ABSTRACT
Background:
There is a need to identify novel markers for CAD, independent of traditional CV risk factors. One of these is gamma-glutamyl transferase (GGT), a marker of increased oxidative stress. Given the high prevalence of CAD in Asian Indians, the link of GGT and CAD in them needs to be studied.
Aim:
To assess GGT in patients with angiographically documented CAD.
Methods and Results:
Two hundred patients aged 58.1 ± 9.95 years, 73% males, hypertension 56%, diabetes 40% were included. Mean GGT was 63.6 ± 44.33 (10–269 U/L). The levels of GGT progressively increased in those with single/double or triple-vessel CAD (36.5, 61.5, and 87 U/L, respectively, P < 0.001). Using objective criteria of CAD burden (SYNTAX and Gensini scores), we reaffirmed these findings. GGT in patients with SYNTAX tertiles 0–22, 23–32, and ≥ 33 was 33, 62, and 97 U/L, respectively and in Gensini tertiles 0–17.65, 17.66–56.65, ≥56.66 was 32, 52, and 88 U/L, respectively, all P < 0.001. SYNTAX score ≥ 23 was present in only 23% patients in GGT tertile 1 (<41 U/L), whereas60% and 94% in GGT tertiles 2 and 3 had SYNTAX ≥ 23. Significant positive correlation was seen between GGT and SYNTAX (r = 0.634) and Gensini score (r = 0.772).
Conclusions:
In this study, GGT had an independent correlation with angiographic severity of CAD and SYNTAX and Gensini scores. Although the existing evidence seems biologically plausible, more studies are needed to explore the potential role of this inexpensive marker for predicting disease burden in patients with CAD.
Keywords: Coronary artery disease, Gamma-glutamyltransferase (GGT), Gensini score, Indians, SYNTAX
INTRODUCTION
Coronary artery disease (CAD) is the major cause of morbidity and mortality worldwide.[1] Although conventional cardiovascular (CV) risk factors such as hypertension (HT), diabetes mellitus (DM), dyslipidemia, and smoking have a strong association with development of CAD, many patients have CAD even without the presence of such risk factors.[2,3] Asian Indians not only have a higher prevalence of CAD as compared to Western counterparts, but the onset is often at a younger age, and the severity of CAD is also much more, highlighting the need to explore additional biomarkers to explain this higher risk and improve risk stratification.[4,5] Despite extensive efforts for the identification of biomarkers for early assessment of CAD, only a limited number of useful biomarkers have been identified, and there is a need to identify and validate novel markers.
Gamma glutamyl transferase (GGT) is an enzyme which is responsible for the extracellular catabolism of glutathione and acts as an important antioxidant in mammalian cells.[6] Whereas increased serum GGT levels are known in patients with hepatobiliary dysfunction, population-based studies have also identified a predictive value of serum GGT for both CV and all-cause mortality.[7,8,9]
Imbalance between oxidant/antioxidant system as well as inflammation have been proposed as the major mechanisms for the association of GGT with pathogenesis of CAD.[10] The presence of catalytically active GGT molecules in human atheromatous plaque and GGT-triggered iron-catalyzed low-density lipoprotein (LDL) oxidation, as well as production of reactive oxygen species are also responsible for this association.[11,12] These events play a central role in the evolution of atherosclerosis including formation of the fibrous cap, cellular apoptosis, plaque erosion and rupture, and enhanced platelet aggregation and thrombosis.
Evidence from various epidemiological studies suggests that higher serum GGT levels are associated with higher incidence of CVD and an increased risk of abnormal vascular stiffness, coronary flow reserve impairment, DM, metabolic syndrome, myocardial infarction, stroke, cardiac, and all cause death.[8,13,14,15]
Data from the third US National Health and Nutrition Examination Survey, 1988–1994 (NHANES III), involving 14,950 adult NHANES participants, revealed that elevated GGT was associated with all-cause mortality [hazard ratio (HR) 1.6] in the age adjusted model, deaths due to diabetes (HR 4.9), death from CVD and cancer (HR 1.5).[16] These HRs were consistent with those reported from an Austrian database as well.[17] An age and gender related gradient of GGT with CV events has also been reported; amongst 28,838 Finnish men and women, GGT-related HRs were higher among individuals under age 60, both for nonfatal heart attack and fatal coronary heart disease (CHD).[18]
Other studies have also reported a dose-response relationship between GGT and CV mortality with higher levels of serum GGT being associated with a 20%, 54%, and 34% increased risk of coronary heart disease (CHD), stroke, and both stroke and CHD, respectively.[19,20,21]
Typically identified as a marker of hepatobiliary dysfunction, GGT appears to be a promising biomarker of oxidative stress and cardiovascular risk. Its association with CAD needs to be explored further, especially in Indian patients, given the exponentially rising trends of CAD in these patients. It is not only important to correlate if serum levels of GGT are elevated in patients with CAD, but it is also pertinent to assess if its levels correlate with the disease burden in patients with CAD, as quantified by single vs multi-vessel disease and established angiographic scores.
Aim
The aim of current study was to investigate the association of GGT with CAD in Indian patients with signs or symptoms of CAD (rest angina/angina on effort/ECG changes) who were scheduled to undergo coronary revascularization.
METHODS
The study was a prospective, single-center study that enrolled 200 patients from January 2019 to December 2020 after an informed written consent, and the study conformed to institutional ethical guidelines. Patients were excluded if they had active liver disease, acute renal failure or chronic kidney disease, cancer, concomitant inflammatory conditions, active infection, or active alcohol consumption.
Detailed history and examination were performed for all enrolled patients, and blood samples were collected before intervention. A 12 lead ECG and trans-thoracic echocardiography were performed in all patients. GGT, HbA1c, renal function tests, and liver function tests were performed on Spectra make, Pro M model Clinical Chemistry Analyzer. Hypertension and diabetes mellitus were defined as per routine standard criteria.
The overall severity of coronary stenosis, complexity, and coronary atherosclerotic burden was quantified by Gensini and the SYNTAX score. The Gensini score was calculated as total of the sum of severity score assigned based on the degree of angiographic luminal stenosis on visual assessment in each segment of the coronary artery. The final GS is the sum of all the lesion scores.[22]
The SYNTAX score was calculated based on coronary artery lesion with diameter stenosis greater than 50%, arterial dominance, coronary segment involved, presence of total occlusion, bifurcation or trifurcation or not, aorto-ostial lesion, lesion length, calcification, tortuosity, and thrombus.[23] The final calculation was based on the online SYNTAX calculator (version 2.28).
Statistical analysis- The appropriate statistical tests were used to analyze the data recorded, like GGT, SYNTAX, and GENSINI score. Numerical variables were reported as the mean ± SD or median (IQR), and categorical variables were represented as frequency (%). Normality of data was tested using Shapiro–Wilk test or Kolmogrov–Smirnov test. For homogenously distributed data, independent t-test and one way ANOVA was used, whereas Mann–Whitney U test and Kruskal––Wallis test was used for skewed data. Chi-square test were used to compare categorical variables between two groups. Correlation among variables was studied using Pearson's correlation coefficient. All test of statistical significance were considered significant at 0.05 levels.
RESULTS
A total of 200 patients were recruited from January 2019 to December 2020. The mean age of the study population was 58.1 ± 9.95 years (range 34–84 years) and of these, 73.7% were males. Hypertension was present in 56% of patients, DM was noted in 40%, and tobacco consumption was present in 23%. The most common presentation was chronic stable angina (CSA), which was seen in 64.7% patients followed by ST-segment elevation myocardial infarction (STEMI) which was seen in 23.7%, and non-STEMI (NSTEMI) was seen in 11.6% of patients. The other biochemical and demographic parameters are listed in Table 1. GGT values ranged from 10 to 269 U/L (mean 63.6 U/L) in the study population. On angiography, most common finding was single-vessel disease (SVD 40%, n = 81) followed by double-vessel disease (DVD 34.7%, n = 69), and 25.3% (n = 50) patients had triple-vessel disease (TVD).
Table 1.
Base line characteristics of study population
| Parameter | Mean+SD |
|---|---|
| Age (years) | 58.1±9.95 |
| Hemoglobin (gm/dL) | 12.7±1.59 |
| Serum creatinine (mg/dL) | 1.1±0.25 |
| HbA1c (%) | 6.1±1.17 |
| Total bilirubin (mg/dL) | 0.8±0.29 |
| SGOT (U/L) | 20.4±7.06 |
| SGPT (U/L) | 25.2±10.41 |
| GGT (U/L) | 63.6±44.33 |
| CRP (mg/L) | 5.9±4.5 |
| Total cholesterol (mg/dL) | 139.8±34.95 |
| HDL (mg/dL) | 45.1±10.81 |
| LDL (mg/dL) | 97.4±27.42 |
| TG (mg/dL) | 144±52 |
| Total Cholesterol (mg/dL) | 186±40 |
| LVEF | 53.9±7.58 |
| SVD | 81 (40%) |
| DVD | 69 (34.7%) |
| TVD | 50 (25.3%) |
| SYNTAX SCORE | 22.87±10.14 |
| GENSINI SCORE | 45.17±35.96 |
SVD: Single vessel disease, DVD: Double vessel disease, TVD: Triple vessel disease
Relation of GGT with angiographic severity of CAD based on SVD/DVD/TVD: We found a significant positive association between serum GGT and patients with angiographically documented CAD. The median level of serum GGT among patients with SVD, DVD, and TVD was 36.5 u/L,61.5 u/L, and 87 u/L, respectively. [Table 2 and Figure 1].
Table 2.
GGT among patients classified according to different angiographic parameters
| SVD | DVD | TVD | P |
|---|---|---|---|
| GGT (U/L) | |||
| SYNTAX GROUP | |||
| <=22 | 23-32 | >=33 | |
| 33 (25-44) | 62 (45-71) | 97 (84-135) | < 0.001 |
| GENSINI GROUP | |||
| 0-17.65 | 17.66-56.65 | >56.66 | |
| 32 (25-44) | 52 (42.5-64) | 88 (76-134) | < 0.001 |
| NO OF VESSELS INVOLVED | |||
| 36.5 (26.5-47) | 61.5 (39-74) | 87 (76-117) | < 0.001 |
SVD: Single vessel disease, DVD: Double vessel disease, TVD: Triple vessel disease
Figure 1.
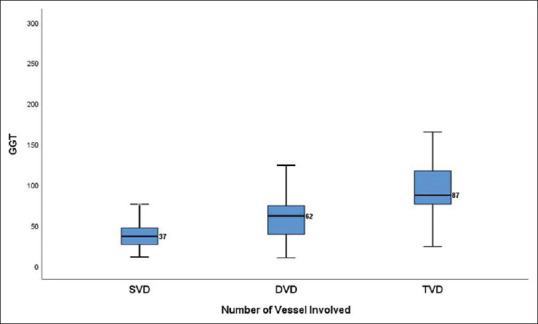
Box plot showing GGT value among different groups based on number of coronary vessels involved
Relation of GGT with angiographic severity of CAD based on angiographic scores: The median level of serum GGT among patients with SYNTAX score (0–22), (23–32), and (≥33) was 33 u/L,62 u/L, and 97 u/L, respectively. [Table 2/Figure 2]. Amongst patients with Gensini score (0–17.65), (17.66–56.65), and (≥56.66), the median GGT level was 32 u/L, 52 u/L, and 88 u/L, respectively. [Table 2 and Figure 3].
Figure 2.
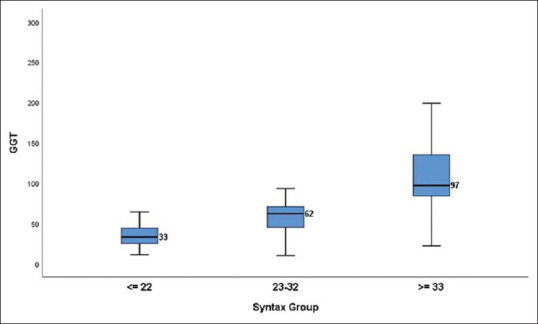
Box plot showing GGT values among different SYNTAX groups
Figure 3.
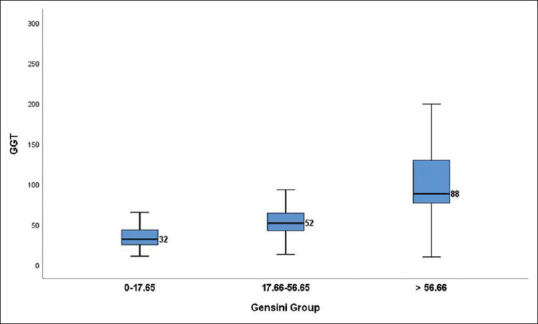
Box plot showing GGT values among different Gensini groups
Logistic regression analysis showed a significant association of GGT with increasing severity of CAD as shown in Table 3. Furthermore, a significant positive correlation was also noted between serum level of GGT and Gensini score (r = 0.772, P < 0.001) and SYNTAX score (r = 0.634, P < 0.001). Figure 4.
Table 3.
Multivariate analysis between GGT and patients classified according to different angiographic parameters
| Number of vessels involved | Odds ratio | 95% CI | P |
|---|---|---|---|
| SVD | |||
| DVD | 1.046 | 1.028-1.064 | <0.001 |
| TVD | 1.066 | 1.046-1.086 | <0.001 |
| SYNTAX Score | |||
| (0-22) | |||
| (23-32) | 1.066 | 1.042-1.091 | <0.001 |
| (≥33) | 1.131 | 1.095-1.168 | <0.001 |
| Gensini Score | |||
| (0-17.65) | |||
| (17.66-56.65) | 1.046 | 1.024-1.069 | <0.001 |
| (≥56.66) | 1.113 | 1.081-1.146 | <0.001 |
Figure 4.
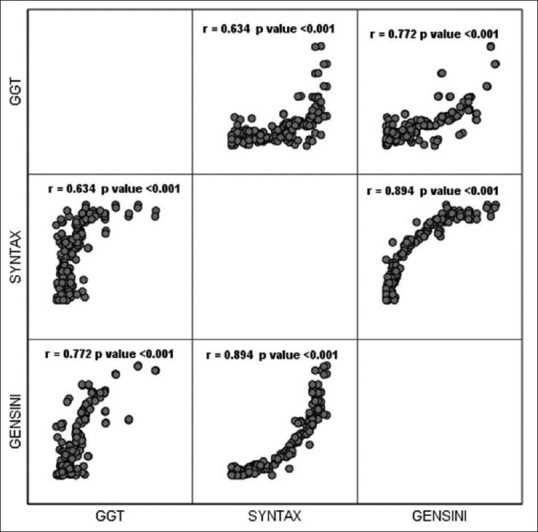
Correlation of GGT with SYNTAX and Gensini score
Stratifying the patient population based on GGT tertiles <41 U/L, 41–71 U/L, and >71 U/L Table 4 revealed that SYNTAX scores was incrementally more severe in those with in the higher GGT teriles (15.2 ± 8.12, 21.2 ± 7.72, and 32 ± 6.22, P < 0.001). In the GGT tertile 1, 78% had SYNTAX score ≤22, and 18.4% and 4.6%, respectively had SYNTAX 23–32 and ≥33. The corresponding % for GGT tertile 2 and 3 were respectively 39.8%, 54.4%, and 5.8% and 5.9%, 26.9%, and 67.2%.
Table 4.
Distribution of SYNTAX and Gensini score patterns amongst GGT tertiles
| GGT valu | <41 (n=65) | 41-71 (n=68) | >71 (n=67) | P |
|---|---|---|---|---|
| SYNTAX | 15.2±8.12 | 21.2±7.72 | 32±6.22 | <0.001 |
| <22 | 50 (78%) | 27 (39.8%) | 4 (5.9%) | |
| 23-32 | 12 (18.4%) | 37 (54.4%) | 18 (26.9%) | |
| >33 | 3 (4.6%) | 4 (5.8%) | 45 (67.2%) | |
| GENSINI | 20.1±18.37 | 31.3±18.92 | 83.3±30.07 | <0.001 |
| 0-17.65 | 43 (66.1%) | 19 (27.9%) | 4 (5.9%) | |
| 17.66-56.65 | 16 (24.6%) | 43 (63.3%) | 9 (13.4%) | |
| >56.66 | 6 (9.3%) | 6 (8.8%) | 54 (80.5%) |
Similarly, Gensini scores increased across the strata of the GGT tertiles (20.1 ± 18.37, 31.3 ± 18.92, and 83.3 ± 30.07, P < 0.001). A Gensini score of 17.66–56.65 and ≥56.66 was noted in 66.1%, 24.6%, and 9.3% in GGT tertile 1, for GGT tertile 2, the % values were 27.9%, 63.3%, and 8.8%, whereas for GGT tertile 3, the corresponding % values were 5.9%, 13.4%, and 80.5%, all P < 0.001
Correlation with other parameters: GGT showed a significant correlation with CRP (r = 0.360, P_ =0.001) and with systolic BP (r = 0.148, P = 0.042), but no significant correlation was observed with other biochemical parameters or conventional CV risk factors like DM, dyslipidemia, and tobacco consumption.
DISCUSSION
In the current study, amongst 200 patients (mean age 58.1 years) who underwent coronary angiography before scheduled revascularization (2/3rd for chronic stable angina), the mean GGT level was 63.6 ± 44.33 U/L. Mean GGT level were slightly higher than what was previously reported in studies by Ghatge et al.[24] (30.94 ± 0.90 U/L), Bijapur et al.[25] (39.48 ± 22.91 U/L), Emdin et al.[26] (39·0 ± 39·2 U/L), and Aksakal et al.[27] (36.7 ± 17.3 U/L), although in the analysis by Arasteh et al.[28] almost similar levels were reported (mean GGT 70.66 U/L).
Serum GGT levels were significantly higher in patients with double/triple vessel CAD as compared to those with single-vessel disease, highlighting the association of GGT with increasing severity of obstructive CAD. Interestingly, the median GGT levels in patients with SVD, DVD, and TVD in our study (36.5 u/L, 61.5 u/L, and 87 u/L, respectively) are almost similar to what was reported by Arasteh et al.[28] (viz 55.6 + 9.7, 71.7 ± 12.7, and 84.7 ± 13.4 U/L), who also noted that GGT levels progressively increased in patients with multi-vessel CAD.
We also analyzed the relationship of GGT levels with angiographic scores like SYNTAX for coronary lesion complexity and Gensini for coronary artery atherosclerotic burden. The level of serum GGT increased significantly with increasing SYNTAX scores in our study (33 u/L, 62 u/L, and 97 u/L, respectively in the tertiles of Synatx <22, 23–32, and ≥ 33). Similar findings were observed by Aksakal et al.,[27] who in a Turkish study cohort, demonstrated an increase in GGT levels from low SYNTAX tertile to high tertile (32.2 ± 16.4, 38.5 ± 18.5, and 42.7 ± 15.9), in patients with stable angina pectoris.
We further attempted to establish the potential link between GGT and coronary artery atherosclerotic burden quantified by Gensini score analysis. Progressively higher GGT levels were seen in patients with higher Gensini scores (32 u/L, 52 u/L, and 88 u/L, respectively in the Gensini tertiles) To the best of our knowledge, none of the previous studies have correlated GGT levels with both measures of angiographic severity (SYNTAX for coronary lesion complexity) and Gensini (for coronary artery atherosclerotic burden).
The association between GGT and coronary lesion complexity (SYNTAX score) was further exemplified as shown in Table 4. SYNTAX score ≥23 was present in only 23% patients in GGT tertile 1 (<41 U/L), whereas 60% and 94% in GGT tertiles 2 and 3 had SYNTAX ≥ 23. Similarly, GGT was linearly associated with coronary artery atherosclerotic burden (Gensini score). Score ≥17.66 was noted in only 34% in GGT tertile 1, as compared to 72% and 94% in GGT tertiles 2 and 3, respectively.
A significant positive correlation between serum level of GGT and SYNTAX score (coronary lesion complexity) and Gensini score (coronary artery atherosclerotic burden) was noted. The correlation was slightly more robust with Gensini score (r = 0.772) compared to SYNTAX score (r = 0.634), although this was not statistically significant. This difference is most likely due to method of calculation of the scores as SYNTAX takes into account only lesions with > 50% stenosis, whereas Gensini score is more closely related to overall atherosclerotic burden and takes into account any degree of stenosis.
Few studies have found a correlation of GGT with other cardiovascular risk factors, such as hypertension, stroke, diabetes, dyslipidemia, and metabolic syndrome, and others have observed its association with inflammatory markers.[24,29] We observed a correlation of GGT with CRP levels (r = 0.148, P = 0.042) and systolic BP (r = 0.148, P = 0.042), but not with presence of other CV risk factors such as DM, lipid levels, or smoking.
Gamma-glutamyl transferase (GGT), an enzyme that regulates intracellular glutathione and is a marker of increased oxidative stress has been linked to the pathogenesis of CAD by way of oxidant/antioxidant imbalance and inflammation.[30] Our study adds to the growing evidence that higher levels of GGT may be associated both with coronary lesion complexity and coronary artery atherosclerotic burden, findings that need to be validated in future studies.
Limitations
Absence of a control arm, a small sample size from a single center, and lack of any outcome data represent important limitations. Due to the COVID pandemic, availability of controls and suboptimal follow up after discharge were important factors in this regard. We also did not measure any other biochemical markers of oxidative stress (due to cost logistics), which could have further added to the strengths of the study. Further studies should also perhaps focus on whether a single point assessment or serial assays are likely to have any clinical implications
CONCLUSION
The present study demonstrated the value of serum level of GGT as a biomarker for predicting severity of CAD amongst patients with documented CAD on coronary angiography. To the best of our knowledge, this is the first study evaluating relationship of serum level of GGT with coronary lesion complexity (SYNTAX score) and coronary artery atherosclerotic burden (Gensini score) in an Indian population. Higher GGT levels potentially denote increased oxidative stress and are hence may be mechanistically linked to development of CAD. As GGT is a low cost and readily available assay, validating these data in a larger cohort and translating the findings into clinical practice would be clinically pertinent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Libby P, Okamoto Y, Rocha VZ, Folco E. Inflammation in atherosclerosis: Transition from theory to practice. Circ J. 2010;74:213–20. doi: 10.1253/circj.cj-09-0706. [DOI] [PubMed] [Google Scholar]
- 2.Greenland P, Knoll MD, Stamler J, Neaton JD, Dyer AR, Garside DB, et al. Major risk factors as antecedents of fatal and nonfatal coronary heart disease events. JAMA. 2003;290:8917. doi: 10.1001/jama.290.7.891. [DOI] [PubMed] [Google Scholar]
- 3.Khot UN, Khot MB, Bajzer CT, Sapp SK, Ohman EM, Brener SJ, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290:898904. doi: 10.1001/jama.290.7.898. [DOI] [PubMed] [Google Scholar]
- 4.Goyal A, Yusuf S. The burden of cardiovascular disease in the Indian subcontinent. Indian J Med Res. 2006;124:23544. [PubMed] [Google Scholar]
- 5.Sharma M, Ganguly NK. Premature coronary artery disease in Indians and its associated risk factors. Vasc Health Risk Manag. 2005;1:21725. [PMC free article] [PubMed] [Google Scholar]
- 6.Meister A. Metabolism and transport of glutathione and other-glutamyl compounds. In: Larsson A, Orrenius S, Holmgren A, Mannervik B, editors. Functions of Glutathione: Biochemical, Toxicological and Clinical Aspects. New York: Raven Press; 1983. pp. 1–22. [Google Scholar]
- 7.Brenner H, Rothenbacher D, Arndt V, Schuberth S, Fraisse E, Fliedner TM. Distribution, determinants, and prognostic value of gammaglutamyltranspeptidase for all-cause mortality in a cohort of construction workers from south Germany. Prev Med. 1997;26:305–10. doi: 10.1006/pmed.1997.0144. [DOI] [PubMed] [Google Scholar]
- 8.Wannamethee G, Ebrahim S, Shaper AG. Gammaglutamyl transferase: Determinants and association with mortality from ischemic heart disease and all causes. Am J Epidemiol. 1995;142:699–708. doi: 10.1093/oxfordjournals.aje.a117699. [DOI] [PubMed] [Google Scholar]
- 9.Peterson B, Trell E, Kristensson H, Fex G, Yettra M, Hood B. Comparison of gammaglutamyltransferase and other health screening tests in average middle-aged males, heavy drinkers and alcohol non-users. Scand J Clin Lab Invest. 1983;43:141–9. doi: 10.1080/00365518309168236. [DOI] [PubMed] [Google Scholar]
- 10.Sener A, Cevik O, Yanikkaya-Demirel G, Apikoglu-Rabus S, Ozsavci D. Influence of platelet γ-glutamyltransferase on oxidative stress and apoptosis in the presence of holo-transferrin. Folia Biol (Praha) 2012;58:193–202. [PubMed] [Google Scholar]
- 11.Paolicchi A, Minotti G, Tonarelli P, Tongiani R, De Cesare D, Mezzetti A, et al. Gammaglutamyltranspeptidase-dependent iron reduction and LDL oxidation: A potential mechanism in atherosclerosis. J Investig Med. 1999;47:151–60. [PubMed] [Google Scholar]
- 12.Dominici S, Valentini M, Maellaro E, Del Bello B, Paolicchi A, Lorenzini E, et al. Redox modulation of cell surface protein thiols in U937 lymphoma cells: The role of gamma-glutamyl transpeptidase-dependent H2O2 production and S-thiolation. Free Radic Biol Med. 1999;27:623–35. doi: 10.1016/s0891-5849(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 13.Whitfield JB. Gamma glutamyl transferase. Crit Rev Clin Lab Sci. 2001;38:263–355. doi: 10.1080/20014091084227. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Qi X, Xu W, Song H, Xu M, Ma W, et al. Serum gamma-glutamyl transferase: A novel biomarker for coronary artery disease. Med Sci Monit. 2014;20:706–10. doi: 10.12659/MSM.890245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koenig G, Seneff S. Gamma-glutamyltransferase: A predictive biomarker of cellular antioxidant inadequacy and disease risk. Dis Markers. 2015;2015:818570. doi: 10.1155/2015/818570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruhl CE, Everhart JE. Elevated serum alanine aminotransferase and γ-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009;136:477–85. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 17.Kazemi-Shirazi L, Endler G, Winkler S, Schickbauer T, Wagner O, Marsik C. Gamma glutamyltransferase and longterm survival: Is it just the liver? Clin Chem. 2007;53:940–6. doi: 10.1373/clinchem.2006.081620. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Silventoinen K, Hu G, Jacobs DR, Jr, Jousilahti P, Sundvall J, et al. Serum gammaglutamyltransferase predicts non-fatal myocardial infarction and fatal coronary heart disease among 28 838 middle-aged men and women. Eur Heart J. 2006;27:2170–6. doi: 10.1093/eurheartj/ehl086. [DOI] [PubMed] [Google Scholar]
- 19.Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H, et al. γ-Glutamyltransferase as a risk factor for cardiovascular disease mortality: An epidemiological investigation in a cohort of 163 944 Austrian adults. Circulation. 2005;112:2130–7. doi: 10.1161/CIRCULATIONAHA.105.552547. [DOI] [PubMed] [Google Scholar]
- 20.Atar AI, Yilmaz OC, Akin K, Selcoki Y, Er O, Eryonucu B. Association between gamma-glutamyltransferase and coronary artery calcification. Int J Cardiol. 2013;167:1264–7. doi: 10.1016/j.ijcard.2012.03.157. [DOI] [PubMed] [Google Scholar]
- 21.Lee W, Ryoo JH, Suh BS, Lee J, Kim J. Association of coronary artery calcification and serum gamma-glutamyltransferase in Korean. Atherosclerosis. 2013;226:269–74. doi: 10.1016/j.atherosclerosis.2012.10.059. [DOI] [PubMed] [Google Scholar]
- 22.Rampidis GP, Benetos G, Benz DC, Giannopoulos AA, Buechel RR. A guide for Gensini Score calculation. Atherosclerosis. 2019;287:181–3. doi: 10.1016/j.atherosclerosis.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Ong AT, Serruys PW, Mohr FW, Morice MC, Kappetein AP, Holmes DR, Jr, et al. The SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery (SYNTAX) study: Design, rationale, and run-in phase. Am Heart J. 2006;151:1194–204. doi: 10.1016/j.ahj.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Ghatge M, Sharma A, Vangala RK. Association of γ-glutamyl transferase with premature coronary artery disease. Biomed Rep. 2016;4:307–12. doi: 10.3892/br.2016.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bijapur SJ, BS A, Nanjappa MC, Reddy PT, Katheria R. Gamma-Glutary Transferase: A Novel Biomarker for Predicting Coronary Artery Disease. J Cardiovasc Med Surg. 2020;6:29–32. [Google Scholar]
- 26.Emdin M, Passino C, Michelassi C, Titta F, L’abbate A, Donato L, et al. Prognostic value of serum gamma-glutamyl transferase activity after myocardial infarction. Eur Heart J. 2001;22:1802–7. doi: 10.1053/euhj.2001.2807. [DOI] [PubMed] [Google Scholar]
- 27.Aksakal E, Tanboga IH, Kurt M, Kaygın MA, Kaya A, Isik T, et al. The relation of serum gamma glutamyltransferase levels with coronary lesion complexity and long-term outcome in patients with stable coronary artery disease. Atherosclerosis. 2012;221:596–601. doi: 10.1016/j.atherosclerosis.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 28.Arasteh S, Moohebati M, Avan A, Esmaeili H, Ghazizadeh H, Mahdizadeh A, et al. Serum level of gamma-glutamyltransferase as a biomarker for predicting stenosis severity in patients with coronary artery disease. Indian Heart J. 2018;70:788–92. doi: 10.1016/j.ihj.2017.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poelzl G, Eberl C, Achrainer H, Doerler J, Pachinger O, Frick M, et al. Prevalence and prognostic significance of elevated gammaglutamyltransferase in chronic heart failure. Circ Heart Fail. 2009;2:294–302. doi: 10.1161/CIRCHEARTFAILURE.108.826735. [DOI] [PubMed] [Google Scholar]
- 30.Emdin M, Passino C, Michelassi C, Donato L, Pompella A, Paolicchi A. Additive prognostic value of gammaglutamyltransferase in coronary artery disease. Int J Cardiol. 2009;136:805. doi: 10.1016/j.ijcard.2008.04.030. [DOI] [PubMed] [Google Scholar]


