Abstract
Chronic fatigue syndrome (CFS) and fibromyalgia (FM) are medically unexplained conditions that share considerable overlapping symptoms, including sleep-related complaints. However, differences between the two conditions have been reported, and we hypothesized that dynamic aspects of sleep, recently attracting scientific interests, would be different in the two groups of patients. We thus study transition probabilities between sleep stages of CFS patients with or without FM. Subjects were 26 healthy controls, 14 CFS patients without FM (CFS alone) and 12 CFS patients with FM (CFS+FM) – all women. We studied transition probabilities between sleep stages (waking, REM sleep and Stage I, Stage II and slow-wave sleep (Stage III+IV)). We found that probabilities of transition from REM sleep to waking were significantly greater in CFS alone than in controls; we have reported previously this sleep disruption as the specific sleep problem for CFS alone [Kishi et al., 2008]. Probabilities of transitions from waking, REM sleep and Stage I to Stage II, and those from slow-wave sleep to Stage I, were significantly greater in CFS+FM than in controls; the former might indicate increased sleep pressure in CFS+FM and the latter may be the specific sleep problem of CFS+FM. These results suggest that CFS and FM are different illnesses associated with different problems of sleep regulation.
I. INTRODUCTION
Chronic fatigue syndrome (CFS) is a medically unexplained condition characterized by persistent or relapsing fatigue lasting at least 6 months, which substantially interferes with normal activity [1]. In addition to severe fatigue, one of the symptoms used for diagnosing CFS is “unrefreshing sleep”, and this sleep-related problem is the most common complaint among CFS patients [2]. Fibromyalgia (FM) is a medically unexplained illness characterized by four quadrant pain and multiple tender points [3], and frequently occurs in conjunction with CFS [4]. In fact, CFS and FM share considerable overlapping symptoms, including sleep-related complaints. However, differences between CFS and FM have been reported, and research focusing on uncovering differences between these medically unexplained illnesses should be helpful to understand them, rather than focusing on their similarities [5].
Polysomnographic studies have shown that sleep problems in CFS and FM are quite similar, for instance, alpha-delta sleep, more arousals, reduced sleep efficiency, prolonged sleep onset, increased Stage I sleep, reduced slow-wave sleep, etc. [6]–[9] However, these observations are not consistent between studies for both CFS and FM, and there are even cases not showing any statistical differences in normal sleep parameters between healthy humans and CFS or FM [2], [10]–[12].
Historically, most sleep studies have been performed based upon sleep staging according to the traditional standardized scoring criteria established by Rechtschaffen and Kales [13]. While this methodology has been extremely useful, sleep stage analysis has been limited to simple descriptive statistics, such as total sleep time, sleep efficiency (the percentage of time asleep relative to the time in bed), the number of awakenings, latencies to sleep onset and rapid eye movement (REM) sleep, and the total duration of each sleep stage. Recently, the importance of dynamical aspects of sleep, such as transition probabilities among sleep stages and duration distributions of each sleep stage, has increasingly been pointed out [14]–[16]. It has been shown that the dynamical transition analysis of sleep stages would be useful in obtaining deeper insights into the pathophysiological aspect of sleep regulations [15].
Therefore, we hypothesized that dynamical aspects of sleep, recently attracting scientific interests, would be different in the two groups of patients. We thus studied transition probabilities between sleep stages of CFS patients with or without FM.
II. METHODS
A. Subjects
The subjects were 52 women – 26 healthy controls (age: 38 ± 8 years), 14 CFS patients without FM (CFS alone) (age: 37 ± 9 years) and 12 CFS patients with FM (CFS+FM) (age: 41 ± 6 years). None of these subjects had clinically evident sleep disorders in the form of restless leg syndrome or sleep disturbed breathing. The patients all fulfilled the 1994 case definition for CFS and thus had neither any medical explanation for their symptoms based on history, physical examination and rule out blood tests nor serious psychiatric diagnoses including schizophrenia, eating disorders, substance abuse, or bipolar disorder [1]; of these patients, 14 also fulfilled the American College of Rheumatology criteria (1990) for FM [3]. Controls all reported their health to be excellent or good and had normal exams and normal blood tests. To reduce variability additionally, menstruating subjects were all studied in the follicular phase of their menstrual cycles.
All the subjects gave their informed consent, approved by the New Jersey Medical School’s Institutional Review Board, to participate in this research. Following instructions to refrain from alcohol and caffeine ingestion and avoid engaging in prolonged and/or strenuous exercise in the daytime before study nights, the subjects underwent one night of polysomnographic recording in a quiet, shaded hospital room. The subjects went to bed at their usual bedtime and awoke the next morning between 7:15 and 8:00 A.M.
B. Polysomnography
Sleep was scored every 30 sec by a single scorer according to standard criteria of Rechtschaffen and Kales [13]. Sleep stages are usually scored by dividing a sleep recording into non-overlapping epochs of equal duration, and a single stage is assigned to each epoch. If more than one sleep stage occurred within an epoch, the sleep stage that occupies the greatest portion of the epoch was scored as the stage of the whole epoch.
C. Data Analysis
The stage transition probabilities were calculated between Awake (W), REM sleep (R), Stage I (S1), Stage II (S2) and Stage III+IV (slow-wave sleep; SS). The transition probabilities were calculated both by dividing the number of transitions between stages by the total number of all transitions (the global transition probability: , and by dividing the number of transitions from a specific stage to one of the other stages by the total number of the transitions from that specific stage to another stage (the normalized transition probability: derived from , where {X, Y, I, J} are derived from {W, R, S1, S2 and SS} and NX→Y is the number of transitions from stages X to Y during the whole night sleep [15].
D. Statistical Analysis
Differences in variables between groups were assessed using a Tukey-Kramer procedure for multiple comparisons, which was performed on transition probabilities for subjects who had non-zero normalized transition probabilities. Also, if statisitical significance was not seen due to multiple comparisons, we noted the statistical significance in non-paired t-test. Statistical significance was accepted when P < 0.05.
III. RESULTS
Traditional descriptive statistics of sleep parameters have been published elsewhere [17]. There was no significant difference between CFS alone and CFS+FM in the normal sleep parameters.
As for transition probabilities, global and normalized probabilities of transitions between five sleep stages (W, R, S1, S2 and SS) of healthy controls, CFS alone and CFS+FM are shown in Fig. 1 and Fig. 2, respectively. Both in the global and normalized transition probabilities, the transitions between Stage I and REM sleep (S1 ↔ R) were significantly greater in healthy controls than in both CFS alone and CFS+FM. In the normalized transition probabilities, the transitions from REM sleep to awake (R → W) were significantly greater in CFS alone than in healthy controls. As for patients with CFS+FM, there were many abnormal patterns of transitions showing significant differences from other groups. Both in the global and normalized transition probabilities, the probabilities of transition from slow-wave sleep to Stage I (SS → S1) were significantly greater in CFS+FM than in both healthy controls and CFS alone. Also, both the global and normalized probabilities of transitions from REM sleep to Stage II (R → S2) were significantly greater in CFS+FM than in healthy controls. Normalized probabilities of transitions from awake to Stage II (W → S2) were significantly greater in CFS+FM than in both healthy controls and CFS alone, and transitions from Stage I to Stage II (S1 → S2) were significantly greater in CFS+FM than in healthy controls. Although the global probabilities of transition from slow-wave sleep to awake (SS → W) and Stage II to slow-wave sleep (S2 → SS) were significantly greater in CFS+FM than in healthy controls, the normalized probabilities in those transitions did not differ significantly when the significant level was adjusted by multiple comparisons; but when assessed by non-paired t-test, the normalized probabilities of transitions from slow-wave sleep to awake (SS → W) and Stage II to slow-wave sleep (S2 → SS) were significantly greater in CFS+FM than in healthy controls.
Fig. 1.
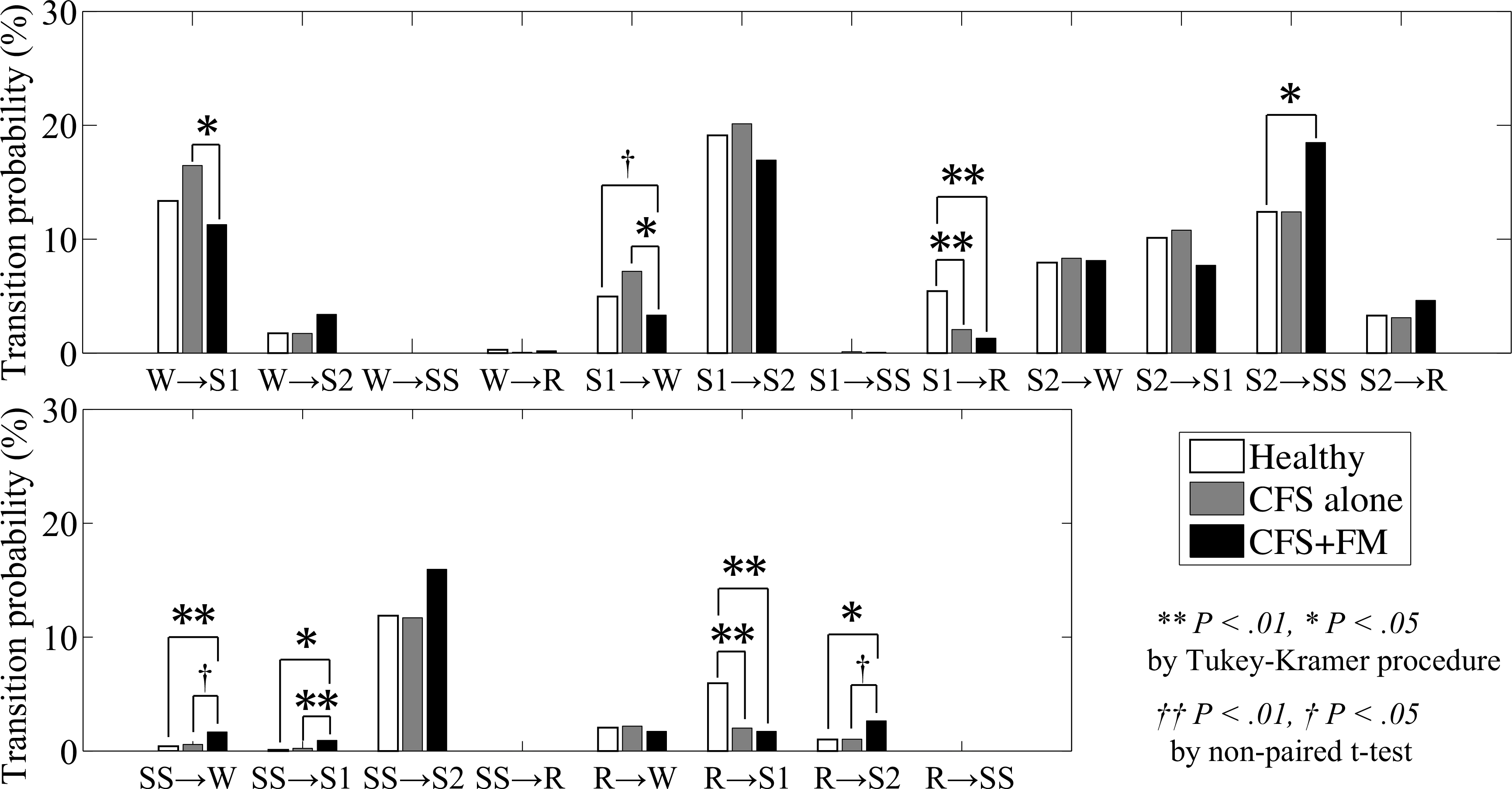
Global transition probabilities between awake (W), REM sleep (R), Stage I (S1), Stage II (S2) and Stage III+IV (SS) for healthy controls (white), CFS alone (gray) and CFS+FM (black). **P < 0.01 and *P < 0.05 by Tukey-Kramer procedure for multiple comparisons. †P < 0.05 by non-paired t-test.
Fig. 2.
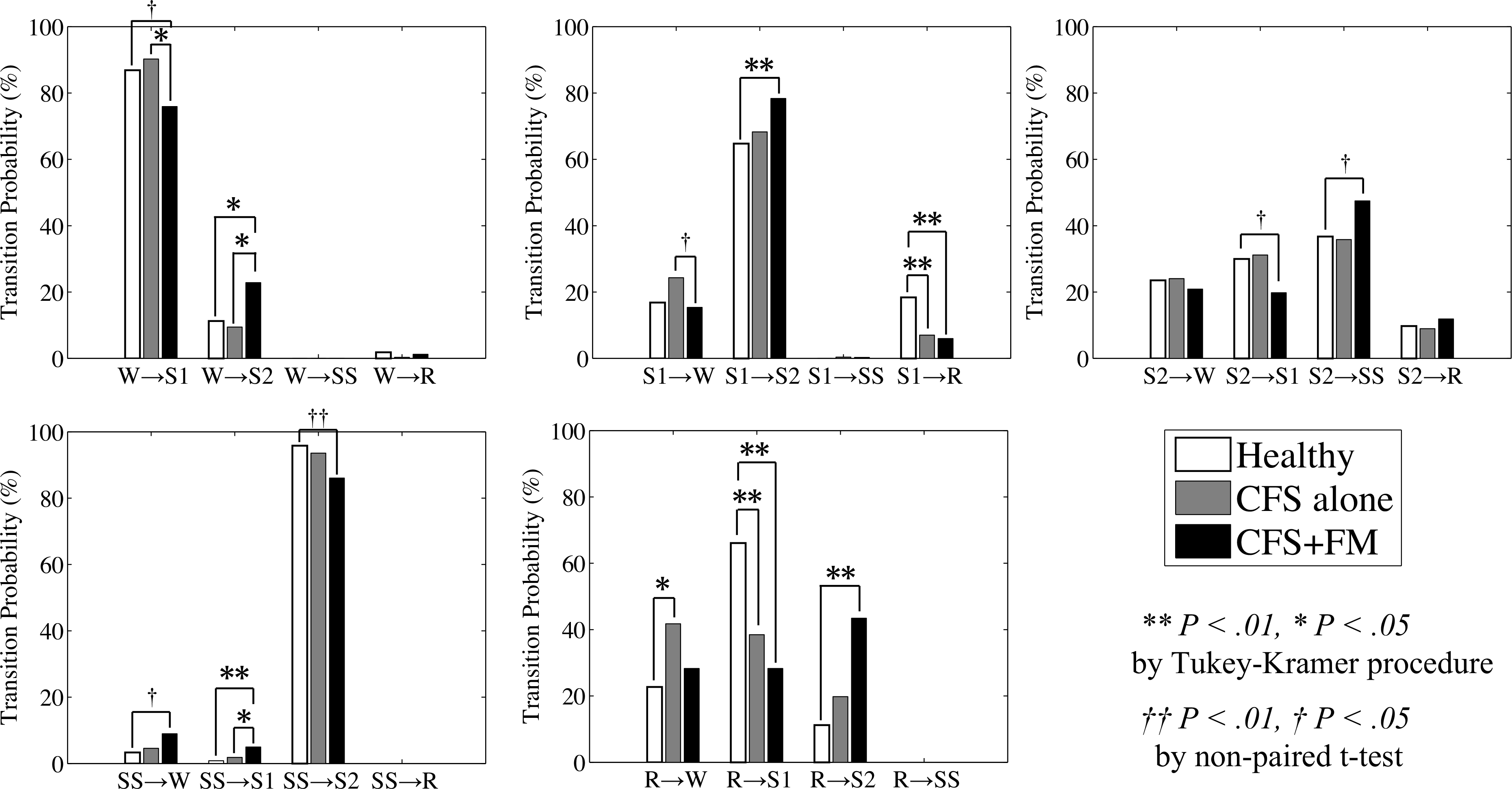
Normalized transition probabilities between awake (W), REM sleep (R), Stage I (S1), Stage II (S2) and Stage III+IV (SS) for healthy controls (white), CFS alone (gray) and CFS+FM (black). **P < 0.01 and *P < 0.05 by Tukey-Kramer procedure for multiple comparisons. ‡P < 0.01 and †P < 0.05 by non-paired t-test.
IV. DISCUSSION
Sleep is a dynamical phenomenon, which emerges from complex interactions among dynamical activities of various neuronal populations, including monoaminergic, cholinergic, orexinergic and GABAergic neuronal systems [18]–[20]. We have recently reported that analyses on dynamical aspects of sleep may provide deeper insights into not only the basic mechanisms of sleep regulations but also the pathophysiological aspect of sleep regulations [15], [21].
Conventional methods to characterize human sleep have been based upon simple descriptive statistics of normal sleep variables. However, those indices did not characterize patients’ sleep in CFS and FM in a consistent manner. Further, such an assessment could not elucidate differences of CFS and FM, whose symptoms are quite similar.
In the present study, we have shown that dynamical aspects of sleep, such as transitions probabilities between sleep stages do differ between CFS alone and CFS+FM. As for transition probabilities, CFS alone has greater probabilities of transitions from REM sleep to awake (R → W) than healthy controls. We have reported previously this sleep disruption as the specific sleep problem for CFS alone [15]. This result could be interpreted as a lower sleep pressure in CFS alone. There is, in fact, a report that sleep latency was not shortened in CFS patients even after the sleep deprivation [22]. In contrast, CFS+FM has greater probabilities of transitions from waking, REM sleep and Stage I to Stage II (W/R/S1 → S2) than healthy controls, suggesting the increased sleep pressure in CFS+FM. Transitions from waking and REM sleep to Stage II (W/R → S2) are unusual transitions in healthy humans [15]. CFS+FM also has greater probabilities of transitions from slow-wave sleep to waking and Stage I (SS → W/S1), suggesting that this may be the specific sleep problem of CFS+FM. This finding might be consistent with a previous study showing that disruptions of slow-wave sleep results in the symptoms resembling FM [23]. Disruptions of deep sleep should certainly lead to an “unrefreshed” state of patients when they wake up. In addition, CFS+FM also has greater probability of transitions from Stage II to slowwave sleep (S2 → SS). This might also indicate the increased sleep pressure of CFS+FM, in addition, that might reflect downregulation of central serotonin in FM patients. There are reports of decreased level of central serotonin in FM [24], [25]. On the other hand, it has been observed that central serotonin responses are upregulated in CFS [5], [10]. We have recently reported that the administration of central monoaminergic (serotonergic and dopaminergic) antagonist alters dynamical sleep stage transitions from Stage II to slowwave sleep (S2 → SS); probability of transition from Stage II to slow-wave sleep were significantly increased when central serotonergic and dopaminergic antagonist was administered [21]. It has also been well known that such monoaminergic systems are closely related with pain modulations [26]. Thus, the imbalance of central monoaminergic (serotonergic) systems in FM patients would lead to abnormalities of pain modulations and sleep regulations.
CFS and FM share considerable overlapping symptoms, including sleep-related complaints. One of the most common of all symptoms in patients with CFS and FM is “unrefreshing” sleep, so the improvement of the sleep quality is quite important for the patients. Although the “unrefreshed” state when patients wake up would superficially be almost the same between CFS and FM, we have shown that underlying dynamical sleep organization which would produce such an undesirable state appears to be different in both cases. We believe that findings in this study should contribute to the understanding of CFS and FM, and also to applications to pharmaceuticals.
In conclusion, in the present study, we have clearly demonstrated that dynamical transition statistics are significantly different between CFS alone and CFS+FM. The fact that the co-exinsting of FM alters dynamical sleep stage transitions of CFS patients suggests that CFS and FM are different illnesses associated with different aspects of sleep regulations.
Acknowledgments
This work was supported in part by NIH AI-54478
Contributor Information
Akifumi Kishi, Educational Physiology Laboratory, Graduate School of Education, The University of Tokyo, Tokyo 113-0033, Japan, Japan Society for the Promotion of Science, Tokyo 102-8472, Japan.
Benjamin H. Natelson, Pain & Fatigue Study Center, Beth Israel Medical Center, New York, NY 10003-3314, USA
Fumiharu Togo, Department of Work Stress Control, National Institute of Occupational Safety and Health, Kawasaki 214-8585, Japan.
Zbigniew R. Struzik, Educational Physiology Laboratory, Graduate School of Education, The University of Tokyo, Tokyo 113-0033, Japan
David M. Rapoport, Department of Medicine, Division of Pulmonary and Critical Care Medicine, NYU School of Medicine, New York, NY 10016, USA
Yoshiharu Yamamoto, Educational Physiology Laboratory, Graduate School of Education, The University of Tokyo, Tokyo 113-0033, Japan.
References
- [1].Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A, International Chronic Fatigue Syndrome Study Group, “The chronic fatigue syndrome: a comprehensive approach to its definition and study”, Ann. Intern. Med, Vol. 12, pp. 1953–959, 1994. [DOI] [PubMed] [Google Scholar]
- [2].Reeves WC et al. , “Sleep characteristics of persons with chronic fatigue syndrome and non-fatigued controls: results from a population-based study”, BMC Neurol, Vol. 6, 41, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wolfe F, et al. , “The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the multicenter criteria committee”, Arthritis. Rheum. Vol. 33 pp. 160–172, 1990. [DOI] [PubMed] [Google Scholar]
- [4].Ciccone DS and Natelson BH, “Comorbit illness in wemen with chronic fatigue syndrome: a test of the single syndrome hypothesis”, Psychosom. Med, Vol. 65, pp.268–275, 2003. [DOI] [PubMed] [Google Scholar]
- [5].Lange G and Natelson BH, “Chronic fatigue syndrome” in Functional pain syndromes: presentation and pathophysiology, Mayer EA and Bushnell MC, Ed. Seattle: IASP Press, 1st ed, 2009. pp. 245–261. [Google Scholar]
- [6].Fischer B, Le Bon O, Hoffmann G, Cluydts R, Kaufman L and De Meirleir K, “Sleep anomalies in the chronic fatigue syndrome. A comorbidity study”, Neuropsychobiology Vol. 35, pp. 115–122, 1997. [DOI] [PubMed] [Google Scholar]
- [7].Sharpley A, Clements A, Hawton K and Sharpe M, “Do patients with “pure” chronic fatigue syndrome (neurasthenia) have abnormal sleep?”, Psychosom. Med, Vol. 59, pp. 592–596, 1997. [DOI] [PubMed] [Google Scholar]
- [8].Van Hoof E, De Becker P, Lapp C, Cluydts R and De Meirleir K, “Defining the occurrence and influence of alpha-delta sleep in chronic fatigue syndrome”, Am. J. Med. Sci, Vol. 333, pp. 78–84, 2007. [DOI] [PubMed] [Google Scholar]
- [9].Moldofsky H, “The significance of the sleeping-waking brain for the understanding of widespread musculoskeletal pain and fatigue in fibromyalgia syndrome and allied syndromes”, Joint Bone Spine, Vol. 75, pp. 397–402, 2008. [DOI] [PubMed] [Google Scholar]
- [10].Afari N and Buchwald D, “Chronic fatigue syndrome: a review”, Am. J. Psychiatry Vol. 160, pp. 221–236, 2003. [DOI] [PubMed] [Google Scholar]
- [11].Fischer B, “Review of clinical and psychobiological dimensions of the chronic fatigue syndrome: differentiation from depression and contribution of sleep dysfunctions”, Sleep Med. Rev, Vol. 3, pp. 131–146, 1999. [DOI] [PubMed] [Google Scholar]
- [12].Chervin RD et al. , “Objective measures of disordered sleep in fibromyalgia”, J. Rheum, Vol. 36, pp. 2009–2016, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rechtschaffen A and Kales A, A manual of standardized terminology, techniques and scoring system for sleep states of human subjects, Washington, DC: US Government Printing Office, 1968. [Google Scholar]
- [14].Comte JC, Ravassard P and Salin PA, “Sleep dynamics: a self-organized critical system”, Phys. Rev. E Stat. Nonlin. Soft. Matter Phys., Vol. 73, 056127, 2006. [DOI] [PubMed] [Google Scholar]
- [15].Kishi A, Struzik ZR, Natelson BH, Togo F and Yamamoto Y, “Dynamics of sleep stage transitions in healthy humans and patients with chronic fatigue syndrome”, Am. J. Physiol. Regul. Integr. Comp. Physiol, Vol. 294, pp. 1980–1987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lo CC, et al. , “Dynamics of sleep-wake transitions during sleep”, Europhys. Lett, Vol. 57, pp. 625–631, 2002. [Google Scholar]
- [17].Togo F, Natelson BH, Cherniack NS, FitzGibbons J, Garcon C and Rapoport DM, “Sleep structure and sleepiness in chronic fatigue syndromewith or without coexisting fibromyalgia”, Arthritis. Res. Ther, Vol. 10, R56, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pace-Schott EF and Hobson JA, “The neurobiology of sleep: genetics, cellular physiology and subcortical networks”, Nat. Rev. Neurosci, Vol. 3, pp. 591–605, 2002. [DOI] [PubMed] [Google Scholar]
- [19].Saper CB, Chou TC and Scammell TE, “The sleep switch: hypothalamic control of sleep and wakefulness”, Trends Neurosci, Vol. 24, pp. 726–731, 2001. [DOI] [PubMed] [Google Scholar]
- [20].Sakurai T, “The neural circuit of orexin (hypocretin): maintaining sleep and wakefulness”, Nat. Rev. Neurosci, Vol. 8, pp. 171–181, 2007. [DOI] [PubMed] [Google Scholar]
- [21].Kishi A, et al. , “Sleep stage transitions in healthy humans altered by central monoaminergic antagonist”, Methods Inf. Med, in press. [DOI] [PubMed] [Google Scholar]
- [22].Nakamura T, Togo F, Cherniack NS, Rapoport DM, Natelson BH, “A subgroup of patients with chronic fatigue syndrome may have a disorder of arousal”, Open Sleep J, Vol. 3, pp. 6–11, 2010. [Google Scholar]
- [23].Moldofsky H, Scarisbrick P, England R and Smythe H, “Musculoskeltal symptoms and non-REM sleep disturbance in patients with “fibrositis syndrome” and healthy subjects”, Psychosom. Med, Vol. 37, pp. 341–351, 1975. [DOI] [PubMed] [Google Scholar]
- [24].Neeck G and Riedel W, “Neuromediator and hormonal perturbations in fibromyalgia syndrome: results of chronic stress?”, Baillière’s Clin. Rheumatol, Vol. 8, pp. 763–775, 1994. [DOI] [PubMed] [Google Scholar]
- [25].Juhl JH, “Fibromyalgia and the Serotonin Pathway”, Altern. Med. Rev, Vol. 3, pp. 367–375, 1998. [PubMed] [Google Scholar]
- [26].Bannister K, Bee LA and Dickenson AH, “Preclinical and early clinical investigations related to monoaminergic pain modulation”, Neurotherapeutics, Vol. 6, pp. 703–712, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


