Abstract
Gonococcal entry into primary human urethral epithelial cells (HUEC) can occur by macropinocytosis. Scanning and transmission electron microscopy revealed lamellipodia surrounding gonococci, and confocal laser scanning microscopy analysis showed organisms colocalized with Mr 70,000 fluorescein isothiocyanate-labeled dextran within the cells. Phosphoinositide 3-kinase inhibitors and an actin polymerization inhibitor prevented macropinocytic entry of gonococci into HUEC.
Neisseria gonorrhoeae colonizes and invades the reproductive mucosal epithelium as well as mucosal epithelial cells from other tissues. Several groups have evaluated gonococcal internalization in primary and immortalized cell systems. Human adenocarcinoma endometrial cells were shown to possess structures with the appearance of coated pits, characteristic of clathrin, adjacent to gonococci interacting with the cell surface (13). Internalization of gonococci into Chang conjunctival epithelial cells has been shown to occur by actin-mediated receptor-dependent endocytosis and to be insensitive to inhibitors of clathrin-mediated endocytosis (8). Mosleh et al. have described the interaction of gonococci with human ureteral tissue explants and have shown tight associations between the cells of the explant and the organisms during internalization, suggestive of a receptor-mediated process (11). We have previously evaluated the interactions of gonococci with human urethral epithelial cells (HUEC) and have reported actin-dependent receptor-mediated invasion in this primary cell culture system (6, 9). Our previous studies have also suggested that clathrin-dependent mechanisms are operative in internalization of gonococci in the cells of infected patients (1).
Endocytosis is a mechanism by which cells take in nutrients and regulate the expression of molecules on the cell surface. The classic endocytotic pathway is clathrin-mediated receptor-dependent endocytosis. Cells can also internalize extracellular material by a process termed macropinocytosis (14). This process involves the actin-dependent formation of lamellipodia, sheet-like plasma membrane extensions supported by a web of actin filaments. Macropinocytosis, as a mechanism of bacterial invasion, has been evaluated in pathogens such as Salmonella enterica serovar Typhimurium, Shigella flexneri, and Haemophilus influenzae (3–5, 7, 10). The entry of both S. enterica serovar Typhimurium and S. flexneri into nonprofessional phagocytic cells involves activation of the bacterial type III secretion system upon cell contact (7). Virulence proteins secreted into host cells stimulate extensive membrane ruffling, which is a form of macropinocytosis. N. gonorrhoeae has not previously been shown to express a type III secretion system or to invade epithelial cells by a process involving this form of membrane ruffling. The macropinocytosis associated with nontypeable H. influenzae invasion of human airway epithelial cells is less extensive than that seen with the ruffling process in Salmonella and Shigella infections. It involves the fusion of several lamellipodia around a single bacterium (10). The purpose of this report is to describe macropinocytosis as a mechanism of gonococcal entry into HUEC that is similar to that seen during Haemophilus infection of human airway cells.
We have investigated models of gonococcal invasion in HUEC derived from membranous urethral tissue explants obtained from men undergoing radical retropubic prostatectomy for prostate cancer (6, 9). These cells were cultured on various collagen-coated tissue culture plates or on round glass coverslips in prostate epithelial growth medium (Clonetics, San Diego, Calif.), as described previously (6, 9). HUEC were subjected to no more than two passages prior to use.
HUEC were challenged with early log-phase cultures of gonococcal strain VP1 or 1291 (2 × 107 CFU/ml) in the presence or absence of Mr 70,000 fluorescein isothiocyanate (FITC)-labeled dextran (dextran-70,000) (Molecular Probes Inc., Eugene, Oreg.). The gonococci expressed Opa, Pil, and 3F11+ lipooligosaccharide. Prior to some of the challenge studies, HUEC were incubated for 2 to 4 h with either wortmannin (1 μM), LY294002 (50 μM), or cytochalasin D (1.5 μM). Postchallenge at various time points, HUEC were washed with sterile phosphate-buffered saline (PBS) and fixed with either 2% paraformaldehyde (30 min at 25°C) or 2.5% glutaraldehyde (30 min at 25°C) in PBS prior to further processing. Trypan blue (0.02%) uptake was used to evaluate HUEC viability. All experiments were duplicated using HUEC derived from at least three different patients. Identical results were obtained with strains 1291 and VP1.
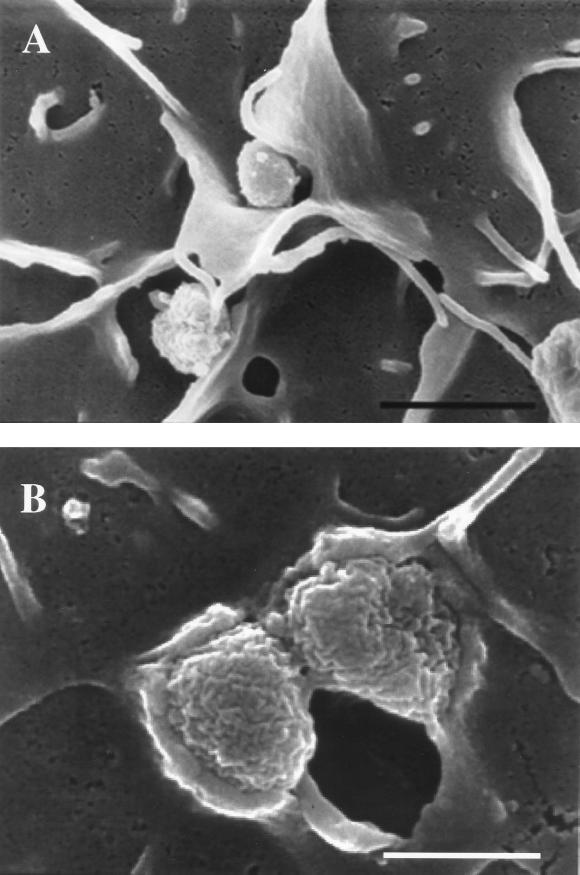
For scanning electron microscopy (SEM), samples were processed and mounted on aluminum stubs, according to previously described methods, prior to viewing with a Hitachi S-4000 scanning electron microscope (Hitachi, Mountain View, Calif.) (10). The results are shown in Fig. 1. At 1 h of infection, lamellipodia surrounded some gonococci on the surface of HUEC. The absence of close association between the eukaryotic cell surface and the membranes of gonococci surrounded by lamellipodia suggests internalization by a non-receptor-mediated process (Fig. 1A). However, the majority of the organisms on the cell surface appeared to be intimately associated with the host cell membrane, indicative of a receptor-mediated process (Fig. 1B).
FIG. 1.
Scanning electron micrographs of gonococci invading HUEC at 1 h postchallenge. (A) Gonococci enveloped by lamellipodia, indicative of macropinocytosis (bar, 2 μm). (B) Gonococci tightly associated with the host cell membrane, characteristic of a receptor-mediated process (bar, 750 nm).
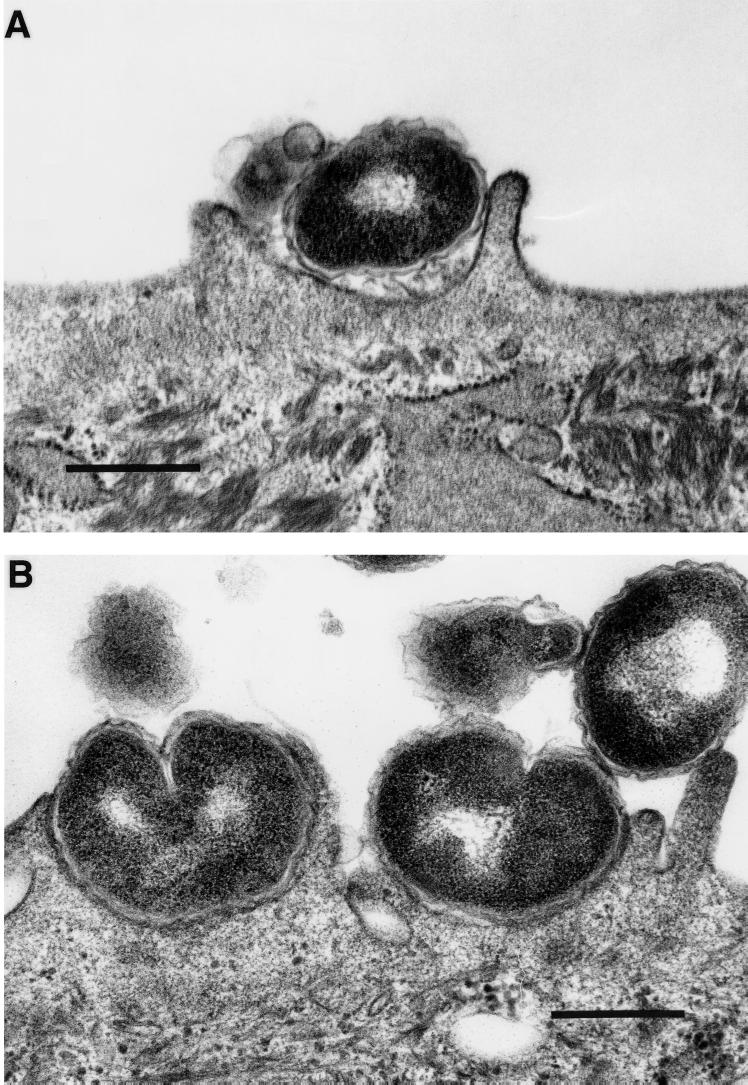
The specimens prepared for transmission electron microscopy (TEM) were treated with 1% osmium tetroxide and dehydrated in a standard ethanol series prior to embedment in Epon acrylic resin and sectioning as previously described (1). The specimens were viewed with an Hitachi H-7000 transmission electron microscope. TEM showed lamellipodia surrounding some gonococci (Fig. 2A). This was in contrast to the majority of the gonococci, where tight bacterial/host cell interactions were observed (Fig. 2B). This is in agreement with the SEM observations.
FIG. 2.
Transmission electron micrographs of gonococci invading HUEC at 1 h postchallenge. (A) Gonococcus with lamellipodium formation beginning to encompass the bacterium (bar, 500 nm). (B) Gonococci in tight association with the host cell membrane (bar, 600 nm).
FITC-labeled dextran-70,000 was used to study macropinocytosis and track macropinocytotic vesicles in eukaryotic cells (12). HUEC and gonococci were counterstained with the nucleic acid stain ethidium bromide (5 μg/ml in PBS for 5 min at 25°C), washed with PBS, and mounted for visualization by confocal laser scanning microscopy (CLSM) using a Bio-Rad MRC 1024 confocal laser scanning microscope (Bio-Rad, Richmond, Calif.). The data are shown in Fig. 3A and B. Dextran was localized inside the cells and was presumed to be in intracellular vesicles. In some of these intracellular vesicles, gonococci were colocalized with dextran, suggesting that the bacteria were taken in with the dextran during a macropinocytotic event. Using N. gonorrhoeae strain 1291 expressing green fluorescent protein (GFP), we viewed eight random fields at ×630 magnification and counted all organisms (green [GFP]) and macropinocytic events (red [Texas red-labeled dextran-70,000]) colocalized and not colocalized. Approximately one macropinocytotic event involving this strain occurred per 20 host epithelial cells. Approximately 2% of the organisms counted in these fields were internalized by macropinocytosis. We observed that the majority of intracellular gonococci were not colocalized with dextran and more likely taken up by other internalization processes. There were also dextran aggregates as well as gonococci on the cell surface that had not yet been internalized.
FIG. 3.
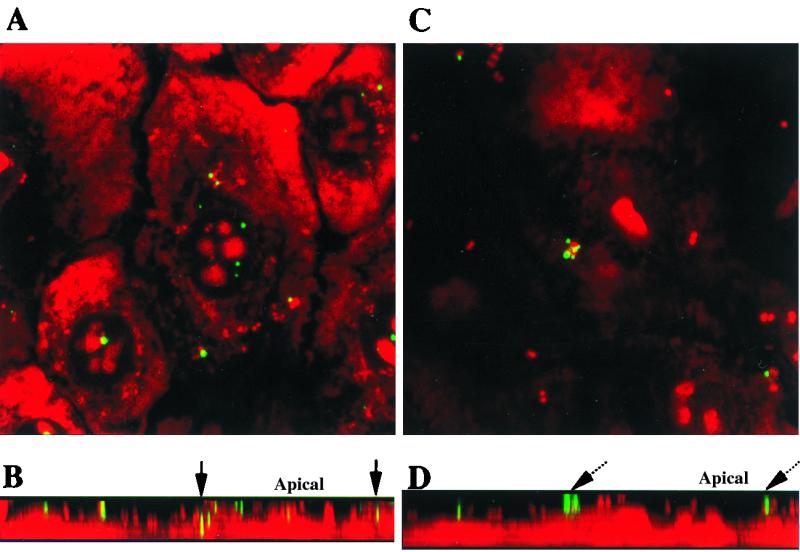
CLSM images of HUEC at 1 h postchallenge with N. gonorrhoeae and FITC-labeled dextran-70,000. Stacked images in the x/y axis (A) and in the z axis (B) show infection in the absence of any inhibitors. Dextrans fluorescing green are observed in continuity with organisms fluorescing red, resulting in yellow fluorescence at sites of colocalization in intracellular vesicles (solid arrow). Panels C and D show HUEC that were incubated with 1 μM wortmannin for 2 h prior to challenge. Stacked images in the x/y axis (C) and in the z axis (D) reveal dextrans and organisms localized to the cell surface with no dextran internalization (dashed arrow). Magnification, ×60.
Figures 3C and D show the results of parallel experiments in which HUEC were incubated with the phosphoinositide (PI) 3-kinase inhibitor wortmannin. PI 3-kinase inhibitors have been shown to prevent complete formation of macropinosomes (2). Dextran was not internalized by HUEC preincubated with wortmannin. These experiments were also performed with LY294002, which is another PI 3-kinase inhibitor, and cytochalasin D, an inhibitor of actin polymerization. These inhibitors also prevented macropinocytosis of gonococci (data not shown). In each of these experiments, there was at least 90% cell viability, as evaluated by trypan blue staining (data not shown).
Our findings suggest that macropinocytosis is one of the mechanisms by which N. gonorrhoeae enters primary HUEC. Based on other studies in our laboratory (data not presented), receptor-mediated endocytosis appears to be the major mechanism for gonococcal entry into these epithelial cells. However, the macropinocytotic process by which cells sample their environment appears to be a means by which a substantial number of gonococci can enter HUEC.
Acknowledgments
We express our appreciation for the assistance by the staff of the Central Microscopy Research Facility of The University of Iowa.
The work described in this paper was supported by NIAID grants AI43924, AI18384, and AI38515.
REFERENCES
- 1.Apicella M A, Ketterer M, Lee F K N, Zhou D, Rice P A, Blake M S. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J Infect Dis. 1996;173:636–646. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- 2.Araki N, Johnson M T, Swanson J A. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clerc P, Sansonetti P J. Entry of Shigella flexeri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987;55:2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Francis C L, Ryan T A, Jones B D, Smith S J, Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993;364:639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-del Portillo F, Finlay B B. Salmonella invasion of nonphagocytic cells induces formation of macropinosomes in the host cell. Infect Immun. 1994;62:4641–4645. doi: 10.1128/iai.62.10.4641-4645.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giardina P C, Williams R, Lubaroff D, Apicella M A. Neisseria gonorrhoeae induces focal polymerization of actin in primary human urethral epithelium. Infect Immun. 1998;66:3416–3419. doi: 10.1128/iai.66.7.3416-3419.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goosney D L, Knoechel D G, Finlay B B. Enteropathogenic E. coli, Salmonella, and Shigella: masters of host cell cytoskeletal exploitation. Emerg Infect Dis. 1999;5:216–223. doi: 10.3201/eid0502.990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grassmé H U C, Ireland R M, van Putten J P M. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harvey H A, Ketterer M R, Preston A, Lubaroff D, Williams R, Apicella M A. Ultrastructural analysis of primary urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect Immun. 1997;65:2420–2427. doi: 10.1128/iai.65.6.2420-2427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ketterer M R, Shao J Q, Hornick D B, Buscher B, Bandi V K, Apicella M A. Infection of primary human bronchial epithelial cells by Haemophilus influenzae: macropinocytosis as a mechanism of airway epithelial cell entry. Infect Immun. 1999;67:4161–4170. doi: 10.1128/iai.67.8.4161-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosleh I M, Boxberger H-J, Sessler M J, Meyer T F. Experimental infection of native human ureteral tissue with Neisseria gonorrhoeae: adhesion, invasion, intracellular fate, exocytosis, and passage through a stratified epithelium. Infect Immun. 1997;65:3391–3398. doi: 10.1128/iai.65.8.3391-3398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oliver J M, Berlin R D, Davis B H. Use of horseradish peroxidase and fluorescent dextrans to study fluid pinocytosis in leukocytes. Methods Enzymol. 1984;108:336–347. doi: 10.1016/s0076-6879(84)08100-3. [DOI] [PubMed] [Google Scholar]
- 13.Shaw J H, Falkow S. Model for invasion of human tissue culture cells by Neisseria gonorrhoeae. Infect Immun. 1988;56:1625–1632. doi: 10.1128/iai.56.6.1625-1632.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swanson J A, Watts C. Macropinocytosis. Trends Cell Biol. 1995;5:424–428. doi: 10.1016/s0962-8924(00)89101-1. [DOI] [PubMed] [Google Scholar]