Abstract
Burkholderia pseudomallei causes melioidosis, a potentially fatal disease whose clinical outcomes include rapid-onset septicemia and relapsing and delayed-onset infections. Like other facultative intracellular bacterial pathogens, B. pseudomallei is capable of survival in human phagocytic cells, but unlike mycobacteria, Listeria monocytogenes, and Salmonella serovar Typhimurium, the species has not been reported to survive as an endosymbiont in free-living amebae. We investigated the consequences of exposing Acanthamoeba astronyxis, A. castellani, and A. polyphaga to B. pseudomallei NCTC 10276 in a series of coculture experiments. Bacterial endocytosis was observed in all three Acanthamoeba species. A more extensive range of cellular interactions including bacterial adhesion, incorporation into amebic vacuoles, and separation was observed with A. astronyxis in timed coculture experiments. Amebic trophozoites containing motile intravacuolar bacilli were found throughout 72 h of coculture. Confocal microscopy was used to confirm the intracellular location of endamebic B. pseudomallei cells. Transmission electron microscopy of coculture preparations revealed clusters of intact bacilli in membrane-lined vesicles inside the trophozoite cytoplasm; 5 × 102 CFU of bacteria per ml were recovered from lysed amebic trophozoites after 60 min of coculture. Demonstration of an interaction between B. pseudomallei and free-living acanthamebae in vitro raises the possibility that a similar interaction in vivo might affect environmental survival of B. pseudomallei and subsequent human exposure. Endamebic passage of B. pseudomallei warrants further investigation as a potential in vitro model of intracellular B. pseudomallei infection.
Burkholderia pseudomallei causes melioidosis, a potentially fatal infection with septicemic, subacute, and chronic forms that is endemic in northern Australia and Southeast Asia. The acute septicemic form has a mortality rate of over 30% (31) and may present after a disease-free interval of several decades (27). Survival of B. pseudomallei within macrophages has been demonstrated in vitro and is proposed as an explanation for delayed-onset disease (17, 24). Survival of B. pseudomallei for extended periods in the absence of nutrients and under acid conditions may allow persistence in a hostile intracellular habitat (18, 32) but does not explain how B. pseudomallei enters or escapes from professional phagocytic cells. A potential explanation is that B. pseudomallei has developed the capacity to invade and survive within free-living amebae in the moist soil and surface water environment where the bacterial species is normally found (5, 15, 29) and amebae are also likely to be present (25).
Several facultative bacterial intracellular pathogens, notably Legionella pneumophila, Mycobacterium avium, and Listeria monocytogenes, have been shown to survive as endosymbionts in free-living amebae such as Acanthamoeba, Hartmanella, and Naegleria (8, 20, 26). It has been suggested that growth in an amebic intracellular environment might assist these bacteria to adapt to survival in mammalian phagocytic cells (3, 7, 11, 26). Moreover, incorporation of bacteria in amebic cysts has also been shown to confer resistance to adverse environmental conditions such as exposure to biocidal agents (1). In addition, amebic endosymbiosis is known to augment the virulence of L. pneumophila and M. avium (6–8). The endocytosis of L. pneumophila by both Acanthamoeba and macrophages has common features including an unusual, coiling form of phagocytosis (3, 14). Although evidence for the direct involvement of amebic endosymbionts in the pathogenesis of human infection has yet to be found, molecular methods and animal models indicate a possible role for free-living amebae as determinants of bacterial virulence (4, 6–8, 11).
The best evidence in support of a putative endosymbiosis involving B. pseudomallei and free-living protozoa is circumstantial: Ralstonia (previously Burkholderia) pickettii, a close phylogenetic relative of B. pseudomallei, has been found in Acanthamoeba species from a hospital environment (23). More recently, intra-amebic survival of Burkholderia cepacia has been demonstrated in Acanthamoeba strains in vitro (21). However, there has been no report of any interaction between B. pseudomallei and free-living amebae to date, nor has there been any report of simultaneous recovery of B. pseudomallei and Acanthamoeba species from the same environmental location. During investigations into a recent cluster of acute melioidosis cases in Western Australia, the strain of B. pseudomallei responsible for the outbreak was isolated from potable water in the affected community (15). Several Acanthamoeba species were also recovered from water specimens collected during the initial outbreak and in further environmental investigations over the following year, prompting the present study into possible interactions between Acanthamoeba species and B. pseudomallei.
MATERIALS AND METHODS
Organisms.
The three amebic species used were Acanthamoeba castellani CCAP 1534/1, A. polyphaga CCAP 1501/3A, and A. astronyxis CCAP 1534/1. These were obtained as axenic strains and maintained in axenic culture media (PYG broth; Excel Laboratory Products, Bentley, Western Australia, Australia) in tissue culture flasks incubated at 30°C. Culture media were changed weekly. The supernatant fluid was kept in a sterile plastic container at 20°C and used within 1 week of harvesting. Amebic suspensions were examined by bright-field and phase-contrast microscopy immediately prior to use. Viable trophozoite counts were obtained with a Fuchs-Rosenthal hemocytometer and trypan blue vital stain.
B. pseudomallei NCTC 10276 was used in all coculture experiments. NCTC 10276 was kept in 20% glycerol broth at −70°C and subcultured via 5% horse blood agar to Trypticase soy broth (Excel Laboratory Products), in which it was grown at 37°C for 18 h. In previous experiments, it had been found that the logarithmic phase of bacterial growth commenced 3 to 4 h after inoculation of the growth medium. The pellicle was lifted aside with a sterile loop and a 1.0-ml aliquot of bacterial suspension transferred to 9.0 ml of fresh Trypticase soy broth. The mixture was incubated at 37°C for 1 h to obtain rapidly motile bacteria in mid-log phase and then for 2 and 24 h. Bacterial motility was checked by examination of bacteria at ×400 magnification under phase-contrast illumination. All aerosol-generating procedures were carried out in a biological safety cabinet.
Light microscopy.
After it was ensured that amebic cells were present in all three identifiable forms (rounded trophozoites, trophozoites with acanthopodia, and thick-walled cysts) and no bacterial contamination had occurred, a 10-μl drop of bacterial suspension was combined with a 10-μl drop of amebic suspension. The interaction was followed under phase contrast at ×400 magnification for 120 min. Separate aliquots were kept in sterile containers at 20°C for 24 h and then reexamined under phase-contrast conditions. Each bacterial-amebic challenge was repeated on at least three occasions with each of the Acanthamoeba spp. The interaction between B. pseudomallei and A. astronyxis was repeated on three further consecutive occasions, when aliquots were taken at time (t) = 0, 20 min, 40 min, 60 min, and 24 h. On each occasion, 20 intact trophozoites were examined for the presence of surface-adherent bacilli, rapidly rotating bacilli, vacuoles containing single bacilli, multibacillary vacuoles (>2 bacilli per vacuole), polar tufts of bacilli (at least five), and extracellular bacterial tangles.
The interaction was followed using a confocal laser scanning microscope with simultaneous differential interference contrast microscopy (Bio-Rad, Hemel-Hempstead, United Kingdom). The vital stains SYTO 9 (1 μl of 3.34 mM in dimethyl sulfoxide; Molecular Probes, Eugene, Oreg.) and propidium iodide (1 μl of 20 mM in dimethyl sulfoxide; Molecular Probes) were added to 1 ml of microbial suspension and incubated for 5 min to assess bacterial viability in unfixed, wet-mounted preparations. Viable cells fluoresced bright green, and nonviable cells fluoresced red. Bacterial suspensions were centrifuged for 5 min at 1,000 × g and resuspended in sterile 0.89% NaCl solution; 24-h-old coincubated preparations were stained by direct addition of 1 μl of 3.34 mM SYTO 9 to 10 μl of cell suspension without a centrifugation or washing step. The internal structure of amebic cells was examined with the microscope's optical sectioning facility to confirm the intracellular location of bacteria. Time-lapse confocal microscopy was used to confirm movement of bacteria within amebic vacuoles.
Transmission electron microscopy.
After confirmation of phagocytosis by phase-contrast microscopy, suspensions of B. pseudomallei cocultured with A. astronxyis were centrifuged at 300 × g for 10 min. The supernatant was removed by pipette and replaced with fresh 2.5% glutaraldehyde. Cell suspensions were fixed in 2.5% glutaraldehyde in 0.05 M cacodylate buffer (pH 7.4) for 24 h, washed several times in 0.2 M cacodylate buffer (pH 7.4), resuspended in 10% albumin solution, placed in a microcentrifuge tube, and centrifuged; the resultant sediment of cells was fixed in 2.5% glutaraldehyde for a further 24 h. The tip of the tube was cut with a single-edged blade, and the block containing cells was postfixed in 1% osmium tetroxide, dehydrated in graded solutions of ethanol, infiltrated with Araldite, and embedded in polyethylene capsules. After retrimming of each block face, 50-nm ultrathin sections were cut, mounted on 200-mesh thin-bar copper grids, double stained in uranyl acetate and lead citrate, and examined in a Philips 410LS transmission electron microscope at an accelerating voltage of 80 kV.
Recovery of intracellular bacteria.
Intracellular bacteria were recovered by a kanamycin-trophozoite lysis procedure as follows. A 5.0-ml coculture volume was set up with a suspension of 105 of trophozoites per ml in 0.9 N NaCl and a 1-h preparation of rapidly motile B. pseudomallei at 107 CFU/ml. Aliquots of 1.0 ml were removed at t = 0 and t = 1 h. These were centrifuged at 300 × g for 5 min, and the pellet was resuspended in 0.9 N NaCl with 100 μg of kanamycin sulfate (Sigma, St. Louis, Mo.) per ml. After 2 h of incubation at 20°C, the aliquot was centrifuged again at 300 × g for 5 min and resuspended in sterile, demineralized water. The sealed container was incubated in a waterbath at 37°C for 30 min and then vortex mixed for 30 s and sonicated for 15 m. The suspension was then centrifuged at 1,000 × g for 5 min and resuspended in 0.9 N NaCl. This suspension was spread in 50-μl aliquots on plate count agar in triplicate, using a spiral plating device. The remaining 850 μl was spread on a further plate count agar plate to detect very low bacterial counts. The same plating procedure was used with aliquots of the uninoculated amebic stock suspension, sterile demineralized water, and 0.9 N NaCl as negative controls. Plates were incubated in air at 37°C for 48 h before counting using a standard template (Don Whitley). Viable counts were obtained using the spiral plater interpretive tables. Viable counts were also performed at t = 0 and t = 60 min after inoculation of 0.9% NaCl with a 1-h B. pseudomallei preparation to determine whether bacterial growth occurred in the coculture medium during this period.
Statistical methods.
Descriptive statistics and the unpaired t test were calculated with the assistance of statistical software (Prism, version 2.01; GraphPad Software, San Diego, Calif.).
RESULTS
Early bacterium-ameba interactions.
When B. pseudomallei was added to a suspension of A. astronyxis, adhesion, endocytosis, and vacuole formation were all observed, in that order. The sequence of cellular events could be observed in individual amebic trophozoites and occurred in a 1-h time frame when a 1-h preparation of highly motile B. pseudomallei was added to acanthapodium-bearing A. astronyxis trophozoites. This sequence of events was quantitated in the timed coculture procedure where bacterial adhesion to the trophozoite surface and rapid bacillary rotation were followed by the appearance of vacuoles containing single bacilli, then multibacillary vacuoles, and finally formation of external bacillary tufts (Table 1). In the initial adhesion event, one end of a highly motile bacillus attached to the cytoplasmic membrane of the amebic trophozoite (Fig. 1a). In most instances, this was followed immediately by high-frequency helicopter blade rotation of the entire bacillus around a fixed point on the trophozoite surface. This lasted no more than a few seconds. Shortly after bacillary rotation ended, adjacent pseudopodia curled round to enclose the bacillus. Formation of a complete cytoplasmic bridge around the bacillus resulted in its inclusion within a membrane-lined vacuole. Pseudopod extension and curling were fast enough to observe in real time. These events occurred repeatedly in individual trophozoites, resulting in a collection of bacterium-containing vacuoles (Fig. 1b). No endocytosis by fully rounded trophozoites or cysts was observed. Using a 1-h motile bacterial preparation, intracellular bacilli were easily demonstrated by uptake of the fluorescent stain SYTO 9 (Fig. 1). Multiple vacuoles packed with bacilli were observed inside rounded intact trophozoites. No green fluorescent bacilli were observed moving freely in the cytoplasm of intact trophozoites. Some intravacuolar bacteria were seen to retain their motility for 1 to 2 h of continuous observation after initial vacuole formation.
TABLE 1.
Cellular features observed during timed coculture of B. pseudomallei and A. astronyxis
| Time (min) | Adherent bacillib | No. of trophozoitesa
|
||||
|---|---|---|---|---|---|---|
| Rotating bacillic | Single-bacillus vacuolesd | Multibacillary vacuolese | Bacillary tuftsf | Bacillary tanglesg | ||
| 0 | 4.3 ± 1.5 | 1.7 ± 0.6 | 0.3 ± 0.6 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 20 | 12.3 ± 3.8 | 6.0 ± 2.6 | 1.7 ± 1.5 | 2.0 ± 3.5 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 40 | 13.7 ± 3.0 | 6.0 ± 3.0 | 3.7 ± 2.1 | 5.7 ± 5.1 | 0.3 ± 0.6 | 0.0 ± 0.0 |
| 60 | 12.0 ± 2.6 | 3.0 ± 2.0 | 4.0 ± 1.7 | 10.3 ± 3.5 | 1.0 ± 1.0 | 0.0 ± 0.0 |
| 1,440 | 20.0 ± 0.0 | 17.7 ± 3.2 | 3.3 ± 1.5 | 12.3 ± 0.6 | 16.3 ± 1.5 | 0.7 ± 0.6 |
At each time interval, 20 trophozoites were examined for the presence of each indicated feature. Values shown are the mean ± standard deviation of the number of trophozoites exhibiting each feature from three consecutive replicate experiments.
Bacilli adherent to external trophozoite surface.
One or more adherent bacilli in high-frequency rotation around point on external trophozoite surface.
One or more trophozoite vacuoles containing single bacilli.
One or more trophozoite vacuoles containing more than one bacillus.
Five or more bacilli in single tuft on external trophozoite surface.
Bacilli in tangled web of chains external to trophozoite or cyst surface.
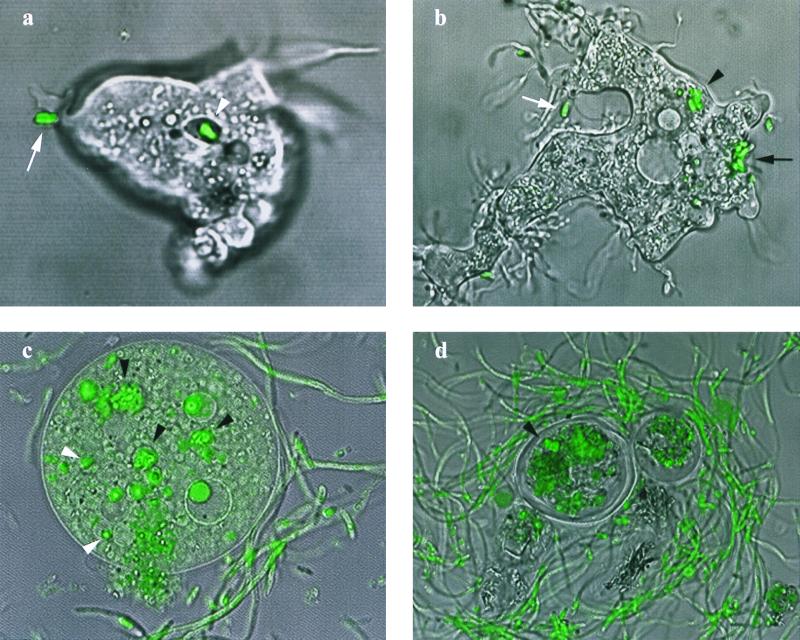
FIG. 1.
Confocal laser scanning microscope images of B. pseudomallei-A. astonyxis coculture stained with epifluorescent dyes SYTO 9 and propidium iodide, superimposed on differential interference contrast images. (a) Adhesion of polar end of bacillus to trophozoite surface (white arrow) leading to inclusion in vacuole (white arrowhead) (magnification, ×1,440); (b) pseudopodia moving to enclose bacillus (white arrow), parasitophorous vacuoles in same trophozoite (black arrowhead), and external bacillary tuft (black arrow) (×1,220); (c) amebic trophozoite with free cytoplasmic bacilli (white arrowheads) and intact parasitophorous vacuoles (black arrowheads) undergoing rupture of external cytoplasmic membrane (×1,130); (d) after 24 h of coincubation, bacilli incorporated in amebic cyst (black arrowhead) and surrounded by bacillary tangle (×1,220). Confocal microscope images were merged and processed using Confocal Assistant, version 4.02 (Bio-Rad) and Photoshop, version 5.0 (Adobe Systems Inc.).
When B. pseudomallei was incubated at 37°C for 2 h, either no or very few bacteria exhibited rapid motility. Very few bacteria from these 2-h preparations attached to the trophozoite surface, and endocytosis was extremely rare. Stationary-phase bacteria obtained after 24 h of incubation at 37°C attached to the external trophozoite surface and rotated but only a few single bacteria vacuoles were formed.
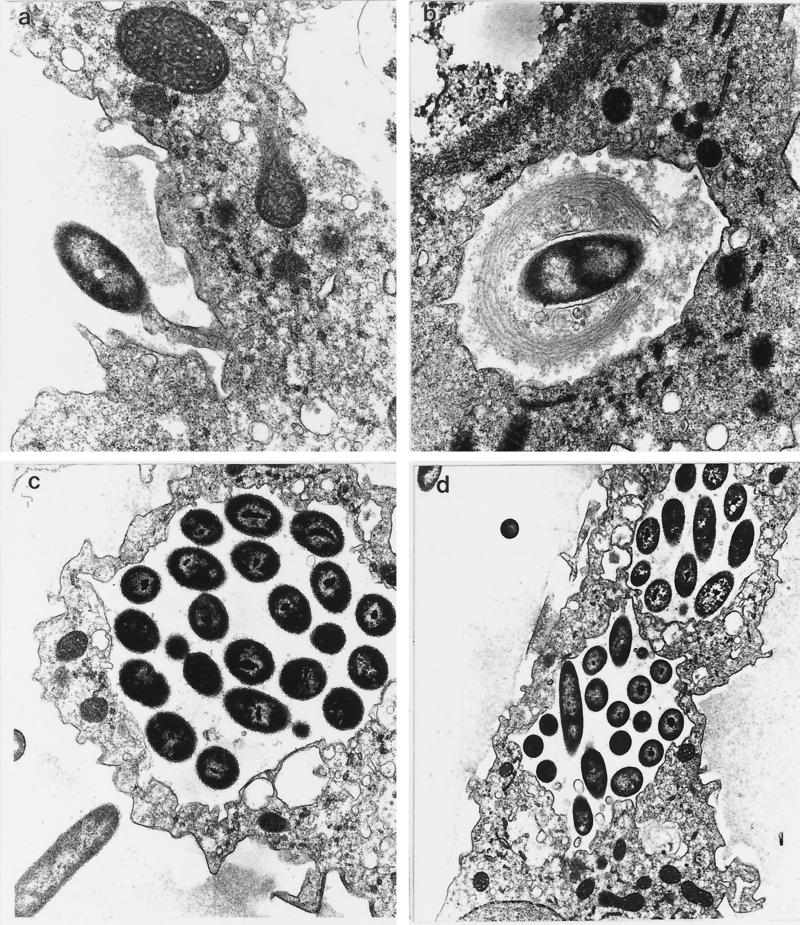
On transmission electron microscopy, pseudopodia were found in contact with one end of each adherent bacillus minutes after the start of coculture (Fig. 2a). Membrane-lined vacuoles containing single bacilli were found inside amebic trophozoites. In some of these vacuoles, bacteria were surrounded by multilamellate membranes consistent with the pseudopodial coils or stacks seen in coiling phagocytosis (Fig. 2b). Membrane-lined vacuoles containing multiple bacilli were found inside amebic trophozoites after 30 min of coculture. Some bacterium-laden vacuoles were apposed to the external trophozoite surface (Fig. 2c), while in other trophozoites multiple bacterium-laden vacuoles could be seen (Fig. 2d).
FIG. 2.
Transmission electron micrographs of B. pseudomallei-A. astronyxis coculture. (a) Pseudopodium in contact with polar end of bacillus shortly after start of coculture (magnification, ×18,700); (b) membrane-lined vacuoles containing single bacillus inside amebic trophozoite, surrounded by multilamellate membrane (×18,700); (c) membrane-lined vacuole containing multiple bacilli apposed to external trophozoite surface (×12,800); (d) multiple bacterium-laden vacuoles formed after 60 min of coculture (×8,000).
Bacterium-ameba interactions after 24 h of coculture.
Twenty-four hours after the start of coculture preparations with bacteria from 2-h preparations, motile bacilli were observed inside endamebic vacuoles that contained three or fewer bacilli. However, in cocultures using bacteria from 1-h preparations, amebae became enclosed in clumps or tangles of bacterial filaments not previously seen in any B. pseudomallei monoculture (Table 1; Fig. 1c and d). Many actively motile intracellular bacilli were present and were observed up to 72 h after coculture began (the maximum period observed). Vortex mixing of coculture suspensions released amebic cysts from bacillary tangles. Bacilli were observed in only a small proportion of amebic cysts (Fig 1d). Some rounded trophozoites were present. These contained vacuoles packed with highly motile bacilli, restricted by the vacuolar membrane (Fig. 1c). Vacuoles packed with bacilli were often observed adjacent to the external plasma membrane. In some cases these protruded or were in the process of releasing bacilli to form an external tuft similar in appearance to the polar tuft formed during endocytosis. In a few trophozoites, single bacilli moved without restriction throughout the cytoplasm outside any vacuoles (Fig. 1c). Trophozoite lysis with spillage of cytoplasmic contents occurred shortly afterwards. These phenomena were not observed in cocultures containing 2-h bacterial preparations or lacking stellate, acanthapodium-bearing trophozoites.
Coincubation with other Acanthamoeba species.
Some of the early features of bacterial endocytosis by A. astronyxis were demonstrated by repetition of the coculture procedure under optimal conditions using A. castellani and A. polyphaga. However, as vacuolation was less pronounced in these species than in A. astronyxis, the sequence of events was more difficult to deduce. No bacterium-containing vacuoles or external bacillary tufts were observed in either species. Initial phagocytosis and production of bacillary tangles were all demonstrated more clearly with A. castellani and A. polyphaga when challenged with B. pseudomallei at 37°C.
Recovery of intracellular bacteria.
A mean bacterial count of 13 CFU/ml (±6.7) was obtained from three replicates from an aliquot taken immediately after commencement of coculture. After 60 min, the mean intracellular bacterial count from three replicates was 507 CFU/ml (±76.9) (t = 6.39, df = 4, P = 0.0031). No growth was obtained from saline and sterile water controls. The mean viable count of B. pseudomallei in control coculture medium was 3.6 × 106 (±2.7 × 105) CFU/ml at t = 0 and 3.9 × 106 at t = 60 min (±1.1 × 106). There was no statistically significant difference between viable counts of controls at t = 0 and t = 60 min in coculture medium.
DISCUSSION
An interaction between B. pseudomallei and Acanthamoeba spp. has not been described previously. In this investigation of a possible interaction between the two species, we observed coiling phagocytosis, the survival of B. pseudomallei in amebic vacuoles, and eventual bacterial escape from vacuoles into amebic cytoplasm and then into the surrounding medium. The optimal conditions for cellular interaction were dependent on the condition of both components of the coculture. A combination of rapidly motile bacilli and acanthapodium-bearing amebae was required to obtain a full range of cellular interactions.
The ability of B. pseudomallei to cause relapsing and late-onset infection up to 26 years after initial exposure has been attributed to an ability of this species to survive in macrophages (17, 24, 27). Intramacrophage survival may be helped by an ability to tolerate prolonged periods of nutrient deprivation and relatively acid conditions as is known to occur with B. pseudomallei (18, 32). It is therefore notable that we were able to recover viable bacteria from amebic trophozoites 1 h after endocytosis and were able to observe motile intracellular bacilli for up to 72 h after coculture began. Similar investigations into other facultative intracellular pathogens such as L. pneumophila have emphasized intracellular replication (2, 7). The formation of extracellular bacillary tufts and tangles provides a major methodological obstacle to using the conventional approach to determining whether or not B. pseudomallei is capable of intraamebic replication. Moreover, the acidic conditions expected in amebic vacuoles may have rendered intracellular bacilli viable but not immediately culturable, as proposed recently (16), further confounding quantitative bacteriology. If late-onset endamebic replication of B. pseudomallei occurs, as has been shown recently with the related species B. cepacia (21), its demonstration will depend on advanced laboratory methods such as those developed recently for investigation of M. avium-Acanthamoeba interactions (28). Nevertheless, the current lack of evidence for endamebic replication of B. pseudomallei does not detract from the analogy between in vitro survival in endamebic vacuoles and survival in mammalian macrophages.
The coexistence of many intact trophozoites with dense clusters, tangles, or mats of B. pseudomallei shows that these species are capable of a nondestructive relationship. Furthermore, the small numbers of intracellular bacteria recovered after 60 min of coincubation with many times that number of bacteria implies a degree of control over bacterial access to the interior of the trophozoite. The observation of coiling phagocytosis, a process noted to occur during endocytosis of L. pneumophila by both Acanthamoeba and human monocytes (3), shows another possible connection between amebic endocytosis and mammalian phagocytosis of B. pseudomallei. The asymmetric, coiling extension of phagocytic pseudopodia by human mononuclear cells was one of the first pieces of evidence linking amebic endocytosis of Legionella to phagocytic events during L. pneumophila infection (14). Coiling phagocytosis of B. pseudomallei by mammalian macrophages has yet to be reported. If the analogy with L. pneumophila infection holds, the recent observation that prior intra-amebic bacterial infection enhances monocyte entry and virulence provides a possible experimental approach to the early stages of cellular interactions (6). The vacuolar and amebic escape of bacilli observed during the later stages of coculture further demonstrates the viability of intracellular bacteria. However, its relevance to human B. pseudomallei infection is speculative since the escape of sequestered bacilli from macrophages in vivo can only be inferred from bacterial replication that must occur in late onset or relapsing infections.
The cellular events that span the interaction between B. pseudomallei and amebic trophozoites bear some resemblance to phenomena previously observed in Legionella-Acanthamoeba cocultures (2, 3, 26). The ecological relationship between these two genera has been described as an endosymbiosis. A commensal relationship between B. pseudomallei and Acanthamoeba is possible in shared environmental habitats, but as we have yet to demonstrate their simultaneous occurrence in vivo or show a mutual codependency, it is not possible to describe the interaction as a true symbiosis. By analogy with L. pneumophila (1–3, 6, 7, 11, 26), it is possible that the capacity of B. pseudomallei to enter, survive within, and exit free-living amebae confers an ability to invade mammalian phagocytic cells, persist within, and escape from them as is thought to occur in relapsing and late-onset melioidosis. By the same token, it is also possible that there are key differences between the mechanisms used during amebic cytoinvasion compared with penetration of human macrophages (12, 13).
The observation of rapid rotation of the entire bacillus while anchored to a fixed point on the external trophozoite surface implies flagellar adhesion. It is of note that mutagenesis and animal model experiments highlight a possible role for the flagellum in the pathogenesis of B. pseudomallei infection (10). Other aspects of the initial interaction between B. pseudomallei and the amebic trophozoite that warrant further attention include expression of acid phosphatase, an enzyme produced by B. pseudomallei under acid conditions and thought to be a virulence factor for intracellular bacterial pathogens (9). This enzyme has been identified as a tyrosine phosphatase (19). It is therefore of note that tyrosine phosphatase activity has been implicated recently in attachment of L. pneumophila to another free-living protozoan, Hartmanella vermiformis (30).
Exposure to B. pseudomallei-contaminated soil or water in the region where the organism is endemic is thought to be the principal means of exposure to infection (5, 15, 29). A detailed appraisal of the possible ecological significance of a B. pseudomallei-Acanthamoeba interaction in vivo is beyond the scope of this investigation. Nevertheless, the remarkable ability of B. pseudomallei to persist in hostile environmental niches could be explained in part by an ability to survive in free-living amebae whose normal habitat lies within the vadosphere and in particular at air/water interfaces in the soil (25). It is possible that detailed study of the microbial ecology of the region where the organism is endemic may provide evidence for a putative role for free-living phagocytic protozoa in the pathogenesis of melioidosis. The observation that Acanthamoeba species such as A. astronyxis can be recovered from the human nasal mucosa (22) make inhalation of endamebic B. pseudomallei a little more plausible and provides a further avenue of investigation.
In conclusion, we have examined the interaction between cells of the facultative intracellular pathogen B. pseudomallei and several Acanthamoeba species. Nondestructive intracellular passage of bacteria and bacterial escape during trophozoite lysis were demonstrated. As yet it is unclear whether these are mutually exclusive or the extremes at either end of a range of outcomes. Whichever interpretation proves to be correct, these observations indicate that B. pseudomallei can adapt to a free-living intracellular habitat such as may be found in acanthamebae. The successful demonstration of an interaction between B. pseudomallei and A. astronyxis that includes coiling phagocytosis and nondestructive bacterial passage via trophozoites may help attempts to demonstrate similar phenomena in mammalian phagocytic cells. Endamebic passage of B. pseudomallei deserves further attention as a possible in vitro model of intracellular B. pseudomallei infection.
ACKNOWLEDGMENT
The Biomedical Confocal Microscopy Research Centre is supported by the Lotteries Commission of Western Australia.
REFERENCES
- 1.Barker J, Scaife H, Brown M R W. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berk S G, Ting R S, Turner G W, Ashburn R J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl Environ Microbiol. 1998;64:279–286. doi: 10.1128/aem.64.1.279-286.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bozue J A, Johnson W. Interaction of Legionella pneumophila with Acanthamoeba castellani: uptake by coiling phagocytosis and inhibition of phagosome-lysosome fusion. Infect Immun. 1996;64:668–673. doi: 10.1128/iai.64.2.668-673.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breiland J K, Fantone J C, Remick D G, LeGendre M, McClain M, Engleberg N C. The role of Legionella pneumophila-infected Hartmanella vermiformis as an infectious particle in a murine model of Legionnaire's disease. Infect Immun. 1997;65:5330–5333. doi: 10.1128/iai.65.12.5330-5333.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambon L. Isolement du bacille Whitmore a partir du milieu exterieur. Ann Inst Pasteur. 1955;89:229–235. [PubMed] [Google Scholar]
- 6.Cirillo J D, Cirillo S L, Yan L, Bermudez L E, Falkow S, Tompkins L S. Intracellular growth in Acanthamoeba castelanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect Immun. 1999;67:4427–4434. doi: 10.1128/iai.67.9.4427-4434.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cirillo J D, Falkow S, Tompkins L S. Growth of Legionella pneumophila in Acanthamoeba castellani enhances invasion. Infect Immun. 1994;62:3254–3261. doi: 10.1128/iai.62.8.3254-3261.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cirillo J D, Falkow S, Tompkins L S, Bermudez L E. Interaction of Mycobacterium avium with environmental amebae enhances virulence. Infect Immun. 1997;65:3759–3767. doi: 10.1128/iai.65.9.3759-3767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dejsirilert S, Butraporn R, Chiewsilp D, Kondo E, Kanai K. High activity of acid phosphatase in Pseudomonas pseudomallei as a possible attribute relating to its pathogenicity. Jpn J Med Sci Biol. 1989;42:39–49. doi: 10.7883/yoken1952.42.39. [DOI] [PubMed] [Google Scholar]
- 10.DeShazer D, Brett P J, Carlyon R, Woods D E. Mutagenesis of Burkholderia pseudomallei with Tn5-OT182: isolation of motility mutants and molecular characterization of the flagellin structural gene. J Bacteriol. 1997;179:2116–2125. doi: 10.1128/jb.179.7.2116-2125.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao L Y, Harb O S, Kwaik Y A. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant host cells, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagele S. Legionella pneumophila kills human phagocytes but not protozoan host cells by inducing apoptotic cell death. FEMS Microbiol Lett. 1998;169:51–58. doi: 10.1111/j.1574-6968.1998.tb13298.x. [DOI] [PubMed] [Google Scholar]
- 14.Horwitz M A. Phagocytosis of the Legionnaire's bacterium (Legionella pneumophila) occurs by a novel mechanism: engulfment within a pseudopod coil. Cell. 1984;36:27–33. doi: 10.1016/0092-8674(84)90070-9. [DOI] [PubMed] [Google Scholar]
- 15.Inglis T J J, Garrow S C, Adams C, Henderson M, Mayo M, Currie B J. Acute melioidosis outbreak in Western Australia. Epidemiol Infect. 1999;123:437–444. doi: 10.1017/s0950268899002964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inglis, T., T. Robertson, M. Wikstrom, B. J. Chang, and B. J. Mee. Does Burkholderia pseudomallei exist in a viable but non-culturable form?, p. 127. In Proceedings of the 9th International Congress of Bacteriology and Applied Microbiology. International Union of Microbiological Societies, Sydney, Australia.
- 17.Jones A L, Beveridge T J, Woods D E. Intracellular survival of Burkholderia pseudomallei. Infect Immun. 1996;64:782–790. doi: 10.1128/iai.64.3.782-790.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanai K, Kondo E, Dejsirilert S, Naigowit P. Growth and survival of Pseudomonas pseudomallei in acidic environment with possible relation to the ecology and epidemiology of melioidosis. In: Puthucheary S D, Malik Y A, editors. Melioidosis: prevailing problems and future directions. Kuala Lumpur, Malaysia: Malaysian Society of Infectious Diseases and Chemotherapy; 1994. pp. 26–38. [Google Scholar]
- 19.Kondo E, Wangroongsaub P, Naigowit P, Kanai K. Separation of antigenic glycoprotein fractions from cell-free homogenate of Pseudomonas pseudomallei and characterization as tyrosine phosphatase. Southeast Asian J Trop Med Public Health. 1994;25:436–442. [PubMed] [Google Scholar]
- 20.Ly T M, Muller H E. Ingested Listeria monocytogenes survive and multiply in protozoa. J Med Microbiol. 1990;33:51–54. doi: 10.1099/00222615-33-1-51. [DOI] [PubMed] [Google Scholar]
- 21.Marolda C L, Hauroder B, John M A, Michel R, Valvano M A. Intracellular survival and saprophytic growth of isolates from the Burkholderia cepacia complex in free-living amoebae. Microbiology. 1999;145:1509–1517. doi: 10.1099/13500872-145-7-1509. [DOI] [PubMed] [Google Scholar]
- 22.Michel R, Rohl R, Schneider H. Isolation of free living amoebae from nasal mucosa of healthy individuals. Zentbl Bakteriol Mikrobiol Hyg. 1982;176:1255–1259. [PubMed] [Google Scholar]
- 23.Michel R, Hauroder B. Isolation of an Acanthamoeba strain with intracellular Burkholderia pickettii infection. Zentbl Bakteriol. 1997;285:541–557. doi: 10.1016/s0934-8840(97)80116-8. [DOI] [PubMed] [Google Scholar]
- 24.Praksachartvuthi S, Aswapokee N, Thankerngpol K. Survival of Pseudomonas pseudomallei in human phagocytes. J Med Microbiol. 1990;31:109–114. doi: 10.1099/00222615-31-2-109. [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Zaragoza S. Ecology of free-living amoebae. Crit Rev Microbiol. 1994;20:225–241. doi: 10.3109/10408419409114556. [DOI] [PubMed] [Google Scholar]
- 26.Rowbotham T. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980;33:1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanford J P, Moore W L. Recrudescent melioidosis: a southeast Asian legacy. Am Rev Respir Dis. 1971;104:452–453. doi: 10.1164/arrd.1971.104.3.452. [DOI] [PubMed] [Google Scholar]
- 28.Steinert M, Birkness K, White E, Fields B, Quinn F. Mycobacterium avium bacilli grow saprozoically in coculture with Acanthamoeba polyphaga and survive within cyst walls. Appl Environ Microbiol. 1998;64:2256–2261. doi: 10.1128/aem.64.6.2256-2261.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strauss J M, Jason S, Mariappan M. Pseudomonas pseudomallei in soil and surface water of Sabah. Med J Malays. 1967;22:31–32. [Google Scholar]
- 30.Venkataraman C, Gao L Y, Bondada S, Kwaik Y A. Identification of putative cytoskeletal protein homologues in the protozoan host Hartmanella vermiformis as substrates for induced tyrosine phosphatase activity upon attachment to the Legionnaire's bacterium, Legionella pneumophila. J Exp Med. 1998;188:505–514. doi: 10.1084/jem.188.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White N J, Dance D A B, Chaowagul W, Wattanagoon Y, Wuthiekanun V, Pitakwatchara N. Halving of mortality of severe melioidosis by ceftazidime. Lancet. 1989;2:697–701. doi: 10.1016/s0140-6736(89)90768-x. [DOI] [PubMed] [Google Scholar]
- 32.Wuthiekanun V, Smith M D, White N J. Survival of Burkholderia pseudomallei in the absence of nutrients. Trans R Soc Trop Med Hyg. 1995;89:491. doi: 10.1016/0035-9203(95)90080-2. [DOI] [PubMed] [Google Scholar]