ABSTRACT
Context:
Viscoelastic hemostatic assays (VHA) are commonly used to identify specific cellular and humoral causes for bleeding in cardiac surgery patients. Cardiopulmonary bypass (CPB) alterations to coagulation are observable on VHA. Citrated VHA can approximate fresh whole blood VHA when kaolin is used as the activator in healthy volunteers. Some have suggested that noncitrated blood is more optimal than citrated blood for point-of-care analysis in some populations.
Aims:
To determine if storage of blood samples in citrate after CPB alters kaolin activated VHA results.
Settings and Design:
This was a prospective observational cohort study at a single tertiary care teaching hospital.
Methods and Material:
Blood samples were subjected to VHA immediately after collection and compared to samples drawn at the same time and stored in citrate for 30, 90, and 150 min prior to kaolin activated VHA both before and after CPB.
Statistical Analysis Used:
VHA results were compared using paired T-tests and Bland–Altman analysis.
Results:
Maximum clot strength and time to clot initiation were not considerably different before or after CPB using paired T-tests or Bland–Altman Analysis.
Conclusions:
Citrated samples appear to be a clinically reliable substitute for fresh samples for maximum clot strength and time to VHA clot initiation after CPB. Concerns about the role of citrate in altering the validity of the VHA samples in the cardiac surgery population seem unfounded.
Keywords: Cardiac surgery, cardiopulmonary bypass, citrate, viscoelastic hemostatic assays
INTRODUCTION
Perioperative bleeding and the use of allogeneic blood products are common in cardiac surgery,[1] although the rate of transfusion varies.[2] In several studies, higher rates of transfusion have been associated with greater morbidity and mortality in cardiac surgery.[3,4] Viscoelastic hemostatic assays (VHAs) have been used to identify specific cellular and humoral causes for postoperative bleeding in cardiac surgery patients.[5] Algorithms based on VHA may allow for the use of fewer blood products.[6,7] VHA measures blood clot formation in a sample of the patient's blood exposed to a clot initiator, most often kaolin. The results of these tests can help determine specific causes for coagulopathy and the appropriate therapy.[8,9] Cardiopulmonary bypass (CPB) alters coagulation independent of the effects of heparin and protamine, and post-CPB hypocoagulation has been described.[10,11,12] This is observable on VHA.[6,13,14,15] The addition of heparinase to a VHA sample catalyzes heparin degradation, thereby exposing intrinsic coagulopathies that might otherwise be masked by the heparin anticoagulative effects. Treating samples with heparinase allows for assessment of post-CPB coagulopathy prior to protamine administration.[16]
VHAs are ideally performed as a point-of-care test soon after the sample is drawn. However, in small centers where the number of personnel certified to use VHA equipment is restricted or during emergencies at times other than regular business hours when assistance is limited, it may be necessary to store samples drawn by any team member until the attention of personnel trained in using VHA can be turned from patient care to performing the test. Blood samples for VHA can be stored in citrate to prevent coagulation and recalcified at the time of testing.[8,9] Studies have tested the validity of citrated compared to fresh blood samples. In the initial studies, VHA using coagulation initiators such as tissue factor showed faster time to clot initiation after as little as 30 min of storage and increased in maximum clot strength as the citrated sample was stored longer.[17,18,19] Subsequent reports of samples from healthy volunteers found that citrated samples activated with kaolin better approximated fresh samples even after 3 h of storage in citrate.[20,21] Despite this, some have suggested that noncitrated blood is more optimal than citrated blood for point-of-care analysis in some populations, such as trauma patients.[22]
We sought to determine whether dilution of samples in citrate and subsequent recalcification altered VHA results in the setting of CPB-induced coagulopathy. Gilman et al.[23] previously observed a decrease in the time to kaolin activated VHA clot initiation for citrated samples from patients after CPB stored for 15 min compared to fresh samples but no differences in the maximum clot strength. Samples from volunteers that did not undergo CPB with heparin added in vitro did not show a faster time to VHA clot initiation with storage in citrate. All samples were treated with heparinase. Given these limited, but potentially significant findings, further examination was warranted. We hypothesized that longer storage times could impact kaolin activated VHA maximum clot strength and the time to clot initiation to a clinically relevant level in a post-CPB sample.
SUBJECTS AND METHODS
The protocol for this study was reviewed and approved by our institutional review board on September 25th, 2019 (IRB #: WRNMMC-2019-0247; Reference #: 914783). After obtaining institutional review board approval, we performed a single-center, prospective, observational study. Our aim was to determine if VHA parameters were altered by the storage of blood samples in citrate tubes compared to fresh samples in cardiac surgery patients both before and after CPB. Informed consent was obtained for all patients. VHA was performed using the TEG® 5000 Thromboelastograph® (Haemonetics®; Boston, MA) system. VHA parameters analyzed were maximum amplitude (MA), reaction time (R-Time), alpha angle, and K Time. Demographic, comorbidity, and surgical parameters were collected by chart review after enrolment of each patient into the study. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Inclusion criterion was all patients having cardiac surgery with CPB at our institution. Patients were excluded if they were unable to personally participate in informed consent, less than 18 years of age, those with a hematocrit below 24%, or were treated with long-acting anticoagulants (e.g., warfarin, lepirudin, bivalrudin, argatroban, dabigatran, rivaroxaban, apixaban, edoxaban, fondaparinux, clopidogrel, ticagrelor, prasugrel, cangrelor, ticlopidine, or greater than 160 mg per day of aspirin). No patients were removed from the study after enrolment.
Perioperative care was standardized per our institutional protocol. Patients received a heparin dose based on Hepcon Hemostasis Management System (Medtronic, Minneapolis, MN) protocol prior to institution of CPB. The target activated clotting time was 480 s. A blood conservation protocol was used to limit allogeneic blood product administration. Acute normovolemic hemodilution was employed based on initial hematocrit and body surface area for all patients as indicated. A tranexamic acid loading dose of 20 mg/kg was administered after the induction of general anesthesia followed by a 2 mg/kg/h infusion intraoperatively. Red blood cell salvage, intraoperative pump salvage from the surgical field, retrograde autologous priming of the cardiopulmonary bypass circuit, microplegia technique, and ultrafiltration to maintain an on CBP hematocrit of 20% or greater during CPB were employed as indicated. Intravenous crystalloid was limited to no more than one liter prior to institution of CPB unless contraindicated by clinical circumstances.
Blood samples for part one of the study were collected after induction of anesthesia but before surgical incision. Samples for part two were collected five minutes after protamine dose completion. Fresh and citrated samples were drawn simultaneously from an arterial catheter using non-heparinized syringes. Fresh samples were tested within 3 min of collection. Citrated blood was stored in commercially available tubes (VACUETTE®; Greiner Bio-one North America Inc., Monroe, NC) containing 0.5 ml of 0.105 mol/l of sodium citrate. Citrated samples were inverted three times to mix nine parts of blood to one part of sodium citrate for a total volume of 3 ml. Citrated blood was stored at room temperature on a countertop until VHA was performed. VHA was performed using 1 ml of either citrated or fresh blood in a manufacturer produced vial (Haemonetics®, Boston, MA) containing premeasured kaolin. A mixture of 360 μl of fresh sample was pipetted into a heparinase containing reaction cup (Haemonetics®, Boston, MA). For citrated blood, 20 μl of calcium chloride was pipetted into the reaction cup prior to adding 340 μl of the kaolin and citrated blood mixture for a total volume of 360 μl.
VHA parameters of fresh samples evaluated immediately after collection (T0) were compared individually to citrated samples stored for 30 min (T30), 90 min (T90), and 150 min (T150) in citrated blood collection tubes. These time points were selected because they lie within previously reported time periods in which no differences in VHA of fresh compared to citrated samples activated with kaolin were observed in healthy volunteers.[20,21] Furthermore, beyond 2.5 h, the clinical impact of the cause of coagulopathy of a patient with ongoing bleeding at a given time point is likely diminished and new samples for VHA analysis would most likely be obtained. Part 1 of this study was designed to confirm previous studies that show similar VHA profiles for fresh and citrated blood when kaolin activation is used in patients not having undergone CPB. Part 2 of the study was designed to similarly evaluate samples after CPB. Because all samples in both parts of the study were drawn at the same time point for each patient (T0), matched samples were considered.
This study was designed to find a clinically relevant difference in MA or R-Time from T0to each time point using paired T-Tests. Using the values reported by Gilman et al.[23] for sample means and the standard deviation of the mean differences in samples, it was possible to obtain 80% power to detect a difference in MA of 4.5 mm with 23 patients and a difference in R-Time of 1.25 min with a sample of 25 patients. Bland–Altman plots were constructed to determine the agreement of VHA at T0compared to each time point both before and after CPB.[24] Normality of the differences in VHA parameters was evaluated using a Shapiro–Wilk test. As an exploratory analysis, the change of VHA from T0to each time point was compared before and after CPB. The absolute value of the difference VHA parameter value at T0to whichever time point was being analyzed was considered the change in VHA. Because VHA parameters in this population could be higher or lower with storage in citrate, using the absolute value of the difference allowed for any change (positive or negative) to be considered statistically significant. All statistical analysis was performed using R version 3.6.1 (R Core Team, Vienna, Austria).
RESULTS
Patient and surgical characteristics for the study sample are described in Table 1. Dichotomous variables are reported as a percentage of the population and continuous variables are described by mean, standard deviation, and range. The population was overwhelmingly male (88%). Cardiac disease was the most common comorbidity with 56% having coronary artery disease and 56% having valvular disease. Chronic renal (8%) and pulmonary (4%) were rare. The average age of patients was 55 years old.
Table 1.
Patient and surgical characteristics
| Characteristic | Yes (%) | ||
|---|---|---|---|
| Male | 22 (88) | ||
| Coronary artery disease | 14 (56) | ||
| Congestive heart failure | 0 (0) | ||
| Chronic obstructive pulmonary disease | 1 (4) | ||
| Obstructive sleep apnea | 8 (32) | ||
| Chronic kidney disease | 2 (8) | ||
| Acute kidney injury | 0 (0) | ||
| Cerebral vascular accident | 1 (4) | ||
| Diabetes miletus | 3 (12) | ||
| Liver disease | 0 (0) | ||
| Valvular disease | 14 (56) | ||
| Coronary artery bypass graft | 11 (44) | ||
| Aortic valve surgery | 11 (44) | ||
| Mitral valve surgery | 4 (16) | ||
|
| |||
| Mean | Standard Deviation | Range | |
|
| |||
| Age (years) | 55.56 | 11.89 | 31-76 |
| Body mass index (kg/m2) | 29.81 | 5.10 | 21.24-42.48 |
| Preoperative platelet count (×109/L) | 211.68 | 57.12 | 138-387 |
| Preoperative prothrombin time (s) | 14.89 | 5.50 | 12.00-36.60 |
| Preoperative partial thromboplastin time (s) | 35.32 | 20.25 | 12.9-105.60 |
| Preop creatinine (mg/dl) | 1.02 | 0.26 | 0.57-1.67 |
| Duration of cardiopulmonary bypass (min) | 131.04 | 39.17 | 55-220 |
| Duration of cross clamp (min) | 100.32 | 29.97 | 43-154 |
| Heparin dose (international units) | 29320 | 6381.74 | 15000-40000 |
| Protamine dose (mg) | 239.44 | 58.32 | 100-400 |
| Postoperative platelets (×109 L) | 143.8 | 40.07 | 82-243 |
| Postoperative prothrombin time (s) | 16.86 | 1.26 | 14.6-19.2 |
| Postoperative partial thromboplastin time (s) | 31.90 | 4.66 | 25.5-46.2 |
| Postoperative creatinine (mg/dl) | 0.92 | 0.22 | 0.43-1.28 |
| Postoperative fibrinogen (mg/dl) | 218.13 | 52.01 | 127-338 |
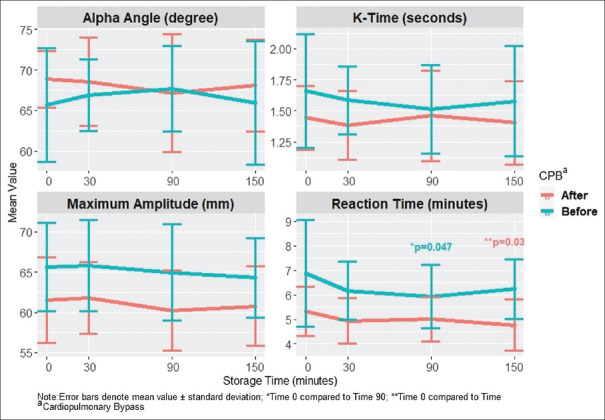
Raw data for VHA times in the fresh and citrated samples at each time point for each patient were collected. Means and standard deviations for each VHA measurement were compared pre- and post-CPB [Figure 1]. A paired T-test was applied to each of the time points to compare for significant difference from the fresh whole blood tested at T0. Only the pre-CPB R-Time sample at 90 min of citrate storage and the post-CPB R-Time sample at 150 min were significantly different. None of the MA, alpha angle, or K Time samples were significantly different from the T0 sample.
Figure 1.
Viscoelastic testing parameter change with storage in citrate blood tubes before and after cardiopulmonary bypass. *= p< 0.05
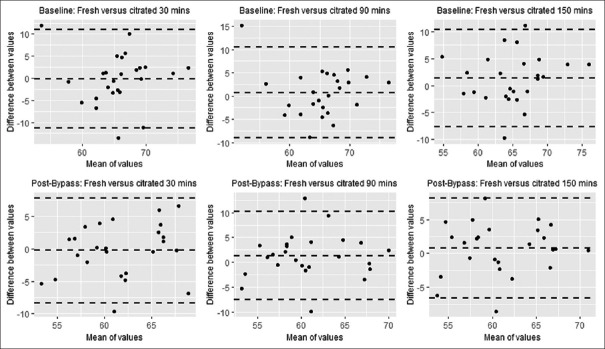
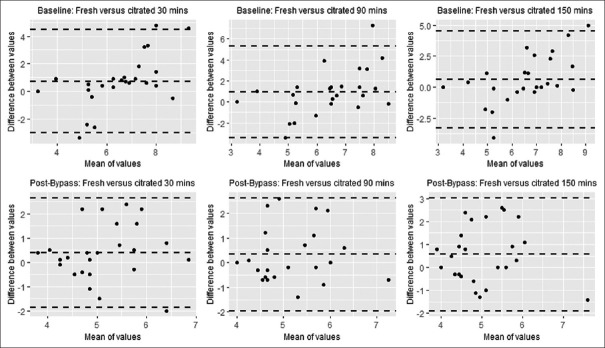
Bland–Altman analyses for MA [Figure 2] and R-Time [Figure 3] at T0to each time point were performed. All values for MA were normally distributed. The values pre-CPB R-Time after 30 min of storage and post-CPB R-Time after 90 min of storage could not be assumed to be normally distributed by the Shapiro–Wilk test. The bias of the values of both MA and R-Time is less than the 4.5 mm and 1.25 min difference, which was considered clinically relevant. There does not appear to be a change in the bias across the range of MA or R-Time values measured noted by visual inspection. The limits of agreement appear low to moderate.
Figure 2.
Bland-Altman plots for maximum amplitude after storage in citrate blood tubes for 30, 90, and 150 minutes before and after cardiopulmonary bypass
Figure 3.
Bland-Altman plots for reaction time after storage in citrate blood tubes for 30, 90, and 150 minutes before and after cardiopulmonary bypass
The change in pre-CPB VHA parameters compared to post-CPB parameters is displayed in Table 2. The mean of the difference for each comparison is listed. This analysis shows that the changes in R-Time at 90 min, K time at 90 min, K time at 150 minutes, and alpha angle at 150 min of storage in citrate tubes were significantly different when comparing samples drawn before to those drawn after CPB. Post hoc power analysis of this comparison found that the power to detect a difference with this test was below 0.65 for all comparisons.
Table 2.
Mean of differences in change of VHAa parameters with storage in citrate before and after CPBb
| 30 min | 90 min | 150 min | |
|---|---|---|---|
| Reaction time | 0.545 | 0.808* | 0.364 |
| K - time | 0.120 | 0.196* | 0.220* |
| Alpha Angle | 1.560 | 2.228 | 3.696* |
| Maximum Amplitude | 0.976 | 0.520 | 0.676 |
aViscoelastic haemostatic assay; bCardiopulmonary Bypass; *P<0.05
DISCUSSION
These data suggest that VHA results for citrated blood are consistent with the results for fresh samples drawn immediately after separation from CPB. Results of part 1 of the study are consistent with and verify previous reports in non-CPB populations. This work expands on the report by Gilman et al.[23] , who stored post-CPB samples in citrate for 15 min prior to obtaining VHA tests. While samples stored for 30 min would have had the same dilutional effects as samples stored longer, they were not subjected to storage and stagnation for a prolonged period. There was no difference in MA in either the previous or current study, suggesting a very low likelihood of a clinically significant alteration existing in post-CPB populations. We were not able to observe a consistent shortening of R-Time after CPB as Gilman et al.[23] described. If a difference does exist, it is unlikely to be clinically relevant.
Bland–Altman analysis comparing MA and R-Time at T0and each time point shows a high level of agreement in the values across time points with biases at or near zero. There does appear to be a moderate amount of variability noted in the limits of agreement for some comparisons. Outliers exist, which may have accounted for some of the variability given our sample size. However, while a maximum range in MA of 10 mm and R-Time of 6 min may alter the interpretation of the citrated samples, the overall clinical picture of coagulopathy will decrease the ambiguity in the interpretation of citrated VHA. For this reason, we feel this was a reasonable value for the limits of agreement of a reliable test.
These findings are relevant to the evaluation of coagulopathy after CPB. Cardiac surgery patients often exhibit a post-CPB coagulopathy.[7,13] While the dilution of CPB is not likely to cause coagulopathy on its own,[25] citrate storage and recalcification requires additional dilution of the blood sample which could theoretically unmask borderline coagulopathy in vitro. Citrate storage introduces another deviation from fresh samples by allowing blood to stagnate in the collection tube. Our findings support that these factors do not appear extensive enough to make citrated VHA discordant from fresh samples.
The TEG 6s,[26] (Haemonetics®, Braintree, MA) ROTEM Sigma,[27] (Instrumentation Laboratory; Barcelona, Spain) and the Quantra Q Plus system[28] (HemoSonics, LLC; Charlottesville, VA) all use samples that are first placed in a citrate tube. This is a deviation from previous generations of VHA, which used both fresh and citrated samples. Interestingly, a VHA modality currently unapproved for use in humans, the Viscoelastic Testing monitor (Entergrion, Inc; Durham, NC), monitors coagulation between two glass plates. Marketing for this test highlights that it does not use chemicals in the test sample “avoiding modifications to the native properties of the patient sample.”[29] Any concerns about the role of citrate in altering the validity of the VHA samples in the cardiac surgery population seem unfounded.
This study has some limitations. R-time has more variability than MA between populations.[30] This means that larger samples are needed to find a difference between samples for R-Time than for MA. Designing a study with separate populations for each VHA parameter may allow for examination of the changes in VHA values that are determined relevant by independent experts. Analysis of the change in VHA parameters before CPB compared to after CPB shows no significant differences in MA changes, and only significance for R-Time at 90 min of storage in citrate. This study was not designed to detect this difference, making this isolated difference difficult to interpret.
In conclusion, the storage of blood in citrate does not appear to affect MA or R-Time to a clinically relevant extent pre- and post-CPB. This may help the understanding and interpretation of abnormal or borderline VHA parameters if multiple samples are discordant, particularly if citrated samples are used. Future studies should continue to independently verify the reliability of VHA modalities in clinical settings by end-users.
This statement also appears in the acknowledgments. It must be included in the published manuscript but only needs to be written once. Please eliminated this and keep the statement in the acknowledgments section.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to thank Cara Olsen and Sorana Raiciulescu for their assistance in selecting statistical tests and in the use of R statistical software. The identification of specific products or scientific instrumentation is considered an integral part of the scientific endeavor and does not constitute an endorsement or implied endorsement on the part of the author, DoD, or any component agency. The views expressed in this article are those of the author and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the US Government.
REFERENCES
- 1.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: An updated report by the American Society of Anesthesiologists Task Force on perioperative blood transfusion and adjuvant therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Bennett-Guerrero E, Zhao Y, O’Brien SM, Ferguson TB, Peterson ED, Gammie JS, et al. Variation in use of blood transfusion in coronary artery bypass graft surgery. JAMA. 2010;304:1568–75. doi: 10.1001/jama.2010.1406. [DOI] [PubMed] [Google Scholar]
- 3.LaPar DJ, Crosby IK, Ailawadi G, Ad N, Choi E, Spiess BD, et al. Blood product conservation is associated with improved outcomes and reduced costs after cardiac surgery. J Thoracic Cardiovasc Surg. 2013;145:796–804. doi: 10.1016/j.jtcvs.2012.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Mazer CD, Whitlock RP, Fergusson DA, Belley-Cote E, Connolly K, Khanykin B, et al. Restrictive or liberal red-cell transfusion for cardiac surgery. N Engl Med. 2017;377:2133–44. doi: 10.1056/NEJMoa1711818. [DOI] [PubMed] [Google Scholar]
- 5.Spiess BD. Thromboelastogram and postoperative hemorrhage. Ann Thorac Surg. 1992;54:810–1. doi: 10.1016/0003-4975(92)91048-e. [DOI] [PubMed] [Google Scholar]
- 6.Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88:312–9. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Serraino GF, Murphy GJ. Routine use of viscoelastic blood tests for diagnosis and treatment of coagulopathic bleeding in cardiac surgery: Updated systematic review and meta-analysis. Br J Anaesth. 2017;118:823–33. doi: 10.1093/bja/aex100. [DOI] [PubMed] [Google Scholar]
- 8.da Luz LT, Nascimento B, Rizoli S. Thrombelastography (TEG®): Practical considerations on its clinical use in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2013;21:29. doi: 10.1186/1757-7241-21-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enriquez LJ, Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br J Anaesth. 2009;103:14–22. doi: 10.1093/bja/aep318. [DOI] [PubMed] [Google Scholar]
- 10.Höffer J, Fries D, Solomon C, Velik-Salchner C, Ausserer J. A Snapshot of coagulopathy after cardiopulmonary bypass. Clin Appl Thromb Hemost. 2016;22:505–11. doi: 10.1177/1076029616651146. [DOI] [PubMed] [Google Scholar]
- 11.Sniecinski RM, Chandler WL. Activation of the hemostatic system during cardiopulmonary bypass. Anesth Analg. 2011;113:1319–33. doi: 10.1213/ANE.0b013e3182354b7e. [DOI] [PubMed] [Google Scholar]
- 12.Besser MW, Ortmann E, Klein AA. Haemostatic management of cardiac surgical haemorrhage. Anaesthesia. 2015;70:87–95. doi: 10.1111/anae.12898. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Kumar S, Tewari P, Pande S, Murari M. Utility of thromboelastography versus routine coagulation tests for assessment of hypocoagulable state in patients undergoing cardiac bypass surgery. Ann Card Anaesth. 2018;21:151–7. doi: 10.4103/aca.ACA_174_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nuttall GA, Oliver WC, Santrach PJ, Bryant S, Dearani JA, Schaff HV, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–81. doi: 10.1097/00000542-200105000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Avidan MS, Alcock EL, Fonseca JD, Ponte J, Desai JB, Despotis GJ, et al. Comparison of structured use of routine laboratory tests or near patient assessment with clinical judgement in the management of bleeding after cardiac surgery. Br J Anaesth. 2004;92:178–86. doi: 10.1093/bja/aeh037. [DOI] [PubMed] [Google Scholar]
- 16.Ortmann E, Rubino A, Altemimi B, Collier T, Besser MW, Klein AA. Validation of viscoelastic coagulation tests during cardiopulmonary bypass. J Thromb Haemost. 2015;13:1207–16. doi: 10.1111/jth.12988. [DOI] [PubMed] [Google Scholar]
- 17.Bowbrick VA, Mikhailidis DP, Stansby G. The use of citrated whole blood in thromboelastography. Anesth Analg. 2000;90:1086–8. doi: 10.1097/00000539-200005000-00015. [DOI] [PubMed] [Google Scholar]
- 18.Zambruni A, Thalheimer U, Leandro G, Perry D, Burroughs AK. Thromboelastography with citrated blood: Comparability with native blood, stability of citrate storage and effect of repeated sampling. Blood Coagul Fibrinolysis. 2004;15:103–7. doi: 10.1097/00001721-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 19.Camenzind V, Bombeli T, Seifert B, Jamnicki M, Popovic D, Pasch T, et al. Citrate storage affects thrombelastograph® analysis. Anesthesiology. 2000;92:1242–9. doi: 10.1097/00000542-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Johansson PI, Bochsen L, Andersen S, Viuff D. Investigation of the effect of kaolin and tissue factor–activated citrated whole blood, on clot forming variables, as evaluated by thromboelastography. Transfusion. 2008;48:2377–83. doi: 10.1111/j.1537-2995.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- 21.Wasowicz M, Srinivas C, Meineri M, Banks B, McCluskey SA, Karkouti K. Technical report: Analysis of citrated blood with thromboelastography: Comparison with fresh blood samples. Can J Anesth. 2008;55:284–9. doi: 10.1007/BF03017205. [DOI] [PubMed] [Google Scholar]
- 22.Kashuk JL, Moore EE, Le T, Lawrence J, Pezold M, Johnson JL, et al. Noncitrated whole blood is optimal for evaluation of postinjury coagulopathy with point-of-care rapid thrombelastography. J Surg Res. 2009;156:133–8. doi: 10.1016/j.jss.2009.03.046. [DOI] [PubMed] [Google Scholar]
- 23.Gilman EA, Koch CD, Santrach PJ, Schears GJ, Karon BS. Fresh and citrated whole-blood specimens can produce different thromboelastography results in patients on extracorporeal membrane oxygenation. Am J Clin Pathol. 2013;140:165–9. doi: 10.1309/AJCPYIQ9JNNSEN4Q. [DOI] [PubMed] [Google Scholar]
- 24.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–60. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 25.Bolliger D, Szlam F, Molarino RJ, Rahe-Meyer N, Levy JH, Tanaka KA. Finding the optimal concentration range for fibrinogen replacement after severe haemodilution. Br J Anaesth. 2009;102:793–9. doi: 10.1093/bja/aep098. [DOI] [PubMed] [Google Scholar]
- 26.Neal MD, Moore EE, Walsh M, Thomas S, Callcut RA, Kornblith LZ, et al. A comparison between the TEG 6s and TEG 5000 Analyzers to assess coagulation in trauma patients. J Trauma Acute Care Surg. 2020;88:279–85. doi: 10.1097/TA.0000000000002545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ziegler B, Voelckel W, Zipperle J, Grottke O, Schöchl H. Comparison between the new fully automated viscoelastic coagulation analyzers TEG 6s and ROTEM Sigma in trauma patients. A prospective observational study. Eur J Anesethesiol. 2019;36:834–42. doi: 10.1097/EJA.0000000000001032. [DOI] [PubMed] [Google Scholar]
- 28.Groves DS, Welsby IJ, Naik BI, Tanaka KA, Hauck JN, Greenberg CS. multicenter evaluation of quantra QPlus system in adult patients undergoing major surgical procedures. Anesth Analg. 2020;130:899–909. doi: 10.1213/ANE.0000000000004659. [DOI] [PubMed] [Google Scholar]
- 29.VCM™ - Viscoelatic Testing Monitor. 2018. [Last accessed on 2020 May 20]. Available from: https://www.entegrion.com/pcm-portable-coagulation-monitor/
- 30.Anderson L, Quasim I, Steven M, Moise SF, Shelley B, Schraag S. Interoperator and intraoperator variability of whole blood coagulation assays: A comparison of thromboelastography and rotational thromboelastometry. J Cardiothor Vasc Anes. 2014;28:1550–7. doi: 10.1053/j.jvca.2014.05.023. [DOI] [PubMed] [Google Scholar]