Abstract
Antiarrhythmic drug therapy has traditionally been centered in modulating the generation or propagation of the cardiac action potential by drugs acting on membrane ion channels. The history of this approach has been disappointing, marked by catastrophic failures such as those of sodium channel blockers or sotalol to treat ventricular arrhythmias in the setting of structural cardiomyopathies, which led to increased mortality, and by modest clinical efficacy in paroxysmal atrial fibrillation. As catheter ablation has become an established effective therapy for most tachyarrhythmias, membrane-acting drugs have been relegated to symptomatic control of benign arrhythmias in normal hearts or to adjunctive treatments of ventricular tachycardia (combined with catheter ablation and cardiac defibrillators) in the setting of cardiomyopathies. Novel targets of biological modulation of arrhythmia substrates beyond the membrane potential appear promising and could represent future opportunities for arrhythmia pharmacotherapy.
Keywords: ethanol, ablation, ventricular vein, ventricular arrhythmias
Introduction
Targeting the membrane potential seemed like a logical foundation to antiarrhythmic therapy. The Vaughan Williams (VW) classification1,2,3 provided a framework of understanding of a group of drugs that targeted the different molecular components of the action potential (Figure 1). Beta-blockers (Class II) were an exception, but their inclusion as antiarrhythmics had a wealth of support as suppressors of adrenergic-dependent arrhythmia. The classification was confusing, incomplete, and inconsistent. It grouped drugs by phenomena (conduction velocity, refractoriness) rather than molecular targets, so it was neither clinically relevant nor mechanistically precise. The Sicilian Gambit,4 devised 20 years later, attempted to add molecular precision to the classification, only to become clinically unmanageable and irrelevant.
Figure 1.
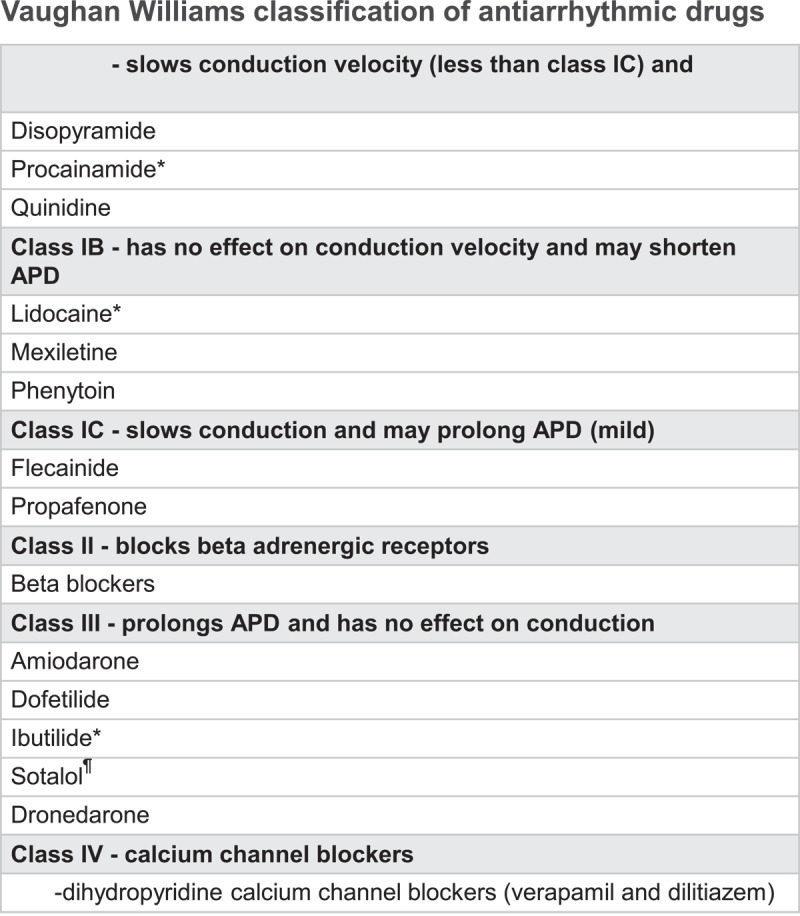
The Vaughan Williams classification provided a framework for understanding antiarrhythmic drugs, which targeted the different molecular components of the membrane action potential. APD: action potential duration
Historic Failures of Antiarrhythmic Drugs
Clinicians needed clarity as to (1) which drug to use, (2) for which arrhythmia, and (3) in which patient. Clarity came first from negative answers. Proarrhythmia and increased mortality were noted with Class I drugs (eg, sodium channel blockers encainide, flecainide, and moricizine) in patients with post-infarction ventricular extrasystoles as shown in the CAST-1 and CAST-2 trials.5,6 D-sotalol, a Class III drug potassium channel blocker, increased mortality in the SWORD trial,7 which studied post-infarction patients with ejection fraction < 40%. In the CASH trial, survivors of cardiac arrest treated with propafenone had increased mortality compared with those treated with an implantable cardioverter defibrillator.8 Most recently, dronedarone in patients with permanent atrial fibrillation (AF) also increased mortality.9
Remaining Clinical Use of Antiarrhythmic Drugs
A practical use of antiarrhythmic drugs is outlined in Figure 2. Class IA and IC drugs are safe and well tolerated in normal hearts, free from prior myocardial infarction or from left ventricular dysfunction. They are useful in AF in such patients and are occasionally used for failed ablations of supraventricular tachycardias. Furthermore, they are effective extrasystole suppressors and are used in patients who failed or refused ablation for ventricular extrasystoles. Concerns remain when prescribed for AF without proper rate control since conduction velocity slowing may lead to slow atrial flutter with paradoxical increase in ventricular response. Dronedarone and sotalol remain valid options for nonpermanent AF in the absence of structural heart disease, and coronary artery disease is an acceptable comorbidity for patients treated with sotalol. Dofetilide is an acceptable choice for AF even in heart failure.10 Although most clinicians may use drugs as their first choice for rhythm control in AF compared with ablation, emerging data support ablation as first line of therapy, and ablation is undisputedly superior in previous drug failure.11,12,13
Figure 2.

Practical use for antiarrhythmic drugs. CHF: congestive heart failure; CAD: coronary artery disease; EF: ejection fraction; ICD: implantable cardioverter defibrillator; AF: atrial fibrillation
Special situations of interest include the use of quinidine for suppression of ventricular arrhythmias in Brugada syndrome.14
Amiodarone is the most potent antiarrhythmic drug.15,16 However, lung, liver, thyroid, eye, and skin toxicities offset its clinical benefits and lead to the need for patient monitoring, requiring periodic toxicity monitoring.17 Thus, management guidelines recommend the use of amiodarone “only after consideration of risks, and when other agents have failed or are contraindicated.”18 Currently it is used for AF and for ventricular tachycardia in the context of structural heart disease.
A particularly complex situation arises when patients with significant cardiomyopathy develop ventricular arrhythmias requiring defibrillator shocks. In this scenario, amiodarone is a commonly used drug. Sotalol can decrease defibrillator shocks. Drug combinations including mexiletine can be effective. Catheter ablation can lead to improved outcomes rather than escalating antiarrhythmic drugs.19
Unclassified Drugs: Ivabradine, Nasal Etripamil
Developed after the VW classification, ivabradine acts on the If current, present in pacemaking cells of the sinus and atrioventricular nodes, with the chief effect of slowing the heart rate. Although ivabradine is approved by the US Food and Drug Administration (FDA) for the treatment of heart failure,20 its main clinical use is for the treatment of symptomatic inappropriate sinus tachycardia.21
Although not FDA approved, etripamil is a calcium channel blocker (thus VW Class IV) that is delivered via nasal spray for the acute termination of supraventricular tachycardia.22
Antiarrhythmic Benefits of Treating Underlying Left Ventricular Dysfunction: “Upstream” Therapies
Most life-threatening arrhythmias arise in the context of some form of heart disease, which determines both the prognostic implication as well as the specific drug treatment. Thus, it is not surprising that treatments targeting underlying left ventricular dysfunction may reduce the incidence of arrhythmias. For example, angiotensin-converting enzyme inhibitors,23 beta-adrenergic blockers,24 mineralocorticoid receptor antagonists,25 sacubitril/valsartan,26 and most recently SGLT2 inhibitors27 have been shown to reduce arrhythmogenic sudden cardiac death—and, in the case of SGLT2 inhibitors, the incidence of AF,28 which was not the case for the other upstream therapies. Optimized treatment of the underlying heart disease is an integral part of arrhythmia management, more so than any membrane-acting antiarrhythmic drug.
The Rise of Catheter Ablation and Device Therapies and Decline of Antiarrhythmics
With improvements in the understanding of cardiac arrhythmia mechanisms, catheter ablation has become the first line of treatment of most supraventricular arrhythmias. Most recently, catheter ablation as a first-line treatment for paroxysmal AF has been shown to have not only improved rhythm control11,29 but also reduced progression to persistent AF on long-term follow-up.13
Similarly, catheter ablation is a guideline-recommended first-line therapy for ventricular tachycardia (VT) in the setting of ischemic heart disease or nonischemic cardiomyopathy.30 Although recent data support ablation early in the course of VT management,31,32,33 most centers resort to ablation after failed antiarrhythmic therapy given the aggressive nature of the procedure, which is considered high risk.
Future Directions
In summary, ablation procedures are at the center of arrhythmia management as potentially curative, mechanistically-driven approaches. Drugs targeting underlying cardiomyopathic processes are mandatory. Antiarrhythmic drugs are relegated to adjuvant or palliative roles.
In terms of future directions, the role of neuromodulatory therapies targeting the cardiac autonomic system is rapidly emerging. This is an opportunity for novel drug targets as much as it is for novel procedural approaches. Gene therapy targeting potential mediators of electrical and neural mediators of AF has shown promising results in preclinical models.34
Key Points
Novel targets of biological modulation of arrhythmia substrates beyond the membrane potential appear promising and could represent future opportunities for arrhythmia pharmacotherapy.
Because most life-threatening arrhythmias arise in the context of some form of heart disease, optimized treatment of the underlying heart disease is an integral part of arrhythmia management, more so than any membrane-acting antiarrhythmic drug.
Catheter ablation has become the first line of treatment of most supraventricular arrhythmias. As a first-line treatment for paroxysmal atrial fibrillation (AF), it has been shown to have improved rhythm control and reduced progression to persistent AF on long-term follow-up. In addition, it is a guideline-recommended first-line therapy for ventricular tachycardia in the setting of ischemic heart disease or nonischemic cardiomyopathy.
Ablation procedures are at the center of arrhythmia management as potentially curative, mechanistically-driven approaches. Drugs targeting underlying cardiomyopathic processes are mandatory. Antiarrhythmic drugs are relegated to adjuvant or palliative roles.
Competing Interests
The author has no competing interests to declare.
References
- 1.Singh BN, Vaughan Williams EM. A third class of anti-arrhythmic action. Effects on atrial and ventricular intracellular potentials, and other pharmacological actions on cardiac muscle, of MJ 1999 and AH 3474. Br J Pharmacol. 1970. Aug;39(4):675-87. doi: 10.1111/j.1476-5381.1970.tb09893.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh BN. A fourth class of anti-dysrhythmic action? Effect of verapamil on ouabain toxicity, on atrial and ventricular intracellular potentials, and on other features of cardiac function. Cardiovasc Res. 1972. Mar;6(2):109-19. doi: 10.1093/cvr/6.2.109 [DOI] [PubMed] [Google Scholar]
- 3.Vaughan Williams EM. A classification of antiarrhythmic actions reassessed after a decade of new drugs. J Clin Pharmacol. 1984. Apr;24(4):129-47. doi: 10.1002/j.1552-4604.1984.tb01822.x [DOI] [PubMed] [Google Scholar]
- 4.The Sicilian gambit. A new approach to the classification of antiarrhythmic drugs based on their actions on arrhythmogenic mechanisms. Task Force of the Working Group on Arrhythmias of the European Society of Cardiology. Circulation. 1991. Oct;84(4):1831-51. doi: 10.1161/01.cir.84.4.1831 [DOI] [PubMed] [Google Scholar]
- 5.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991. Mar 21;324(12):781-8. doi: 10.1056/NEJM199103213241201 [DOI] [PubMed] [Google Scholar]
- 6.Cardiac Arrhythmia Suppression Trial II Investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. New Engl J Med. 1992. Jul 23;327(4):227-33. doi: 10.1056/NEJM199207233270403 [DOI] [PubMed] [Google Scholar]
- 7.Waldo AL, Camm AJ, deRuyter H, et al. Effect of d-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival With Oral d-Sotalol. Lancet. 1996. Jul 6;348(9019):7-12. doi: 10.1016/s0140-6736(96)02149-6 [DOI] [PubMed] [Google Scholar]
- 8.Siebels J, Kuck KH. Implantable cardioverter defibrillator compared with antiarrhythmic drug treatment in cardiac arrest survivors (the Cardiac Arrest Study Hamburg). Am Heart J. 1994. Apr;127(4 Pt 2):1139-44. doi: 10.1016/0002-8703(94)90101-5 [DOI] [PubMed] [Google Scholar]
- 9.Connolly SJ, Camm AJ, Halperin JL, et al.; PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med. 2011. Dec 15;365(24):2268-76. doi: 10.1056/NEJMoa1109867 [DOI] [PubMed] [Google Scholar]
- 10.Torp-Pedersen C, Moller M, Bloch-Thomsen PE, et al. Dofetilide in patients with congestive heart failure and left ventricular dysfunction. Danish Investigations of Arrhythmia and Mortality on Dofetilide Study Group. N Engl J Med. 1999. Sep 16;341(12):857-65. doi: 10.1056/NEJM199909163411201 [DOI] [PubMed] [Google Scholar]
- 11.Wazni OM, Dandamudi G, Sood N, et al.; STOP AF First Trial Investigators. Cryoballoon Ablation as Initial Therapy for Atrial Fibrillation. N Engl J Med. 2021. Jan 28;384(4):316-324. doi: 10.1056/NEJMoa2029554 [DOI] [PubMed] [Google Scholar]
- 12.Andrade JG, Wells GA, Deyell MW, et al.; EARLY-AF Investigators. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N Engl J Med. 2021. Jan 28;384(4):305-315. doi: 10.1056/NEJMoa2029980 [DOI] [PubMed] [Google Scholar]
- 13.Andrade JG, Deyell MW, Macle L, et al.; EARLY-AF Investigators. Progression of Atrial Fibrillation after Cryoablation or Drug Therapy. N Engl J Med. 2022. Nov 7. doi: 10.1056/NEJMoa2212540. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Anguera I, Garcia-Alberola A, Dallaglio P, et al. Shock Reduction With Long-Term Quinidine in Patients With Brugada Syndrome and Malignant Ventricular Arrhythmia Episodes. J Am Coll Cardiol. 2016. Apr 5;67(13):1653-1654. doi: 10.1016/j.jacc.2016.01.042 [DOI] [PubMed] [Google Scholar]
- 15.Singh BN, Singh SN, Reda DJ, et al.; Sotalol Amiodarone Atrial Fibrillation Efficacy Trial Investigators. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005. May 5;352(18):1861-72. doi: 10.1056/NEJMoa041705 [DOI] [PubMed] [Google Scholar]
- 16.Le Heuzey JY, De Ferrari GM, Radzik D, Santini M, Zhu J, Davy JM. A short-term, randomized, double-blind, parallel-group study to evaluate the efficacy and safety of dronedarone versus amiodarone in patients with persistent atrial fibrillation: the DIONYSOS study. J Cardiovasc Electrophysiol. 2010. Jun 1;21(6):597-605. doi: 10.1111/j.1540-8167.2010.01764.x [DOI] [PubMed] [Google Scholar]
- 17.Santangeli P, Di Biase L, Burkhardt JD, et al. Examining the safety of amiodarone. Expert Opin Drug Saf. 2012. Mar;11(2):191-214. doi: 10.1517/14740338.2012.660915 [DOI] [PubMed] [Google Scholar]
- 18.January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2019. Jul 9;74(1):104-132. doi: 10.1016/j.jacc.2019.01.011 [DOI] [PubMed] [Google Scholar]
- 19.Sapp JL, Wells GA, Parkash R, et al. Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016. Jul 14;375(2):111-21. doi: 10.1056/NEJMoa1513614 [DOI] [PubMed] [Google Scholar]
- 20.Swedberg K, Komajda M, Bohm M, et al.; SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010. Sep 11;376(9744):875-85. doi: 10.1016/S0140-6736(10)61198-1 [DOI] [PubMed] [Google Scholar]
- 21.Koruth JS, Lala A, Pinney S, Reddy VY, Dukkipati SR. The Clinical Use of Ivabradine. J Am Coll Cardiol. 2017. Oct 3;70914:1777-1784. doi: 10.1016/j.jacc.2017.08.038 [DOI] [PubMed] [Google Scholar]
- 22.Stambler BS, Dorian P, Sager PT, et al. Etripamil Nasal Spray for Rapid Conversion of Supraventricular Tachycardia to Sinus Rhythm. J Am Coll Cardiol. 2018. Jul 31;72(5):489-497. doi: 10.1016/j.jacc.2018.04.082 [DOI] [PubMed] [Google Scholar]
- 23.Teo KK, Mitchell LB, Pogue J, Bosch J, Dagenais G, Yusuf S, HOPE Investigators. Effect of ramipril in reducing sudden deaths and nonfatal cardiac arrests in high-risk individuals without heart failure or left ventricular dysfunction. Circulation. 2004. Sep 14;110(11):1413-7. doi: 10.1161/01.CIR.0000141729.01918.D4 [DOI] [PubMed] [Google Scholar]
- 24.Al-Gobari M, El Khatib C, Pillon F, Gueyffier F. β-Blockers for the prevention of sudden cardiac death in heart failure patients: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2013. Jul 13;13:52. doi: 10.1186/1471-2261-13-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bapoje SR, Bahia A, Hokanson JE, et al. Effects of mineralocorticoid receptor antagonists on the risk of sudden cardiac death in patients with left ventricular systolic dysfunction: a meta-analysis of randomized controlled trials. Circ Heart Fail. 2013. Mar;6(2):166-73. doi: 10.1161/CIRCHEARTFAILURE.112.000003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohde LE, Chatterjee NA, Vaduganathan M, et al. Sacubitril/Valsartan and Sudden Cardiac Death According to Implantable Cardioverter-Defibrillator Use and Heart Failure Cause: A PARADIGM-HF Analysis. JACC Heart Fail. 2020. Oct;8(10):844-855. doi: 10.1016/j.jchf.2020.06.015 [DOI] [PubMed] [Google Scholar]
- 27.Curtain JP, Docherty KF, Jhund PS, et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J. 2021. Sep 21;42(36):3727-3738. doi: 10.1093/eurheartj/ehab560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pandey AK, Okaj I, Kaur H, et al. Sodium-Glucose Co-Transporter Inhibitors and Atrial Fibrillation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J Am Heart Assoc. 2021. Sep 7;10(17):e022222. doi: 10.1161/JAHA.121.022222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade JG, Deyell MW, Verma A, et al. Association of Atrial Fibrillation Episode Duration With Arrhythmia Recurrence Following Ablation: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2020. Jul 1;3(7):e208748. doi: 10.1001/jamanetworkopen.2020.8748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018. Oct;15(10):e190-e252. doi: 10.1016/j.hrthm.2017.10.035 [DOI] [PubMed] [Google Scholar]
- 31.Arenal A, Avila P, Jimenez-Candil J, et al. Substrate Ablation vs Antiarrhythmic Drug Therapy for Symptomatic Ventricular Tachycardia. J Am Coll Cardiol. 2022. Apr 19;79(15):1441-1453. doi: 10.1016/j.jacc.2022.01.050 [DOI] [PubMed] [Google Scholar]
- 32.Tung R, Xue Y, Chen M, et al. First-Line Catheter Ablation of Monomorphic Ventricular Tachycardia in Cardiomyopathy Concurrent with Defibrillator Implantation: The PAUSE-SCD Randomized Trial. Circulation. 2022. May 4;145:1839-1849. doi: 10.1161/CIRCULATIONAHA.122.060039 [DOI] [PubMed] [Google Scholar]
- 33.Della Bella P, Baratto F, Vergara P, et al. Does Timing of Ventricular Tachycardia Ablation Affect Prognosis in Patients With an Implantable Cardioverter Defibrillator? Results From the Multicenter Randomized PARTITA Trial. Circulation. 2022. Jun 21;145(25):1829-1838. doi: 10.1161/CIRCULATIONAHA.122.059598 [DOI] [PubMed] [Google Scholar]
- 34.Yoo S, Geist GE, Pfenniger A, Rottmann M, Arora R. Recent advances in gene therapy for atrial fibrillation. J Cardiovasc Electrophysiol. 2021. Oct;32(10):2854-2864. doi: 10.1111/jce.15116 [DOI] [PMC free article] [PubMed] [Google Scholar]


