CASE PRESENTATION
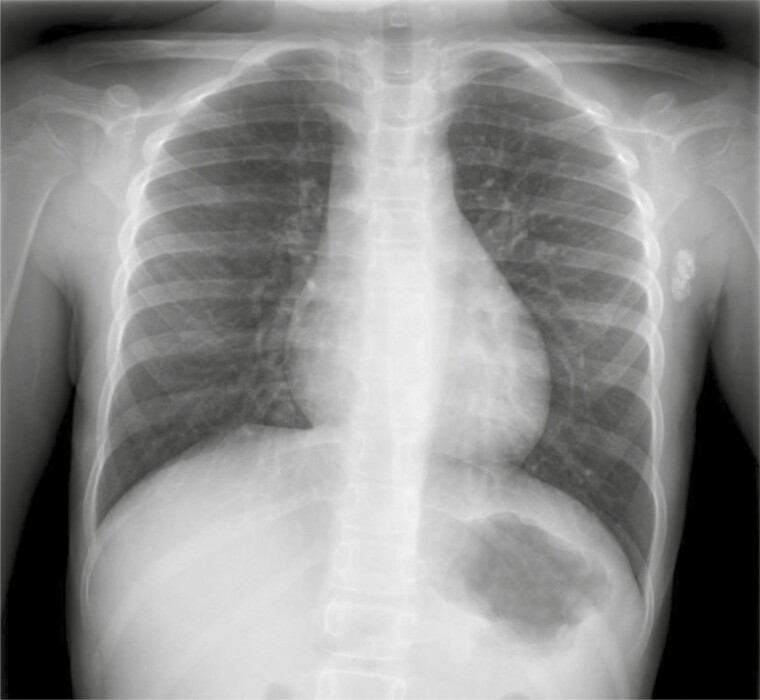
A 3-year-old girl of Tunisian descent was referred to a paediatric clinic for assessment of a persistent cough for the last 2 months. A chest-x-ray performed in that context revealed a calcified left axillary lymph node (Figure 1). Consequently, her physician performed a tuberculin skin test (TST), which at 48 hours produced an induration of 20 mm. She was referred to a paediatric infectious disease clinic.
Figure 1. .
Chest X-ray displaying calcified left axillary lymph nodes. No mediastinal lymphadenopathy and normal lung parenchyma.
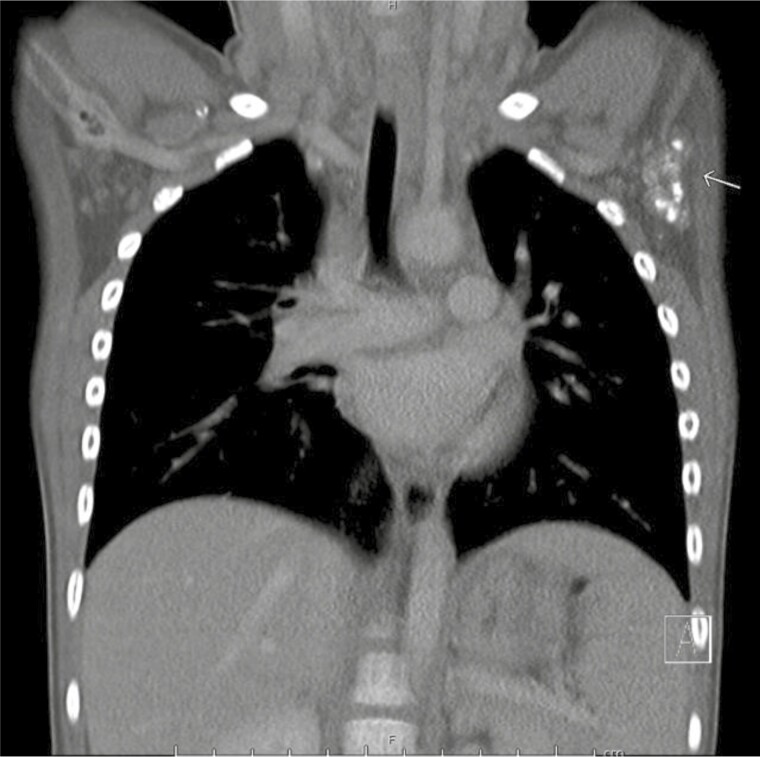
History revealed that she was born at term in Tunisia following a healthy pregnancy. She had emigrated to Canada at the age of 23 months. She was taking no medications and her immunizations were up to date according to provincial standards. Her cough had resolved post-introduction of asthma therapy. Her parents denied any symptoms compatible with tuberculosis (TB) or any known TB contacts. Physical examination revealed her weight to be 14.3 kg (75%ile). She was afebrile and had a normal respiratory exam. A small, non-tender <1 cm lymph node was found on examination of the left axilla. A chest CT-scan performed to rule-out additional adenopathy or parenchymal abnormalities revealed multiple calcified left axillary lymph nodes measuring between 7 and 8 mm (Figure 2).
Figure 2. .
Chest CT-scan (coronal view) with multiple calcified axillary lymph nodes (left). Three dominant calcified left axillary lymph nodes were measured at 8 mm, 8 mm, and 7 mm. No mediastinal lymphadenopathy or parenchymal changes were noted.
An additional physical finding and test established the patient’s diagnosis.
DISCUSSION
Upon further examination, a Bacille Calmette-Guérin (BCG) vaccine scar was found on the ipsilateral side of her calcified axillary lymph nodes. An interferon gamma release assay (IGRA), the Quantiferon-TB Gold Plus, was sent to rule-out latent tuberculosis infection (LTBI) and was found to be negative. Her positive TST and left axillary lymph node calcifications were both attributed to prior BCG vaccination. She continued to evolve well 3 years post-initial presentation.
The BCG vaccine is one of the most widely administered vaccines worldwide (1). BCG is a live attenuated strain of Mycobacterium bovis, originally developed by Albert Calmette and Camille Guérin. In countries with high prevalence of TB, BCG vaccination has relatively high protective efficacy against meningeal and miliary TB (85% to 92%), with a lower protective efficacy for pulmonary TB (74%) (1).
Following intradermal injection of BCG, a normal local reaction occurs with swelling and redness at the site of injection, which later develops into a small pustule or ulcer. Thereafter, a small permanent scar appears at the site of injection. Uncommonly, BCG can result in adverse reactions. The most commonly described adverse reactions are subcutaneous abscesses at the site of injection, typically occurring within 2 months post-vaccination, and regional lymphadenopathy, typically occurring within 6 months of vaccination (1). Regional lymphadenopathy, which may present as a calcified lymph node at a later age, are directly due to replication of attenuated M. bovis and clearance by the regional lymph node. The differential of axillary lymph node calcifications include other granulomatous disease (mycobacterial tuberculosis complex, histoplasmosis, and sarcoidosis) and malignancies (2).
The most feared complication, fatal disseminated infection, occurs primarily in children who are severely immunocompromised, such as children with poorly controlled human immunodeficiency virus or severe combined immunodeficiency. Antituberculosis agents should be used for disseminated infection but are not required for local adverse reactions such as regional lymphadenopathy in immunocompetent patients (1).
This case was diagnostically challenging for several reasons. First, the patient presented with a history of persistent cough and had recently immigrated from a region of high TB endemicity. Second, she had a 20 mm TST. The resolution of her cough with no additional findings on CT-scan, as well as the absence of other clinical manifestations, went against a diagnosis of active TB. The negative IGRA went against a diagnosis of LTBI in this patient.
The TST is an indirect test utilized for detection of a previous M. tuberculosis complex exposure. This test is performed by injecting intradermally a small amount of purified protein derivative (PPD) from heat-inactivated M. tuberculosis. If previously sensitized, a delayed cell-mediated reaction will occur and be observed as an increased area of induration at the site of injection. Lack of a reaction to TST does not exclude LTBI nor active TB. False positives may occur due to BCG immunization due to a cross reaction of the PPD antigen with M. bovis (attenuated strain) found in the vaccine (1).
IGRAs measure the interferon-gamma production in response to stimulation with a polypeptide mixture. An advantage of the IGRA is that there is no cross-reaction with antigens found in the BCG. The most recent Canadian Tuberculosis Standards recommends preferentially using IGRA testing in children older than 2 years old previously vaccinated with the BCG vaccine (1).
Paediatricians should be aware of common adverse effects of the BCG vaccine given its use worldwide. Children vaccinated in their countries of origin may present with benign local reactions or may be found to have calcified lymph node incidentally. Ruling out LTBI and active TB remains of essence, but knowledge of these common reactions can help reduce unnecessary testing in asymptomatic children.
CLINICAL PEARLS
BCG is a live attenuated vaccine that is widely used in areas with high TB prevalence worldwide.
BCG may result in benign adverse local reactions, most commonly local abscesses at the site of injection and regional lymphadenopathy (suppurative, non-suppurative or calcified); expectant management is adequate in immunocompetent children.
Children above 2 years of age who were previously vaccinated with BCG should preferentially be tested with an IGRA to rule-out LTBI, if locally available.
Contributor Information
Esther Vaugon, Division of Pediatric Infectious Diseases, Department of Pediatrics, CHU Sainte-Justine, Université de Montreal, Montreal, Quebec, Canada.
Ana C Blanchard, Division of Pediatric Infectious Diseases, Department of Pediatrics, CHU Sainte-Justine, Université de Montreal, Montreal, Quebec, Canada.
Matthew Magyar, Division of Pediatric Infectious Diseases, Department of Pediatrics, CHU Sainte-Justine, Université de Montreal, Montreal, Quebec, Canada; Division of Microbiology, Department of Laboratory Medicine, CHU Sainte-Justine, Université de Montreal, Montreal, Quebec, Canada.
FUNDING
There are no funders to report for this manuscript.
POTENTIAL CONFLICTS OF INTEREST
All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
INFORMED CONSENT
Written informed consent was obtained from the patient and her family.
REFERENCES
- 1. Dwilow R, Hui C, Kakkar F, Kitai I.. Chapter 9: Pediatric tuberculosis. Can J Respir Crit Care Sleep Med. 2022;6(supp1):129–48. [Google Scholar]
- 2. Mello GGN, Aguillar VLN, Chala LF, Maciel AA, Aracava M, Shimizu C, editors. Differential diagnosis of calcific axillary lymphadenopathy. European Congress of Radiology. Vienna: European Society of Radiology; 2017. [Google Scholar]