Abstract
A Vibrio cholerae cytotoxin, designated VcVac, was found to cause vacuolation in Vero cells. It was originally detected in the pathogenic O1 Amazonia variant of V. cholerae and later shown to be produced in environmental strains and some El Tor strains. Comparison of VcVac production in various strains suggested that hemolysin was responsible for the vacuolating phenotype. Genetic experiments established a firm correlation between vacuolation and hemolysin production. The mammalian cell vacuolating activity of the V. cholerae hemolysin is a new property of this protein and points to a previously unknown type of interaction between V. cholerae and its host.
The cholera epidemic in Latin America, from 1991 to 1993, started in Peru and was caused by an El Tor O1 strain. Some clinical strains were obtained in northern Brazil at the beginning of the epidemic. A subgroup within these strains, coming from a cluster of small villages, proved to be different from the prevailing El Tor. DNA fingerprints by the randomly amplified polymorphic DNA (RAPD) method (5) showed a distinct profile; after further characterization, these strains were denominated the Amazonia variant of Vibrio cholerae, a group of O1 pathogenic strains with a similar RAPD pattern that do not belong to the El Tor or classical biotype (3, 4). These strains do not secrete the cholera toxin (i.e., are phenotypically CT−) and do not produce other known virulence factors such as the toxin-coregulated pilus, the thermostable toxin, or the Zot toxin. It was found, however, that they secrete a cytotoxic vacuolating activity.
Vacuolation of mammalian cells in response to bacterial secreted proteins has been described for Helicobacter pylori (14), through the action of the secreted cytotoxin VacA (6). The aerolysin of Aeromonas hydrophila and the hemolysin of Serratia marcescens have also been recently described as causing cell vacuolation (1, 10).
In this paper we describe the vacuolating phenotype of V. cholerae supernatants on Vero mammalian cells. We also show the results of screening a number of V. cholerae strains for the presence of this vacuolating cytotoxin and demonstrate a correlation of this activity with the expression of hemolysin.
The vacuolating phenotype.
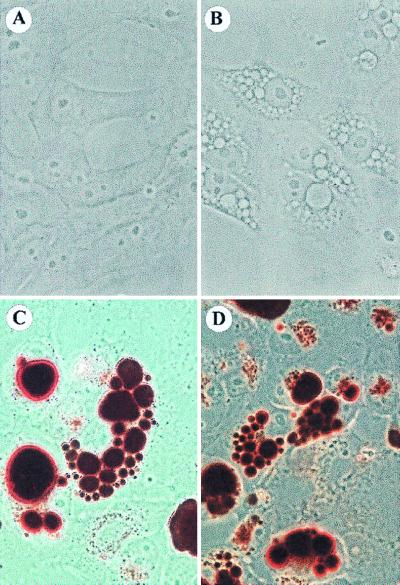
V. cholerae Amazonia supernatants exhibited a cytotoxic and vacuolating activity when applied to Vero cells. The vacuoles started to appear at 2 h after addition of the supernatant and were quantified at 24 h. Vacuoles became very large, taking almost all of the cell space, with the nucleus still clearly visible. Figure 1B shows vacuolated cells, photographed under phase-contrast microscopy after fixation (22), compared to untreated cells (Fig. 1A). Under vacuolating conditions, cells retained their shape and adherence. The number of vacuoles per cell varied, from one large vacuole to 40 or 50 small and some large ones.
FIG. 1.
Vacuolation of mammalian cells caused by a cytotoxin secreted by V. cholerae. (A) Control normal Vero cells. (B) Vacuolated cells after treatment with the supernatant of a V. cholerae strain (Amazonia), seen by phase-contrast microscopy (magnification, ×320). (C and D) Vacuolated cells after a neutral red uptake assay, done after 24 h of incubation with the cytotoxin. The weak base neutral red is taken up by the vacuoles.
Vero (green monkey kidney) cells were grown in minimum essential medium α medium (GIBCO/BRL) in 80-cm2 flasks, at 37°C, under an atmosphere of 5% CO2. Bacterial growth conditions used for the cytotoxin production were CAYE medium (26) at 30°C for 18 to 24 h, in small volumes, typically 10 ml in 50-ml flasks. Bacteria were then centrifuged for 30 min at 14,500 rpm (30,000 × g in a JA-17 rotor). The supernatants were filtered through 0.2-μm-pore-size Acrylex low-protein-binding filters (Millipore). They were kept frozen at −70°C without a noticeable loss of activity. Concentrated preparations of the vacuolating toxin were obtained by precipitation with a final concentration of 50% ammonium sulfate. Table 1 shows the characteristics and vacuolating activity titers of the strains used.
TABLE 1.
Characteristics and effect on Vero cells of strains used
| Strain | Descriptiona | VcVac | Titerb | No vacuolation |
|---|---|---|---|---|
| V. cholerae | ||||
| O1 Amazonia | 20/20c | |||
| FG1066, FG1068 and 18 others | Amazonas, Brazil, 91-92 | + | ||
| FG1066 | S, 1/640; C, 1/10,240 | |||
| O1 El Tor | 5/18 | 13/18 | ||
| E7946 | Ogawa, Bahrain, 78 | S, C | ||
| ANCO223 | Ogawa, Peru | S, C | ||
| 0872 | Inaba, Peru | + | S, 1/120; C, 1/2,560 | |
| N16961 | Inaba, Bangladesh, 75 | + | S, 1/30; C, 1/320 | |
| N16117 | Ogawa, Bangladesh | S, C | ||
| P27459 | Inaba, Bangladesh, 76 | S, C | ||
| 4 strains | Brazil, 92-94 | S, C | ||
| T19479 | Bangladesh, 79 | S, C | ||
| 26-3 | Ogawa, Philippines, 61 | + | S, 1/60; C, 1/480 | |
| 30167 | Bangladesh, 76 | S, C | ||
| 62746 | Bangladesh, 76 | S, C | ||
| N.2 | Inaba, China, 86 | + | S, 1/20; C, 1/160 | |
| N.5 | Ogawa, China, 77 | + | S, 1/960; C, >1/2,560 | |
| 62-6-91 | Tanzania | S, C | ||
| LA-M-644 | Nigeria | S, C | ||
| O1 Gulf of Mexico El Tor | 3/3 | |||
| E506 | Texas | + | S, 1/20; C, 1/80 | |
| SGN7277 | Sewage, Louisiana, 80 | + | S, 1/20 | |
| 4808-78 | Patient, Louisiana, 78 | + | S, 1/20 | |
| O1 classical | 6/6 | |||
| 0395 | India, 64 | S, C | ||
| AMS 20-A-73 (VRL3) | China, 45 | S, C | ||
| N32089 | Bangladesh, 82 | S, C | ||
| N19073 | Bangladesh, 82 | S, C | ||
| N19812 | Bangladesh, 82 | S, C | ||
| N22646 | Bangladesh, 82 | S, C | ||
| Environmental, non-O1, non-0139 | 7/10 | 3/10 | ||
| FG1130 | Water, RJ, Brazil, 97 | + | S, 1/320 | |
| FG1131 | Water, RJ, Brazil, 97 | + | S, 1/1920 | |
| FG1132 | Mollusk, RJ, Brazil, 97 | S, C | ||
| FG1133 | Mollusk, RJ, Brazil, 97 | S, C | ||
| FG1134 | Mollusk, RJ, Brazil, 97 | + | S, 1/20 | |
| FG1138 | Mollusk, RJ, Brazil, 95 | + | S, 1/80 | |
| FG1139 | Crab, RJ, Brazil, 96 | S, C | ||
| FG1140 | Crab, RJ, Brazil, 97 | + | S, 1/30 | |
| FG1135 | Water, RJ, Brazil, 97 | + | S, 1/40 | |
| FG1137 | Crab, RJ, Brazil, 95 | + | S, 1/240 | |
| E. colid | ||||
| DH5α | ||||
| DH5αλpir | From A. Camilli, Tufts University | |||
| EK322 | MM294/pRK2013 | |||
| VJ737 | MC4100/pKAS32 from R. Taylor, Dartmouth University | |||
| FG1155 | DH5αλpir/pFG300, this work | |||
| FG1221 | DH5α/pFG350, this work | |||
| Other O1 V. cholerae strains | ||||
| JBK56 | Inaba, derived from N16961, Δctx Apr | |||
| JBK70 | Inaba, derived from N16961, Δctx Hgr | |||
| CVD104 | Inaba, N16961 Δctx ΔhlyA | |||
| CVD113 | Ogawa, E7946 Δvirulence cassette ΔhlyA::mer ctxB, Δlec | |||
| FG1106 | FG1066 Smr, this work | |||
| FG1153 | FG1106 hlyA::pFG300, Apr, this work | |||
| FG1173 | Hemolytic revertant of FG1153, this work | |||
| FG1167 | Smr revertant of FG1153, this work | |||
| FG1229 | FG1153/pFG350, this work |
Numerals indicate years (91-92 denotes 1991 to 1992, etc.) of isolation at the location given.
S, filtered supernatant of culture; C, 80-fold volume concentrate prepared from S by precipitating with 50% ammonium sulfate.
Fraction of VcVac-positive strains in each group.
Either strains have not been tested on cells or their results are presented in a separate table.
Cytotoxin quantification.
Vacuolating activity was quantified by two different methods. One was direct observation with an inverted microscope. Vero cells were transferred to 96-multiwell plates (100 μl, 2 × 103 cells/well) and incubated overnight (37°C, 5% CO2). Cytotoxin dilutions in the same medium were prepared in a separate plate, from supernatants or concentrates, starting with a dilution of 1/20 and diluted twofold, generally up to 1/2,560. Cytotoxin dilutions were substituted for the old medium in the multiwell plates and were incubated at 37°C in the CO2 incubator. The dilution with maximal vacuolation at 24 h was taken as a measure of activity. The second quantification method was that of neutral red uptake, extensively used in the study of vacuolation by the VacA cytotoxin of H. pylori (18). Figures 1C and D show examples of cells with the vacuoles filled with neutral red, photographed on a Zeiss Axioplan 2 microscope. The uptake of the weak base neutral red by the vacuoles indicates that they are acidic, as are vacuoles caused by VacA. The two quantification methods agreed with each other, and the dilution with maximal vacuolation was used as a measure for activity comparisons.
High concentrations of toxin, generally from 1/20 to 1/160 dilutions of the supernatants, were lethal to Vero cells, which appeared as round, flat cells, isolated or in groups, that detached from the plastic and could be easily washed away. Intense vacuolation occurred at intermediate concentrations, with no visible effect at lower concentrations. Typical experiments showed maximal vacuolation at a 1/640 dilution of the supernatants. Even more concentrated activity was obtained after ammonium sulfate precipitation of the supernatant proteins (Table 1). The possibility remains that there are two distinct activities, vacuolation and cytotoxicity, but we favor the hypothesis that the vacuolating activity results in cell death at high concentrations, as the hlyA mutants described below are deficient for both vacuolation and cytotoxicity.
The vacuolating activity is protein mediated.
Temperature sensitivity was tested by heating samples of a filtered concentrated supernatant for 30 min at different temperatures. Vacuolating activity was tested as described above. Vacuoles were detected with filtrates subjected to a temperature up to 50°C, although after treatment at this temperature the activity was already reduced about eightfold. With treatment above this temperature (60°C) no vacuolation or cytotoxicity was observed, which represents a decrease in activity of at least 20-fold. Proteinase K sensitivity was also tested. Supernatants were treated with 10 or 25 μg of proteinase K per ml at 37°C and tested at various times. Treatment with 10 μg/ml for 10 min reduced the activity 8-fold, while treatment with 25 μg/ml reduced it 16-fold. Other properties observed were a relative resistance to trypsin (only twofold decrease in activity after treatment with 500 μg/ml for 2 h at room temperature) and retention of the activity in ultrafiltration membranes (Centriplus; Millipore) with a cutoff size of 100 kDa, pointing to a protein or an oligomer with a high molecular weight, which we denote VcVac.
Bafilomycin A sensitivity tests.
Vacuole production by VacA from H. pylori is inhibited by bafilomycin A (18), a specific inhibitor of vacuolar-type H+-ATPases at concentrations below 10 μM. Bafilomycin A was used to determine if the vacuolation caused by the V. cholerae cytotoxin was also inhibited by this compound. Vero cell monolayers were prepared in 96-well plates and incubated overnight as described above. Fresh medium was substituted for the spent medium (100 μl per well), and 1-μl aliquots of various concentrations of bafilomycin A in dimethyl sulfoxide were added. The final concentrations used were 100, 50, 25, and 0 nM. The cells were incubated for 30 min at 37°C; 10 μl from each dilution of the toxin being tested (1/20 to 1/2,560 of supernatants) was then added to the wells, in duplicate, and incubation was continued for 24 h. As a control, supernatants from VacA+ H. pylori were assayed for vacuolation of Vero cells in the presence and absence of bafilomycin A.
Contrary to the results with VacA, VcVac was not inhibited by bafilomycin A. Cell toxicity and vacuolation were seen, in the same amount, in cells treated with the vacuolating toxin of V. cholerae and bafilomycin A and in cells treated only with the cytotoxin (titer of 1/1,280 in both cases). VacA without bafilomycin A presented maximum vacuolation at 1/60 dilution, and with bafilomycin A it did not show any vacuolation (titer, <1/20). Bafilomycin A alone (100 nM) and dimethyl sulfoxide (1 μl) had no visible effect on the cells at the concentrations used. These results indicate a different mode of action of these two toxins.
Presence of the vacuolating activity in other V. cholerae strains.
The 20 Amazonia strains that we tested produce the vacuolating activity. In addition, 37 other strains were tested (Table 1): 18 El Tor, 3 Gulf of Mexico El Tor, 6 classical, and 10 environmental strains from the state of Rio de Janeiro, Brazil (C. Barros and A. Coelho, unpublished data). Vacuolating activity was found in five of the El Tor strains, the three Gulf of Mexico strains, and seven of the environmental strains. All strains positive for vacuolation were known to be hemolytic. This prompted us to test the hypothesis that the hemolysin is involved in the vacuolating phenotype.
El Tor strain N16961, whose genome is being sequenced, has been used in the construction of a series of strains designed as potential cholera vaccines (13). This series of strains (N16961, JBK56, JBK70, and CVD104), each lacking one or more virulence genes, was tested for vacuolating cytotoxic activity. The results with filtrate supernatants and ammonium sulfate concentrates are presented in Table 2. Deletion of the ctx genes did not affect production of the vacuolating cytotoxin. On the other hand, deletion of a region from the hemolysin gene, hlyA, was sufficient to completely inactivate the vacuolating activity in both supernatants and ammonium sulfate concentrates. This result indicates the involvement of the hemolysin in vacuolating activity. Strain CVD113, an hlyA mutant with a deletion in the lecithinase lec gene, was also negative for vacuolating activity.
TABLE 2.
Comparison of vacuolating activities of Amazonia and vaccine strains derived from the El Tor strain N16961
| Fraction | Dilution yielding maximum vacuolating activity
|
||||
|---|---|---|---|---|---|
| FG1066 (Amazonia) | N16961 (El Tor, wild type) | JBK56 (CT− Apr) | JBK70 (CT− Hgr) | CVD104 (CT− HlyA−) | |
| Supernatant | 1/640 | 1/20 | 1/20 | 1/20 | <1/20 |
| Concentrate | 1/5120 | 1/320 | 1/320 | 1/320 | <1/20 |
Presence of the hlyA gene and hemolytic activity in the Amazonia strains.
Primers surrounding a central portion of the hlyA gene of the El Tor strain N16961 were used to amplify the same region from several Amazonia strains. A fragment with the predicted size (0.6 kb) was amplified (data not shown). Our first report on these strains described them as nonhemolytic (4) due to inadequate filtration of the supernatants used in tube tests (20). In this study they were found to be hemolytic both on 5% sheep blood plates (PML Microbiologicals) and in tube tests (20) done after filtering with low-protein-binding filters (data not shown).
DNA sequencing.
The sequence of the complete hlyA gene from Amazonia strain FG1066 was obtained by automated laser fluorescence sequencing of three overlapping PCR fragments, amplified with the Expand High Fidelity PCR system (Boehringer). The size of the gene is 2,226 bp as for other El Tor and environmental hemolysins. A comparison was made to the sequence of the hemolytic El Tor strain N16961, obtained from The Institute for Genomic Research website (http://www.tigr.org). Fifty-five base substitutions were found, a 2.5% difference. The hemolysin protein sequence, obtained by translation of the DNA sequence, has 741 amino acids. Six amino acid changes are present, all in the carboxy-terminal half of the sequence, the region purportedly associated with hemolytic activity (2).
Construction of hlyA insertion knockout mutants of an Amazonia strain.
To confirm that the hemolysin is required for cytotoxic vacuolating activity, the hlyA gene was disrupted by insertion of a suicide plasmid. A 0.6-kb central fragment of the hemolysin gene of Amazonia strain FG1066 was amplified by PCR with the primers hemf (5′-GAGCGTAATGCGAAGAATGC-3′) and hemr (5′-GAGTCAGGTTTTGGTTACAGG-3′) and cloned in the suicide plasmid pKAS32 (21). The resulting plasmid, pFG300, was introduced by mating into V. cholerae Amazonia strain FG1106, a streptomycin-resistant (Smr) derivative of FG1066.
The ampicillin-resistant (Apr) strains obtained were confirmed to have the insertion into the hlyA gene by Southern blotting, using as a probe the same Amazonia hlyA fragment amplified for the construction of plasmid pFG300. The single PstI 6.5-kb fragment of the Amazonia strain is lost after the insertion and replaced by two new fragments with sizes of 3.2 and 5.5 kb. These are the expected sizes for the chromosomal junctions with the two partial copies of the hlyA gene.
Cytotoxic activity of the hlyA mutants compared to the wild-type strain is shown in Table 3. A striking effect was observed, with the complete loss of cytotoxicity and vacuolating activity in the mutants. The experiment was done both with filtered supernatants and with ammonium sulfate concentrates. Whereas a maximum vacuolating effect with a dilution of 1/4,096 was observed in the wild-type strain, no effect at only a 1/20 dilution in the mutant supernatants was detected. A total of six mutants were tested with the same result. These mutants also lost their hemolytic activity, as indicated both on blood plates and in tube tests (data not shown).
TABLE 3.
Comparison of vacuolating cytotoxic activities from various strains in hlyA inactivation, reversion, cloning, and complementation experimentsa
| Fraction | Dilution yielding maximum vacuolating activity
|
||||||
|---|---|---|---|---|---|---|---|
| FG1106 | FG1153 | FG1173 | FG1167 | DH5α | FG1221 | FG1229 | |
| Supernatant | 1/212 | <1/20 | 1/162 | 1/125 | <1/20 | <1/20 | 1/853 |
| Concentrate | 1/4,096 | <1/20 | 1/7,680 | 1/5,120 | <1/10 | 1/30 | 1/40,960 |
See Table 1 for descriptions of the strains used.
Reversion of the insertion mutations restores the vacuolation activity and hemolysis.
Homologous recombination leading to reversion of the insertion mutants FG1153 was obtained by two methods. In the first, a few spontaneous revertants of the nonhemolytic strain appearing on sheep blood plates as hemolytic colonies were purified. In another approach not dependent on the hemolytic phenotype, the insertion mutant FG1153 was plated on streptomycin (1 mg/ml), and bacteria that had lost the wild-type rpsL allele present on the inserted suicide vector were selected. Six of these revertants were tested for the vacuolating cytotoxin activity and hemolysis. The results for the cytotoxin test in Table 3 show a restoration of the activity after excision of the inserted plasmid. Hemolytic activity was also fully restored (data not shown).
Cloning of the hlyA region of the Amazonia strain.
A 6.5-kb PstI DNA fragment from Amazonia strain FG1066 was cloned into the positive kanamycin resistance (Kmr) selection vector pKil194T (9). E. coli DH5α was electroporated with the ligation products, and recipients were selected by plating on Luria broth (LB) supplemented with kanamycin. Colonies were then screened on sheep blood agar. One hemolytic colony carried a plasmid, pFG350, with the 6.5-kb fragment previously found in other strains to carry the hlyA and hlyB genes (16). This insert hybridized on a Southern blot to a probe consisting of the whole hlyA gene (data not shown). The E. coli strain carrying this plasmid was grown in the conditions described for cytotoxin production, and filtered supernatants and concentrates were prepared. Vacuolating activity in the supernatant was at the lower limit of detection and was found only in two out of four experiments at the 1/20 dilution, but the concentrates from the four experiments contained a vacuolating activity with an average maximum at a 1/30 dilution (Table 3). This activity was not found in the parental strain DH5α tested in the same experiments.
Complementation of the hlyA insertion mutant for VcVac production and hemolysis.
Plasmid pFG350 was transferred into the Apr V. cholerae hlyA insertion mutant strain FG1153 to test for complementation of the hlyA mutation. The transfer was done in a triparental mating with EK222, DH5α/pFG350, and FG1153. The colonies obtained on LB-kanamycin-ampicillin plates were tested on blood agar plates, as Kmr is present both on the conjugative plasmid pRK2013 and on plasmid pFG350 with the hlyA gene. The vast majority of the colonies obtained were hemolytic (>90%). Plasmid preparations confirmed the presence of the 11-kb pFG350 in these transconjugants (FG1229). Supernatants and concentrates were obtained from six such cultures and tested for vacuolation (Table 3). Complementation occurred, and these strains had a high level of VcVac, yielding higher titers (1/40,960 from the concentrate) than the original Amazonia strains in the two experiments done. This is probably due to an increased copy number of the hlyA gene.
Vacuolation with purified El Tor hemolysin.
Purified El Tor hemolysin (kindly provided by A. Zitzer) was tested in our cell system to determine whether it would produce the same vacuolation effect. Twofold dilution series from the original preparation were tested. Hemolysin was very cytotoxic to the cells at a high concentration, giving rise to vacuoles similar to the ones described above, in dilutions of approximately 1/800. This corresponds to 0.5 ng of purified El Tor HlyA.
In summary, we have described a new cell-vacuolating activity, VcVac, which is associated with hemolysin (HlyA) production by V. cholerae. Vacuolation by Amazonia strains was noticed previously (4), but in this work we investigated the source of this activity and its presence in other strains. An insertion mutation in the hlyA gene abolished both hemolytic and vacuolating activity. This mutation should not be polar on downstream genes, as the mRNA for hlyA alone is produced separately from the immediately downstream gene hlyB, coding for a chemotactic transducer (24). An E. coli strain with a plasmid carrying the hlyA and hlyB of the V. cholerae Amazonia strain yielded a small amount of active vacuolating toxin in the supernatant. As V. cholerae hemolysin is not secreted by E. coli (16), the vacuolating activity that we detect in the supernatant of E. coli FG1221 is probably due to lysis of some cells and processing by some proteolytic activity present in the supernatant or the residual trypsin in the cell culture medium (25). This experiment with E. coli and the cloned hlyA gene links the vacuolation activity directly to HlyA. Finally, purified El Tor HlyA was confirmed to cause vacuolation in the same conditions. The vacuolating phenotype is a new activity of V. cholerae hemolysin, and cell vacuolation is a previously unknown type of interaction of V. cholerae with mammalian cells in culture. Our study shows that many strains, including epidemic hemolytic El Tor, Gulf Coast El Tor, the Amazonia variant, and environmental strains, are proficient in vacuolation.
The role of hemolysin in pathogenesis has been the subject of considerable debate. One human volunteer study indirectly indicated that hemolysin does not have a role in the pathogenesis of V. cholerae (15). On the other hand, there is ample evidence for both cytotoxic (11, 17, 27) and enterotoxic (12, 17) activities of hemolysin. A 30-fold increase in the 50% lethal dose in infant mice has been reported from an El Tor hlyA mutant (23). It is possible that in the case of the Amazonia strains, which lack other known virulence factors, hemolysin could play a more important role in the disease, perhaps as a result of higher expression or as a consequence of the six amino acid differences in the carboxy half leading to increased activity. In the case of VacA of H. pylori, the presence of the vacuolating toxin is strongly associated with pathogenesis, being linked to peptic ulcers and gastric cancer.
The comparative mechanisms of vacuole formation by VacA of H. pylori and HlyA of V. cholerae merit further study. Both toxins interact with cell membranes as monomers which lead to the formation of oligomeric pores (7, 27). These two toxins have apparently different modes of action: VacA binds to the membrane but acts in the cytosol after endocytosis (8), and HlyA acts on the membrane, forming pentamers and leading to pore formation (27). A difference between the two toxins was also found in the study of susceptibility of the vacuolating phenotype to bafilomycin A. While VacA vacuolation is markedly inhibited by this agent (18), the V. cholerae cytotoxin-mediated vacuolation and cell death were not affected. The behavior of HlyA seems more similar to that of aerolysin from A. hydrophila. Aerolysin, a hemolysin, interacts with the cell membrane, forming heptameric pores. Without apparently entering the cytoplasm, it is capable of causing the formation of vacuoles derived from the endoplasmic reticulum (1), in contrast to VacA vacuoles originated from late endosomal/prelysosomal compartments (19). It will be important to determine the site of HlyA action. The cell vacuolation in itself should be further investigated, particularly in relation to membrane trafficking in the cell.
Nucleotide sequence accession number.
The sequence reported can be found in GenBank under accession no. AF194418.
Acknowledgments
We thank M. Swanson for the use of cell culture facilities, J. Swanson and S. Koszycki for assistance with photography, the Instituto Noel Nutels for epidemic strains from Brazil, C. Barros and J. Kaper for Vibrio strains, M. de Bernard for protocols and strains, T. Cover for supernatants of H. pylori, and A. Zitzer for purified El Tor HlyA.
A. Coelho is the recipient of a postdoctoral training grant from CNPq, Brazil.
REFERENCES
- 1.Abrami L, Fivaz M, Glauser P-E, Parton R G, van der Goot F G. A pore-forming toxin interacts with a GPI-anchored protein and causes vacuolation of the endoplasmic reticulum. J Cell Biol. 1998;140:525–540. doi: 10.1083/jcb.140.3.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm R A, Mayrhofer G, Kotlarski I, Manning P A. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine. 1991;9:588–594. doi: 10.1016/0264-410x(91)90247-4. [DOI] [PubMed] [Google Scholar]
- 3.Baptista M A, Andrade J R, Vicente A C, Salles C A, Coelho A. The Amazonia variant of Vibrio cholerae: molecular identification and study of virulence genes. Mem Inst Oswaldo Cruz. 1998;93:601–607. doi: 10.1590/s0074-02761998000500008. [DOI] [PubMed] [Google Scholar]
- 4.Coelho A, Andrade J R C, Vicente A C P, Salles C A. New variant of Vibrio cholerae O1 from clinical isolates in Amazonia. J Clin Microbiol. 1995;33:114–118. doi: 10.1128/jcm.33.1.114-118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coelho A, Vicente A C P, Baptista M A S, Momen H, Santos F R W, Salles C A. The distinction of pathogenic Vibrio cholerae groups using arbitrarily primed PCR fingerprints. Res Microbiol. 1995;146:671–683. doi: 10.1016/0923-2508(96)81064-3. [DOI] [PubMed] [Google Scholar]
- 6.Cover T L, Blaser M J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J Biol Chem. 1992;267:10570–10575. [PubMed] [Google Scholar]
- 7.Czajkowsky D M, Iwamoto H, Cover T L, Shao Z. The vacuolating toxin from Helicobacter pylori forms hexameric pores in lipid bilayers at low pH. Proc Natl Acad Sci USA. 1999;96:2001–2006. doi: 10.1073/pnas.96.5.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bernard M, Arico B, Papini E, Rizzuto R, Grandi G, Rappuoli R, Montecucco C. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol. 1997;26:665–674. doi: 10.1046/j.1365-2958.1997.5881952.x. [DOI] [PubMed] [Google Scholar]
- 9.Gabant P, Szpirer C Y, Couturier M, Faelen M. Direct selection cloning vectors adapted to the genetic analysis of gram-negative bacteria and their plasmids. Gene. 1998;207:87–92. doi: 10.1016/s0378-1119(97)00610-0. [DOI] [PubMed] [Google Scholar]
- 10.Hertle R, Hilger M, Weingardt-Kocher S, Walev I. Cytotoxic action of Serratia marcescens hemolysin on human epithelial cells. Infect Immun. 1999;67:817–825. doi: 10.1128/iai.67.2.817-825.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Honda T, Finkelstein R A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979;26:1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichinose Y, Yamamoto K, Nakasome N, Tanabe M J, Takeda T, Miwatani T, Iwanaga M. Enterotoxicity of El Tor-like hemolysin of non-O1 Vibrio cholerae. Infect Immun. 1987;55:1090–1093. doi: 10.1128/iai.55.5.1090-1093.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaper J B, Mobley H L T, Michalski J M, Herrington D A, Levine M M. Recent advances in developing a safe and effective live oral attenuated Vibrio cholerae vaccine. In: Ohtomo N, Sack R B, editors. Advances in research on cholera and related diarrheas. Vol. 6. Tokyo, Japan: KTK Scientific Publishers; 1988. pp. 161–167. [Google Scholar]
- 14.Leunk R D, Johnson P T, David B C, Kraft W G, Morgan D R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988;26:93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- 15.Levine M M, Kaper J B, Herrington D, Losonsky G, Morris J G, Clements M L, Black R E, Tall B, Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manning P A, Brown M H, Heuzenroeder M W. Cloning of the structural gene (hly) for the haemolysin of Vibrio cholerae El Tor strain 017. Gene. 1984;31:225–231. doi: 10.1016/0378-1119(84)90213-0. [DOI] [PubMed] [Google Scholar]
- 17.McCardell B A, Madden J M, Shah D B. Isolation and characterization of a cytolysin produced by Vibrio cholerae serogroup non-O1. Can J Microbiol. 1985;31:711–720. doi: 10.1139/m85-135. [DOI] [PubMed] [Google Scholar]
- 18.Papini E, Bugnoli M, de Bernard M, Figura N, Rappuoli R, Montecucco C. Bafilomycin A1 inhibits Helicobacter pylori-induced vacuolization of HeLa cells. Mol Microbiol. 1993;7:323–327. doi: 10.1111/j.1365-2958.1993.tb01123.x. [DOI] [PubMed] [Google Scholar]
- 19.Papini E, Satin B, de Bernard M, Molinari M, Arico B, Galli C, Telford J R, Rappuoli R, Montecucco C. Action site and cellular effects of cytotoxin VacA produced by Helicobacter pylori. Folia Microbiol. 1998;43:279–284. doi: 10.1007/BF02818613. [DOI] [PubMed] [Google Scholar]
- 20.Richardson K, Michalski J, Kaper J B. Hemolysin production and cloning of two hemolysin determinants from classical Vibrio cholerae. Infect Immun. 1986;54:415–420. doi: 10.1128/iai.54.2.415-420.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skorupski K, Taylor R K. Positive selection vectors for allelic exchange. Gene. 1996;169:47–52. doi: 10.1016/0378-1119(95)00793-8. [DOI] [PubMed] [Google Scholar]
- 22.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams S G, Attridge S R, Manning P A. The transcriptional activator HlyU of Vibrio cholerae: nucleotide sequence and role in virulence gene expression. Mol Microbiol. 1993;9:751–760. doi: 10.1111/j.1365-2958.1993.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 24.Williams S G, Manning P A. Transcription of the Vibrio cholerae haemolysin gene, hlyA, and cloning of a positive regulatory locus, hlyU. Mol Microbiol. 1991;5:2031–2038. doi: 10.1111/j.1365-2958.1991.tb00825.x. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto K, Ichinose Y, Shinagawa H, Makino K, Nakata A, Iwanaga M, Honda T, Miwatani T. Two-step processing for activation of the cytolysin/hemolysin of Vibrio cholerae O1 biotype El Tor: nucleotide sequence of the structural gene (hlyA) and characterization of the processed products. Infect Immun. 1990;58:4106–4116. doi: 10.1128/iai.58.12.4106-4116.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K, Takeda Y, Miwatani T, Craig J P. Purification and some properties of a non-O1 Vibrio cholerae enterotoxin that is identical to cholera enterotoxin. Infect Immun. 1983;39:1128–1135. doi: 10.1128/iai.39.3.1128-1135.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zitzer A, Wassenaar T M, Walev I, Bhakdi S. Potent membrane-permeabilizing and cytocidal action of Vibrio cholerae cytolysin on human intestinal cells. Infect Immun. 1997;65:1293–1298. doi: 10.1128/iai.65.4.1293-1298.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]