Abstract
Introduction
Given prior work showing racial differences on baseline social determinants of health (SDoH) and 10‐year trajectories of everyday functioning, we examined associations between SDoH and longitudinal everyday functioning performance in Black/African American and White older adults.
Methods
Participants were 2505 older adults (Mage = 73.5; 28% Black/African American) without dementia. SDoH included economic stability/status, education access/quality, health‐care access, neighborhood/built environment, and social/community contexts. The Observed Tasks of Daily Living (OTDL) measured everyday functioning and was administered at baseline and 1‐, 2‐, 3‐, 5‐, and 10‐year visits.
Results
Across the sample, social and community context and economic stability/status were associated with steeper age‐related OTDL declines (βs = 0.05 to 0.07, Ps < 0.001). Lower levels of social and community context (β = 0.08, P = 0.002) and economic stability/status (β = 0.07, P = 0.04) were associated with OTDL linear age declines in Black/African American participants, but not in White participants (Ps > 0.30).
Discussion
Inequities across SDoH accelerate age‐related declines in everyday functioning among Black/African American older adults.
Keywords: ACTIVE study, disparities, everyday functioning, social determinants of health
1. INTRODUCTION
As we move toward engaging in more inclusive clinical care and research efforts that better address and characterize pathological aging in minoritized older adults, it is imperative that we acknowledge the important role that social and structural factors play in determining late‐life cognitive and functional outcomes. The life course perspective to aging emphasizes that cognitive and brain health are fundamentally shaped by the social and physical environments an individual interacts with throughout early, mid‐, and late life. 1 , 2 , 3 However, there are incredible differences in social and environmental conditions across racial/ethnic groups within the United States. Black/African American individuals have been systematically and intentionally excluded from opportunities to accrue wealth, attain quality education, and pursue occupations of prestige and power. Although the Fair Housing Act was passed in 1968, segregation and other systematic barriers that have had a disproportionately negative impact on minoritized individuals continue to be pervasive and impact these community members today. Minoritized individuals are more likely to be exposed to harmful conditions (e.g., economic inequality, lower resourced neighborhoods and schools) and social situations (e.g., discrimination). 4 , 5 , 6 , 7 The impact of these negative experiences may accumulate over time, can become biologically embedded, lead to reduced cognitive and brain reserve, and subsequently increase dementia risk. 1 , 5
RESEARCH IN CONTEXT
Systematic Review: Authors reviewed literature on life course theory and social determinants of health (SDoH). SDoH contribute to inequities experienced throughout one's life that increase late‐life dementia risk by activating biological pathways that reduce cognitive and brain reserve. While greater cumulative disadvantage across SDoH has been linked to poor functional outcomes, whether SDoH differentially relate to longitudinal trajectories of everyday functioning across racial groups has yet to be fully characterized.
Interpretation: In a large multi‐site study of community‐dwelling older adults without dementia, (1) SDoH are associated with age‐related declines in everyday functioning across the sample and (2) the impact of SDoH was greater in Black compared to White participants.
Future Directions: Although we identify key intervention targets that may reduce inequitable functional outcomes in older adults, future actions must center on dismantling upstream drivers of SDoH‐related inequities attributable to unjust systems of power.
Social and environmental risk factors are commonly referred to as social determinants of health (SDoH) and the Healthy People 2030 campaign has delineated five distinct categories of risk that include (1) economic stability/status, (2) education access and quality, (3) health‐care access, (4) neighborhood and built environment, and (5) social and community contexts. 6 However, it is worthwhile to note there are several National Institutes of Health (NIH)–related frameworks with slight variations in the conceptualization of these factors. 5 , 7 These SDoH can be measured on the individual or larger societal level and have consistently been linked to cognitive and brain health outcomes in late adulthood. 8 For example, economic instability/status as measured by lower levels of income, wealth, or home ownership has been linked to faster rates of cognitive decline and reduced brain volume in older adult samples. 9 , 10 , 11 , 12 Poor educational access and quality in early life has also been linked to an increased risk for dementia in late life. 13 , 14 Furthermore, neighborhood and community characteristics in the form of higher density housing and lower walkability are associated with poorer cognitive performance in older adult samples. 15 , 16
In addition to cognitive and brain health, recent evidence suggests that SDoH also play an important role in instrumental activities of daily living (IADLs). 17 IADLs encompass a wide array of complex functional tasks (e.g., managing finances, completing household chores) and are essential for living independently. 18 , 19 Assessment of IADLs is also a crucial component of clinical decision making surrounding dementia diagnosis. 20 , 21 One recent study in a large sample of respondents from the National Health and Nutrition Survey revealed that cumulative disadvantage across multiple SDoH (e.g., poverty, food insecurity) was associated with greater IADL impairment and poorer health in older adults. 17 Furthermore, results revealed that Hispanic and Black/African American respondents within the study were more likely to have higher cumulative disadvantage across multiple SDoH relative to non‐Hispanic White respondents, possibly contributing to increased risk for dementia and poor late‐life functional outcomes in these minoritized racial/ethnic groups. Importantly, there is an urgent need to clarify the role of SDoH as targets for the promotion and preservation of independence in older adults while society continues to confront and remedy the unjust systems of power and structure that ultimately lead to health disparities. Nevertheless, some structural SDOH (e.g., education inequalities) might be offset by compensatory strategies (e.g., adult education, cognitive training) in the immediate interim.
Prior work in the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial has demonstrated that across a large community‐based sample of Black/African American and White older adults, SDoH are linked to baseline composite scores of processing speed, memory, and reasoning and health‐related quality of life, 22 and that with increasing age, Black/African American participants experience accelerated decline on the Observed Tasks of Daily Living (OTDL) relative to White participants. 23 However, the independent effects of SDoH on longitudinal everyday functioning and whether this differs across Black/African American and White older adults remains less clear. The current project sought to enhance our understanding of the impact of SDoH on everyday functioning by examining (1) the association between baseline SDoH scores and 10‐year change in OTDL across the sample, (2) race by SDoH interactions on OTDL trajectories, and (3) associations between SDoH and OTDL trajectories stratified within each racial group. Our goal was to clarify which SDoH negatively impact everyday functioning within Black/African American and White older adults to inform the development of immediate targeted interventions centered on preserving independence in late life.
2. METHODS
2.1. Participants
Data were obtained from the ACTIVE study, a multi‐site randomized controlled trial of cognitive training interventions in older adults that also included longitudinal cognitive, functional, and health assessments. 24 , 25 ACTIVE participants consisted of community‐dwelling adults aged 65 and older that were recruited from six sites throughout the United States (University of Alabama at Birmingham, Hebrew Rehabilitation Center in Boston, Indiana University, Johns Hopkins University, Wayne State University, and Pennsylvania State University) between March 1998 and October 1999. Participants were excluded from ACTIVE if they demonstrated a Mini‐Mental State Examination (MMSE) score <23; reported impairment in basic activities of daily living; self‐reported diagnoses of Alzheimer's disease, stroke, or cancer; displayed communication difficulties; or had <20/50 visual acuity.
Each participant was randomized to one of the 10‐week cognitive training intervention groups (memory, reasoning, speed, no‐contact control). Assessments were completed prior to (baseline) and immediately after training, and at 1‐, 2‐, 3‐, 5‐, and 10‐year follow‐up visits. ACTIVE was approved by the institutional review boards of all participating sites and written informed consent was obtained for all study participants.
The present study consisted of 2505 participants that (1) had baseline SDoH composite data available, (2) completed longitudinal performance‐based functional assessments, and (3) self‐identified as White or Black.
2.2. SDoH composites
Previous work within the cohort has used self‐report baseline study questionnaire, US Census information, and North American Industry Classification System data to characterize social and structural determinants that have been linked to health.22,26 Prior analyses used a principal components extraction method to delineate five orthogonal components that explain roughly 68% of the shared variance among the 16 variables. These principal components correspond to the social determinants of health outlined by Healthy People 2030 that included (1) economic stability/status, (2) education access and quality, (3) health‐care access and quality, (4) neighborhood and built environment, and (5) social and community context (see Thomas and Marsiske 23 for more detail). The economic stability/status factor included the percentage of individuals within the neighborhood with a college degree, the presence of sports and recreation instruction facilities, median home value, and median rent. The education access and quality consisted of years of education, reflected occupational “data” codes, reflected occupational “people” codes, and reflected occupational “thing” codes. The health‐care access and quality factor consisted of the number of pharmacies and drug stores, physician offices, services for elderly and disabled, and supermarkets or grocery stores. The neighborhood and built environment factor consisted of owner occupancy and single unit dwellings. Finally, the social and community context factor consisted of the number of golf courses and country clubs, percentage of White residents in the neighborhood, and the number of supermarkets and grocery stores. See Table 1 for a complete listing of determinants of interest and how these items loaded onto SDoH factors. Higher scores on the components represent higher levels of the named dimensions and have also been linked to better cognitive performance.
TABLE 1.
Variables underlying SDoH factors
| Economic Stability/Status Factor (16.7%) | Healthcare Access and Quality (14.2%) | Educational Access and Quality (14.0%) | Neighborhood and Built Environment (12.2%) | Social and Community Context (10.6%) | |
|---|---|---|---|---|---|
| % with college degree | X | ||||
| Sports and recreation instruction | X | ||||
| Median home value | X | ||||
| Median rent | X | ||||
| Pharmacies and drug stores | X | ||||
| Physician offices | X | ||||
| Services for elderly and disabled | X | ||||
| Supermarkets and other grocery stores | X | –X | |||
| Years of education | X | ||||
| Reflected occupational “data” codes | X | ||||
| Reflected occupational “people” | X | ||||
| Reflected occupational “thing” | –X | ||||
| Owner occupancy | X | ||||
| Single unit dwellings | X | ||||
| Golf courses and country clubs | X | ||||
| % White residents in neighborhood | X |
Note: See Clay et al. 22 for complete listing of factor loading weights.
Abbreviations: SDoH, social determinants of health; X, indicated positively loaded onto factor; –X, indicates negatively loaded on factor.
2.3. Everyday functioning
The OTDL is a performance‐based measure that was used to characterize everyday functioning. 27 Participants completed nine tasks pertaining to medication and telephone use and financial management. Higher OTDL scores have been linked to better performance on cognitive measures 27 , 28 and lower scores have been shown to distinguish between participants with and without mild cognitive impairment (MCI). 29 OTDL total scores for baseline and 1‐, 2‐, 3‐, 5‐, and 10‐year visits were used in the present study. While cognitive training benefits were found for self‐reported IADL functioning in ACTIVE, no improvements were found for the OTDL. 24 , 30 , 31
2.4. Covariates
The models described below included multiple covariates including sex (dummy‐coded as male and female), vocabulary score as a proxy for literacy, 32 general cognition defined using the MMSE, 33 general health subscale from the 36‐Item Short Form Health Survey (SF‐36), 34 depressive symptoms measured using the Center for Epidemiological Studies–Depression‐12 (CES‐D), 35 and visual acuity to adjust for other variables contributing to OTDL performance as well as selective attrition. 24 Further, while no intervention effects of the memory, reasoning, or speed training have been detected on OTDL, 30 intervention group and whether someone received booster training (along with the interactions with time) were included to adjust for any disturbances in the longitudinal change trajectories due to training. Finally, study site and replicate (six testing cohorts were included to manage data collection workflow) were included in the models.
2.5. Statistical analyses
We followed the comprehensive framework and guidelines of the Ward et al. 36 in our explorations of the effects of SDoH on change in everyday functioning over time in older adults. We started by exploring group‐specific differences in (1) SDoH (exposure prevalence) and (2) OTDL performance over time (outcome prevalence), 24 and then we (3) subsequently examined race x SDoH interactions on OTDL trajectories and race‐stratified SDoH and OTDL associations. As noted by Ward et al., exploring the relationship between exposure, outcomes, and race‐by‐exposure interactions are critical for disparity investigations and evaluating the interaction term alone may not be sufficient. 36
Analyses of variance and chi‐squared analyses examined racial group differences in sample characteristics. Analyses of covariance adjusting for age, sex, level of education, vocabulary score, CES‐D depression score, MMSE score, recruitment wave, and study site examined racial group differences across all five SDoH domains. 36
Mixed‐effect models using age as the time scale were used to examine (1) the associations of SDoH with 10‐year change in OTDL across the sample, (2) whether race moderated the SDoH associations with OTDL trajectories, and (3) the associations of SDoH and OTDL trajectories within race. Models were run separately for each SDoH domain. Race x SDoH models included all main effects, SDoH x linear age and SDoH x age2, race x linear age and race x age2, and a three‐way interaction term for race x SDoH x linear age. Models initially included the three‐way interactions with both linear and quadratic effects of age; however, the interaction term with age2 was not significant for any SDoH and model fit per log‐likelihood, Akaike information criterion, and Bayesian information criterion was optimized with the exclusion of the age2 three‐way interaction for four out of five SDoH. Therefore, for model parsimony, this parameter (race x SDoH x quadratic age) was excluded. Race‐stratified models included the following predictors of interest: main effect of SDoH, main effect of linear age, main effect of age2, and interaction between SDoH by linear age. The interaction between SDoH by age2 was not significant for any SDoH and was excluded for model parsimony. Covariates described above were included in all mixed effects models. Intercept, age, and age2 were included as random effects. All predictor variables were centered around the mean. Models were estimated using full‐information log likelihood maximization and used a continuous autoregressive covariance structure. General purpose optimization was used with a maximum of 150 iterations to achieve model convergence. Analyses were conducted in R version 3.5.0 (https://cran.r‐project.org/).
3. RESULTS
3.1. Sample characteristics
Participant characteristics are presented in Table 2. On average, relative to the Black/African American group, the White group was older, had more years of education, higher vocabulary scores and MMSE scores, and endorsed better health (Ps > 0.001). The Black/African American group had more females and higher rates of MCI (P < 0.008) relative to the White group.
TABLE 2.
Participant characteristics
| Overall | White | Black/African American | F or χ2 | P | |
|---|---|---|---|---|---|
| Age | 73.53 (5.83) | 74.05 (5.93) | 72.20 (5.35) | 52.16 | <0.001 |
| Sex, % female | 76% | 73.2% | 83.5% | 29.30 | <0.001 |
| Education | 13.53 (2.70) | 13.76 (2.68) | 12.93 (2.65) | 49.54 | <0.001 |
| Vocabulary total score | 12.37 (3.96) | 14.40 (3.33) | 9.73 (4.19) | 526.97 | <0.001 |
| MCI diagnosis, % yes | 19% | 18% | 22% | 7.1 | 0.008 |
| MMSE total score | 27.30 (2.01) | 27.59 (1.91) | 26.57 (2.07) | 134.68 | <0.001 |
| CES‐D total score | 5.20 (5.25) | 5.18 (4.75) | 5.20 (5.11) | 0.008 | 0.928 |
| SF‐36 general health total | 69.16 (19.15) | 70.05 (19.06) | 66.84 (19.20) | 13.91 | <0.001 |
Abbreviations: CES‐D, Center for Epidemiological Studies—Depression‐12; MCI, mild cognitive impairment; MMSE, Mini‐Mental State Examination; SF‐36, 36 Item Short Form Health Survey.
3.2. Racial differences in SDoH
There were significant racial group differences in economic stability/status (β = –0.17, 95% confidence interval [CI: –0.21, –0.13], t = –19.53, P < 0.001), access to health care (β = 0.09, 95% CI [0.06, 0.12], t = 13.88, P < 0.001), neighborhood and built environment (β = –0.13, 95% CI [–0.18, –0.09], t = –13.36, P < 0.001), educational/occupational complexity (β = –.24, 95% CI [–0.28, –0.20], t = –24.57, P < 0.001), and social and community context (β = –0.40, 95% CI [–0.44, –0.36], t = –42.31, P < 0.001). Black/African American older adults had significantly lower scores across all SDoH with the exception of health‐care access. See Figure 1.
FIGURE 1.
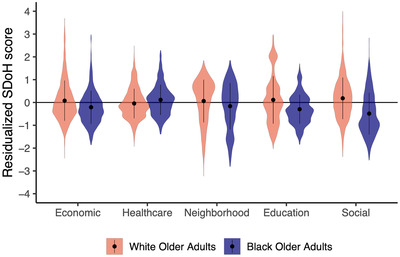
Residualized social determinants of health (SDoH) scores for Black/African American and White older adults. Black/African American older adults are represented by Blue and White older adults are represented by red. On the y‐axis SDoH are listed with economic = economic stability/status, healthcare = health‐care access and quality, neighborhood = neighborhood and built environment, education = education access and quality, social = social and community context.
3.3. OTDL trajectories
The overall sample demonstrated a significant linear and quadratic effect of decline in OTDL trajectories over time (linear β = –0.21, 95% CI [–0.23, –0.18], t = –17.36, P < 0.001; quadratic β = –0.006, 95% CI [–0.009, –0.004], t = 5.01, P < 0.001). Significant race x time (age) interactions were observed for age‐related OTDL declines (race x linear age: β = –0.25, 95% CI [–0.30, –.019], t = –8.89, P < 0.001; race x quadratic age: β = –0.009, 95% CI [–0.02, –0.004], t = –3.28, P = 0.001). When stratified by race, there was a significant linear and quadratic effect of age on OTDL trajectories over time among Black/African American older adults (linear β = –0.42, 95% CI [–0.48, –0.36], t = –13.69, P < 0.001; quadratic β = ‐0.01, 95% CI [–0.02, –0.008], t = –4.74, P < 0.001) and White older adults (linear β = –0.16, 95% CI [–0.18, –0.13], t = –13.59, P < 0.001; quadratic β = –0.005, 95% CI [–0.007, –0.002], t = –3.84, P < 0.001). See Figure 2.
FIGURE 2.
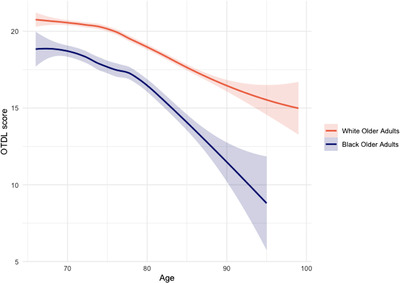
Racial group trajectories of predicted Observed Tasks of Daily Living (OTDL) scores over time. Trajectory of Black/African American older adults (navy) and White older adults (red).
3.4. SDoH on OTDL trajectories
Across the sample, there was a significant linear effect of economic stability/status (β = 0.04, 95% CI [0.02, 0.06], t = 3.84, P < 0.001), and social and community context (β = 0.04, 95% CI [0.02, 0.06], t = 4.13, P < 0.001) on OTDL trajectories such that lower levels of these SDoH were associated with a faster decline in OTDL scores over time. There was no significant effect of educational/occupational access, neighborhood and built environment, or health‐care access on OTDL trajectories (all Ps > 0.05).
3.5. Race, SDoH, and OTDL trajectories
There was no significant race x economic stability/status x OTDL linear age trajectories (β = 0.05, 95% CI [–0.005, 0.11], t = 1.79, P = 0.07). However, when stratified across racial groups, there was a significant economic stability/status x OTDL on linear age trajectories within Black/African American older adults (β = 0.07, 95% CI [0.002, 0.13], t = 2.00, P = 0.046), such that lower levels of economic stability/status were associated with a steeper age‐related decline in OTDL scores. No significant association between economic stability/status and OTDL linear age trajectories were observed in White older adults (β = 0.01, 95% CI [–0.009, 0.03], t = 1.04, P = 0.30). See Figure 3 for the effect of economic stability/status on OTDL declines in Black/African American and White older adults.
FIGURE 3.
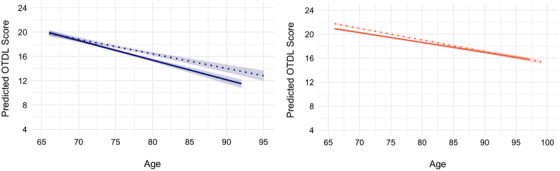
Effect of economic stability/status on Observed Tasks of Daily Living (OTDL) trajectories within Black/African American older adults and White older adults. High versus low economic stability/status was determined based on median split: dotted line, high; solid line, low. Black/African American older adults are represented by Blue on the left‐hand side and White older adults are represented by red on right‐hand side.
There was a significant race x social and community context x OTDL linear age trajectories (β = 0.07, 95% CI [0.03, 0.12], t = 3.02, P = 0.003). When stratified across racial groups, there was a significant effect of social and community context on OTDL linear age trajectories among Black/African American older adults (β = 0.08, 95% CI [0.03, 0.13], t = 3.09, P = 0.002), such that lower social and community context was associated with a steeper decline on the OTDL scores. No significant associations between social and community context on OTDL linear age trajectories were observed in White older adults (β = 0.003, 95% CI [–0.02, 0.02], t = 0.24, P = 0.81; see Figure 4). Finally, no other race x SDoH interactions on OTDL age trajectories were observed (Ps > 0.05). Results for the remaining mixed effects models are shown in Table 3.
FIGURE 4.
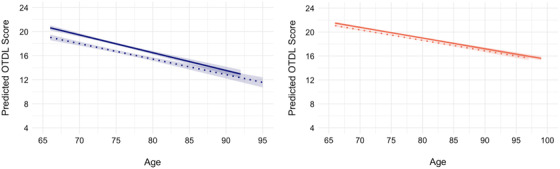
Effect of social and community context on Observed Tasks of Daily Living (OTDL) trajectories within Black/African American older adults and White older adults. High versus low social and community context was determined based on median split: dotted line, high; solid line, low. Black/African American older adults are represented by Blue on the left‐hand side and White older adults are represented by red on right‐hand side.
TABLE 3.
Estimates for SDoH and OTDL trajectory models
| Economic stability/status | Social/community | Education | Health‐care access | Neighborhood | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | β | SE | β | SE | β | SE | β | SE | |
| Intercept | 18.15*** | 0.08 | 18.10*** | 0.08 | 18.14*** | 0.07 | 18.12*** | 0.07 | 18.09*** | 0.07 |
| Race | –1.14*** | 0.20 | –1.46*** | 0.20 | –1.18*** | 0.20 | –1.30*** | 0.19 | –1.21*** | 0.19 |
| SDoH | –0.06 | 0.08 | –0.12 | 0.08 | –0.08 | 0.08 | 0.11 | 0.10 | 0.27*** | 0.07 |
| Age | –0.22*** | 0.01 | –0.22*** | 0.01 | –0.22*** | 0.01 | –0.22*** | 0.01 | –0.22*** | 0.01 |
| Age2 | –0.008*** | 0.001 | –0.008*** | 0.001 | –0.007*** | 0.001 | –0.007*** | 0.001 | –0.007** | 0.001 |
| Age x race | –0.22*** | 0.03 | –0.21*** | 0.03 | –0.25*** | 0.03 | –0.25*** | 0.03 | –0.26*** | 0.03 |
| Age2 x race | –0.009** | 0.003 | –0.01** | 0.003 | –0.01** | 0.003 | –0.01** | 0.003 | –0.01*** | 0.003 |
| Age x SDoH | 0.03* | 0.01 | 0.03* | 0.01 | –0.01 | 0.01 | –.009 | 0.01 | –0.01 | 0.01 |
| Age2 x SDoH | 0.001 | 0.001 | 0.001 | 0.001 | –0.001 | 0.001 | <0.001 | 0.001 | –0.001 | 0.001 |
| Race x SDoH | 0.40* | 0.20 | –0.05 | 0.18 | 0.24 | 0.24 | 0.06 | 0.15 | –0.15 | 0.16 |
| Age x race x SDoH | 0.05 | 0.03 | 0.07** | 0.03 | 0.02 | 0.03 | –0.04 | 0.02 | –0.02 | 0.02 |
Notes: Model parameter estimates for race (White, Black/African American) x SDoH x age analyses. Covariates included main effects of sex, level of education, vocabulary score, CES‐D depression score, visual acuity, MMSE total score, recruitment wave, study site, training group, and booster training, as well as training group and booster training interactions with age.
*P < 0.05.
**P < 0.01.
***P < 0.001.
Abbreviations: β, standardized regression coefficient; CES‐D, Center for Epidemiological Studies—Depression‐12; MMSE, Mini‐Mental State Examination; OTDL, Observed Tasks of Daily Living; SDoH, social determinants of health; SE, standard error of the estimate.
4. DISCUSSION
Consistent with prior work, 23 there were significant racial group differences across all SDoH. White older adults displayed higher levels of economic stability/status, neighborhood and built environment, educational/occupational access, and social and community context relative to Black/African American older adults. In contrast, Black/African American older adults displayed higher levels of health‐care access relative to White older adults. We also replicated the findings that Black/African American older adults experienced faster age‐related declines on the OTDL over time relative to White older adults. 24 Finally, lower levels of economic stability/status and social and community context were two SDoH associated with faster linear age‐related declines on OTDL performance across the sample and specifically within Black/African American older adults. Findings revealed that SDoH negatively impact age‐related functional outcomes in older Black/African American adults, which may be the consequence of increased susceptibility to poor outcomes that is tied to disproportionate and repeated exposure to poor social and structural conditions as a consequence of systemic racism.
Unsurprisingly, we found significant racial group disparities across all SDoH composites within this community‐based sample. These results are largely consistent with a previous investigation that found racial group differences in the individual factors that went into these composites, 22 with the exception that Black/African American older adults displayed significantly higher health‐care access on our composite metric relative to White older adults. This inconsistency could be related to a number of covariates considered in our analyses as well as the fact that the ACTIVE inclusion criteria may have resulted in a more selected (e.g., cognitively healthy, more highly educated) sample, particularly Black/African American participants, than the general population. Nevertheless, there is a robust body of literature highlighting that social and structural factors disproportionately affect minoritized and marginalized groups. 37 , 38 While characterizing inequalities in SDoH are critical to understanding potential causal factors underlying late‐life health disparities, it is imperative we move away from merely identifying group differences in risk factors to acting upon upstream drivers of these inequities. This requires addressing centuries of unjust systems of power, policies, and institutional barriers that are largely designed to intentionally disadvantage Black/African American people and other minority/marginalized groups. 37 , 39
Our study revealed that ACTIVE participants experienced declines on OTDL that were disproportionately faster at older ages, and we replicated previous findings noting that Black/African American participants experience faster rates of age‐related declines in everyday functioning relative to White participants. 23 Results also align well with another cross‐sectional study demonstrating that Black/African American older adults display and report poorer everyday functioning relative to White older adults. 40 Given this evidence of age‐related racial disparities in trajectories of everyday functioning, it is possible that Black/African American older adults may face higher health‐care costs and expenses at earlier ages that only serve to exacerbate inequities in late life. Importantly, Thomas and Marsiske 23 found that racial group differences in declines on the OTDL were mitigated when participants were given a verbal prompt during the task. Thus, one simple intervention technique that may be immediately translatable and prove useful for this group is to offer to more cues and structured assistance surrounding IADL tasks. Nevertheless, concerted efforts toward preventing initial drivers and accelerators of age‐related declines in everyday functioning are needed.
Our study extends previous findings by demonstrating there are significant associations between SDoH and age‐related declines in everyday functioning across the sample that has up to 10 years of longitudinal data. Although results indicated there was no evidence of a race x economic stability/status interaction on OTDL trajectories across the sample, race‐stratified analyses showed lower levels of economic stability/status were associated with a steeper decline in OTDL scores over time within Black/African American older adults. A potential reason for the discrepancy between the non‐significant interaction by race and the significant within‐group effect of economic stability/status for Black/African American participants may be due to variance explained by the covariates differing by race. Additionally, while the non‐significant interaction indicates the effects of economic stability did not necessarily differ across the Black/African American and White racial groups, it is important to acknowledge the significant within‐group effect may be the consequence of historical policies like the National Housing Act of 1933, which restricted homeownership and educational opportunities, promoted redlining and disinvestment, and prevented the accrual and passing of wealth across generations within Black/African American communities. Nevertheless, this work expands upon a robust body of literature illustrating that economic stability/status is not only related to poor cognitive and functional outcomes in late adulthood, 9 , 10 , 11 , 21 but is associated with that rate of age‐related functional decline as well.
Results revealed there was a significant race x social and community context interaction on change in everyday functioning. Although Black/African American older adults with lower levels of community‐level social support appear to have slightly higher levels of baseline everyday functioning, they experienced greater age‐related declines over time. However, there was no association between social and community context and age‐related declines in White older adults. Interestingly, the number of golf courses and country clubs and percentage of White residents loaded positively, whereas the number of supermarkets and grocery stores loaded negatively on the social and community context component. Importantly, Black/African American older adults may have less access to beneficial community‐level resources that promote or enhance independence as a result of policy‐driven racial segregation that have intentionally deprived community‐level resources within predominantly minority and “redlined” neighborhoods. Furthermore, the measures underlying the social and community context component may also be reflective of resources that differ as a function of living in urban versus rural/suburban environments. Nevertheless, results suggest that targeted prevention efforts centered on promoting positive social and community contexts may help with maintaining independence in late adulthood. While no other direct associations between the other SDoH and OTDL trajectories were found, it is possible that these other SDoH work indirectly to impact biological pathways that also affect functional outcomes. Indeed, several studies have identified that Black/African American older adults display higher levels of inflammation, 41 have more severe cardiovascular disease, 42 and experience DNA methylation changes indicative of accelerated biological aging 43 relative to White older adults. While early intervention and proper management of these health conditions are downstream factors necessary to mitigate disparities in late‐life cognitive and functional health outcomes, society must address upstream drivers—that is, root causes of inequities (i.e., racism, White Supremacy, classism) and institutional reinforcement of these broken systems of power—to make true progress toward health equity. 44 , 45
It is worthwhile to note that the individual and society level factors underlying these SDoH constructs can be measured in a variety of ways. For example, there is no agreed upon consensus as to how to best measure socioeconomic status and some metrics of community‐level resources (e.g., number golf and country clubs or gentrification) may not adequately reflect nuanced lived experiences that may play a role in observed health disparities. Further complicating matters some of these SDoH may interact with each other throughout the life course and the influence of these factors are not monotonic—while the negative effects of SDoH may be observed independent of race, it is critical to recognize that these negative effects may disproportionately affect specific groups that may be more vulnerable due their marginalization within society. Importantly, recent efforts to collect structured unified data querying elements of SDoH are being made at Alzheimer's Disease Research Centers in an effort to better inform and guide clinical care practice initiatives. 7 Furthermore, the NIH has several other cultural and group‐specific frameworks designed to highlight other potential factors of influence in characterizing SDoH. 5 , 46 While these serve as important guides, it is important to recognize that interventions across multiple SDoH throughout the life course are likely necessary to reduce age‐related health disparities.
Strengths of the study include use of data from a large community‐dwelling sample that includes a large proportion of Black/African American older adults that were recruited from six sites across the United States; longitudinal modeling of everyday functioning across 10 years; and the use of a performance‐based measure of everyday functioning which may be more ecologically valid than other standardized testing and less susceptible to additional factors (e.g., depressive symptoms, self‐efficacy) than self‐report measures. However, there are several important limitations to our study that warrant careful consideration. First, this study sample did not include older adults from other minoritized/marginalized identities (e.g., disability status, sex and gender minorities, Latinx or Indigenous racial groups) and findings should not directly be extrapolated to these groups without investigation given their unique experiences within society. Second, we did not directly assess cumulative disadvantage or discrimination across the life course, which may be provide important context for understanding the larger impact of SDoH on functional outcomes in Black/African American adults across time. Third, there were limited individual‐level self‐report measures used in the statistical derivation of the SDoH factors and the inclusion of additional measures of perceived socioeconomic status and various other neighborhood factors (e.g., social cohesion, social disorder) may have been important for additional characterization of elements underlying SDoH. Finally, we did not directly examine whether our SDoH factors interacted with one another, which should be considered in the future given this could exacerbate 10‐year age‐related functional declines.
5. CONCLUSION
There are significant racial differences in SDoH and 10‐year trajectories of everyday functioning. Across all the SDoH composites, results revealed that lower levels of economic stability/status and social and community context were associated with steeper age‐related declines in everyday functioning in Black/African American adults. Future work should examine the role of cumulative disadvantage or interactions across these SDoH and include the direct influence of these factors on brain metrics.
CONFLICTS OF INTEREST
Dr. Marsiske has received an in‐kind contribution of 72 software licenses from the Posit Science Company (licensees of the Useful Field of View testing and training programs described in this manuscript) for another study (funded by the Robert Wood Johnson Foundation). All other authors do not have any conflicts of interest to disclose. Author disclosures are available in the Supporting Information.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The current study is supported by NIA R01 AG056486. The ACTIVE Cognitive Training Trial was supported by grants from the National Institutes of Health to six field sites and the coordinating center, including: Hebrew Senior‐Life, Boston (NR04507); the Indiana University School of Medicine (NR04508); the Johns Hopkins University (AG014260); the New England Research Institutes (AG014282); the Pennsylvania State University (AG14263); the University of Alabama at Birmingham (AG14289); and the University of Florida (AG014276). Dr. Thomas is supported by the US Department of Veterans Affairs Clinical Sciences Research and Development Service (Career Development Award‐2 1IK2CX001865) and NIH/NIA (R03 AG070435). This work was further supported by a Shiley‐Marcos Alzheimer Disease Research Education Center Grant to Dr. Clark and Dr. Thomas (P30AG062429) and a National Science Foundation Graduate Research Fellowship Program awards to doctoral student A.J.W. (DGE‐1650012). Dr. Thomas, Dr. Clark, and Ms. Weigand are also supported by an Alzheimer's Association Research Grant (AARF‐22‐723000). Dr. Marsiske has received an in‐kind contribution of 72 software licenses from the Posit Science Company (licensees of the Useful Field of View testing and training programs described in this manuscript) for another study (funded by the Robert Wood Johnson Foundation). Effort for Dr. Clay is also supported by the University of Alabama at Birmingham Alzheimer's Disease Research Center (P20AG068024). The opinions expressed here are those of the authors and do not necessarily reflect those of the funding agencies; academic, research, governmental institutions; or corporations involved.
Clark AL, Weigand AJ, Clay OJ, et al. Associations between social determinants of health and 10‐year change in everyday functioning within Black/African American and White older adults enrolled in ACTIVE. Alzheimer's Dement. 2022;14:e12385. 10.1002/dad2.12385
REFERENCES
- 1. Glymour MM, Manly JJ. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol Rev. 2008;18(3):223‐254. 10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- 2. Kuh D. Life course epidemiology. J Epidemiol Community Health. 2003;57(10):778‐783. 10.1136/jech.57.10.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Komp K, Johansson S. Population ageing in a lifecourse perspective: developing a conceptual framework. Ageing Soc. 2016;36(9):1937‐1960. 10.1017/S0144686X15000756 [DOI] [Google Scholar]
- 4. Hummer RA. Black‐white differences in health and mortality: a review and conceptual model. Sociol Q. 1996;37(1):105‐125. 10.1111/j.1533-8525.1996.tb02333.x [DOI] [Google Scholar]
- 5. Hill CV, Pérez‐Stable EJ, Anderson NA, Bernard MA. The national institute on aging health disparities research framework. Ethn Dis. 2015;25(3):245‐254. doi: 10.18865/ed.25.3.245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gómez CA, Kleinman DV, Pronk N, et al. Addressing health equity and social determinants of health through healthy people 2030. J Public Health Manag Pract. 2021;27(Suppl 6):S249‐S257. 10.1097/PHH.0000000000001297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stites SD, Midgett S, Mechanic‐Hamilton D, et al. Establishing a Framework for Gathering Structural and Social Determinants of Health in Alzheimer's Disease Research Centers. Meeks S, ed. The Gerontologist. 2022;62(5):694‐703. 10.1093/geront/gnab182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bronfenbrenner U, Evans GW. Developmental science in the 21 st century: emerging questions, theoretical models, research designs and empirical findings. Soc Dev. 2000;9(1):115‐125. 10.1111/1467-9507.00114 [DOI] [Google Scholar]
- 9. Hussenoeder FS, Riedel‐Heller SG. Primary prevention of dementia: from modifiable risk factors to a public brain health agenda? Soc Psychiatry Psychiatr Epidemiol. 2018;53(12):1289‐1301. 10.1007/s00127-018-1598-7 [DOI] [PubMed] [Google Scholar]
- 10. Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: childhood socioeconomic status influences adult hippocampal size. Ann Neurol. 2012;71(5):653‐660. 10.1002/ana.22631 [DOI] [PubMed] [Google Scholar]
- 11. Sisco SM, Marsiske M. Neighborhood influences on late life cognition in the ACTIVE study. J Aging Res. 2012;2012:1‐11. 10.1155/2012/435826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steptoe A, Zaninotto P. Lower socioeconomic status and the acceleration of aging: an outcome‐wide analysis. Proc Natl Acad Sci U S A. 2020;117(26):14911‐14917. 10.1073/pnas.1915741117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hyun J, Hall CB, Katz MJ, et al. Education, occupational complexity, and incident dementia: a COSMIC collaborative cohort study. J Alzheimers Dis. 2022;85(1):179‐196. 10.3233/JAD-210627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kivimäki M, Walker KA, Pentti J, et al. Cognitive stimulation in the workplace, plasma proteins, and risk of dementia: three analyses of population cohort studies. BMJ. 2021;374:n1804. 10.1136/bmj.n1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Besser LM, Brenowitz WD, Meyer OL, Hoermann S, Renne J. Methods to address self‐selection and reverse causation in studies of neighborhood environments and brain health. Int. J. Environ. Res. Public Health. 2021;18(12):6484. 10.3390/ijerph18126484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wight RG, Aneshensel CS, Miller‐Martinez D, et al. Urban neighborhood context, educational attainment, and cognitive function among older adults. Am. J. Epidemiol. 2006;163(12):1071‐1078. 10.1093/aje/kwj176 [DOI] [PubMed] [Google Scholar]
- 17. Rhee TG, Lee K, Schensul JJ. Black–White disparities in social and behavioral determinants of health index and their associations with self‐rated health and functional limitations in older adults. Newman AB, ed. J. Gerontol. 2021;76(4):735‐740. 10.1093/gerona/glaa264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andersen CK, Wittrup‐Jensen KU, Lolk A, Andersen K, Kragh‐Sørensen P. Ability to perform activities of daily living is the main factor affecting quality of life in patients with dementia. Health Qual. Life Outcomes. 2004;2:52. 10.1186/1477-7525-2-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawton MP, Brody EM. Assessment of older people: self‐maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179‐186. [PubMed] [Google Scholar]
- 20. De Lepeleire J, Aertgeerts B, Umbach I, et al. The diagnostic value of IADL evaluation in the detection of dementia in general practice. Aging & Mental Health. 2004;8(1):52‐57. 10.1080/13607860310001613338 [DOI] [PubMed] [Google Scholar]
- 21. Smith GE. Healthy cognitive aging and dementia prevention. Am Psychol. 2016;71(4):268‐275. 10.1037/a0040250 [DOI] [PubMed] [Google Scholar]
- 22. Clay OJ, Ball KK, Wheeler KM, et al. Evaluating social determinants of health domains and their predictive validity within Black/African American and white older adults from the active trial. J Aging Health. June 2022:089826432211112. 10.1177/08982643221111205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thomas KR, Marsiske M. Age trajectories of everyday cognition in African American and White older adults under prompted and unprompted conditions. Neuropsychol. Rehabil.. 2017;27(4):522‐539. 10.1080/09602011.2015.1092453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ball K, Berch DB, Helmers KF, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271. 10.1001/jama.288.18.2271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gross AL, Rebok GW, Unverzagt FW, Willis SL, Brandt J. Cognitive predictors of everyday functioning in older adults: results from the active cognitive intervention trial. J. Gerontol. ‐ B Psychol. Sci. 2011;66B(5):557‐566. 10.1093/geronb/gbr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Owens, J. H. , Jones, R. , Marsiske, M . (in press). The effects of occupational complexity on late life cognition in ACTIVE: examining the mediating and moderating effects of race. J Aging Health.</bib> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diehl M, Marsiske M, Horgas AL, Rosenberg A, Saczynski JS, Willis SL. The revised observed tasks of daily living: a performance‐based assessment of everyday problem solving in older adults. J Appl Gerontol. 2005;24(3):211‐230. 10.1177/0733464804273772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmitter‐Edgecombe M, Parsey C, Cook DJ. Cognitive correlates of functional performance in older adults: comparison of self‐report, direct observation, and performance‐based measures. J Int Neuropsychol Soc. 2011;17(05):853‐864. 10.1017/S1355617711000865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas KR, Marsiske M. Verbal prompting to improve everyday cognition in MCI and unimpaired older adults. Neuropsychology. 2014;28(1):123‐134. 10.1037/neu0000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rebok GW, Ball K, Guey LT, et al. Ten‐year effects of the advanced cognitive training for independent and vital elderly cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62(1):16‐24. 10.1111/jgs.12607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Willis SL, Tennstedt SL, Marsiske M, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805‐2814. 10.1001/jama.296.23.2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ekstrom, R. B. , French, J. W. , Harman, H. , & Derman, D . (1976). Kit of Factor‐Referenced Cognitive Tests Rev. Educational Testing Service. [Google Scholar]
- 33. Folstein MF, Folstein SE, McHugh PR. “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- 34. Ware JE, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473‐483. [PubMed] [Google Scholar]
- 35. Scale Radloff LS. The CES‐D: A self‐report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385‐401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- 36. Ward JB, Gartner DR, Keyes KM, Fliss MD, McClure ES, Robinson WR. How do we assess a racial disparity in health? Distribution, interaction, and interpretation in epidemiological studies. Ann Epidemiol. 2019;29:1‐7. 10.1016/j.annepidem.2018.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gee GC, Ford CL. Structural racism and health inequities: old issues, new directions. Du Bois Rev. 2011;8(1):115‐132. 10.1017/S1742058X11000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lavizzo‐Mourey RJ, Besser RE, Williams DR. Understanding and mitigating health inequities — past, current, and future directions. N Engl J Med. 2021;384(18):1681‐1684. 10.1056/NEJMp2008628 [DOI] [PubMed] [Google Scholar]
- 39. Braveman P, Gottlieb L. The social determinants of health: it's time to consider the causes of the causes. Public Health Rep. 2014;129 Suppl 2:19‐31. 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Morgan AA, Marsiske M, Whitfield KE. Characterizing and explaining differences in cognitive test performance between african american and European American older adults. Exp Aging Res. 2008;34(1):80‐100. 10.1080/03610730701776427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Boots EA, Feinstein DL, Leurgans S, et al. Acute versus chronic inflammatory markers and cognition in older black adults: results from the minority aging research study. Brain, Behavior, and Immunity. 2022;103:163‐170. 10.1016/j.bbi.2022.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Aiken Morgan AT, Sims RC, Whitfield KE. Cardiovascular health and education as sources of individual variability in cognitive aging among African Americans. J Aging Health. 2010;22(4):477‐503. 10.1177/0898264310361627 [DOI] [PubMed] [Google Scholar]
- 43. Levine ME, Crimmins EM. Evidence of accelerated aging among African Americans and its implications for mortality. Soc. Sci. Med. 2014;118:27‐32. 10.1016/j.socscimed.2014.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Maybank A, De Maio F, Lemos D, Derige DN. Embedding racial justice and advancing health equity at the American Medical Association. Am. J. Med. 2022;135(7):803‐805. 10.1016/j.amjmed.2022.01.058 [DOI] [PubMed] [Google Scholar]
- 45. Noren Hooten N, Pacheco NL, Smith JT, Evans MK. The accelerated aging phenotype: the role of race and social determinants of health on aging. Ageing Res. Rev. 2022;73:101536. 10.1016/j.arr.2021.101536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Office of Disease Prevention and Health Promotion (n.d.‐b). Social Determinants of Health. Healthy People 2020. U.S. Department of Health and Human Services. https://Www.Healthypeople.Gov/2020/Data‐Search/Search‐the‐Data#topic‐Area=3499
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


