Abstract
Background
Patients with moderate and severe traumatic brain injury (TBI) are admitted to general hospitals (GHs) without neurosurgical services, but few studies have addressed the management of these patients. This study aimed to describe these patients, the rate of and reasons for managing patients entirely at the GH, and differences between patients managed entirely at the GH (GH group) and patients transferred to the regional trauma centre (RTC group). We specifically examined the characteristics of elderly patients.
Methods
Patients with moderate (Glasgow Coma Scale score 9–13) and severe (score ≤ 8) TBIs who were admitted to one of the seven GHs without neurosurgical services in central Norway between 01.10.2004 and 01.10.2014 were retrospectively identified. Demographic, injury-related and outcome data were collected from medical records. Head CT scans were reviewed.
Results
Among 274 patients admitted to GHs, 137 (50%) were in the GH group. The transferral rate was 58% for severe TBI and 40% for moderate TBI. Compared to the RTC group, patients in the GH group were older (median age: 78 years vs. 54 years, p < 0.001), more often had a preinjury disability (50% vs. 39%, p = 0.037), and more often had moderate TBI (52% vs. 35%, p = 0.005). The six-month case fatality rate was low (8%) in the GH group when transferral was considered unnecessary due to a low risk of further deterioration and high (90%, median age: 87 years) when neurosurgical intervention was considered nonbeneficial. Only 16% of patients ≥ 80 years old were transferred to the RTC. For this age group, the in-hospital case fatality rate was 67% in the GH group and 36% in the RTC group and 84% and 73%, respectively, at 6 months.
Conclusions
Half of the patients were managed entirely at a GH, and these were mainly patients considered to have a low risk of further deterioration, patients with moderate TBI, and elderly patients. Less than two of ten patients ≥ 80 years old were transferred, and survival was poor regardless of the transferral status.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13049-022-01050-0.
Keywords: Traumatic brain injuries; Craniocerebral trauma; General hospitals; Trauma centers; Tertiary care centers; Referral and consultation; Mortality; Aged, 80 and over
Background
Traumatic brain injury (TBI) is frequent, with an estimated 4 000 hospital admissions per year in Norway [1]. In low-income countries, the incidence of TBI has increased due to more traffic accidents, while in high-income countries, more elderly people sustain TBIs due to falls [2]. Approximately 15% of all hospital-referred TBIs are moderate or severe [1], with outcomes varying from complete restitution to severe disability or death.
Several guidelines for the initial management of TBI in Scandinavia have been published in recent decades [3–5]. In 2008, the Scandinavian Neurotrauma Committee (SNC) published summarised guidelines for the prehospital management of severe TBI based on previously developed guidelines by the Brain Trauma Foundation (BTF) [4, 6]. These guidelines recommend that all cases of suspected severe TBI (defined by a Glasgow Coma Scale (GCS) score of 3–8) should be stabilised and transported promptly to the nearest neurosurgical department for specialised neurointensive management. Notably, the SNC and BTF have not addressed the management of elderly patients with TBI specifically, even though these patients present with a greater burden of comorbid conditions and a higher risk of death and may not benefit from aggressive neurosurgical treatment [7].
For patients with a TBI not considered severe, the decision on primary admission to the regional trauma centre (RTC) or the closest local trauma centre (general hospital (GH)) is based on evaluations performed by the prehospital emergency personnel, taking a range of factors into consideration. The benefit of direct transportation has been extensively studied, with conflicting results, and a systematic review did not find a clear benefit of direct transportation to an RTC in this patient group [8]. Hence, the Norwegian National Trauma Plan states that if transportation time to the RTC is 45 min or more, transportation to the closest trauma centre should be considered [9].
The initial in-hospital management of minimal, mild, and moderate TBI in adults is described in guidelines that were developed by the SNC in 2000 and revised in 2013 [3, 5]. According to the guidelines applied in the period of the current study, all patients with signs of moderate TBI should have an early head CT scan and be admitted to the hospital for further observation for ≥ 12 h. In cases with abnormal head CT scans, consultation with a neurosurgical department is recommended. However, the guidelines do not specify which patients should be managed at the GH and which patients should be managed in a neurosurgical department.
Most studies of treatment and outcome of moderate and severe TBI originate from university hospitals with neurosurgical services [10]; hence, there is a shortage of knowledge about patients managed entirely at GHs.
This study aimed to describe adult patients with moderate and severe TBI admitted to GHs in a defined health region, focusing on patients who were never transferred (the GH group). We investigated the rate of and reason for managing patients entirely at the GH and explored differences between patients managed entirely at the GH and patients transferred to the RTC for further treatment, including differences in survival. Furthermore, we aimed to specifically describe the transferral rate and survival in elderly patients.
Methods
Study setting and study participants
The study comprised patients admitted primarily to GHs without neurosurgical services in central Norway, one of the four health regions in the country. Central Norway covered a population of approximately 670,000 inhabitants in the study period, and the distance between the GHs and the RTC ranged from 42 to 348 km by car. At the time of the study, the region held two levels of hospitals [11]: seven GHs meeting the requirements for level III trauma centres (for details see Additional file 1: Table S1) and one level I/II trauma centre, St. Olavs Hospital, with a neurosurgical department, denoted the RTC. The RTC also serves as a GH for approximately 45% of the population in the region, and most patients admitted there with TBI in need of hospitalisation for more than 24 h are admitted to the neurosurgical department, regardless of TBI severity. Patients belonging to the catchment area of St. Olavs Hospital were therefore not included in this study. As the RTC and the GHs lie within the same health region in central Norway, the standards of the prehospital care were the same for all patients and were in line with the guidelines applicable at the time they were admitted [3–5].
Study procedures and inclusion and exclusion criteria
Patients admitted to one of the seven GHs of the region between 01.10.2004 and 01.10.2014, with a main or secondary diagnosis registered as ICD-10 code(s) S06.1-S06.9 or S09.7-S09.9 (International Classification of Diseases, 10th revision), were screened for inclusion by review of their medical records. The ICD-10 coding system does not have unique codes for the severity of TBI, but moderate or severe TBI would most likely be coded as one or several of the abovementioned codes [12]. The Trondheim moderate and severe (ms)TBI study served as an additional source of patient identification. This is a prospective cohort study of all patients with moderate or severe TBI admitted to St. Olavs Hospital, including those transferred from GHs, from 01.10.2004 onwards [13].
We included patients with moderate and severe TBIs upon admission to the emergency department (ED), as well as patients with mild TBI in the ED who deteriorated to moderate or severe TBI during the acute phase to capture the entire burden of moderate and severe TBI managed at the GHs. TBI was classified as mild (GCS scores 14–15), moderate (GCS scores 9–13), or severe (GCS scores 3–8) [14]. Patients with a GCS score of 13 were included as moderate TBI because of the higher frequency of intracranial lesions and risk of adverse outcomes [15, 16], in accordance with the Head Injury Severity Scale, commonly used in Scandinavia [17]. The acute phase was defined as the time from admission to the GH to discharge from any ward responsible for the management of the trauma or related complications. If the patient was transferred to the RTC, discharge from the RTC denoted the end of the acute phase. A subsequent stay at a GH or rehabilitative institution was not considered part of the acute phase.
Intoxicated patients with an initially low GCS score who regained normal consciousness within 12 h were considered to have mild TBI and were excluded [18]. Patients who did not reside in Norway were excluded. Children < 17 years old were also excluded, as we have previously shown that most of these patients are transferred to the RTC [19]. Patients with a chronic subdural haematoma and patients where a diagnosis of TBI was considered unlikely or uncertain were also excluded (e.g., patients with stroke), as well as patients who presented to the ED > 72 h after the trauma.
Medical records and head CT scans for the entire acute phase were reviewed, and patients were categorised into patients managed entirely at the GH (GH group) and patients transferred to the RTC (RTC group).
Study variables
Preinjury disability was classified as yes if the patient had any known concurrent condition that affected daily functioning at the time of injury and was further categorised into neurological condition (including dementia), alcohol abuse, cardiopulmonary disease, psychiatric disorder, substance abuse, cancer, developmental disorder, other, or several. Preinjury antithrombotic medication was recorded as yes if the patient was using platelet inhibitors or anticoagulants (including heparins or direct-acting oral anticoagulants).
Alcohol intoxication upon admission was recorded as yes if clinical suspicion was described in the medical records or if the patient had a measured serum ethanol ≥ 2.2 mmol/L [20]. Injury-related variables were the cause of injury (fall, motor vehicle accident, or other cause including violence), admission GCS score (the lowest recorded GCS score in the ED) and severity of TBI at admission (mild, moderate, or severe). In cases with prehospital intubation, the last recorded GCS before sedation was used. Trauma team activation (TTA) at admission, extracranial injuries, extracranial surgery, and admission to the intensive care unit (ICU) were also registered. For patients transferred to the RTC, any neurosurgical procedure performed, including intracranial pressure (ICP) monitoring, was recorded.
Head CT scans for the entire acute phase (first and later CT scans) were reviewed by a resident in radiology (EHF), who was blinded to the patients’ medical records except for the CT referral information. CT findings were recorded dichotomously as yes or no for the variables epidural haematoma (EDH), subdural haematoma (SDH), traumatic subarachnoid haemorrhage (tSAH), single contusion/haematoma, multiple contusions/haematomas, intraventricular haemorrhage, general oedema (one or both hemispheres, or cerebellum), skull fracture with or without impression, and intracranial air.
Whether the consultant neurosurgeon or resident in neurosurgery on call at the RTC was consulted while the patient was in the ED, or was consulted later, was registered. When the patient was not transferred to the RTC, the main reason for keeping the patient at the GH after stabilisation in the ED was derived from the patient’s medical records and assigned to one of four predefined categories: unsalvageable was used for patients with a low GCS score, bilateral fixed semidilated pupils and/or a head CT scan demonstrating signs of severe herniation. Neurosurgical intervention considered nonbeneficial was selected when the risk associated with a neurosurgical procedure or intensive care was considered to outweigh the potential benefits associated with these interventions. Presumed low risk of further deterioration was selected in cases where the patient had a relatively high/stable GCS score upon arrival and modest head CT findings or in cases where alcohol intoxication was suspected to influence the GCS score [18]. Another reason was selected when the reason for keeping the patient at the GH did not fall into the other specified categories, for instance, severe trauma to other organ systems requiring immediate surgery at the GH or cases suggestive of isolated traumatic axonal injury (TAI) assuming no need for ICP measurement.
Outcome variables were the length of the acute hospital stay, discharge destination (nursing home, own home, specialised rehabilitation, other rehabilitative institution, or another hospital), death during the acute phase (i.e., in-hospital fatality), and death within 6 months and 12 months postinjury (6- and 12-month fatality). The cause of in-hospital fatality was categorised as intracranial hypertension when the clinical and radiological characteristics indicated increased ICP resulting in tamponade. Complications of TBI was selected when the patient died from complications or severe neurological impairments secondary to TBI. Other trauma, or unrelated to the injury, was selected when death was caused by trauma to a different organ system or was deemed unrelated to the TBI (e.g., a myocardial ischaemic infarction).
Statistical analyses
Descriptive data are reported as the mean with standard deviation when normally distributed and the median with interquartile range (IQR) when nonnormally distributed. Comparisons between the GH group and the RTC group were performed using the independent samples t test for normally distributed continuous variables and the Mann‒Whitney U test for ordinal and nonnormally distributed continuous data. Proportions were compared using the Pearson χ2 test, and the exact z-pooled test when the expected count was less than 5 for any cell, as recommended [21]. Cases with missing values were not included in the analyses. Proportions are reported as a percentage of the total included cases, regardless of the missing status, except for reported CT findings. All CT variables were missing in seven patients, and proportions of all cases with nonmissing status were reported. Missing data are noted in all tables, except when the frequency of missing values was < 5%. The significance level was set to α = 0.05. All statistical analyses were performed using IBM SPSS Statistics version 25.
Results
Included patients
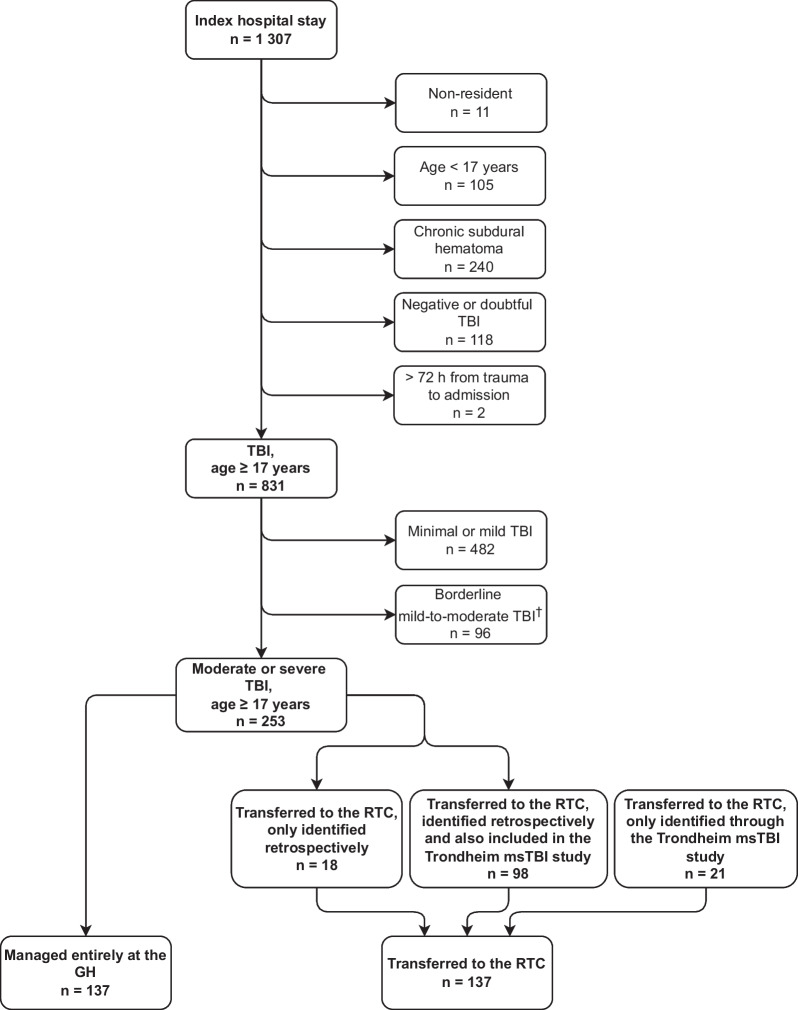
In total, 1307 index hospital stays at the seven GHs were reviewed. Of these, 253 patients met the inclusion criteria for moderate or severe TBI (including deterioration from mild TBI in the acute phase). In addition, 19 patients were included from the Trondheim msTBI study’s database (Fig. 1). These were not in the retrieved lists of ICD-10 codes, for instance, because very short stays at the GH had not been coded or because other ICD-10 codes had been used, for instance, I61 intracerebral haemorrhage. Hence, the total study population was 274 patients.
Fig. 1.
Flowchart of the inclusion process. Index hospital stay refers to any admission to a general hospital with ICD-10 code S06.1-S06.9, S09.7-S09.9 during the study period. GH General hospital, msTBI moderate and severe TBI, RTC Regional trauma centre, TBI Traumatic brain injury. †Categorised as borderline mild-to-moderate due to the inability to accurately classify these patients’ TBI severity. For instance, intoxicated patients with an initially low GCS score who regained normal consciousness within 12 h, and severely demented patients with an already reduced GCS score preinjury
The overall transferral rate was 50%, leaving 137 in the GH group and 137 in the RTC group. There were no major differences between the seven GHs in transferral rate (Additional file 1: Table S1). The transferral rate was 58% for patients with severe TBI (n = 125) and 40% for patients with moderate TBI (n = 119). The patients transferred to the RTC had a median length of stay of 2.8 h (IQR 1.9–5.2 h) at the GH before transferral.
Patient characteristics, fatality, and discharge destination in the GH and RTC groups
There were more patients with moderate TBI (52% vs. 35%, p = 0.005) in the GH group. The patients in the GH group were significantly older than the patients in the RTC group (median age: 78 years vs. 54 years, p < 0.001) and were more often injured in falls (Table 1). In addition, patients in the GH group more often had preinjury disability than did patients in the RTC group (50% vs. 39%, p = 0.037) and more often received antithrombotic medication (48% vs. 28%, p < 0.001). Platelet inhibitors were the most common antithrombotic medication for both groups (Additional file 1: Table S2). Anticoagulants were used by 14% of patients in the GH group and 9% in the RTC group (p = 0.106) (Table 1). In the GH group, fewer patients were admitted with TTA (35% vs. 57%, p < 0.001), received extracranial surgery (13% vs. 32%, p < 0.001), or were admitted to the ICU (80% vs. 93%, p < 0.001).
Table 1.
Patients characteristics in the GH group and the RTC group (n = 274)
| GH group | RTC groupa | p-value | |
|---|---|---|---|
| n = 137 | n = 137 | ||
| Age in years, median [IQR] | 78 [55, 87] | 54 [33, 68] | < 0.001 |
| Age in years, mean (SD)b | 68 (23) | 52 (21) | N/A |
| Patients ≥ 80 years old, n (%) | 57 (42) | 11 (8) | < 0.001 |
| Male, n (%) | 90 (66) | 108 (79) | 0.015 |
| Preinjury functional disability, n (%) yes (%) | 69 (50) | 54 (39) | 0.037 |
| Missing | 8 (6) | 4 (3) | N/A |
| Antithrombotic medication, n (%) | 66 (48) | 38 (28) | < 0.001 |
| Missing | 11 (8) | 1 (1) | N/A |
| Anticoagulant use, n (%) | 19 (14) | 12 (9) | 0.106 |
| Missing | 13 (9) | 1 (1) | N/A |
| Alcohol intoxication, n (%) | 25 (18) | 44 (32) | 0.009 |
| Cause of injury, n (%) | |||
| Fall | 111 (81) | 75 (55) | < 0.001 |
| Motor vehicle accident | 17 (12) | 41 (30) | < 0.001 |
| Other cause | 7 (5) | 17 (12) | N/A |
| Admission GCS score, median [IQR] | 10 [5, 12] | 8 [5, 13] | 0.827 |
| Missing, n (%) | 30 (22%) | 4 (3%) | N/A |
| Admission GCS score of 13, n (%) | 13 (9%) | 17 (12%) | 0.883 |
| Severity of TBI at admission, n (%) | |||
| Severe TBI | 53 (39) | 72 (53) | 0.021 |
| Moderate TBI | 71 (52) | 48 (35) | 0.005 |
| Mild TBI | 13 (9) | 17 (12) | 0.439 |
| Trauma team activation, n (%) | 48 (35) | 78 (57) | < 0.001 |
| Extracranial injury, n (%) | 58 (42) | 68 (50) | 0.224 |
| Extracranial surgery, n (%) | 18 (13) | 44 (32) | < 0.001 |
| ICU stay, n (%) | 109 (80) | 128 (93) | < 0.001 |
| Neurosurgical procedure, n (%) | – | 94 (69) | – |
Number of missing cases not stated when frequency < 5%
GCS Glasgow coma scale, GH General hospital, IQR Interquartile range, N/A Not applicable, RTC Regional trauma centre, SD Standard deviation, TBI Traumatic brain injury
Bold font indicates statistical significance
aTwo patients were transferred to a different RTC than St. Olavs Hospital due to extraordinary circumstances
bStatistical analyses were not conducted due to non-normality in the two groups
EDH, multiple contusions, and general oedema were more frequent in the RTC group (p < 0.001, p = 0.026 and p < 0.001, respectively Table 2). SDH and tSAH were frequent in both groups. In the RTC group, 94 patients (69%) had a neurosurgical procedure (Table 1).
Table 2.
Main findings at head CT scans in the GH group and the RTC group
| GH group | RTC group | p-value | |
|---|---|---|---|
| n = 131a | n = 136b | ||
| Normal first CT, n (%) | 2 (1) | 1 (1) | 0.540 |
| Epidural haematoma, n (%) | 6 (4) | 26 (19) | < 0.001 |
| Subdural haematoma, n (%) | 92 (67) | 93 (68) | 0.744 |
| Subarachnoid haemorrhage, n (%) | 92 (67) | 90 (66) | 0.477 |
| Single contusion/haematoma, n (%) | 33 (24) | 18 (13) | 0.013 |
| Multiple contusions/haematomas, n (%) | 45 (33) | 65 (47) | 0.026 |
| Intraventricular haemorrhage, n (%) | 32 (23) | 36 (26) | 0.702 |
| General oedema, n (%) | 4 (3) | 48 (35) | < 0.001 |
| Skull fracture without impression, n (%) | 53 (39) | 72 (53) | 0.041 |
| Skull fracture with impression, n (%) | 5 (4) | 10 (7) | 0.210 |
| Intracranial air, n (%) | 18 (13) | 39 (28) | 0.003 |
CT Computed tomography, GH General hospital, RTC Regional trauma centre
Bold font indicates statistical significance
aThere was missing data for 6 cases in the GH group, for all variables except epidural haematoma of which there were 7 missing cases
bThere was missing data for 1 case in the RTC group, for all variables
More patients died in the acute phase in the GH group than in the RTC group (44% vs. 16%, p < 0.001) (Table 3) but less often from intracranial hypertension (70% vs. 95%, p = 0.015). The six-month case fatality rate (CFR) was higher in the GH group than in the RTC group (54% vs. 25%, p < 0.001).
Table 3.
Fatality and discharge destinations in the GH group and the RTC group
| GH group | RTC group | p-value | |
|---|---|---|---|
| n = 137 | n = 137 | ||
| Length of stay in days, median [IQR] | 5 [2, 12] | 6 [3, 12] | 0.169 |
| In-hospital fatality, n (%) | 60 (44) | 22 (16) | < 0.001 |
| Cause of in-hospital fatality, n (%) | 60 (44) | 22 (16) | |
| Intracranial hypertension | 42 (70) | 21 (95) | 0.015 |
| Complications of TBI | 17 (28) | 1 (5) | 0.021 |
| Unrelated to the injury | 1 (2) | 0 (0) | N/A |
| Other trauma | 0 (0) | 0 (0) | N/A |
| Discharge destination, n (%) | 77 (56) | 115 (84) | |
| Nursing home | 25 (32) | 0 (0) | < 0.001 |
| Home | 22 (29) | 2 (2) | < 0.001 |
| Specialised rehabilitation | 18 (23) | 8 (7) | 0.001 |
| Other rehabilitative institution | 6 (8) | 0 (0) | N/A |
| Other hospital | 6 (8) | 105 (91) | < 0.001 |
| 6-month fatality, n (%) | 74 (54) | 34 (25) | < 0.001 |
| Missing | 0 (0) | 8 (6) | N/A |
| 12-month fatality, n (%) | 79 (58) | 38 (28) | < 0.001 |
| Missing | 0 (0) | 8 (6) | N/A |
GH General hospital, IQR Interquartile range, N/A Not applicable, RTC Regional trauma centre, TBI Traumatic brain injury
Bold font indicates statistical significance
Patients in the GH group were discharged to a variety of settings, including specialised rehabilitation (23%) and nursing homes (32%). In the RTC group, most (91%) were discharged to their respective GHs.
Reasons for managing patients at the GH and relation to fatality
A consultant neurosurgeon or resident in neurosurgery on call at the RTC was consulted from the ED in 110 cases (80%) in the GH group. In addition, 12 cases (8%) were discussed later in the acute phase. All patients categorised as unsalvageable died during the acute phase (Table 4). Among patients for whom neurosurgical intervention was considered nonbeneficial, 17% presented with severe TBI at admission, and 90% died within 6 months. Among patients with a presumed low risk of further deterioration, four patients (8%) died within 6 months (age range: 71–94 years). These four patients died during the acute phase or were discharged to palliative care. One was later diagnosed with a severe concurrent somatic disease, while the remaining three patients were > 70 years old, none had preinjury disability, all had a mild TBI in the ED phase, and all were on antithrombotic medications.
Table 4.
Reasons for managing patients entirely at the GH (n = 137), with corresponding severity of TBI and fatality
| Unsalvageable | Neurosurgical intervention considered non-beneficial | Presumed low risk of further deterioration | Another reason | Unknown reason | |
|---|---|---|---|---|---|
| n = 32 (23%) | n = 42 (31%) | n = 49 (36%) | n = 8 (6%) | n = 6 (4%) | |
| Age, median [IQR] | 79 [69, 85] | 87 [82, 90] | 58 [41, 71] | 48 [37, 65] | 43 [25, 78] |
| Patients ≥ 65 years old, n (%) | 24 (75) | 40 (95) | 16 (33) | 2 (25) | 2 (33) |
| Patients ≥ 80 years old, n (%) | 14 (44) | 35 (83) | 7 (14) | 0 (0) | 1 (17) |
| Severe TBI at admission, n (%) | 31 (97) | 7 (17) | 8 (16) | 4 (50) | 3 (50) |
| Moderate TBI at admission, n (%) | 1 (3) | 31 (74) | 32 (65) | 4 (50) | 3 (50) |
| Mild TBI at admission, n (%) | 0 (0) | 4 (10) | 9 (18) | 0 (0) | 0 (0) |
| In-hospital fatality, n (%) | 32 (100) | 27 (64) | 1a (2) | 0 (0) | 0 (0) |
| 6-month fatality, n (%) | 32 (100) | 38 (90) | 4 (8) | 0 (0) | 0 (0) |
ED Emergency department, GH General hospital, IQR Interquartile range, TBI Traumatic brain injury
aTwo additional patients > 70 years of age were discharged to palliative care and died, thus fatal outcome was observed in the acute phase in 6% of patients in this category
Elderly patients
The percentage of patients transferred to the RTC decreased with increasing age, from 64% of patients < 65 years old to 16% of patients ≥ 80 years old (Table 5). Patients ≥ 80 years old constituted 25% of the total study population: 42% of the GH group versus 8% of the RTC group (p < 0.001) (Table 1). In the GH group, 63% of the patients ≥ 80 years old had a preinjury functional disability, most commonly a neurologic condition.
Table 5.
Characteristics of elderly patients in the GH group and the RTC group
| < 65 years old | ≥ 65 years old | ≥ 80 years old | ||||
|---|---|---|---|---|---|---|
| GH group | RTC group | GH group | RTC group | GH group | RTC group | |
| n = 53 | n = 95 | n = 84 | n = 42 | n = 57 | n = 11 | |
| Preinjury functional disability, n (%) | 20 (38) | 34 (36) | 49 (58) | 20 (48) | 36 (63) | 4 (36) |
| Missing | 4 (8) | 4 (4) | 4 (5) | 0 (0) | 2 (4) | 0 (0) |
| Antithrombotic medication, n (%) | 5 (9) | 8 (8) | 61 (73) | 30 (71) | 41 (72) | 9 (82) |
| Missing | 6 (11) | 1 (1) | 5 (6) | 0 (0) | 3 (5) | 0 (0) |
| Cause of injury, n (%) | ||||||
| Fall | 35 (66)* | 43 (45) | 76 (90)* | 32 (76) | 53 (93)* | 7 (64) |
| Motor vehicle accident | 11 (21) | 32 (34) | 6 (7)* | 9 (21) | 3 (5)** | 4 (36) |
| Other cause | 5 (9) | 16 (17) | 2 (2) | 1 (2) | 1 (2) | 0 (0) |
| Severity of TBI at admission, n (%) | ||||||
| Severe TBI | 20 (38)* | 53 (56) | 33 (39) | 19 (45) | 20 (35) | 7 (64) |
| Moderate TBI | 32 (60)** | 33 (35) | 39 (46) | 15 (36) | 31 (54)*** | 0 (0) |
| Mild TBI | 1 (2) | 9 (9) | 12 (14) | 8 (19) | 6 (11) | 4 (36) |
| Trauma team activation, (%) | 24 (45)* | 59 (62) | 24 (29) | 19 (45) | 12 (21) | 4 (36) |
| Reason for managing patient at the GH, n (%) | ||||||
| Unsalvageable | 8 (15) | – | 24 (29) | – | 14 (25) | – |
| Neurosurgical intervention considered non-beneficial | 2 (4) | – | 40 (48) | – | 35 (61) | – |
| Presumed low risk of further deterioration | 33 (62) | – | 16 (19) | – | 7 (12) | – |
| Another reason | 6 (11) | – | 2 (2) | – | 0 (0) | – |
| Unknown reason | 4 (8) | – | 2 (2) | – | 1 (2) | – |
| Neurosurgical procedure, n (%) | – | 70 (74) | – | 24 (57) | – | 6 (55) |
| In-hospital fatality, n (%) | 8 (15) | 12 (13) | 52 (62)*** | 10 (24) | 38 (67) | 4 (36) |
| 6-month fatality, n (%) | 10 (19) | 16 (17) | 64 (76)*** | 18 (43) | 48 (84) | 8 (73) |
| Missing | 0 (0) | 7 (7) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
| 12-month fatality, n (%) | 11 (21) | 18 (19) | 68 (81)*** | 20 (48) | 49 (86) | 8 (73) |
| Missing | 0 (0) | 7 (7) | 0 (0) | 1 (2) | 0 (0) | 0 (0) |
Number of missing cases not stated when frequency < 5%
ED Emergency department, GCS Glasgow coma scale, GH General hospital, RTC Regional trauma centre, TBI Traumatic brain injury
*p < 0.05, **p < 0.01, ***p < 0.001 in statistical analyses between the GH group and the RTC group for this age category
In the category unsalvageable, 75% of patients were ≥ 65 years old, and 44% of patients were ≥ 80 years old. In the category neurosurgical intervention considered nonbeneficial, 95% of patients were ≥ 65 years old, and 83% of patients were ≥ 80 years old (Table 4).
In patients ≥ 65 years old, the in-hospital CFR was higher in the GH group than in the RTC group (62% vs. 24%, p < 0.001), as was the 6-month CFR (76% vs. 43%, p < 0.001) (Table 5). In patients ≥ 80 years old, the in-hospital CFR seemed to be higher in the GH group than in the RTC group, although not significantly different (67% and 36%, p = 0.062), whereas the CFR at 6 months was similar (84% and 73%, p = 0.420).
Discussion
This is the first Scandinavian study describing adult patients with moderate and severe TBIs admitted to GHs, focusing on patients never transferred to RTCs. Of all patients initially admitted to a GH, 60% of patients with moderate and 42% of patients with severe TBI were managed entirely at the GH. Most (94%) of the patients presumed to have a low risk of further deterioration remained stable and survived the acute phase. In addition to a large proportion of patients considered unsalvageable (23%), we found that the status neurosurgical intervention considered nonbeneficial, especially among elderly individuals with preinjury disability, was a common cause of not transferring the patient. More patients died in the acute phase in the GH group than in the RTC group, potentially reflecting the higher number of elderly patients in the GH group. Overall, half of the patients were managed entirely at the GH. The decision regarding the appropriate level of care for the patient was mostly made in cooperation with a neurosurgeon. Most patients with EDH, multiple contusions or general oedema were transferred. Most of the transferred patients received neurosurgical procedures, indicating that patients evaluated as likely to benefit from neurosurgery were transferred.
We identified two main categories of patients managed entirely at the GHs: patients considered safe to manage at the GH level and elderly patients considered either unsalvageable or unlikely to benefit from neurosurgical intervention.
First, few patients with a presumed low risk of further deterioration died during the acute phase, including patients with a GCS score ≤ 8 at admission, indicating that communication between the managing physician at the GH and the on-call neurosurgeon, with shared access to the CT scan, may provide an accurate estimate of the patient’s prognosis; this finding is in line with other studies [22, 23]. The four patients in this category who deteriorated and died or entered a palliative setting within six months after the injury were all elderly and receiving antithrombotic medication or were later diagnosed with a severe concurrent disease. It remains uncertain whether the four deaths could have been prevented by transferral to the RTC. This study extends the current knowledge regarding patients with moderate TBI [24], as we have demonstrated that 60% of the patients admitted to GHs were managed entirely at the GH. Several studies have investigated the opportunities for the management of patients with TBI without transfer to a neurosurgical department, but most of these studies have only included patients with mild TBI or have health care systems not readily comparable to the Scandinavian health care model [25–27]. On both the national and international levels, there is a lack of guidelines offering nuanced recommendations for the management of moderate and severe TBI at the GH level.
The second group included elderly patients considered either unsalvageable or unlikely to benefit from neurosurgical intervention. Patients managed entirely at the GH were older than patients transferred to the RTC, and only 16% of patients ≥ 80 years old were transferred to the RTC. Even among patients with severe TBI, almost half were never transferred to the RTC, despite a high degree of conferring with the RTC and current guidelines recommending transfer [4, 28]. The most common rationale for not transferring patients with severe TBI was that the patients were considered unsalvageable, and most (75%) of these were ≥ 65 years old. It is likely that younger patients, even those with no hope of survival, were admitted directly to or rapidly transferred to the RTC, for instance, when involved in road traffic accidents [29]. Nevertheless, even when excluding patients categorised as unsalvageable, one-fourth of patients with severe TBI were managed entirely at the GH, many of whom were elderly patients where neurosurgical intervention was considered nonbeneficial. Elderly patients in the GH group had a high rate of preinjury disability, which increased with increasing age. Age and comorbidity have been associated with less aggressive management [7, 30, 31] and lower rates of transferral [32–34] in previous studies, consistent with our findings.
The choice of management level for elderly patients has been insufficiently studied since many studies exclude elderly patients with preinjury disability or significant comorbidity [35]. One study did not find an association between transfer to a level I or II trauma centre and long-term functional outcomes in older patients with all-severity TBI and concluded that routine transfer is not warranted [36]. Considering the increasingly older population in high-income countries and a demonstrated increase in TBIs in elderly individuals, this is an important topic that needs further investigation [7].
CFRs were high in the elderly patients in the GH group for both patients≥ 65 years and ≥ 80 years old but also in patients ≥ 80 years old in the RTC group. Studies regarding the benefits of neurosurgical intervention in the elderly are conflicting [37–42]. Moreover, these studies were conducted at neurosurgical referral centres and were likely biased towards cases considered suitable for surgical intervention. The in-hospital CFR was higher for patients ≥ 80 years old in the GH group versus the RTC group; however, the 6-month CFR was very high in both groups. This increase in fatality rate during the first few months demonstrates that studies on elderly patients must also consider post-discharge status when investigating outcomes.
Whether the high CFR among elderly patients indicates a nihilistic approach or well-considered treatment-limiting decisions was not investigated in this study. The former possibility has been explored in several previous studies, which found that less aggressive management in elderly patients might increase mortality rates [31, 43]. In the latter study, increasing age was associated with reduced management intensity, irrespective of head injury severity, and an association between low management intensity and increased risk of 30-day mortality was found. However, in some cases, palliative care would be in the best interest of the patient [44], and our study shows that treatment-limiting decisions are common for elderly patients with severe TBI in our region, consistent with another Norwegian study [45]. Interestingly, a German and Austrian study found that even if neurosurgeons were willing to perform an emergency operation on an elderly patient with a life-threatening TBI [46], the elderly patients themselves might not wish for a life-prolonging intervention if it would involve severe disability [47].
As in geriatric medicine, the focus on frailty in the field of TBI has increased. Frailty reflects the individual’s physiological vulnerability beyond that of chronological age, and a TBI-specific frailty index was recently developed and validated [48]. Management guidelines for TBI could probably be improved by incorporating the assessment of frailty [49].
Limitations
Although the population-based design, covering all the GHs without neurosurgical services in the region over a ten-year period, was a strength of this study, the retrospective nature of this study entails some limitations of the data material. First, the long inclusion period and the time passed since the last included patients, imply that the study results might not generalize to the current situation at general hospitals. Second, ICD-10 codes to be reviewed were limited as described in the methods section, thereby excluding cases coded with S06.0 (concussion). We might therefore have missed a few patients with a normal head CT scan who clinically suffered a moderate or severe TBI due to isolated TAI. We might also have missed cases due to incorrect coding [12]. Third, the GCS score was in some cases not documented or could not be validly obtained from the medical records, for instance, due to concomitant alcohol intoxication, extracranial severe injuries affecting respiratory or circulatory function, dementia, or other neurologic comorbidities. Moreover, reasons for managing patients entirely at the GH were not always accurately described and can be especially difficult to derive retrospectively, which was also the case for the cause of death; the results must therefore be interpreted with caution.
An additional limitation is that the present study used the HISS classification of moderate TBI (GCS scores of 9–13), instead of the range of 9–12 used in many current studies. Last, patients residing in the area where the RTC also serves as the GH were not included in this study. Consequently, the present study did not include all cases of moderate and severe TBI in central Norway; instead, it attempted to investigate the situation from the perspective of GHs without neurosurgical services.
Conclusions
GHs manage many patients with moderate or severe TBI, and there is a high degree of communication between the GHs and the RTCs about these patients. The patients managed entirely at the GHs differed from the patients transferred to the RTC by being older and having more preinjury disabilities. Hence, studies conducted at hospitals with a neurosurgical department will not be representative of all patients with moderate or severe TBI. Two main groups of patients were identified in the GH group: patients considered to have a low risk of further deterioration—a mostly accurate prediction, and elderly patients dying upon arrival or presenting with severe comorbidities considered not to benefit from neurosurgical intervention due to assumed poor prognosis. Notably, few patients ≥ 80 years old were transferred to the RTC, and the 6-month CFR in this age group was high regardless of transferral. Transferral to the RTC may therefore not be in the best interest of older patients with high premorbid frailty. Future guidelines should describe the management of moderate and severe TBI in the elderly population in greater detail, especially regarding the treatment level and benefit of transferral.
Supplementary Information
Additional file 1: Table S1 Patients with moderate or severe TBI in the GHs of central Norway 01.10.2004–01.10.2014. Table S2 Preinjury functional disability and antithrombotic medication usage in the GH group versus the RTC group.
Acknowledgements
The authors would like to thank Beate Mærk Voll, Torun Elsie Wanvik Farnes, Stine Borgen Lund, and Oddrun Sandrød for management of the Trondheim msTBI study database.
Abbreviations
- BTF
Brain Trauma Foundation
- CFR
Case fatality rate
- CT
Computed tomography
- ED
Emergency department
- EDH
Epidural haematoma
- GCS
Glasgow Coma Scale
- GH
General hospital
- ICD-10
International Classification of Diseases, 10th revision
- ICP
Intracranial pressure
- ICU
Intensive care unit
- msTBI
Moderate and severe TBI
- RTC
Regional trauma centre
- tSAH
Traumatic subarachnoid haemorrhage
- SDH
Subdural haematoma
- SNC
Scandinavian Neurotrauma Committee
- TAI
Traumatic axonal injury
- TBI
Traumatic brain injury
- TTA
Trauma team activation
Author contributions
SR and TS contributed to the design of the study, participated in data collection and analysis, and drafted the manuscript. EL and AV contributed to the design of the study. EHF read the CT scans. RP participated in data collection. All authors edited the manuscript and approved the final version.
Funding
Open access funding provided by Norwegian University of Science and Technology.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Regional Committee for Medical Research Ethics approved the Trondheim msTBI study (reference number 2009/2328), with the present study approved as an extension. The study was approved without the requirement of patient consent for the patients in the GH group. For the Trondheim msTBI study, written informed consent was obtained from the surviving patients or their next of kin for incapacitated patients. Permission was obtained from the Norwegian Directorate of Health to use data from deceased patients without consent from their next of kin. All data access committees of the individual GHs have approved the access to medical journal records (reference numbers 2017/1523, 17336/2017, 2017/1033-8, and 13/1373).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shavin Rahim, Email: shavinrah@gmail.com.
Eivor Alette Laugsand, Email: eivor.a.laugsand@ntnu.no.
Even Hovig Fyllingen, Email: even.h.fyllingen@ntnu.no.
Vidar Rao, Email: vidar.rao@stolav.no.
Rabea Iris Pantelatos, Email: rabea.i.pantelatos@ntnu.no.
Tomm Brostrup Müller, Email: tomm.brostrup.muller@stolav.no.
Anne Vik, Email: anne.vik@ntnu.no.
Toril Skandsen, Email: toril.skandsen@ntnu.no.
References
- 1.Andelic N, Sigurdardottir S, Brunborg C, Roe C. Incidence of hospital-treated traumatic brain injury in the Oslo population. Neuroepidemiology. 2008;30(2):120–128. doi: 10.1159/000120025. [DOI] [PubMed] [Google Scholar]
- 2.Maas AIR, Menon DK, Adelson PD, Andelic N, Bell MJ, Belli A, Bragge P, Brazinova A, Buki A, Chesnut RM, et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12):987–1048. doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 3.Unden J, Ingebrigtsen T, Romner B, Scandinavian Neurotrauma C. Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 2013;11:50. doi: 10.1186/1741-7015-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sollid S, Sundstrom T, Kock-Jensen C, Juul N, Eskesen V, Bellander BM, Wester K, Romner B. Scandinavian guidelines for prehospital management of severe traumatic brain injury. Tidsskr Nor Laegeforen. 2008;128(13):1524–1527. [PubMed] [Google Scholar]
- 5.Ingebrigtsen T, Romner B, Kock-Jensen C. Scandinavian guidelines for initial management of minimal, mild, and moderate head injuries. Scand Neurotrauma Committee J Trauma. 2000;48(4):760–766. doi: 10.1097/00005373-200004000-00029. [DOI] [PubMed] [Google Scholar]
- 6.Gabriel EJ, Ghajar J, Jagoda A, Pons PT, Scalea T, Walters BC. Guidelines for prehospital management of traumatic brain injury. J Neurotrauma. 2002;19(1):111–174. doi: 10.1089/089771502753460286. [DOI] [PubMed] [Google Scholar]
- 7.Gardner RC, Dams-O'Connor K, Morrissey MR, Manley GT. Geriatric traumatic brain injury: epidemiology, outcomes, knowledge gaps, and future directions. J Neurotrauma. 2018;35(7):889–906. doi: 10.1089/neu.2017.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sewalt CA, Gravesteijn BY, Menon D, Lingsma HF, Maas AIR, Stocchetti N, Venema E, Lecky FE. Primary versus early secondary referral to a specialized neurotrauma center in patients with moderate/severe traumatic brain injury: a CENTER TBI study. Scand J Trauma Resusc Emerg Med. 2021;29(1):113. doi: 10.1186/s13049-021-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norwegian National Advisory Unit on Trauma. Identifying the severely injured patient. In: National Trauma Plan. 2016.
- 10.Steyerberg EW, Wiegers E, Sewalt C, Buki A, Citerio G, De Keyser V, Ercole A, Kunzmann K, Lanyon L, Lecky F, et al. Case-mix, care pathways, and outcomes in patients with traumatic brain injury in CENTER-TBI: a European prospective, multicentre, longitudinal, cohort study. Lancet Neurol. 2019;18(10):923–934. doi: 10.1016/S1474-4422(19)30232-7. [DOI] [PubMed] [Google Scholar]
- 11.Dehli T, Gaarder T, Christensen BJ, Vinjevoll OP, Wisborg T. Implementation of a trauma system in Norway: a national survey. Acta Anaesthesiol Scand. 2015;59(3):384–391. doi: 10.1111/aas.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Organization WH: Chapter XIX Injury, poisoning and certain other consequences of external causes (S00-T98) In: International statistical classification of diseases and related health problems 10th revision—ICD-10 Version: 2019. 2020.
- 13.Bjarko VV, Skandsen T, Moen KG, Gulati S, Helseth E, Nilsen TIL, Vik A. Time of injury and relation to alcohol intoxication in moderate-to-severe traumatic brain injury: a decade-long prospective study. World Neurosurg. 2019;122:e684–e689. doi: 10.1016/j.wneu.2018.10.122. [DOI] [PubMed] [Google Scholar]
- 14.Maas AI, Stocchetti N, Bullock R. Moderate and severe traumatic brain injury in adults. Lancet Neurol. 2008;7(8):728–741. doi: 10.1016/S1474-4422(08)70164-9. [DOI] [PubMed] [Google Scholar]
- 15.Stein SC, Ross SE. Moderate head injury: a guide to initial management. J Neurosurg. 1992;77(4):562–564. doi: 10.3171/jns.1992.77.4.0562. [DOI] [PubMed] [Google Scholar]
- 16.Mena JH, Sanchez AI, Rubiano AM, Peitzman AB, Sperry JL, Gutierrez MI, Puyana JC. Effect of the modified Glasgow Coma Scale score criteria for mild traumatic brain injury on mortality prediction: comparing classic and modified Glasgow Coma Scale score model scores of 13. J Trauma. 2011;71(5):1185–92. doi: 10.1097/TA.0b013e31823321f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein SC, Spettell C. The Head Injury Severity Scale (HISS): a practical classification of closed-head injury. Brain Inj. 1995;9(5):437–444. doi: 10.3109/02699059509008203. [DOI] [PubMed] [Google Scholar]
- 18.Rundhaug NP, Moen KG, Skandsen T, Schirmer-Mikalsen K, Lund SB, Hara S, Vik A. Moderate and severe traumatic brain injury: effect of blood alcohol concentration on Glasgow Coma Scale score and relation to computed tomography findings. J Neurosurg. 2015;122(1):211–218. doi: 10.3171/2014.9.JNS14322. [DOI] [PubMed] [Google Scholar]
- 19.Olsen M, Vik A, Lund Nilsen TI, Uleberg O, Moen KG, Fredriksli O, Lien E, Finnanger TG, Skandsen T. Incidence and mortality of moderate and severe traumatic brain injury in children: a ten year population-based cohort study in Norway. Eur J Paediatr Neurol. 2019;23(3):500–506. doi: 10.1016/j.ejpn.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Department of medical biochemistry SOH. Ethanol (ethyl alcohol) in plasma and serum. In: Lab analyses and tests. 2020.
- 21.Lydersen S, Fagerland MW, Laake P. Recommended tests for association in 2×2 tables. Stat Med. 2009;28(7):1159–1175. doi: 10.1002/sim.3531. [DOI] [PubMed] [Google Scholar]
- 22.Ashkenazi I, Haspel J, Alfici R, Kessel B, Khashan T, Oren M. Effect of teleradiology upon pattern of transfer of head injured patients from a rural general hospital to a neurosurgical referral centre. Emerg Med J. 2007;24(8):550–552. doi: 10.1136/emj.2006.044461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashkenazi I, Zeina AR, Kessel B, Peleg K, Givon A, Khashan T, Dudkiewicz M, Oren M, Alfici R, Olsha O. Effect of teleradiology upon pattern of transfer of head injured patients from a rural general hospital to a neurosurgical referral centre: follow-up study. Emerg Med J. 2015;32(12):946–950. doi: 10.1136/emermed-2014-203930. [DOI] [PubMed] [Google Scholar]
- 24.Einarsen CE, van der Naalt J, Jacobs B, Follestad T, Moen KG, Vik A, Haberg AK, Skandsen T. Moderate traumatic brain injury: clinical characteristics and a prognostic model of 12-month outcome. World Neurosurg. 2018;114:e1199–e1210. doi: 10.1016/j.wneu.2018.03.176. [DOI] [PubMed] [Google Scholar]
- 25.Capron GK, Voights MB, Moore HR, 3rd, Wall DB. Not every trauma patient with a radiographic head injury requires transfer for neurosurgical evaluation: application of the brain injury guidelines to patients transferred to a level 1 trauma center. Am J Surg. 2017;214(6):1182–1185. doi: 10.1016/j.amjsurg.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Yun BJ, White BA, Benjamin Harvey H, Prabhakar AM, Sonis JD, Glover M, Vallillo E, Choi S, Borczuk P, Raja AS. Opportunity to reduce transfer of patients with mild traumatic brain injury and intracranial hemorrhage to a Level 1 trauma center. Am J Emerg Med. 2017;35(9):1281–1284. doi: 10.1016/j.ajem.2017.03.071. [DOI] [PubMed] [Google Scholar]
- 27.Joseph B, Friese RS, Sadoun M, Aziz H, Kulvatunyou N, Pandit V, Wynne J, Tang A, O'Keeffe T, Rhee P. The BIG (brain injury guidelines) project: defining the management of traumatic brain injury by acute care surgeons. J Trauma Acute Care Surg. 2014;76(4):965–969. doi: 10.1097/TA.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 28.Carney N, Totten AM, O'Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, Bratton SL, Chesnut R, Harris OA, Kissoon N, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. 2017;80(1):6–15. doi: 10.1227/NEU.0000000000001432. [DOI] [PubMed] [Google Scholar]
- 29.Moen KG, Klepstad P, Skandsen T, Fredriksli OA, Vik A. Direct transport versus interhospital transfer of patients with severe head injury in Norway. Eur J Emerg Med. 2008;15(5):249–255. doi: 10.1097/MEJ.0b013e3282f4d111. [DOI] [PubMed] [Google Scholar]
- 30.Roe C, Skandsen T, Anke A, Ader T, Vik A, Lund SB, Mannskow U, Sollid S, Sundstrom T, Hestnes M, et al. Severe traumatic brain injury in Norway: impact of age on outcome. J Rehabil Med. 2013;45(8):734–740. doi: 10.2340/16501977-1198. [DOI] [PubMed] [Google Scholar]
- 31.Skaansar O, Tverdal C, Ronning PA, Skogen K, Brommeland T, Roise O, Aarhus M, Andelic N, Helseth E. Traumatic brain injury-the effects of patient age on treatment intensity and mortality. BMC Neurol. 2020;20(1):376. doi: 10.1186/s12883-020-01943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma S, Gomez D, de Mestral C, Hsiao M, Rutka J, Nathens AB. Emergency access to neurosurgical care for patients with traumatic brain injury. J Am Coll Surg. 2014;218(1):51–57. doi: 10.1016/j.jamcollsurg.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Visca A, Faccani G, Massaro F, Bosio D, Ducati A, Cogoni M, Kraus J, Servadei F. Clinical and neuroimaging features of severely brain-injured patients treated in a neurosurgical unit compared with patients treated in peripheral non-neurosurgical hospitals. Br J Neurosurg. 2006;20(2):82–86. doi: 10.1080/02688690600682416. [DOI] [PubMed] [Google Scholar]
- 34.Munro PT, Smith RD, Parke TR. Effect of patients' age on management of acute intracranial haematoma: prospective national study. BMJ. 2002;325(7371):1001. doi: 10.1136/bmj.325.7371.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson HJ, Dikmen S, Temkin N. Prevalence of comorbidity and its association with traumatic brain injury and outcomes in older adults. Res Gerontol Nurs. 2012;5(1):17–24. doi: 10.3928/19404921-20111206-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nishijima DK, Gaona SD, Faul M, Tancredi DJ, Waechter T, Maloney R, Bair T, Blitz A, Elms AR, Farrales RD, et al. The association of trauma center transport and long-term functional outcomes in head-injured older adults transported by emergency medical services. Acad Emerg Med. 2020;27(3):207–216. doi: 10.1111/acem.13915. [DOI] [PubMed] [Google Scholar]
- 37.Herou E, Romner B, Tomasevic G. Acute traumatic brain injury: mortality in the elderly. World Neurosurg. 2015;83(6):996–1001. doi: 10.1016/j.wneu.2015.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Dang Q, Simon J, Catino J, Puente I, Habib F, Zucker L, Bukur M. More fateful than fruitful? Intracranial pressure monitoring in elderly patients with traumatic brain injury is associated with worse outcomes. J Surg Res. 2015;198(2):482–488. doi: 10.1016/j.jss.2015.03.092. [DOI] [PubMed] [Google Scholar]
- 39.Trevisi G, Sturiale CL, Scerrati A, Rustemi O, Ricciardi L, Raneri F, Tomatis A, Piazza A, Auricchio AM, Stifano V, et al. Acute subdural hematoma in the elderly: outcome analysis in a retrospective multicentric series of 213 patients. Neurosurg Focus. 2020;49(4):E21. doi: 10.3171/2020.7.FOCUS20437. [DOI] [PubMed] [Google Scholar]
- 40.Wutzler S, Lefering R, Wafaisade A, Maegele M, Lustenberger T, Walcher F, Marzi I, Laurer H, TraumaRegister DGU. Aggressive operative treatment of isolated blunt traumatic brain injury in the elderly is associated with favourable outcome. Injury. 2015;46(9):1706–1711. doi: 10.1016/j.injury.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 41.Won SY, Dubinski D, Brawanski N, Strzelczyk A, Seifert V, Freiman TM, Konczalla J. Significant increase in acute subdural hematoma in octo- and nonagenarians: surgical treatment, functional outcome, and predictors in this patient cohort. Neurosurg Focus. 2017;43(5):E10. doi: 10.3171/2017.7.FOCUS17417. [DOI] [PubMed] [Google Scholar]
- 42.Taussky P, Hidalgo ET, Landolt H, Fandino J. Age and salvageability: analysis of outcome of patients older than 65 years undergoing craniotomy for acute traumatic subdural hematoma. World Neurosurg. 2012;78(3–4):306–311. doi: 10.1016/j.wneu.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 43.Kirkman MA, Jenks T, Bouamra O, Edwards A, Yates D, Wilson MH. Increased mortality associated with cerebral contusions following trauma in the elderly: bad patients or bad management? J Neurotrauma. 2013;30(16):1385–1390. doi: 10.1089/neu.2013.2881. [DOI] [PubMed] [Google Scholar]
- 44.Hwang F, Pentakota SR, Glass NE, Berlin A, Livingston DH, Mosenthal AC. Older patients with severe traumatic brain injury: national variability in palliative care. J Surg Res. 2020;246:224–230. doi: 10.1016/j.jss.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Robertsen A, Forde R, Skaga NO, Helseth E. Treatment-limiting decisions in patients with severe traumatic brain injury in a Norwegian regional trauma center. Scand J Trauma Resusc Emerg Med. 2017;25(1):44. doi: 10.1186/s13049-017-0385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unterhofer C, Hartmann S, Freyschlag CF, Thome C, Ortler M. Severe head injury in very old patients: to treat or not to treat? Results of an online questionnaire for neurosurgeons. Neurosurg Rev. 2018;41(1):183–187. doi: 10.1007/s10143-017-0833-0. [DOI] [PubMed] [Google Scholar]
- 47.Unterhofer C, Ho WM, Wittlinger K, Thome C, Ortler M. "I am not afraid of death"—a survey on preferences concerning neurosurgical interventions among patients over 75 years. Acta Neurochir (Wien) 2017;159(8):1547–1552. doi: 10.1007/s00701-017-3240-y. [DOI] [PubMed] [Google Scholar]
- 48.Galimberti S, Graziano F, Maas AIR, Isernia G, Lecky F, Jain S, Sun X, Gardner RC, Taylor SR, Markowitz AJ, et al. Effect of frailty on 6-month outcome after traumatic brain injury: a multicentre cohort study with external validation. Lancet Neurol. 2022;21(2):153–162. doi: 10.1016/S1474-4422(21)00374-4. [DOI] [PubMed] [Google Scholar]
- 49.Newcombe VFJ, Chow A. The features of the typical traumatic brain injury patient in the ICU are changing: what will this mean for the intensivist? Curr Opin Crit Care. 2021;27(2):80–86. doi: 10.1097/MCC.0000000000000814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1 Patients with moderate or severe TBI in the GHs of central Norway 01.10.2004–01.10.2014. Table S2 Preinjury functional disability and antithrombotic medication usage in the GH group versus the RTC group.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.