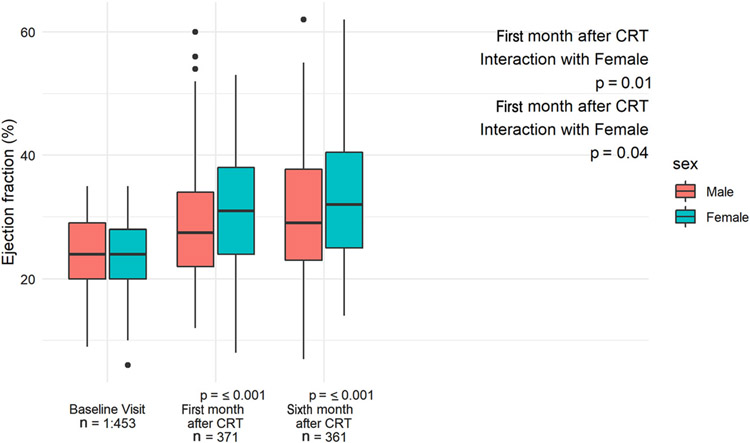
On aggregate (including both genders), LVEF was similar by gender at baseline but demonstrated divergence by gender at 1-month after CRT (Figure 1). Women exhibited a mean absolute change of +7.5 (95% confidence interval [CI] 5.9 to 8.7) (from mean 25.3% to 32.3%) in LVEF during the first month and a further increase of 1.8 (95% CI 1.2 to 2.4) (from mean 32.3% to 34.1%) between 1 and 6 months. Men exhibited a mean absolute change of +4.6 (95% CI 3.0 to 5.0) (from mean 25.5% to 30.1%) during the first month and mean absolute change of 1.5 (95% CI 1.3 to 1.7) (from mean 30.1% to 31.6%) between 1 and 6 months (Figure 1). In the linear mixed effects regressions adjusted for the confounders that were different between men and women that were previously associated with CRT response, notably ischemic etiology of HF, LBBB, and the presence of AF,10,22 we found a statistically significant interaction between time after CRT and gender (Table 2), suggesting a greater increase in LVEF for women both early at the 1-month time point that was sustained at the 6-month time point.
Figure 1.
Echocardiographic response to CRT in men and women at 1 and 6 months after implantation. Comparison of LVEF at baseline before implantation and at 1- and 6-month timepoints after implantation in male and female patients. Interaction of female gender with post–CRT LV remodeling was analyzed using adjusted mixed effect model (Table 2).
Table 2.
Mixed effect adjusted model for echocardiographic ventricular remodeling after CRT assessing the effect of sex on CRT response (n = 411 for patients with ECHOs at all time-points).
| Estimate | SE | P-value | |
|---|---|---|---|
| Change of LVEF at 1st month | 4.65 | 1 | <0.0001 |
| Change of LVEF at 6th month | 6.49 | 1.04 | <0.0001 |
| Sex | −0.049 | 0.71 | 0.94 |
| Etiology of heart failure | −0.21 | 0.69 | 0.72 |
| Age | 0.05 | 0.02 | 0.01 |
| Diabetes | −0.59 | 0.51 | 0.25 |
| Hypertension | 0.33 | 0.47 | 0.51 |
| Smoking | −0.05 | 0.46 | 0.97 |
| Fibrillation | −0.43 | 0.49 | 0.38 |
| QRS at baseline | 0.01 | 0.008 | 0.24 |
| QRS at baseline 3150ms | −0.45 | 0.85 | 0.59 |
| Baseline LVEF | 0.85 | 0.04 | <0.0001 |
| LBBB | 0.03 | 0.65 | 0.95 |
| Female Sex # 1st month (interaction) | 2.69 | 0.94 | 0.001 |
| Female Sex # 6th month (interaction) | 1.97 | 0.95 | 0.03 |
| Ischemic etiology # 1st month (interaction) | −2.58 | 0.90 | 0.001 |
| Ischemic etiology # 6th month (interaction) | −4.45 | 0.90 | <0.0001 |
| LBBB # 1st month (interaction) | 0.11 | 0.88 | 0.9 |
| LBBB # 6th month (interaction) | 2.97 | 0.89 | <0.001 |
| QRS> 150ms 1st month (interaction) | 1.70 | 0.86 | 0.04 |
| QRS> 150ms 6th month (interaction) | 1.16 | 0.87 | 0.18 |
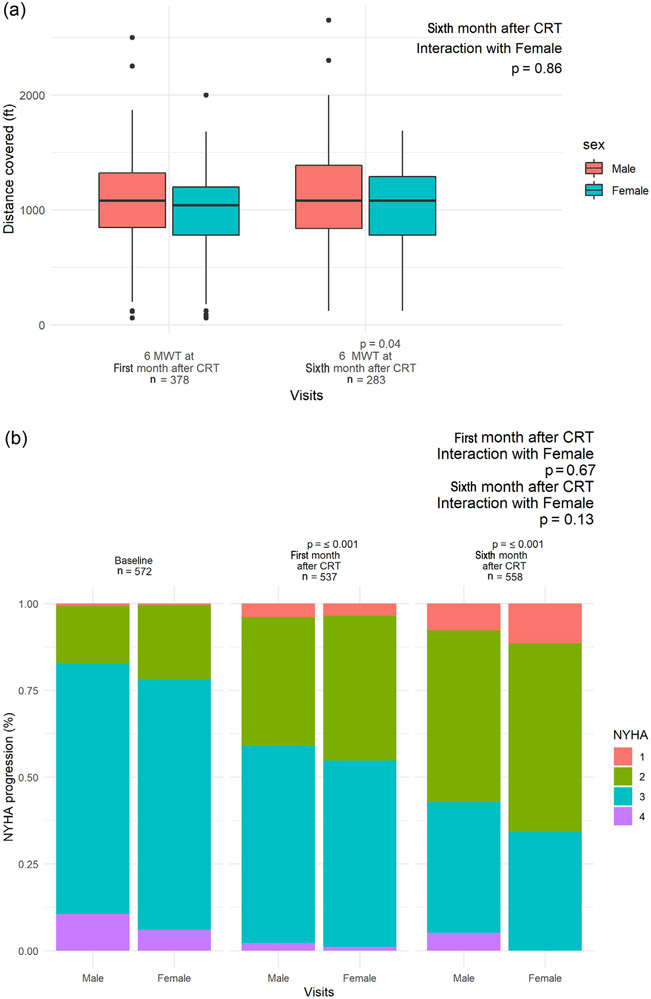
We next sought to assess functional and clinical response to CRT by assessment of 6-minute walk distance and NYHA class. For 6MWT, we were restricted to observations made after CRT implantation, as baseline 6MWT before implantation was not indicated for clinical care and hence, not obtained for most patients. The distribution of 6-minute walk distance (stratified by gender) is shown in Figure 2. Between 1 and 6 months, we observed a mean improvement of 50 ft during 6MWT in the whole cohort (paired t test p = 0.01) (from 1,030 to 1,080, SD 386). Although crude analysis suggested that women may have greater improvements between 1 and 6 months (men: 1,063 to 1,104 ft, p = 0.1; women: 947 to 1,027 ft, p = 0.03) these differences were not supported in adjusted mixed effects models (Supplementary Table 1), with age and diabetes being statistically significant negative predictors of functional response after CRT. We observed similar results for NYHA class (Figure 2), with modest changes in NYHA class noted across the entire sample (baseline 2.76 ± 0.58; first month 2.37 ± 0.68; sixth month 2.11 ± 0.71), which was not associated with gender (Supplementary Table 2).
Figure 2.
Functional and clinical response to CRT in men and women at 1- and 6 month after implantation. (A) Functional status as measured by 6MWT at 1 and 6 months after implantation. (B) NYHA class at baseline, 1 and 6 months.
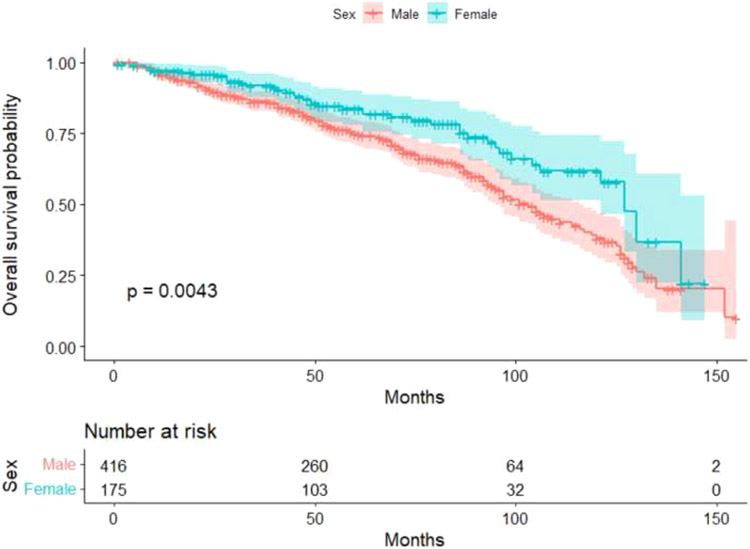
We observed 592 patients, of whom 204 died at a median follow-up time of 62 months (IQR 35 to 89 months). Unadjusted survival analysis demonstrated that female gender is associated with better outcomes after CRT (Figure 3). However, in an adjusted Cox model, gender was not associated with survival (Table 3); although, expectedly, ischemic etiology of HF was the only variable significantly association with death, relative risk 2.19 (95% CI 1.44 to 3.32, p <0.001)
Figure 3.
Mortality after CRT implantation in men and women. Kaplan–Meier curve is shown by gender (n = 592).
Table 3.
Cox proportional Hazard survival analysis
| RR | CI 95% | P-Value | |
|---|---|---|---|
| Log LVEF at baseline | 0.94 | 0.78-1.28 | 0.51 |
| Age | 1.14 | 0.93-1.4 | 0.18 |
| Male sex | 1.04 | 0.69-1.58 | 0.83 |
| Ischemic etiology | 2.19 | 1.44-3.32 | <0.001 |
| Diabetes | 1.39 | 0.98-1.95 | 0.06 |
| Hypertension | 0.85 | 0.60-1.21 | 0.38 |
| Atrial fibrillation | 1.12 | 0.79-1.58 | 0.5 |
| QRS >150ms | 1.15 | 0.82-1.63 | 0.40 |
| LBBB | 0.88 | 0.61-1.25 | 0.48 |
Discussion
In this study, we demonstrate key differences in clinical, demographic, and echocardiographic features between men and women referred for CRT. First, women referred for CRT have a shorter QRS duration, less ischemic etiology of HF, and more frequently have a LBBB. Importantly, men were more likely to obtain CRT implants without LBBB and with AF (both class II indications) than women. Although the LVEF at baseline was similar by gender, women experienced a greater, early, and sustained benefit in LVEF after CRT than men; although differences in walk distance, functional class, and all-cause mortality were not different by gender. These findings were robust to adjustment by etiology of HF and other important confounders of CRT response different by gender. Collectively, these findings highlight the benefits of CRT on cardiac function in women and the similar benefits on functional status and survival across gender, suggesting the importance of inclusion of women in CRT studies and clinical deployment of this important strategy in advanced HF care.
Although women continue to be under-represented in CRT populations and clinical trials,6,10,23,24 women may derive equivalent (or even enhanced) benefits in echocardiographic response to CRT. In a study of 752 patients referred for CRT, Hsu et al25 reported a nearly twofold higher odds of “super-response” in women (defined as an increase in echocardiographic LVEF ≥14.5% between preimplant and 12 months after CRT), with a subsequently lower mortality. Similar sex-based heterogeneity in LV structure and function have been seen in CRT: in a study of 550 patients referred for CRT, Levya et al26 reported a nearly 48% reduced hazard of cardiovascular death in women (independent of known confounders) and a 62% rate of reverse LV remodeling (defined by 15% reduction in LV end-systolic volume; compared with 44% in men). Some of these responses to CRT may be driven by the balance of disease co-morbidity in men compared with women: in a large, hospital-based sample of >300,000 patients who underwent CRT implant across the United States between 2006 and 2012, women referred for CRT were more likely to harbor predictors of CRT response (nonischemic etiology of HF, LBBB, no renal disease, or AF),26 similar to the findings from our cohort. Importantly, even after adjusting for these confounders, women continued to demonstrate an improved echocardiographic response to CRT in our study.
Our present results are not only consistent with these findings but also extend the time frame for CRT response to early post-CRT (e.g., within 1 month), suggesting that benefits that have been linked to long-term survival may accrue early and in a sustained fashion, particularly in women. It is likely that the higher prevalence of these same favorable clinical characteristics that portend improved LV reverse remodeling after CRT in women may also translate to improved mortality as shown in Figure 3. When adjusted for these known predictors of favorable CRT response, we noted a lack of functional or outcome differences by gender in our sample. Previous studies have demonstrated that women may enjoy improved HF-free survival after CRT in some cases more than men as noted in the Multicenter InSync Randomized Clinical Evaluation (MIRACLE27), Multicenter Automatic Defibrillator Implantation With Cardiac Resynchronization Therapy (MADIT-CRT28), and REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction(REVERSE29) studies and have attributed this to the increased presence of nonischemic cardiomyopathy in women referred for CRT. Strikingly, the benefits of CRT to women extend to class II indications, where referrals may be even less common: in a meta-analyses of major CRT randomized studies (MADIT-CRT, RAFT, and REVERSE), Zusterzeel et al30 reported a >75% reduction in the risk of death with CRT-D implant in women with milder degrees of HF and LBBB with a QRS duration between 130 and 149 ms. These results are especially striking, given the mean QRS duration for women included in our study was near 150 ms (Table 1), with a higher proportion of patients with LBBB and class I indication for CRT. Collectively, these findings call for broader application of CRT therapies regardless of gender, with specific attention to biologic differences by gender (e.g., shorter QRS duration)31 that may modify clinical referral patterns and current indications for CRT.
The abstraction of data was performed retrospectively and relied on clinical diagnoses, leading to potential ascertainment bias. As the registry covered patients over 13 years, we must also recognize that both the medical treatment of HF (with more recent addition of Entresto and sodium-glucose cotransporter inhibitors to regimens) and advances in device therapies may affect the interpretation of this study. Of note, the use of algorithms, including adaptive pacing and measurement of ‘effective’ resynchronization therapy, the use of quadripolar leads to achieve better anatomic positions for LV pacing, and the use of multipoint pacing particularly for nonresponders may apply nonuniformly to the population and may act as confounders. Although our study was not powered to assess how these changes may have affected men and women separately, this would be a fruitful analysis in the future. Finally, the absence of a survival difference in the adjusted model may reflect the duration of follow-up in the entire cohort (median 62 months, IQR 54-71) or a greater representation of ischemic etiology in men referred for CRT; nevertheless, previous work in this space with longer follow-up has shown gender-based heterogeneity in outcome.
In conclusion, women referred for CRT implantation and follow-up at our institution are more likely to have class I indication for CRT. Women exhibit increases in LVEF as early as 1 month after CRT, to a greater extent than men, and with sustained benefit to 6 months. These results demonstrate the temporal dynamics of echocardiographic reverse remodeling in women and extend present data, suggesting equivalent (or improved) benefits for women after CRT. Our study suggests that women benefit from widespread adaptation of CRT to forestall HF progression. Our work in a real-world cohort suggests that CRT may be an underutilized therapy in women even though women demonstrate more pronounced reverse remodeling that is initiated early after CRT and appears to be sustained. The mechanism for favorable structural remodeling and clinical outcome in women may in part be explained by the makeup of the referral population (more likely to be nonischemic with LBBB); however, even when adjusted for these confounders, women appear to favorably remodel more rapidly and in a more sustained manner than men. Although our study along with the analysis of other clinical trials demonstrate a favorable response to CRT in women with HF with dyssynchrony, the inclusion of women in both clinical trials and in a real-world cohort remains suboptimal. Further studies on the outcomes of CRT in women with class II indications with longer-term follow-up and mechanistic investigations to understand the gender differences in CRT response are warranted.
Supplementary Material
Footnotes
Disclosures
Dr. Das is a founding member and holds equity for LQT Therapeutics and Switch Therapeutics and receives research grant from Abbott. Dr. Singh has consulted for Abbott, Boston Scientific, Biotronik, CVRx, Cardiologs, Medtronic, Microport, EBR, Impulse Dynamics, Nopras Inc, National Century Health, Sanofi Inc., and Toray Inc. Dr. Heist has consulted for Boston Scientific, Pfizer, Medtronic, and Abbott; has equity in Oracle Health and receives research grant from Biotronik. The remaining authors have no conflicts of interest to declare.
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.amjcard.2022.03.021.
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J, Study Group MIRACLE. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002;346:1845–1853. [DOI] [PubMed] [Google Scholar]
- 2.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004;350(21):2140–2150. [DOI] [PubMed] [Google Scholar]
- 3.Auricchio A, Stellbrink C, Sack S, Block M, Vogt J, Bakker P, Huth C, Schöndube F, Wolfhard U, Böcker D, Krahnefeld O, Kirkels H. Pacing Therapies in Congestive Heart Failure (PATH-CHF) Study Group. Long-term clinical effect of hemodynamically optimized cardiac resynchronization therapy in patients with heart failure and ventricular conduction delay. J Am Coll Cardiol 2002;39:2026–2033. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappen-berger L, Tavazzi L. Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005;352:1539–1549. [DOI] [PubMed] [Google Scholar]
- 5.Mooyaart EAQ, Marsan NA, van Bommel RJ, Thijssen J, Borleffs CJ, Delgado V, van der Wall EE, Schalij MJ, Bax JJ. Comparison of long-term survival of men versus women with heart failure treated with cardiac resynchronization therapy. Am J Cardiol 2011;108:63–68. [DOI] [PubMed] [Google Scholar]
- 6.Adams KF Jr, Dunlap SH, Sueta CA, Clarke SW, Patterson JH, Blauwet MB, Jensen LR, Tomasko L, Koch G. Relation between gender, etiology and survival in patients with symptomatic heart failure. J Am Coll Cardiol 1996;28:1781–1788. [DOI] [PubMed] [Google Scholar]
- 7.Frazier CG, Alexander KP, Newby LK, Anderson S, Iverson E, Packer M, Cohn J, Goldstein S, Douglas PS. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction: a pooled analysis of 5 randomized control trials. J Am Coll Cardiol 2007;49:1450–1458. [DOI] [PubMed] [Google Scholar]
- 8.Regitz-Zagrosek V Therapeutic implications of the gender-specific aspects of cardiovascular disease. Nat Rev Drug Discov 2006;5:425–438. [DOI] [PubMed] [Google Scholar]
- 9.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, MADIT-CRT, Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–1338. [DOI] [PubMed] [Google Scholar]
- 10.Goldenberg I, Moss AJ, Hall WJ, Foster E, Goldberger JJ, Santucci P, Shinn T, Solomon S, Steinberg JS, Wilber D, Barsheshet A, McNitt S, Zareba W, Klein H, Committee MADIT-CRT Executive. Predictors of response to cardiac resynchronization therapy in the multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy (MADIT-CRT). Circulation 2011;124:1527–1536. [DOI] [PubMed] [Google Scholar]
- 11.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117:2608–2616. [DOI] [PubMed] [Google Scholar]
- 12.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 13.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA 3rd, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Tracy CM, Epstein AE, Darbar D, DiMarco JP, Dunbar SB, Estes NA 3rd, Ferguson TB Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD. American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61:e6–75. [DOI] [PubMed] [Google Scholar]
- 14.Mihos CG, Santana O, Yucel E, Capoulade R, Upadhyay GA, Orencole MP, Singh JP, Picard MH. The effects of cardiac resynchronization therapy on left ventricular and mitral valve geometry and secondary mitral regurgitation in patients with left bundle branch block. Echocardiography 2019;36:1450–1458. [DOI] [PubMed] [Google Scholar]
- 15.Martin DO, Lemke B, Birnie D, Krum H, Lee KL, Aonuma K, Gasparini M, Starling RC, Milasinovic G, Rogers T, Sambelashvili A, Gorcsan J 3rd, Houmsse M. Adaptive CRT Study Investigators. Investigation of a novel algorithm for synchronized left-ventricular pacing and ambulatory optimization of cardiac resynchronization therapy: results of the adaptive CRT trial. Heart Rhythm 2012;9:1807–1814. [DOI] [PubMed] [Google Scholar]
- 16.Kandala J, Altman RK, Park MY, Singh JP. Clinical, laboratory, and pacing predictors of CRT response. J Cardiovasc Transl Res 2012;5:196–212. [DOI] [PubMed] [Google Scholar]
- 17.Altman RK, Parks KA, Schlett CL, Orencole M, Park MY, Truong QA, Deeprasertkul P, Moore SA, Barrett CD, Lewis GD, Das S, Upadhyay GA, Heist EK, Picard MH, Singh JP. Multidisciplinary care of patients receiving cardiac resynchronization therapy is associated with improved clinical outcomes. Eur Heart J 2012;33:2181–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh JP, Ruskin JN. Cardiac resynchronization therapy: the MGH experience. Ann Noninvasive Electrocardiol 2005;10(suppl):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, Berman LB. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 20.Guyatt GH, Thompson PJ, Berman LB, Sullivan MJ, Townsend M, Jones NL, Pugsley SO. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis 1985;38:517–524. [DOI] [PubMed] [Google Scholar]
- 21.Ellenbogen KA, Gold MR, Meyer TE, Fernndez Lozano I, Mittal S, Waggoner AD, Lemke B, Singh JP, Spinale FG, Van Eyk JE, Whitehill J, Weiner S, Bedi M, Rapkin J, Stein KM. Primary results from the SmartDelay determined AV optimization: a comparison to other AV delay methods used in cardiac resynchronization therapy (SMART-AV) trial: a randomized trial comparing empirical, echocardiography-guided, and algorithmic atrioventricular delay programming in cardiac resynchronization therapy. Circulation 2010;122:2660–2668. [DOI] [PubMed] [Google Scholar]
- 22.Zusterzeel R, Curtis JP, Caños DA, Sanders WE, Selzman KA, Pina IL, Spatz ES, Bao H, Ponirakis A, Varosy PD, Masoudi FA, Strauss DG. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT: results from the NCDR. J Am Coll Cardiol 2014;64:887–894. [DOI] [PubMed] [Google Scholar]
- 23.Costanzo MR. Cardiac resynchronization therapy in women. Card Electrophysiol Clin 2015;7:721–734. [DOI] [PubMed] [Google Scholar]
- 24.Al-Khatib SM, Hellkamp AS, Hernandez AF, Fonarow GC, Thomas KL, Al-Khalidi HR, Heidenreich PA, Hammill S, Yancy C, Peterson ED. Get With the Guidelines Steering Committee and Hospitals. Trends in use of implantable cardioverter-defibrillator therapy among patients hospitalized for heart failure: have the previously observed sex and racial disparities changed over time? Circulation 2012;125:1094–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, Moss AJ, Foster E, Committee MADIT-CRT Executive. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol 2012;59:2366–2373. [DOI] [PubMed] [Google Scholar]
- 26.Chatterjee NA, Borgquist R, Chang Y, Lewey J, Jackson VA, Singh JP, Metlay JP, Lindvall C. Increasing sex differences in the use of cardiac resynchronization therapy with or without implantable cardioverter-defibrillator [published correction appears in Eur Heart J 2018;39:1077] Eur Heart J 2017;38:1485–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo GW, Petersen-Stejskal S, Johnson JW, Conti JB, Aranda JA Jr, Curtis AB. Ventricular reverse remodeling and 6-month outcomes in patients receiving cardiac resynchronization therapy: analysis of the MIRACLE study. J Interv Card Electrophysiol 2005;12:107–113. [DOI] [PubMed] [Google Scholar]
- 28.Biton Y, Zareba W, Goldenberg I, Klein H, McNitt S, Polonsky B, Moss AJ, Kutyifa V, MADIT-CRT Executive Committee. Sex differences in long-term outcomes with cardiac resynchronization therapy in mild heart failure patients with left bundle branch block. J Am Heart Assoc 2015;4:e002013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gold MR, Daubert JC, Abraham WT, Hassager C, Dinerman JL, Hudnall JH, Cerkvenik J, Linde C. Implantable defibrillators improve survival in patients with mildly symptomatic heart failure receiving cardiac resynchronization therapy: analysis of the long-term follow-up of remodeling in systolic left ventricular dysfunction (REVERSE). Circ Arrhythm Electrophysiol 2013;6:1163–1168. [DOI] [PubMed] [Google Scholar]
- 30.Zusterzeel R, Selzman KA, Sanders WE, Caños DA, O'Callaghan KM, Carpenter JL, Piña IL, Strauss DG. Cardiac resynchronization therapy in women: US Food and Drug Administration meta-analysis of patient-level data. JAMA Intern Med 2014;174:1340–1348. [DOI] [PubMed] [Google Scholar]
- 31.Narasimha D, Curtis AB. Sex differences in utilisation and response to implantable device therapy. Arrhythm Electrophysiol Rev 2015;4:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.