Abstract
A persistent Staphylococcus epidermidis infection in mice around a subcutaneous polyvinylpyrrolidone-grafted silicon elastomer catheter (SEpvp) but not around a conventional silicon elastomer catheter was observed. With SEpvp pericatheter tissue, protracted and exaggerated interleukin-1β (IL-1β) production was found. Apparently, sustained levels of IL-1β are associated with enhanced susceptibility to biomaterial-associated S. epidermidis infection.
Infection of catheters and other implanted biomedical devices is most frequently due to Staphylococcus epidermidis (2, 6). It is assumed that bacterial adherence is the initial step, but alterations in the host response may also play a role in the pathogenesis of catheter-associated infection (CAI) (2, 6, 18). Around a novel polyvinylpyrrolidone-grafted silicon elastomer catheter (SEpvp) (Bioglide; Medtronic PS Medical, Goleta, Calif.) developed to reduce bacterial adherence, abscesses and persistent infection were observed in rabbits and mice (3) in the presence of low numbers of S. epidermidis. Although the number of adherent bacteria found in vitro on a conventional silicon elastomer catheter (SE) (Medtronic PS Medical) was nine times higher than on SEpvp (4), no SE-associated infection developed. Both catheters are used in clinical settings. We sought to determine whether specific changes in tissue response and local cytokine production around subcutaneously inserted SEpvp are associated with the enhanced susceptibility to abscess formation and persistent infection in mice.
(Part of these data was presented at the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Diego, Calif., 24 to 28 September, 1998.)
Female C57BL/6 mice, 6 to 8 weeks old and weighing 15 to 20 g, were used. They were divided into seven groups of 24 mice each and one group of 8 mice (baseline controls). Two groups received SE segments and two groups received SEpvp segments, implanted subcutaneously under antiseptic conditions, as described previously (3). Two groups of mice received sham operations (sham controls). We used an inoculum of 106 CFU of S. epidermidis strain RP62a (ATCC 35984), prepared from a logarithmic culture in Trypticase soy broth (Difco, Detroit, Mich.) by dilution with pyrogen-free isotonic saline, which reproducibly induced persistent infection around SEpvp (3). One group of mice with implanted SEs and one group with SEpvps received the inoculum delivered in a 0.025-ml volume (model 4001-025; Tridak division, Stepper, Brookfield, Conn.) subcutaneously along the inserted segments. Implant controls were mice with implanted SE or SEpvp segments that received a saline injection. The two sham control groups received an injection of either the bacterial inoculum or saline in the subcutaneous wound. The seventh group of 24 mice, which had not been subjected to surgery, received only the bacterial inoculum (injection control). After 1 h, 6 h, 2 days, 5 days, 14 days, or 60 days, four mice from each group were sacrificed. The implantation sites were inspected for purulence and standardized biopsies (φ, 12 mm) were taken from the implantation sites as previously described (3). The right-side biopsy from each mouse was fixed in 10% buffered formaldehyde (pH 7.3), embedded in methyl methacrylate-butyl methacrylate (Merck Schuchart, Hohenbrunn, Germany), sectioned, and stained with hematoxylin-eosin. Slides were examined for four histological features characteristic for tissue reactions after implantation of a foreign body (1): (i) infiltration of inflammatory cells polymorphonuclear leukocytes or mononuclear cells; (ii) foreign-body giant-cell (FBGC) formation; (iii) fibrosis, characterized by inflammatory cells, fibroblasts, and newly formed collagen; and (iv) encapsulation of the foreign body. Features were scored independently and in a blinded fashion by three of the authors, using a scale of 0 (not observed) to 3 (maximally present). From the left-side biopsy, the catheter segment (if present) was separated from the tissue, washed, sonicated to dislodge adherent bacteria, and quantitatively cultured as previously described (3). The tissue samples were weighed (125 and 160 mg), and homogenized (Tissue Tearer model 985-370; Biospec Products, Bartlesville, Okla.) in a volume of pyrogen-free isotonic saline corresponding to four times the tissue sample weight at 4°C. Fifty microliters of the homogenates was quantitatively cultured. The sonicated segments as well as 50 μl of the homogenate were cultured in 80 ml of thioglycolate broth for 72 h at 37°C. For statistical purposes, it was assumed that 1 CFU per 1-cm segment or per 50 μl of the homogenate had been present, in case only the broth culture was positive.
The homogenates, reduced by 100 μl for culture, were diluted in 1 volume of lysis buffer (9), incubated on ice for 1 h, and centrifuged at 130,000 × g for 15 min at 4°C to remove cell debris. The cell supernatants were frozen at −80°C, thawed, centrifuged at 5000 × g to remove macroaggregates, and stored in aliquots of 35 μl each at −80°C until use. Levels of interleukin-1β (IL-1β) (R & D Systems, Minneapolis, Minn.), IL-6 (PharMingen, San Diego, Calif.), IL-10 (R & D Systems), tumor necrosis factor alpha (TNF-α) (PharMingen), and gamma interferon (IFN-γ) (R & D Systems) were measured by commercially available enzyme-linked immunosorbent assay kits and expressed as the number of picograms per milliliter of homogenate. All values are expressed as means ± standard error. Two sample comparisons were made by analysis of variance, and comparisons over time between cytokine levels of groups were made by analysis of variance in a general linear-general factorial model. The significance of differences for proportional data-set analysis was determined by the chi-square test. P values of <0.05 were considered significant.
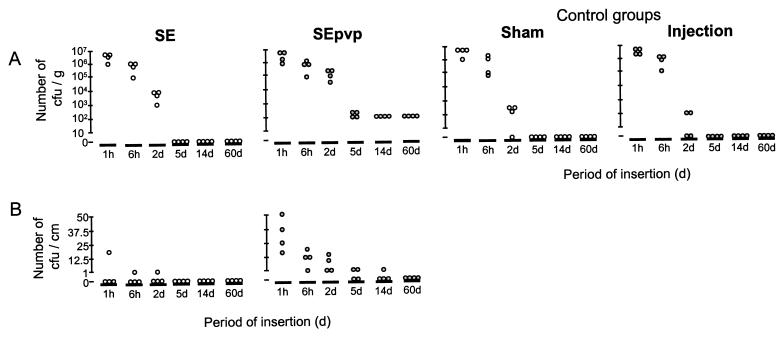
Abscesses were only seen in mice with SEpvp challenged with S. epidermidis from 2 to 60 d. SEpvp segments were significantly more often positive for S. epidermidis RP62a by culture than were SE segments (15 of 24 SEpvp segments versus 6 of 24 SE segments; P < 0.05) (Fig. 1B). At 48 h, significantly more colonies were cultured from SEpvp pericatheter tissue (126,000 ± 11,400 CFU/g) than from SE pericatheter tissue (8,800 ± 2,400 CFU/g; P < 0.001) (Fig. 1A), sham-operated tissue (800 ± 640 CFU/g), or inoculum injection control tissue (88 ± 80 CFU/g) (Fig. 1A).
FIG. 1.
(A) Numbers of CFU of S. epidermidis in tissue around subcutaneously inserted SE and SEpvp catheter segments challenged with 106 CFU of S. epidermidis RP62a along these segments, in tissue of sham-operated mice challenged with the same inoculum, and in tissue of mice who received the inoculum injection only, at various time points. (B) Number of adherent CFU of S. epidermidis on SE and SEpvp segments at various time points after inoculation.
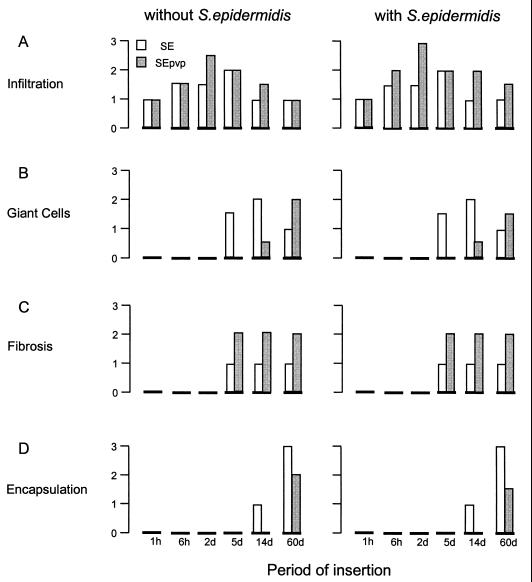
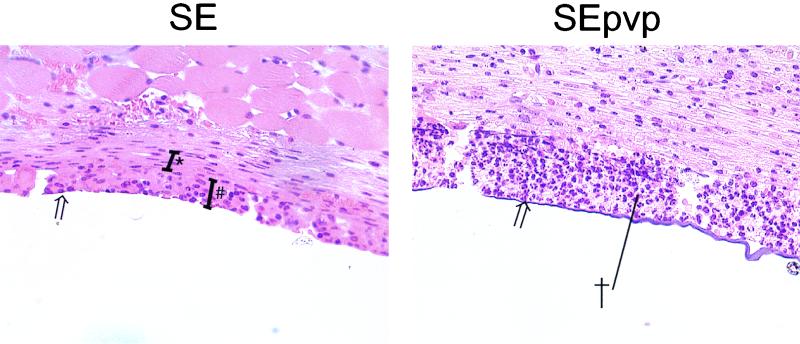
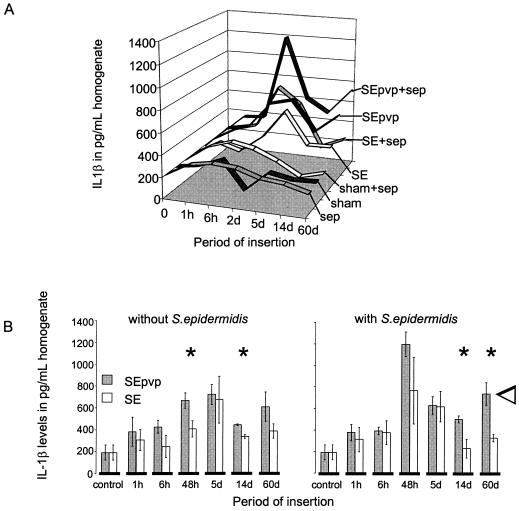
Generally, the implantation of a catheter results in injury, initiation of inflammatory responses (acute and chronic), and finally a foreign-body reaction, characterized by FBGC formation around the implant after 4 d and encapsulation after 10 d (1). Histologically, no difference in infiltration was seen between tissue around SE in the presence or the absence of S. epidermidis at any of six time points (Fig. 2). Infiltration around SEpvp was more extensive than around SE for up to 60 d. Also, chronic inflammation around SEpvp was more extensive, judging by the thicker layer of fibrosis around SEpvp. In comparison to SE, which induced a normal foreign-body reaction, FBGC formation and encapsulation around SEpvp were delayed (Fig. 2 and 3). Apparently, subcutaneously inserted SEpvp induced a higher influx of inflammatory cells than did SE and a delay in the onset of a proper foreign-body reaction. Implantation of sterile SE or SEpvp segments induced a bimodal production of TNF-α, IL-6, and IFN-γ (data not shown). The first peak (1 h) was most likely caused by the surgical procedure itself, since in biopsies of sham-operated mice a similar peak was observed. A second peak, at 5 days, was not seen in sham control and injection control animals and is therefore due to the implanted catheters. SE and SEpvp with S. epidermidis induced higher levels of TNF-α and IFN-γ levels over time than the corresponding implant controls, but no differences between SE and SEpvp were found (data not shown). For IL-10, no significant differences were observed, irrespective of the catheter type or the presence of S. epidermidis. In contrast, local IL-1β production around sterile SEpvp increased earlier, and levels remained elevated for a longer period than around SE (Fig. 4). Injection of S. epidermidis along SE or SEpvp was associated with a marked increase in IL-1β concentrations. Significantly higher levels of IL-1β were found at 2, 14, and 60 d and for the over-time profile. Apparently, the persistence of S. epidermidis around SEpvp is associated with sustained levels of IL-1β production.
FIG. 2.
Mean scores of the blind examination of sections of tissue around SE and SEpvp in presence and absence of S. epidermidis. Each section was scored by three independent investigators for four of the six histological features characteristic for tissue reactions after implantation of a foreign body: infiltration (A), giant-cell formation (B), fibrosis (C), and encapsulation (D).
FIG. 3.
Histological examination of tissue surrounding subcutaneously implanted SE and SEpvp 14 d after implantation and challenge of 106 CFU. #, a layer of giant cells; ∗, the fibrous capsule; †, the abscess associated with SEpvp; open arrow, the catheter-tissue interface. Magnification, ×100.
FIG. 4.
(A) Production of IL-1β over time in tissue surrounding the subcutaneously implanted SE and SEpvp catheter segments and in tissue of the control groups (sham-operated mice and injection only) in the presence or absence of S. epidermidis (sep). (B) Detailed representation of production over time of IL-1β (means ± standard error) in tissue surrounding the subcutaneously implanted SE and SEpvp catheter segments in the absence and presence of S. epidermidis. Significant differences between SE and SEpvp at the individual time points are indicated by an asterisk (∗), and significant differences between levels over time are indicated by an open arrow.
The present study showed that a biomaterial inducing a more extensive inflammation has a higher susceptibility to infection. This is in accordance with other studies (8, 19). Biomaterials like SEpvp, which can be regarded as biocompatible in the absence of bacteria, can enhance the inflammatory reaction in the presence of bacteria, resulting in abscess formation and persistent infection (3). As a sustained IL-1β level was associated with persistent infection, IL-1 may be a marker for testing bioincompatibility in vitro or in vivo.
At the later time points, S. epidermidis was cultured from the tissue homogenates and not from the implanted SEpvp. This contradicts the contention that adherence of bacteria to and growth on the biomaterial surface are of decisive importance in the pathogenesis of CAI (2, 18). The survival of bacteria around SEpvp could have been associated with the abscess (7, 13). Alternatively, sustained local inflammation, as observed in mice with implanted SEpvp segments, may compromise the resolution of an infection by priming for intracellular and extracellular growth of bacteria that exceeds the clearance ability of the host (12, 16). Various observations in humans support this speculation. Patients with enhanced local or circulating levels of proinflammatory cytokines, such as (i) patients suffering from acute respiratory distress syndrome (10, 15), (ii) patients with long-term exposure to intravascular catheters (14), and (iii) patients who underwent extracorporal circulation (5) or receiving hemodialysis (11, 17) are highly susceptible to infection. Thus, protracted proinflammation, as characterized by sustained IL-1β levels in our mouse model, may well play a role in the pathogenesis of CAI. This could definitively be assessed by using mice deficient for IL-1 or for the IL-1 receptor.
Acknowledgments
T. van der Poll is a fellow of the Royal Netherlands Academy of Arts and Sciences.
REFERENCES
- 1.Anderson J M. Inflammatory response to implants. Trans Am Soc Artif Intern Organs. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Bisno A L, Waldvogel F A. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C.: ASM Press; 1994. [Google Scholar]
- 3.Boelens, J. J., S. A. Zaat, J. Meeldijk, and J. Dankert. Subcutaneous abscess formation around catheters induced by viable and non-viable Staphylococcus epidermidis as well as by small amounts of bacterial cell wall components. J. Biomed. Mater. Res., in press. [DOI] [PubMed]
- 4.Boelens, J. J., W. F. Tan, J. Dankert, and S. A. Zaat. Antibacterial activity of antibiotic-soaked polyvinylpyrrolidone-grafted silicon elastomer hydrocephalus shunts. J. Antimicrob. Chemother., in press. [DOI] [PubMed]
- 5.Cameron D. Initiation of white cell activation during cardiopulmonary bypass: cytokines and receptors. J Cardiovasc Pharmacol. 1996;27(Suppl):S1–S5. doi: 10.1097/00005344-199600001-00004. [DOI] [PubMed] [Google Scholar]
- 6.Dankert J, Hogt A, Feijen J. Biomedical polymers: bacterial adhesion, colonization, and infection. Crit Rev Biocompat. 1986;2:219–301. [Google Scholar]
- 7.Deighton M A, Borland R, Capstick J A. Virulence of Staphylococcus epidermidis in a mouse model: significance of extracellular slime. Epidemiol Infect. 1996;117:267–280. doi: 10.1017/s0950268800001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edlich R F, Panek P H, Rodeheaver G T, Turnbull L D, Kurtz L D, Edgerton M T. Physical and chemical configuration of sutures in the development of surgical infection. Ann Surg. 1973;177:679. doi: 10.1097/00000658-197306000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberger M J, Streiter R M, Kunkel S L, Danforth J M, Goodman R E, Standiford T J. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 10.Headley A, Tolley E, Meduri G. Infections and the inflammatory response in acute respiratory distress syndrome. Chest. 1997;111:1306–1321. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 11.Herbelin A, Urena P, Ngugen T, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39:954–960. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 12.Kanangat S, Meduri G, Tolley E, Patterson D, Meduri C, Pak C, et al. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect Immun. 1999;67:2834–2840. doi: 10.1128/iai.67.6.2834-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long J, Kapral F. Host response to coagulase-negative staphylococci in abscesses induced within mice. J Med Microbiol. 1993;39:191–195. doi: 10.1099/00222615-39-3-191. [DOI] [PubMed] [Google Scholar]
- 14.Martin L, Varv T, Davis P, Munger B, Lynch J, Spangler S, et al. Intravascular plastic catheters. How they potentiate tumor necrosis factor release and exacerbate complications associated with sepsis. Arch Surg. 1991;126:1087–1093. doi: 10.1001/archsurg.1991.01410330041005. [DOI] [PubMed] [Google Scholar]
- 15.Meduri G. The role of the host defense response in the progression and outcome of ARDS: pathophysiological correlations and response to glucocorticoid treatment. Eur Respir J. 1996;9:2650–2670. doi: 10.1183/09031936.96.09122650. [DOI] [PubMed] [Google Scholar]
- 16.Porat R, Clark B D, Wolff S M, Dinarello C A. Enhancement of growth of virulant strains of Escherichia coli by interleukin-1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 17.Schindler R, Lonnemann G, Shaldon S, Koch M K, Dinarello C A. Transcription, not synthesis, of interleukin-1 and tumor necrosis factor by complement. Kidney Int. 1990;37:85–93. doi: 10.1038/ki.1990.12. [DOI] [PubMed] [Google Scholar]
- 18.Seifert H, Jansen B, Farr B. Catheter-related infections. New York, N.Y: Marcel Dekker Press; 1997. [Google Scholar]
- 19.Tomford J W, Hershey C O, McLaren C E, Porter D K, Cohen D I. Intravenous therapy team and peripheral venous catheter-associated complications. A prospective controlled study. Arch Intern Med. 1984;144:1191. [PubMed] [Google Scholar]