Abstract
Background
Recently, numerous novel bioactive fungal metabolites have been identified that possess broad therapeutic activities including anti-inflammatory, antibiotic, antioxidant, and antitumor. The fungal mycochemicals as well as extracts have increased the interest of the scientific community in drug discovery research through a combination approach such as, molecular metabolic, pharmacological and computational techniques. Therefore, the natural fungus Aspergillus ficuum (A. ficuum) (FCBP-DNA-1266) was selected for metabolic and pharmacological profiling in this study.
Results
The metabolic profile of A. ficuum was explored for the first time and revealed the presence of bioactive compounds such as choline sulfate, noruron, hydroxyvittatine, aurasperone D, cetrimonium, kurilensoside, heneicosane, nonadecane and eicosane. Similarly, a pharmacological screen of A. ficuum was performed for the first time in in vivo and in vitro models. Interestingly, both the ethyl acetate and n-hexane fractions of A. ficuum were found to be more active against Bacillus subtilis among five tested bacteria with their zone of inhibition (ZOI) values of 21.00 mm ±1.00 and 23.00 mm ±1.00, at a concentration of 150 μgmL-1 respectively. Similarly, a significant decrease (P<0.001) and (P<0.01) in paw edema was observed in A. ficuum-treated animals at doses of 50 and 150 mgkg-1, respectively, reflecting its potent anti-inflammatory effect. Furthermore, the docking results supported the antibacterial and anti-inflammatory effects of A. ficuum. In addition, the crude extract demonstrated no acute toxicity and the highest percent radical scavenging was recorded for both n-hexane and ethyl acetate extracts.
Conclusion
The metabolic profile of A. ficuum indicated the presence of biological relevant compounds. A. ficuum extract exhibited potent antibacterial and anti-inflammatory effects supported by docking results. Furthermore, A. ficuum extract demonstrated the highest percentage of radical scavenging activity along with no acute toxicity.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12866-022-02693-w.
Keywords: Metabolic Profiling, Pharmacological screening, Molecular docking, Aspergillus ficuum
Introduction
Human life is under threat worldwide as mortality increases in developing countries. The spread and development of infectious diseases and drug-resistant pathogens has stimulated the search for new pharmaceutical agents [1]. The development of novel bioactive drugs is not only required against microbial resistance but also very important to overcome degenerative diseases. As a degenerative disease, problems are caused by the reactive oxygen species (ROS) and are detrimental to various biological processes involved in human health [2]. A natural product, schisandrin A, which belongs to the lignan class, significantly attenuated DNA damage and H2O2-induced cytotoxicity by blocking ROS accumulation [3]. Similarly, triterpenoids are diverse molecules and are considered ROS modulators [4]. Since the discovery of penicillin from Penicillium, the exploration of antibiotics and other drugs from microorganisms like fingolimod, cyclosporine, lovastatin, caspofungin, etc. has become popular in the pharmaceutical industry. Fungi have been reported for the production and development of different classes of bioactive metabolites [5].
Recently, numerous new bioactive fungal metabolites have been discovered that have wide-ranging therapeutic properties, such as: anti-inflammatory, antibacterial, antioxidant and anti-cancer properties. The scientific community's interest in drug discovery research has grown as a result of the use of fungal mycochemical chemicals and extracts for various purposes [6].
The genus Aspergillus consists of several fungi known in the pharmaceutical industry for their important therapeutic role. They have diverse chemical applications as shown by genome sequencing. The fungal secondary metabolites are controlled by the genes present in the biosynthetic gene clusters (BGCs), which are chromosomal architectures encoding the required synthetases and/or synthases in the fungal genome [7]. In recent years, more than 315 bioactive metabolites based on the unique chemical structure of fungal species of the genus Aspergillus have been documented [8]. Therefore, A. ficuum is selected for metabolic and pharmacological profiling for the first time in this study. A. ficuum is known as black Aspergilli which belongs to the Niger group of fungi. Since 1923, the Niger group has been used explicitly for commercial purposes in the production of cosmetics, pharmaceutical agents, and citric acid. A large number of secondary metabolites and mycotoxins are reported in niger clad including ochratoxins, fumonisins, naphtha-pyrones, bicoumarins, malformins, asperazines, and alkaloids [9, 10]. Recently, a protein profile study of Aspergillus niger was performed by using advanced technique of nLC-qTOF mass spectrometry; a total number of 108 protein molecules were reported from Aspergillus niger [11].
Our work is related to uncover the untargeted metabolites of less-studied A. ficuum that are biosynthesized in liquid culture through Liquid chromatography quadruple time-of-flight mass spectrometry (LC-QToF-MS) and Gas chromatography-mass spectrometry (GC-MS) analysis. Further, the pharmacological potential such as, anti-inflammatory, acute oral toxicity, antibacterial and free radical scavenging potential of both ethyl acetate and the n-hexane fractions of A. ficuum were also investigated applying in vivo and in vitro models, respectively. In addition, the proposed mechanism through which tentatively identified mycocompounds interact with DNA-polymerase enzyme of the Bacillus subtilis and the inflammation supporting enzyme cyclooxygenase-2 was also investigated by molecular docking analysis.
Materials and Methods
Fungal strains and animals
The fungal strain Aspergillus ficuum (FCBP-DNA-1266) was purchased from the First Culture Bank of Pakistan, University of Punjab, Lahore [12]. Mice of both sexes (BALB/c) 25-35 g were purchased from the National Institute of Health (NIH), Islamabad, Pakistan. They were bread and maintained on a light/dark cycle spanning 12 h/12 h at 22 °C, with water and food provided via ad libitum throughout the study. The Animal Scientific Procedures Act, NIH (the UK, 1986) guidelines were followed throughout the experiments for the care and laboratory use of the animals [13]. The study protocols for in vivo studies were approved from the Ethical Committee FAHV&S, under the number 7196/LM/UoA, The University of Agriculture Peshawar, Pakistan.
Extraction and fractionations
Spores (105 conidia/mL) from the actively growing culture of A. ficuum were transferred to sterilized 30 L of Potato Dextrose Broth (PDB) in multiple 500 mL Erlenmeyer flasks. The flasks were held static at 28 °C ± 2 for 21 days. The mycelium obtained from each flask was dried and crushed into powder form. The powdered mycelia were extracted with ethyl acetate (EtOAc) (3×300 mL) followed by fractionation with n-hexane (3×300 mL) (solvent-solvent extraction). The fractionation was performed using a separatory funnel. The ethyl acetate fraction, which is denser than n-hexane, was removed first, followed by the n-hexane fraction. Both the fractions were condensed under reduced pressure using a rotary evaporator (Buchi, Germany, Model R-300). Both the dried fractions ethyl acetate (2.3 g) and (1.7 g) were stored in the refrigerator at 4 °C for further analysis [14].
LC-QToF-MS analysis
Untargeted metabolic profiling of A. ficuum was performed by liquid chromatography quadruple time-of-flight mass spectrometry (LC-QToF-MS). A crude extract of 0.5 mg was dissolved in 1 mL of methanol and loaded to the column. Before analysis, the crude extract solution was filtered through a 0.45 micron filter. The assembly consisted of a diode array detector, quaternary pump, autosampler cooled chambers (4 °C), degasser, and controlled temperature column (25 °C) coupled to quadruple time-of-flight (QToF) mass spectrometer (MS) (Agilent 6530) with steam ionization source of a dual jet (Agilent Technologies, Australia). The positive ionization mode was acquired with the help of the full scan mass spectrum. C18 (Poroshell column) with the guard column (Agilent Technologies) was used for chromatographic separation. Subsequent separation was done on column (Kinetex HILIC) (Phenomenex, USA). The flow rate of the mobile phase was 0.3 mL min-1. The columns were equilibrated for 40 min before analysis. The gradients of solvent A (LCMS grade) [water (Milli-Q, Germany) + 0.1% formic acid (Sigma-Aldrich)] and solvent B [acetonitrile (95% HPLC grade, Thailand) + 0.1% formic acid (Sigma-Aldrich)] was used for separating the flow rate maintained at 0.3 mL min-1. The solvent gradient was as follows: 5% B for 0.5 min, increasing to 100% B over the next 16.5 min, then holding at 100% B for 23 min, and returning to 5% B from 23.1 min to 29 min. DAD monitored the absorbance in the wavelengths ranging from 210-635 nm. The QToF-MS was standardized at psi 35. Fragmentor and capillary voltage were kept at 135 V and 3500 V, respectively. The injection volume for crude ethyl acetate extract of A. ficuum was 10 μL. Three collision energies were used to record MS/MS spectra by using Auto MS/MS mode. UHPLC-QToF-MS was used for compound quantification. The algorithm set for matching secondary metabolites with Personal Compound Database Library (PCDL) (Agilent Technologies, CA, USA) was entities having similar accurate mass and retention time [15].
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analysis of crude A. ficuum extracts was performed on an Agilent 7890A/5975C. A stock solution of crude extract 10 μgmL-1 was prepared. For analysis, 50 μL of the solution was filtered and loaded to the column using injection. The capillary column was operated in EI mode. An MS column HP-5 (30 m×250 m×0.25 m) was used as the stationary phase. A helium carrier gas with a flow rate of 1.2 mLmin-1 was used as a mobile phase. The peak area expresses the concentration of the chemical components in the extract, while the retention indices help identify the compounds. A different runtime was provided. Mass spectra (EI) were examined at 70 eV keeping the scan range between 35-650 amu. The obtained mass spectrum was coordinated with NIST 08. L mass spectrum libraries [16]. The result was thoroughly inspected and the impurity peaks were removed. Also, the repetition of compounds or match names at different retention times was removed and the obtained data were simplified.
Antibacterial assay
Pathogenic strains, including gram-negative bacteria Escherichia coli, Xanthomonas campestris, and the gram-positive bacteria Clavibacter michiganensis, Bacillus subtilis, Staphylococcus aureus were investigated for antibacterial activity. Fresh bacterial cultures were grown in nutrient broth at 37 °C for 24 h. Sterilized disks were soaked in 60 μL fungal extract of ethyl acetate and n-hexane having a concentration of 150 μgmL-1. Streptomycin was considered a positive control and dimethyl sulfoxide (DMSO) as a negative control. The antimicrobial assay was assessed by measuring the zone of inhibition in millimeters. The extent of inhibition compared to the control was calculated as follows [17].
DPPH radical scavenging assay
Percent radical scavenging activity was evaluated by DPPH (2, 2`-Diphenyl-1-picrylhydrazyl) protocol [18]. Different concentrations of fungal extracts from A. ficuum of 25, 50, 75, and 100 g mL-1 were prepared in DMSO. A DPPH solution (0.1 mM) with a volume of 2.96 mL was prepared in methanol and was added to both fungal extracts. The solution was kept in the dark for half an hour and the absorbance maxima were noted at 517 nm using a spectrophotometer (6800 UV-VIS spectrophotometer). The percentage (%) of DPPH radical scavenging was measured using the following formula:
Where As= sample absorbance
Ac= negative control absorbance
Anti-inflammatory assay
The carrageenan-induced paw edema test was used to evaluate the anti-inflammatory activity of ethyl acetate extract of A. ficuum. The animals were divided into four groups such as, vehicle, vehicle + carrageenan, aspirin + carrageenan, and A. ficuum + carrageenan. The ethyl acetate extract of A. ficuum was orally administered in two different doses of 50 mgkg-1 and 150 mgkg-1 together with aspirin (control) at a dose concentration of 150 mgkg-1 to the respective groups of mice. The doses of A. ficuum tested for anti-inflammatory activity were estimated for consideration from the reported studies [19, 20]. These concentrations were optimized for important variables and assessed to identify the stated values. After one hour, 5 mL of a 1% carrageenan solution was administered to the left hind paw in the subplantar area. The anti-inflammatory effect was assessed using calipers by measuring the paw thickness of the respective animals. This process of measuring paw thickness was repeated every hour for a total of 5 hours of study. The percentage anti-inflammatory effect was evaluated by the following Equation [21].
Acute oral toxicity
Acute oral toxicity of A. ficuum extract was performed on mice. Random groups were formed, each consisting of six animals (25-30 g). They were subjected to 12-hour fasting. Different doses of the extract i.e. 10 mLkg-1, 15 mLkg-1, and 20 mLkg-1 dissolved in normal saline were administered orally via a gavage. The control group received normal saline at a concentration of 10 mLkg-1. All animals were given free access to the water. Feeding and observation were carried out in two phases, viz. initially six hours and then 72 hours for the presence of acute toxicity manifestations. Daily visual observations of growth, general behavior and physical activity, mortality, morbidity, and general health were identified and documented [21]. From the published research [22, 23], estimates were made for the A. ficuum doses tested for acute oral toxicity. These concentrations were evaluated to determine the reported values and optimized for significant variables.
Molecular docking analysis
Mycocompounds tentatively identified through LC-QToF-MS investigation were selected for docking study against DNA-polymerase (PDB ID: 4TR6) enzyme of Bacillus subtilis and the inflammation supporting enzyme cyclooxygenase-2 (COX-2) (PDB ID: 5JVZ) [24, 25]. High-resolution X-rays crystal structures of the selected enzymes were imported from Protein Data Bank (HTTP:// www.rcsb.org/pdb). Further protein molecules were prepared for docking analysis by removing water molecules, adding missing hydrogen atoms, assigning of correct hybridization state to each atom in each residue, and correcting charges applying the preparation program embedded in Molecular Operating Environment (MOE) software. Active sites inside the selected proteins were identified through the active site finder tool of MOE software. Finally, tentatively identified compounds were docked inside the active binding sites of the selected pathogenic proteins employing the docking program of MOE software. Thirty conformations were generated for each compound through selected torsion angles for all the rotatable bonds of the compound. London dock scoring function implanted in MOE was applied to find out the binding energy for each protein-ligand complex system [26, 27].
Results & Discussion
Metabolites profiling through LC-QToF MS analysis
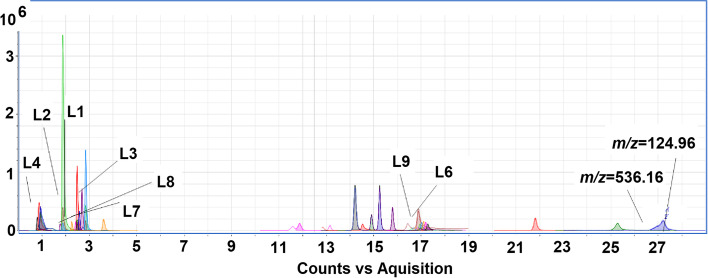
In this study, an untargeted metabolic profiling of A. ficuum was investigated for the first time, showing satisfactory data quality with high specificity and sensitivity (Table S1). In the positive ion mode of the A. ficuum extract, 35 distinct chromatographic peaks were observed (Fig. 1). Twenty-one compounds were identified using METLIN and Personal Compound Database Library (PCDL), while the remaining fourteen peaks remained unidentified. In this research, we have elaborated the 9 major secondary metabolites identified by LC-QToF-MS analysis on the basis of 99% match score of molecular mass with the libraries (Table 1).
Fig. 1.
LC- QToF-MS (ESI+) chromatogram of A. ficuum
Table 1.
LC-QToF-MS analysis of ethyl acetate extract of Aspergillus ficuum
| Compoundsa | Name | Formula | RT | m/z |
|---|---|---|---|---|
| L1 | 11-Hydroxyvittatine | C16 H17 N O4 | 2.485 | 288.1232 |
| L2 | Noruron | C13 H22 N2 O | 1.882 | 223.1806 |
| L3 | Aurasperone D | C31 H24 O10 | 3.601 | 557.1446 |
| L4 | Choline sulfate | C5 H14 N O4 S | 0.947 | 184.0639 |
| L5 | 4-[[5-[[(cyclopentyloxy)carbonyl] amino]-1-methyl-1H-indol-3-yl] methyl]-3-methoxy benzoic acid | C24 H26 N2 O5 | 2.842 | 423.1922 |
| L6 | Kurilensoside F | C33 H58 O11 | 17.108 | 648.432 |
| L7 | 17-phenyl-trinor-PGE2 | C23 H30 O5 | 2.487 | 409.1971 |
| L8 | 4-Benzyloxy-2'-hydroxy-3',4',5',6'-tetramethoxychalcone | C26 H26 O7 | 2.839 | 901.3418 |
| L9 | Cetrimonium | C19 H42 N | 16.888 | 284.3316 |
aCompounds=Ligands
It was also observed that some metabolites were previously only reported as being of plant origin. Most species of fungi have a mutual relationship with plants. According to the study, plant enzymes have the potential to alter fungal gene expression in a mutual relationship [28]. This fungal-host relationship is responsible for the production of different compositions of metabolites [29]. The compound 11-hydroxyvittatin is a phyto-alkaloid and has already been described from many plant species [30]. Benzyloxy-2'-hydroxy-3',4',5',6' tetramethoxychalcone belongs to the class of flavonoids. Many flavonoids are reported from endophytic fungi. Flavonoids from endophytic fungi are known to play an important role in the production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) that help plants protect themselves from microbial attacks [31].
Interestingly, to the best of our knowledge, some metabolites such as, Kurilensoside F, beclomethasone, 4-[[5-[[(cyclopentyloxy)carbonyl]amino]-1-methyl-1H-indol-3-yl]methyl]-3-methoxy-benzoic acid (zafirlukast) and 4,4',6-trimethylangelicin have not previously been reported from microbial or plant origin and are being reported from this species for the first time. This change might be due to geographic origin, ecological, climatic, and nutritional needs [32]. Kurilensoside F is a prominent important biological compound which was first documented from starfish [33]. Two other important anti-asthmatic compounds were beclomethasone and 4-[[5-[[(cyclopentyloxy)carbonyl]amino]-1-methyl-1H-indol-3-yl]methyl]-3-methoxy-benzoic acid (zafirlukast) used as medicines against lung diseases [34, 35]. Additionally, 4,4',6-trimethylangelicin is a furocoumarin that appears to be a promising drug for photochemotherapy of psoriasis [36].
Some peaks with m/z values of 239.2118, 129.1388, 665.2874, 457.1769, 571.1605, 736.4843, 692.4578, 604.4055, 560.3798, 536.1659 and 124 .9642 were not identified by the METLIN and PTDC. The data obtained showed that some chemical constituents of A. ficuum are already been reported from Niger clad. The Niger clade consists of different species such as, A. uvarum, A. japonicus, A. homomorphus, A. aculeatus, A. aculeatinus and A. ellipticus [9]. These compounds include aurasperone D and deoxysappanone B from the naphtho-ɣ-pyrones class and are best known for their potent cytotoxic potential [37]. Given the need to discover new medicines, synthetic and plant-derived medicines are not enough to meet the specific needs of today's world. Therefore, A. ficuum could be a promising candidate to take on its role in the pharmaceutical industry for a future drug discovery program.
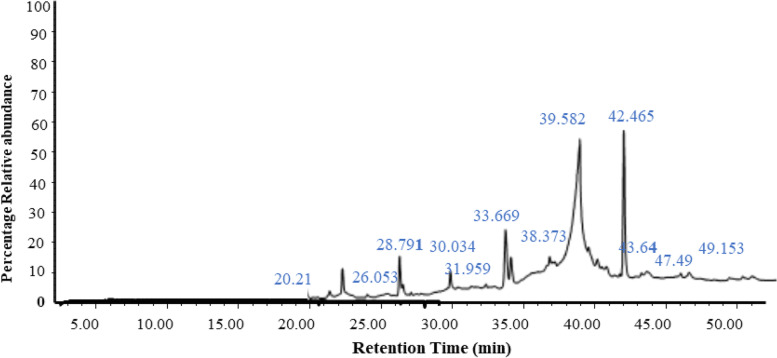
GC-MS analysis
A well-defined total ion chromatogram (TIC) of A. ficuum ethyl acetate extract represents compounds of different classes (Fig. 2) such as, alkanes, alkenes, alcohols, amides, aromatics, and organosulphur (Table S2).
Fig. 2.
GCMS Chromatogram of A. ficuum
Bioactive compounds (Table 2) potentially having broad therapeutic and other beneficial properties present in ethyl acetate extract were tentatively identified by GC-MS analysis. Compounds such as, heneicosane, nonadecane, eicosane and furan derivatives known for their pheromonic, antimicrobial activities and fumigating properties have been identified [38, 39].
Table 2.
GC-MS analysis of ethyl acetate extract of Aspergillus ficuum
| Retention time | Number of compounds | Match name | M.wt (amu) |
|---|---|---|---|
| 21.675 | 1 | 1-Tetradecene | 196.219 |
| 26.053 | 1 | 1-Octadecene | 252.282 |
| 28.791 | 2 | E-15-Heptadecenal | 252.245 |
| Hexadecanoic acid, methyl ester | 270.256 | ||
| 30.034 | 1 | Cycloeicosane | 280.313 |
| 31.959 | 7 | 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | 294.256 |
| 33.669 | 11 | 1-Docosene | 308.344 |
| 1-Eicosene | 280.313 | ||
| 1-Pentadecanethiol | 244.222 | ||
| 1-Nonadecene | 244.222 | ||
| n-Nonadecanol-1 | 284.308 | ||
| Tridecanoic acid, methyl ester | 228.209 | ||
| 9,17-Octadecadienal, (Z) | 264.245 | ||
| Heptadecanoic acid, 16-methyl-, methyl ester | 298.287 | ||
| 1-Heneicosanol | 312.339 | ||
| 1-Hexadecanol | 242.261 | ||
| 11-Tricosene | 322.36 | ||
| 39.582 | 2 | 1,2-Benzenedicarboxylic acid, mono(2-ethylhexyl) ester | 278.152 |
| 38.373 | 1 | 7-Pentadecyne | 208.219 |
A study conducted on 9,12-Octadecadienoic acid found that it inhibited glucose production in H4IIE cells at 25 μM, which is a good sign for antidiabetic studies [40]. Insecticidal activity has been reported from the essential oil containing heneicosane, eicosane and other fatty acid methyl ester [41]. Previously, various compounds were tentatively identified by GC-MS analysis in numerous species of the Aspergillus genus such as, A. clavatonanicus and A. niger [42, 43]. However, this study also reported 26 bioactive molecules in A. ficuum-a specie belonging to the same genus Aspergillus.
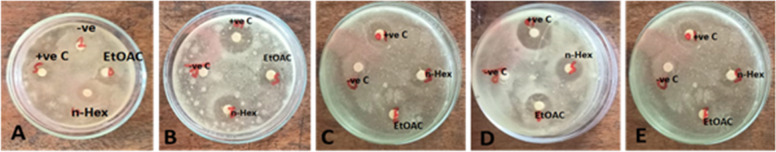
Antibacterial activity
Bioactive mycocompounds were tentatively identified in A. ficuum extract through LC-QToF-MS and GC-MS analysis. Therefore, a preliminary antibacterial screening was performed on crude n-hexane and ethyl acetate fractions of A. ficuum against five standard pathogenic bacteria (Fig. 3). Zones of inhibition values were obtained ranging from 16-23 mm and 13-21 mm for n-hexane and ethyl acetate fractions, respectively (Table 3). Both n-hexane and ethyl acetate fractions of A. ficuum inhibited the growth of all pathogens; they proved to be more effective against Bacillus subtilis with their zone of inhibitions (ZOI) of 21.00 ±1.00 and 23.00 ±1.00, respectively. These results are attributed to the antimicrobial potency of mycocompounds present in both fractions of A. ficuum. The standard streptomycin was found more active against Clavibacter michiganensis with a zone of inhibition of 29.25 mm. As diverse antimicrobial activity has previously been documented from fatty acid methyl ester, the existence of 9,12-Octadecadienoic acid and 9,12-octadecadienoic acid can be linked to the powerful antibacterial impact of n-hexane [44]. The genus Aspergillus is an active biosynthesizer of essential pharmacological and industrial products [45]. Many bioactive compounds such as, aurasperone H, butenolide, aspergivones, asperchondols have been reported from Aspergillus [46, 47]. The ethyl acetate extract of aspergillus niger was also reported to be active against standard bacterial pathogens [48–50].
Fig. 3.
Antibacterial activities of EtOAc and n-Hexane extract of Aspergillus ficuum, A Escherichia coli, B Staphylococcus aureus, C Clavibacter michiganensis, D=Bacillus subtilis, E Xanthomonas campestris
Table 3.
Antibacterial inhibitory activity of fractions of Aspergillus ficuum
| Sample | Conc | Bacterial strains | n-Hexane | Ethyl acetate | Streptomycin |
|---|---|---|---|---|---|
| Zone of Inhibition (mm) | |||||
| Aspergillus ficuum | 10 μgml-1 | Escherichia coli | 16.00 ±1.00 | 13.00 ± 1.00 | 28 ± 0.288 |
| Staphylococcus aureus | 17.67 ±1.53 | 17.00 ±2.00 | 26 ± 0.25 | ||
| Clavibacter michiganensis | 22.67 ±2.52 | 20.67 ±1.15 | 29.25 ± 1.06 | ||
| Bacillus subtilis | 23.00 ±1.00 | 21.00 ±1.00 | 24.66 ± 0.7 | ||
| Xanthomonas campestris | 17.67 ±0.29 | 12.00 ±1.00 | 28 ± 0.7 | ||
DPPH Radical scavenging activity
Taking into account the antioxidant potential of Aspergillus species, the scavenging potency of both n-hexane and ethyl acetate extracts of A. ficuum was evaluated in a concentration-dependent manner using an in vitro model. The results showed that both n-hexane and ethyl acetate fractions of A. ficuum have the potential to scavenge the free radicals at all concentrations ranging between 25-100 μg mL-1 (Table 4).
Table 4.
DPPH radical scavenging activity of Aspergillus ficuum
| Sample | Concentration (μgmL-1) | n-Hexane | Ethyl acetate | Ascorbic acid |
|---|---|---|---|---|
| Percentage Radical Scavenging Activity | ||||
| Aspergillus ficuum | 25 | 5.19±0.13 | 10.82±0.04 | 45.06±0.03 |
| 50 | 16.01±0.08 | 21.64±0.22 | 63.16±0.08 | |
| 75 | 27.05±0.21 | 32.46±0.08 | 77.22±0.12 | |
| 100 | 37.89±0.27 | 43.29±0.12 | 88.02±0.07 | |
Both the n-hexane and ethyl acetate fractions had the highest antioxidant potential at 100 μg mL-1, i.e., 37.89% and 43.29%, respectively. The radical scavenging nature of both extracts can be credited to the presence of mycocompounds that can donate their electron or proton to stabilize the DPPH radical. In addition, the results also demonstrated that the radical scavenging potential of ethyl acetate extract was higher than that of n-hexane; this is attributed to the presence of a higher concentration of radical scavengers in the ethyl acetate extract. Both the ethyl acetate and n-hexane fractions of A. ficuum showed a linear increase with an increase in concentration (Fig. S2). While in the case of standard (ascorbic acid), the highest percent radical scavenging activity was 88.02% at concentration of 100 μg mL-1. Microbes are a great source of antioxidant compounds. Secondary metabolites isolated from Aspergillus species are reported to inhibit lipid peroxidation, linoleic acid oxidation for hydrogen peroxide, ABTS, and DPPH radical scavenging activities [51–54].
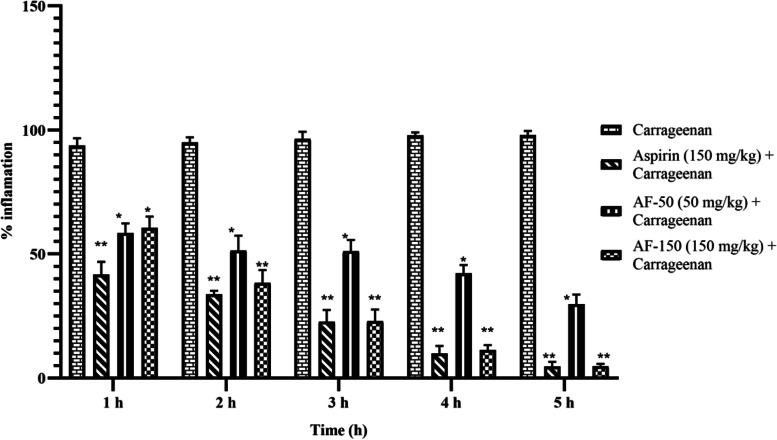
Anti-inflammatory activity
The effects of A. ficuum ethyl acetate extract on temporal inflammation in the hind paw of mice were evaluated and induced by intraplantar injection of carrageenan. The results showed a significant inhibitory, dose-dependent response in paw edema compared to vehicle+carrageenan-treated mice or control groups (Fig. 4, S3). A significant decrease (P < 0.001) and (P < 0.01) in the edematous paw was observed in A. ficuum-treated mice at doses of 50 and 150 mg kg-1 respectively, during the 5 hour study as compared to carrageenan (Table 5).
Fig. 4.
Graphical representation of the anti-inflammatory effect of A. ficuum extract
Table 5.
Anti-inflammatory activity of ethyl acetate extract of Aspergillus ficuum
| Percent Inflammation (%) | ||||
|---|---|---|---|---|
| Time | Carrageenan | Aspirin (150 mg kg-1) + Carrageenan | A. ficuum (50 mg kg-1) + Carrageenan | A. ficuum (150 mg kg-1) + Carrageenan |
| 1 h | 93.644±3.006 | 41.71**±5.1 | 58.44*±3.821 | 60.38*±4.648 |
| 2 h | 94.994±2.048 | 33.81**±1.385 | 51.31*±6.028 | 38.35**±5.212 |
| 3 h | 96.405±2.861 | 22.76**±4.669 | 51.03*±4.563 | 22.93**±4.709 |
| 4 h | 97.723±1.296 | 9.94**±2.966 | 42.27*±3.282 | 11.32**±1.911 |
| 5 h | 97.959±1.662 | 4.68**±1.811 | 29.73*±3.905 | 4.71**±0.998 |
The effects of A. ficuum on paw edema induced by carrageenan. The values are presented as mean ± SEM, the complete randomized design was used and the p-value was calculated using one-way ANOVA. * Represent the p-value <0.001 and ** represent p-value <0.01 as compared to carrageenan. n = 6 mice per group
This clearly shows that A. ficuum extract possesses certain chemical constituents that counteract the release of serotonin, kinins, and histamine. In addition, the anti-inflammatory effect of A. ficuum was also confirmed by an increase in the percentage inhibition of inflammation. Treatment with aspirin (standard) at a dose of 150 mg kg-1 resulted in a significant decrease (P < 0.01) in carrageenan time-induced paw edema. Relatively speaking, the aspirin (standard) and A. ficuum at a dose level of 150 mgkg-1 exhibited a robust anti-inflammatory effect, which is in line with the results of a decrease (P < 0.01) in thickness in paw edema and an increase in the percentage inhibition of inflammation (P < 0.01), related to the vehicle-treated carrageenan injected animals’ group (Fig. 4).
Several secondary metabolites such as phloroglucinol and glyceollin derived from Aspergillus species have been reported for their anti-inflammatory effect [55, 56]. The polyketides isolated from A. rugulosa exhibited anti-inflammatory effects better than the positive control [57]. Similarly, a review of Aspergillus genus metabolites revealed that a rich source of anti-inflammatory metabolites are synthesized by Aspergillus genus species such as, polyketides, terpenoids, butenolides, and alkaloids [58]. This study will help expand the database of Aspergillus anti-inflammatory metabolites and increase knowledge of the mechanism of action of these fungal metabolites.
Acute toxicity
The acute toxicity of A. ficuum extract in mice was studied using an in vivo model (Table 6). Intraperitoneal injection of Territerum from A. terreus demonstrated its lethality at doses of 17.60 1.91, 9.06 1.07 and 6.28 1.56 mgkg-1. Therefore, no mortality was recorded for either the negative control or the mushroom extracts in the 72-hour study at doses of 10, 15 or 20 mlkg-1. Further studies at higher doses are needed to confirm the safe use of A. ficuum extract in therapeutics.
Table 6.
Acute toxicity of ethyl acetate extract of Aspergillus ficuum
| Treatment | Dose (ml kg-1) | Replication | Time Interval (h) | No of the mice died |
|---|---|---|---|---|
| Negative control | 10 | 1 | 72 | 0/5 |
| A. ficuum | 10 | 1 | 72 | 0/5 |
| 15 | 2 | 72 | 0/5 | |
| 20 | 3 | 72 | 0/5 |
The genus Aspergillus is known to produce mycotoxins which are responsible for the lethality of fungal species. Filamentous fungi produce a range of mycotoxins; Ochratoxin A is only considered because of its toxicity [59]. The most potent Aspergillus-derived mycotoxins include ochratoxins, aflatoxins, sterigmatocystin, patulin, gliotoxin, and fumonisins. Mycotoxins are believed to have cytotoxic, nephrotoxic, teratogenic, carcinogenic and genotoxic properties that can lead to immunocompromised conditions, renal dysfunction and liver cancer [23]. The biological activity of A. ficuum without toxic effects indicates that this fungus has the potential to be used as an active pharmaceutical ingredient in future drug discovery.
Docking studies
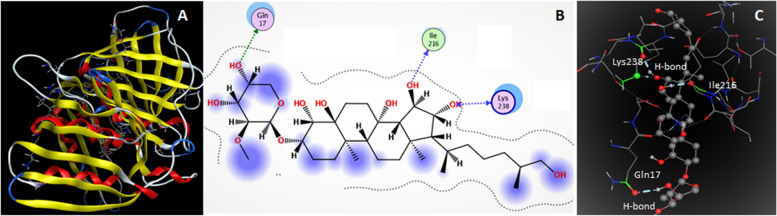
For supporting antibacterial and anti-inflammatory activities, tentatively identified mycocompounds and receptor molecules i.e. DNA-polymerase enzyme of Bacillus subtilis and inflammation supporting enzyme namely cyclooxygenase-2 (COX-2) were docked. The DNA-polymerase enzyme mainly catalyzes the process of replication of the genome accurately and efficiently; further it is responsible for the maintenance and transmission of genetic information. COX-2 catalyzes the process of conversion of arachidonic acid to thromboxanes and prostaglandins upon the induction of inflammation that might harm the tissues. Docking results (Table 7) reveal that myco-molecules (L1-L9) show strong interactions with both enzymes 4TR6 and 5JVZ. Among all compounds, ligand L6 (kurilenoside F) has the highest binding energy of -9.9476 Kcalmol-1 and established four H-bonding interactions with DNA-polymerase molecule indicating its strong inhibitory effect (Fig. 5). One H-bond was established between the hydrogen atom of a hydroxyl group of L6 and the carbonyl oxygen of residue GLN17; the hydrogen atom of another hydroxyl group of L6 was involved in the second H-bond with the carbonyl oxygen of residue ILE216. The third H-bond was developed between the hydrogen atom of the third hydroxyl group of L6 and the carbonyl oxygen of residue LYS238; the fourth H-bond was formed between the oxygen atom of the same third hydroxyl group of L6 and the α-hydrogen atom of residue LYS238 (Table S3). L5 showed three physical interactions with the receptor 4TR6 molecule resulting in binding energy of -8.6359 Kcalmol-1 (Fig. S4).
Table 7.
Docking results of tentatively identified compounds (L1-L9) against receptor 4TR6
| Receptors | Ligands | Number of interactions | Binding energy (Kcalmol-1) | Interacting residues |
|---|---|---|---|---|
| 4TR6 |
L1 L2 L3 L4 L5 L6 L7 L8 L9 |
02 01 02 05 03 04 05 03 03 |
-6.2235 -5.7973 -7.3578 -5.5456 -8.6359 -9.9476 -8.4604 -8.2522 -7.0680 |
LYS190, GLN260 ASP18 LEU217, ASP218 ILE216, ASP18, LYS238, ASP218, ASP18 LYS 238, LYS 238, LEU223 ILE216, LYS238, GLN17, LYS238 2ASP18, LYS21, LEU217, ASP218 LEU217, LYS21, ASP218 ILE216, LYS238, ILE216 |
Fig. 5.
(A) 3D structures of 4TR6, (B) 2D and (C) 3D interactions of L6 with DNA-polymerase enzymes of Bacillus subtilis (D stands for dimensional)
Secondary metabolites L7, L8, and L9 displayed 5, 3, and 3 physical interactions with receptor 4TR6 molecule, respectively with their binding energies of -8.4604, -8.2522, and -7.0680 Kcalmol-1, respectively (Fig. S5). These results infer that these ligands also have strong potential for the inhibition of the DNA-polymerase enzyme. Compounds L1, L2, L3, and L4 exhibited 2, 1, 2, and 5 interactions with DNA-polymerase molecules, respectively (Fig. S6). These physical interactions resulted binding energies of -6.2235, -5.7973, -7.3578, and -5.5456 Kcal mol-1, respectively [26, 27].
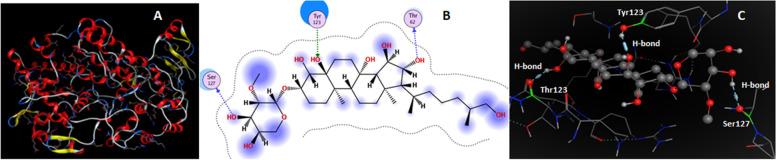
Similarly, compound L6 generated three H-bonding interactions with the COX-2 enzyme resulting the highest binding affinity of -9.9476 Kcal mol-1 among all docked compounds (Fig. 6). One H-bond was developed between the hydrogen atom of a hydroxyl group of L6 and the carbonyl oxygen of SER127; Second H-bond was established between the oxygen atom of a hydroxyl group of L6 and the hydrogen atom of the hydroxyl group of residues TYR123. Carbonyl oxygen of residue THR62 and hydrogen atom of a hydroxyl group of L6 were involved in the third H-bond formation (Table S4). Strong physical interactions and binding affinity make L6 a potential candidate for the inhibition of the COX-2 enzyme. Compounds L7, L8, and L9 have high binding energies of -8.4604, -8.2522, and -7.0680 Kcalmol-1 as well as 4, 2, 1 physical interaction values with COX-2, respectively (Fig. S7). Binding energies of L7, L8, and L9 indicate their high inhibitory effects. Similarly, mycocompounds L1-L5 possessed reasonable physical interactions (Fig. S8) and binding affinities with COX-2 protein (Table 8).
Fig. 6.
(A) 3D structure of COX-2, (B) 2D and (C) 3D interactions of L6 with COX-2
Table 8.
Docking results of tentatively identified compounds (L1-L9) against receptor 5JVZ
| Receptor | Ligands | Number of interactions | Binding energy (Kcal mol-1) |
Interacting residues |
|---|---|---|---|---|
| 5JVZ |
L1 L2 L3 L4 L5 L6 L7 L8 L9 |
01 01 03 07 03 03 04 02 01 |
-6.2235 -5.7973 -8.0984 -5.5456 -8.6359 -9.9476 -8.4604 -8.2522 -7.0680 |
TYR123 SER127 LYS79, ASP126, SER127 CYS41, GLU466, ASN43, 2LYS469, ARG44, GLU466 PHE372, ASP126, SER127 THR62, SER127, TYR123 TYR123, LYS469, SER472, ARG44 SER472, ASP126 TYR123 |
Docking results deduce that mycocompound L6 has the strongest potential for the inhibition of both DNA-polymerase of Bacillus subtilis and inflammation supporting enzyme cyclooxygenase-2. Other compounds also carry reasonable inhibitory effects [26, 27, 60]. Keeping in view their strong physical interactions and binding affinities, tentatively identified compounds support the antibacterial and anti-inflammatory effects of A. ficuum.
Conclusions
The drug discovery procesas been advanced by exploring fungi for their distinctive pharmacological and other beneficial aspects. In this study, we examined the metabolic profile of A. ficuum for the first time using GC-MS and LC-QToF-MS techniques. The metabolic profile indicates the presence of several compounds of pharmacological and biological importance. Similarly, we screened A. ficuum pharmacologically in an in vivo and in vitro model for the first time. Preliminary results revealed that both ethyl acetate and n-hexane fractions significantly inhibit the growth of standard bacterial pathogens. A. ficuum extract also possessed the highest DPPH radicals scavenging effect. Ethyl acetate extract displayed the highest anti-inflammatory effect on paw edema in mice, which was further confirmed by observing an increase in the percentage inhibition of inflammation. Complimentary molecular docking analysis of tentatively identified compounds with the DNA polymerase enzyme of Bacillus subtilis and the pro-inflammatory enzyme COX-2 also supported the antibacterial and anti-inflammatory activities of the A. ficuum extract. Ligand L6 showed strong interactions with both target proteins and could therefore be used as a potent inhibitor against the various pathogenic enzymes. In addition, no acute toxicity was reported for A. ficuum. These results suggest that Aspergillus has the potential to be used as a promising therapeutic for drug discovery in the future.
Supplementary Information
Additional file 1: Supplementary Figure S1. Structures of docked ligands (L1-L9). Figure S2. Percentage antioxidant activity of n-hexane and ethyl acetate fractions. Figure S3. (A) Oral administration of AF extract, (B) Injection of carrageenan in paw, (C, D, E) measurement of Paw edema at regular intervals. Figure S4. (A) 2D and (B) 3D interactions of L5 with DNA-polymerase enzymes of bacillus subtilis. Figure S5. (A, C, E) 2D and (B, D, F) 3D interactions of L7, L8 and L9 with DNA-polymerase enzymes of bacillus subtilis, respectively. Figure S6. (A, C, E, G) 2D and (B, D, F, H) 3D interactions of L1, L2, L3 and L4 with DNA-polymerase enzymes of bacillus subtilis, respectively. Figure S7. (A, C, E) 2D and (B, D, F) 3D interactions of L7, L8, L9 and COX-2, respectively. Figure S8. A, C, E, G) 2D and (B, D, F, H) 3D interactions of L1, L2, L3, L4 and L5 with COX-2, respectively.
Additional file 2: Table S1. Complete detail of LC-MS-QTOF analysis of Aspergillus ficuum.
Additional file 3: Table S2. Complete detail of GC-MS analysis of Aspergillus ficuum.
Additional file 4: Table S3. Nature, distance and energy of interactions of secondary metabolites (L1-L9) with receptor 4TR6.
Additional file 5: Table S4. Nature, distance and energy of interactions of secondary metabolites (L1-L9) with receptor 5JVZ.
Acknowledgements
The authors acknowledge the support of the School of Animal and Veterinary Sciences, Charles Sturt University, Wagga, Australia for LC-QToF-MS analysis.
Authors’ contributions
Z.A.S: conceptualization, methodology; K.K: investigation, supervision, results analysis; H.U.R: writing—review and editing; T.S: formal analysis, investigation, methodology; M.J: Molecular docking, formal analysis, editing; Z.I: formal analysis, investigation, methodology. The authors read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
The animal experiments were reviewed and approved by The Animal Care and Use Committee (approval no. 7196/LM/UoA) of Agriculture University of Peshawar and were carried out in accordance with the guidelines given in Animal Scientific Procedures Act, National Institutes of Health (UK, 1986) for Care and Use of Laboratory Animals [13]. Further this study is reported in accordance with ARRIVE guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Khalid Khan, Email: drkhalidchem@yahoo.com.
Mariusz Jaremko, Email: Mariusz.jaremko@kaust.edu.sa.
References
- 1.Vasundhara M, Reddy MS, Kumar AJN. Elsevier b: Chapter 18-Secondary metabolites from endophytic fungi and their biological activities. New and future developments in microbial biotechnology and bioengineering; 2019. p. 237-258.
- 2.Raunsai M, Wulansari D, Fathoni A, Agusta A. Antibacterial and antioxidant activities of endophytic fungi extracts of medicinal plants from Central Sulawesi. Journal of Applied Pharmaceutical Science. 2018;8(8):69–74.
- 3.Choi YHJB, Pharmacotherapy: Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomedicine & Pharmacotherapy. 2018;106:902-909. [DOI] [PubMed]
- 4.Ling T, Boyd L, Rivas F. Triterpenoids as Reactive Oxygen Species Modulators of Cell Fate. Chemical Research and Toxicology. 2022;35(4):569–84. [DOI] [PMC free article] [PubMed]
- 5.Saxena S, Chhibber M, Singh IP. Fungal bioactive compounds in pharmaceutical research and development. Current Bioactive Compounds. 2019;15(2):211–31.
- 6.Devi R, Kaur T, Guleria G, Rana KL, Kour D, Yadav N, Yadav A, Saxena AJN. Elsevier b: Chapter 9-Fungal secondary metabolites and their biotechnological applications for human health. New and future developments in microbial biotechnology and bioengineering. 2020. p. 147-161.
- 7.Keller NP. Fungal secondary metabolism: regulation, function and drug discovery. Nature Reviews Microbiology. 2019;17(3):167–80. [DOI] [PMC free article] [PubMed]
- 8.Zhang X, Li Z, Gao J. Chemistry and biology of secondary metabolites from Aspergillus genus.The Natural Product Journal. 2018;8(4):275–304.
- 9.Nielsen KF, Mogensen JM, Johansen M, Larsen TO, Frisvad JC. chemistry b: Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Analytical and Bioanalytical Chemistry. 2009;395(5):1225–42. [DOI] [PubMed]
- 10.Frisvad JC, Møller LL, Larsen TO, Kumar R, Arnau JJAM. Biotechnology: Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Applied Microbiology and Biotechnology. 2018;102(22):9481–515. [DOI] [PMC free article] [PubMed]
- 11.Shankar JJB. Insight into the metabolic changes during germination of Aspergillus niger conidia using nLC-qTOF. Biologia. 2022:1-14.
- 12.Bajwa RJM. Scope of first fungal culture bank of Pakistan. Mycopath. 2006;4:41-43.
- 13.Council N. Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press (US); 2011. [PubMed]
- 14.Ali Shah Z, Khan K, Iqbal Z, Masood T, Hemeg HA, Rauf A. Metabolic and pharmacological profiling of Penicillium claviforme by a combination of experimental and bioinformatic approaches. Annals of Medicine. 2022;54(1):2102–14. [DOI] [PMC free article] [PubMed]
- 15.Latif S, Weston PA, Barrow RA, Gurusinghe S, Piltz JW, Weston LA. Metabolic Profiling Provides Unique Insights to Accumulation and Biosynthesis of Key Secondary Metabolites in Annual Pasture Legumes of Mediterranean Origin. Metabolites. 2020;10(7):267. doi: 10.3390/metabo10070267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji M, Yu Z, Chen G, Masood T, Ma F. Chemical Constituents and Biological Functions of Different Extracts of Millettia speciosa Leaves. J Food Nutr Res. 2020;8(9):506–515. doi: 10.12691/jfnr-8-9-7. [DOI] [Google Scholar]
- 17.Klančnik A, Piskernik S, Jeršek B, Možina SS. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J Microbiol Methods. 2010;81(2):121–126. doi: 10.1016/j.mimet.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Wong SP, Leong LP, JHW K. Antioxidant activities of aqueous extracts of selected plants. 2006;99(4):775–83.
- 19.Govindappa M, Naga SS, Poojashri M, Rappa CP. Antimicrobial, antioxidant and in vitro anti-inflammatory activity of ethanol extract and active phytochemical screening of Wedelia trilobata (L.) Hitchc. Journal of Medicinal Plants Research. 2011;3(3):43–51.
- 20.Yin ZN, Wu WJ, Sun CZ, Liu HF, Chen WB, Zhan QP, Lei ZG, Xuan X, Juan J, Kun YJB, et al. Antioxidant and anti-inflammatory capacity of ferulic acid released from wheat bran by solid-state fermentation of Aspergillus niger. Biomed Environ Sci. 2019;32(1):11–21. doi: 10.3967/bes2019.002. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad N, Subhan F, Islam NU, Shahid M, Rahman FU, Fawad K. A novel pregabalin functionalized salicylaldehyde derivative afforded prospective pain, inflammation, and pyrexia alleviating propensities. Arch Pharm. 2017;350(6):e201600365. doi: 10.1002/ardp.201600365. [DOI] [PubMed] [Google Scholar]
- 22.Gbodi T, Nwude N, Aliu Y, Ikediobi C, Chineme CO. Toxicology H. Acute toxicity of crude extracts of Aspergillus quadrilineatus isolated from acha (Digitaria exilis Stapf). Veterinary and human toxicology. 1991;33(1):27–31. [PubMed]
- 23.Ráduly Z, Szabó L, Madar A, Pócsi I, Csernoch L. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Frontiers in Microbiology. 2020;10:2908. [DOI] [PMC free article] [PubMed]
- 24.Wolff P, Amal I, Oliéric V, Chaloin O, Gygli G, Ennifar E, Lorber B, Guichard G, Wagner J, Dejaegere A. Differential modes of peptide binding onto replicative sliding clamps from various bacterial origins. J Med Chem. 2014;57(18):7565–7576. doi: 10.1021/jm500467a. [DOI] [PubMed] [Google Scholar]
- 25.Dong L, Yuan C, Orlando BJ, Malkowski MG, Smith WL. Fatty acid binding to the allosteric subunit of cyclooxygenase-2 relieves a tonic inhibition of the catalytic subunit. J Biol Chem. 2016;291(49):25641–25655. doi: 10.1074/jbc.M116.757310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmad N, Rehman AU, Badshah SL, Ullah A, Mohammad A, Khan K. Molecular dynamics simulation of zika virus NS5 RNA dependent RNA polymerase with selected novel non-nucleoside inhibitors. J Mol Struct. 2020;1203:127428. doi: 10.1016/j.molstruc.2019.127428. [DOI] [Google Scholar]
- 27.Ahmad N, Farman A, Badshah SL, Rahman AU, ur Rashid H, Khan K. Molecular modeling, simulation and docking study of ebola virus glycoprotein. J Mol Graph Model. 2017;72:266–271. doi: 10.1016/j.jmgm.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Hardoim P, Van Overbeek L, Berg G, Pirttilä AM, Compant S, Campisano A, et al. The Hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev. 2015;79:293–320. doi: 10.1128/MMBR.00050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludwig-Müller J. Plants and endophytes: equal partners in secondary metabolite production? Biotechnol Lett. 2015;37(7):1325–1334. doi: 10.1007/s10529-015-1814-4. [DOI] [PubMed] [Google Scholar]
- 30.Masi M, Mubaiwa B, Mabank T, Karakoyun C, Cimmino A, Van Otterlo W, Green I, Evidente A. Alkaloids isolated from indigenous South African Amaryllidaceae: Crinum buphanoides (Welw. ex Baker), Crinum graminicola (I. Verd.), Cyrtanthus mackenii (Hook. f) and Brunsvigia grandiflora (Lindl) South African J Botany. 2018;118:188–191. doi: 10.1016/j.sajb.2018.07.021. [DOI] [Google Scholar]
- 31.George TK, Devadasan D, Jisha M. Chemotaxonomic profiling of Penicillium setosum using high-resolution mass spectrometry (LC-Q-ToF-MS) Heliyon. 2019;5(9):e02484. doi: 10.1016/j.heliyon.2019.e02484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Zhang X, Ye L, Kang Z, Jia D, Yang L, Zhang B. LC-MS-based metabolomic approach revealed the significantly different metabolic profiles of five commercial truffle species. Front Microbiol. 2019;10:2227. doi: 10.3389/fmicb.2019.02227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kicha AA, Ivanchina NV, Kalinovsky AI, Dmitrenok PS, Stonik VA. Steroidal monoglycosides from the Far Eastern starfish Hippasteria kurilensis and hypothetic pathways of polyhydroxysteroid biosynthesis in starfish. Steroids. 2009;74(2):238–244. doi: 10.1016/j.steroids.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 34.Jentzsch NS, Camargos P, Sarinho ES, Bousquet J. Adherence rate to beclomethasone dipropionate and the level of asthma control. Respir Med. 2012;106(3):338–343. doi: 10.1016/j.rmed.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Goverdhan G, Reddy AR, Srinivas K, Himabindu V, Reddy GM. Identification, characterization and synthesis of impurities of zafirlukast. J Pharm Biomed Anal. 2009;49(4):895–900. doi: 10.1016/j.jpba.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 36.Baccichetti F, Carlassare F, Bordinα F, Guiotto A, Rodighiero P, Vedaldi D, Tamaro M, DallAcqua F. 4, 4', 6-Trimethylangelicin, a new very photoreactive and non skin-phototoxic monofunctional furocoumarin. Photochem Photobiol. 1984;39(4):525–529. doi: 10.1111/j.1751-1097.1984.tb03887.x. [DOI] [PubMed] [Google Scholar]
- 37.Bouras N, Mathieu F, Coppel Y, Lebrihi A. Aurasperone F–a new member of the naphtho-gamma-pyrone class isolated from a cultured microfungus, Aspergillus niger C-433. Nat Prod Res. 2005;19(7):653–659. doi: 10.1080/14786410412331286955. [DOI] [PubMed] [Google Scholar]
- 38.Lee S, Hung R, Yap M, Bennett JW. Age matters: the effects of volatile organic compounds emitted by Trichoderma atroviride on plant growth. Arch Microbiol. 2015;197(5):723–727. doi: 10.1007/s00203-015-1104-5. [DOI] [PubMed] [Google Scholar]
- 39.Nemčovič M, Jakubíková L, Víden I, Farkaš V. Induction of conidiation by endogenous volatile compounds in Trichoderma spp. FEMS Microbiol Lett. 2008;284(2):231–236. doi: 10.1111/j.1574-6968.2008.01202.x. [DOI] [PubMed] [Google Scholar]
- 40.Yoshida J, Uesugi S, Kawamura T, Kimura K-i, Hu D, Xia S, et al. (4Z, 15Z)-Octadecadienoic acid inhibits glycogen synthase kinase-3β and glucose production in H4IIE cells. 2017;52(3):295–301. [DOI] [PubMed]
- 41.Funmilola AS, Babatunde OO, Victor O, Segun A, Folake OI, Ganiyu O. Involvement of Cholinergic and Redox Impairments in Insecticidal Properties of Essential Oils from Fertility Tree and Horseradish Tree Leaves in Fruit Fly (Drosophila melanogaster). Journal of Oleo Science. 2020;69(8):941–50. [DOI] [PubMed]
- 42.Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K. Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol. 2007;25(2):221–231. doi: 10.1038/nbt1282. [DOI] [PubMed] [Google Scholar]
- 43.Blumenthal CZ. Production of toxic metabolites in Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei: justification of mycotoxin testing in food grade enzyme preparations derived from the three fungi. Regul Toxicol Pharmacol. 2004;39(2):214–228. doi: 10.1016/j.yrtph.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 44.Agboke AA, Attama AA. Bioactive components and antibacterial activities of n-hexane extract of Moringa oleifera root bark on clinical isolates of methicilin resistant Staphylococcus aureus. International Journal of Current Research in Chemistry and Pharmaceutical Sciences. 2016;3(3):1-9.
- 45.Wang Y-T, Xue Y-R, Liu C-H. A brief review of bioactive metabolites derived from deep-sea fungi. Mar Drugs. 2015;13(8):4594–4616. doi: 10.3390/md13084594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang W, Liao Y, Tang C, Huang X, Luo Z, Chen J, Cai P. Cytotoxic and antibacterial compounds from the coral-derived fungus Aspergillus tritici SP2-8-1. Mar Drugs. 2017;15(11):348. doi: 10.3390/md15110348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li W-t, Luo D, Huang J-N, Ll W, Zhang FG, Xi T, Liao JM, Lu YY. Antibacterial constituents from Antarctic fungus, Aspergillus sydowii SP-1. Nat Prod Res. 2018;32(6):662–667. doi: 10.1080/14786419.2017.1335730. [DOI] [PubMed] [Google Scholar]
- 48.Kalyani P, Hemalatha K. In vitro antimicrobial potential of Aspergillus niger (MTCC-961) Int J Chem Tech Res. 2017;10(4):430–435. [Google Scholar]
- 49.Ratnaweera PB, de Silva ED, Williams DE, Andersen RJ. Antimicrobial activities of endophytic fungi obtained from the arid zone invasive plant Opuntia dillenii and the isolation of equisetin, from endophytic Fusarium sp. BMC Complement Altern Med. 2015;15(1):1–7. doi: 10.1186/s12906-015-0722-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Akinyemi A. Antimicrobial activities of secondary metabolites from fungal endophytes. IOSR J Pharm Biol Sci. 2017;12(6):13–17. [Google Scholar]
- 51.Yen G-C, Chang Y-C, Sheu F, Chiang H-C. Isolation and characterization of antioxidant compounds from Aspergillus candidus broth filtrate. J Agric Food Chem. 2001;49(3):1426–1431. doi: 10.1021/jf001109t. [DOI] [PubMed] [Google Scholar]
- 52.Yen G-C, Wu J-Y. Antioxidant and radical scavenging properties of extracts from Ganoderma tsugae. Food Chem. 1999;65(3):375–379. doi: 10.1016/S0308-8146(98)00239-8. [DOI] [Google Scholar]
- 53.Yagi R, Doi M. Isolation of an antioxidative substance produced by Aspergillus repens. Biosci Biotechnol Biochem. 1999;63(5):932–933. doi: 10.1271/bbb.63.932. [DOI] [PubMed] [Google Scholar]
- 54.Lage GA. Medeiros FdS, Furtado WdL, Takahashi JA, Filho JDdS, Pimenta LPS: The first report on flavonoid isolation from Annona crassiflora Mart. Nat Prod Res. 2014;28(11):808–811. doi: 10.1080/14786419.2014.885518. [DOI] [PubMed] [Google Scholar]
- 55.Cedrón JC, Gutiérrez D, Flores N, Ravelo ÁG, Estévez-Braun A. Synthesis and antimalarial activity of new haemanthamine-type derivatives. Bioorg Med Chem. 2012;20(18):5464–5472. doi: 10.1016/j.bmc.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 56.Carere A, Ortali V, Cardamone G, Morpurgo G. Mutagenicity of dichlorvos and other structurally related pesticides in Salmonella and Streptomyces. Chem Biol Interact. 1978;22(2-3):297–308. doi: 10.1016/0009-2797(78)90134-5. [DOI] [PubMed] [Google Scholar]
- 57.Xu Q, Qiao Y, Zhang Z, Deng Y, Chen T, Tao L, Xu Q, Liu J, Sun W, Ye Y: New polyketides with anti-Inflammatory activity from the Fungus Aspergillus rugulosa. 2021, 12. [DOI] [PMC free article] [PubMed]
- 58.Ferreira MS, Katchborian-Neto A, Cruz JC, de Jesus Nicácio K, Dias DF, Chagas-Paula DA, Soares MG: Systematic Review of Anti-inflammatory Agents from Aspergillus Species. 2021:1-12.
- 59.Moss MO. Mycotoxin review-1. aspergillus and penicillium. Mycologist. 2002;16(3):116–119. doi: 10.1017/S0269915X02003014. [DOI] [Google Scholar]
- 60.Rahman MM, Saha T, Islam KJ, Suman RH, Biswas S, Rahat EU, Hossen MR, Islam R, Hossain MN, Mamun AA. Virtual screening, molecular dynamics and structure–activity relationship studies to identify potent approved drugs for Covid-19 treatment. J Biomol Struct Dyn. 2021;39(16):6231–6241. doi: 10.1080/07391102.2020.1794974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplementary Figure S1. Structures of docked ligands (L1-L9). Figure S2. Percentage antioxidant activity of n-hexane and ethyl acetate fractions. Figure S3. (A) Oral administration of AF extract, (B) Injection of carrageenan in paw, (C, D, E) measurement of Paw edema at regular intervals. Figure S4. (A) 2D and (B) 3D interactions of L5 with DNA-polymerase enzymes of bacillus subtilis. Figure S5. (A, C, E) 2D and (B, D, F) 3D interactions of L7, L8 and L9 with DNA-polymerase enzymes of bacillus subtilis, respectively. Figure S6. (A, C, E, G) 2D and (B, D, F, H) 3D interactions of L1, L2, L3 and L4 with DNA-polymerase enzymes of bacillus subtilis, respectively. Figure S7. (A, C, E) 2D and (B, D, F) 3D interactions of L7, L8, L9 and COX-2, respectively. Figure S8. A, C, E, G) 2D and (B, D, F, H) 3D interactions of L1, L2, L3, L4 and L5 with COX-2, respectively.
Additional file 2: Table S1. Complete detail of LC-MS-QTOF analysis of Aspergillus ficuum.
Additional file 3: Table S2. Complete detail of GC-MS analysis of Aspergillus ficuum.
Additional file 4: Table S3. Nature, distance and energy of interactions of secondary metabolites (L1-L9) with receptor 4TR6.
Additional file 5: Table S4. Nature, distance and energy of interactions of secondary metabolites (L1-L9) with receptor 5JVZ.
Data Availability Statement
The datasets generated and/or analyzed during this study are available from the corresponding author upon reasonable request.