Abstract
The role of the surface (S)-layer proteins of Campylobacter fetus subsp. fetus has been investigated using an ovine model of abortion. Wild-type strain 23D induced abortion in up to 90% of pregnant ewes challenged subcutaneously. Isolates recovered from both dams and fetuses expressed S-layer proteins with variable molecular masses. The spontaneous S-layer-negative variant, strain 23B, neither colonized nor caused abortions in pregnant ewes. A series of isogenic sapA and recA mutants, derived from 23D, also were investigated in this model. A mutant (501 [sapA recA+]) caused abortion in one of five challenged animals and was recovered from the placenta of a second animal. Another mutant (502 [sapA recA]) with no S-layer protein expression caused no colonization or abortions in challenged animals but caused abortion when administered intraplacentally. Mutants 600(2) and 600(4), both recA, had fixed expression of 97- and 127-kDa S-layer proteins, respectively. Two of the six animals challenged with mutant 600(4) were colonized, but there were no abortions. As expected, all five strains recovered expressed a 127-kDa S-layer protein. In contrast, mutant 600(2) was recovered from the placentas of all five challenged animals and caused abortion in two. Unexpectedly, one of the 16 isolates expressed a 127-kDa rather than a 97-kDa S-layer protein. Thus, these studies indicate that S-layer proteins appear essential for colonization and/or translocation to the placenta but are not required to mediate fetal injury and that S-layer variation may occur in a recA strain.
Campylobacter fetus, comprising two subspecies, C. fetus subsp. fetus and C. fetus subsp. venerealis, are important pathogens of humans and animals. In humans, C. fetus subsp. fetus may cause an acute intestinal illness, or systemic disease, especially in immunocompromised hosts (4). Infections of veterinary importance are manifest as two distinct diseases of breeding: C. fetus subsp. venerealis causes enzootic infertility in cattle, while C. fetus subsp. fetus is associated with sporadic epizootic abortion in cattle and sheep (17, 37). Ovine abortion is a worldwide problem, of particular importance in those countries where lamb is the predominant meat food source or is of economic significance (1, 31). About 11% of ovine abortions diagnosed in Great Britain are campylobacter related, mostly C. fetus associated. Although the prevalence of disease can vary substantially, between 1993 and 1996 it increased by over 150% (3). The potential of C. fetus subsp. fetus infections to cause abortion has been previously demonstrated using ovine experimental models (13, 14, 22, 32, 40). The natural route of transmission is considered to be fecal-oral, and asymptomatic intestinal carriage is believed to occur frequently (38). However, infection of susceptible, pregnant ewes within the last 3 months of pregnancy results in pathology to the placenta (27).
Little is known about the bacterial mechanisms involved in the pathological events associated with C. fetus-associated ovine abortion. However, early studies identified a “loosely-attached capsular envelope” from C. fetus subsp. fetus which was later shown to mediate protection against phagocytosis and serum killing (6, 28, 44). This material comprises a family of highly antigenic proteins with variable molecular masses (97 to 147 kDa) (9, 34, 43) existing in a complex with lipopolysaccharide (12, 45). These proteins exhibit the characteristics of surface layers (S-layers) with the protein subunits arranged to form a two-dimensional paracrystalline surface array. Each S-layer protein is encoded by one of multiple sapA homologs (7, 16). Evidence indicates that DNA reciprocal recombination, including DNA inversion, enables high-frequency generation of S-layer protein variants in C. fetus (10). Recent studies have shown that these events are RecA dependent (11). Such phenotypic changes also mediate antigenic variation, potentially providing a bacterial mechanism for survival in an immunologically hostile host environment and enabling persistence of infection (15, 43).
The role of the S-layer proteins during C. fetus infection is not completely understood. Recent experiments using bovine and mouse models suggest that the S-layer is a dominant virulence factor enabling persistence in the genital tract (18) and systemic infection (5, 33). To investigate the role of S-layer proteins in ovine abortion, an in vivo model has been developed using the subcutaneous or intraplacental administration of C. fetus subsp. fetus strain 23D to pregnant sheep. The abortifacient activities of an S-layer-deficient spontaneous variant, 23B, and a series of isogenic mutants with defined effects on S-layer protein and/or RecA expression have been investigated. The results clearly show that the expression of at least one S-layer protein is essential for systemic infection and thereby for the induction of ovine abortion by C. fetus subsp. fetus, but that this virulence factor does not cause the fetopathogenic effects resulting in abortion.
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains and mutants used in this study are described in Table 1. C. fetus subsp. fetus 23D was originally isolated from bovine vagina (28). The spontaneous variant 23B does not express any S-layer protein (41) due to the absence of a 9-kb fragment including the sapA promoter region (10, 41). The construction and characterization of the sapA and recA deletion mutants have been previously described (11).
TABLE 1.
C. fetus subsp. fetus strains and mutants used
| C. fetus subsp. fetus strain | Origin | Major S-layer protein (kDa) expressed | Antibiotic resistance cassette | Genotype
|
Reference | |
|---|---|---|---|---|---|---|
| sapA | recA | |||||
| 23D | Wild type | 97 | None | sapA+ | recA+ | 28 |
| 23B | Spontaneous mutant of 23D | None | None | 9-kb segment including sapA promoter is absent | recA+ | 41 |
| 23D:600(2) | Defined mutant of 23D | 97 | Kanamycin | sapA+ | recA | 11 |
| 23D:600(4) | Defined mutant of 23D | 127 | Kanamycin | sapA+ | recA | 11 |
| 23D:501 | Defined mutant of 23D | None | Chloramphenicol | sapA | recA+ | 11 |
| 23D:502 | Defined mutant of 23D | None | Kanamycin-chloramphenicol | sapA | recA | 11 |
All strains were cultured on Columbia blood agar (Oxoid Ltd.) at 37°C for 48 h under microaerobic conditions. For the defined mutants, culture medium contained kanamycin (40 μg/ml), chloramphenicol (50 μg/ml), or both. Strains were stored at −70°C in FBP medium (19) with 15% (vol/vol) glycerol until required.
Antibodies and antisera.
The production of the rabbit polyclonal antiserum directed against S-layer proteins has been previously described (34). Mouse monoclonal antibody (MAb) 1D1 is directed against some S-layer proteins (43) and was used to visualize altered sapA expression during in vivo passage. Mouse MAb CF15 recognizes a genus-specific epitope of campylobacter flagellin (30).
Ovine abortion model.
Female Welsh mountain sheep were used throughout these studies. Prior to experimental treatment, vaginal and fecal swabs were taken from all ewes to demonstrate absence of natural infection with C. fetus subsp. fetus. Feces samples were enriched in TEM (24, 25) for 24 h at 37°C in microaerobic conditions before inoculation onto agar plates containing selective antibiotics (36) and cultured as described above. The reproductive cycles of the ewes were synchronized using standard techniques (21). After mating, ewes were examined using an ultrasound scanner to confirm pregnancy. At days 105 to 126 of pregnancy, 108 CFU of C. fetus cells suspended in FBP broth were administered by the subcutaneous or intraplacental route. For the purpose of this study, infectious abortion was defined as occurring in any animal that produced a dead fetus, or one that died within 12 h of birth, and in which C. fetus subsp. fetus was isolated from the products of parturition.
Blood was collected for serum on a weekly basis until a few weeks after lambing. Vaginal swabs and fecal samples were obtained on a biweekly basis. All fetal membranes at parturition were sampled by swabbing. Dead fetuses were necropsied, and swabs were obtained from the fetal liver abomasum, jejunum, and placenta. Feces were cultured after enrichment in TEM medium as described above. All other swabs were inoculated directly onto selective agar (36), or Columbia blood agar with appropriate additional antibiotics for the mutants, and cultured as above. Isolated campylobacters were identified to the species level as previously described (26).
SDS-PAGE and Western blotting.
The total bacterial protein profile was determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on 10% (wt/vol) polyacrylamide gels (23) in a Mini-Protean II apparatus (Bio-Rad Ltd.). Gels were stained with 0.01% (wt/vol) Coomassie blue. For Western blotting, electrophoresed whole-cell lysates were electrotransferred onto nitrocellulose (39). The blots were blocked with 0.5% (wt/vol) gelatin in Tris-buffered saline (pH 7.5) containing 0.05% (vol/vol) Tween 20 (TBS-Tween) for 1 h at room temperature. Blots were incubated with sheep serum (diluted 1:50 in TBS-Tween), rabbit antiserum (diluted 1:100), or MAb (diluted 1:4) for 1 h at room temperature. After washing, bound antibodies were detected by incubation with appropriate peroxidase-conjugated antisera (diluted 1:1,000; DAKO [Glostrup, Denmark] immunoglobulins) for 60 min at room temperature. The bound peroxidase-labeled antibodies were visualized using the substrate 4-chloro-1-naphthol.
RESULTS
Characterization of the study strains.
SDS-PAGE confirmed that wild-type strain 23D, but not variant 23B, expressed an S-layer protein with a molecular mass of 97 kDa (Fig. 1); however, several other protein band differences also were observed. At least one of these differences was shown to be in the flagellin protein, which had molecular masses of 68 kDa in strain 23D and 58 kDa in strain 23B, as demonstrated by Western blotting with MAb CF15 (data not shown).
FIG. 1.
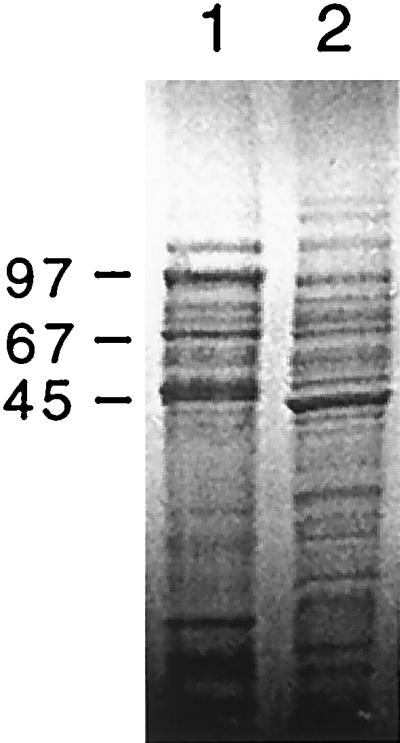
SDS-polyacrylamide gel (10%) of the total protein profile of wild-type C. fetus subsp. fetus 23D (lane 1) and the spontaneous variant 23B (lane 2). Sizes are indicated in kilodaltons.
Challenge of pregnant sheep with C. fetus subsp. fetus strains 23D and 23B.
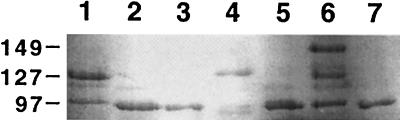
Eleven ewes were subcutaneously challenged at day 105 of pregnancy with 108 CFU of the wild-type C. fetus subsp. fetus strain 23D. Each of the animals challenged excreted C. fetus subsp. fetus in their feces for up to 42 days postchallenge. This excretion was intermittent, but isolates were recovered from most animals on the majority of sampling occasions. Ten (91%) of these 11 ewes aborted. Abortion occurred 10 to 25 days postchallenge. Aborted fetuses showed gross pathology of the liver, with necrotic lesions in 2 (20%) of the 10 fetal livers examined (Fig. 2). The fetal abomasum and jejunum were red and inflamed. C. fetus subsp. fetus was recovered from all aborted fetal tissues. In each case, isolates were recovered from one or more of the fetal sites sampled (liver, jejunum, and abomasum). Isolates also were recovered from the placentae of all the corresponding dams. The S-layer proteins expressed by isolates recovered from fecal specimens (data not shown) and fetal and placental tissues (Fig. 3) varied significantly in molecular mass during the infection, from 97 to 149 kDa. All ewes challenged with strain 23D developed serum antibody responses directed against all of the S-layer proteins expressed, as detected by Western blotting (data not shown).
FIG. 2.
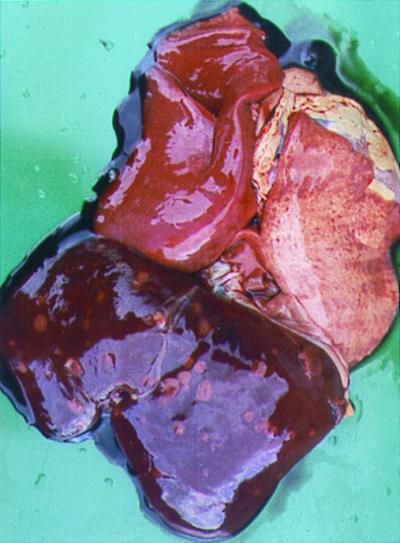
Gross pathological features of lung and liver from an aborted fetus, of a ewe challenged with C. fetus subsp. fetus strain 23D, showing consolidation of one lung and necrotic foci in the liver and lung.
FIG. 3.
SDS-polyacrylamide gel (7%) of the total protein profiles of C. fetus subsp. fetus strain 23D isolated from ewes and abortion products following challenge. Isolates were recovered from placentae (lanes 1, 2, and 4) and fetal livers (lanes 3, 5, and 6). The challenge strain is shown in lane 7. Sizes are indicated in kilodaltons.
Seven pregnant ewes were similarly challenged with 108 CFU of C. fetus subsp. fetus strain 23B. In comparison to the results of challenge with strain 23D, no excretion was observed in any of the ewes challenged with strain 23B. Moreover, all of these ewes lambed normally, and C. fetus subsp. fetus was not recovered from the placentae of any of them. None of the ewes challenged with strain 23B developed detectable circulating anti-S-layer protein antibodies (data not shown).
Effect of mutation of sapA and/or recA on the abortifaciant activity of C. fetus subsp. fetus.
We next asked whether S-layer protein expression alone was essential for ovine abortion and, if so, whether the ability to vary the S-layer protein expressed was important. To address these questions, at day 126 of pregnancy, ewes were challenged subcutaneously with approximately 108 CFU of strain 23D or the defined mutants 501, 502, 600(2), and 600(4) (Table 2). In this experiment, for all five ewes challenged with strain 23D, the placentae were colonized, and the ewes developed circulating antibody responses similar to those described in the previous experiment. However, only one of five animals aborted. As expected, none of the five animals challenged with mutant 502 (sapA recA) were colonized, aborted, or developed detectable antibody responses to the S-layer proteins. In contrast, mutant strain 23D:501 (sapA recA+), in which the ability to switch to expression of an alternative sapA homolog was not compromised, colonized the placentae of two (33%) of the six animals tested and caused abortion in one animal. C. fetus was recovered from the feces of only one animal on one sampling occasion. All isolates recovered from the colonized animals expressed a 97-kDa S-layer protein which expressed an epitope recognized by MAb 1D1. Thus, in vivo infection selected for colonization with an S-layer-positive strain. All six ewes challenged with this mutant elicited a circulating immune response directed against S-layer protein, as demonstrated by Western blotting (data not shown).
TABLE 2.
Effect of subcutaneous challenge of pregnant ewes with wild-type C. fetus subsp. fetus strain 23D or its defined mutants
| C. fetus subsp. fetus strain | No. of animals challenged | No. of culture-positive placentae | No. excreting C. fetus subsp. fetus in feces | No. of abortions or stillbirths |
|---|---|---|---|---|
| 23D | 5 | 5 | 1 | 1 |
| 23D:502 | 5 | 0 | 0 | 0 |
| 23D:501 | 6 | 2 | 1 | 1 |
| 23D:600(2) | 5 | 5 | 2 | 2 |
| 23D:600(4) | 5 | 5 | 2 | 2 |
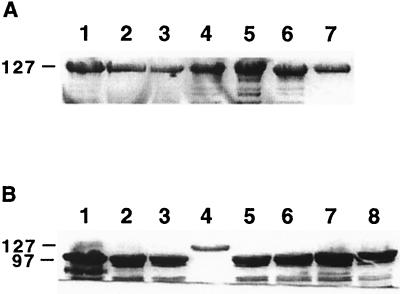
We then hypothesized that S-layer protein variation was not essential to induce abortion. Therefore, we next investigated the abortifacient activity of the two mutants [23D:600(2) and 23D:600(4)] in which the particular S-layer protein expressed was fixed by defined interruption of recA. The molecular masses of the S-layer proteins expressed by these mutants were 97 and 127 kDa, respectively. Mutant 23D:600(2) colonized the placentae of all animals tested and induced abortions in two of these ewes. In contrast, strain 23D:600(4) colonized only two of the six ewes tested and failed to cause any abortions. As expected for a recA strain, all isolates recovered from animals infected with strain 23D:600(4) expressed the original 127-kDa S-layer protein (Fig. 4A). Similarly, the majority (15 of 16) isolates recovered from animals infected with strain 23D:600(2) expressed a 97-kDa S-layer protein identical to that for the challenge strain. However, unexpectedly for a recA strain, the remaining isolate expressed a 127-kDa S-layer protein (Fig. 4B). This isolate colonized the placenta but did not cause abortion. All ewes colonized with mutants expressing S-layer proteins developed serum anti-S-layer protein antibodies (data not shown).
FIG. 4.
Immunoblots (10% polyacrylamide) of isolates, incubated with rabbit anti-S-layer antisera. (A) Isolates from the vaginas of ewes 136, 147, and 140 (two isolates 1 week apart) (lanes 2, 3, 6, and 7, respectively) or the placentae of ewes 147 and 140 (lanes 4 and 5, respectively) challenged with the defined mutant C. fetus subsp. fetus 23D:600(4) (lane 1). (B) Isolates from the placentae of ewes 45, 133, and 142 (lanes 2, 4, and 8, respectively) or the fetal gastrointestinal tract (including jejunum and abomasum) or liver of ewes 45, 133, and 150 (lanes 3, 5, 6, and 7, respectively) challenged with the defined mutant C. fetus subsp. fetus 23D:600(2) (lane 1). Only the isolate from the placentae of ewe 133 expressed an S-layer protein differing in molecular mass from the challenge strain. Sizes are indicated in kilodaltons.
Next, we examined whether the S-layer protein is essential for systemic spread to occur but not necessarily for abortion. To do so, a ewe at day 105 of pregnancy was intraplacentally injected with the S-layer-negative mutant 23D:502 (sapA recA). This ewe aborted 14 days later. C. fetus subsp. fetus was isolated from the aborted fetal tissues (liver, jejunum, and abomasum) and the placenta and vagina of the dam. All five of these isolates were S-layer negative, as demonstrated by immunoblotting (data not shown). Similarly, a ewe intraplacentally injected, on day 105 of pregnancy, with wild-type strain 23D aborted 7 days postchallenge. In contrast, a ewe intraplacentally injected with FBP broth only gave birth to a live lamb at full term. Neither of the dams challenged intraplacentally with strain 502 or 23D excreted C. fetus in their feces, nor did detectable genital tract infection persist. As measured by ELISA, the ewe challenged intraplacentally with strain 23D increased its response to purified S-layer proteins from 0.24 to 1.97 optical density units, whereas the ewe challenged with broth alone showed little change (0.21 to 0.34 optical density units).
DISCUSSION
C. fetus is an important veterinary pathogen with host tissue specificity for both the gastrointestinal and genital tracts and can cause abortion and infertility in cattle and sheep (17, 37). C. fetus cells express S-layer proteins that form paracrystalline structures and which confer protection from host phagocytes and from complement-mediated killing mechanisms (6). Multiple S-layer proteins can be expressed in vitro and in vivo, as reflected in observed differences in molecular masses and antigenicity (8, 15, 18). Such differences mediate antigenic variation, which is one bacterial mechanism for avoiding host immune responses and ensuring chronicity of infection (15, 43). Both the antigenic variation and complement resistance conferred by the S-layer proteins indicate their potential importance as virulence factors for this obligately extracellular pathogen.
We now report establishing an ovine model for C. fetus subsp. fetus-mediated abortion that reproduces the outcome of the naturally acquired infection. In this model, the route of administration of the pathogen is subcutaneous rather than the presumed natural oral route. However, previous studies showed that abortion could be enhanced from 20% after oral dosing to about 80% using this subcutaneous route (20). C. fetus subsp. fetus strain 23D, administered by this subcutaneous route, consistently colonized both the ovine gastrointestinal and genital tracts and could cause abortion. The route by which the organism reached gastrointestinal sites from a subcutaneous challenge is unknown but may have involved translocation from the blood through the biliary tract to colonize the gallbladder (4, 13). Importantly, abortion rates appear to be influenced by the timing of the challenge, even within the last 3 months of pregnancy; challenge at 105 days of pregnancy caused nearly every ewe to abort, but after challenge 3 weeks later, the rate was only 20%. The explanations for this are not known but may reflect increasing immune competence of the fetus.
We used this ovine model to assess the role of S-layer proteins in the pathogenicity of C. fetus-induced abortion. Strain 23B, the spontaneous variant of wild-type strain 23D, neither colonized nor caused abortion when injected subcutaneously. The lack of S-layer protein expression in 23B is caused by a 9-kb chromosomal deletion including the promoter of sapA (10, 41). However, SDS-PAGE analysis of strains 23D and 23B indicated that expression of the S-layer protein was not the only difference detectable. In particular, differences in the flagellins expressed were detected with MAbs. Based on the genomic map for C. fetus subsp. fetus, there is no obvious relationship between the flaA/B and the sapA loci (35). Because of the multiple differences in protein expression between strains 23D and 23B, defined mutants were required to confirm the role of the S-layer protein. Each S-layer protein is encoded by a separate structural gene. In strain 23D, there are believed to be eight homologs of these genes (sapA1 to sapA8) but only a single promoter (10). Reciprocal recombination events, involving the various sapA homologs, lead to expression of the different S-layer proteins (42), at least in part due to inversions of the DNA element containing the unique sap promoter (10). RecA plays an important role in these events, since when recA was inactivated, no rearrangements were detected in in vitro studies (11). A series of mutants that express no S-layer proteins or express only a single definable S-layer protein with no detectable variation (11) were used to definitively assess the role of S-layer proteins in the colonization of pregnant sheep and subsequent abortion.
In comparison with strain 23D, the mutant strain 23D:502 (sapA recA), which is unable to express S-layer proteins under all in vitro conditions tested (11), neither colonized at any site tested nor caused abortion after subcutaneous challenge. This finding supports the evidence from previous experiments with a spontaneous mutant strain, 23B, that the S-layer protein is important in the colonization and thus the disease process. Whether this effect is due to lack of colonization and/or translocation or to loss of fetopathogenic activity was investigated by direct intraplacental challenge which clearly indicated that for fetal injury, and subsequent abortion, the S-layer protein was not required.
The results of these studies also suggest that the molecular mass of the S-layer protein expressed may affect the virulence, since the mutant expressing a 97-kDa S-layer protein colonized better than the mutant expressing a 127-kDa S-layer protein and also could cause abortion. Most C. fetus subsp. fetus isolates from natural ovine infections express the 97-kDa protein, fewer express the 127-kDa protein, and the 149-kDa surface layer protein is rarely seen (R. Grogono-Thomas, unpublished data). The less frequent occurrence of the high-molecular-weight S-layer proteins suggests that they are preferentially produced under as yet undefined conditions, perhaps optimizing survival in hostile environments and/or colonization of specialized microenvironmental niches. Such specialized advantages could be related to physical properties since, for example, the 97-kDa S-protein forms a hexagonal lattice whereas the 127- and 149-kDa proteins form tetragonal lattices (15). One unexpected finding of this study was the shift in molecular mass of one of 16 isolates of mutant 600(2) following in vivo passage. Southern hybridization analyses indicate that the recA genotype of this mutant was unaffected (K. C. Ray and M. J. Blaser, unpublished data). Since RecA was not produced, the size shift should not result from homologous recombination of the sapA homologs, but the actual events are currently unknown.
The role of antigenic variation in campylobacter-associated ovine abortion remains unclear. That both C. fetus subsp. fetus strains 23D:600(2) and 23D:600(4) caused placental colonization and abortion suggests that S-layer variation is not required for disease, at least in this rather acute (10 to 25 days) model of infection. This observation does not exclude the possibility that antigenic variation is required for persistent gut carriage. Long-lived immunity is established following abortion caused by C. fetus subsp. fetus infection (22, 29), and as shown in our study, infected sheep develop a substantial systemic antibody response directed against the S-layer antigen. Such observations indicate that the S-layer proteins may be candidates for subunit vaccines against ovine abortion.
C. fetus subsp. fetus abortions in flocks with enzootic disease often occurs in waves every 4 to 5 years (2). Such patterns may be explained by animals with asymptomatic persistent low-level shedding acting as reservoirs of infection. Then, as either immunity wanes or new susceptible ewes are introduced into a flock, a gradual loss of herd immunity would occur, permitting increased transmission, and given the appropriate stage of pregnancy, disease symptoms become observed. As the S-layer is the predominant surface protein antigen, antigenic variation may enable avoidance of host immune response permitting chronic infection, perhaps at mucosal sites such as the gallbladder (13). Future studies of the ability of the sapA+ recA mutants, with absent S-layer variation, to sustain gut colonization would indicate the role of antigenic variation in persistence. Such studies will be informative if S-layer proteins are to be considered potential vaccine candidates against ovine abortion.
ACKNOWLEDGMENTS
We gratefully acknowledge the financial support of the Wellcome Trust, the Ministry of Agriculture Fisheries and Foods (Great Britain) and the National Institutes of Health (grant RO1 AI 24145).
REFERENCES
- 1.Aldomy F M M A. A study of perinatal mortality in small ruminants in Jordan. Ph.D. thesis. London, England: University of London; 1992. [Google Scholar]
- 2.Anonymous. Veterinary Investigation Data Analysis (VIDA). New Haw, Surrey, England: Central Veterinary Laboratory; 1995. [Google Scholar]
- 3.Anonymous. Veterinary Investigation Data Analysis (VIDA). New Haw, Surrey, England: Central Veterinary Laboratory; 1996. [Google Scholar]
- 4.Blaser M J. Campylobacter fetus—emerging infection and model system for bacterial pathogenesis at mucosal surfaces. Clin Infect Dis. 1998;27:256–258. doi: 10.1086/514655. [DOI] [PubMed] [Google Scholar]
- 5.Blaser M J, Pei Z. Pathogenesis of Campylobacter fetus infections: critical role of high molecular-weight S-layer proteins in virulence. J Infect Dis. 1993;167:372–377. doi: 10.1093/infdis/167.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Blaser M J, Smith P F, Repine J E, Joiner K A. Pathogenesis of Campylobacter fetus infections. Failure of encapsulated Campylobacter fetus to bind C3b explains serum and phagocytosis resistance. J Clin Investig. 1988;81:1434–1444. doi: 10.1172/JCI113474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blaser M J, Wang E, Tummuru M K, Washburn R, Fujimoto S, Labigne A. High-frequency S-layer protein variation in Campylobacter fetus revealed by sapA mutagenesis. Mol Microbiol. 1994;14:453–462. doi: 10.1111/j.1365-2958.1994.tb02180.x. [DOI] [PubMed] [Google Scholar]
- 8.Dubreuil J D, Kostrzynska M, Logan S M, Harris L A, Austin J W, Trust T J. Purification, characterization, and localization of a protein antigen shared by thermophilic campylobacters. J Clin Microbiol. 1990;28:1321–1328. doi: 10.1128/jcm.28.6.1321-1328.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubreuil J D, Logan S M, Cubbage S, Eidhin D N, McCubbin W D, Kay C M, Beveridge T J, Ferris F G, Trust T J. Structural and biochemical analyses of a surface array protein of Campylobacter fetus. J Bacteriol. 1988;170:4165–4173. doi: 10.1128/jb.170.9.4165-4173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dworkin J, Blaser M J. Generation of Campylobacter fetus S-layer protein diversity utilizes a single promoter on an invertible DNA segment. Mol Microbiol. 1996;19:1241–1253. doi: 10.1111/j.1365-2958.1996.tb02469.x. [DOI] [PubMed] [Google Scholar]
- 11.Dworkin J, Shedd O L, Blaser M J. Nested DNA inversion of Campylobacter fetus S-layer genes is recA dependent. J Bacteriol. 1997;179:7523–7529. doi: 10.1128/jb.179.23.7523-7529.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dworkin J, Tummuru M K, Blaser M J. A lipopolysaccharide-binding domain of the Campylobacter fetus S-layer protein resides within the conserved N terminus of a family of silent and divergent homologs. J Bacteriol. 1995;177:1734–1741. doi: 10.1128/jb.177.7.1734-1741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Firehammer B D, Hawkings W W. The pathogenicity of Vibrio fetus isolated from ovine bile. Cornell Vet. 1964;52:21–33. [PubMed] [Google Scholar]
- 14.Frank F W, Bialey J W, Heithecker D. Experimental oral transmission of vibrionic abortion of sheep. J Am Vet Med Assoc. 1957;131:472–473. [PubMed] [Google Scholar]
- 15.Fujimoto S, Takade A, Amako K, Blaser M J. Correlation between molecular size of the surface array protein and morphology and antigenicity of the Campylobacter fetus S layer. Infect Immun. 1991;59:2017–2022. doi: 10.1128/iai.59.6.2017-2022.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujita M, Morooka T, Fujimoto S, Moriya T, Amako K. Southern blotting analyses of strains of Campylobacter fetus using the conserved region of sapA. Arch Microbiol. 1995;164:444–447. doi: 10.1007/BF02529743. [DOI] [PubMed] [Google Scholar]
- 17.Garcia M M, Eaglesome M D, Rigby C. Campylobacters important in veterinary medicine. Vet Bull. 1983;53:793–818. [Google Scholar]
- 18.Garcia M M, Lutze-Wallace C L, Denes A S, Eaglesome M D, Holst E, Blaser M J. Protein shift and antigenic variation in the S-layer of Campylobacter fetus subsp. venerealis during bovine infection accompanied by genomic rearrangement of sapA homologs. J Bacteriol. 1995;177:1976–1980. doi: 10.1128/jb.177.8.1976-1980.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.George H A, Hoffman P S, Smibert R M, Krieg N R. Improved media for growth and aerotolerance of Campylobacter fetus. J Clin Microbiol. 1978;8:36–41. doi: 10.1128/jcm.8.1.36-41.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grogono-Thomas R, Woodland R M. An experimental model of Campylobacter fetus subsp. fetus induced abortion in sheep. In: Newell D G, Ketley J M, Feldman R A, editors. Campylobacters, helicobacters and related organisms. New York, N.Y: Plenum Press; 1996. pp. 351–354. [Google Scholar]
- 21.Henderson D C. The reproductive cycle and its manipulation. In: Martin W B, Aitken I D, editors. Diseases of sheep. 2nd ed. Oxford, England: Blackwell Scientific Publications; 1991. pp. 25–33. [Google Scholar]
- 22.Jensen R, Miller V A, Hammerlund M A, Graham W R. Vibrionic abortion in sheep. I. Transmission and immunity. Am J Vet Res. 1957;18:326–329. [PubMed] [Google Scholar]
- 23.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 24.Lander K P. The application of a transport and enrichment medium to the diagnosis of Campylobacter fetus infections in bulls. Br Vet J. 1990;146:334–340. doi: 10.1016/s0007-1935(11)80026-6. [DOI] [PubMed] [Google Scholar]
- 25.Lander K P. The development of a transport and enrichment medium for Campylobacter fetus. Br Vet J. 1990;146:327–333. doi: 10.1016/s0007-1935(11)80025-4. [DOI] [PubMed] [Google Scholar]
- 26.Lander K P, Gill K P W. Campylobacters. In: Collins C H, Grange J H, editors. Isolation and identification of micro-organisms of medical and veterinary importance. London, England: Academic Press; 1985. pp. 123–142. [Google Scholar]
- 27.Lindenstruth R W, Ashcroft J B, Ward B Q. Studies on vibrionic abortion of sheep. J Am Vet Med Assoc. 1949;114:204–209. [PubMed] [Google Scholar]
- 28.McCoy E C, Doyle D, Burda K, Corbeil L B, Winter A J. Superficial antigens of Campylobacter (Vibrio) fetus: characterization of antiphagocytic component. Infect Immun. 1975;11:517–525. doi: 10.1128/iai.11.3.517-525.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinershagen B S, Frank D V M, Hulet C V, Price D A. Immunity in ewes resulting from natural exposure to Vibrio fetus. Am J Vet Res. 1969;31:1773–1777. [PubMed] [Google Scholar]
- 30.Newell D G. Monoclonal antibodies directed against the flagella of Campylobacter jejuni: cross-reacting and serotypic specificity and potential use in diagnosis. J Hyg (London) 1986;96:377–384. doi: 10.1017/s0022172400066134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orr M. Animal health laboratory network. Review of diagnostic cases. Surveillance. 1994;21:3–6. [Google Scholar]
- 32.Osborne J C, Smibert R M. Vibrio fetus. I. Hypersensitivity and abortifacient action. Cornell Vet. 1963;54:561–572. [PubMed] [Google Scholar]
- 33.Pei Z, Blaser M J. Pathogenesis of Campylobacter fetus infections. Role of surface array proteins in virulence in a mouse model. J Clin Investig. 1990;85:1036–1043. doi: 10.1172/JCI114533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pei Z, Ellison III R T, Lewis R V, Blaser M J. Purification and characterization of a family of high molecular weight surface-array proteins from Campylobacter fetus. J Biol Chem. 1988;263:6416–6420. [PubMed] [Google Scholar]
- 35.Salama S M, Newnham E, Chang N, Taylor D E. Genome map of Campylobacter fetus subsp. fetus ATCC 27374. FEMS Microbiol Lett. 1995;132:239–245. doi: 10.1016/0378-1097(95)00316-w. [DOI] [PubMed] [Google Scholar]
- 36.Skirrow M B. Campylobacter enteritis: a “new” disease. Br Med J. 1977;2:9–11. doi: 10.1136/bmj.2.6078.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skirrow M B. Diseases due to Campylobacter, Helicobacter and related bacteria. J Comp Pathol. 1994;111:113–149. doi: 10.1016/s0021-9975(05)80046-5. [DOI] [PubMed] [Google Scholar]
- 38.Smibert R M. Vibrio fetus var intestinalis isolated from fecal and intestinal contents of clinically normal sheep: isolation of microaerophilic Vibrios. Am J Vet Res. 1965;26:315–319. [Google Scholar]
- 39.Towbin H T, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tucker J O, Robertstad G W. Experimental vibriosis in sheep. J Am Vet Med Assoc. 1956;129:511–513. [PubMed] [Google Scholar]
- 41.Tummuru M K, Blaser M J. Characterization of the Campylobacter fetus sapA promoter: evidence that the sapA promoter is deleted in spontaneous mutant strains. J Bacteriol. 1992;174:5916–5922. doi: 10.1128/jb.174.18.5916-5922.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tummuru M K, Blaser M J. Rearrangement of sapA homologs with conserved and variable regions in Campylobacter fetus. Proc Natl Acad Sci USA. 1993;90:7265–7269. doi: 10.1073/pnas.90.15.7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang E, Garcia M M, Blake M S, Pei Z, Blaser M J. Shift in S-layer protein expression responsible for antigenic variation in Campylobacter fetus. J Bacteriol. 1993;175:4979–4984. doi: 10.1128/jb.175.16.4979-4984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Winter A J, McCoy E C, Fullmer C S, Burda K, Bier P J. Microcapsule of Campylobacter fetus: chemical and physical characterization. Infect Immun. 1978;22:963–971. doi: 10.1128/iai.22.3.963-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang L Y, Pei Z H, Fujimoto S, Blaser M J. Reattachment of surface array proteins to Campylobacter fetus cells. J Bacteriol. 1992;174:1258–1267. doi: 10.1128/jb.174.4.1258-1267.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]