Abstract
Cryopreservation of epididymal sperm collected after euthanasia is a common method to preserve and distribute valuable mouse models worldwide. However, the euthanasia method used prior to sperm collection must not adversely affect sperm quality. The most common method of euthanasia in mice is CO2 asphyxiation, but its effect on the quality of sperm collected postmortem is largely unknown. The objective of this study was to determine the effects of CO2 euthanasia of C57BL/6 mice on both freshly recovered sperm and sperm subjected to freezing and thawing. First, sperm concentration, progressive motility, curvilineal velocity (VCL), average path velocity (VAP), and progressive velocity (VSL) were analyzed for mice euthanized by cervical dislocation (CD), high flow CO2 (100%), or low flow CO2 (30%) displacement/minute, respectively. Then, in-vitro fertilization and embryonic development rates were determined using frozen-thawed sperm from each euthanasia method. Neither fresh nor frozen-thawed sperm showed significant differences in sperm concentration, progressive motility, VAP, or VCL when compared to CD and CO2 groups. However, frozen-thawed sperm collected from CD mice had higher VCL values than did those collected from the low flow mice (P = 0.039). VCL was not different in fresh or frozen-thawed sperm collected after mouse euthanasia by CD as compared with high flow CO2 or by high flow as compared with low flow CO2. Frozen-thawed sperm showed no differences among the 3 euthanasia groups for fertilization (P = 0.452) or blastocyst development rates (P = 0.298). The results indicate that CO2 euthanasia can be used as an alternative to CD to obtain optimal quality mouse sperm for cryopreservation while remaining compliant with welfare requirements.
Abbreviations: ART, assisted reproductive technologies; CD, cervical dislocation; IVF, in vitro fertilization; VAP, average path velocity; VCL, curvilineal velocity; VSL, progressive velocity
Introduction
Mice are by far the animals most commonly used by biomedical researchers25 and the most commonly used animals for the generation of genetically engineered models of human disease and disorders.41 To this end, researchers worldwide have created thousands of genetically modified mouse strains to investigate the genetic origins of human diseases and disorders.23 The numbers of these rodent strains continue to increase and can be costly to create and maintain. Scientists perform a wide spectrum of phenotypic characterization on these mice to characterize the strain for the biomedical community. Germplasm cryopreservation is an effective means of preventing loss of these strains during catastrophic events.3 Furthermore, scientific collaboration among different institutions, often across the globe, require safe transfer of these strains. Shipping live mice can be challenging and sometimes impossible, both domestically and internationally, due to logistics and international trade laws. Therefore, a more cost effective, efficient, and convenient strategy is to transport cryopreserved germplasm for future rederivation at receiving institutions.
Postmortem collection of germplasm (for example spermatozoa, oocytes, embryos, ovarian, or testicular tissues) from mice requires great care to ensure the quality and functional viability of the recovered cells. High quality germplasm is important to obtaining successful rederivation via assisted reproductive technologies (ART) such as in vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI) and embryo transfers. In addition, one of the main requirements for the use of animals in biomedical research is to comply with the 3Rs of replacement, reduction, and refinement. These principles are employed to improve animal welfare and scientific vigor where the use of animals cannot be avoided.16,19,29,34 Prioritizing efficient postmortem recovery of germplasm greatly reduces the number of donor mice that are necessary for germplasm bio-banking. Therefore, the methods used to euthanize mice for germplasm harvest must not reduce its quality for future ARTs used to rederive specific strains.
Euthanasia for postmortem germplasm collection for cryopreservation and subsequent recovery of live mice via IVF is commonly performed by either cervical dislocation (CD) (mice) or CO2 asphyxiation (rats).43,47 Sperm is abundant, easy to collect, relatively easy to cryopreserve and can be collected from sick, aged or even dead animals. Sperm can be used to preserve transgenic mouse lines that have a single mutation on an inbred background. While the AVMA recognizes CD as an approved method of euthanasia for mice, its guidelines state that CD is technically challenging and requires scientific justification.5 One study found that CD was unsuccessful in 21% of cases, even when performed by highly trained technicians.10 In addition, euthanasia via CD is aesthetically displeasing and may distress personnel and contribute to compassion fatigue.44 Thus, CO2 asphyxiation is the most commonly used euthanasia method for many rodent users due to its convenience, ease and cost effectiveness.7 Researchers who use CO2 asphyxiation as a euthanasia method should first confirm it does not have deleterious effects and is suitable for achieving research objectives.
Cryopreservation can have serious detrimental effects on spermatozoa including osmotic, physical, hypothermic, and chemical toxicity of the cryoprotective agent.51 Epididymal mouse sperm, particularly when derived from the inbred mouse strains (for example C57BL/6, FVB or 129, BALB/C) are more sensitive to these effects than other species, including bull, boar, and ram.51 Sperm may lose functional integrity if they are subjected to suboptimal conditions either in vivo or in vitro and may then no longer be able to complete fertilization and trigger further embryonic development. Thus, maintaining high quantitative and qualitative yield is important for achieving rederivation using cryopreserved spermatozoa. Germplasm quality is critical for cryo-resuscitation and for minimizing experimental confounds during the development of effective cryopreservation protocols.
Under normal conditions, the body can balance the ions that control pH within the required physiologic levels.18 In the case of hypoventilation, such as that caused by a prolonged period of CO2 asphyxiation, respiratory acidosis can occur because the lungs cannot eliminate adequate amounts of CO2 from the peripheral blood. This excess CO2 causes the pH of the circulating blood and, potentially, of other bodily fluids to fall, producing an acidic environment. However, mouse oocytes and sperm must remain within the range of 7.3 to 7.4 to obtain optimal fertility both in the female reproductive tract and during IVF.6,34,39 One study reported that the circulating blood pH of the C57BL/6 female mice euthanized by low-flow CO2 asphyxiation caused significant reduction in circulating blood pH to 6.5 as compared with 7.3 in mice euthanized by CD and reduced IVF success when using C57BL/6 sperm.24
Our group recently reported that CD yields superior quality fertilizable oocytes compared with either low- or high-flow CO2 asphyxiation.52 This in-depth subcellular investigation showed that higher rates of premature cortical granule exocytosis (PCGE) are largely responsible for reduced fertilization rates for the oocytes collected from donor mice euthanized by CO2 asphyxiation before the IVF procedure. These findings collectively suggest that an acidic environment in the oviduct due to CO2-induced asphyxiation prior to oocyte recovery impaired successful IVF.24,53
To date, few studies have examined the effects of CO2 euthanasia on rodent epididymal sperm motility characteristics.9,46 None of those studies found adverse effects of CO2 asphyxiation euthanasia on rat spermatozoa, but to our knowledge effects of CO2 euthanasia procedure on mouse spermatozoa have not been investigated. In the current study, C57BL/6 male mice were euthanized by high flow CO2, low flow CO2, or CD, and then motility characteristics of fresh and thawed sperm were assessed. Furthermore, IVF and subsequent embryo culture experiments were performed using either fresh or thawed sperm to effects on IVF and in vitro embryo developmental competence.
Materials and Methods
All chemicals used in this study were obtained from Sigma chemical company (St. Louis, MO) or ThermoFisher Scientific (Waltham, MA) unless stated otherwise.
Animals.
This study was conducted at an AAALAC-accredited facility under an IACUC-approved protocol and in compliance with the 8th edition of the Guide for the Care and Use of Laboratory Animals.28 Twelve- to 14-wk-old male C57BL/6J (n = 42) and 7- to 8-wk-old female C57BL/6J (n = 56) Mus musculus were obtained from an inhouse breeding colony. The housing environment was maintained at 22 ± 2°C, with a relative humidity of 30% to 70% on a 14:10-h light:dark cycle (lights on, 700 CST). Mice were either single housed in standard polypropylene shoebox cages (7.25 in. L × 11.75 in. W × 5 in. H [18.4 cm × 29.8 cm × 12.7 cm], Allentown, Allentown, NJ) or group housed in groups of 8 to 10 in large shoebox caging (10.5 in. × 19 in. × 6 in. [26.7 cm × 48.3 cm × 15.2 cm]) on aspen bedding (Specialty Papers, Watertown, TN) and had unrestricted access to a commercial rodent diet (Formulab Diet 5008, Purina, St. Louis, MO) and water. Colony health was evaluated every 3 mo through sentinel exposure to dirty bedding. All sentinels were seronegative for Mouse hepatitis virus, Minute virus of mice, Mouse parvovirus, Parvovirus NS-1, Theiler murine encephalomyelitis virus, Murine rotavirus, Mycoplasma pulmonis, Sendai virus. PCR testing was negative for fur mites and pinworms.
Euthanasia.
Mice in CO2 euthanasia groups were placed in a clean 14.6-L polyurethane box connected to a CO2 tank (38.5 cm L, 19.5 cm W, 19.5 cm H). The flow rate was 30% displacement volume/minute for mice in the low flow CO2 group and 100% displacement volume/minute for the high flow CO2 group via Western Medica CO2 flow meter (Westlake, OH). The percentages were chosen to represent the lowest acceptable flow rate in the 2020 AVMA euthanasia guidelines and the maximal rate at which CO2 could be displaced.5 Mice remained in the box until they had stopped breathing for 1 min. Mice were removed from the box and cervically dislocated as a secondary method of euthanasia; the box was then cleaned with 70% ethanol. The time from the start of euthanasia to time of sperm collection was recorded for each mouse. Mice in the CD group were euthanized by trained personnel by simply grasping the skin on the back of the neck by the thumb and forefinger and immediately pulling on the base of the tail in an opposite upward direction from the head in accordance with 2020 AVMA guidelines. The immediate dislocation of the spinal column from the brain ensured sudden death in a few seconds. All female donors for oocyte collection for IVF were euthanized via CD only.
Computer-Assisted Sperm Motility Analysis (CASA).
Computer-assisted sperm motility analysis (Hamilton Thorne Biosciences, M2030, Beverly, MA) was used to determine sperm motility characteristics in an 80-µm deep dual sided chamber (2× CELL, Hamilton Thorne Biosciences) at 37°C. After capacitation, 10-µL sperm samples were assessed on an IVOS (Hamilton Thorne sperm analysis system). One fresh and one frozen-thawed sample was assessed from each mouse. Ten representative areas from each sample were used on each slide to measure concentration, progressive motility, curvilineal velocity (VCL), average path velocity (VAP), and progressive velocity (VSL) for each sample.
Sperm collection and freezing.
After euthanasia, the cauda epididymis was removed, washed in HEPES buffered follicle holding medium (FHM), and external blood and fat removed with clean tissue paper. Each epididymis was secured using fine tweezers, and a small cut was made using sterile microscissors. Densely packed sperm was gently teased from each epididymis using fine tweezers. For freezing, sperm were exposed to 1.2 mL 18% raffinose pentahydrate (w/v) and 3% skim milk (w/v) freezing solution as previously described.50 After 10 min, freezing solution containing the sperm was loaded into 0.5-mL French straws and placed in a Styrofoam box containing LN2. Straws were placed and cooled in vapor phase of LN2 on a mesh wire 8 in. (20.3 cm) above LN2 for 5 min, and then the straws were plunged into LN2 for long-term storage.
Motility of fresh sperm was analyzed from each donor mouse prior to cryopreservation by diluting a small portion of fresh sperm in HEPES buffered FHM media and using the CASA system. To determine motility characteristics of frozen-thawed sperm, the straws containing frozen sperm were taken directly from LN2 storage, placed in a warm water bath (37°C) and allowed to thaw. Freezing solution containing sperm was then expelled from the straw into a 1.5-mL microfuge tube with 1 mL of FHM media for motility analysis. Tubes were spun down in a microcentrifuge at 0.3 relative centrifugal field (RCF) for 5 min. After centrifugation, the supernatant was carefully removed without disturbing the pellet, and 70 µL of FHM was added to the pellet. Tubes were then placed in a hot bead bath at 37°C and allowed to capacitate for 30 min before motility analysis was performed using the CASA system.
Superovulation.
Each mouse was injected with 5 IU pregnant mare serum gonadotropin (PMSG) intraperitoneally followed by 5 IU human chorionic gonadotropins (hCG) 48 h later. Clutches of cumulus-oocyte complexes (COCs) were collected from the oviducts 14 to 15 h after hCG injection.
In vitro fertilization.
The straws containing frozen sperm were removed from LN2 and thawed in a water bath at 37°C. The content of the straw was gently expelled on top of 1 mL FHM media containing BSA (4 mg/mL) in microfuge tube and then centrifuged for 5 min at 300 g. The supernatant was removed, and sperm pellet was gently pipetted out and placed into pre-equilibrated 95-mL FERTIUP drop for sperm capacitation for 30 min. IVF was performed as previously described.48,49 Both FERTIUP and CARD (for in vitro fertilization) media were pre-equilibrated in a humidified incubator containing 5% CO2 in air under mineral oil at 37°C in 35 mm culture dishes. For IVF, the dissected oviducts from the donor females were placed separately into mineral oil next to a CARD drop under a stereomicroscope. The clutches of COCs from the donors were first released into the mineral oil using fine tweezers and a 28 gauge needle. They were then gently pulled into 90-µL CARD drops. For IVF, thawed or fresh sperm was capacitated in FERTIUP and then gently added (approximately 350 × 105 motile sperm/mL) to CARD drops that contained the clutches of COCs collected after the 3 euthanasia methods. After about 6 to 8 h of sperm and egg coincubation, the presumptive zygotes were denuded from cumulus cells by pipetting, washed in FHM media containing BSA (4 mg/mL), transferred into KSOM amino acid culture drops, and allowed to develop in an incubator at 37°C and 5% CO2.26,48 Development rates were assessed at 24 h for 2-cell, 72 h for morula and 96 h for blastocyst development.
Statistical analysis.
Statistical analysis was performed using SigmaPlot version 14.0 (Systat Software, Palo Alto, CA) and P values < 0.05 were considered significant. All values are given as mean ± SEM and represented by error bars in the figures. An unpaired t test was used to compare the time from onset of CO2 to completed euthanasia for the 2 methods of CO2 euthanasia. A 2-way ANOVA with a Tukey posthoc analysis was used to determine whether euthanasia method affected on sperm quantity and motility parameters, including sperm concentration, progressive motility, VAP, VCL, and VSL. To determine whether exposure to CO2 affected fresh or frozen sperm quality, an ANOVA was performed on both fresh and thawed samples for each motility parameter (progressive motility, VAP, VSL, and VCL) samples. An ANOVA was used to test for differences between groups. A 2-way ANOVA was used to determine whether euthanasia time affected measures of sperm quality. A one-way ANOVA was used to test whether CO2 euthanasia of mice affected the embryo developmental competence (2-cell, morula, and blastocyst) of their thawed sperm.
Results
Euthanasia time.
Mice (n = 12 per treatment group) in the low-flow rate CO2 group had a significantly longer euthanasia time (340 s) as compared with the high flow rate group (52 s) (P < 0.001). However, euthanasia method did not significantly affect fresh sperm recovery from cauda epididymis (P = 0.939). Recoveries for CD, low-flow, and high-flow rate CO2, and CD euthanasia methods were, respectively, 16 million/mL, and 17 million/mL, 18 million/mL.
Fresh and frozen-thawed sperm motility.
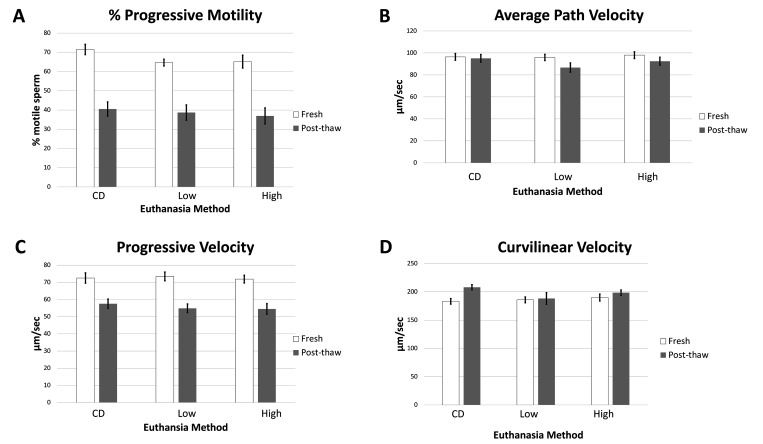
To determine whether euthanasia via CO2 asphyxiation affected sperm motility parameters (progressive motility, VSL, VAP, and VCL), sperm was analyzed by CASA (Figure 1A through D). Progressive motility of freshly collected sperm were65%, and 65%, and 71% for low-flow, and high-flow rate CO2, and CD respectively, with no significant differences between groups (P = 0.105). In thawed sperm, progressive motility values were39%, and 37%, and 40% respectively, for low-flow, and high-flow rate CO2, and CD with no significant differences between groups (P = 0.817). VAP, VSL, and VCL values were also measured for fresh and thawed sperm. VAP values for the freshly collected sperm were 96, 98, and 96 µm/sec for low-flow, and high-flow rate CO2, and CD respectively, with no significant differences between groups (P = 0.877). For the thawed sperm, VAP values were 87, 92, and 95 µm/sec for low-flow, and high-flow rate CO2, and CD respectively, with no significant differences between groups (P = 0.295).
Figure 1.
The C57BL/6 cauda epididymal fresh sperm and post thaw progressive motility (A) velocity parameters; curvilineal velocity (B), average path velocity (C), and progressive velocity (D) for either fresh or frozen-thawed C57BL/6 sperm. The results are presented as averages across groups (n = 12 per treatment group). CD: cervical dislocation. Low: low-flow CO2. High: high-flow CO2. Error bars represent standard error of mean. * indicates significance.
VSL values for freshly collected sperm were 72, 73, and 72 µm/sec for low-flow, and high-flow rate CO2, and CD respectively, with no significant differences between groups (P = 0.873). For thawed sperm, VSL values were 55, 54, and 58 µm/sec, respectively, for low-flow and high-flow rate CO2, and for CD, with no significant differences between groups (P = 0.710). VCL values for freshly collected sperm were186, 190, and 183 µm/sec for low-flow, and high-flow rate CO2, and CD respectively, with no significant differences between groups (P = 0.673). For thawed sperm, VCL values were 188, 198, and 208 µm/sec for low-flow, and high-flow rate CO2, and CD respectively. Thawed sperm collected from the CD group had significantly higher VCL values than did sperm collected from the low-flow group (P = 0.039), with no significant differences between sperm collected from the CD and with high-flow groups (P = 0.344) or between high-flow and low-flow groups (P = 0.548). No significant differences were found for progressive motility, VSL, or VAP between groups for either fresh or thawed samples. No significant differences were found for VCL between groups for freshly collected sperm, but thawed sperm samples from mice euthanized by CD were significantly faster than those obtained from mice euthanized via low-flow CO2 (P = 0.039).
Embryonic development competency.
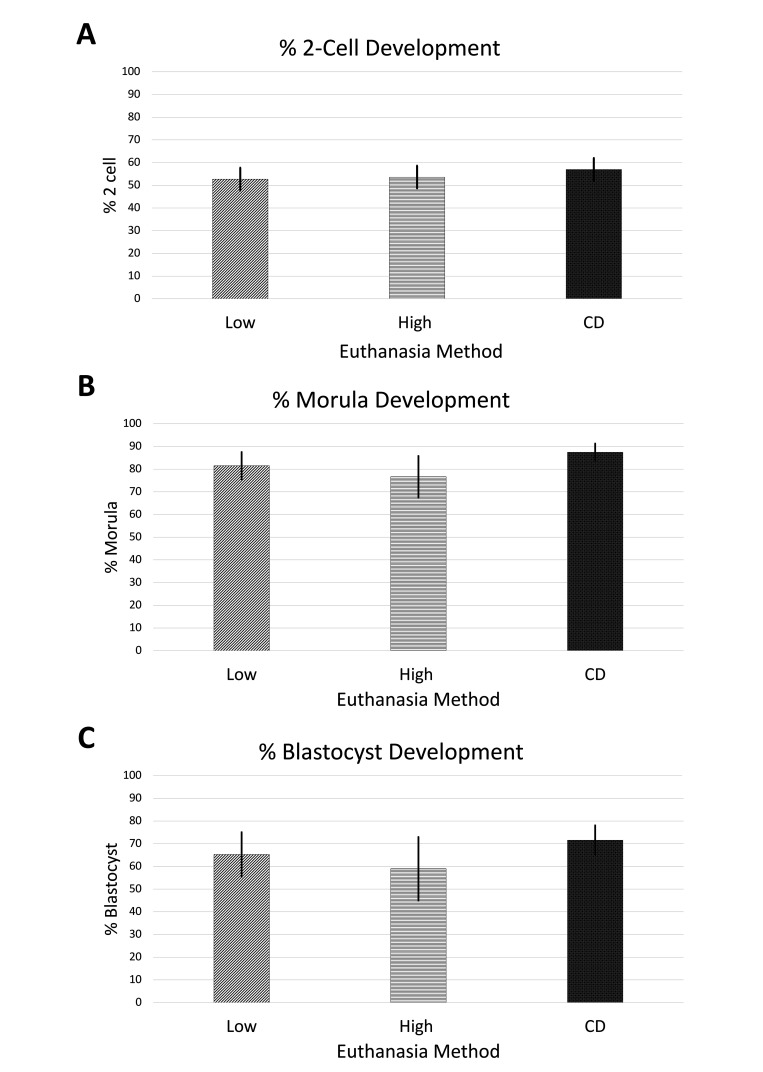
To determine whether sperm collected from mice euthanized with CO2 were altered with regard to IVF and in vitro embryo development, the percentage of embryos that reached developmental milestones were calculated (Figure 2). The percentage of oocytes that developed to 2-cell stage embryos was first calculated for thawed sperm. This value was then used to calculate morula and blastocyst development percentages (Figure 2). The percentages of 2-cell development for high-flow, low-flow CO2, or CD were 54%, 53%, and 57%, respectively. Morula development rates for high flow, low flow CO2, or CD were, respectively, 77%, 81%, and 87%, while blastocyst stage development rates were 59%, 65%, and 72%. No significant differences were detected between groups for IVF (P = 0.452), morula development rates (P = 0.310), or blastocyst development rates (P = 0.298) using thawed sperm collected after the 3 euthanasia methods.
Figure 2.
(A) Percent in vitro fertilization rate, (B) morula development rate, and (C) blastocyst development rate (C) using C57BL/6 sperm collected after cervical dislocation, low flow CO2 and high flow CO2 euthanized donors following in-vitro fertilization using C57BL/6 frozen-thawed sperm. The results are presented as averages across the groups based on replicated 6 replications). Error bars represent standard error of mean. CD: cervical dislocation. Low: low-flow CO2. High: high-flow CO2.
Discussion
Obtaining high quality spermatozoa and oocytes is integral to efficient in vitro fertilization, intracytoplasmic sperm injection and high-quality embryo production for reproductive studies and germplasm cryopreservation. In contrast to other domestic farm and companion animals, the most common method of recovering mouse sperm or oocytes for in vitro reproductive studies or genome cryo-banking requires postmortem collection of spermatozoa or oocytes from the cauda epididymis or oviduct of previously superovulated donor mice, respectively.2 The euthanasia method is an important determinant of the future developmental competence of the germplasm, and should provide optimal recovery of spermatozoa, oocytes, or preimplantation embryos. Fertilization is a critical period for preimplantation embryonic development in mammals. Thus, the exposure of oocytes or sperm to suboptimal conditions such as hypothermia, acidosis, or various physical stresses, either before or during the course of fertilization, could impair fertilization and ultimately result in lower rates of embryonic development.21,52,53 One group showed that the blood pH of the female C57BL/6 mice euthanized by using CO2 at a displacement rate of 20%/L/min was significantly lower (pH 6.5) than that of mice euthanized by CD (pH 7.3).24 One of our recent studies demonstrated that CD preserved the intact distribution of cortical granules (CGs) and F-actin of metaphase II (MII) mouse oocytes, leading to significantly higher IVF success rates and better embryo development to the blastocyst stage as compared with either high-flow or low-flow rate CO2.53 This suggested that the key factors for harvesting good quality oocytes were maintaining blood and intracellular pH at approximately 7.3 and rapid collection of oocytes after euthanasia by CD. While CD appears necessary to collect quality oocytes, it has been unclear if it was necessary to collect quality sperm.53
The current study extended our investigation to compare the effects of low-flow or high-flow CO2 asphyxiation and CD euthanasia on mouse cauda epididymal sperm motility, in vitro fertilization, and embryonic developmental competence. Several in vitro studies from a number of species suggest that hydrogen ions, bicarbonate ions, and CO2 can influence sperm motility, viability, and metabolism and thus optimal pH is necessary not only for sperm maturation, but also for proper motility and acrosome reactions upon fertilization.35,36 The basis of our study was that respiratory acidosis caused by CO2 retention would detrimentally affect epididymal sperm and impair their fertility after postmortem recovery because these physiologic and subcellular parameters are important in sperm maturation and motility.20,24,53 Sperm production is protected by the blood–testes barrier, which creates an immune privileged site within the testes and epididymis and prevents the accumulation of harmful substances.13,15 A previous study showed that sperm maturation in the epididymis is regulated by both absorptive and secretory activities of the epithelial lining.14 After spermatozoa production in the seminiferous tubules in the testis, sperm transit along the length of the caput, corpus, and cauda epididymis, and are subjected to constant morphologic, ionic, pH and osmotic changes. One group found that 96% of the testicular fluid output and bicarbonate was reabsorbed in the efferent ducts before transit into caput epididymis.35 In that study, luminal pH and bicarbonate levels in the efferent ducts of the rat are higher than those found in the epididymis, where low pH and bicarbonate contribute to sperm quiescence during storage. The high rate of bicarbonate reabsorption in the efferent ducts is considered a major contributor to the establishment of the low pH and bicarbonate milieu of the epididymis; bicarbonate has a role in role in balancing pH both in vivo and in vitro.
In vivo studies in rats found significant acidification in caput and cauda epididymis whereas little acidification was detected in the seminiferous tubules in the testis.31,32 Later, in vivo micropuncture studies in the rat seminiferous tubules and epididymal duct were devised to study acidification by direct measurement of intraluminal pH by using a highly reliable pH microelectrode.8 The study found that pH in the proximal caput (pH 6.6), middle caput (pH 6.59), and proximal cauda epididymis (pH 6.8) were significantly more acidic than testicular artery (pH 7.4) or systemic arterial blood (pH 7.4). A considerable amount of information suggests that pH and the bicarbonate concentration in the lumen of the extra testicular ducts have a direct physiologic role in reproductive function. Several previous studies indicated that these factors are involved in the regulation of sperm metabolism and motility.11,12,30,37 For instance, lower intracellular pH suppresses sperm metabolism and thus motility,11 while bicarbonate has a role in activating sperm adenylyl cyclase,38 and thus increases cAMP production. Other reports indicate that pH and bicarbonate levels are regulated in the lumen of the extratesticular ducts and decline along the epididymis.4,8,42 The low pH and bicarbonate concentration in the cauda epididymis keeps sperm in a quiescent state during tightly packed epididymal storage.1,4,12,30,38
Computer-aided sperm analysis (CASA) systems allow analysis of large numbers of spermatozoa and provide values that are representative of the sample, minimizing subjective bias in choosing sperm for motion characterization. During sperm motion analysis, the most important characteristics are the progressive motility and various velocity parameters like curvilinear velocity (VCL, total distance traveled during observation period), straight-line velocity (VSL, net space gain during observation period), and average path velocity (VAP, distance traveled in average direction of movement). The percentage of progressively motile sperm in any given population is a sensitive indicator of adverse effects on sperm motion and ultimately determines how many sperm will reach the oocytes and perform proper fertilization. In addition, VCL, VSL, and VAP are useful and sensitive indicators of adverse effects on sperm motion. For example, decreased VSL values are associated with less in in vivo fertilization, and a fall in VSL predicts that sperm will travel a shorter distance.45
The results of the current study indicate that mice that have been euthanized with either high flow or low flow CO2 have few changes in sperm recovery or motility as compared with mice euthanized by CD. Although the VCL was significantly lower in thawed samples from mice euthanized by high flow CO2 as compared with the CD group, the biologic relevance of this difference is minimal. Our study tested the 30% displacement/minute flow rate recommended by the AVMA and a 100% displacement/minute as this is the highest flow rate possible if had wanted to titrate the flow rate to determine the optimal rate for preserving sperm quality. However, we emphasize to readers that if CO2 is used to euthanize mice for sperm collection, then a 30% flow rate should be used as it is in accordance with the 2020 AVMA Guidelines for the Euthanasia of Animals, and has been demonstrated to not impact sperm quality.5 In addition, based on our study we can infer that a flow rate between 30% to 70% should not affect sperm quality, however, studies using 70% displacement per minute would give further guidance. Our data indicate that CO2 euthanasia is a viable alternative to CD when collecting sperm for cryopreservation and other downstream applications.
Several early studies investigated the effects of euthanasia using CO2 and various inhalation agents on Sprague–Dawley rat epididymal sperm motility characteristics. One group tested decapitation, ether or halothane inhalation, and high or low rate CO2 asphyxiation, but found no significant differences among the treatments with regards to sperm motility.46 Similarly, another group euthanized male rats using either high-flow rate CO2, decapitation, and inhalation anesthetics enflurane, halothane, isoflurane, or sevoflurane, but did not detect any differences in progressive motility among the treatments.46 Later, another study compared isoflurane and low flow rate CO2 asphyxiation on rat sperm and did not find significant effects on sperm motility even after 10 min of CO2 exposure. Thus, our study in mice is consistent with these previous studies on rat sperm collected after CO2 euthanasia.9
One of the most important considerations in any animal experimentation is that the method of euthanasia should not adversely influence the immediate postmortem viability and the function of the collected cells or tissues needed for other in vitro experimentation. This would ultimately ensure quantitative and qualitative assessment of the data collected. Successful assisted reproductive techniques, such as cryopreservation, IVF, or sperm microinjection, require germplasm of high quality and quantity. To date, relatively few studies have investigated the influence of euthanasia methods on postmortem sperm, oocyte and embryos.24,27,39,43,53 One study examined the influence of delayed dissection of oviducts after CD on MII mouse oocyte viability and embryo developmental competence in vitro.22 The study determined that retaining the MII oocytes or pre‐implantation embryos in the oviduct for up to 30 min resulted in lower oocyte viability upon recovery and adversely affected the in vitro developmental potential. Two studies have shown adverse effects of CO2 euthanasia on IVF ability for C57BL/6 oocytes due to largely premature exocytosis of cortical granules.24,53 Another group examined the quality of mouse zygotes derived from 3 strains of mice (C57BL/6, B6SJLF1 and FVB/N) after euthanasia by either high-flow rate CO2 (100% displacement) or CD.27 They found no difference between high CO2 and CD with regard to the morphologically normal zygote yield among the 3 mouse strains. In addition, they found no significant difference between high flow CO2 and CD euthanasia on the in vitro development potential of the zygotes to blastocyst stage. Our study did not find a significant difference in terms of fertilization ability of spermatozoa in development of 2-cell stage embryos between either CO2 euthanasia method or CD; this further demonstrates their functional viability at the cellular level, as indicated by mitochondrial, plasma membrane and acrosome integrity and is consistent with the progressive motility values, which were also not affected by the euthanasia methods in our study.
The blastocyst stage embryo development rates in our study were also comparable between CO2 and CD euthanasia. Sperm DNA must be specifically organized in a unique condensed state that differs considerably from that of somatic cells or oocytes. Sperm chromatin undergoes extensive modifications during spermatogenesis, and the addition of protamines lead to very tightly packed chromatin.33 Thus, the chromatin contained in the nucleus of a fully developed spermatozoa is a very stable structure. Therefore, the chromatin of mature sperm is much more resistant to damages caused by potentially harmful agents than that of spermatogonia in seminiferous tubules, somatic cells, or oocytes.17 To date, few other studies have examined the effects of euthanasia method on in vitro embryonic developmental competence.26,27 A previous, related study investigated the quality of one-cell embryos derived from 3 strains of mice (C57BL/6, FVB/N, and B6SJLF1) that were euthanized by either high-flow rate CO2 or CD.27 That study found no difference between CO2 and CD in terms of the morphologically normal zygote yield and in vitro development potential of the one-cell embryos to blastocyst stage. These studies clearly suggest that once fertilization take place, development to later stages such as 2-cell to morula and blastocyst are developmentally more robust and thus are more tolerant of suboptimal conditions.26,27
The use of CO2 rather than CD is beneficial in many ways. One obvious benefit is better animal welfare. As stated earlier, even highly trained professionals can perform incomplete CD euthanasia. In addition, becoming proficient at CD requires practice that typically involves live animals.10 Replacing CD with CO2 euthanasia fulfills the 2 of the 3Rs by refining euthanasia methods while conserving sperm quality and by reducing the overall number of animals used in research by choosing a technique that does not require extensive training on live animals.16 Finally, replacing CD with CO2 euthanasia can help to reduce compassion fatigue in research staff. Compassion fatigue is a well-known issue for persons those who work with laboratory animals, especially those who perform euthanasia.40 Replacing a physical method of euthanasia with a more aesthetically pleasing method can perhaps help to reduce compassion fatigue in these individuals.
This study showed that, unlike in oocyte collection from female mice, euthanasia of male mice by CO2 asphyxiation is suitable for collection of epididymal sperm for cryopreservation or other ARTs. We suggest that various innate biologic mechanisms, including the blood–testes barrier, relatively low pH, and higher osmotic pressure in the cauda epididymis maintain the sperm in a relatively acidic and dehydrated state and likely protect mouse sperm from potential damage at the nuclear, cytoplasmic, and cellular level. Our study used C57BL/6 mice because they are one of the most commonly used strains in research, and a common background strain for transgenic mice. We can infer that our results apply to other mouse strains, but future studies using other strains would provide further insight. Thus, for the purpose of collecting mouse sperm for cryopreservation or other ARTs, euthanasia via CO2 asphyxiation can be used as an alternative method in circumstances where CD is not preferred due to personnel distress and the potential development of compassion fatigue.
Acknowledgments
Technical support was provided by Corinne Piotter, Christine Bethune and Jennifer Walls.
Support for SG was provided in part by the Joseph E Wagner Fellowship in Laboratory Animal Medicine. Mice were provided by the MMRRC supported by the NIH grant U42OD010918.
References
- 1.Acott TS, Carr W. 1984. Inhibition of bovine spermatozoa by caudal epididymal fluid: II. Interaction of pH and a quiescence factor. Biol Reprod 30:926–935. 10.1095/biolreprod30.4.926. [DOI] [PubMed] [Google Scholar]
- 2.Agca Y, Agca C. 2021. Cryopreservation of Mouse Sperm for Genome Banking, p 401–412. In Wolkers WF, Oldenhof H, editors. Cryopreservation and Freeze-Drying Protocols. Methods in Molecular Biology, 2180. New York (NY): Humana. 10.1007/978-1-0716-0783-1_17. [DOI] [PubMed] [Google Scholar]
- 3.Agca Y. 2012. Genome resource banking of biomedically important laboratory animals. Theriogenology 78:1653–1665. 10.1016/j.theriogenology.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Au CL, Wong PYD. 1980. Luminal acidification perfused rat cauda epididymis. J Physiol 309:419–427. 10.1113/jphysiol.1980.sp013517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AVMA Guidelines for the Euthanasia of Animals. 2020. [Cited November 8, 2019] Available at: https://www.avma.org/sites/default/files/2020-01/2020-Euthanasia-Final-1-17-20.pdf.
- 6.Bath ML. 2003. Simple and efficient in vitro fertilization with cryopreserved C57BL/6J mouse sperm. Biol Reprod 68:19–23. 10.1095/biolreprod.102.007344. [DOI] [PubMed] [Google Scholar]
- 7.Boivin GP, Hickman D, Creamer-Hente M, Pritchett-Corning K, Bratcher N. 2017. Review of CO2 as a euthanasia agent for laboratory rats and mice. J Am Assoc Lab Anim Sci 56:491–499. [PMC free article] [PubMed] [Google Scholar]
- 8.Caflisch CR, DuBose TD. 1990. Direct evaluation of acidification by rat testis and epididymis. Am J Physiol 258:E143–E150. [DOI] [PubMed] [Google Scholar]
- 9.Campion SN, Cappon GD, Chapin RE, Jamon RT, Winton TR, Nowland WS. 2012. Isoflurane reduces motile sperm counts in the Sprague-Dawley rat. Drug Chem Toxicol 35:20–24. 10.3109/01480545.2011.564182. [DOI] [PubMed] [Google Scholar]
- 10.Carbone L, Carbone ET, Yi EM, Bauer DB, Lindstrom KA, Parker JM, Austin JA, Seo Y, Gandhi AD, Wikerson JD. 2012. Assessing cervical dislocation as a humane euthanasia method in mice. J Am Assoc Lab Anim Sci 51:352–356. [PMC free article] [PubMed] [Google Scholar]
- 11.Carr DW, Acott TS. 1989. Intracellular pH regulates bovine sperm motility and protein phosphorylation. Biol Reprod 41:907–920. 10.1095/biolreprod41.5.907. [DOI] [PubMed] [Google Scholar]
- 12.Carr DW, Ysselman MC, Acott TS. 1985. Effects of pH, lactate, and viscoelastic drag on sperm motility: a species comparison. Biol Reprod 33:588–595. 10.1095/biolreprod33.3.588. [DOI] [PubMed] [Google Scholar]
- 13.Cheng CY, Mruk DD. 2012. The blood-testis barrier and its implications for male contraception. Pharmacol Rev 64:16–64. 10.1124/pr.110.002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooper TG. 1986. The epididymis, sperm maturation, and fertilization. Heidelberg, Germany: Springer Berlin. [Google Scholar]
- 15.Dolores D, Cheng C. 2015. The mammalian blood-testis barrier: Its biology and regulation. endrocr rev. 36:564–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopkins E. 2020. Physiology, Acid Base Balance. StatPearls. [PubMed] [Google Scholar]
- 17.Evenson DP, Higgins PJ, Grueneberg D, Ballachey BE. 1985. Flow cytometric analysis of mouse spermatogenic function following exposure to ethylnitrosourea. Cytometry 6:238–253. 10.1002/cyto.990060311. [DOI] [PubMed] [Google Scholar]
- 18.Fenwick N, Danielson P, Griffin G. 2011. Survey of Canadian animal-based researchers’ views on the Three Rs: replacement, reduction and refinement. PLoS One 6:e22478. 10.1371/journal.pone.0022478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fenwick N, Griffin G, Gauthier C. 2009. The welfare of animals used in science: How the “Three Rs” ethic guides improvements. Can Vet J 50:523–530. [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher S, Burgess WL, Hines KD, Mason GL, Owiny JR. 2016. Inter strain differences in CO2-induced pulmonary hemorrhage in mice. J Am Assoc Lab Anim Sci 55:811–815. [PMC free article] [PubMed] [Google Scholar]
- 21.Fuku E, Xia L, Downey BR. 1995. Ultrastructural changes in bovine oocytes cryopreserved by vitrification. Cryobiology 32:139–156. 10.1006/cryo.1995.1013. [DOI] [PubMed] [Google Scholar]
- 22.George MA, Doe BG. 1989. The influence of handling procedures during mouse oocyte and embryo recovery on viability and subsequent development in vitro. J In Vitro Fert Embryo Transf 6:69–72. 10.1007/BF01130728. [DOI] [PubMed] [Google Scholar]
- 23.Gurumurthy CB, Kent Lloyd C. 2019. Generating mouse models for biomedical research: technological advances. Disease Mls & Mechanisms 12(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazzard KC, Watkins-Chow DE, Garrett LJ. 2014. Method of euthanasia influences the oocyte fertilization rate with fresh mouse sperm. J Am Assoc Lab Anim Sci 53:641–646. [PMC free article] [PubMed] [Google Scholar]
- 25.Hickman DL, Johnson J, Vemulapalli TH, Crisler JR, Shepher R. 2017. Chapter 7 - Commonly used animal models, p 117–175. In: Suckow MA, Stewart KL, editors. Principles of animal research for graduate and undergraduate students. San Diego (CA): Elsevier. [Google Scholar]
- 26.Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. 1995. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev 41:232–238. 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- 27.Howell RL, Donegan CL, Pinkert CA. 2003. Mouse embryo yield and viability after euthanasia by CO2 inhalation or cervical dislocation. Comp Med 53:510–513. Available at https://www.ncbi.nlm.nih.gov/books/NBK507807/. [PubMed] [Google Scholar]
- 28.Institute for Laboratory Animal Research. 2011.Guide for the care and use of laboratory animals 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 29.Jackson KV, Kiessling AA. 1989. Fertilization and cleavage of mouse oocytes exposed to the conditions of human oocyte retrieval for in vitro fertilization. Fertil Steril 51:675–681. 10.1016/S0015-0282(16)60620-9. [DOI] [PubMed] [Google Scholar]
- 30.Jones RC, Murdoch RN. 1996. Regulation of the motility and metabolism of spermatozoa for storage in the epididymis of eutherian and marsupial mammals. Reprod Fertil Dev 8:553–568. 10.1071/RD9960553. [DOI] [PubMed] [Google Scholar]
- 31.Levine N, Kelly H. 1978. Measurement of pH in the rat epididymis in vivo. J Reprod Fertil 52:333–335. 10.1530/jrf.0.0520333. [DOI] [PubMed] [Google Scholar]
- 32.Levine, Marsh DJ. 1971. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. Journal of Physiology 213:557–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manandhar G, Sutovsky P. 2007. Comparative histology and subcellular structures of mammalian spermatogenesis and spermatozoa, p 81–98. In: Schatten H, Constantinescu GH, editors. Comparative reproductive biology. Ames (IA): Blackwell Publishing. 10.1002/9780470390290.ch3c [DOI] [Google Scholar]
- 34.Miyamoto H, Toyoda Y, Chang MC. 1974. Effect of hydrogen-ion concentration on in vitro fertilization of mouse, golden hamster, and rat eggs. Biol Reprod 10:487–493. 10.1095/biolreprod10.4.487. [DOI] [PubMed] [Google Scholar]
- 35.Newcombe N, Clulow J, Man SY, Jones RC. 2000. 0pH and bicarbonate in the ductuli efferentes testis of the rat. Int J Androl 23:46–50. 10.1046/j.1365-2605.2000.00204.x. [DOI] [PubMed] [Google Scholar]
- 36.Nishigaki T, José O, González-Cota AL, Romero F, Treviño CL, Darszon A. 2014. Intracellular pH in sperm physiology. Biochem Biophys Res Commun 450:1149–1158. 10.1016/j.bbrc.2014.05.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okamura N, Tajima Y, Soejima A, Musuda H, Sugita Y. 1985. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J Biol Chem 260:9699–9705. 10.1016/S0021-9258(17)39295-5. [DOI] [PubMed] [Google Scholar]
- 38.Okamura N, Tajima Y, Sugita Y. 1987. Regulation of mammalian sperm activity by bicarbonate in genital fluids, 197–203. In: Mohri H, editor. New horizons in sperm cell research. Japan Scientific Societies Press. [Google Scholar]
- 39.Puissant F, Degueldre M, Buisson L, Leroy F. 1986. Effects of carbon dioxide acidification of mouse oocytes before in vitro fertilization, culture, and transfer. Gamete Res 13:223–230. 10.1002/mrd.1120130305. [DOI] [Google Scholar]
- 40.Randall MS, Moody CM, Turner PV. 2021. Mental wellbeing in laboratory animal professionals: A cross-sectional study of compassion fatigue, contributing factors, and coping mechanisms. J Am Assoc Lab Anim Sci. 60:54–63. 10.30802/AALAS-JAALAS-20-000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perlman RL. 2016. Mouse models of human disease. Evol Med Public Health 2016: 170–176. 10.1093/emph/eow014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Martinez H, Ekstedt E, Einarsson S. 1990. Acidication of the epididymal fluid in the boar. Int J Androl 13:238–243. 10.1111/j.1365-2605.1990.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 43.Roustan A, Perrin J, Berthelot-Ricou A, Lopez E, Botta A, Courbiere B. 2012. Evaluating methods of mouse euthanasia on the oocyte quality: Cervical dislocation versus isoflurane inhalation. Lab Anim 46:167–169. 10.1258/la.2012.011115. [DOI] [PubMed] [Google Scholar]
- 44.Scotney R. 2016. Occupational stress and compassion fatigue: The effects on workers in animal-related occupations. Phychology. Ph.D. Thesis, University of Queensland, Queensland, Australia [Google Scholar]
- 45.Slott VL, Linder RE, Dyer CJ. 1994. Method of euthanasia does not affect sperm motility in the laboratory rat. Reprod Toxicol 8:371–374. 10.1016/0890-6238(94)90053-1. [DOI] [PubMed] [Google Scholar]
- 46.Stutler SA, Johnson EW, Still KR, Schaeffer DJ, Hess RA, Arfsten DP. 2007. Effect of method of euthanasia on sperm motility of mature Sprague-Dawley rats. J Am Assoc Lab Anim Sci 46:13–20. [PubMed] [Google Scholar]
- 47.Summers MC. 2014. A brief history of the development of the KSOM family of media. Hum Fertil (Camb) 17 sup1:12–16. 10.3109/14647273.2014.919185. [DOI] [PubMed] [Google Scholar]
- 48.Takeo T, Nakagata N. 2011. Reduced glutathione enhances fertility of frozen/thawed C57BL/6 mouse sperm after exposure to methyl-Betacyclodextrin. Biol Reprod 85:1066–1072. 10.1095/biolreprod.111.092536. [DOI] [PubMed] [Google Scholar]
- 49.Takeo T, Hoshii T, Kondo Y, Toyodome H, Arima H, Yamamura KI, Irie T, Nakagata N. 2008. Methyl-beta-cyclodextrin improves fertilizing ability of C57BL/6 mouse sperm after freezing and thawing by facilitating cholesterol efflux from the cells. Biol Reprod 78:546–551. 10.1095/biolreprod.107.065359. [DOI] [PubMed] [Google Scholar]
- 50.Varisli O, Uguz C, Agca C, Agca Y. 2009. Various physical stress factors on rat sperm motility, integrity of acrosome, and plasma membrane. J Androl 30:75–86. 10.2164/jandrol.107.004333. [DOI] [PubMed] [Google Scholar]
- 51.Varisli O, Yguz C, Agca C, Agca Y. 2009. Effect of chilling on the motility and acrosomal integrity of rat sperm in the presence of various extenders. J Am Assoc Lab Anim Sci 48:499–505. [PMC free article] [PubMed] [Google Scholar]
- 52.Wuri L, Agca C, Agca Y. 2019. Euthanasia via CO2 inhalation causes premature cortical granule exocytosis in mouse oocytes and influences in vitro fertilization and embryo development. Mol Reprod Dev 86:825–834. 10.1002/mrd.23167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloter E, Schmid TE, Marchetti F, Eskenazi B, Nath J, Wyrobek AJ. 2006. Quantitative effects of male age on sperm motion. Hum Reprod 21:2868–2875. 10.1093/humrep/del250. [DOI] [PubMed] [Google Scholar]