Abstract
Purpose of Review:
Orthopaedic trauma is a major cause of morbidity and mortality worldwide. Although many fractures tend to heal if treated appropriately either by nonoperative or operative methods, delayed or failed healing, as well as infections, can lead to devastating complications. Tissue engineering is an exciting, emerging field with much scientific and clinical relevance in potentially overcoming the current limitations in the treatment of orthopaedic injuries.
Recent Findings:
While direct translation of bone tissue engineering technologies to clinical use remains challenging, considerable research has been done in studying how cells, scaffolds, and signals may be used to enhance acute fracture healing and to address the problematic scenarios of nonunion and critical-sized bone defects. Taken together, the research findings suggest that tissue engineering may be considered to stimulate angiogenesis and osteogenesis, to modulate the immune response to fractures, to improve the biocompatibility of implants, to prevent or combat infection, and to fill large gaps created by traumatic bone loss. The abundance of preclinical data supports the high potential of bone tissue engineering for clinical application, although a number of barriers to translation must first be overcome.
Summary:
This review focuses on the current and potential applications of bone tissue engineering approaches in orthopaedic trauma with specific attention paid to acute fracture healing, nonunion, and critical-sized bone defects.
Keywords: Orthopaedic trauma, bone fracture, bone tissue engineering, stem cells, biomaterials
Introduction
Orthopaedic trauma is a common [1, 2] and significant cause of morbidity and disability [3, 4]. Injuries occur frequently in all demographics [2, 5], so understanding the biological response to fractures is essential to more effectively treat them. Any displaced fracture has an element of mechanical disruption, and careful attention must be paid to provide either relative or absolute stability, depending on the fracture pattern and location, and on whether the goal is to promote endochondral or intramembranous ossification [6]. However, even when fractures are treated with the appropriate level of nonoperative or operative stabilization, delayed unions, nonunions, and infections can frequently occur and lead to serious complications [7]. Bones can fail to heal for a number of reasons, including patient factors such as comorbid conditions, malnutrition, alcohol intake, and smoking, but also injury-related factors such as fracture comminution, soft tissue injury, and the creation of critical-sized bone defects [8]. Though advances in knowledge and technology have led to improvements in the care of orthopaedic trauma patients, many still have poor outcomes due to the limitations of current treatments.
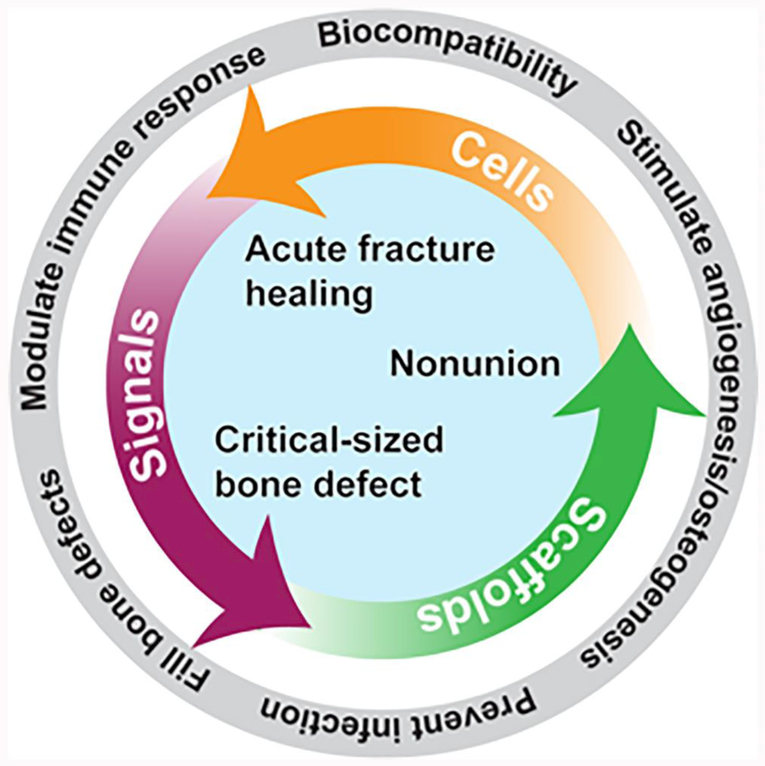
Bone tissue engineering as a field has grown exponentially over the last several decades, and it can play a relevant role in orthopaedic trauma and fracture care (Fig. 1) [9, 10]. Tissue engineering approaches use either cells, scaffolds, signals, or any combination of these in order to generate bone tissue [11]. Specifically, when there is an insufficient natural healing response to injury, bone tissue engineering seeks to create materials that are osteoconductive (allow bone growth onto surface), osteoinductive (recruit osteoprogenitor cells to an area), and osteogenic (stimulate osteoprogenitor cells to develop into osteoblasts) [12]. However, although preclinical studies investigating tissue engineering techniques abound, there has been limited translation of products to the clinic [13]. The barriers to clinical adoption [14] include scientific and technical limitations [9], the unfamiliarity of many scientists and clinicians with the regulatory processes involved in translating basic science breakthroughs to clinical products, and stringent regulatory and quality assurance guidelines by the Food and Drug Administration (FDA) [15]. Notwithstanding these hurdles, there are many promising applications for bone tissue engineering in the field of orthopaedic trauma, and this review will highlight those relevant to acute fracture healing, nonunion, and critical-sized bone defects.
Fig. 1.
Potential applications and functional characteristics of bone tissue engineering technologies in orthopaedic trauma.
Applications of bone tissue engineering in acute fracture healing
The implants currently in use to treat fractures in the acute setting are often metal plates, rods, and screws made of stainless steel, titanium, or various alloys. These implants are advantageous in that they provide excellent mechanical stability and often allow patients to return to functional activities of daily living relatively quickly. It is desirable that any implants used are non-toxic, non-inflammatory, non-allergenic, and non-carcinogenic [16]. In addition, implants should not only provide mechanical support but should also allow for, and ideally stimulate, bone healing. To accomplish this, implants are frequently created with a rough surface that produces an increased surface area for host-implant interaction and with materials that stimulate osteoblast adhesion and differentiation [17, 18, 19, 20, 21, 22]. In the acute setting, various types of autologous or allogenic bone grafts are also frequently used to stimulate bone healing.
The key elements of bone tissue engineering in fracture repair are well summarized by the diamond concept as proposed by Giannoudis et al. [23]. They described four strategies to stimulate acute fracture healing: the provision of osteogenic cells, osteoconductive or osteoinductive scaffolds, growth factors, and an appropriate mechanical environment. In this context, tissue vascularity is also critical to healing and is intimately tied to the other factors [24]. Specific approaches have recently focused on the stimulation of angiogenesis and osteogenesis, immunomodulation to promote tissue regeneration, design of implants with improved biocompatibility, and the delivery of bioactive compounds to prevent and/or treat infection [16, 25, 26, 27]. While the majority of fractures do heal naturally, these methods have the potential to accelerate the response, and could be particularly valuable in patients with an impaired baseline healing response, such as those with comorbid medical conditions, malnutrition, or unhealthy lifestyles [28].
Angiogenesis and Osteogenesis
Angiogenesis and osteogenesis are integral to acute fracture healing and have been an important component of studies in bone tissue engineering. Although historically thought of as distinct processes, recent research has demonstrated significant crosstalk between angiogenic and osteogenic signaling pathways [29, 30]. Angiogenic growth factors, such as vascular endothelial growth factor (VEGF), fibroblast growth factor-2 (FGF-2), platelet derived growth factor (PDGF), insulin-like growth factor (IGF), and angiopoietins, play a role in both angiogenesis and osteogenesis by increasing blood flow to tissues, and by inducing stem cell and osteoblastic differentiation [26]. However, their clinical translation for use in acute fracture healing has been limited thus far, due to difficulty in ascertaining optimal and safe dosing and delivery strategies [30]. Nonetheless, human studies have been performed, and a notable example is the randomized, controlled trial by Kawaguchi and colleagues in which they delivered FGF-2 in a gelatin hydrogel for tibial shaft fractures, and found an acceleration in bone healing in patients who received the formulation [31]. Importantly, they observed no difference in adverse effects in those who did or did not receive FGF-2.
The primary osteogenic cells utilized in bone tissue engineering are mesenchymal stem cells (MSCs) [32]. They are a common type of multipotent stem cell able to differentiate along osteoblastic, chondrogenic, and adipogenic lineages. Furthermore, they are able to stimulate bone healing through angiogenic, immunomodulatory, and paracrine effects, and are naturally recruited to the site of injury in the physiological acute fracture healing process [26, 33]. For bone tissue engineering purposes, both autologous or allogeneic MSCs have been the focus of a considerable amount of research. Allogeneic cells are advantageous in that there is no donor site morbidity, but they may be less efficacious than autologous cells, and have the potential to elicit an immune reaction in the host. It is for this reason that there are major regulatory challenges associated with their use. Autologous MSCs are most frequently derived either from bone marrow (also called bone marrow stromal cells) or adipose tissue. Because of the relatively low number of cells in these tissues, autologous MSCs must be harvested, isolated, and expanded in vitro prior to use, as a large number of cells is thought to be required for therapeutic effect. This process can take days to weeks, and is far from ideal in the clinical setting because it would necessitate multiple operations. Thus, alternative point-of-care options, where the process is more rapid but results in a lower yield of cells, are currently being explored [34]. The method of delivery of MSCs is an important consideration, and general options include physical entrapment, electrostatic binding, or via microparticles [26].
Various scaffolds and growth factors are currently being studied for the optimal delivery of stem cells. While much work has been done in preclinical models, optimal knowledge of cell differentiation, processing, and dosing must be ascertained prior to routine application in patients [10]. Scaffolds may be acellular or be seeded with MSCs, and there is some preclinical evidence that scaffolds seeded with MSCs may lead to enhanced bone healing and a more rapid integration with host tissue compared with acellular scaffolds [35]. In order to deliver MSCs effectively, scaffolds must be able to provide structural support and allow MSC survival, migration, differentiation, and more work is necessary to create and test scaffolds with these characteristics. Growth factors can be used to supplement MSC cultures and have been shown to promote the differentiation of osteoprogenitor cells, but this can have the unwanted effect of reducing the number of viable MSCs [36].
Bone morphogenetic proteins (BMPs), first discovered by Urist in 1965 [37], are well characterized growth factors of the transforming growth factor beta (TGF-β) superfamily [38]. A randomized, controlled study that generated much excitement was published in 2002 by Govender et al., who found that in patients with open tibial fractures, the application of recombinant human BMP-2 (rhBMP-2) in an absorbable collagen sponge scaffold led to enhanced healing and decreased the need for secondary surgery [39]. BMP-2 subsequently was afforded premarket approval by the FDA for use with acute open tibial fractures, but studies performed since that time have sobered expectations. In other randomized controlled trials, Aro et al. [40] and Lyon et al. [41] found no acceleration of healing when rhBMP-2 was used for the treatment of open or closed tibial shaft fractures, respectively. In these trials, MSCs were not delivered in the treatment, and thus bone healing relied on cell invasion into the scaffolds. Importantly, rhBMP-2 is also FDA approved for use in certain spinal fusion procedures, and has been shown to lead to improved union rates in anterior lumbar interbody fusion [42].
Immunomodulation
Fracture healing is a complex, multifactorial process that involves many body systems. As thoroughly reviewed elsewhere, it can be divided into three main biological events, the inflammatory phase, the repair phase, and the remodeling phase [43, 44, 45]. The inflammatory response to fracture is closely interlinked with the vasculature. Blood flow to the fracture site initially decreases due to local soft tissue trauma and disruption of endosteal and/or periosteal blood vessels, but the blood flow subsequently increases due to increased arterial circulation [44]. While healing can still occur with the initial transient decrease in blood flow, angiogenesis and revascularization are necessary for normal bone healing. In fact, the fracture hematoma is an important source of leukocytes, pro- and anti-inflammatory cytokines, and angiogenic growth factors [45]. Osteoprogenitor cells are produced and stimulated both by angiogenesis and by the interactions between inflammatory and bone cells [46]. Numerous studies highlight the interaction between bone and immune cells as well as their overlapping regulatory mechanisms [46]. Most prominent is the interaction between osteoblasts and osteoclasts that regulate bone formation and degradation, respectively, and is vital to bone growth, remodeling, and healing. Osteoclasts, the bone-resorbing cells, are derived from the same myeloid precursor cells that give rise to macrophages and myeloid dendritic cells. Osteoblasts, the bone-forming cells, regulate hematopoietic stem cell niches from which all blood and immune cells are derived [6]. Many of the soluble mediators of immune cells regulate the activities of both cells types in a tightly controlled manner. While inflammatory mediators are necessary for bone healing to occur, healing can be impaired when there is a chronically upregulated immune system, such as with rheumatoid arthritis, diabetes, or systemic lupus erythematosus, or with acute dysregulation of the immune system, as in the setting of polytrauma or sepsis [44].
Immunomodulation is a relatively new area of study in bone tissue engineering, but has the potential for important applications in acute fracture healing. As previously discussed, inflammation plays a critical role in fracture healing because of the crosstalk between bone and the immune system [46], which affects host integration of the cells, growth factors, and/or scaffolds used in bone tissue engineering [33]. MSCs can both stimulate and suppress the immune system through variable interactions with macrophages, T and B cells, natural killer cells, and dendritic cells, and if these immunomodulatory effects can be understood and harnessed, new treatments to stimulate fracture healing could be studied and developed [33].
Biocompatibility of implants
Other areas of focus in bone tissue engineering and biomaterials research as they relate to acute fracture healing are an improvement in the biocompatibility of current prostheses and fixation devices, and the creation of tissue-engineered implants and bone scaffolds [47]. Methods to stimulate bone healing and increase biocompatibility have included increasing the hydrophilicity of implants [48], and coating surfaces with hydroxyl and amine chemical groups [49], bisphosphonates [50], or growth factors such as BMP-2 or VEGF [51]. Much work has been done by bone tissue engineers on polymer scaffolds, which could theoretically be used in combination with or in lieu of metallic implants, and would confer the advantage of matching the mechanical properties of bone more closely [17]. Various polymers, including polyglycolic acid (PGA) and poly-L-lactic acid (PLLA), have been used to create resorbable screws, and they provide the advantage of having no retained metal hardware [52]. In clinical studies, these screws have thus far been used to achieve favorable outcomes in calcaneal fractures [53] and tibiofibular syndesmotic injuries [54]; the observation of occasional immune-mediated mild to moderate inflammatory foreign body reactions has been reported and is an area of active investigation [55, 56]. In addition, there is evidence indicating that osteoprogenitor cells resident in or recruited to the implantation site are also essential to prevent implant loosening [57].
Prevention of Infection
In orthopaedics, peri-implant infections are most frequently discussed and studied in the context of joint replacements, but they are highly relevant in orthopaedic trauma, as they can inhibit fracture healing and lead to chronic wound infections, osteomyelitis, reoperation, and other serious complications [58]. The current treatment options include systemic combined with local administration of antibiotics with antibiotic-coated nails and antibiotic beads, cement, or powder [59]. Tissue engineering approaches have also been used to address the issue of peri-implant infections. Engineered implant surfaces have been created to prevent bacterial adhesion to implants, such as surfaces that are antiadhesive or are intrinsically antibacterial [60]. Furthermore, various natural and synthetic bactericidal coatings have been studied that can release antibiotics in either a triggered-release or slow-release fashion [61]. A promising area of study is the use of scaffolds to prevent peri-implant infections, as they can release either antibiotics, silver, antimicrobial peptides, or bacteriophages [62]. While recent advances have occurred with antimicrobial scaffolds in preclinical models [25, 63], these have not yet been translated to the clinical setting. Finally, MSCs have been shown to have antimicrobial effects, and cell-based therapies may have a future role in preventing or treating orthopaedic infections [64, 65].
Applications of bone tissue engineering in nonunion
The prevalence of nonunion, which is defined as a failure of bone to heal, is generally considered to be between 5 and 10% in long bone fractures, with rates being higher in the tibia than in the femur or the long bones of the upper extremity [66]. While a recent study showed that this percentage may be an overestimate [67], nonunions remain a significant cause of pain and disability in many orthopaedic trauma patients. The development of nonunion is multifactorial, and many studies have investigated the risk factors associated with it [68, 69]. These include injury characteristics such as multiple fractures, open fractures, fractures with significant communication or high initial displacement, and those located in the tibia or in a portion of bone with inadequate vascularity. Patient-related risk factors for nonunion include advanced age, diabetes, obesity, smoking, and the use of non-steroidal anti-inflammatory medications, anticonvulsants, and anticoagulants [68, 69].
Nonunions can be either septic or aseptic, and aseptic nonunions are further categorized as being either atrophic, hypertrophic, or somewhere in between (oligotrophic). Atrophic nonunion tissue has a low cell density as compared with hypertrophic nonunion tissue, while evidence of vascularity is often observed in both types of nonunion tissue. Interestingly, MSCs and BMPs are frequently present in nonunion tissue, although their inhibitors may also be present in the nonunion site, thus preventing MSC differentiation [70]. The treatments currently being utilized for aseptic nonunions include nonoperative and operative methods. Nonsurgical options include extracorporeal shock wave therapy, low-intensity pulsed ultrasound, electrical stimulation, or functional bracing. Surgical management is often necessary, with options that may include exchange intramedullary nailing, adjunctive plate fixation to improve mechanical stability, intramedullary nail removal followed by plate fixation, or external fixation, and these are often combined with autologous bone grafting (Fig. 2) [71, 72]. When an infected or septic nonunion occurs, it is usually necessary to perform multiple-stage revision procedures because the infection must be cleared prior to any definitive fixation, and achieving bony union in these patients is often a difficult and prolonged process [73].
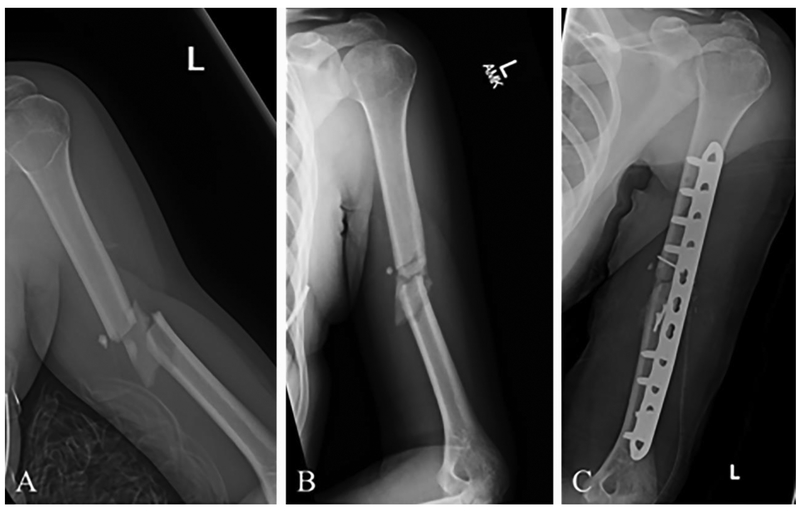
Fig. 2.
X-rays of a 23-year-old female who sustained a midshaft fracture of her left humerus in a fall. (A) X-ray at the time of injury. (B) X-ray after months of nonoperative treatment using an arm brace, showing nonunion of the fracture site. (C) Postoperative X-ray after open reduction and internal fixation using plates and screws with supplementary iliac crest bone grafting. This is the current standard of care for this fracture in cases of nonunion.
Because the presence of a nonunion indicates a failure of the body’s natural healing response to fracture, nonunions have been of interest to bone tissue engineers, and many animal studies and clinical trials utilizing cells, scaffolds, or signals (such as growth factors) have been performed. Regarding septic nonunions, there is a great deal of potential for tissue engineering strategies to alter clinical practice and assist clinicians in these complex patient scenarios. Studies on the bacterial biofilms that form on implants are helping to elucidate the causative mechanisms underlying peri-implant infections and septic nonunions [74]. Anti-infective scaffolds that can release antibiotics, silver, bacteriophages, or antimicrobial peptides are being actively investigated in animals but, to our knowledge, there have been no clinical studies with these methods in orthopaedic trauma patients with septic nonunion [62].
MSCs and growth factors, either alone or in combination, have been the primary bone tissue engineering techniques investigated for the treatment of aseptic nonunions. Most clinical studies that have used bone tissue engineering methods for the treatment of aseptic nonunions have simply tested the safety and efficacy of supplementing the regular treatment methods with either osteogenic cells or growth factors, and have not replaced the standard therapies described above. In relatively small studies ranging from 30 to 68 patients, BMP-7 supplementation was shown to be safe and led to a high union rate in aseptic nonunions of the femur [75], tibia [76], and upper and lower extremity long bones [77, 78]. In a clinical trial comparing outcomes of BMP-7 in a collagen type I carrier versus the standard autologous bone grafting for tibial nonunions, there was no difference in union rates or adverse effects between the two groups, and the authors suggested that BMP-7 could be a suitable treatment that would prevent donor site morbidity associated with autologous bone grafts [79]. A clinical trial comparing the standard treatment for aseptic tibial and femoral nonunions to treatment supplemented with platelet rich fibrin containing MSCs showed a faster union time and improved radiographic consolidation in the group receiving platelet rich fibrin supplementation [80]. Combination methods have also had successful outcomes. Giannoudis and colleagues showed that union occurred in 63 of 64 patients with long bone nonunions who were treated with reamer irrigator aspirator graft, BMP-7, and bone marrow aspirate, and all patients returned to their activities of daily living [81]. For patients with nonunion of the forearm, Calori et al. showed that those who underwent polytherapy with MSCs, BMP-7, and a scaffold had a higher union rate (89%) than those undergoing treatment with only one of those components (63% union rate) [82]. Challenges to clinical translation remain, as individualized therapy is required for each patient due to unique host and nonunion characteristics, but clinical trials are underway around the world that are moving bone tissue engineering techniques closer to becoming a reality for the clinical treatment of nonunions [83].
Applications of bone tissue engineering in critical-sized bone defects
Traumatic bone loss and critical-sized bone defects are challenging problems that orthopaedic surgeons encounter regularly given the prevalence of falls, motor vehicle collisions, gunshot wounds, and other high-energy injuries. While historically, many fractures with significant bone loss would have necessitated amputation, advances in surgical and medical management have allowed for limb salvage to be pursued for increasing number of injury patterns [8]. Bone loss can occur either during the initial trauma in cases of severe open fractures, or be necessitated intraoperatively. During a limb salvage or reconstruction procedure, devitalized bone fragments that are completely devoid of soft tissue attachment must be removed, as they are avascular and can serve as a nidus for infection.
In current clinical practice, a variety of treatments may be utilized for the reconstruction of critical-sized bone defects. Depending on the size of the defect, internal or external fixation may be combined with autologous, allogenic, or vascularized bone grafts, or with demineralized bone matrix (Fig. 3) [84]. Distraction osteogenesis (also known as the Ilizarov technique) is a treatment option, and involves a lengthy process of transporting a segment of bone to an area where there is a defect using an external fixation frame, and progressively allowing the area of the defect to consolidate while gradually pulling the bone fragments apart with the frame [85]. An alternative approach, the induced membrane technique (also known as the Masquelet technique), involves a two-stage procedure where a spacer is placed in the defect for 6-8 weeks, allowing a fibrous membrane to form [86]. At the end of this time, the spacer is removed and either autologous or allogenic bone graft is used to fill the defect. The membrane serves as a source of growth factors and prevents resorption of the bone graft, which stimulates bone healing. While these methods of treating critical-sized bone defects can be effective, they have their limitations, and complications such as deep infection, re-fracture, and neurovascular complications are relatively common [87, 88].
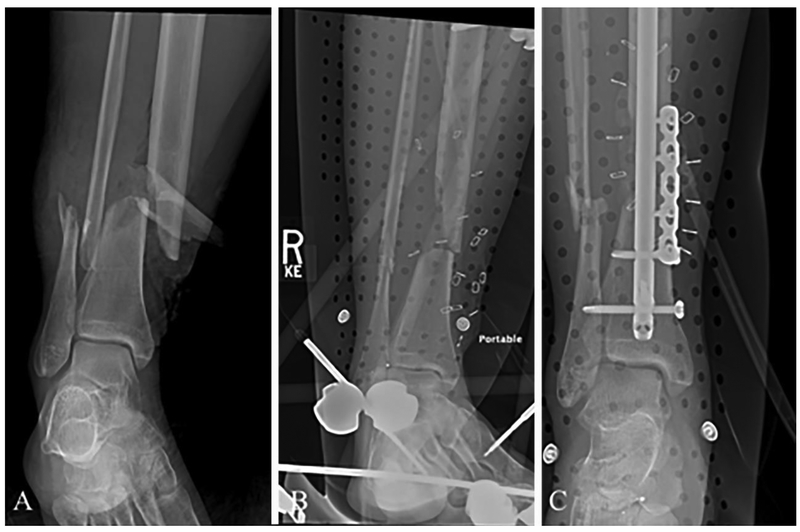
Fig. 3.
X-rays of a 53-year-old woman who sustained multiple injuries in a motor vehicle collision, including open fractures of her distal tibia and fibula. (A) X-ray at the time of injury. (B) Postoperative X-ray after initial management with external fixation. A large devitalized fragment of tibial bone was removed during this surgery. (C) Postoperative X-ray several days later after internal fixation with an intramedullary rod along with a plate and screw construct, as well as insertion of antibiotic beads. This is the current standard of care for staged management of this injury.
Critical-sized bone defects in the orthopaedic trauma setting are problematic generally because of a large fracture gap and not due to an abnormal or dysfunctional bone healing process. To combat this, a large number of studies in bone tissue engineering have focused on using either natural or synthetic polymeric scaffolds to fill these defects. Natural polymeric scaffolds are derived from sources such as collagen, hyaluronate, chitosan, and alginate. They are biocompatible and do not generally elicit an inflammatory response. However, there is variability from one scaffold to the next, their supply may be limited, and their long-term viability may be inadequate. Synthetic polymeric scaffolds are advantageous because of their availability, their uniformity from one sample to the next, and their ability to be mechanically altered for different needs. However, they run the risk of being less biocompatible, absence of bioactive epitopes, and their degradation can also be problematic in some circumstances [89, 90]. Scaffolds must have the appropriate mechanical properties and be loaded with either growth factors or osteogenic cells [87].
Open tibial shaft fractures are an example of injuries that often have a large bone defect due to the subcutaneous location of the bone and the frequently high-energy nature of these injuries. As previously discussed, clinical trials have studied BMPs delivered with various scaffolds (but without MSCs) as a possible method to stimulate healing in these injuries, and have been met with variable success [39, 40, 91]. However, although extensively studied, much of the research that has investigated tissue engineering methods specifically for critical-sized bone defects has been performed in animal models. For example, Baker and colleagues successfully treated mouse femoral segmental bone defects using a self-deploying memory polymer scaffold [92]. Decambron et al. applied BMP-2 and MSCs onto a coral scaffold, and found it to be effective in treating critical-sized metatarsal bone defects in a sheep model [93]. In a rat femoral defect model, Bosemark et al. showed that bridging bone healing could be achieved effectively through the Masquelet technique with the combination of a synthetic scaffold, BMP-7, and bisphosphonate treatment [94]. These are just a few examples of the encouraging studies that have been performed in animals.
Future opportunities in bone tissue engineering for critical-sized bone defects lie in the clinical translation of methods that have been successful in preclinical models. In addition to the methods of scaffolds plus cells or growth factors described above, the printing of bone substitutes is a promising experimental field that has a potential future role in the treatment of large bone defects [95]. Barriers that will need to be overcome in applying bone tissue engineering techniques to the treatment of critical-sized bone defects involve the need for vascularity in the region of the defect, scaffold integration, safety, and cost effectiveness. Additionally, because many of these therapies are combination products, obtaining approval for use in humans from the FDA and other regulatory agencies is a difficult and lengthy process. Sources of controversy for the use of new materials include their degradation rates and by-products, their effect on the surrounding tissues, and their performance in situ. The optimal treatment method for addressing critical-sized bone defects needs to be individualized for each patient, but will likely lie in supplementing current treatment methods with multiple bone tissue engineering techniques.
Conclusion
Orthopaedic trauma afflicts patients unexpectedly and is often life-altering, particularly when injuries are severe. While treatments for fractures have evolved and advanced considerably over the past several decades, technology and treatment approaches continue to change. Bone tissue engineering techniques are actively being investigated to help improve the treatment of orthopaedic trauma patients, whether it be to stimulate acute fracture healing in routine injuries or to help address the difficult issues of nonunion or critical-sized bone defects. Persistence and multidisciplinary collaboration are essential to overcome the scientific, practical, and regulatory hurdles in order to translate bone tissue engineering techniques to clinical therapies.
Acknowledgments
Funding
This work was supported by in part by CASIS (Grant no. GA-2016-236), U.S. Department of Defense (DOD W81XWH-14-2-0003, DOD W81XWH-14-1-0217, DOD W81XWH-15-2-0011), the National Institutes of Health (1UG3 TR002136), and the Ri.MED Foundation, Palermo, Italy.
Footnotes
Conflict of Interest
The authors (Peter N. Mittwede, Riccardo Gottardi, Peter G. Alexander, Ivan S. Tarkin, and Rocky S. Tuan) declare that they have no conflicts of interest.
Compliance with Ethics Guidelines
Human and Animal Rights and Informed Consent
This review article does not contain any human or animal studies performed by any of the authors.
References
Papers of particular interest that have been published recently have been highlighted as:
•Of importance
••Of outstanding importance
- 1.Amin S, Achenbach SJ, Atkinson EJ, et al. Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res 2014;29:581–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwal-Harding KJ, Meara JG, Greenberg SLM, et al. Estimating the global incidence of femoral fracture from road traffic conditions. J Bone Jt Surg 2015;31:1–9. [DOI] [PubMed] [Google Scholar]
- 3.Belmont PJ, Garcia EJ, Romano D, et al. Risk factors for complications and in-hospital mortality following hip fractures: a study using the National Trauma Data Bank. Arch Orthop Trauma Surg 2014;134:597–604. [DOI] [PubMed] [Google Scholar]
- 4.Patel KV, Brennan KL, Davis ML, et al. High-energy femur fractures increase morbidity but not mortality in elderly patients. Clin Orthop Relat Res 2014;472:1030–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Odén A, McCloskey EV, Kanis JA, et al. Burden of high fracture probability worldwide: secular increases 2010–2040. Osteoporos Int 2015;26:2243–8. [DOI] [PubMed] [Google Scholar]
- 6.Marsell R, Einhorn TA. The biology of fracture healing. Injury 2011;42:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hak DJ, Fitzpatrick D, Bishop JA, et al. Delayed union and nonunions: Epidemiology, clinical issues, and financial aspects. Injury 2014;45:S3–7. [DOI] [PubMed] [Google Scholar]
- 8.Keating JF, Simpson AH, Robinson CM. The management of fractures with bone loss. J Bone Joint Surg Br 2005;87:142–50. [DOI] [PubMed] [Google Scholar]
- 9.Quarto R, Giannoni P. Bone tissue engineering: past–present–future. In: Methods in molecular biology (Clifton, N.J.).Vol 1416., 2016:21–33. [DOI] [PubMed] [Google Scholar]
- 10.Marcucio RS, Nauth A, Giannoudis PV, et al. Stem cell therapies in orthopaedic trauma. J Orthop Trauma 2015;29:24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatara AM, Mikos AG. Tissue engineering in orthopaedics. J Bone Joint Surg Am 2016;98:1132–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schroeder JE, Mosheiff R. Tissue engineering approaches for bone repair: Concepts and evidence. Injury 2011;42:609–13. [DOI] [PubMed] [Google Scholar]
- 13.Evans CH. Barriers to the clinical translation of orthopedic tissue engineering. Tissue Eng Part B Rev 2011;17:437–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies BM, Rikabi S, French A, et al. Quantitative assessment of barriers to the clinical development and adoption of cellular therapies: A pilot study. J Tissue Eng 2014;5:2041731414551764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bara JJ, Herrmann M, Evans CH, et al. Improving translation success of cell-based therapies in orthopaedics. J Orthop Res 2016;34:17–21. [DOI] [PubMed] [Google Scholar]
- 16.Bauer S, Schmuki P, von der Mark K, et al. Engineering biocompatible implant surfaces. Part I: Materials and surfaces. Prog Mater Sci 2012;58:261–326. [Google Scholar]
- 17.Agarwal R, García AJ. Biomaterial strategies for engineering implants for enhanced osseointegration and bone repair. Adv Drug Deliv Rev 2015;94:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivares-Navarrete R, Raines AL, et al. Osteoblast maturation and new bone formation in response to titanium implant surface features are reduced with age. J Bone Min Res 2012;27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groessner-Schreiber B, Tuan RS. Enhanced extracellular matrix production and mineralization by osteoblasts cultured on titanium surfaces in vitro. J Cell Sci 1992; 101 (Pt 1:209–17. [DOI] [PubMed] [Google Scholar]
- 20.Shah A, Sinka R, Hickok N, et al. High-resolution morphometric analysis of human osteoblastic cell adhesion on clinically relevant orthopedic alloys. Bone 1999;24:499–506. [DOI] [PubMed] [Google Scholar]
- 21.Sinha R, Tuan R. Regulation of human osteoblast integrin expression by orthopedic implant materials. Bone 1996;18:451–7. [DOI] [PubMed] [Google Scholar]
- 22.Sinha R, Morris F, Shah S, et al. Surface composition of orthopaedic implant metals regulates cell attachment, spreading, and cytoskeletal organization of primary human osteoblasts in vitro. Clin Orthop Relat Res 1994;305:258–72. [PubMed] [Google Scholar]
- 23.Giannoudis PV, Einhorn TA, Marsh D. Fracture healing: The diamond concept. Injury 2007;38:3–6. [DOI] [PubMed] [Google Scholar]
- 24.Giannoudis PV, Einhorn TA, Schmidmaier G, et al. The diamond concept - open questions. Injury 2008;39:5–8. [DOI] [PubMed] [Google Scholar]
- 25. •Lu M, Liao J, Dong J, et al. An effective treatment of experimental osteomyelitis using the antimicrobial titanium/silver-containing nHP66 (nano-hydroxyapatite/polyamide-66) nanoscaffold biomaterials. Sci Rep 2016;6:39174. This study shows that in a rabbit model, a scaffold containing silver ions and oxidized titanium has antibacterial effect and is effective in combating osteomyelitis.
- 26.Almubarak S, Nethercott H, Freeberg M, et al. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016;83:197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiernan CH, Wolvius EB, Brama PAJ, et al. The immune response to allogeneic differentiated mesenchymal stem cells in the context of bone tissue engineering. Tissue Eng Part B Rev 2017:ten.TEB.2017.0175. [DOI] [PubMed] [Google Scholar]
- 28.Copuroglu C, Calori GM, et al. Fracture non-union: who is at risk? Injury 2013;44:1379–82. [DOI] [PubMed] [Google Scholar]
- 29.Cui Q, Dighe AS, Irvine JI Jr. Combined angiogenic and osteogenic factor delivery for bone regenerative engineering. Curr Pharm Des 2013;19:3374–83. [DOI] [PubMed] [Google Scholar]
- 30.Bayer EA, Gottardi R, Fedorchak MV, et al. The scope and sequence of growth factor delivery for vascularized bone tissue regeneration. J Control Release 2015;219:129–40. [DOI] [PubMed] [Google Scholar]
- 31.Kawaguchi H, Oka H, Jingushi S, et al. A local application of recombinant human fibroblast growth factor 2 for tibial shaft fractures: A randomized, placebo-controlled trial. J Bone Miner Res 2010;25:2459–67. [DOI] [PubMed] [Google Scholar]
- 32.Grayson WL, Bunnell BA, Martin E, et al. Stromal cells and stem cells in clinical bone regeneration. Nat Rev Endocrinol 2015;11:140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina ER, Smith BT, Shah SR, et al. Immunomodulatory properties of stem cells and bioactive molecules for tissue engineering. J Control Release 2015;219:107–18. [DOI] [PubMed] [Google Scholar]
- 34.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dupont KM, Sharma K, Stevens HY, et al. Human stem cell delivery for treatment of large segmental bone defects. Proc Natl Acad Sci 2010;107:3305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vo TTN, Kasper FK, Mikos AGA. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv Drug Deliv Rev 2012;64:1292–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urish MR. Bone: formation by autoinduction. Science 1965;150:893–9. [DOI] [PubMed] [Google Scholar]
- 38.Hankenson KD, Gagne K, Shaughnessy M. Extracellular signaling molecules to promote fracture healing and bone regeneration. Adv Drug Deliv Rev 2015;94:3–12. [DOI] [PubMed] [Google Scholar]
- 39.Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am 2002;84-A:2123–34. [DOI] [PubMed] [Google Scholar]
- 40.Aro HT, Govender S, Patel AD, et al. Recombinant uhman bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone Joint Surg 2011;93:801. [DOI] [PubMed] [Google Scholar]
- 41.Lyon T, Scheele W, Bhandari M, et al. Efficacy and safety of recombinant human bone matrix for closed tibial diaphyseal fracture. J Bone Joint Surg Am 2013;95:2088–97. [DOI] [PubMed] [Google Scholar]
- 42.Galimberti F, Lubelski D, Healy AT, et al. A systematic review of lumbar fusion rates with and without the use of rhBMP-2. Spine (Phila Pa 1976) 2015;40:1132–9. [DOI] [PubMed] [Google Scholar]
- 43.Einhorn TA, Gerstenfeld LC. Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 2014;11:45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Claes L, Recknagel S, Ignatius A. Fracture healing under healthy and inflammatory conditions. Nat Rev Rheumatol 2012;8:133–43. [DOI] [PubMed] [Google Scholar]
- 45.Kolar P, Schmidt-Bleek K, Schell H, et al. The early fracture hematoma and its potential role in fracture healing. Tissue Eng Part B Rev 2010;16:427–34. [DOI] [PubMed] [Google Scholar]
- 46.Loi F, Córdova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone 2016;86:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu S, Liu X, Yeung KWK, et al. Biomimetic porous scaffolds for bone tissue engineering. Mater Sci Eng R Reports 2014;80:1–36. [Google Scholar]
- 48.Rupp F, Scheideier L, Olshanska N, et al. Enhancing surface free energy and hydrophilicity through chemical modification of microstructured titanium implant surfaces. J Biomed Mater Res - Part A 2006;76:323–34. [DOI] [PubMed] [Google Scholar]
- 49.Keselowsky BG, Collard DM, Garcia AJ. Integrin binding specificity regulates biomaterial surface chemistry effects on cell differentiation. Proc Natl Acad Sci U S A 2005;102:5953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wermelin K, Aspenberg P, Linderbäck P, et al. Bisphosphonate coating on titanium screws increases mechanical fixation in rat tibia after two weeks. J Biomed Mater Res - Part A 2008;86:220–7. [DOI] [PubMed] [Google Scholar]
- 51.Ramazanoglu M, Lutz R, Rusche P, et al. Bone response to biomimetic implants delivering BMP-2 and VEGF: An immunohistochemical study. J Cranio-Maxillofacial Surg 2013;41:826–35. [DOI] [PubMed] [Google Scholar]
- 52.Böstman OM, Laitinen OM, Tynninen O, et al. Tissue restoration after resorption of polyglycolide and poly-laevo-lactic acid screws. J Bone Joint Surg Br 2005;87:1575–80. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Ebraheim N, Lause GE, et al. A comparison of absorbable screws and metallic plates in treating calcaneal fractures : A prospective randomized trial. 2009;72:106–10. [DOI] [PubMed] [Google Scholar]
- 54.Sun H, Luo CF, Zhong B, et al. A prospective, randomised trial comparing the use of absorbable and metallic screws in the fixation of distal tibiofibular syndesmosis injuries: Mid-term follow-up. Bone Joint J 2014;96 B:548–54. [DOI] [PubMed] [Google Scholar]
- 55.Böstman OM, Pihlajamäki HK. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res 2000:216–27. [PubMed] [Google Scholar]
- 56.Ramot Y, Haim-Zada M, Domb AJ, Nyska A. Biocompatibility and safety of PLA and its copolymers. Adv Drug Deliv Rev 2016;107:153–62. [DOI] [PubMed] [Google Scholar]
- 57.Tuan R Role of adult stem/progenitor cells in osseointegration and implant loosening. Int J Oral Maxillofac Implant 2011;26 Suppl:50–62. [PubMed] [Google Scholar]
- 58.Trampuz A, Widmer AF. Infections associated with orthopedic implants. Curr Opin Infect Dis 2006;19:349–56. [DOI] [PubMed] [Google Scholar]
- 59.Hake ME, Young H, Hak DJ, et al. Local antibiotic therapy strategies in orthopaedic trauma: practical tips and tricks and review of the literature. Injury 2015;46:1447–56. [DOI] [PubMed] [Google Scholar]
- 60.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013;34:8533–54. [DOI] [PubMed] [Google Scholar]
- 61.Raphel J, Holodniy M, Goodman SB, et al. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016;84:301–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson CT, García AJ. Scaffold-based anti-infection strategies in bone repair. Ann Biomed Eng 2015;43:515–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qureshi AT, Terrell L, Monroe WT, et al. Antimicrobial biocompatible bioscaffolds for orthopaedic implants. J Tissue Eng Regen Med 2014;8:386–95. [DOI] [PubMed] [Google Scholar]
- 64.Hou T, Xu J, Li Q, et al. In vitro evaluation of a fibrin gel antibiotic delivery system containing mesenchymal stem cells and vancomycin alginate beads for treating bone infections and facilitating bone formation. Tissue Eng Part A 2008; 14:1173–82. [DOI] [PubMed] [Google Scholar]
- 65.Chamberlain G, Fox J, Ashton B, et al. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007;25:2739–49. [DOI] [PubMed] [Google Scholar]
- 66.Tzioupis C, Giannoudis PV. Prevalence of long-bone non-unions. Injury 2007;38. [DOI] [PubMed] [Google Scholar]
- 67.Mills LA, Aitken SA, Simpson AH. The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults. Acta Orthop 2017;88:434–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zura R, Mehta S, Della Rocca GJ, et al. Biological risk factors for nonunion of bone fracture. JBJS Rev 2016;4:1–12. [DOI] [PubMed] [Google Scholar]
- 69.Santolini E, West R, Giannoudis PV. Risk factors for long bone fracture non-union: A stratification approach based on the level of the existing scientific evidence. Injury 2015;46:S8–19. [DOI] [PubMed] [Google Scholar]
- 70.Panteli M, Pountos I, Jones E, et al. Biological and molecular profile of fracture non-union tissue: Current insights. J Cell Mol Med 2015;19:685–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hak DJ. Management of aseptic tibial nonunion. J Am Acad Orthop Surg 2011; 19:563–73. [DOI] [PubMed] [Google Scholar]
- 72.Kadhim M, Holmes L Jr, Gesheff MG, et al. Treatment options for nonunion with segmental bone defects: systematic review and quantitative evidence synthesis. J Orthop Trauma 2017;31:111–9. [DOI] [PubMed] [Google Scholar]
- 73.Babhulkar S, Pande K, Babhulkar S. Nonunion of the diaphysis of long bones. Clin Orthop Relat Res 2005;NA;50–6. [DOI] [PubMed] [Google Scholar]
- 74.Metsemakers WJ, Kuehl R, Moriarty TF, et al. Infection after fracture fixation: current surgical and microbiological concepts. Injury 2016;1383(16)30470. [DOI] [PubMed] [Google Scholar]
- 75.Kanakaris NK, Lasanianos N, Calori GM, et al. Application of bone morphogenetic proteins to femoral non - unions : A 4 - year multicentre experience. Injury 2009;40:54–61. [DOI] [PubMed] [Google Scholar]
- 76.Kanakaris NK, Calori GM, Verdonk R, et al. Application of BMP-7 to tibial non-unions: a 3-year multicenter experience. Injury 2008;39. [DOI] [PubMed] [Google Scholar]
- 77.Giannoudis PV, Kanakaris NK, Dimitriou R, et al. The synergistic effect of autograft and BMP-7 in the treatment of atrophic nonunions. Clin Orthop Relat Res 2009;467:3239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Papanna MC, Al-Hadithy N, Somanchi BV, et al. The use of bone morphogenic protein-7 (OP-1) in the management of resistant non-unions in the upper and lower limb. Injury 2012;43:1135–40. [DOI] [PubMed] [Google Scholar]
- 79.Friedlaender GE, Perry CR, Cole JD, et al. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions: A prospective, randomized clinical trial comparing RHOP-1 with fresh bone autograft. J Bone Joint Surg 2001;83:SI51–8. [PMC free article] [PubMed] [Google Scholar]
- 80.Daliari D, Rani N, Sabbioni G, et al. Radiological assessment of the PRF/BMSC efficacy in the treatment of aseptic nonunions: A retrospective study on 90 subjects. Injury 2016;47:2544–50. [DOI] [PubMed] [Google Scholar]
- 81.Giannoudis PV, Gudipati S, Harwood P, et al. Long bone non-unions treated with the diamond concept: A case series of 64 patients. Injury 2015;46:S48–54. [DOI] [PubMed] [Google Scholar]
- 82.Calori GM, Colombo M, Mazza E, et al. Monotherapy vs. polytherapy in the treatment of forearm non-unions and bone defects. Injury 2013;44. [DOI] [PubMed] [Google Scholar]
- 83.Gómez-Barrena E, Rosset P, Lozano D, et al. Bone fracture healing: Cell therapy in delayed unions and nonunions. Bone 2015;70:93–101. [DOI] [PubMed] [Google Scholar]
- 84.Mauffrey C, Barlow BT, Smith W. Management of segmental bone defects. J Am Acad Orthop Surg 2015;23:143–53. [DOI] [PubMed] [Google Scholar]
- 85.Aronson J Current concepts review - limb-lengthening, skeletal reconstruction, and bone transport with the Ilizarov method*. J Bone Joint Surg Am 1997;79:1243–58. [DOI] [PubMed] [Google Scholar]
- 86.Masquelet AC, Begue T. The concept of induced membrane for reconstruction of long bone defects. Orthop Clin North Am 2010;41:27–37. [DOI] [PubMed] [Google Scholar]
- 87.Chimutengwende-Gordon M, Mbogo A, Khan W, et al. Limb reconstruction after traumatic bone loss. Injury 2017;48:206–13. [DOI] [PubMed] [Google Scholar]
- 88.Papakostidis C, Bhandari M, Giannoudis PV. Distraction osteogenesis in the treatment of long bone defects of the lower limbs: effectiveness, complications and clinical results; a systematic review and meta-analysis. Bone Joint J 2013;95-B: 1673–80. [DOI] [PubMed] [Google Scholar]
- 89.Balasundaram G, Webster TJ. An overview of nano-polymers for orthopedic applications. Macromol Biosci 2007;7:635–42. [DOI] [PubMed] [Google Scholar]
- 90.O’Brien FJ. Biomaterials & scaffolds for tissue engineering. Mater Today 2011; 14:88–95. [Google Scholar]
- 91.Lyon T, Scheele W, Bhandari M, et al. Efficacy and safety of recombinant human bone matrix for closed tibial diaphyseal fracture. J Bone Joint Surg Am 2013;95:2088–97. [DOI] [PubMed] [Google Scholar]
- 92. ••Baker RM, Tseng LF, Iannolo MT, et al. Self-deploying shape memory polymer scaffolds for grafting and stabilizing complex bone defects: A mouse femoral segmental defect study. Biomaterials 2016;76:388–98. This study shows that shape memory polymer grafts are effective in integrating into mouse femoral defects and are able to provide torsional stability at the location of the defect.
- 93.Decambron A, Fournet A, Bensidhoum M, et al. Low-dose BMP-2 and MSC dual delivery onto coral scaffold for critical-size bone defect regeneration in sheep. J Orthop Res 2017:1–9. [DOI] [PubMed] [Google Scholar]
- 94. •Bosemark P, Perdikouri C, Pelkonen M, et al. The masquelet induced membrane technique with BMP and a synthetic scaffold can heal a rat femoral critical size defect. J Orthop Res 2015;33:488–95. This article shows that treatment with three agents combined (BMP-7, a tricalcium phosphate hydroxyapatite scaffold, and systemic bisphosphanate) was more effective in filling a critical-sized rat femoral defect than with one or two of the agents.
- 95.Arealis G, Nikolaou VS. Bone printing: new frontiers in the treatment of bone defects. Injury 2015;46:S20–2. [DOI] [PubMed] [Google Scholar]