Abstract
Differences in cage microenvironments may contribute to variation in data and affect the outcome of animal studies involving metabolic diseases. To study this, we compared the effects 3 types of bedding—corncob bedding, hardwood bedding, and hardwood bedding plus a cardboard enrichment item—on baseline fasting and nonfasting blood glucose and body weight in mice. Mice housed on corncob bedding showed significantly higher fasting blood glucose than did mice housed on hardwood bedding, with or without the enrichment item. None of the groups showed an effect of bedding type on nonfasting blood glucose levels or body weight. This information informs the choice of bedding substrates for studies that measure fasting blood glucose and potentially mitigates a variable that could confound research outcomes.
Abbreviations: FBG, fasting blood glucose; NFBG, nonfasting blood glucose
Introduction
The use of mice for studying metabolic diseases, including diabetes and obesity, has prompted standardization of methodologies for key metabolic techniques.3 Cage microenvironments, including bedding substrates, are potential variables that could affect experimental treatments and outcomes.6 Research facilities use many types of bedding, including hardwood, paper, and corncob. An ideal bedding substrate is highly absorptive, inedible, tactilely desirable and comfortable for resting, nontraumatic, nontoxic, safe for personnel, free of dust and splinters, cost effective and offering opportunities for nesting, digging and insulation from temperature fluctuations.6,7,13,23 In addition, cage cleaning elicits numerous physiologic and behavioral responses that may indicate aversive responses in mice.21,22 Therefore, by minimizing detectable ammonia and extending the interval between cage changes, bedding with high absorptive capacity could reduce operating costs, improve animal welfare, and reduce workers’ exposure to allergens and pathogens.21,22 The choice of bedding type may be influenced by the purpose of the study in which the mice will be used.26
One substrate that we assessed in the current study was corncob bedding. The raw stock for corncob bedding is 100% corncob, and the 1/8-in. product is produced from the woody-ring portion of the cob. During production, whole corncobs are ground, dried, sifted, and aspirated to make very uniform particles that are essentially dust free.8 Corncob bedding is more absorbent than many other products, providing a drier microenvironment and reducing the amount of ammonia,7,19 thereby potentially extending the interval between cage changes. Other advantages of corncob include its ability to reduce the spread of allergens.25 In one study, corncob supported the lowest unfavorable microbial load among the substrates evaluated.4 Another study found that commercially available rodent beddings, especially corncob and mixes of corncob and paper, had high bacterial counts including coliforms and pathogenic agents, leading to the recommendation that they undergo autoclaving or irradiation prior to their use in strict barrier facilities.31 In addition, mice may ingest corncob bedding, which contains digestible material that reduces the efficiency of feed conversion in mice fed a high-fat diet.1 Rats housed in solid-bottom cages ate so much corncob bedding that the material was seen in centrifuged feces, leading to concerns that the bedding might interfere with nutritional studies, particularly those investigating the health benefits of dietary fiber.14 The potential biologic effects of corncob bedding include its high levels of estrogenic compounds,28 which may induce endocrine disruption and thus cause variability in breast and prostatic cancer studies16 or alter rodent behavior.28
The other bedding used in the current study is made from 100% virgin Great Lakes aspen hardwood. Aspen hardwood bedding has a low rate of contamination from tars and resin, does not significantly affect liver enzymes, and has not been linked to any observed physiologic or behavioral confounding effects.10 However, wood-based beddings generally display poor fluid absorption and ammonia control,27 thereby potentially limiting the interval between cage changes as compared with other bedding substrates, like corncob.
Fasting blood glucose (FBG) levels are commonly measured for metabolic and nutritional studies. Fasting levels may be more consistent because food is removed for a designated amount of time for all mice involved. Because mice primarily eat at night, overnight fasting depletes liver glycogen stores and leads to a catabolic state in mice. Overnight fasting can result in an approximately 15% loss of lean body mass.3 After a 4- to 6-h fast, overtly healthy nondiabetic mice typically have an FBG of 80 to 100 mg/dL.9 Data in the Mouse Phenome Database include the serum glucose levels obtained from 41 strains of mice fed a standard diet; blood samples were collected from 7- to 9-wk-old male and female mice after a 4-h fast. The overall mean blood glucose level after this brief fast was 179 ± 31 mg/dL17 but varied with age, sex, and strain. Females of a given strain tended to have lower levels than males, with LP/J mice showing the lowest values (female, 125 ± 22 mg/dL; male, 146 ± 19 mg/dL) and C57B1/10J mice showing the highest values (female, 230 ± 25 mg/dL; male, 263 ± 57 mg/dL). In mice, serum glucose levels decrease between the 3rd and 12th month of age; in C57BL/6 and BALB/c strains, the glucose level rises again after 24 months.15,20 We measured both fasting and nonfasting blood glucose (NFBG) levels in the current study.
Body weight is a useful, nonspecific indicator of mouse health.24 In one study, the presence of environmental enrichment had no significant effect on the body weight of mice, but strain-associated differences remained until 11 wk of age.29
Just as bedding substrates could alter the effects of treatment outcomes, so too could environmental enrichment. However, scientific evidence is scarce regarding whether these items pose a variable that affects research results. One approach to environmental enrichment is to provide animals with manipulanda that promote species-typical behaviors.5 Environmental enrichment may improve the wellbeing of research rodents2 but should not negatively influence their health or safety or alter the outcome of the study.5
The current study focused on assessing the effects of 2 bedding substrates on indicators of metabolism; it also included an additional treatment group in which mice on hardwood bedding received an autoclaved cardboard toilet paper roll as environmental enrichment.11 Researchers engaged in studies of metabolism at our institution raised concerns about the possibility that mice might ingest some of the glue while chewing on these rolls. Information provided by the vendor indicated that the adhesive for the cardboard toilet paper roll contains food-grade ingredients including gelatin, glycerin, water, Epsom salt, and corn sugar. We therefore sought to determine whether this form of enrichment altered the indicators of metabolism that were assessed in the current study.
Materials and Methods
Animals and housing.
C57BL/6NCrl female mice (n = 30; age, 42 d) were purchased from a commercial vendor (Charles River, Wilmington, MA). The mice were housed in the University of Hawaii Animal and Veterinary Services’ vivarium. The program is AAALAC-accredited and adheres to the standards set forth in the Guide for the Care and Use of Laboratory Animals.12 The University of Hawaii’s IACUC approved the experimental procedures described (protocol no. 18-2876). Mice were SPF for viruses including EDIM, MHV, MPV, MVM, LCMV, and TMEV and for pinworms and fur mites. Mice were housed in IVC (model 1285, Tecniplast USA, West Chester, PA) set at 75 air changes per hour. IVC motors were tested every 4 mo. The temperature set point for the room was 70 to 74 °F (21° to 23 °C) and relative humidity ranged from 30% to 70%. Mice were housed in 14:10-h light:dark cycle with lights on at 0600 and 27 ft. candles measured at 1200. Mice were fed irradiated chow (Teklad 2919, Envigo, Indianapolis, IN) and given municipal water without restriction from water bottles with stainless-steel sipper tubes. Cages and accessories were sanitized at 180 °F (82 °C). Cages were changed weekly in a biosafety cabinet. Bedding substrates included autoclaved 1/8-in. corncob (Teklad 7092, Envigo) and pelleted aspen hardwood bedding (Teklad 7086G, Envigo). Some mice on pelleted aspen hardwood bedding received environmental enrichment in the form of autoclaved cardboard toilet paper rolls (Proctor and Gamble, Cincinnati, OH) that contained food-grade adhesives (gelatin, glycerin, water, Epsom salt, and corn sugar).
Collection of blood samples and body weights.
Samples of whole blood (5 µL) were collected by using a lancet (EasyTouch, Fairfield, OH) to nick the tail vein of mice during restraint in a plastic tube holder. Whole blood was placed directly onto test strips. NFBG samples were collected every Monday between 0800 and 1000 and FBG samples collected every Friday between 0800 and 1000, and immediately measured by using a glucometer (EasyTouch). Mice were weighed twice a week on Mondays and Fridays between 0800 and 1000 by using an electronic scale (Tanita, Tokyo, Japan).
Timeline.
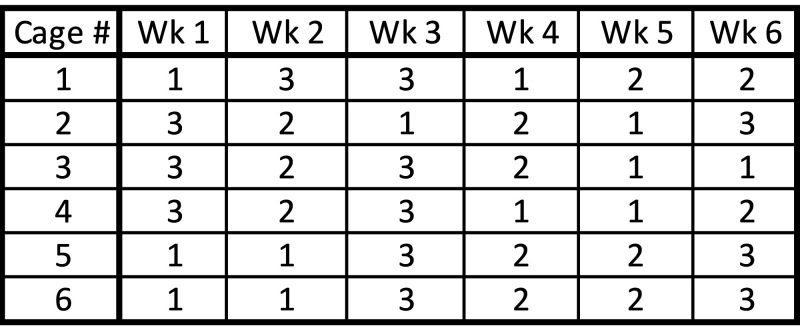
Beginning on arrival and continuing until they reached 8 wk of age, mice were acclimated to corncob bedding in IVC cages, with unrestricted access to food and water. After the acclimation period, a blood sample was collected from all mice for baseline NFBG measurement. After this, groups of 5 mice were randomly assigned to each of 6 cages. Each cage was subjected to 3, week-long treatments that were replicated twice for each treatment over a 6-wk period (Figure 1). The 3 cage treatments were corncob bedding, hardwood bedding plus an autoclaved cardboard toilet paper roll for environmental enrichment, and hardwood bedding only. The order of treatment of each cage was randomized to eliminate effects due to the age of the mice or the order of exposure. Mice were assigned to cages on day 0 between 0800 and 1000 and were acclimated for 72 h before NFBG samples were collected. Blood samples were collected from the tail vein of every mouse on day 3 between 0800 and 1000 for measurement of NFBG. On day 6, food was removed from cages between 1900 and 2100, for a 14-h overnight fast, with water available without restriction. A new cardboard toilet paper roll was placed on day 6, when food was removed for the overnight fast. On day 7 between 0800 and 1000, blood samples were collected from the tail vein for measurement of FBG. After blood sampling, each group of mice was transferred to a new treatment cage with either corn cob bedding, hardwood bedding plus an autoclaved toilet paper roll, or hardwood bedding only. For each cage, the 7-d process was repeated weekly (2 iterations per treatment) for 6 wk. Mice were weighed twice a week, on days 3 and 7, between 800 and 1000.
Figure 1.
Schematic of randomized-block cage treatment schedule. Each cage contained 5 randomly assigned mice, for a total of 6 cages. The 3 cage treatments were: 1) hardwood bedding; 2) hardwood bedding plus an autoclaved cardboard toilet paper roll for enrichment; and 3) corncob bedding. Each treatment exposure period lasted 1 wk; each cage was exposed twice to each 1-wk treatment, for a total of 6 wk.
Statistical analysis.
Statistical analysis of FBG and NFBG levels and body weights collected over a 6-wk period was performed using R (GNU General Public License, R Core Team, Vienna, Austria) for the Shapiro–Wilk normality test, Kruskal–Wallis rank sum test, and Dunn All-Pairwise test (Prism 7, GraphPad Software, La Jolla, CA). A P value of less than 0.01 was considered to be statistically significant. Data are reported as medians with lower and upper quartiles.
Results
Comparison of FBG.
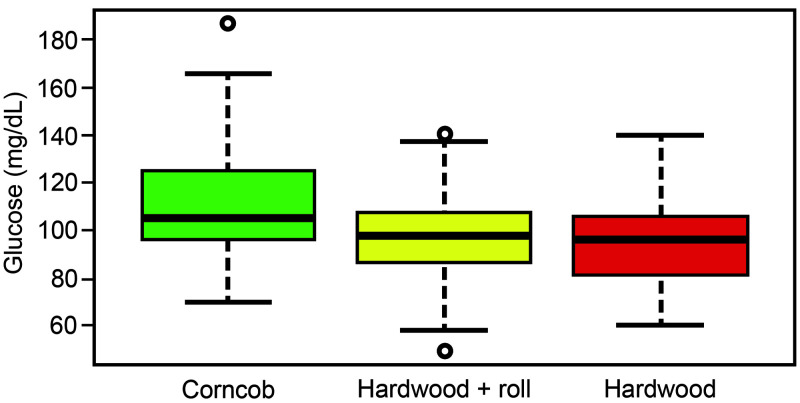
Box plots for the 3 cage treatments revealed that the FBG data for corncob bedding were more dispersed than for the other 2 treatments (Figure 2). The Shapiro–Wilk normality test revealed the distribution of the FBG data to be nonnormal, so a Kruskal–Wallis test (a conceptual analog of ANOVA) was used to determine whether the data distributions differed. At least one treatment yielded FBG levels that differed statistically from the others (χ2 = 18.823, df = 2, P = 8.177 × 10–5). A Dunn test to separate the medians showed that the FBG for the corncob bedding (median, 105 mg/dL) was higher than those for hardwood bedding, with and without enrichment (median, 98 and 96 mg/dL, respectively). The FBG distribution for the hardwood bedding values was not significantly affected by enrichment.
Figure 2.
Box plot distribution of FBG levels in mice exposed to 3 cage treatments, with median values and reference intervals. These box plots revealed differences in FBG data for corncob bedding, which were more dispersed and slightly different (P = 8.177 × 10–5) from those for the other 2 treatments.
Baseline NFBG and body weight.
Kruskal–Wallis rank sum tests detected no significant treatment-related differences in baseline NFBG (χ2 = 3.176, df = 2, P = 0.2043) or body weight (χ2 = 4.8169, df = 2, P = 0.08995).
Discussion
The present study found that mice housed on corncob bedding had higher FBG levels than did mice on hardwood bedding, and that FBG did not differ between mice housed on hardwood bedding with or without enrichment. The higher FBG in mice housed on corncob bedding suggested that they might ingest the bedding during the overnight fast. Follow-up observations of home cage behavior would confirm whether mice did ingest corncob during fasting or nonfasting periods.
This study has several limitations. We used only a single sex (female) and strain (C57BL/6NCrl) of mice; both sex and strain could influence results. We evaluated 8 to 14 wk old mice in this study. NFBG, FBG, and body weight might show age-related differences. Aging in humans can naturally lead to significant changes in weight, body fat percentage, and glucose metabolism and may be difficult to translate from mice to humans.18 Also, we evaluated only 2 types of bedding; other types of bedding could be studied to determine any interaction between bedding substrate and environmental enrichment. None of the treatment groups in our study differed with regard to NFBG. The differences noted in the Mouse Phenome Database17 in the serum glucose levels of 41 strains of mice indicates that blood glucose levels vary with age, sex, and stain. Studying both sexes and multiple strains and ages of mice would provide more information on whether sex and strain are biologic variables in terms of NFBG and bedding type.
None of the treatment groups in our study showed differences in body weight. In a previous study, strain-associated differences in body weight were apparent until mice were 11 wk old.29 Therefore, future studies could include more strains to assess effects of bedding substrate (with or without environmental enrichment) on body weight.
The enrichment item used in our study (an autoclaved cardboard toilet-paper roll) had no effect on FBG, NFBG, or body weight in mice housed on the hardwood bedding, even though the rolls were always present in the cages, including during the 14-h overnight fast. Based on information supplied by the vendor, the item contained food-grade adhesives (gelatin, glycerin, water, Epson Salts, and corn sugar). This variation of a tube-shaped cardboard enrichment device is commonly used at our institution to promote species appropriate behaviors in mice at this institution.11 This lack of difference between groups indicated that this enrichment item did not alter the FBG, NFBG, or body weight of mice on hardwood bedding.
In conclusion, the reproducibility of animal research has profound implications for scientific progress and ethical use of animals. The reason for failures in reproducibility are likely multifactorial and include microenvironmental factors, such as the type of bedding used substrates in the primary enclosure. In addition to potential effects on FBG, the source of raw materials, production processes, and microbial contaminates of bedding substrates may confound research results. Many husbandry practices, including the bedding substrates used, are based on industry standards and operational considerations. However, these choices may influence research results, leading to wasted resources.30 Bedding substrates such as corncob may mask treatment effects in metabolic studies that measure FBG, such as research on diabetes. The information from the current study may inform the choice of bedding substrates used.
Acknowledgments
We acknowledge Drs J Zahorsky-Reeves and LW Castellani for their 2010 abstract on this topic, which served as the basis for this study. We thank Dr. Steven Seifried (formerly with the Department of Cell and Molecular Biology, University of Hawaii, Honolulu) for his statistical analyses and the staff of the Office of Research Compliance (Animal and Veterinary Services Program, University of Hawaii) for their assistance with this study. The work was supported in part by the University of Hawaii Undergraduate Research Opportunities Program, Office of the Vice Provost for Research and Scholarship (University of Hawaii, Manoa). The study was conducted in accordance with IACUC protocol no. 18-2876.
References
- 1.Ambery AG, Tackett L, Penque BA, Hickman DL, Elmendorf JS. 2014. Effect of corncob bedding on feed conversion efficiency in a high-fat–diet-induced prediabetic model in C57Bl/6J mice. J Am Assoc Lab Anim Sci 53:449–451. [PMC free article] [PubMed] [Google Scholar]
- 2.André V, Gau C, Scheideler A, Aguilar-Pimentel JA, Amarie OV, Becker L, Garrett L, Hans W, Hölter SM, Janik D, Moreth K, Neff F, Östereicher M, Racz I, Rathkolb B, Rozman J, Bekeredjian R, Graw J, Klingenspor M, Klopstock T, Ollert M, Schmidt-Weber C, Wolf E, Wurst W, Gailus-Durner V, Brielmeier M, Fuchs H, Hrabé de Angelis M. 2018. Laboratory mouse housing conditions can be improved using common environmental enrichment without compromising data. PLoS Biol 16:e2005019. 10.1371/journal.pbio.2005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP. 2010. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model & Mech 3:525–534. 10.1242/dmm.006239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu AP, Prasad H, Venkateswaran G, Muthukumar SP. 2013. Evaluation of micro-environment and microbiological monitoring of various bedding materials for laboratory rodents. Res Anim 56-61. [Google Scholar]
- 5.Bayne K. 2018. Environmental enrichment and mouse models: Current perspectives. Animal Model Exp Med 1:82–90. 10.1002/ame2.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blom HJM, Van Tintelen G, Van Vorstenbosch CJAHV, Baumans V, Beynen AC. 1996. Preferences of mice and rats for types of bedding material. Lab Anim 30:234–244. 10.1258/002367796780684890. [DOI] [PubMed] [Google Scholar]
- 7.Burn CC, Mason GJ. 2005. Absorbencies of 6 different rodent beddings: Commercially advertised absorbencies are potentially misleading. Lab Anim 39:68–74. 10.1258/0023677052886592. [DOI] [PubMed] [Google Scholar]
- 8.Envigo. [Internet]. 2017. Corncob bedding. [Cited 30 September 2022]. Available at: https://insights.envigo.com/hubfs/resources/data-sheets/7092-7097-CornCob-Bedding-US-2017.pdf
- 9.Fontaine D. [Internet]. 2019. Five common questions for diabetic models. [Cited 23 September 2019]. Available at: https://www.jax.org/news-and-insights/jax-blog/2019/september/5-common-questions-for-diabetic-models
- 10.Jackson E, Demarest K, Eckert WJ, Cates-Gatto C, Nadav T, Cates LN, Howard H, Roberts AJ. 2015. Aspen shaving versus chip bedding: Effects on breeding and behavior. Lab Anim 49:46–56. 10.1177/0023677214553320. [DOI] [PubMed] [Google Scholar]
- 11.Kondo S. 1997. “Roll” out the mouse enrichment. Tech Talk 2:2. [Google Scholar]
- 12.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 13.Kraft LM. 1980. The manufacture, shipping and receiving, and quality control of rodent bedding materials. Lab Anim Sci 30:366–376. [PubMed] [Google Scholar]
- 14.Le Leu RK, Conlon MA, Bird AR, Clarke JM. 2015. Housing experimental rats in solid-based cages with digestible bedding may confound outcomes of nutritional studies. J Sci Food Agric 95:2155–2158. 10.1002/jsfa.6919. [DOI] [PubMed] [Google Scholar]
- 15.Loeb W. 1997. Clinical biochemistry of laboratory rodents and rabbits, p 845–899. In: Kaneko JJ, Harvey JW, Brass ML, editors. Clinical biochemistry of domestic animals. San Diego (CA): Academic Press. 10.1016/B978-012396305-5/50030-0 [DOI] [Google Scholar]
- 16.Markaverich B, Mani S, Alejandro MA, Mitchell A, Markaverich D, Brown T, Velez-Trippe C, Murchison C, O’Malley B, Faith R. 2002. A novel endocrine-disrupting agent in corn with mitogenic activity in human breast and prostatic cancer cells. Environ Health Perspect 110:169–177. 10.1289/ehp.02110169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naggert JK, Svenson KL, Smith RV, Paigen B, Peters LL. 2022. [Internet]. Diet effects on bone mineral density and content, body composition, and plasma glucose, leptin, and insulin levels. MPD 143. Mouse Phenome Database Website, The Jackson Laboratory. [Cited 30 September 2022]. Available at: https://phenome.jax.org/projects/Naggert1.
- 18.National Institute on Aging. [Internet]. 2021. Fasting blood glucose levels in mouse models might not translate to age-related metabolic changes in humans. [Cited 18 November 2021]. Available at: https://www.nia.nih.gov/news/fasting-blood-glucose-levels-mouse-models-might-not-translate-age-related-metabolic-changes
- 19.Perkins SE, Lipman NS. 1995. Characterization and quantification of microenvironmental contaminants in isolator cages with a variety of contact beddings. Contemp Top Lab Anim Sci 34:93–98. [PubMed] [Google Scholar]
- 20.Quimby FW, Luong RH. 2007. Chapter 6: Clinical chemistry of the laboratory mouse, p 171–216. In: Fox JG, Quimby FW, Newcomer CE, Davisson MT, Barthold SW, Smith AL. The mouse in biomedical research, 2nd edition. San Diego (CA): Academic Press. 10.1016/B978-012369454-6/50060-1 [DOI] [Google Scholar]
- 21.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73. https://doi.org/10.1258/0023677011911381. [DOI] [PubMed] [Google Scholar]
- 22.Reed B, Hawkins P, Latham N, Westwood K, Van Driel K, Battram C, Golledge H, Farmer AM, Osborne N, Jennings M, Hubrecht R. 2008. Report of the 2006 RSPCA/UFAW Rodent Welfare Group meeting. Lab Anim (NY) 37:216–222. 10.1038/laban0508-216. [DOI] [PubMed] [Google Scholar]
- 23.Reinhardt V, Reinhardt A, editors. 2002. Comfortable quarters for laboratory animals, 9th ed. Washington (DC): Animal Welfare Institute. [Google Scholar]
- 24.Rosenbaum MD, VandeWoude S, Johnson TE. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. J Am Assoc Lab Anim Sci 48:763–773. [PMC free article] [PubMed] [Google Scholar]
- 25.Sakaguchi M, Inouye S, Miyazawa H, Kamimura H, Kimura M, Yamazaki S. 1990. Evaluation of countermeasures for reduction of mouse airborne allergens. Lab Anim Sci 40:613–615. [PubMed] [Google Scholar]
- 26.Smith E, Stockwell JD, Schweitzer I, Langley SH, Smith AL. 2004. Evaluation of cage microenvironment of mice housed on various types of bedding materials. Contemp Top Lab Anim Sci 43:12–17. [PubMed] [Google Scholar]
- 27.Tataryn NM, Buckmaster CA, Schwiebert RS, Swennes AG. 2021. Comparison of 4 beddings and ammonia control in individually ventilated mouse cages. J Am Assoc Lab Anim Sci 60:37–43. 10.30802/AALAS-JAALAS-20-000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thigpen JE, Setchell KDR, Kissling GE, Locklear J, Caviness GF, Whiteside T, Belcher SM, Brown NM, Collins BJ, Lih FB, Tomer KB, Padilla-Banks E, Camacho L, Adsit FG, Grant M. 2013. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol A studies. J Am Assoc Lab Anim Sci 52:130–141. [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai PP, Pachowsky U, Stelzer HD, Hackbarth H. 2002. Impact of environmental enrichment in mice. 1: Effect of housing conditions on body weight, organ weights, and haematology in different strains. Lab Anim 36:411–419. 10.1258/002367702320389071. [DOI] [PubMed] [Google Scholar]
- 30.Van de Weerd HA, Aarsen EL, Mulder A, Kruitwagen CLJJ, Hendriksen CFM, Baumans V. 2002. Effects of environmental enrichment for mice: Variation in experimental results. J Appl Anim Welf Sci 5:87–109. 10.1207/S15327604JAWS0502_01. [DOI] [PubMed] [Google Scholar]
- 31.Whiteside TE, Thigpen JE, Kissling GE, Grant MG, Forsythe DB. 2010. Endotoxin, coliform, and dust levels in various types of rodent bedding. J Am Assoc Lab Anim Sci 49:184–189. [PMC free article] [PubMed] [Google Scholar]